-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
: Trypanosomatids Adapted to Plant Environments
Over 100 years after trypanosomatids were first discovered in plant tissues, Phytomonas parasites have now been isolated across the globe from members of 24 different plant families. Most identified species have not been associated with any plant pathology and to date only two species are definitively known to cause plant disease. These diseases (wilt of palm and coffee phloem necrosis) are problematic in areas of South America where they threaten the economies of developing countries. In contrast to their mammalian infective relatives, our knowledge of the biology of Phytomonas parasites and how they interact with their plant hosts is limited. This review draws together a century of research into plant trypanosomatids, from the first isolations and experimental infections to the recent publication of the first Phytomonas genomes. The availability of genomic data for these plant parasites opens a new avenue for comparative investigations into trypanosomatid biology and provides fresh insight into how this important group of parasites have adapted to survive in a spectrum of hosts from crocodiles to coconuts.
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004484
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1004484Summary
Over 100 years after trypanosomatids were first discovered in plant tissues, Phytomonas parasites have now been isolated across the globe from members of 24 different plant families. Most identified species have not been associated with any plant pathology and to date only two species are definitively known to cause plant disease. These diseases (wilt of palm and coffee phloem necrosis) are problematic in areas of South America where they threaten the economies of developing countries. In contrast to their mammalian infective relatives, our knowledge of the biology of Phytomonas parasites and how they interact with their plant hosts is limited. This review draws together a century of research into plant trypanosomatids, from the first isolations and experimental infections to the recent publication of the first Phytomonas genomes. The availability of genomic data for these plant parasites opens a new avenue for comparative investigations into trypanosomatid biology and provides fresh insight into how this important group of parasites have adapted to survive in a spectrum of hosts from crocodiles to coconuts.
Introduction
The trypanosomatids are a monophyletic group of single-celled eukaryotic parasites that are spread between multicellular hosts predominantly by insects. Globally, these parasites cause a considerable burden on human health and welfare, with an estimated 20 million people infected with trypanosomatid pathogens [1], as well as several devastating diseases of livestock. Thus, trypanosomatids have attracted significant attention from disparate academic communities for their importance to global human welfare. In more recent years, trypanosomatids have also drawn interest for use as model organisms because of their streamlined and often extreme biology. In this context, the study of trypanosomatid biology has greatly contributed to our understanding of several biological phenomena, including mechanisms of immune evasion [2], glycosylphosphatidylinositol anchors [3], RNA editing [4], polycistronic transcription [5], trans-splicing [6], chromosome segregation [7], and the eukaryotic cilium [8]. Though trypanosomatids have been the focus of many studies, one large and diverse subgroup of plant-infecting trypanosomatids known as Phytomonas are relatively poorly understood. Little is known of their biology, life cycle, or how they have adapted to life inside plants. This review discusses what is known about Phytomonas in terms of disease biology, morphology, and metabolism in the light of emerging genome resources for this globally distributed group.
Phytomonas: Endophytes and Pathogens
Over 100 years have passed since the first plant trypanosomatids were isolated from the latex of Euphorbia pilulifera on the island of Mauritius by Lafont in 1909 [9]. The discovery of these trypanosomatids, initially named Leptomonas davidi, was confirmed in E. pilulifera in Madras (4,400 kilometres away) later the same year by Donovan [10]. It was recognised from the outset that the plant trypanosomatids were morphologically distinct from mammalian infective species [10], and it was on this basis that Donovan suggested a new genus termed Phytomonas for their classification [10]. The first reports of Phytomonas infections of plants initially described the host plants as exhibiting poor growth and wilt [9]. Subsequent observations in Euphorbia segetalis and Euphorbia peplus agreed that infection with Phytomonas was deleterious to the plant [11], and reported that infected plants exhibited a depletion of starch granules from the latex and surrounding parenchyma and a reduction in the viscosity of the latex [12]. However, in contrast to this, other authors stated that infections in different Euphorbiaceae produced no detectable effects on plant growth or yield. Moreover, they observed that parasitised and nonparasitised plants were indistinguishable without examination of the latex by microscopy [12,13]. Thus, from the outset there was contention as to whether these parasites were pathogenic or endophytic (nondetrimental) in their plant hosts.
