-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
The ubiquitous mold A. fumigatus is isolated in over 80% of all patients with invasive aspergillosis (IA). A. nidulans is a relatively non-pathogenic species that rarely causes IA except in patients with chronic granulomatous disease (CGD), a hereditary disease characterized by impaired neutrophil function due to mutations in the NADPH oxidase complex. Here, we demonstrate that one factor underlying the differences in the intrinsic virulence between A. fumigatus and A. nidulans is the amount of the exopolysaccharide galactosaminogalactan that is associated with the cell wall of these species. A. fumigatus produces higher amounts of cell wall-associated galactosaminogalactan and is more resistant than A. nidulans to neutrophil killing by NADPH-oxidase dependent extracellular traps (NETs). Increasing cell wall-associated galactosaminogalactan in A. nidulans enhanced resistance to NETs and increased the virulence of this species to the same level as A. fumigatus in mice with intact NET formation. Collectively, these data suggest that A. nidulans is more sensitive than A. fumigatus to NADPH-oxidase dependent NETosis due to lower levels of cell wall-associated GAG.
Published in the journal: . PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005187
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005187Summary
The ubiquitous mold A. fumigatus is isolated in over 80% of all patients with invasive aspergillosis (IA). A. nidulans is a relatively non-pathogenic species that rarely causes IA except in patients with chronic granulomatous disease (CGD), a hereditary disease characterized by impaired neutrophil function due to mutations in the NADPH oxidase complex. Here, we demonstrate that one factor underlying the differences in the intrinsic virulence between A. fumigatus and A. nidulans is the amount of the exopolysaccharide galactosaminogalactan that is associated with the cell wall of these species. A. fumigatus produces higher amounts of cell wall-associated galactosaminogalactan and is more resistant than A. nidulans to neutrophil killing by NADPH-oxidase dependent extracellular traps (NETs). Increasing cell wall-associated galactosaminogalactan in A. nidulans enhanced resistance to NETs and increased the virulence of this species to the same level as A. fumigatus in mice with intact NET formation. Collectively, these data suggest that A. nidulans is more sensitive than A. fumigatus to NADPH-oxidase dependent NETosis due to lower levels of cell wall-associated GAG.
Introduction
Invasive aspergillosis (IA) is the most common invasive mold infection in humans. In immunocompromised patients, the inhalation of airborne spores of Aspergillus species leads to a necrotizing fungal pneumonia that can disseminate hematogenously to the brain and other organs [1]. Although the genus Aspergillus is comprised of over 250 members, Aspergillus fumigatus is responsible for more than 80% of invasive aspergillosis cases [2]. On the other hand, Aspergillus nidulans is a model, non-pathogenic organism used extensively for the study of eukaryotic cell biology. Interestingly, A. nidulans is rarely a cause of invasive disease, except in patients with chronic granulomatous disease (CGD), a genetic disorder of the NADPH oxidase system that results in impaired production of reactive oxygen species by phagocytes [3–6]. The predominance of A. fumigatus as a cause of invasive disease in patients without CGD is not reflected in air or environmental sampling studies in which A. fumigatus accounts for only a minority of the total Aspergillus species recovered [7]. These observations suggest that A. fumigatus possesses unique virulence traits that enhance its ability to cause human infection. Disruption of a number of putative virulence factors of A. fumigatus results in attenuated virulence of this species (reviewed in [7,8]). However, none of these factors have been demonstrated to confer increased virulence on less pathogenic Aspergillus species such as A. nidulans. The identification of this type of transferable virulence factor would improve our understanding of the pathogenesis of IA, and could potentially guide the development of novel therapeutic strategies.
Recently, we and others have reported that the secreted and cell wall exopolysaccharide galactosaminogalactan (GAG) is required for normal virulence of A. fumigatus [9,10]. GAG is an α-1,4-linked linear heteroglycan composed of a variable combination of galactose and N-acetyl-galactosamine (GalNAc). Although the pathways governing GAG synthesis are not fully understood, two UDP-glucose 4-epimerases, Uge5 and Uge3, are required for GAG production. Uge5 mediates the conversion of UDP-glucose to UDP-galactose, while Uge3 is a bifunctional epimerase that can mediate both the interconversion of UDP-glucose to UDP-galactose and of UDP-N-acetylglucosamine to UDP-GalNAc [11]. Deletion of uge5 results in the production of GAG with a lower galactose content, while deletion of uge3 completely abrogates GAG synthesis [10,11]. GAG plays a number of roles in host-pathogen interactions [9,10]. This glycan mediates adherence to a variety of substrates, including host cells, and is required for normal biofilm formation [12]. Also, GAG covers the surface of hyphae to conceal β-1,3-glucans from recognition by the pattern recognition receptor dectin-1 leading to decreased pulmonary inflammation [10]. Purified, soluble GAG also induces natural killer (NK) cell-mediated apoptosis of neutrophils in vitro [13], and administration of soluble GAG is immunosuppressive through the induction of IL-1RA production [14]. Consistent with the pathogenic function of GAG, a GAG-deficient A. fumigatus mutant was found to have attenuated virulence in a mouse model of invasive aspergillosis [10]. Synthesis of linear α-1,4-linked GAG has been reported in other Aspergillus species, including A. parasiticus [15], A. niger [16], and A. nidulans [17,18], although the quantity of GAG from these species has not been compared. In light of the important role that GAG plays in the virulence of A. fumigatus, we investigated whether differences in GAG production or composition might contribute to the spectrum of virulence observed among Aspergillus species.
Results
A. fumigatus GAG contains higher levels of GalNAc than GAG produced by other Aspergillus species
To determine if different species of Aspergillus produce different levels of secreted GAG, we investigated two medically relevant species, A. fumigatus as well as A. flavus, which is the second most common Aspergillus isolate in IA patients [19]. We also studied the less pathogenic A. niger and A. nidulans. When these organisms were grown under GAG-inducing conditions, all species secreted similar amounts of GAG into the medium, as recovered by ethanol precipitation (S1A Fig). However, scanning electron microscopy (SEM) of hyphae from each of these Aspergillus species demonstrated dramatic differences in the amount of GAG-associated decorations on the cell wall of hyphae (Fig 1A) [10]. A. fumigatus displayed abundant cell wall-bound decorations, while the other species more closely resembled the previously described A. fumigatus ΔstuA mutant, which produces very low levels of GAG (Fig 1A) [10]. To confirm that the alterations in cell wall morphology reflected changes in the amount of cell wall-associated GAG, the amount of cell wall-bound GAG was examined by staining hyphae of each of the Aspergillus species with the GalNAc specific lectin, soybean agglutinin (SBA) [10,11,20,21]. Staining of cell wall-bound GalNAc by SBA was strongest with A. fumigatus, followed by A. flavus, while A. niger and A. nidulans exhibited minimal SBA binding (Fig 1B). Importantly, similar levels of hyphal cell wall SBA staining were observed with the commonly used A. nidulans laboratory strain A26 and three other clinical isolates of this species (S1B and S1C Fig),. Interestingly, the amount of GalNAc-rich GAG and cell wall decorations produced by these species paralleled their frequency of recovery from patients with invasive aspergillosis. Collectively, these results suggest that Aspergillus species exhibit significant differences in the amount of cell wall-associated GAG. Moreover, these differences correlate with the reported intrinsic virulence of these species. In light of these findings, A. fumigatus and A. nidulans A26 were selected for further study as representatives of highly pathogenic and minimally pathogenic species of Aspergillus species that display significant differences in cell wall-bound GAG.
Fig. 1. Production of GalNAc-rich GAG correlates with reported virulence of Aspergillus spp. 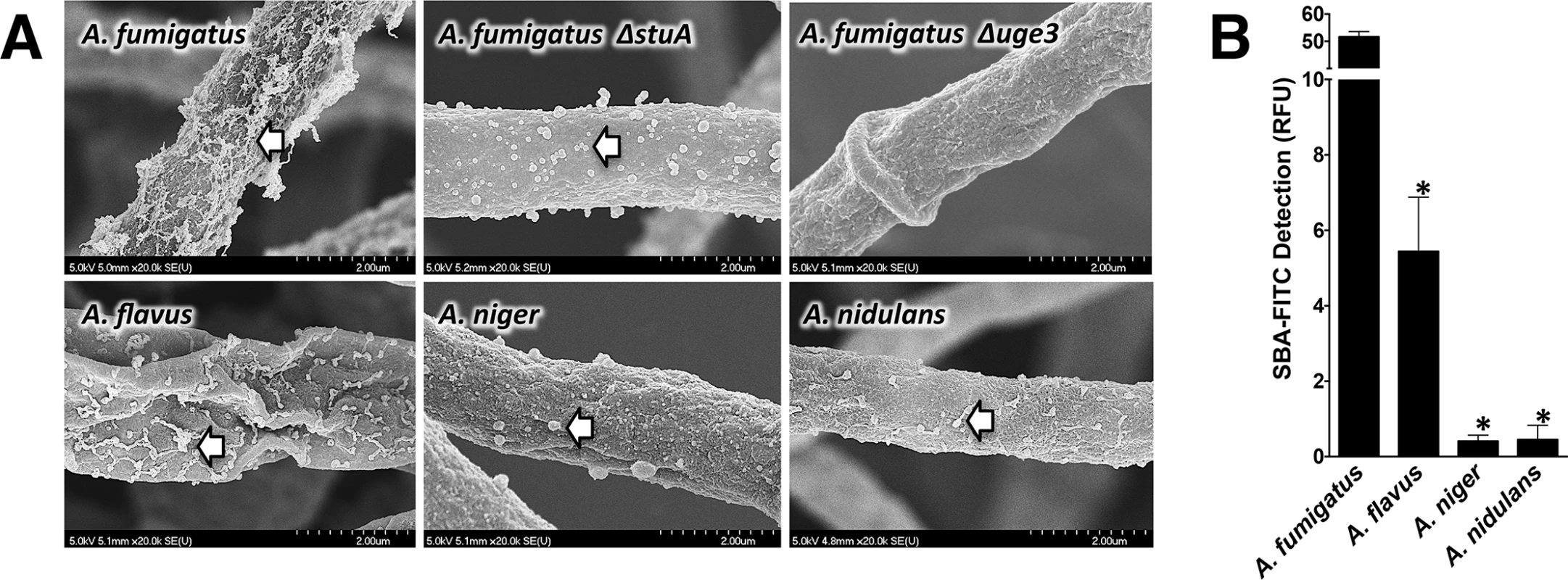
(A) Scanning electron micrograph of hyphae of indicated species at 20,000X magnification. Arrows indicate surface decorations associated with cell wall-bound GAG. The GAG deficient A. fumigatus Δuge3 mutant [10] and the A. fumigatus ΔstuA mutant [49] which produces only minimal amounts of GAG are included for comparison purposes. (B) Cell wall GalNAc staining with FITC-conjugated soybean agglutinin (SBA). SBA binding to mature hyphal mats of the indicated species was quantified by fluorometry. Data are represented as mean +/- SEM. * indicates a significant difference between A. fumigatus and other species, p<0.05 by ANOVA and pairwise comparison. A. nidulans produces GalNAc-poor GAG and has impaired biofilm formation as compared with A. fumigatus
To test if the degree of cell wall-bound GAG could reflect differences in the composition of GAG produced by A. fumigatus and A. nidulans, monosaccharide analysis of secreted GAG from both species was performed by gas chromatography. Secreted GAG produced by A. nidulans contained significantly less GalNAc and more galactose as compared with A. fumigatus GAG (Fig 2A).
