-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
Stereotaktická radioterapie jaterních metastáz kolorektálního karcinomu; časné výsledky
Východiska:
Extrakraniální stereotaktické radioterapie (SBRT) je dobře proveditelná a účinná metoda léčby jaterních metastáz kolorektálního karcinomu.Materiál a metody:
Od září 2009 do prosince 2011 bylo extrakraniální stereotaktickou radioterapií pomocí lineárního urychlovače Varian Clinac iX léčeno 11 pacientů s 15 inoperabilními jaterními metastázami. Jednalo se o 6 mužů a 5 žen ve věku od 51 do 81 let (medián 68 let). Použité dávky záření v rozmezí od 40 do 56 Gy (medián 54 Gy) byly aplikovány ve třech až osmi frakcích.Výsledky:
Lokální kontrola ve 2, 4, 6, 9 a 12 měsících od ukončení SBRT byla 100 %, 91 %, 91 %, 67 % a 50 %. Bez progrese onemocnění přežívalo ve 2, 4, 6, 9 a 12 měsících 82 %, 82 %, 64 %, 50 % a 50 % pacientů. Medián sledování byl 15 měsíců. Žádné závažné nežádoucí účinky léčby nebyly pozorovány.Závěr:
Naše studie hodnotila proveditelnost SBRT ve vybraném souboru pacientů s 1–3 jaterními metastázami kolorektálního karcinomu neřešitelnými jinými metodami léčby. Dosáhli jsme vynikající lokální kontroly za současných velmi mírných akutních i pozdních nežádoucích účinků léčby. Nejčastější příčinou relapsu onemocnění po provedené SBRT se staly vzdálené metastázy. SBRT vykazuje vynikající lokální kontrolu a umožňuje u vybraných pacientů dlouhodobé přežití bez vážných nežádoucích účinků léčby.Klíčová slova:
kolorektální karcinom – metastázy – játra – radioterapie – extrakraniální stereotaktická terapie
Authors: P. Burkoň 1; P. Slampa 1,3; T. Kazda 1; M. Slavik 1; T. Prochazka 2; M. Vrzal 2
Authors place of work: Department of Radiation Oncology, Masaryk Memorial Cancer Institute, Brno and Faculty of medicine, Masaryk University, Brno, Czech Republic 1; Department of Radiological Physics, Masaryk Memorial Cancer Institute, Brno, Czech Republic 2; Regional Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 3
Published in the journal: Klin Onkol 2012; 25(Supplementum 2): 93-97
Práce byla podpořena Evropským fondem pro regionální rozvoj a státním rozpočtem České republiky (OP VaVpI – RECAMO, CZ.1.05/2.1.00/03.0101).
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 23. 9. 2012
Přijato: 29. 10. 2012Summary
Background:
Stereotactic body radiation therapy (SBRT) is well feasible and effective method for treatment of colorectal cancer liver metastases.Materials and Methods:
From September 2009 to December 2011, 11 patients with 15 inoperable liver metastases of colorectal cancer were treated by SBRT using Varian Clinac iX linear accelerator. We treated 6 men and 5 women of age from 51 to 81 years (median 68). SBRT doses ranged from 40 to 56 Gy (median 54 Gy) and were administered in 3 to 8 fractions.Results:
Local control rates at 2, 4, 6, 9 and 12 months after completion of SBRT were 100%, 91%, 91%, 67% and 50%, respectively. Disease progression-free survival rates at 2, 4, 6, 9 and 12 months were 82%, 82%, 64%, 50% and 50%, respectively. Median follow-up was 15 months. No severe side effects were attributed to the therapy. Conclusion:
Our study assessed the feasibility of SBRT in selected group of patients with 1 to 3 colorectal cancer liver metastases with no other treatment option. We achieved excellent local control and very moderate acute and late side effects. Distant metastases were the most common recurrence form after SBRT. SBRT demonstrated excellent local control and resulted in occasional long-term survivors without any serious side effects of therapy.Key words:
colorectal neoplasms – neoplasm metastasis – liver – radiation therapy – stereotactic body radiotherapyIntroduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in Europe, with an annual incidence of 400.000 cases and an annual mortality of more than 200.000 patients [1]. The incidence in the Czech Republic is one of the highest worldwide. The most frequent site of metastases of CRC is the liver. Almost 70% of CRC patients develop liver metastases during the course of disease [2]. However, although the median survival of patients with untreated disease ranges from 6 to 12 months, the addition of an optimal chemotherapy regimen improves median survival only to 20 months. Further improvement is achieved by implementation of molecular targeted therapy into the treatment [3–6].