Since these initial investigations into the pathogenicity of Phytomonas parasites, two species have been definitively shown to cause plant disease. The first species, Phytomonas staheli, is the aetiologic agent of “hartrot (fatal wilt) of coconut palm (Cocos nucifera), “marchitez sorpresiva” (sudden wilt), and slow wilt of oil palm (Elaeis guineensis) [14,15], both diseases are acute lethal wilts that begin in the leaves and progress to the rotting of the spear and root [16], although this rotting may be the result of secondary infection by bacteria and other organisms [14]. The second pathogenic species, Phytomonas leptovasorum, causes coffee phloem necrosis in both Liberica and Arabica coffee [17]. A common feature of both of these pathogenic Phytomonas species is that they exclusively inhabit the phloem during the plant stage of their life cycle [14,17]. A third species, Phytomonas françai, inhabits the latex ducts of cassava (Manihot esculenta) and has been linked with yield loss diseases known as “chochamento de raizes,” or empty root syndrome. This disease is characterised by poor root development and chlorosis of the leaves [18,19]. Several attempts have been made to show experimentally that P. françai is the aetiological agent of empty root syndrome; however, in experimental infections, infected plants appeared identical to uninfected individuals [13,19,20]. Moreover, the Unha cultivar of cassava that may have exhibited pathology when infected with Phytomonas is no longer widely farmed in Brazil and therefore there have been no reports of empty root syndrome in cassava since 1980 [16]. Thus, it appears that P. françai poses little or no risk to food security in this crop.
Though there are only two proven pathogenic species, those species pose some economic risk in South America. P. leptovasorum (the aetiological agent of coffee phloem necrosis) has been isolated in Suriname [21] and Brazil [22]. With the rapid expansion of coffee plantations across South America and the resulting change in the distribution of crop pathogens and pests [23], coffee phloem necrosis poses a potential risk to these expanding coffee economies. For example, Brazil is the world’s largest exporter of green coffee beans trading more than 1,700,000 tonnes in 2011 at a value of 8,000 million dollars (http://faostat.fao.org/). P. staheli (the aetiological agent of wilt diseases of oil and coconut palm) has previously been isolated from Ecuador, Suriname, Venezuela, Brazil, Costa Rica, Honduras, and Colombia [24]. In these regions, oil palm is a major commercial crop with 397,000 tonnes of palm oil exported by Colombia and Ecuador alone in 2011 at a value of 493 million dollars (http://faostat.fao.org/). Currently, there are no effective treatments of these Phytomonas plant diseases, and strategies for infection control comprise the felling of diseased plants or the removal of the infected plant material. Attempts to develop effective chemical controls have suffered from a lack of genome resources and have focused on exploiting targets that are conserved in related trypanosomatid species [25]. These include perturbation of shared metabolic pathways such as aspects of nucleic acid biosynthesis [25].
Host Range and Phylogeny
Phytomonas as a group are globally distributed (Fig. 1A) and have been repeatedly isolated from disparate plant hosts that span the angiosperm tree-of-life (Fig. 1B). S1 Table is a list of all published Phytomonas isolations known at the time of publication of this article and is summarised in Fig. 1. Within their plant hosts Phytomonas species have been isolated form a variety of different tissues including phloem, latex ducts, fruit, flowers, and seeds (Fig. 1C & S1 Table) and thus have evolved to inhabit both extracellular and intracellular plant environments [16].