Fig. 2. nidulans produces GalNAc-poor GAG which is associated with non-adherence. 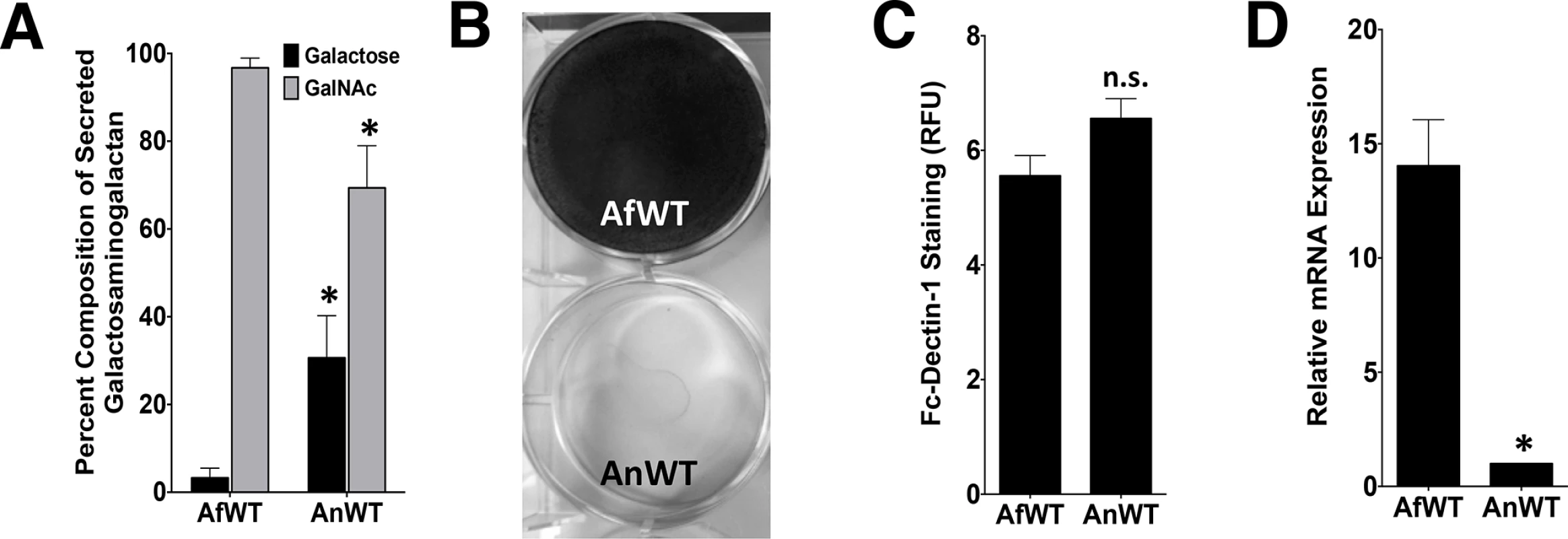
A. (A) Galactose and GalNAc content of secreted GAG from either A. fumigatus or A. nidulans as identified by gas chromatography and quantified by hexose or hexosamine assays. (B) Formation of adherent biofilms on tissue culture-treated polystyrene plates by A. fumigatus and A. nidulans. After 24 hours growth, biofilms were washed and visualized by staining with 0.1% crystal violet. (C) Detection of β-1,3-glucan exposure on the surface of hyphae by immunostaining with Fc-dectin-1 antibody by fluorometry. (D) Relative expression of ugeB in A. nidulans and uge3 in A. fumigatus, during growth in Brian medium as measured by real-time RT-PCR. Expression of tef1 from each respective species was used as an internal reference gene. Primer efficiency was verified, and was not different between species (S2E Fig). For all panels: data are represented as mean +/- SEM. AfWT indicates A. fumigatus, and AnWT indicates A. nidulans. * indicates a significant difference between A. fumigatus and A. nidulans, p<0.05 by Student t test or ANOVA with Tukey’s test for pairwise comparison, where applicable. To test if the production of lower GalNAc-containing GAG by A. nidulans could affect the known virulence-associated properties of GAG, A. nidulans was compared with A. fumigatus with respect to its ability to form adherent biofilm and to mask hyphal β-glucan exposure. A. nidulans A26 and three clinical isolates of A. nidulans formed less adherent biofilms (Figs 2B and S1) as compared with A. fumigatus. In contrast, β-glucan binding by recombinant Fc-dectin-1 was not different between these two species (Fig 2C). These observations suggest that the quantity of cell wall bound GAG as well as the ability of GAG to mediate biofilm formation are dependent on the GalNAc content of GAG. In contrast, β-glucan masking appears to be GalNAc-independent, or at least require a lower amount of this hexosamine.
A. nidulans produces GAG with a lower GalNAc content due to low levels of expression of the glucose epimerase UgeB
The synthesis of the GalNAc component of GAG in A. fumigatus results from the activity of the UDP-glucose 4-epimerase, Uge3 [10,11]. A search of the A. nidulans genome identified ugeB as the gene whose product has the closest homology to A. fumigatus Uge3 (85% amino acid identity). As with the A. fumigatus uge3 gene [10], deletion of ugeB in A. nidulans resulted in a strain whose hyphae lacked detectable GalNAc by SBA staining (S2A Fig), confirming that Uge3 and UgeB both mediate GalNAc synthesis. Consistent with a previous report that ugeB expression is extremely low in A. nidulans [22], real-time RT-PCR demonstrated that the expression of ugeB in A. nidulans was significantly lower than the expression of uge3 in A. fumigatus (Fig 2D), suggesting that the lower GalNAc content of GAG produced by A. nidulans may be due to the lower expression levels of ugeB. As expected, expression of uge3 was not detected in A. nidulans (S2B Fig).
Overexpression of uge3 or ugeB in A. nidulans increases the GalNAc content and cell wall binding of GAG, as well as enhances biofilm formation
To test the hypothesis that the low GalNAc content of A. nidulans GAG results from low expression of ugeB, strains of A. nidulans were constructed in which the A. fumigatus uge3 or A. nidulans ugeB genes were expressed under the constitutively active gpdA promoter to produce strains An-Uge3 and An-UgeB, respectively (Fig 3A). Overexpression of either uge3 or ugeB had no significant effect on total secreted GAG production (Fig 3B). However, increased expression of either gene resulted in an increase in the GalNAc content of secreted GAG (Fig 3C) to levels similar to that found in A. fumigatus. Even more dramatically, overexpression of either gene markedly increased the amount of cell wall-bound GAG as detected by SBA lectin binding (Fig 3D) and SEM to levels indistinguishable from A. fumigatus (Fig 3E). These data suggest that the GalNAc content of GAG is important in determining the amount of GAG that binds to the hyphal cell wall.
