-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
Filoviruses (Ebola and Marburg viruses) and arenaviruses (Lassa and Junín viruses) are high-priority pathogens that hijack host proteins and pathways to complete their replication cycles and spread from cell to cell. Here we provide genetic and pharmacological evidence to demonstrate that the host calcium channel protein Orai1 and ER calcium sensor protein STIM1 regulate efficient budding and spread of BSL-4 pathogens Ebola, Marburg, Lassa, and Junín viruses. Our findings are of broad significance as they provide new mechanistic insight into fundamental, immutable, and conserved mechanisms of hemorrhagic fever virus pathogenesis. Moreover, this strategy of targeting highly conserved host cellular protein(s) and mechanisms required by these viruses to complete their life cycle should elicit minimal drug resistance.
Published in the journal: . PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005220
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005220Summary
Filoviruses (Ebola and Marburg viruses) and arenaviruses (Lassa and Junín viruses) are high-priority pathogens that hijack host proteins and pathways to complete their replication cycles and spread from cell to cell. Here we provide genetic and pharmacological evidence to demonstrate that the host calcium channel protein Orai1 and ER calcium sensor protein STIM1 regulate efficient budding and spread of BSL-4 pathogens Ebola, Marburg, Lassa, and Junín viruses. Our findings are of broad significance as they provide new mechanistic insight into fundamental, immutable, and conserved mechanisms of hemorrhagic fever virus pathogenesis. Moreover, this strategy of targeting highly conserved host cellular protein(s) and mechanisms required by these viruses to complete their life cycle should elicit minimal drug resistance.
Introduction
There is an urgent and unmet need for safe and effective therapeutics against high priority pathogens, including filoviruses (Ebola and Marburg) and arenaviruses (Lassa fever and Junín), which can cause fatal infections in humans. We and others have established that enveloped RNA viruses, including hemorrhagic fever viruses, exhibit a common requirement for host pathways, most notably ESCRT pathway functions, for efficient budding [1–7]. Indeed as host dependent budding mechanisms are highly conserved within and sometimes across virus families, they represent innovative and immutable antiviral targets for inhibiting virus transmission and disease progression [8–11]. Importantly, high mutation rates of RNA viruses in general are a factor in their ability to develop resistance to therapeutics that target specific viral proteins or functions [3, 12–23]. Consequently, strategies that target specific host mechanisms required by viruses should reduce the development of resistance.
As a number of these host mechanisms, including steps in ESCRT protein function, are targets of calcium regulation, the focus of this study was to determine whether and how hemorrhagic fever viruses mobilize calcium in host cells and whether calcium so mobilized regulates virus budding. Here we reveal a novel and fundamental requirement for host STIM1 - and Orai-mediated Ca2+ entry that regulates late steps of filovirus and arenavirus egress from mammalian cells. Orai activation is typically linked to either tyrosine kinase or G-protein coupled receptors that activate phospholipase C (PLC) and generate diacylglycerol and inositol 1,4,5-triphoshate (IP3) from membrane phospholipids. IP3 activates receptor/channels on the endoplasmic reticulum (ER) to allow Ca2+ to exit from the ER. The subsequent drop in ER Ca2+ below the KD (400–600μM, [24]) for the N-terminal EF hands of the ER membrane-resident protein STIM1 initiates a conformational change that promotes STIM1 oligomerization and localization to ER regions adjacent to the plasma membrane. At the plasma membrane, STIM1 interacts with and activates Calcium-Release Activated Calcium (CRAC) channels through which extracellular Ca2+ enters the cell (reviewed in [25]). CRAC channels are encoded by the Orai family of proteins (Orai1, 2, & 3; [26–28]) that provide a pathway for sustained extracellular Ca2+ entry to regulate a range of cell functions including gene expression, subcellular trafficking, and the regulation of cell shape and motility [29–31].
Herein, we demonstrate that both filovirus (VP40) and arenavirus (Z) matrix proteins trigger Orai dependent Ca2+ entry in mammalian cells. In addition, suppression of STIM1 expression and genetic inactivation or pharmacological blockade of Orai inhibits Ebolavirus (EBOV), Marburgvirus (MARV), Lassa Virus (LASV), and Junín Virus (JUNV) VLP and infectious virion production and transmission in cell culture. Together, these data establish a novel and critical role for STIM1 - and Orai-mediated Ca2+ entry in late steps of hemorrhagic fever virus egress and establish STIM1 and Orai inhibitors as potential broad-spectrum anti-viral targets for regulation of these and possibly other enveloped RNA viruses that bud by similar mechanisms.
Results
Viral VP40 and Z matrix protein expression mobilizes cytoplasmic Ca2+
While we previously implicated Ca2+ in EBOV VP40-dependent VLP generation [32] our initial objective here was to understand if and how hemorrhagic fever virus matrix proteins trigger a change in cytosolic calcium in host cells. To do this we measured intracellular calcium in cells during an extended time course of EBOV and MARV VP40 (eVP40 and mVP40, respectively) and JUNV Z matrix protein-mediated VLP production. Calcium levels (R-GECO-1 fluorescence, [33]) measured in HEK293T cells under physiological conditions for 18–24 hours revealed that eVP40, mVP40, and JUNV Z protein expression each induced a time-dependent increase in Ca2+ concentration (Fig 1, blue), while the GFP-vector backbone induced a negligible Ca2+ increase that plateaued at a low amplitude or declined to baseline levels (Fig 1, magenta). To probe the role of Orai1 in these responses we performed identical measurements in an HEK293T line that stably expresses a dominant negative mutant Orai1 having a glutamic acid (E) to alanine (A) substitution in its ion selectivity filter (E106A). Incorporation of even a single Orai1 E106A subunit into endogenous WT Orai channels exerts a dominant negative block of its Ca2+ permeation [34]. Importantly, both WT and E106A Orai HEK293T cells exhibited a similar transient Ca2+ elevation following treatment with the membrane permeant SERCA pump inhibitor thapsigargin in Ca2+ free bath solution, indicating that ER stores were intact in E106A Orai1 expressing HEK293T (S1 Fig). The absence of a secondary increase in cytoplasmic Ca2+ (S1 Fig, left panel) following reperfusion of HEK293T Orai1 E106A cells with Ca2+-containing Ringers solution (S1 Fig, right panel) verified the Orai permeation defect of this line. Significantly, in permeation defective Orai1 E106A cells neither eVP40, mVP40, JUNV Z protein (Fig 1, yellow), nor GFP vector (Fig 1, orange) triggered any change in cytoplasmic Ca2+ levels indicating that Ca2+ elevations initiated by expression of EBOV, MARV, and JUNV matrix proteins required and resulted from Ca2+ entry though Orai channels. Consistent with these results from Orai E106A HEK293T cells and specifically the role of Orai, the Orai inhibitor Synta66 similarly blocked the eVP40-mediated increase in cytoplasmic Ca2+ (Fig 1, lower right panel) in WT HEK293T cells.