Fig. 1. Summary of all known Phytomonas isolates. 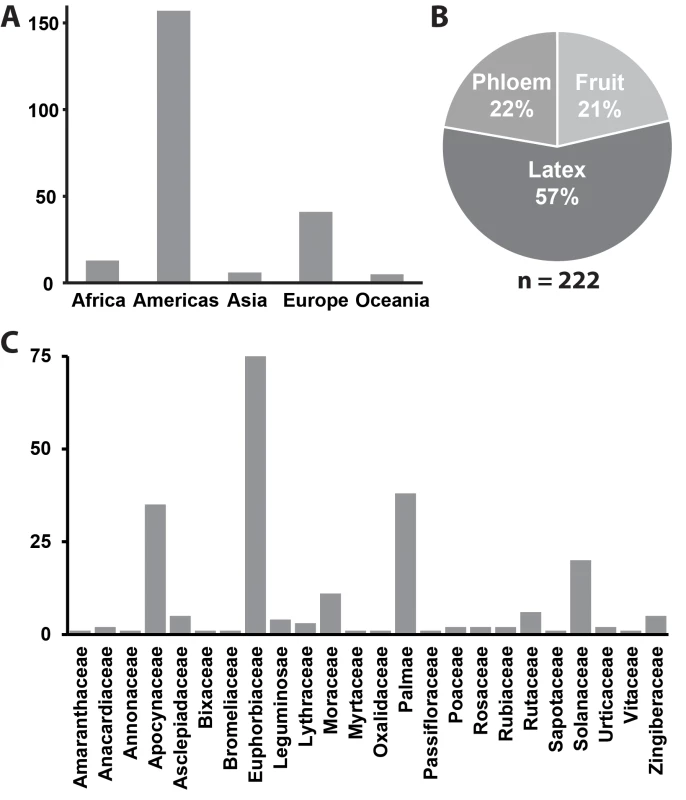
A) A bar chart depicting the number of reported isolations by continent. B) A pie chart depicting the plant host environment from which these isolations were made. C) A bar chart depicting the plant families from which these isolations were made. For further details see S1 Table. The first attempt to rationalise the phylogeny and subgroupings of Phytomonas used isoenzyme analysis [26]. This was extended using molecular markers, including the internal transcribed spacer of the ribosomal RNA locus, kinetoplast DNA sequence, and the spliced leader RNA gene array [27–30]. Collectively, these studies revealed that Phytomonas are monophyletic and that the pathogenic phloem-limited Phytomonas species formed a discrete group termed the phloemicola that are distinct from nonpathogenic trypanosomatid parasites of fruit and latex [28]. Outside of this association, molecular data revealed that neither habitat nor host species was predictive of phylogenetic subgroupings within Phytomonas [30]. Moreover, it was found that the same plant species could harbour distantly related Phytomonas species. For example, the genome strain Phytomonas serpens 9T isolated from tomato fruit (Solanum esculentum) in Brazil belongs to a different group than Phytomonas isolated from tomatoes from Southern Spain [30]. Similarly, the Phytomonas species isolated from plant latex in Europe, Africa, and India were serologically and molecularly distinct from parasites isolated from latex from South America [30]. However, the low quantities of data available precluded the complete resolution of the evolutionary relationship between all phylogenetic groups.
The first Phytomonas genomes to be published were those of P. serpens 9T [31], Phytomonas sp. HART1 [32], and Phytomonas EM1 [32]. P. serpens 9T was isolated from a tomato fruit in South America [33], Phytomonas HART1 was isolated from the phloem of a diseased coconut palm in Guiana [30,32], and Phytomonas EM1 was isolated from the latex of an asymptomatic Euphorbia in the south of France [30,32]. Fig. 2 is a phylogenetic tree constructed from 959 single copy nuclear genes, adapted from [34] with the inclusion of L. tarentole, L. donovani, Phytomonas HART1, and Phytomonas EM1, showing the evolutionary relationship of the sequenced Phytomonas species to the several pathogenic and nonpathogenic taxa from Leishmania and Trypanosoma. Interestingly, these Phytomonas genomes are much smaller than those of their Leishmania or Trypanosoma relatives; for example, the genome of EM1 is 17.8 mega bases (Mb) [32] in comparison to 32.9 Mb of Leishmania major [35], 62.3 Mb of Trypanosoma brucei [36] and 32.5 Mb of Trypanosoma cruzi [37]. A unifying feature of phylogenetic analyses of Phytomonas is that there appears to be large diversity within the group. For example, the kDNA sequence data shows that the divergence between the phloem limited Phytomonas HART1 and the fruit parasite P. serpens is greater than that spanning all sampled Leishmania species and similar to the divergence between T. cruzi and T. brucei [32]. This finding is also supported by studies of other molecular markers such as small subunit rRNA and glycosomal GAPDH [38,39] as well as the concatenated protein sequence phylogeny presented in Fig. 2.