Fig. 3. Overexpression of uge3 or ugeB in A. nidulans increases the GalNAc content of GAG and enhances the formation of adherent biofilms. 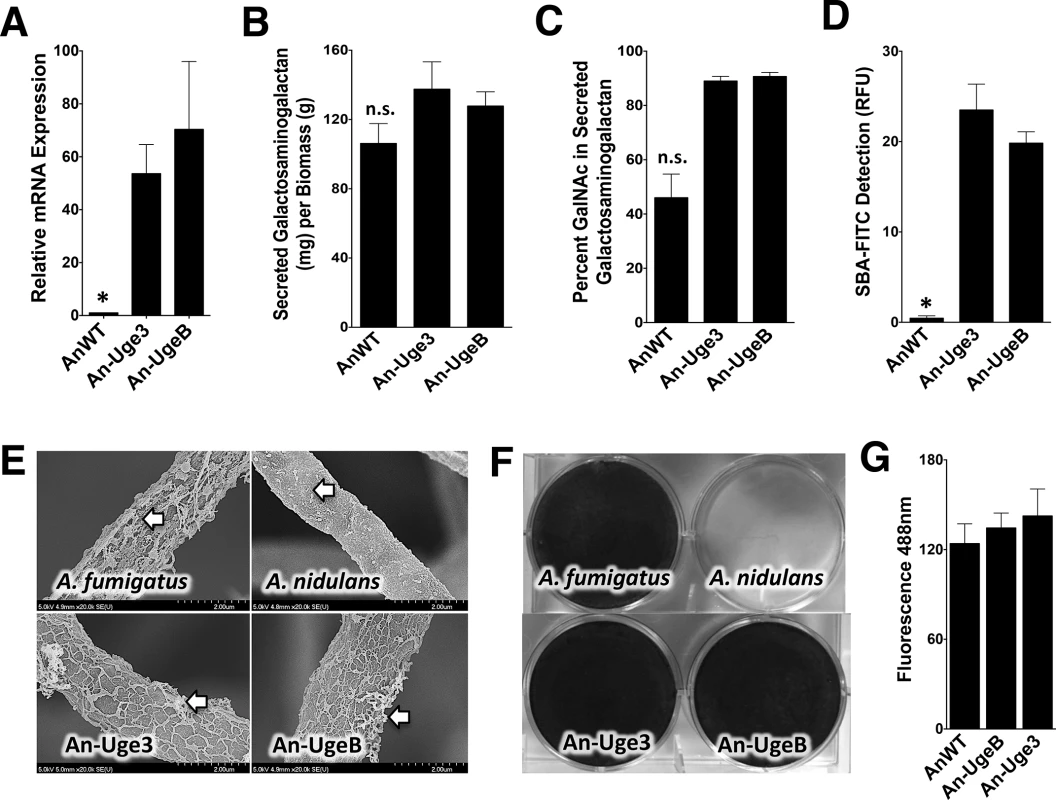
(A) Relative expression of uge3 in the An-Uge3 strain and ugeB in the An-UgeB strain compared to the expression level of ugeB in wild-type A. nidulans grown in Brian medium and as measured by real-time RT-PCR. (B) Total amount of secreted GAG from the indicated strains. (C) GalNAc content of secreted GAG from the indicated strains as determined by gas chromatography and quantified by hexose or hexosamine assays. (D) Cell wall GalNAc staining with FITC-conjugated soybean agglutinin (SBA). SBA binding to mature hyphal mats of the indicated strains was quantified by fluorometry. (E) Scanning electron micrograph of hyphae of indicated species at 20,000X magnification. Arrows indicate surface decorations associated with cell wall-bound GAG. (F) Formation of adherent biofilms on tissue culture treated polystyrene plates by the indicated strains. After 24 hours growth, biofilms were washed and visualized by staining with 0.1% crystal violet. (G) Detection of β-1,3-glucan exposure on the surface of hyphae by immunostaining with Fc-dectin-1 antibody labeled with FITC secondary antibody and quantified by fluorometry at 495 nm. For all panels: An-Uge3 indicates the A. nidulans overexpressing uge3 strain; An-UgeB indicates the A. nidulans overexpressing ugeB strain; and AnWT indicates wild type A. nidulans. Data are represented as mean +/- SEM and * indicates a significant difference between A. nidulans, and both overexpression strains, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. Augmenting the GalNAc content of GAG in A. nidulans resulted in a marked increase in biofilm formation (Fig 3F), but had no effect on the size or germination of the conidia (S2C and S2D Fig), β-1,3-glucan masking (Figs 3G and S2F), or hyphal growth rate (S2G Fig). Collectively, these data suggest that increasing GalNAc content of A. nidulans GAG by overexpressing either the native A. nidulans ugeB or A. fumigatus uge3 gene resulted in a strain of A. nidulans that resembled A. fumigatus, in vitro.
Increasing the GalNAc content of A. nidulans GAG increases the virulence of A. nidulans
To determine if the increase in cell wall-bound GalNAc-rich GAG resulting from overexpression of either uge3 or ugeB could result in increased virulence, corticosteroid-treated Balb/c and C57BL/6 mice were infected intranasally with wild-type A. fumigatus, wild-type A. nidulans, An-Uge3, or An-UgeB. As expected, mice infected with wild-type A. nidulans had a longer median survival than those infected with A. fumigatus (Fig 4A and 4B). Also, 10–25% of mice infected with wild-type A. nidulans survived to the end of the experiment, whereas none of the animals infected with A. fumigatus survived. In contrast, the onset of death, median survival and overall mortality of mice infected with An-Uge3 or An-UgeB were similar to mice infected with A. fumigatus. Similar findings were observed in Balb/c (Fig 4A) and C57BL/6 mice (Fig 4B), suggesting these differences in virulence were not mouse strain specific. Thus, increasing the GalNAc content of GAG in A. nidulans by increasing expression of a heterologous or endogenous GalNAc epimerase significantly enhanced the virulence of this minimally pathogenic Aspergillus species.
Fig. 4. Overexpression of uge3 in A. nidulans increases virulence in mice. 
(A) Survival of corticosteroid treated BALB/c mice infected with the indicated conidial species and strains. Data represent the results from two independent experiments for total N = 20 for A. nidulans or An-Uge3; N = 10 for A. fumigatus or An-UgeB; N = 8 for PBS control. (B) Survival of corticosteroid treated C57BL/6 mice infected with the indicated conidial species and strains. Five or more mice were infected for each group. * indicates a significant difference in survival of A. nidulans compared with A. fumigatus, An-Uge3, and An-UgeB overexpression strains as determined by the Mantel-Cox log-rank test with pairwise comparison applying Bonferroni correction. Increasing the GalNAc content of A. nidulans GAG resulted in increased tissue invasion
To examine the mechanisms underlying the increased virulence seen with overexpression of uge3 or ugeB in A. nidulans, corticosteroid-treated mice were infected with wild-type A. nidulans or An-Uge3 and their lungs examined after 4 days of infection. The pulmonary fungal burden of mice infected with An-Uge3 was significantly higher than in mice infected with wild-type A. nidulans, as measured by galactomannan content (Fig 5A) and quantitative morphometric analysis of histopathology sections (Fig 5B). Histopathologic examination of lungs from these mice revealed striking differences in the degree of pulmonary invasion between wild-type A. nidulans (Fig 5C, top) and An-Uge3 (Fig 5C, bottom). Hyphae of wild-type A. nidulans were largely restricted to the airway lumen with minimal penetration into the pulmonary parenchyma. In contrast, hyphae of the An-Uge3 strain were markedly more invasive and penetrated significantly deeper into pulmonary tissues. Quantitative morphometric analysis of histopathology sections confirmed that hyphae of the An-Uge3 strain invaded much further from the airways than the parental A. nidulans strain (Fig 5D). Importantly, the magnitude of tissue invasion by the An-Uge3 strain (5-fold (Fig 5D)) was much greater than the increase in total lesion size (1.5-fold (Fig 5B)), suggesting that the increased invasiveness was not simply a reflection of increased lesion size. In fact, in mice infected with the An-Uge3 overexpression strain, only 2 out of 51 lesions remained confined within the airway lumen compared to 18 out of 51 lesions from mice infected with wild-type A. nidulans. Further histological examination by immunohistochemistry with anti-GAG antibody revealed that the hyphal surface of An-Uge3 hyphae in these lesions was associated with increased cell wall-bound GAG, consistent with in vitro observations (Fig 5E). These results suggest that increasing the GalNAc content of GAG in A. nidulans increases cell wall-associated GAG, and enhances the growth of hyphae within pulmonary tissues.