Fig. 1. Viral matrix protein expression mobilizes cytoplasmic Ca2+. 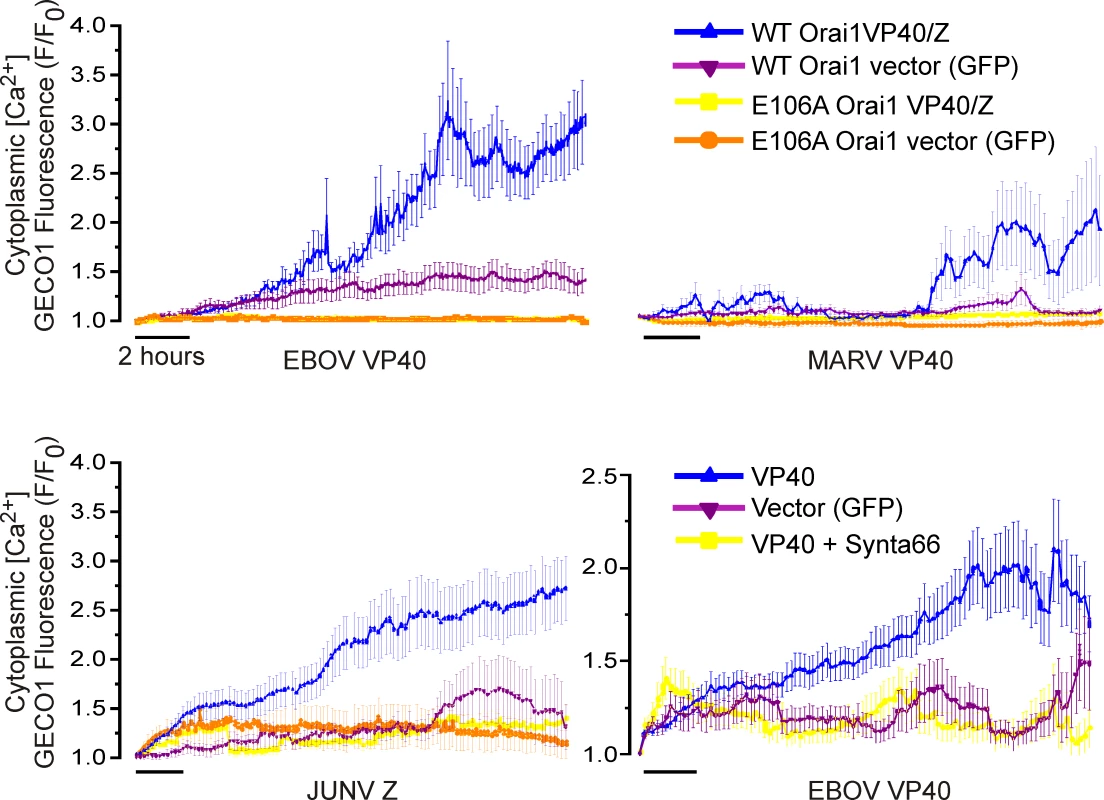
Viral matrix proteins or vector controls and the genetically encoded Ca2+ indicator R-GECO-1 were co-expressed in WT HEK293T cells, HEK293T cells stably expressing the dominant negative permeation defective Orai (E106A) mutant, or WT HEK293T cells treated with the Orai inhibitor Synta66 (50 μM, lower right). Cytoplasmic R-GECO-1 fluorescence emission (580nM) was monitored between 6 and 24 hours post transfection from cells cultured in an environmentally controlled chamber on the microscope stage. Each trace represents the average +/- SEM of at least two experiments of at least 20 cells. Orai1 regulates filovirus and arenavirus VLP production
Given the ability of EBOV, MARV, and JUNV matrix proteins to initiate an Orai-dependent Ca2+ signal in HEK293T cells, we assessed the role of Orai1-mediated calcium signals in eVP40, mVP40, LASV Z or JUNV Z mediated VLP production in WT and Orai1 E106A HEK293T cells. Consistent with a role for Ca2+ entry via Orai1 in VLP production, we found that Orai1 E106A cells did not support efficient filovirus or arenavirus VLP production (Fig 2). Indeed, levels of eVP40 VLPs from Orai1 E106A cells were ~50 fold lower than that from WT cells (Fig 2A, VLPs). Similarly, production of mVP40 VLPs exhibited an even greater dependence on Orai1-mediated calcium entry (Fig 2B, VLPs), as mVP40 VLPs from Orai1 E106A HEK293T cells were ~100 fold lower than that from WT cells (Fig 2B). Orai similarly regulated JUNV Z (Fig 2C) and LASV Z (Fig 2D) VLP production as both JUNV Z and LASV Z protein-mediated VLP production from E106A cells was ~100 fold lower than that from WT HEK293T cells. In all instances, cellular levels of VP40 or Z were similar in WT and E106A cells, indicating no general requirement for Orai1-mediated Ca2+ entry in viral protein expression (Fig 2A–2D; Cells). Together, these data point to a conserved and selective role for Orai-mediated Ca2+ entry in hemorrhagic fever virus budding.
Fig. 2. Budding of filovirus and arenavirus VLPs from WT and E106A cells. 
WT or Orai1 E106A mutant HEK293T cells were transfected as indicated with eVP40 (A), mVP40 (B), JUNV-Z (C), or LASV-Z (D) for 24 hours and VP40 or Z protein levels in cells and VLPs were quantified by immunoblot analysis. Expression of cellular actin served as a loading control. Results are representative of three independent experiments. STIM1 expression is required for eVP40 VLP egress
Implicit in this common critical role for Orai1-mediated Ca2+ entry in EBOV, MARV, JUNV, and LASV VLP production is an upstream requirement for STIM1, the only known trigger for Orai activation in mammalian cells. STIM1 is a single pass ER membrane protein whose activity is regulated by ER Ca2+ binding. Ca2+ dissociation from STIM1 following a decrease in ER concentration triggers a N-terminal conformational change that initiates its multimerization and relocalization within the ER membrane to domains juxtaposed to the plasma membrane [35–37]. The resulting subplasmalemmal STIM1 clusters physically activate Orai channels to allow extracellular Ca2+ to enter the cell [25]. Using eVP40 VLP budding as our model, we probed the role of STIM1 in VLP formation by assessing VLP production from STIM1 suppressed HEK293T cells. eVP40 VLP budding from STIM1 suppressed cells was reduced by approximately 10 fold relative to that from cells receiving random siRNAs or no siRNA (Fig 3A), and the loss of STIM1 had no effect on cellular expression of eVP40 or actin (Fig 3A; Cells). To further confirm this requirement for STIM1 in VLP formation, we utilized a bicistronic vector to suppress endogenous STIM1 (by targeting the 5’ UTR) and rescued its expression with exogenous human STIM1 translated from a shRNA resistant cDNA (shSTIM1-STIM1 plasmid) (Fig 3B). HEK293T WT cells expressing a fixed amount of eVP40 were transfected with increasing amounts of the shSTIM1-suppression vector or empty vector (Fig 3B). While cellular eVP40 expression levels were equivalent under all conditions (Fig 3B, Cells), progressive suppression of STIM1 expression led to a dose-dependent decrease in eVP40 VLP production (Fig 3B, VLPs, middle panel). Importantly, STIM1 re-expression in suppressed cells fully rescued eVP40 VLP production across all levels of STIM1 suppression (Fig 3B, VLPs, bottom panel). Similar to results with STIM1 shRNA, budding of eVP40 VLPs was significantly reduced (Fig 3C, VLP) following siRNA mediated STIM1 suppression (>90%, Fig 3C, middle panel); and over-expression of exogenous STIM1 restored eVP40 VLP production (Fig 3C). Taken together with results from experiments performed on E106A HEK293T cells, these data definitively establish a role for STIM1/Orai dependent Ca2+ signals in regulation of VLP egress.
Fig. 3. STIM1 suppression inhibits egress of eVP40 VLPs. 