Fig. 2. A maximum likelihood protein sequence phylogenetic tree of trypanosomatids. 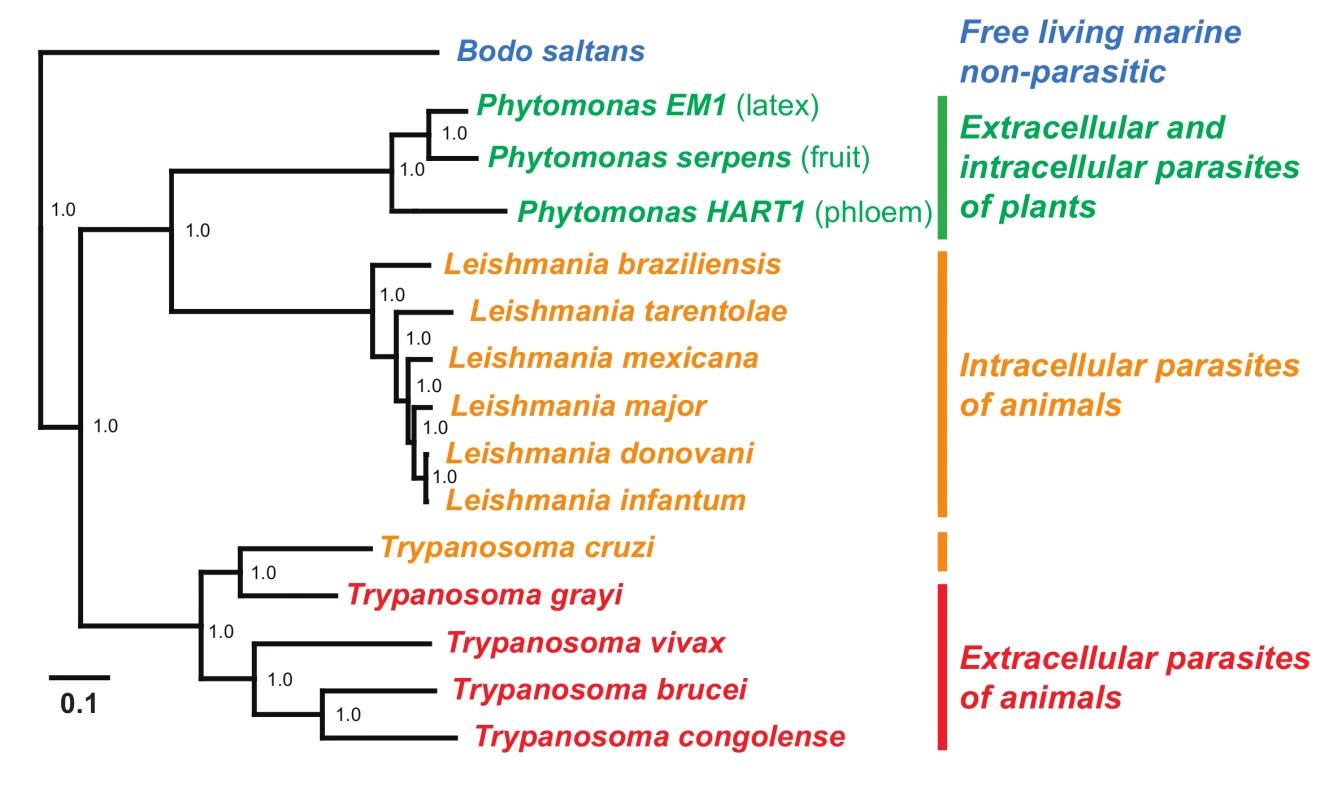
The protein sequence files for a subset of available trypanosomatid genomes were downloaded from TriTrypDB [95]. These were combined with the newly predicted protein sequences from the Phytomonas genomes and subjected to clustering using OrthoMCL [96]. Clusters that contained only single copy genes in each of the species were selected (n = 959). These single copy gene families were aligned using MergeAlign [97] and concatenated to form a super alignment. This concatenated alignment was subject to phylogenetic inference using maximum likelihood implemented in the FastTree algorithm [98]. The tree was inferred utilizing the JTT (Jones, Taylor & Thornton) model of amino acid substitution and the CAT model of rate heterogeneity. The topology received 100% support by approximate SH-like support values. Scale bar indicates number of changes per site. Phytomonas Cell Morphology
Consistent with their sister grouping to Leishmania, the majority of Phytomonas species isolated to date exhibit promastigote morphology [16,43], characterised by a long cell body with a detached flagellum that protrudes from a flagellar pocket at the cell anterior. This morphology is similar to Leishmania parasites in the insect host and many monoxenous trypanosomatid insect parasites. In Leishmania and Trypanosoma, cell morphologies are replicated with high fidelity through the cell-division cycle and altered with high fidelity when transitioning between different developmental stages depending on the host and organ or type of tissue colonised [43,44]. From the earliest descriptions of Phytomonas, it was noted these parasites exhibited extreme morphological polymorphism [11,45]. Polymorphism in cell body width, cell body length, and flagellum length is typical of trypanosomatids isolated from their hosts, common to morphologies with a free (liberform) and attached (juxtaform) flagellum [44]. Whether the observed variation is an adaptation to changes in physiological conditions or uncharacterised life cycle stages is unknown. However, it has been hypothesised [44], based on division of the Leishmania mexicana promastigote [46], that one contributing factor to polymorphism of trypanosomatids with a detached flagellum is the pattern of morphogenesis through the cell cycle. In L. mexicana, the cell body increases in length during G1 and decreases post-S phase, and flagellar length is highly variable, in particular at cell division where the old flagellum is significantly longer than the new flagellum [46].