Fig. 5. Increase in virulence of the A. nidulans strain overexpressing uge3 is associated with increase in fungal burden and pulmonary tissue invasion. 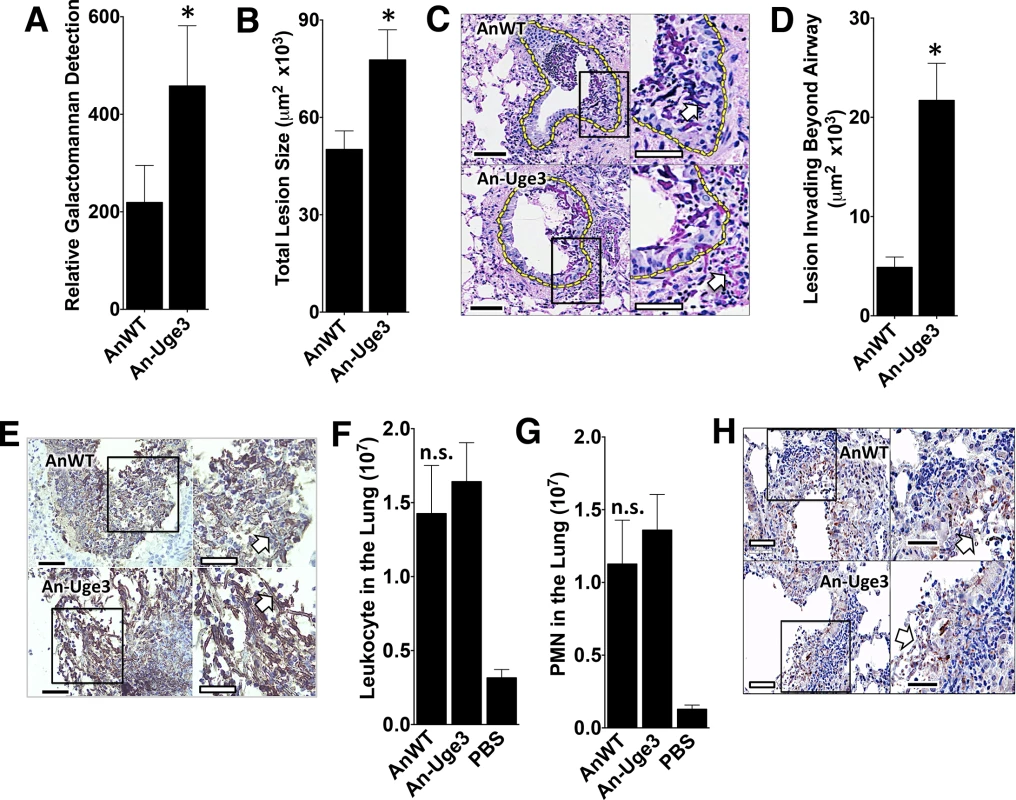
(A) Pulmonary fungal burden measured by relative galactomannan content in the lungs of mice infected with the indicated strains, N = 10 Balb/c mice for each fungal strain. (B) Total fungal lesion size as determined by morphometric analysis of lung histopathology for the indicated strains, N = 4 Balb/c mice for each fungal strain. (C) Pulmonary histopathology sections from Balb/c mice infected with indicated strains and stained with PAS for visualization of fungi. The yellow dotted line indicates the limit of the airway used for morphometric analysis. White arrow indicates fungal elements outside the airway and invading into pulmonary tissues. Scale bar represents 100 μm (black) or 50 μm (white). (D) Lesion invasion beyond the airway as determined by morphometric analysis of infected mouse lung histopathology for the indicated strains, N = 4 Balb/c mice for each fungal strain. (E) Pulmonary histopathology sections from Balb/c mice infected for 4 days with the indicated strains and labeled with an anti-GAG antibody to visualize GAG on hyphae. Arrow indicates hyphae. Scale bar represents 80 μm (black) or 30 μm (white). (F) Total pulmonary leukocytes from mice 4 days after infection with the indicated strains as measured by CD45 detection by flow cytometry. N = 10 Balb/c mice for each fungal strain, N = 5 for PBS control. (G) Total pulmonary neutrophils from of mice 4 days after infection with the indicated strains as measured by Ly6G+, CD11bhigh, and CD11clow detection using flow cytometry. N = 10 Balb/c mice for each fungal strain, N = 5 Balb/c mice for PBS control. (H) Pulmonary histopathology sections from Balb/c mice 4 days after infection with the indicated strains and labeled with anti-caspase-3 antibody to visualize host cells undergoing apoptosis. Arrow indicates hyphae. Scale bar represents 100 μm (black) or 50 μm (white). For all panels: An-Uge3 indicates the A. nidulans overexpressing uge3 strain and AnWT indicates wild type A. nidulans. For panel A, B, D, F, and G, data are represented as median with interquartile ranges and * indicates a significant difference between A. nidulans and the An-Uge3 strain, p<0.05 by Kruskal-Wallis test with Dunn’s test for pairwise comparison. Cell wall-associated GAG has been reported to modulate immune responses through the masking of hyphal PAMPs [10]. Additionally, soluble GAG has been found to directly induce neutrophil apoptosis and the induction of IL-1RA production by macrophages [9,14]. Multiple parameters of the host inflammatory response were therefore analyzed in lungs of infected mice to determine if An-Uge3 induced a different response than wild-type A. nidulans. No significant differences were observed between mice infected with A. nidulans or the An-Uge3 strain with respect to total leukocyte (Fig 5F) or neutrophils (Fig 5G) in the lungs of infected mice. Analysis of the bronchoalveolar lavage fluid also revealed no significant differences between mice infected with A. nidulans or the An-Uge3 strain with respect to total leukocytes or neutrophils, (S3A and S3B Fig); or total lung myeloperoxidase, TNF-α, IL-1β, IL-17 and IL-1RA levels (S3C–S3G Fig). In addition, no differences in the number of live or dead leukocytes or neutrophils were found between mice infected with wild-type A. nidulans or the An-Uge3 strain (S3H and S3I Fig). Further, histopathological examination of fungal lesions did not identify any differences in leukocyte nuclear fragmentation surrounding fungal lesions suggestive of differences in apoptosis (S3J Fig), and immunohistochemical staining for caspase-3 did not demonstrate differences between lesions resulting from these two strains (Fig 5H). Taken together, these results suggest that altering the amount of cell wall-bound GAG in A. nidulans does not alter virulence by altering the host inflammatory response.
Increasing cell wall-associated GAG enhances resistance to neutrophil killing in vitro
Increasing the GalNAc content of GAG in A. nidulans markedly increased the amount of polysaccharide bound to the hyphal surface and was associated with increased fungal growth and invasion in vivo. In light of these findings, we hypothesized that cell wall-associated GAG might function like a capsule and mediate resistance to neutrophil damage during infection. We therefore examined the ability of primary human neutrophils to damage hyphae of A. fumigatus, A. nidulans and the An-Uge3 strain. Consistent with our observations in vivo, A. nidulans was more susceptible to damage by neutrophils than A. fumigatus or the An-Uge3 strain (Fig 6A), suggesting that cell wall-associated GAG enhances resistance to neutrophil killing.
Fig. 6. Increasing cell wall-associated GAG enhances the resistance of A. nidulans to NADPH oxidase-dependent neutrophil extracellular traps (NETs) but not reactive oxygen species. 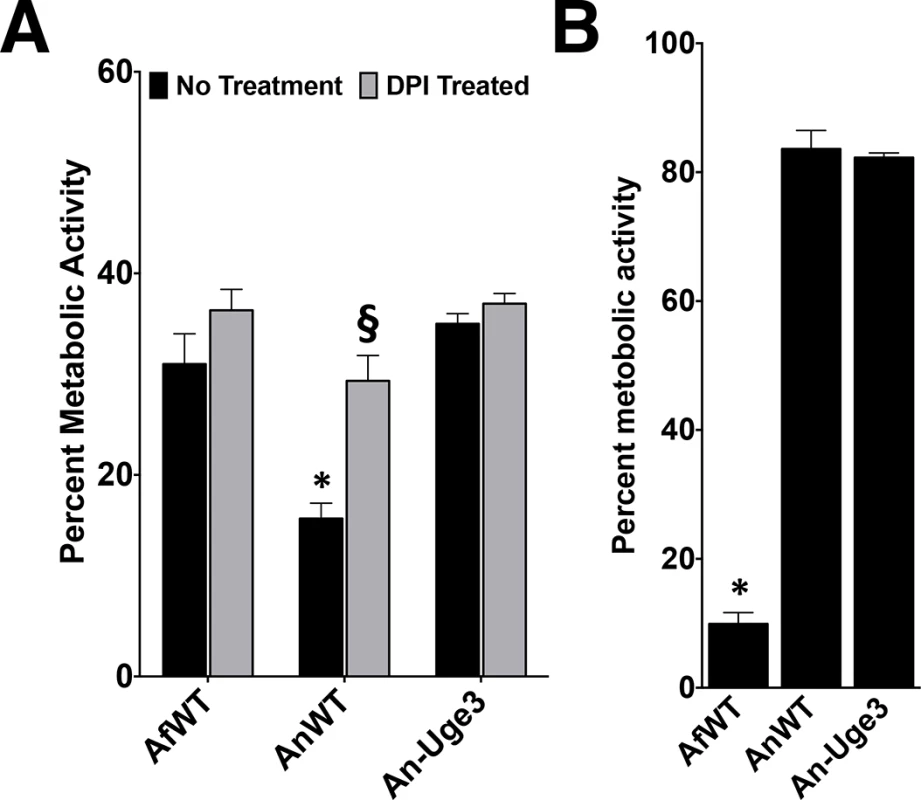
(A) Fungal injury by primary human neutrophils (PMN) either untreated (black bars) or treated with the NADPH oxidase inhibitor diphenyleneiodonium (DPI) (gray bars). (B) Susceptibility of the indicated fungal strains to injury after treatment with 3.2 mM hydrogen peroxide. * indicates a significant difference between A. nidulans and A. fumigatus or An-Uge3 strains, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. § indicates a significant difference in killing of A. nidulans between DPI-treated and untreated neutrophils, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. Increasing cell wall-associated GAG enhances resistance to ROS-independent, NADPH oxidase-dependent neutrophil damage
To probe the mechanism underlying the enhanced resistance of GAG-producing Aspergillus to neutrophil-mediated damage, primary human neutrophils were treated with the NADPH oxidase inhibitor, diphenylene iodonium (DPI), and incubated with the fungal strains. Differences in fungal strain susceptibility to neutrophil mediated damage were lost when neutrophils were treated with DPI (Fig 6A). This observation suggests that the cell wall-associated GAG mediates resistance to NADPH oxidase-dependent neutrophil killing. Since NADPH oxidase mediates production of reactive oxygen species, hyphal susceptibility to hydrogen peroxide was examined. Surprisingly. A. nidulans and the An-Uge3 strain were equally resistant to oxidative stress and significantly more resistant than A. fumigatus (Fig 6B). These findings suggest that cell wall-associated GAG mediates resistance to NADPH oxidase-dependent neutrophil killing, but not by directly enhancing resistance to reactive oxygen species.