In most cases local palliative treatment leads to both local tumour control and survival improvement. Surgical data show that local treatment of liver tumours – especially hepatocellular carcinoma and liver metastases – might be curative in up to 25–30% of patients if patient selection is appropriate [7]. Nevertheless a significant proportion of patients are not suitable for surgery because of age, medical comorbidities, or unfavourable intrahepatic localisation of the tumour (bilobar, adjacent to large vessels/portal structures). For these cases, SBRT might be a good treatment option [8].
Stereotactic Body Radiation Therapy (SBRT) is one of the most advanced methods of radiation treatment characterised by limited number of fractions using highly focused ablative beams for treating cancer lesions outside of the skull. Recent results of clinical studies using SBRT in the treatment of liver metastases are emerging. Phase I and II studies have demonstrated excellent local control and occasional long-term survivors. These studies showed actuarial local control rates of at least 80% after 2 years (see Tab. 1) [9–16].
Tab. 1. SBRT of colorectal liver metastases, recent publications. 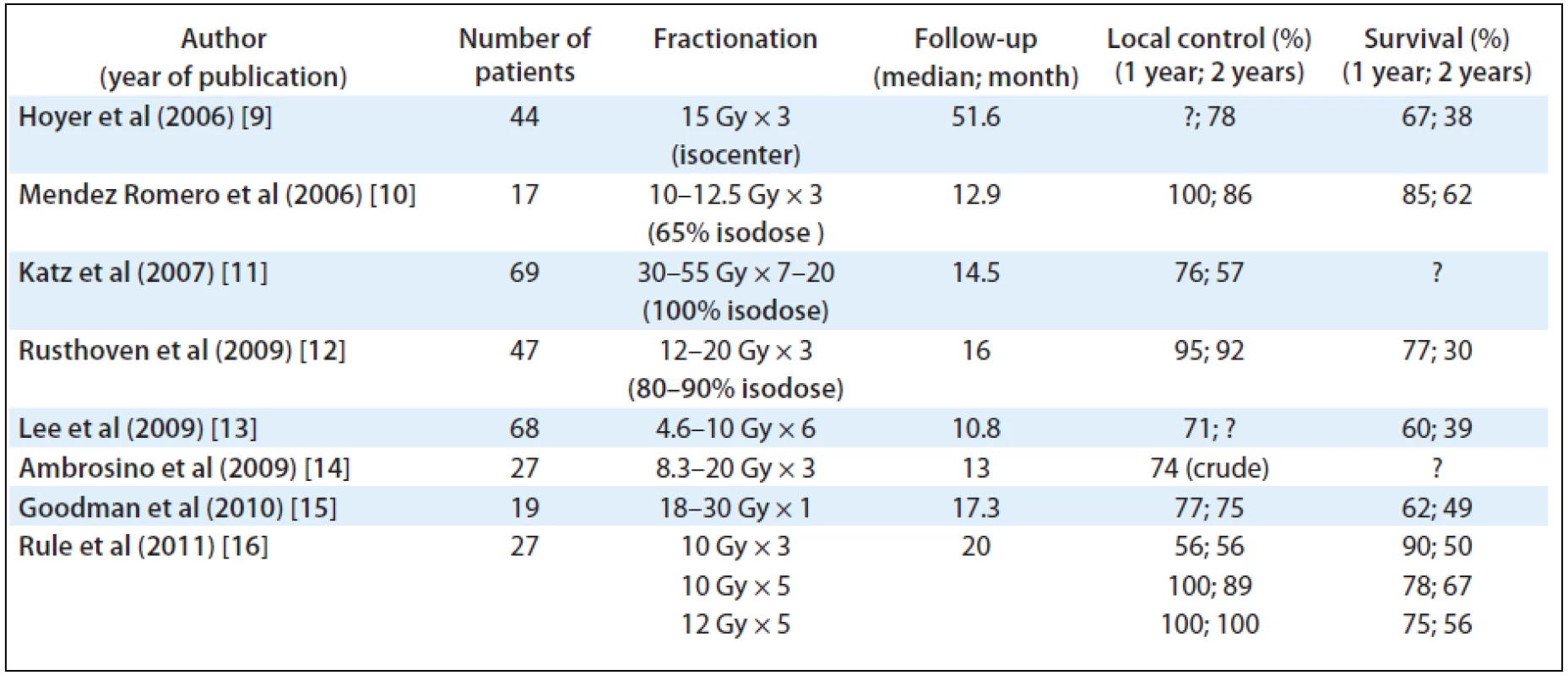