A. VP40 was expressed in control and STIM1 suppressed HEK293T cells as indicated and eVP40 VLP production quantified by immunoblot analysis demonstrates significant inhibition of eVP40 production in STIM1 suppressed cells (n = 4 independent experiments). B. Immunoblot analysis of cell extracts and VLPs from HEK293T cells expressing eVP40 in cells co-transfected with indicated amounts of control vector (top panel), STIM1 suppression plasmid (middle panel), or shSTIM1-STIM1 suppression-rescue plasmid (bottom panel). C. Cellular VP40 and STIM1 levels were measured in mock-transfected HEK293T cells, or cells transfected with the indicated plasmids and siRNAs. Results are representative of three independent experiments. Pharmacological block of Orai channels inhibits eVP40 and mVP40 VLP production
Genetic approaches outlined above to modulate STIM1 expression and Orai1 permeation establish a novel and common critical role for STIM1 and Orai1 in filovirus and arenavirus budding. Given the broad utility of targeting ion channels to regulate a range of cell physiological functions, we asked whether pharmacological blockade of Orai might represent an effective strategy for regulating filovirus and arenavirus budding. Although high affinity Orai1 blockers for in vivo applications are not available at present, we tested several commercially available inhibitors including Synta66 and 2-APB, both of which inhibit Orai-mediated Ca2+ entry in HEK293T cells at micromolar levels (10–50 μM) [38, 39] without impacting calcium release from the ER (S2 Fig). Both 2-APB and Synta66 inhibited eVP40 - (Fig 4A and 4B) and mVP40-induced (Fig 4C and 4D) VLP production with identical potency as inhibition of Orai-mediated calcium entry, and neither drug affected cellular expression of VP40 or actin (Fig 4A–4D). A concentration of 2-APB that fully blocks Orai1 channels (50 μM) [38] inhibited eVP40 VLP production (Fig 4A, right panel) by ~5 fold and mVP40 VLP production (Fig 4C, right panel) by ~50 fold. Likewise, Synta66 (50μM) substantially inhibited eVP40 (~5 fold) and mVP40 VLP (~10 fold) production (Fig 4B and 4D) with no effect on steady state levels of cellular VP40 or actin and without altering membrane localization of viral proteins (S3 Fig). Importantly, as neither 2-APB (Fig 4E) nor Synta66 (Fig 4F) exerted cytotoxic effects on cells under conditions of these measurements (cell viability, cellular production of VP40, or VP40 membrane localization, Fig 4 and S3 Fig), their anti-budding activity can be attributed to inhibition of Orai-mediated Ca2+ entry. Finally, an additional Orai selective inhibitor (RO2959 [40]), which recently became commercially available, also blocks eVP40 VLP formation (~10-fold) with a potency that parallels its inhibition of calcium permeation of the channel (S4 Fig, IC50 = ~2.5μM). Thus, the sensitivity of budding to three chemically distinct Orai inhibitors, at the same concentration that blocks calcium permeation of Orai, further substantiates the critical role of Orai-mediated Ca2+ entry in VLP production.
Fig. 4. Pharmacological effect of Synta66 and 2-APB on egress of filovirus VLPs. 
HEK293T expressing eVP40 (A and B) or mVP40 (C and D) were treated with indicated concentrations of 2-APB or Synta66. eVP40 and mVP40 levels in cell extracts and VLPs were detected by immunoblot analysis and quantified in VLPs (bar graphs). 2-APB (E) and Synta66 (F) cytotoxicity was assessed with an MTT viability assay under conditions that mimicked those used for the VLP experiments. In all experiments, cellular actin served as a loading control. Relative protein bands densities were normalized to untreated sample densities (0 μM). Plotted values represent the average (+/- S.E.M) of 3–6 independent experiments and were analyzed with either Welch's t-test or Wilcoxon rank-sum test (*p = 0.01, **p = 0.05). Pharmacological block of Orai channels inhibits arenavirus budding
We next sought to validate VLP findings by examining the effect of the Orai1 inhibitors Synta66 and 2-APB on budding of the live attenuated Candid-1 JUNV vaccine strain [41, 42]. Briefly, VeroE6 cells infected with live attenuated Candid-1 JUNV were cultured in the absence or presence of Orai inhibitors, and infectious virions produced from these cells were quantified in a focus forming assay (Fig 5). Enumeration of JUNV foci revealed a statistically significant, dose-dependent reduction in JUNV virus production following treatment with Synta66 (Fig 5A) or 2-APB (Fig 5B). Moreover, neither compound affected the viability of cells cultured under conditions mimicking those used for JUNV infection experiments (Fig 5A and 5B, right panels), nor affected the synthesis of JUNV GP in infected VeroE6 cells at any concentration tested (Fig 5A and 5B, Western blots). Together, these findings corroborate results of VLP budding assays (Fig 2) and demonstrate that Orai1-mediated calcium entry is required for efficient budding of infectious JUNV.
Fig. 5. Synta66 and 2-APB inhibit egress of live JUNV (Candid-1 strain) from VeroE6 cells. 
JUNV foci were visualized and the number of all detectable foci was plotted for each Synta66 (A) or 2-APB concentration (B). Statistical significance was analyzed by one way ANOVA (*p < 0.01 for Synta66) (*p < 0.05, **p < 0.01 for 2-APB). MTT based cell viability measurements demonstrate no toxicity of Synta66 (A) or 2-APB (B) under identical conditions as those used for JUNV infection of VeroE6 cells. Each value is normalized to cells treated with DMSO alone. Comparable levels of JUNV GP were detected by immunoblot analysis in JUNV infected VeroE6 cells in the absence (DMSO alone) or presence of Synta66 (A) or 2-APB (B). Orai1 regulates budding of infectious pathogenic strains of EBOV, MARV, LASV, and JUNV
Based on the general requirement we identify for Orai channels in filovirus and arenavirus VLP production and JUNV (Candid-1) budding, we next sought to determine whether Orai channels regulate spread of infectious pathogenic strains of EBOV, MARV, LASV, and JUNV. We first examined viral spread, an indicator of efficient viral budding, in HEK293T cells that constitutively express the dominant negative permeation defective variant of Orai1 (E106A) used in VLP assays described above (Fig 2). These cells were infected at a low multiplicity of infection (MOI), which resulted in the infection of approximately 2–5% of the cells. Cells were then incubated for a period of time that equates to several rounds of viral replication, allowing us to assess viral spread. We observed that the percent of Orai1 E106A expressing cells infected with live BSL-4 variants of EBOV, MARV, JUNV, or LASV was significantly lower than Orai WT cells infected with the same viruses (Fig 6). These results are consistent with a role for Orai in the spread of infectious filoviruses and arenaviruses.
Fig. 6. Genetic inactivation of Orai Ca2+ permeation inhibits egress of authentic filoviruses and arenaviruses. 
Wild type HEK293T cells and a Ca2+ permeation defective HEK293T line that stably overexpresses the dominant negative Orai E106A mutant were infected with LASV (MOI 0.05), JUNV (MOI 0.1), MARV (MOI 0.1), or EBOV (MOI 0.5). Cellular virus levels were detected by immunofluorescence staining of fixed cells at 72 (LASV) or 96 (JUNV, MARV, EBOV) hours post infection. The percent of infected cells relative to total viable cells per condition is plotted and error bars represent standard error of mean (SEM). Statistical significance was determined by student t test, two-tailed (*** p < 0.0001, **** p < 0.0001 as indicated) We next assessed the effect of the Orai blocker Synta66 on the spread of these BSL-4 pathogens, because it is a more consistent Orai blocker than 2-APB. Viral spread was assessed by infecting HeLa cells with LASV, JUNV, MARV, or EBOV at a low MOI and then treating with vehicle or Synta66 at the indicated concentrations beginning 1 hour post infection and for the duration of experiments. Seventy two (LASV, JUNV) or 96 (MARV, EBOV) hours post infection we quantified the percentage of cells infected with virus.