Increasing cell wall-associated GAG enhances resistance to neutrophil extracellular traps (NETs)
In addition to mediating the production of reactive oxygen species that directly injure fungi, phagocyte NADPH oxidase also mediates antifungal host defense by inducing the coordinated release of DNA and antimicrobial peptides from neutrophil granules to form neutrophil extracellular traps (NETs) [23,24]. NADPH-oxidase dependent NET formation has been reported in response to both A. fumigatus and A. nidulans, in vitro and in vivo [25–28]. To determine if NET formation correlated with the differences in GAG-dependent susceptibility of strains to neutrophil killing, primary neutrophils were co-cultured with A. fumigatus, A. nidulans and An-Uge3 and examined for NET formation (Fig 7A). Nuclear de-condensation and the formation of propidium iodide staining NETs were observed during co-culture with all three organisms (Fig 7A). Although the number of neutrophils undergoing NETosis was not different in response to the different fungal strains, more propidium iodide positive NETs were bound by hyphae of wild-type A. nidulans compared with A. fumigatus or An-Uge3 (Fig 7A). Treatment of primary human neutrophils with micrococcal nuclease (MNase), which degrades DNA, prevented the formation of NETs as previously reported [29], and reduced the differences in susceptibility to neutrophil-mediated damage among the three Aspergillus strains (Fig 7B), mirroring the effects of DPI treatment. Similarly, neutrophils isolated from a patient with CGD failed to form NETs, and did not exhibit enhanced killing of A. nidulans relative to A. fumigatus or An-Uge3 (Fig 7C), reproducing the effects of DPI and MNase treatment. Consistent with the differences in strain virulence seen in the corticosteroid-treated mouse model, corticosteroid treated primary neutrophils formed NETs and retained their ability to kill wild-type A. nidulans to a greater degree than A. fumigatus or An-Uge3, (Fig 7D). Finally, wild-type A. nidulans was also found to be more susceptible to primary neutrophil lysates than A. fumigatus or the An-Uge3 strain (Fig 8A), confirming that cell wall-bound GAG directly mediates resistance to damage by neutrophil contents contained within NETs. Interestingly, similar patterns of differential susceptibility to damage were also seen with lysates collected from DPI-treated human neutrophils, as well as primary neutrophils from C57BL/6 mice and primary neutrophils from NADPH-oxidase deficient mice (Fig 8C and 8D). These observations suggest that, under these conditions, neutrophil NADPH oxidase exerts its antifungal activity largely by orchestrating release of neutrophil intracellular contents, rather than influencing their composition or activity. Collectively these data suggest that cell wall-bound GAG enhances resistance to NADPH oxidase-dependent neutrophil extracellular damage by directly increasing resistance to NETs.
Fig. 7. Inhibition or disruption of NETs attenuates the susceptibility of A. nidulans to killing by human neutrophil-mediated killing. 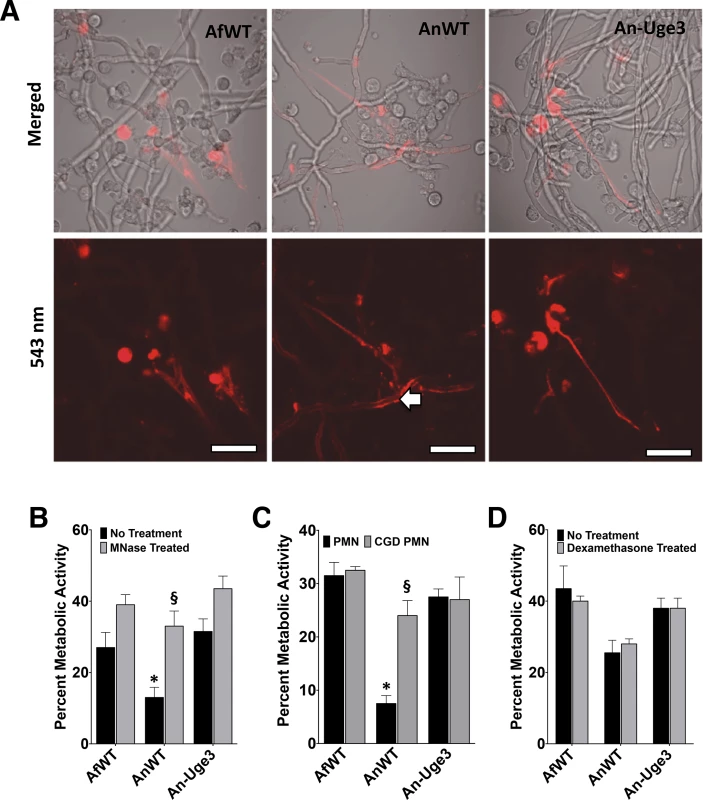
(A) Neutrophil extracellular traps formation by primary human PMN as visualized by the DNA intercalating agent propidium iodide. Arrows indicate the increased binding of propidium iodide stained NETs on the surface of wild-type A. nidulans hyphae. Images were acquired using a 543 nm laser and detected by confocal microscopy at 600X magnification with 4X digital zoom. Scale bar represents 10 μm. (B) Susceptibility of fungal strains to injury by PMNs in the presence (gray bars) or absence (black bars) of micrococcal nuclease (MNase). (C) Susceptibility of fungal strains to injury by PMNs from healthy donor (black bars) or from a CGD patient (grey bars). (D) Susceptibility of fungal strains to injury by PMNs pre-treated with 10 μM dexamethasone (grey bars) or untreated (black bars). * indicates a significant difference between A. nidulans and A. fumigatus or An-Uge3 strains, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. § indicates significant difference treatment groups of PMNs co-incubated with A. nidulans, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. All data are represented as mean +/- SEM. Fig. 8. GAG-mediated resistance to neutrophil killing is dependent on neutrophil lysate content. 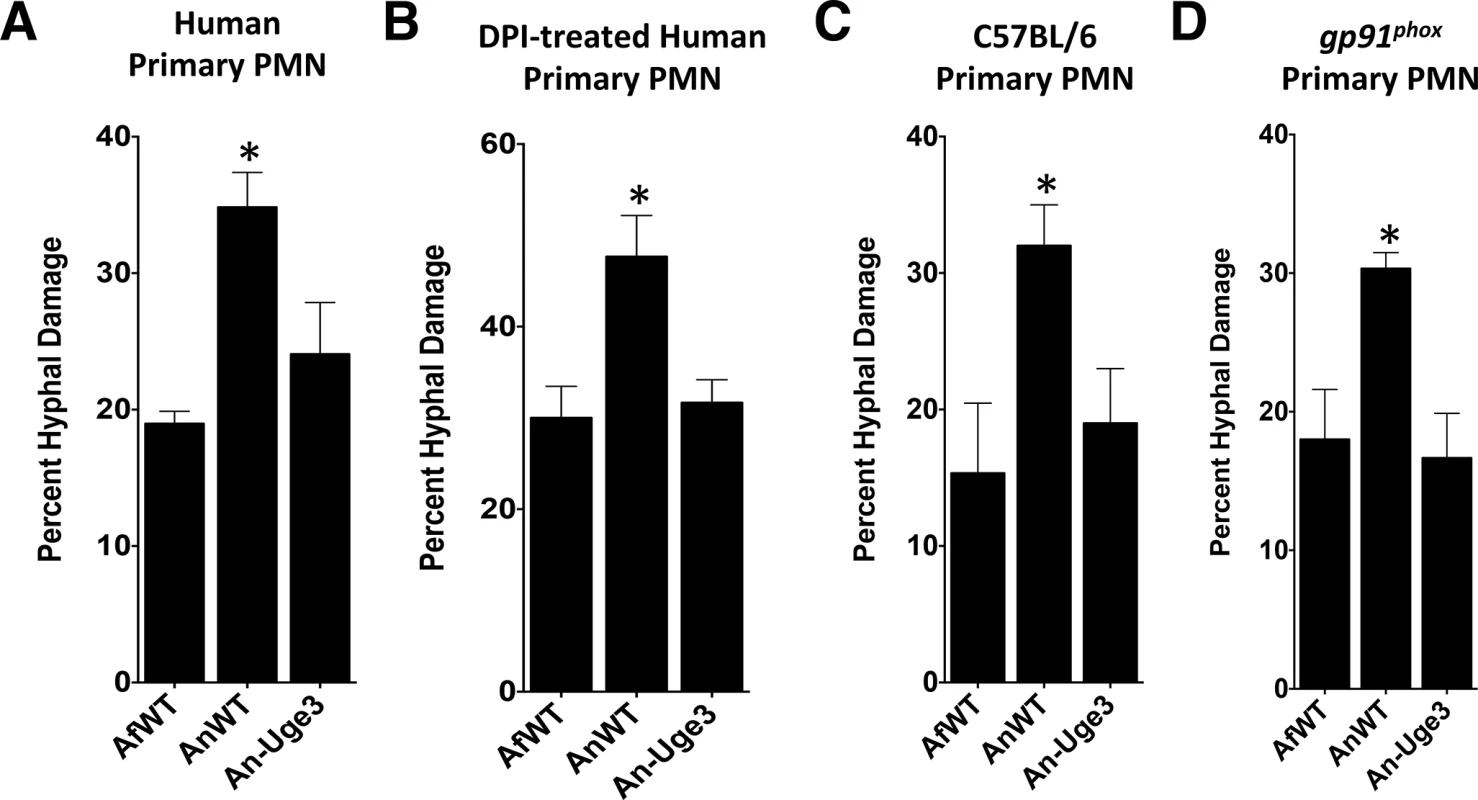
Susceptibility of fungal strains to injury by lysates derived from, (A) primary human neutrophils, (B) primary human neutrophils treated with DPI., (C) primary C57BL/6 mouse neutrophils or gp91phox deficient (CGD) mouse neutrophils. * indicates a significant difference between A. nidulans and A. fumigatus or An-Uge3 strains, p<0.05 by ANOVA with Tukey’s test for pairwise comparison. To test if GAG-mediated resistance to NETs underlies the increased virulence of the An-Uge3 strain (Fig 4), we compared the virulence of wild-type A. nidulans and the An-Uge3 strain in two groups of mice that cannot make NETs in response to Aspergillus infection: neutropenic mice and gp91phox deficient mice, that lack functional NADPH oxidase [26]. Consistent with our in vitro findings, increasing cell wall-associated GAG expression in the An-Uge3 strain did not increase A. nidulans virulence in either of these NET deficient mice (Fig 9A and 9B). These data strongly suggest that the expression of cell wall-bound GAG by Aspergillus mediates virulence through enhancing resistance of hyphae to NADPH oxidase-dependent NET formation.