SBRT is a non-invasive short time treatment method, with no need of hospitalisation whereby meets the criteria of high-quality palliative care of patients with metastatic disease. Because of its’ precision, SBRT delivers a higher dose to the tumour and causes less damage to surrounding normal tissues. Reported acute toxicity is moderate. Clinically relevant subacute or late toxicities are not reported, if organs at risk (OAR) are kept out of the high dose region. Radiation-induced liver disease (RILD) is rare after SBRT of liver metastases, while fibrosis of those portions of the liver included in the high dose volume is common, with subsequent compensatory hypertrophy of liver tissue spared from radiation. When dose limits to the adjacent organs are exceeded (oesophagus, stomach, duodenum or large bowel) possible late side effects might appear (e.g. gastrointestinal bleeding, small bowel obstruction, gastric outlet obstruction or fistula formation) [9,17]. The most common toxicities found in the literature are listed in Tab. 2.
Tab. 2. Possible acute and late toxicity listed in the literature. 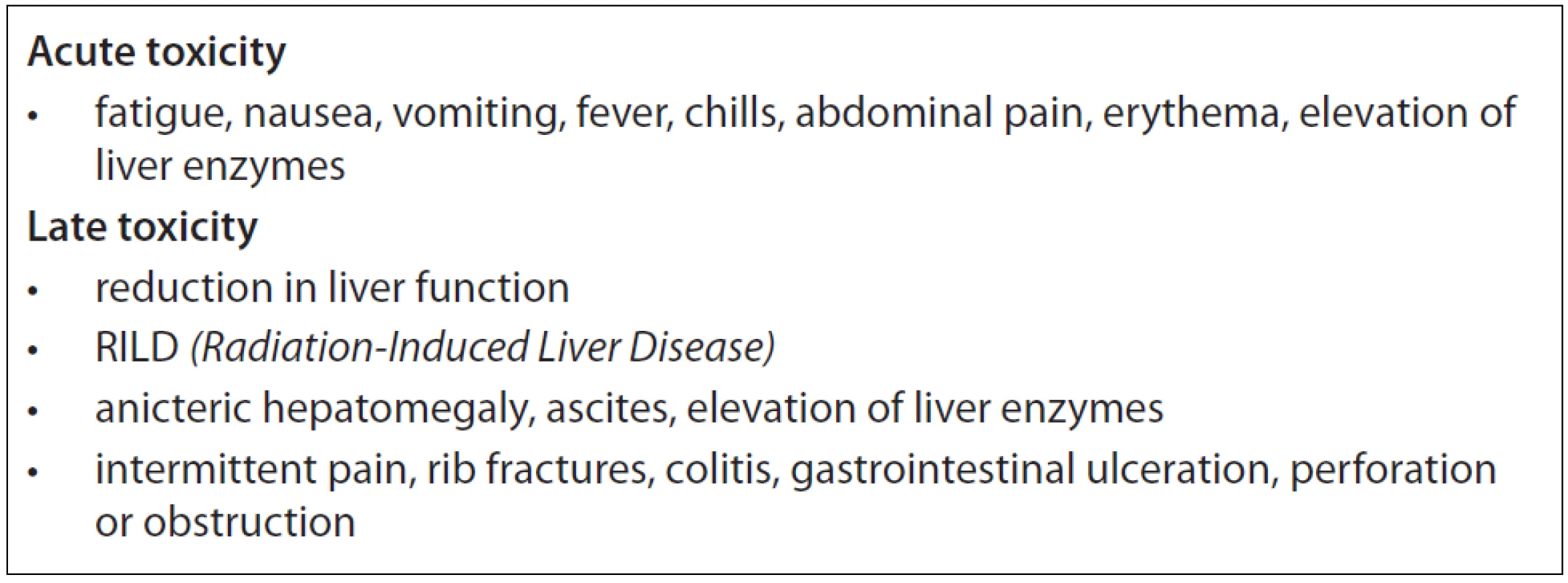
In our present work, we focused on the feasibility and efficacy of SBRT in the treatment of colorectal cancer liver oligometastases. Even though the number of patients included is relatively small, we attempted to determine local control rate, disease free survival and failure patterns after SBRT retrospectively.