For each virus, we observed a significant Synta66 dose-dependent decrease in the percentage of cells infected (Fig 7A). Consistent with inhibition of viral spread, we also observed a general decrease in the number and size of infected cell clusters with increasing Synta66 concentration (Fig 7B). Similar to the more potent inhibition by Synta66 of mVP40 - versus eVP40-mediated VLP production (Fig 4), Synta66 also exerted more potent inhibition of live MARV than EBOV. Interestingly, the spread of both arenaviruses was more sensitive to Orai inhibition than either filovirus (Fig 7A). In general, cultures treated with higher concentrations of Synta66 and for a prolonged period of time (72–96 hours) contained fewer cells than vehicle control treated cultures as measured by the number of nuclei (Fig 7B). For this reason, we normalized the results as the percent of cells infected at the time of analysis for each condition. The decrease in cell numbers, however, does not appear to reflect toxicity (see Figs 4F and 5A). Indeed, as HeLa cells autonomously divide, fewer cells more likely reflects an effect of prolonged Synta66 treatment on cell proliferation. Nonetheless, we evaluated Synta66 induced toxicity by two separate methodologies. Cell-titer Glo “viability” measurements revealed that prolonged Synta66 produced a dose-dependent decrease in ATP (S5 Fig) that is attributed to a decrease in the overall number of cells in cultures and not an effect of Synta66 on cell viability (see Fig 7B). We then utilized an Alamar Blue assay to assess the metabolic health of Synta66 treated cells. Indeed, cellular oxidation-reduction potential of Synta66 treated and vehicle control treated cells were equivalent, confirming comparable metabolic activity (S5 Fig). Thus, while prolonged Synta66 treatment resulted in lower overall cell numbers, those cells that are present are metabolically healthy and are fully capable of producing virus.
Fig. 7. Synta66 inhibits egress of authentic filoviruses and arenaviruses. 
A. HeLa cells were infected with LASV (MOI 0.01), JUNV (MOI 0.1), MARV (MOI 0.1), or EBOV (MOI 0.1) and treated with Synta66 at indicated concentrations. Cellular virus levels were detected by immunofluorescence staining of fixed cells at 72 (LASV, JUNV) or 96 (MARV, EBOV) hours post infection with virus specific antibodies. The percent of cells infected (relative to total cells) was determined using Harmony High Content Imaging and Analysis software (PerkinElmer). Data is expressed relative to vehicle (DMSO) control treated cells for each virus. The percent of infected cells for vehicle control treatment was as follows (LASV = 12% ± 2.69%, JUNV = 9% ± 1.11%, MARV = 20% ± 1.92%, EBOV = 15% ± 1.55%). Error bars indicate standard error of mean (SEM) and statistical significance was determined by two way ANOVA with Bonferroni multiple comparisons (** p < 0.01, **** p < 0.0001). B. Representative images from a single live virus experiment demonstrate Synta66 dose (0, 5, 10, 30, 50μM) dependent inhibition of virus spread. For each condition, respective viruses were detected with virus specific antibodies (green). The value in the upper left hand corner of each image is the percentage of total cells infected. For each condition, the total number of cells was determined by staining nuclear DNA with Hoechst DNA dye. We next sought to definitively establish that the effect of Synta66 on virus spread is due to inhibition of virus egress and not entry. We first pretreated HeLa cells with Synta66 and then infected with a high MOI of LASV, JUNV, MARV, or EBOV. Cells were then fixed after only 2–3 viral replication cycles. Infecting with a high MOI and fixing soon after infection ensured that the extent of infection minimally involves spread between cells and rather reflects the extent of primary infection. Under these high MOI conditions, we observed relatively little effect of Synta66 on infection levels with only modest inhibition of infection evident at high Synta66 concentrations (S6 Fig). Further confirmation that Synta66 blocks egress of live filoviruses and arenaviruses was obtained by assessing the amount of virus released into culture supernatants. Supernatants were collected between 48 and 96 hours post-infection with JUNV, LASV, MARV, or EBOV from Synta66 or vehicle treated HeLa cells. Consistent with all of our VLP (Figs 2–4) and live virus data (Figs 5, 6, 7A and 7B) we found that Synta66 (30μM) significantly reduced the titer of infectious Lassa, Junín, Marburg, and Ebola virion particles in culture supernatants (Fig 8). Taken together, this data provide a clear and comprehensive demonstration that Synta66 treatment significantly impairs authentic filovirus and arenavirus budding and release from infected cells.
Fig. 8. Synta66 decreases the number of viral particles in supernatants. 
A virus plaque assay used to quantify infectious virions in supernatants of virus infected cells demonstrates that Synta66 (30 μM) significantly inhibits the budding and spread of live LASV, JUNV, MARV, and EBOV. Error bars indicate standard error of mean (SEM) and statistical significance was determined by two way ANOVA with Bonferroni multiple comparisons (**p<0.01, ***p < 0.001, ****p < 0.0001). In summary, our results clearly establish that 1) Orai1-mediated Ca2+ entry is a critical virus-triggered host signal that regulates filovirus and arenavirus budding, and 2) STIM1 and Orai1 represent novel targets for broad-spectrum control of these emerging and often fatal viruses. Indeed, the conserved role for Orai mediated calcium entry among these four hemorrhagic fever viruses raises the interesting possibility that Orai inhibitors may have general utility for broad spectrum control of these and other enveloped RNA viruses that bud by similar Ca2+ dependent mechanisms.
Discussion
The recent catastrophic outbreak of EBOV in West Africa highlights the need to develop therapeutics for EBOV and other hemorrhagic fever viruses. Indeed, much progress has been made toward the development of candidate vaccines and therapies against EBOV that are currently in clinical trials. Nevertheless, it is critically important that we improve our understanding of the mechanisms of hemorrhagic fever virus pathogenesis not only to identify novel viral targets, but also to identify host targets and common mechanisms that these viruses require for completion of their life cycles as these could lead to the development of broad spectrum host oriented therapeutics.
A key advantage of therapeutics that target conserved host pathways required broadly by families of viruses for transmission is the potential for broad spectrum efficacy compared with drugs that target strain specific viral targets. Moreover, host targets should be essentially immutable and thereby insensitive to selective pressures that normally allow pathogens to develop drug resistance [3, 12–23]. Here we focused on the second messenger Ca2+ and the host proteins responsible for its mobilization and asked whether calcium signals within host cells orchestrate virus assembly and budding.
While calcium has been implicated generally in EBOV and HIV-1 budding [32, 43, 44], previous efforts have not addressed if and how matrix proteins encoded by filoviruses or arenaviruses might trigger changes in Ca2+ concentration in host cells. Herein, we demonstrate for the first time that the filovirus matrix protein VP40 and JUNV Z protein trigger STIM1/Orai activation and that the resulting influx of extracellular Ca2+ controls both VLP formation and production of infectious filovirus and arenavirus progeny. Moreover, using Orai channel inhibitors, Orai permeation defective lines, and by suppressing STIM1 expression, we establish STIM1 and Orai as effective host targets for pharmacological regulation of virus egress. It should be noted; however, that we cannot rule out a role for other Orai isoforms (Orai2 and Orai3) in to the residual budding observed for live virus from E106A or Synta66 treated cells.