Interestingly, Phytomonas polymorphism extends beyond promastigote size and shape to include amastigote (no external flagellum) forms. The earliest drawings of parasites isolated from Euphorbia latex clearly show cells with a small round cell body and no external flagellum [9,20,47–49] reminiscent of amastigote stages of Leishmania parasites [50]. Amastigote-like cells have also been repeatedly extracted from fruit [16]. This is consistent with the hypothesis that there are two major classes of trypanosomatid morphology, those with a free flagellum (including the Phytomonas sister taxa Leishmania), and those with a laterally attached flagellum, that have conserved pathways for morphogenesis through their cell cycles [44]. However, the biological similarity of these Phytomonas amastigote forms to amastigote forms of Leishmania parasites and the mechanism(s) regulating differentiation remain completely unknown. In this context it is noteworthy that the functional significance of the promastigote and amastigote cell forms is also unknown, although in Leishmania these cell types have been linked with a requirement for motility and sensory functions of the flagellum respectively [51].
Transmission and Life Cycle
Though trypanosomatids that parasitize fish [52], frogs [53], turtles [53], and possibly several Australian vertebrates [54,55] are transmitted by leeches, the overwhelming majority of described trypanosomatid species are spread between hosts by insects [54,55]. Very shortly after the initial discovery of Phytomonas, it was shown in caged insect experiments that the phytophagous insect Nysius euphorbiae, a hemipteran that feeds on multiple species of Euphorbia, was able to transmit the parasites from infected plants to uninfected hosts [50]. Further work by Bouet and Rouband (1911) reviewed in [12] demonstrated that the latex-feeding insect Dieuches humilis could also act as the insect host; however, the natural host was shown to be the nocturnal coreid spurgebug Dicranocephalus agilis [11]. Taken together, these early investigations suggested that individual Phytomonas species may be spread between plant hosts by a broad range of different insects. Consistent with these early reports, all subsequent evidence suggests that Phytomonas are transmitted by phytophagous insects [45,56,57]. Though the relationship between plant host, parasite, and insect host appears simple (S1 Table), in reality it is unknown to what extent different Phytomonas species can be spread by different insects or can colonise different plants. This relationship between plant host, parasite, and insect host is further complicated by the nomenclature of the parasites. For example, the name P. serpens has been applied to Phytomonas parasites that have been isolated from the fruit of multiple different tomato cultivars (S1 Table) in the Americas, Africa, and Europe [30,45]. Moreover, it has also been applied to isolates from multiple different insects, for example, from the salivary glands of Nezara viridula in South Africa [45] and from Phthia picta in Brazil [58]. These multiple disparate isolations may represent different species and thus there may be some inflation of the true host and insect host range for an individual species.