Materials and Methods
Patients’ Characteristics
From September 2009 to December 2011, 11 patients with 15 liver metastases of colorectal cancer were treated with SBRT using the linear accelerator Varian Clinac iX (Varian Systems, USA) at our institution. Patient eligibility criteria for SBRT for liver metastases of colorectal cancer were as follows:
- inoperable disease or refusal of surgery
- progression after chemotherapy +/ – targeted therapy
- another local treatment modality (such as radiofrequency ablation) not indicated
- fewer than four hepatic lesions, tumour not attached or close (< 3 mm) to the oesophagus, stomach or duodenum
- good performance status (Karnofsky index > 70, life expectancy > 6 months, Child-Pugh score class A)
- no extra-hepatic active lesion detected by positron emission tomography (PET) or PET/CT
Tab. 3 summarises patient and tumour characteristics. We treated 6 men and 5 women of age from 51 to 81 years (median 68 years). Primary tumours were located in the colon in 7 cases and the rectum in 4 cases. All patients had a localised primary tumour at the time of diagnosis, all of them suffered from new dissemination after adjuvant therapy. Chemotherapy regimen combined with targeted therapy if indicated has been administrated for all of patients. Ethical permission was granted after review at the Masaryk Memorial Cancer Institute and all patients gave written consent.
Tab. 3. Patients and treatment characteristics, response and survival. 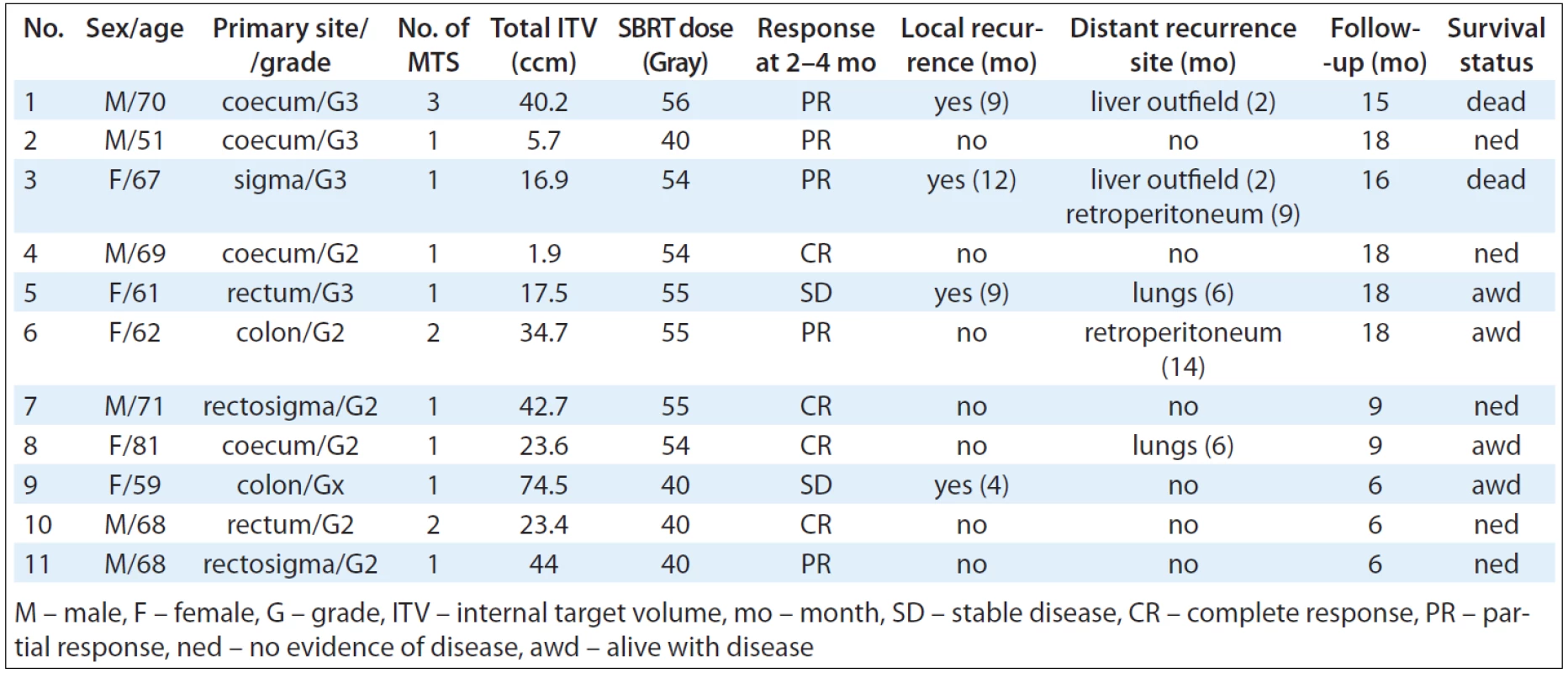
SBRT
Safe daily SBRT treatment was achieved by ensuring reliable and reproducible immobilisation, accurate planning and treatment correlation, pre-treatment quality assurance using daily imaging and management of tumour and organ intrafraction motion in each treatment session. To minimise rotational shifts, the stereotactic body frame was used for