Fig. 9. GAG mediated enhancement of A. nidulans virulence requires functional leukocyte NADPH oxidase. 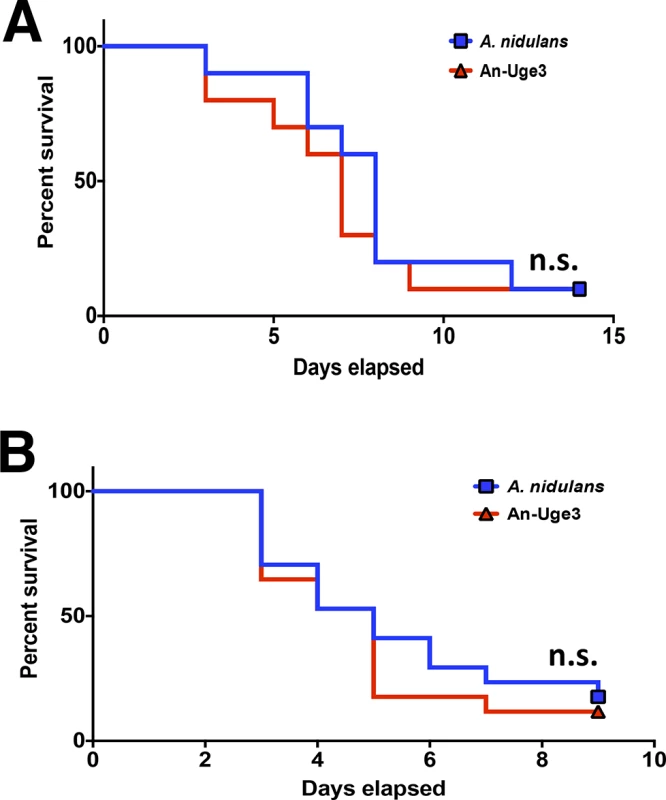
(A) Survival of leukopenic mice infected with either A. nidulans or An-Uge3 conidia. N = 9 per infection group. (B) Survival of gp91phox deficient mice lacking functional NADPH oxidase infected with either A. nidulans or An-Uge3 conidia. N = 17 per infection group. For all panels: An-Uge3 indicates the A. nidulans overexpressing uge3 strain; AnWT indicates wild type A. nidulans; and AfWT indicates wild type A. fumigatus. n.s. indicates no significant difference in survival of A. nidulans compared with the An-Uge3, strain as determined by the Mantel-Cox log-rank test with pairwise comparison applying Bonferroni correction. Discussion
This study establishes a role for cell wall-associated GAG in mediating resistance to killing of Aspergillus by NETs and provides the first example of a virulence factor of A. fumigatus that is able to mediate enhanced virulence when expressed in a less pathogenic Aspergillus species. GAG has been reported to mediate a number of functions in vitro that could influence virulence, including host cell adherence, masking of β-glucan, the modulation of host immune responses and the induction of neutrophil apoptosis [10,13,14,34]. While determining the contribution of each of these mechanisms to virulence is challenging, the present study provides some insights into this question. First, β-glucan masking was not different among A. fumigatus A. nidulans and the An-Uge3 strains in our study, suggesting that differences in β-glucan masking and dectin-1 activation do not contribute to the difference in virulence between these strains. Second, the similar levels of virulence exhibited by wild-type A. nidulans and the An-Uge3 strain in neutropenic and NADPH-oxidase deficient mice suggests that increased biofilm formation by the overexpression strain does not play a significant role in virulence. Finally, increasing the amount of cell wall-bound GAG enhanced Aspergillus virulence in the absence of any detectable difference in inflammation or immune response, including pulmonary IL-1RA levels, neutrophil recruitment to site of infection, and the induction of neutrophil apoptosis, which have been observed during treatment with soluble GAG [13,14]. The failure to observe changes in these responses in the present study likely reflects the fact that these effects of GAG were observed in response to treatment with soluble GAG. In the experiments reported here, the amount of shed, soluble GAG was not different between the three strains of Aspergillus and only the quantity of cell wall-bound GAG differed among these strains.
Multiple lines of evidence suggest that increased cell wall-associated GAG production augments virulence in non-neutropenic mice through enhancing resistance to neutrophil contents released as extracellular traps. The higher levels of cell wall-bound GAG in A. fumigatus and An-Uge3 increased resistance to damage by neutrophils and by neutrophil lysates, but not by toxic reactive oxygen species. Moreover, differences in susceptibility to neutrophil mediated damage among A. fumigatus, wild-type A. nidulans, and the An-Uge3 strain were not seen with neutrophils deficient in NETosis, isolated either from a patient with CGD, or healthy human neutrophils treated with two inhibitors of NETosis (DPI and MNase). Finally, the increased virulence of the An-Uge3 strain was lost in mice with defective NADPH-oxidase. These data are in agreement with multiple prior studies examining the interactions of A. fumigatus and A. nidulans with neutrophils. A previous report suggested that killing of A. nidulans by human neutrophils occurs predominately via a non-oxidative mechanism [35]. Similarly, while susceptibility of A. nidulans to killing by NETs has been observed [26], A. fumigatus growth was found to be only minimally inhibited by NET formation [27]. Our findings suggest that differences in the expression of cell wall-associated GAG between A. nidulans and A. fumigatus account for the differential susceptibility to NETs reported in these two studies.
Collectively, the results of these experiments suggest a model in which cell wall-bound GalNAc-rich GAG functions as an extracellular capsule to enhance resistance to NETs, analogous to bacterial capsular exopolysaccharide [11,36–38]. Although the mechanism by which exopolysaccharide mediates resistance to NETs has not yet defined, the increased binding of NETs to A. nidulans suggests that GAG may directly inhibit NET binding to hyphae. We have previously reported that the GalNAc component of GAG undergoes partial deacetylation, rendering GAG a cationic glycan [39]. It is likely that this positive charge inhibits binding of cationic antimicrobial peptides and histones within NETs resulting in increased resistant to NETosis. A similar mechanism of resistance to cationic antimicrobial peptides by exopolysaccharide-mediated electrostatic repulsion has been described in Staphylococcus epidermidis [40]. Further work to clarify this mechanism is clearly required.
Why has A. fumigatus evolved this change in the composition of an exopolysaccharide and the resulting increase in cell wall-associated GAG? As A. fumigatus is an environmental organism and only an incidental opportunistic pathogen of immunocompromised hosts, we speculate that selection for GalNAc-rich GAG was mediated through environmental pressures that are unique to A. fumigatus. One hypothesis is that the production of a capsule-like hyphal sheath could offer protection against competing microorganisms in the complex microbial environment of decomposing organic matter, where A. fumigatus is commonly found. Studies comparing the production and composition of GAG in strains of different environmental origin may be helpful in shedding light on this question.
Invasive aspergillosis can develop in non-neutropenic patients who are immunocompromised due to corticosteroid or other immunosuppressive therapies, as well as in patients with quantitative or qualitative defects in neutrophil function. The findings of this study are most relevant to invasive aspergillosis in corticosteroid-treated or other non-neutropenic patients in whom NET production is preserved. It is likely that there are other factors that contribute to the spectrum of intrinsic virulence of Aspergillus species in different hosts. For example, GAG-mediated resistance to NETs clearly plays no role in the pathogenesis in invasive aspergillosis associated with neutropenia. In CGD patients, who are distinctly more susceptible to A. nidulans, the inability to form NETs may have a greater effect on increasing susceptibility to infection with A. nidulans relative to A. fumigatus. However, further studies comparing the pathogenesis of A. fumigatus and A. nidulans infection in CGD mice, or in other models of invasive aspergillosis, are required to understand the role that GAG and other fungal factors play in the pathogenesis of this disease.
Although our data suggests that cell wall-bound GAG may contribute to differences in intrinsic virulence in species other than A. fumigatus and A. nidulans, more work is needed to confirm this hypothesis. The biochemical, antibody and lectin studies used to quantify GAG were optimized with A. fumigatus and it is therefore possible that some differences in GAG composition and structure other than GalNAc content could be missed by these techniques. Also, while we observed similar low levels of cell wall-bound GAG in multiple laboratory and clinical isolates of A. nidulans strains, a larger screen of clinical and environmental isolates will be required to more firmly establish the association between GalNAc-rich, cell wall-bound GAG and intrinsic virulence. In addition, further mechanistic studies modulating GAG composition in these other species are required to confirm that the observations made in A. nidulans are applicable to other species that produce GalNAc-poor GAG.
Modulating the composition of a single exopolysaccharide significantly enhanced the virulence of a relatively non-pathogenic Aspergillus species in corticosteroid-treated mice through enhancing resistance to NETs. This study highlights the importance of GAG as a key virulence factor of A. fumigatus in this population and suggests that targeting this exopolysaccharide may be an effective antifungal approach.
Materials and Methods
Fungal strains and growth conditions
A. nidulans strain A26 (Fungal Genetics Stock Center, Kansas City, MO, USA) was used as the parent wild-type strain for all molecular manipulations. Other strains used in this study include A. fumigatus strain Af293 (a generous gift from P. Magee, University of Minnesota, St. Paul, MN, USA), the A. fumigatus Δuge3 mutant [10], ΔstuA mutant [41] and clinical isolates of A. flavus and A. niger (obtained from the McGill University Health Center, Montreal, QC, Canada) and A. nidulans isolates from CGD patients (generous gifts from A. Warris and S. Henriett, Radboud University Medical Center, Nijmegen, The Netherlands, and Adrian Zelazny US National Institute of Health, USA). Unless otherwise noted, A. fumigatus strains and A. flavus were maintained on YPD agar (Fisher Scientific), A. niger on potato-dextrose agar (Fisher Scientific), and A. nidulans strains on Aspergillus minimum medium agar [10] at 37°C. For growth in liquid medium, Brian medium [9] and phenol red-free RPMI 1640 (Wisent, Inc.) were used as indicated. All growth media for A. nidulans strains were supplemented with biotin (Fisher Scientific).