In insect-spread mammalian infections of Leishmania and Trypanosoma, parasite cells undergo differentiation to metacyclic forms that are capable of infecting the mammalian host. In Trypanosoma brucei, these infective metacyclic cells express a specialised variant surface glycoprotein on their cell surface [59] while in Leishmania major, the molecular marker for mammalian infective metacyclic cells is a specialised lipophosphoglycan (LPG) [60,61]. There is no evidence for metacyclic variant surface glycoproteins or metacyclic LPG in Phytomonas. Moreover, the metacyclic specific LPG found in L. major is not found in other Leishmania species, and there are no other known molecular markers of metacylogenesis. Though there are descriptions of metacyclic Phytomonas cells in the literature [45], these descriptions are based solely on cell morphology and it is unknown whether they represent a true specialised plant infective stage of the life cycle. In this context it is noteworthy that there have been successful infections of tomato fruit with insect isolated P. serpens cells grown in culture and from allowing infected insects to feed on fruit [45,62]. This is in contrast to Leishmania and Trypanosoma where cultured insect adapted cells are not infective to mammalian hosts and metacylogenesis must be induced prior to infection [63]. Though a preadapted metacyclic stage may not be present in the Phytomonas life cycle, P. serpens cells have been observed to attach to insect salivary glands [56] indicative of a true developmental stage inside the insect host.
Some studies have begun to emerge on the effect of Phytomonas infection on insect hosts. It has been demonstrated that P. serpens infection is countered by phagocytosis by hemocytes in the milkweed bug Oncopeltus fasciatus [56]. However, the responses of the hemocytes were not sufficient to prevent the parasite from attaching to the salivary glands within 24 hours post infection [56]. Future studies may reveal whether infection of insect hosts leads to alteration in host behaviour. For example, Leishmania infection of the sandfly Lutzomyia longipalpis results in increased biting persistence and a concomitant increase in transmission efficiency [64]. Similarly, some bacterial pathogens increase their transmission efficiency by modification of their plant host. For example, infection with some Phytoplasma species can cause yellowing of infected plant tissue resulting in increased transmission as phloem-feeding insects prefer young green/yellow leaves [65]. It has yet to be shown whether Phytomonas infection leads to alteration of plant or insect host for enhanced transmission. However, emerging data suggests that Phytomonas serpens is able to produce auxin and thus may interfere with plant hormone signalling [66].
The Phytomonas Cell Surface
The cell surface of Phytomonas, like all trypanosomatids, consists of at least three discrete domains. The domain with the largest surface area is the cell body surface; there is also the flagellar surface and next to the flagellum a distinct portion of the cell body membrane that is invaginated to form the flagellar pocket. In all trypanosomatids, these cellular surfaces lack an outer cell wall and instead are coated with membrane-anchored proteins and glycoinositol phospholipids [67]. This exterior cell surface acts to protect the parasite cell from host responses and from changing environmental conditions. The three domains are visible when whole Phytomonas cells are labelled with biotin and incubated in the presence of a streptavidin conjugated fluorescent dye as shown in Fig. 3.