patient fixation (Elekta stereotactic body frame). Abdominal compression served to reduce diaphragmatic expansion during breathing cycle [18]. Treatment plans were created using Eclipse planning system (Varian, v.8.6) with AAA algorithm and delivered by Rapid Arc technology (see Fig. 1). Daily pre-treatment imagings by cone-beam computed tomography (CBCT) on-board imagine system of our linear accelerator [19]. To ensure patient safety each plan was verified using gamma analysis.
Fig. 1. 62-year old female with 2 liver metastases; dose distribution by Rapid Arc technology. Fractionation and dose – given in Grays. 
To capture tumour movements during breathing cycle a four-dimensional CT (4DCT) scanning was used. This technology has been recently introduced to correlate CT image acquisition with the breathing cycle, allowing better analysis of variables that affect respiratory motion [20,21].
CT scans of 2–3 mm slices including target respiratory movements were performed and sent to the planning system. The data from MRI and FDG PET helped us to identify target volume. No gold fiducials were used.
We used an internal target volume (ITV) concept for target volume definition. Gross tumour volume (GTV) was outlined as a tumour visible in CT or CT/MRI fusion without any margins. We contoured an inspiration GTV100, expiration GTV0 and mid GTV50 of normal breathing cycle. Then we created an ITV encompassing all these GTVs. Clinical target volume (CTV) was created volumetrically by 2 mm expansion from ITV to take a subclinical tumour spread into account. Planning target volume (PTV), which includes set-up and internal margin errors was outlined automatically with another 5 mm margin in all dimensions [8]. Where a PTV and OAR are close or even overlap, a responsible clinical decision about relative risks of tumour relapse or normal tissue damage had to be made.