While we have established a critical role for Orai-mediated calcium entry in budding of hemorrhagic fever viruses, the mechanism by which Ca2+ does so remains an important question and the focus of ongoing efforts. Indeed, a number of critical steps implicated in efficient budding of enveloped RNA viruses have been linked to cellular Ca2+ signals, including the activation and localization of specific ESCRT components. Although not absolutely required, the ESCRT pathway has been shown to play a key role in efficient budding of a plethora of RNA viruses including filoviruses, arenaviruses, rhabdoviruses, and retroviruses [5]. It is tempting to speculate that the observed calcium regulation of budding described here may be linked mechanistically to the role of ESCRT during virus egress. For example, the structure, activation, and interactions of ESCRT-related proteins such as Tsg101, Nedd4, and Alix have been shown to be regulated in part by calcium [44–47]. Additionally, given that Ca2+ control of membrane repair reflects ESCRT induced shedding of damaged membrane [48], one might also speculate that Ca2+ dependent mechanisms are similarly triggered by insertion of viral proteins in the plasma membrane. Studies underway are thus focused on determining whether Ca2+ controls budding through regulation of ESCRT pathway function.
STIM1 and Orai1 mediated Ca2+ signals have been implicated in distinct steps of the life cycle of other viruses including the replication of Rotaviruses, which are non-enveloped RNA viruses that do not bud from the plasma membrane. Constitutive STIM1 (and Orai1) activation observed in rotavirus-infected cells reflects an effect of its nonstructural protein 4 (NSP4) on endoplasmic reticulum Ca2+ permeability [49]. Indeed, ongoing efforts within our group to understand the mechanisms by which hemorrhagic fever virus matrix proteins trigger STIM1/Orai activation include testing whether VP40 might likewise trigger Ca2+ leak from the ER by inhibiting SERCA pump activity. Furthermore, Ca2+ influx also seems to regulate entry of West Nile virus [50, 51], Coxsackievirus [52, 53], Hepatitis B virus [54], and Epstein Barr virus [55, 56].
Recently it was shown that subunits of a functionally distinct family of voltage-gated calcium channels (VDCCs) also play a role in JUNV and Mouse Mammary Tumor pseudovirus entry and infection [57] and that the VDCC blockers nifedipine and verapamil suppressed host cell entry by these viruses. Surprisingly; however, in this instance the involvement of VDCC subunits seemed to be distinct from any role in regulating Ca2+ levels. How VDCC inhibitors might operate independently of any action on VDCC Ca2+ permeation is unclear, but could reflect the promiscuous affinity of VDCC inhibitors for channels including voltage-gated potassium (Kv) channels. Indeed, verapamil and nifedipine also block voltage-gated potassium channels that set the membrane potential of non-excitable cells [58, 59]. Depolarization of the plasma membrane as a result of Kv channel blockade could indirectly block calcium entry by dissipating the electrical driving force (membrane potential) required for calcium permeation of Orai [60–62].
While these studies cumulatively point to additional roles for Orai1-mediated and independent Ca2+ influx in steps of infection and replication used by a range of disparate viruses, these roles are distinct from the selective requirement we identify for Orai-dependent calcium entry in budding of filoviruses and arenaviruses. However, Orai might represent a conserved target for regulating budding of additional enveloped RNA viruses, including retroviruses such as HIV-1, which buds by similar mechanisms. Indeed, similar to hemorrhagic fever viruses, the HIV-1 matrix protein Gag directs HIV-1 budding in part, via well-characterized L-domain interactions with ESCRT proteins, and Gag mediated VLP formation also exhibits dependence on Ca2+ regulation [43]. Further study is needed to fully assess the role for calcium in the HIV-1 lifecycle because, unlike filoviruses and arenaviruses, Gag-mediated VLP production was found to be insensitive to high concentrations of 2-APB (up to 200uM) that fully block Ca2+ permeation of Orai channels [44].
In conclusion, we provide the first direct evidence that host Ca2+ signals, triggered by virus activation of STIM1 and Orai, are among key host mechanisms that orchestrate late steps of filovirus and arenavirus assembly and budding. Importantly, from a therapeutic perspective, Orai channels are ubiquitously expressed and like ion channels in general, they represent pharmacologically accessible (cell surface) therapeutic targets. While Orai1 inhibitors by themselves appear to have broad spectrum efficacy, an exciting possibility raised by our results is that drug cocktails formulated to target sequential steps in the virus life cycle, including entry, L-domain/host interactions, and other steps involved in budding, could produce enhanced potency, coverage and efficacy over approaches targeting any one host dependent step in the virus life cycle. Thus, while other calcium channel modulators identified may have distinct targets and even calcium independent effects, they may synergize with Orai1, and also L-domain inhibitors we’ve described previously that block VP40 and Z protein L-domain interactions with host Nedd4 and Tsg101 [42, 63].
Finally, the ability of certain individuals to survive hemorrhagic fever virus infection seems to reflect their capacity to mount a robust anti-viral immune response. In the context of the severity and the acute nature of these viral diseases, the impact of side effects and even minor effects on cell proliferation that might be associated with long term administration of Orai inhibitors that would be required for immune suppression and immune modulation, may be tolerable in the context of infection with these highly pathogenic and often fatal viruses. Indeed, there is no evidence from murine models that the loss of STIM or Orai activity or function would affect antigen induced lymphocyte activation required for an antiviral immune response [64, 65]. Thus our prediction is that administration of Orai1 or STIM1 inhibitors, or cocktails that could also include L-domain inhibitors, would slow or dampen virus transmission within and between individuals, and thereby could provide infected individuals additional time needed to mount a protective adaptive immune response. Although Synta66 and the more potent compound RO2959 are no longer being developed as therapeutics, several smaller pharmaceutical companies and academic groups persist in efforts to develop potent Orai1 inhibitors to suppress the pathogenesis of chronic immune-mediated and inflammatory diseases. If and when these become available, direct inhibition of enveloped RNA virus budding from host cells and transmission between individuals may represent an entirely novel use for these channel blockers.
Materials and Methods
Cell lines, plasmids, and reagents
HEK293T, HeLa, and VeroE6 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml)/streptomycin (100μg/ml) at 37°C in a humidified 5% CO2 incubator. The stable HEK293T Orai1 E106A mutant-expressing cell line was maintained in DMEM with 10% FCS, penicillin (100 U/ml)/streptomycin (100μg/ml) in the presence of 500 μg/ml of G418. HeLa cells were maintained in Minimal Essential Medium (MEM) with 5% fetal bovine serum, penicillin (100 U/ml)/streptomycin (100μg/ml) at 37°C in a humidified 5% CO2 incubator. The pCAGGS based plasmids expressing EBOV VP40, MARV VP40, JUNV Z, LASV Z, and GFP-eVP40 have been described previously [42, 63, 66]. mVP40 and JUNZ Z protein are flag tagged while eVP40 was detected using an anti-eVP40 polyclonal antibody previously described [67]. mVP40 and JUNV Z protein were detected with an anti-flag monoclonal antibody (Sigma-Aldrich), and STIM1 was detected with a rabbit anti-STIM1 specific polyclonal antibody (gift of Dan Billadeau, Mayo Clinic). 2-aminoethoxy diphenyl borate (2-APB, Sigma Aldrich), Synta66, and RO2959 (Glixx Labs, Southborough, MA) were freshly prepared from stock solutions in DMSO.