Molecular and genetic manipulations
Heterologous overexpression of uge3 and endogenous overexpression of ugeB in A. nidulans was performed as previously described [42,43], with minor modifications. The open reading frames of uge3 or ugeB were amplified by PCR from genomic DNA of A. fumigatus or A. nidulans, respectively. The resulting PCR products were cloned downstream of the constitutive gpdA promoter in plasmid pGFP-Phleo [42] [44] by replacing the GFP coding regions with either uge3 or ugeB to yield plasmids pUge3-OX and pUgeB-OX, respectively (S1 Table). A. nidulans was then transformed by spheroplasting, as previously described [45]. Gene overexpression was confirmed by real time RT-PCR [11].
Mutant characterization
Purification and analysis of GAG was performed as previously described [11]. Hyphae were grown for 72h, and GAG was precipitated from culture supernatants with 2.5 volumes of ethanol. The GalNAc-rich insoluble fraction was collected by filtration on Nylon membrane and washed with 60% ethanol (2.5 volume). Other volumes of ethanol precipitation was tested with similar results. Composition of GAG was determined by gas chromatography after derivatization to its alditol acetate, and quantified by hexose and hexosamine assays [9]. Conidial size was measured by FACS analysis (BD LSR Fortessa) of conidia of each strain fixed with 4% paraformaldehyde. Germination kinetics assay was performed by inoculating 1x105 conidia per well in a 6-well plate, and incubating at 37°C, 5% CO2. At every hour, the number of germinated fungi was counted. For hyphal growth measurements, 1x106 conidia of indicated strains were inoculated on agar plates and incubated at 37°C. Thallus diameter was measured daily. Biofilm adherence was assessed on 24h grown hyphae of indicated strains by rigorously washing and staining with 0.1% crystal violet for visualization, as previously described [10]. For scanning electron microscopy (SEM), fluorescein labeled soybean agglutinin (FITC-SBA) lectin binding, and Fc-dectin-1 binding experiments, fungi were grown for the indicated times in phenol red-free RPMI at 37°C, 5% CO2 incubation, fixed with either 2.5% gluteraldehyde or 4% PFA, and further processed [10]. Briefly, for SEM, samples were sequentially dehydrated in ethanol, critical point dried, coated in Au-Pd, and imaged at 20,000X magnification (Hitachi, Inc, or FEI Company), as previously described [10]. For FITC-SBA lectin or Fc-dectin-1 binding, fungi were grown in 96-well opaque plates (Thermo Fisher Scientific, Inc.) or clear-bottom plates (Corning, Inc.) for 9–12 h, and immunostained with FITC-SBA (Vector Labs, Inc.), or Fc-dectin-1 (a generous gift from Dr. G.D. Brown, Aberdeen, UK) followed by FITC labeled anti-human IgG, FCγ fragment specific (Jackson ImmunoResearch Laboratories, Inc.). Fluorescence of labeled samples was imaged by confocal microscopy (Olympus, Inc.) or relative fluorescence measured at 495 nm excitation and 515 nm emission using Spectramax fluorimeter (Molecular Devices, LLC) or Infinite M1000 (Tecan, Inc.).
Virulence studies
For virulence studies in non-neutropenic mice, BALB/c or C57BL/6 mice, 6–8 weeks old, were immunosuppressed with 5 doses of 10 mg of cortisone acetate per mouse (Sigma-Aldrich), administered subcutaneously every other day starting on day -4 relative to infection [46]. For studies in neutropenic mice, 6–8 weeks old C57BL/6 mice were treated on days -2 and +3 with 250 mg/kg of cortisone acetate (Sigma-Aldrich) intraperitoneally, and 250 mg/kg on day -2 and 200 mg/kg on day +3 of cyclophosphamide (Western Medical Supply). To prevent bacterial infection, enrofloxacin was added to the drinking water. For studies in an NADPH oxidase-deficient host, 8–12 week old male and female gp91phox deficient mice lacking functional NADPH oxidase were used. All mice were infected intranasally with 1x106 conidia from A. nidulans, An-Uge3, A. fumigatus or An-UgeB strains or treated with PBS alone as uninfected controls. Mice were monitored for a period of 2 weeks for signs of illness, and moribund animals were euthanized or were sacrificed 4 days after infection for determination of fungal burden and inflammation markers. At the time of sacrifice, bronchoalveolar lavage (BAL) fluid was collected and then lungs were harvested and homogenized [10]. To determine pulmonary leukocyte recruitment, Balb/c mice infected with the indicated fungal strains were sacrificed 4 days after infection and lungs were removed for collagenase (Sigma-Aldrich) digestion. Excised lungs were washed in PBS, minced, and digested for 1 hour at 37°C in RPMI (Wisent) and 150 U/mL collagenase (Sigma-Aldrich) supplemented with 5% fetal bovine serum (Wisent). After digestion, cells were washed and stained as mentioned, and post-fixed in 2% PFA. Cells from BAL fluid or digested lungs were labeled with CD45, CD11b, CD11c, and Ly6G (BD Biosciences), stained with Cell Viability Stain (eBiosciences), and counted using LSR Fortessa cell analyzer (BD Biosciences) and analyzed using FlowJo VX software (Tree Star). Total leukocytes were gated as CD45+ cells, while neutrophils were gated as CD11bhigh, CD11clow, and Ly6G+ cells. For histopathology studies, lungs from a subset of immunosuppressed BALB/c mice were fixed in formalin. Thin sections were stained with hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) to visualize fungal elements. Caspase-3 immunohistopathology was performed using an anti-capsase-3 antibody (Institute for Research in Immunology and Cancer, Montreal, Canada). Immunohistochemistry staining with an anti-GAG antibody (Fontaine et al., 2011) was performed to detect GAG in pulmonary sections (Institute for Research in Immunology and Cancer, Montreal, Canada). Pulmonary fungal burden was measured by determining relative galactomannan levels by EIA (BioRad) using an internal standard [10]. Myeloperoxidase activity (Hycult Biotech), or cytokine production of TNF-α (eBiosciences, Inc.), IL-1RA (R&D Systems, Inc), or IL-1β (Sigma-Aldrich) were measured by commercial EIA following the manufacturer’s instructions. All procedures involving mice were approved by the Los Angeles Biomedical Research Institute Animal Use and Care Committee; the Montana State University Animal Care Facility; or the McGill University Animal Care Committee, and followed the National Institutes of Health guidelines for animal housing and care and the guidelines established by the Canadian Council on Animal Care.
Morphometric analysis
PAS stained lung sections were digitally scanned at up to 400X magnification (Goodman Cancer Center, McGill University). Fungal lesions were identified by visual inspection and lesion size and distance from airways were calculated using Spectrum (Apio, Inc) software. To ensure unbiased data collection, readers were blinded to strain identity.
Neutrophil damage experiments
Indicated fungal strains were grown at 37°C, 5% CO2 for 6–9 h in either Brian medium or Iscove’s Modified Dulbecco Medium (IMDM) adjusted to pH 6.5 (Life Technologies, Inc.) in a 24-well tissue culture treated plate (Corning, Inc.). For co-incubation experiments with neutrophils, blood samples obtained from healthy donors or from an individual with granulomatous disease were purified to obtain polymorphonuclear cells (PMN), as previously described [47]. Briefly, white blood cells were separated using Ficoll gradation, followed by dextran sedimentation, and red blood cell lysis. Neutrophils isolated from donors were added to wells with young hyphae and co-incubated for 12 h. After 12 h co-incubation, wells were washed with PBS, and remaining neutrophils were lysed with distilled, endotoxin-free water (Fisher, Inc.). To quantify hyphal injury, metabolic activity of fungi was measured by reduction of the tetrazolium reagent XTT (Bioshop, Inc.), as previously described [48]. To inhibit NADPH-oxidase activity, neutrophils were incubated with 25 μM diphenyleneiodonium (Sigma-Aldrich) for 1 h and washed twice in medium prior to co-incubation with fungi. To test the ability of DNAse to disrupt neutrophil extracellular traps, 10 Units of micrococcal nuclease (Sigma-Aldrich) were added to each well at the time of co-incubation of hyphae and neutrophils. To ascertain the effects of corticosteroid on neutrophil function in vitro, neutrophils were treated with 10μM dexamethasone (Sigma-Aldrich) for 30 minutes prior to co-incubation. Effect of dexamethasone treatment on neutrophil function was measured by fungal killing quantified by reduction of XTT and normalized to the metabolic activity of dexamethasone treated hyphae for each strain.
To assess non-oxidative hyphal killing, 6–9 h grown young hyphae of indicated strains were incubated with neutrophil lysates for 12 h, followed by XTT reduction assay, as previously described [48]. Neutrophil lysates were prepared by subjecting the cells through a freeze-thaw cycle, re-suspending in Brian medium pH 5.4 supplemented with 2% protease-free bovine serum albumin (Bioshop, Inc.). Neutrophils were incubated with 25 μM DPI (Sigma, Inc.) for 1 h for the preparation of DPI-treated lysates. After mechanical disruption lysates were separated from cell debris by centrifugation. The multiplicity of infection used for all assays, including PMN lysates, was 1 : 100 (conidia to neutrophil). All procedures involving human subjects were approved by the McGill University Research Ethics Board.
To test susceptibility to reactive oxygen species, hyphae of the indicated strains were incubated for 12 h with various concentrations of t-butyl peroxide (Sigma-Aldrich) ranging from 0 to 20 mM. Hyphal killing by t-butyl peroxide was measured by XTT reduction assay.