For dose calculation we used a “risk adaptive concept” – dose was reduced during treatment planning when normal tissue constraints consideration contraindicated the use of primarily prescribed dose. According to this concept, total SBRT doses ranged from 40 to 56 Gy (median 54 Gy) and were delivered in 3 to 8 fractions. Six patients from our group had “ablative dose” of radiation, i.e. biological equivalent dose (BED) > 105 Gy and in 5 patients the dose had to be reduced, i.e. a BED dose was < 105 Gy [22].
Adequate target coverage was achieved when 98–100% of planning target volume was covered by 95% of prescribed dose while the mean dose was 100% of prescribed dose. Dose gradient was also controlled and the treatment plans should meet a number of organs at risk dose constraints. Tab. 3 summarises ITV and SBRT dosage details.
Responses and Toxicity Assessment
Patients were followed up and tumour response assessment performed by computed tomography at 2, 4, 6, 9, 12, 15, 18 and 24 months after SBRT (when possible, we performed PET/CT scan at 6, 12, 18 and 24 months after SBRT; see Fig. 2). Local progression was defined as a new tumour lesion in the irradiated area or an increase in tumour size of more than 20%. Disease progression free survival was defined as a time from SBRT treatment termination to date of local progression, regional progression (new lesion in the liver outside the irradiated area) or distant metastasis (progression outside the liver tissue). Acute and late toxicities were sorted by the time of 3 months after treatment completion. RTOG Toxicity Criteria was used to evaluate acute and late side effects of the treatment.
Fig. 2. 62-year old female with 2 liver metastases; both metastases shrinkage and no PET/CT activity after 12 months. mo – months, size of metastases – given in mm 
Results
Response to SBRT was evaluated using CT scans performed between 2 and 4 months after SBRT completion. The number of patients with complete regression was 4 (36%), there were 5 patients (45%) with partial response and 2 patients (18%) showed stable disease. During follow-up, 6 patients (55%) experienced local recurrence, distant metastases, or both. Both local and distant failure occurred in 3 patients (27%), distant failure alone in 2 patients (18%) and local failure alone occurred in 1 patient (9%). Distant metastasis was the first form of recurrence. Patients underwent additional salvage or palliative treatments according to the failure pattern after recurrence.
Local control rates at 2, 4, 6, 9 and 12 months after completion of SBRT were 100%, 91%, 91%, 6% and 50%, respectively. Disease progression-free survival rates at 2, 4, 6, 9 and 12 months were 82%, 82%, 64%, 50% and 50%, respectively and median follow-up was 15 months. Three patients of the total number of six who were followed up more than 12 months, were alive with no evidence of disease. There were 2 deaths according to the distant progression after 15 and 16 months after SBRT completion.
Acute grade 1 toxicity occurred in 4 of the 11 patients (mainly nausea, fatigue, fever), and there were no grade 3 or 4 acute side effects. No radiation-induced liver disease (RILD) was observed, 2 patients suffered from temporary intercostals nerve irritation, 1 from asymptomatic ascites. No other late toxicities were observed.
Conclusion
High precision focused ablative hypofractionated radiation therapy is a very effective and safe palliative treatment modality suitable for patients presenting one to three liver metastases of CRC. Due to its good common tolerability and minimal toxicity, SBRT is a suitable option for patients who cannot undergo surgery for any reason. This method showed excellent local control and also occasional long-term survivors without any serious side effects of therapy. Indeed, the optimal doses, fractionation schemes and the indications have to be established in the future by numerous prospective trials.
The work was supported by the European Regional Development Fund and the State Budget of the Czech Republic (RECAMO, CZ.1.05/2.1.00/03.0101).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Petr Burkon, M.D., Ph.D.
Department of radiation oncology
Masaryk Memorial Cancer Institute
Zluty kopec 7
656 53 Brno
Czech Republic
e-mail: burkon@mou.cz
Submitted: 23. 9. 2012
Accepted: 29. 10. 2012
Zdroje
1. Ferlay J, Autier P, Boniol M et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007; 18(3): 581–592.
2. Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer 2002; 38(7): 1023–1033.
3. Saltz LB, Clarke S, Díaz-Rubio E et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26(12): 2013–2019.
4. Saltz LB. Metastatic colorectal cancer: is there one standard approach? Oncology (Williston Park) 2005; 19(9): 1147–1160.