Viability assays
Cell viability in VLP budding and live virus infection assays was examined using an MTT assay (Amresco). 5×103 HEK293T or VeroE6 cells were plated in collagen-coated 96-well tissue culture plates in triplicate. Cells were transfected with empty vector using Lipofectamine for 6 hours, and incubated in serum-free or 2% FCS in phenol-red-free OPTI-MEM in the presence of Synta66 or 2-APB at the indicated concentrations for 20 hours, which mimics the transfection and treatment conditions for VP40 VLP budding. 20μl of MTT solution (5mg/ml in PBS) was added into each well and cells were incubated for 3.5 hours. Media was discarded and 150 μl DMSO was added. Absorbance was determined by spectrophotometry using a wavelength of 590 nm. For experiments with BSL-4 variants of filoviruses and arenaviruses, HeLa cells were seeded in 96 well plates ~24 hours prior to addition of Synta66 or vehicle control at indicated concentrations. Cells were incubated for 72 or 96 hour at 37°C in a humidified 5% CO2 incubator and viability was assessed using either CellTiter-Glo assay (Promega), or AlamarBlue assay (Life Technologies), in accordance with manufacturer’s instructions.
Calcium imaging
HEK 293T Orai1-wild type and Orai1-E106A mutant cells (kind gift from Dr. Jonathan Soboloff, Temple University) were plated at 5x105 cells/well in a 2-chambered Lab-Tek II Chambered #1.5 slide (Nunc, Rochester, NY) and grown overnight at 37°C, 5% CO2 in Dulbecco’s modified Eagle’s Medium supplemented with 4.5g/L glucose, L-glutamine, sodium pyruvate (Mediatech, Inc., Manassas, VA), 10% FBS (Gibco), and 1% Penicillin/Streptomycin (Gibco). Cells were transfected with 1μg of R-GECO-1 plasmid (Addgene, Cambridge, MA) using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions in phenol-red-free OPTI-MEM. The next day, cells were transfected with GFP-eVP40 fusion plasmid (GFP-eVP40) using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions in phenol-red-free OPTI-MEM. Six hours post transfection, fresh phenol-red-free OPTI-MEM was added to the wells, and cells were imaged at 37°C and 5% CO2 in a custom environmental chamber for the duration of the imaging on a Leica DMI4000 with Yokagawa CSU-X1 Spinning Disk Microscope with a 20X dry objective. Cellular R-GECO-1 fluorescence was imaged every 4 seconds for 1 minute periods, repeated every 10 minutes over 18 hours with a Hamamatsu 16-bit cooled EMCCD camera. Imaging and data analysis were performed using the Metamorph 7.6 imaging suite. Normalized fluorescence intensity (F/F0, where F0 is calculated as the average fluorescence intensity for the initial 1 minute interval) was calculated for each region of interest (ROI) in the time-series.
Virus like particle budding assay
WT HEK293T or HEK293T E106A cells were seeded in collagen-coated six-well plates and transfected with 0.5μg of the indicated expression plasmids using Lipofectamine (Invitrogen) and the protocol of the supplier. At 6 hours post-transfection, cells were incubated in serum-free OPTI-MEM media or 2% FCS DMEM for 20–24 hours. Cells were incubated with vehicle (DMSO) alone, Synta66, or 2-APB at the indicated concentrations. Culture medium was harvested and centrifuged at 2500 rpm for 10 minutes to remove cellular debris, layered over a 20% sucrose cushion in STE buffer and centrifuged at 220,000xG for 2 hours at 4°C. The VP40 VLP-containing pellet was suspended in 50 μl of STE buffer at 4°C overnight. Cells were lysed in RIPA buffer as described above. Viral proteins in VLPs and cell lysates was detected by SDS-PAGE and Western-blot using primary rabbit anti-VP40 antibody for Ebola VP40, mouse anti-flag antibody for Marburg VP40, or mouse anti-HA antibody for LASV and JUNV Z followed by an appropriate HRP-conjugated secondary antibody.
siRNA and shRNA mediated protein suppression
Human STIM1 siRNAs were purchased from Dharmacon SMARTpools. ON-TARGETplus STIM1 siRNA (Thermo SCIENTIFIC) is a mixture of 4 siRNAs to specifically silence the target gene. HEK293T cells in OPTI-MEM media in collagen-coated six-well plate were transfected twice with either control siRNA or STIM1 siRNA at a final concentration of 200nM using Lipofectamine (Invitrogen) at 2-day intervals. The final transfection included both siRNAs and 0.5μg of Ebola VP40 expression plasmid. VLPs and cell lysates were harvested at 48 hours following the last transfection as described above. VP40 protein levels in VLPs and VP40 and STIM1 levels in cell extracts were analyzed by Western-blot with rabbit anti-VP40 antibody, or rabbit anti-STIM1 antibody, followed by anti-rabbit IgG HRP-conjugated secondary antibody. STIM1 suppression and rescue was accomplished using bicistronic vectors developed in house. STIM1 shRNA generated against the 5’ untranslated region of human STIM1 was expressed using the H1 promoter and human STIM1 cDNA expressed from a CMV promoter in the same construct. VLP samples and cell lysates were harvested at 48 hours after last transfection and analyzed by Western-blot.
Candid-1 Junin virus and infection
The Candid-1 vaccine strain of JUNV was kindly provided by Robert B. Tesh (U.T.M.B., Galveston, TX) via Susan R. Ross (Univ. of Penn., Philadelphia, PA), and was propagated in VeroE6 cells as described previously [41]. For JUNV infection, VeroE6 cells were infected with JUNV (Candid-1) at an MOI of 0.02 for 42 hours at 37°C. Supernatants were removed and the cells were washed 3X with 1X PBS. Cells were then treated with DMSO alone, or the indicated concentrations of Synta66 for an additional 30 hours. Virions were harvested from the supernatant samples as described above for VLPs, and then used to infect fresh monolayers of VeroE6 cells for 48 hours for quantification of all foci detected in 6-well plates using fluorescence microscopy as described previously [42].
Authentic virus infection of HEK293T cells
For all experiments using authentic viruses, Ebola virus (Kikwit isolate), Marburg virus (Ci67 isolate), Lassa virus (Josiah isolate), and Junin virus (Espindola isolate) were used. Wild-type HEK293T cells and Orai1 E106A mutant-expressing HEK293T cells, seeded in 96 well black plates (Corning BioCoat Cellware, Collagen Type I), were incubated with EBOV (MOI = 0.5), MARV (MOI = 0.1), JUNV (MOI = 0.1), or LASV (MOI = 0.05) in a Biosafety Level 4 laboratory located at USAMRIID. Following 1 hour absorption, virus inoculum was removed and growth media was added. Cells were then incubated at 37°C, 5% CO2, 80% humidity for 72 (LASV) or 96 (EBOV, MARV, JUNV) hours, at which time the cells were washed once with PBS and submerged in 10% formalin prior to removal from the BSL4 laboratory. Formalin was removed and cells were washed 3 times with PBS. For LASV infection only, cells were treated with 300mM NaOH for 20 minutes at room temperature prior to the blocking step. Cells were blocked by adding 3% BSA/PBS to each well and incubating at 37°C for 2 hours. EBOV GP-specific mAb KZ52 (kind gift from Kartik Chandran, Albert Einstein College of Medicine, Bronx, NY), MARV GP-specific mAb 9G4 (USAMRIID), LASV GP-specific mAb 52-161-6 (USAMRIID), and JUNV GP-specific mAb GD01-AG02 (BEI Resources), diluted in 3% BSA/PBS, were added to appropriate wells containing infected cells and incubated at room temperature for 2 hours. Cells were washed 3 times with PBS prior to addition of goat anti-mouse or goat anti-human IgG-AlexaFluor 594 (Invitrogen, Molecular Probes) secondary antibody. Following 1 hour incubation with secondary antibody, cells were washed 3 times prior to addition of Hoechst 33342 (Invitrogen, Molecular Probes) diluted in PBS. Cells were imaged and percent of virus infected cells calculated using the Operetta High Content Imaging System (PerkinElmer) and Harmony High Content Imaging and Analysis Software (PerkinElmer). Statistical significance between wild-type cells and Orai1 E106A mutant cells was determined using student t test, two-tailed.