NET formation by neutrophils was detected by live imaging using confocal microscopy. Differentiated HL-60 cells were co-incubated for 10–12 h with 6–9 h young hyphae of indicated strains grown on coverslips. The coverslips were placed on slides containing 6–10 μL of phenol red-free RPMI (Wisent, Inc.) containing 5 μg/mL of propidium iodide (Invitrogen, Inc.) and imaged at an excitation wavelength of 543 nm laser using an Olympus Fluoview confocal microscope (Olympus, Corp.). To ensure consistency between replicate imaging, all image acquisitions were performed at room temperature, within 10–15 minutes.
Statistical analysis
For all mouse lung data analysis, unless indicated on the legend, the Mantel-Cox log-rank test with pairwise comparison using Bonferroni correction was applied. For non-parametric data with multiple comparisons, the Krustal-Wallis rank analysis, was performed, followed by Dunn’s test for pairwise comparison. For all other data analysis, statistical significance was determined by applying one-way ANOVA, partitioned with pairwise comparison using Tukey’s test, and Bonferroni correction applied where applicable. All statistical analyses were performed either with Prism (GraphPad, Inc.) or SAS (SAS Institute, Inc.) with significance determined at p < 0.05. For all statistical analysis, n.s. denotes statistically no significant differences found.
Supporting Information
Zdroje
1. Garcia-Vidal C, Upton A, Kirby KA, Marr KA (2008) Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 47 : 1041–1050.
2. Geiser DM, Klich MA, Frisvad JC, Peterson SW, Varga J, et al. (2007) The current status of species recognition and identification in Aspergillus. Studies in Mycology 59 : 1–10. doi: 10.3114/sim.2007.59.01 18490947
3. Lucas GM, Tucker P, Merz WG (1999) Primary Cutaneous Aspergillus nidulans Infection Associated with a Hickman Catheter in a Patient with Neutropenia. Clinical Infectious Diseases 29 : 1594–1596. 10585834
4. Henriet SS, Verweij PE, Warris A (2012) Aspergillus nidulans and chronic granulomatous disease: a unique host-pathogen interaction. J Infect Dis 206 : 1128–1137. doi: 10.1093/infdis/jis473 22829648
5. Song E, Jaishankar GB, Saleh H, Jithpratuck W, Sahni R, et al. (2011) Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy 9 : 10. doi: 10.1186/1476-7961-9-10 21624140
6. Henriet S, Verweij PE, Holland SM, Warris A (2013) Invasive fungal infections in patients with chronic granulomatous disease. Adv Exp Med Biol 764 : 27–55. 23654055
7. Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, et al. (2010) What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27 : 155–182. doi: 10.1016/j.riam.2010.10.003 20974273
8. Sales-Campos H, Tonani L, Cardoso CR, Kress MR (2013) The immune interplay between the host and the pathogen in Aspergillus fumigatus lung infection. Biomed Res Int 2013 : 693023. doi: 10.1155/2013/693023 23984400
9. Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, et al. (2011) Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. pp. e1002372. doi: 10.1371/journal.ppat.1002372 22102815
10. Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, et al. (2013) Aspergillus Galactosaminogalactan Mediates Adherence to Host Constituents and Conceals Hyphal β-Glucan from the Immune System. PLoS pathogens. doi: 10.1371/journal.ppat.1003575 23990787
11. Lee MJ, Gravelat FN, Cerone RP, Baptista SD, Campoli PV, et al. (2014) Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J Biol Chem 289 : 1243–1256. doi: 10.1074/jbc.M113.522516 24257745
12. Gravelat FN, Ejzykowicz DE, Chiang LY, Chabot JC, Urb M, et al. (2010) Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol 12 : 473–488. doi: 10.1111/j.1462-5822.2009.01408.x 19889083
13. Robinet P, Baychelier F, Fontaine T, Picard C, Debre P, et al. (2014) A Polysaccharide Virulence Factor of a Human Fungal Pathogen Induces Neutrophil Apoptosis via NK Cells. J Immunol. doi: 10.4049/jimmunol.1303180 24790151
14. Gresnigt MS, Bozza S, Becker KL, Joosten LA, Abdollahi-Roodsaz S, et al. (2014) A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 10: e1003936. doi: 10.1371/journal.ppat.1003936 24603878
15. Ruperez P, Leal JA (1981) Extracellular galactosaminogalactan from Aspergillus parasiticus. Transactions of the British Mycological Society 77 : 621–625.
16. Bardalaye PC, Nordin JH (1976) Galactosaminogalactan from cell walls of Aspergillus niger. Journal of bacteriology 125 : 655–669. 173713
17. Gorin PAJ, Eveleigh DE (1970) Extracellular 2-acetamido-2-deoxy-D-galacto-D-galactan from Aspergillus nidulans. Biochemistry 9 : 5023–5027. 5480165
18. Leal JA, Ruperez P (1978) Extracellular polysaccharide production by Aspergillus nidulans. Transactions of the British Mycological Society 70 : 115–120.
19. Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007) Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology (Reading, England) 153 : 1677–1692.
20. Rao VS, Lam K, Qasba PK (1998) Three dimensional structure of the soybean agglutinin Gal/GalNAc complexes by homology modeling. Journal of biomolecular structure & dynamics 15 : 853–860.9619508
21. Dam TK, Gerken TA, Cavada BS, Nascimento KS, Moura TR, et al. (2007) Binding studies of alpha-GalNAc-specific lectins to the alpha-GalNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. The Journal of biological chemistry 282 : 28256–28263. 17652089
22. Paul BC, El-Ganiny AM, Abbas M, Kaminskyj SGW, Dahms TES (2011) Quantifying the importance of galactofuranose in Aspergillus nidulans hyphal wall surface organization by atomic force microscopy. Eukaryotic cell 10 : 646–653. doi: 10.1128/EC.00304-10 21335527
23. Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, et al. (2002) Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416 : 291–297. 11907569
24. Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23 : 197–223. 15771570
25. Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, et al. (2010) NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5: e9631. doi: 10.1371/journal.pone.0009631 20300512
26. Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, et al. (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114 : 2619–2622. doi: 10.1182/blood-2009-05-221606 19541821
27. Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, et al. (2010) Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6: e1000873. doi: 10.1371/journal.ppat.1000873 20442864
28. Rohm M, Grimm MJ, D'Auria AC, Almyroudis NG, Segal BH, et al. (2014) NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun 82 : 1766–1777. doi: 10.1128/IAI.00096-14 24549323
29. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5: e1000639. doi: 10.1371/journal.ppat.1000639 19876394
30. Lamarre C, Beau R, Balloy V, Fontaine T, Wong Sak Hoi J, et al. (2009) Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cellular microbiology 11 : 1612–1623. doi: 10.1111/j.1462-5822.2009.01352.x 19563461
31. Kwon-Chung KJ, Sugui JA (2013) Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 9: e1003743 doi: 10.1371/journal.ppat.1003743 24348239
32. Schaffner A (1985) Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest 76 : 1755–1764. 3932471
33. Brummer E, Maqbool A, Stevens DA (2001) In vivo GM-CSF prevents dexamethasone suppression of killing of Aspergillus fumigatus conidia by bronchoalveolar macrophages. J Leukoc Biol 70 : 868–872. 11739548
34. Lamarre C, Beau R, Balloy V, Fontaine T, Wong Sak Hoi J, et al. (2009) Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cellular microbiology 11 : 1612–1623. doi: 10.1111/j.1462-5822.2009.01352.x 19563461
35. Henriet SS, Hermans PW, Verweij PE, Simonetti E, Holland SM, et al. (2011) Human leukocytes kill Aspergillus nidulans by reactive oxygen species-independent mechanisms. Infect Immun 79:: 767–773. doi: 10.1128/IAI.00921-10 21078850
36. Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, et al. (2004) Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun 72 : 7107–7114. 15557634
37. Liu HZ M.; Zhu S. (2011) Persistence of Antibiotic Resistance and Capsule in E. coli B23 after Removal from Sublethal Kanamycin Treatment JEMI 15 : 43–46.
38. Bales PM, Renke EM, May SL, Shen Y, Nelson DC (2013) Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS One 8: e67950. 23805330
39. Lee MJ, Geller AM, Gravelat FN, Liu H, Snarr BD, et al. Deacetylation of Aspergillus fumigatu galactosaminogalactan is required for adherence and virulence; 2014; Madrid, Spain.
40. Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, et al. (2004) A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem 279 : 54881–54886. 15501828
41. Sheppard DC, Doedt T, Chiang LY, Kim HS, Chen D, et al. (2005) The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol Biol Cell 16 : 5866–5879. 16207816
42. Campoli P, Al Abdallah Q, Robitaille R, Solis NV, Fielhaber JA, et al. (2011) Concentration of antifungal agents within host cell membranes: a new paradigm governing the efficacy of prophylaxis. Antimicrob Agents Chemother 55 : 5732–5739. doi: 10.1128/AAC.00637-11 21930891
43. Twumasi-Boateng K, Yu Y, Chen D, Gravelat FN, Nierman WC, et al. (2009) Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot Cell 8 : 104–115. doi: 10.1128/EC.00265-08 19028996
44. Choe SI, Gravelat FN, Al Abdallah Q, Lee MJ, Gibbs BF, et al. (2012) Role of Aspergillus niger acrA in arsenic resistance and its use as the basis for an arsenic biosensor. Appl Environ Microbiol 78 : 3855–3863. doi: 10.1128/AEM.07771-11 22467499
45. Gravelat FN, Askew DS, Sheppard DC (2012) Targeted gene deletion in Aspergillus fumigatus using the hygromycin-resistance split-marker approach. Methods in molecular biology (Clifton, NJ) 845 : 119–130.
46. Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE Jr., et al. (2004) Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 48 : 1908–1911. 15105158
47. Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM (2010) Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol 125 : 1389–1390. doi: 10.1016/j.jaci.2010.01.047 20392475
48. Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr., Mowat E, et al. (2008) A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3 : 1494–1500. doi: 10.1038/nport.2008.141 18772877
49. Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE Jr., et al. (2004) Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 48 : 1908–1911. 15105158
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