5. Fong Y, Fortner J, Sun RL et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230(3): 309–321.
6. Cunningham D, Pyrhonen S, James RD et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 1998; 352(9138): 1413–1418.
7. Lehnert T, Golling M. Indikationen und Ergebnisse der Lebermetastasenresektion. Radiologe 2001; 41(1): 40–48.
8. Nagata Y, Wulf J, Lax I et al. Stereotactic Radiotherapy of Primary Lung Cancer and Other Targets: Results of Consultant Meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2011; 79(3): 660–669.
9. Hoyer M, Roed H, Traberg HA et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006; 45(7): 823–830.
10. Mendez Romero A, Wunderink W, Hussain SM et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase I-II study. Acta Oncol 2006; 45(7): 831–837.
11. Katz AW, Carey-Sampson M, Muhs AG et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys 2007; 67(3): 793–798.
12. Rusthoven KE, Kavanagh BD, Cardenes H et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009; 27(10): 1572–1578.
13. Lee MT, Kim JJ, Dinniwell R et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009; 27(10): 1585–1591.
14. Ambrosino G, Polistina F, Constantin G et al. Image-guided robotic stereotactic radiosurgery for unresectable liver metastases: preliminary results. Anticancer Res 2009; 29(8): 3381–3384.
15. Goodman KA, Wiegner EA, Maturen KE et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignances. Int J radiat Oncol Biol Phys 2010; 78(2): 486–493.
16. Rule W, Timmerman R, Tong L et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011; 18(4): 1081–1087.
17. Herfarth KK, Debus J, Lohr F et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001; 19(1): 164–170.
18. Nagata Y, Takayama K, Matsuo Y et al. Clinical outcomes of a phase I/II of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005; 63(5): 1427–1431.
19. Ong CL, Verbakel WF, Cuijpers JP et al. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010; 97(3): 437–442.
20. Heinzerling JH, Anderson JF, Papiez L et al. Four-Dimensional Computed Tomography Scan Analysis of Tumor and Organ Motion at Varying Levels of Abdominal Compression During Stereotactic Treatment of Lung and Liver. Int J radiat Oncol Biol Phys 2008; 70(5): 1571–1578.
21. Purdie TG, Bissonnette JP, Franks K et al. Cone-beam Computed Tomography for On-line Image Guidance of Lung Stereotactic Radiotherapy: Localization, Verification, and Intrafraction Tumor Position. Int J Radiat Oncol Biol Phys 2007; 68(1): 243–252.
22. Onishi H, Shirato H, Nagata Y et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2010; 81(5): 1352–1358.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2012 Číslo Supplementum 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Hojení análních fisur urychlí čípky a gel
-
Všechny články tohoto čísla
- p63 – an Important Player in Epidermal and Tumour Development
- Detection of Cancer Stem Cell Markers in Sarcomas
- NKT-like Cells are Expanded in Solid Tumour Patients
- Cancer as a Metabolic Disease and Diabetes as a Cancer Risk?
- PThe Regulation of p53 Synthesis
- Protein Quality Control and Cancerogenesis
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- The Role of Platelets in Tumour Growth
- Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
- A Combined Immunoprecipitation and Mass Spectrometric Approach to Determine ΔNp63-Interacting Partners
- Identification and Characterisation of Pro-metastatic Targets, Pathways and Molecular Complexes Using a Toolbox of Proteomic Technologies
- The Biobanking Research Infrastructure BBMRI_CZ: a Critical Tool to Enhance Translational Cancer Research
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- Development and Use of Non-FDG PET Radiopharmaceuticals at the Masaryk Memorial Cancer Institute
- New Mechanisms for an Old Drug; DHFR- and non-DHFR-mediated Effects of Methotrexate in Cancer Cells
- Stereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
- Phase I Trial in Oncology – Theory and Practice
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- p63 – an Important Player in Epidermal and Tumour Development
- NKT-like Cells are Expanded in Solid Tumour Patients
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- Phase I Trial in Oncology – Theory and Practice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