Authentic virus infection of Synta66 treated HeLa cells
For immunofluorescence based assays, HeLa cells, seeded in 96 well black plates (Greiner Bio-One Cellcoat), were incubated with EBOV (MOI 0.1), MARV (MOI 0.1), LASV (MOI 0.01), or JUNV (MOI 0.1) for 1 hour at 37°C, 5% CO2, 80% humidity. Virus inoculum was removed and cells were washed once with PBS. Synta66 was diluted in HeLa cell culture media at indicated concentrations and added to cells. An equivalent percentage of DMSO in HeLa media served as the vehicle control. Cells were then incubated at 37°C, 5% CO2, 80% humidity for 72 (LASV and JUNV) or 96 (EBOV and MARV) hours at which time, the cells were washed once with PBS and submerged in 10% formalin prior to removal from the BSL4 laboratory. As described above, virus specific antibodies were added to appropriate wells containing infected cells and samples processed as previously described, except that goat anti-mouse or goat anti-human IgG-AlexaFluor 488 (Invitrogen, Molecular Probes) was used as the secondary antibody. Statistical significance was determined by two way ANOVA with Bonferroni multiple comparisons relative to vehicle control treated cells. For viral titer analysis, HeLa cells were seeded in 6 well plates ~24 hours prior to infection with LASV (MOI = 0.01), JUNV (MOI = 0.1), MARV (MOI = 0.1), or EBOV (MOI = 0.1). One hour after infection, cells were treated with Synta66 or vehicle control at indicated concentrations. Culture supernatants were collected at 48 (MARV), 72 (LASV, JUNV) or 96 (EBOV) hours and cell debris removed by centrifugation. Viral titers of clarified supernatants were determined by routine plaque assay as previously described [68–70]. All data is a graphical representation of at least two independent, replicate experiments. Statistical significance of log transformed data was determined by two way ANOVA with Bonferroni multiple comparisons relative to vehicle control treated cells.
Supporting Information
Zdroje
1. Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology. 2008;372(2):221–32. 18063004
2. Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;344(1):64–70. 16364737
3. Harty RN. No exit: targeting the budding process to inhibit filovirus replication. Antiviral Res. 2009;81(3):189–97. doi: 10.1016/j.antiviral.2008.12.003 19114059
4. Jasenosky LD, Kawaoka Y. Filovirus budding. Virus research. 2004;106(2):181–8. 15567496
5. Liu Y, Harty RN. Viral and host proteins that modulate filovirus budding. Future Virol. 2010;5(4):481–91. 20730024
6. Urata S, de la Torre JC. Arenavirus budding. Advances in virology. 2011;2011 : 180326. doi: 10.1155/2011/180326 22312335
7. Neumann G, Ebihara H, Takada A, Noda T, Kobasa D, Jasenosky LD, et al. Ebola virus VP40 late domains are not essential for viral replication in cell culture. Journal of Virology. 2005;79(16):10300–7. 16051823
8. Feldmann H, Klenk HD. Filoviruses. In: Baron S, editor. Medical Microbiology. 4th ed. Galveston (TX)1996.
9. Feldmann H, Klenk HD. Marburg and Ebola viruses. Advances in virus research. 1996;47 : 1–52. 8895830
10. Grant A, Seregin A, Huang C, Kolokoltsova O, Brasier A, Peters C, et al. Junin virus pathogenesis and virus replication. Viruses. 2012;4(10):2317–39. doi: 10.3390/v4102317 23202466
11. Yun NE, Walker DH. Pathogenesis of Lassa fever. Viruses. 2012;4(10):2031–48. doi: 10.3390/v4102031 23202452
12. Lingappa UF, Wu X, Macieik A, Yu SF, Atuegbu A, Corpuz M, et al. Host-rabies virus protein-protein interactions as druggable antiviral targets. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):E861–8. doi: 10.1073/pnas.1210198110 23404707
13. Adamson CS, Freed EO. Novel approaches to inhibiting HIV-1 replication. Antiviral research. 2010;85(1):119–41. doi: 10.1016/j.antiviral.2009.09.009 19782103
14. Aman MJ, Kinch MS, Warfield K, Warren T, Yunus A, Enterlein S, et al. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral research. 2009;83(3):245–51. doi: 10.1016/j.antiviral.2009.06.001 19523489
15. Andrei G, De Clercq E. Molecular approaches for the treatment of hemorrhagic fever virus infections. Antiviral research. 1993;22(1):45–75. 8250543
16. Connor JH, McKenzie MO, Parks GD, Lyles DS. Antiviral activity and RNA polymerase degradation following Hsp90 inhibition in a range of negative strand viruses. Virology. 2007;362(1):109–19. 17258257
17. Garcia M, Cooper A, Shi W, Bornmann W, Carrion R, Kalman D, et al. Productive Replication of Ebola Virus Is Regulated by the c-Abl1 Tyrosine Kinase. Science translational medicine. 2012;4(123):123ra24. doi: 10.1126/scitranslmed.3003500 22378924
18. Kinch MS, Yunus AS, Lear C, Mao H, Chen H, Fesseha Z, et al. FGI-104: a broad-spectrum small molecule inhibitor of viral infection. American journal of translational research. 2009;1(1):87–98. 19966942
19. Kolokoltsov AA, Adhikary S, Garver J, Johnson L, Davey RA, Vela EM. Inhibition of Lassa virus and Ebola virus infection in host cells treated with the kinase inhibitors genistein and tyrphostin. Archives of virology. 2012;157(1):121–7. doi: 10.1007/s00705-011-1115-8 21947546
20. Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12978–83. 14563923
21. Tavassoli A, Lu Q, Gam J, Pan H, Benkovic SJ, Cohen SN. Inhibition of HIV budding by a genetically selected cyclic peptide targeting the Gag-TSG101 interaction. ACS chemical biology. 2008;3(12):757–64. doi: 10.1021/cb800193n 19053244
22. Urata S, Ngo N, de la Torre JC. The PI3K/Akt Pathway Contributes To Arenavirus Budding. J Virol. 2012;86(8):4578–85. doi: 10.1128/JVI.06604-11 22345463
23. Warren TK, Warfield KL, Wells J, Enterlein S, Smith M, Ruthel G, et al. Antiviral activity of a small-molecule inhibitor of filovirus infection. Antimicrobial agents and chemotherapy. 2010;54(5):2152–9. doi: 10.1128/AAC.01315-09 20211898
24. Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. JBiolChem. 2006;281(47):35855–62.
25. Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28 : 491–533. doi: 10.1146/annurev.immunol.021908.132550 20307213
26. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312(5777):1220–3. 16645049
27. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–85. 16582901
28. Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. ProcNatlAcadSciUSA. 2006;103(24):9357–62.
29. Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol. 2009;10(1):3–5. doi: 10.1038/ni0109-3 19088731
30. Babich A, Burkhardt JK. Coordinate control of cytoskeletal remodeling and calcium mobilization during T-cell activation. Immunological reviews. 2013;256(1):80–94. doi: 10.1111/imr.12123 24117814
31. Freedman BD. Mechanisms of calcium signaling and function in lymphocytes. Critical Reviews in Immunology. 2006;26(2):97–111. 16700648
32. Han Z, Harty RN. Influence of calcium/calmodulin on budding of Ebola VLPs: implications for the involvement of the Ras/Raf/MEK/ERK pathway. Virus Genes. 2007;35(3):511–20. 17570046
33. Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PK, et al. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nature methods. 2013;10 : 1105–07. doi: 10.1038/nmeth.2647 24056873
34. Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–13. doi: 10.1126/science.1228757 23180775
35. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. CurrBiol. 2005;15(13):1235–41.
36. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. JCell Biol. 2005;169(3):435–45.
37. Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–5. 16208375
38. Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. JPhysiol. 2001;536(Pt 1):3–19.
39. Ng SW, Di CJ, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. JBiolChem. 2008;283(46):31348–55.
40. Chen G, Panicker S, Lau KY, Apparsundaram S, Patel VA, Chen SL, et al. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Molecular immunology. 2013;54(3–4):355–67. 23357789
41. Cuevas CD, Lavanya M, Wang E, Ross SR. Junin virus infects mouse cells and induces innate immune responses. Journal of virology. 2011;85(21):11058–68. doi: 10.1128/JVI.05304-11 21880772
42. Lu J, Han Z, Liu Y, Liu W, Lee MS, Olson MA, et al. A host-oriented inhibitor of Junin Argentine hemorrhagic fever virus egress. J Virol. 2014;88(9):4736–43. doi: 10.1128/JVI.03757-13 24522922
43. Ehrlich LS, Carter CA. HIV Assembly and Budding: Ca(2+) Signaling and Non-ESCRT Proteins Set the Stage. Mol Biol Int. 2012;2012 : 851670. doi: 10.1155/2012/851670 22761998
44. Ehrlich LS, Medina GN, Carter CA. ESCRT machinery potentiates HIV-1 utilization of the PI(4,5)P(2)-PLC-IP3R-Ca2+ signaling cascade. Journal of molecular biology. 2011;413(2):347–58. 21875593
45. Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W. Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem. 2010;285(16):12279–88. doi: 10.1074/jbc.M109.086405 20172859
46. Bissig C, Lenoir M, Velluz M-CC, Kufareva I, Abagyan R, Overduin M, et al. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Developmental cell. 2013;25(4):364–73. doi: 10.1016/j.devcel.2013.04.003 23664863
47. Maki M, Suzuki H, Shibata H. Structure and function of ALG-2, a penta-EF-hand calcium-dependent adaptor protein. Sci China Life Sci. 2011;54(8):770–9. doi: 10.1007/s11427-011-4204-8 21786200
48. Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, et al. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun. 2014;5 : 5646. doi: 10.1038/ncomms6646 25534348
49. Hyser JM, Utama B, Crawford SE, Broughman JR, Estes MK. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. Journal of virology. 2013;87(24):13579–88. doi: 10.1128/JVI.02629-13 24109210
50. Siddharthan V, Wang H, Davies CJ, Hall JO, Morrey JD. Inhibition of west nile virus by calbindin-D28k. PLoS One. 2014;9(9):e106535. doi: 10.1371/journal.pone.0106535 25180779
51. Scherbik SV, Brinton MA. Virus-induced Ca2+ influx extends survival of west nile virus-infected cells. J Virol. 2010;84(17):8721–31. doi: 10.1128/JVI.00144-10 20538858
52. de Jong AS, Visch HJ, de Mattia F, van Dommelen MM, Swarts HG, Luyten T, et al. The coxsackievirus 2B protein increases efflux of ions from the endoplasmic reticulum and Golgi, thereby inhibiting protein trafficking through the Golgi. J Biol Chem. 2006;281(20):14144–50. 16540472
53. van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, et al. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16(12):3519–32. 9218794
54. Yang B, Bouchard MJ. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochondrial calcium uptake. J Virol. 2012;86(1):313–27. doi: 10.1128/JVI.06442-11 22031934
55. Dellis O, Arbabian A, Papp B, Rowe M, Joab I, Chomienne C. Epstein-Barr virus latent membrane protein 1 increases calcium influx through store-operated channels in B lymphoid cells. J Biol Chem. 2011;286(21):18583–92. doi: 10.1074/jbc.M111.222257 21454636
56. Dellis O, Arbabian A, Brouland JP, Kovacs T, Rowe M, Chomienne C, et al. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Molecular cancer. 2009;8 : 59. doi: 10.1186/1476-4598-8-59 19650915
57. Lavanya M, Cuevas CD, Thomas M, Cherry S, Ross SR. siRNA screen for genes that affect Junín virus entry uncovers voltage-gated calcium channels as a therapeutic target. Science translational medicine. 2013;5(204).
58. Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, et al. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. MolPharmacol. 1994;45(6):1227–34.
59. Verheugen JA, Korn H. A charybdotoxin-insensitive conductance in human T lymphocytes: T cell membrane potential is set by distinct K+ channels. JPhysiol (Lond). 1997;503 (Pt 2):317–31.
60. Freedman BD, Price MA, Deutsch CJ. Evidence for voltage modulation of IL-2 production in mitogen - stimulated human peripheral blood lymphocytes. JImmunol. 1992;149(12):3784–94.
61. Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. JImmunol. 1997;158(11):5120–8.
62. Hess SD, Oortgiesen M, Cahalan MD. Calcium oscillations in human T and natural killer cells depend upon membrane potential and calcium influx. JImmunol. 1993;150 : 2620–33.
63. Han Z, Lu J, Liu Y, Davis B, Lee MS, Olson MA, et al. Small-Molecule Probes Targeting the Viral PPxY-Host Nedd4 Interface Block Egress of a Broad Range of RNA Viruses. J Virol. 2014;88(13):7294–306. doi: 10.1128/JVI.00591-14 24741084
64. Fuchs S, Rensing-Ehl A, Speckmann C, Bengsch B, Schmitt-Graeff A, Bondzio I, et al. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. JImmunol. 2012;188(3):1523–33.
65. Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9(4):432–43. doi: 10.1038/ni1574 18327260
66. Licata JM, Simpson-Holley M, Wright NT, Han Z, Paragas J, Harty RN. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J Virol. 2003;77(3):1812–9. 12525615
67. Lu J, Qu Y, Liu Y, Jambusaria R, Han Z, Ruthel G, et al. Host IQGAP1 and Ebola virus VP40 interactions facilitate virus-like particle egress. J Virol. 2013;87(13):7777–80. doi: 10.1128/JVI.00470-13 23637409
68. Moe JB, Lambert RD, Lupton HW. Plaque assay for Ebola virus. J Clin Microbiol. 1981;13(4):791–3. 7014628
69. Tomori O, Johnson KM, Kiley MP, Elliott LH. Standardization of a plaque assay for Lassa virus. Journal of medical virology. 1987;22(1):77–89. 3585290
70. Bushar G, Sagripanti JL. Method for improving accuracy of virus titration: standardization of plaque assay for Junin virus. Journal of virological methods. 1990;30(1):99–107. 1707890
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