Fig. 3. Epifluorescence microscopy to show cell surface biotin labelling of Phytomonas serpens. 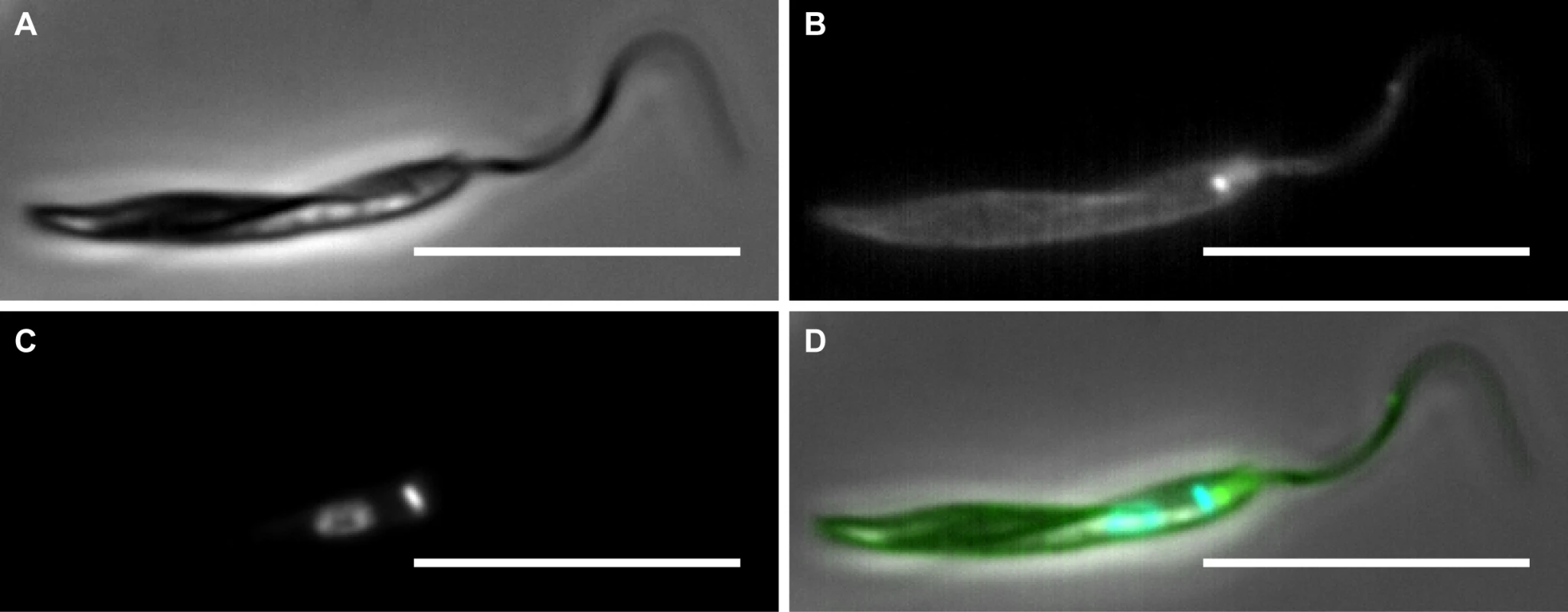
Cells were labelled with EZ-link-sulfo-NHS-SS-Biotin (Thermo Scientific) and fixed in 4% paraformaldehyde. Biotin was detected using streptavidin AlexaFlour488 (green) (Invitrogen). DNA was stained using Hoechst (blue). A) Phase contrast image of a P. serpens cell. B) Distribution of biotin on the cell surfaces to show at least three cell membrane domains. C) DNA. D) Merge of all channels. Scale bar 10μm. To date, few molecular components of the Phytomonas cell surface have been characterised. Those that have been so far identified are those that are conserved in other trypanosomatids. One major component is the zinc-metalloprotease Major Surface Protease (MSP), also known as gp63 [68]. This protein is likely to be pan-eukaryotic [69] and is found ubiquitously among the trypanosomatids in both a secreted form and a form anchored to the plasma membrane by glycosylphosphatidylinsoitol (GPI) [70,71]. The role of gp63 in the plant host has yet to be interrogated; however, there is some evidence to suggest that gp63 is important in the insect stage of the Phytomonas life cycle [68]. Treatment of P. serpens with antibodies raised to gp63 perturbs parasite morphology and results in loss of adhesion to the salivary glands of the insect host, a step necessary for the establishment of infection in the insect host [33,72]. Along with gp63, cysteine-proteases related to cruzipain of T. cruzi have also been found in P. serpens [33]. Both of these proteins have been shown to be secreted by P. serpens, as well as existing in a form anchored to the cell surface by GPI [72]. Consistent with this observation, interrogation of the Phytomonas genomes revealed the presence of the pathways for GPI anchor biosynthesis [32].
Analysis of the genome sequences also revealed the surprising finding that HART1 contains an expansion in the gp63 gene family with over 20 members in contrast to 2 genes in EM1 [32]. This expansion suggests that HART1 may have a more complex cell surface that might facilitate survival in the hostile environment of the plant phloem [73,74]. This increased diversity may also be further enhanced through post-translational modification as the genes required for post-translational addition of glucose, mannose, galactose, N-acetylglucosamine, glucuronic acid, xylose, and fucose are also present in one or both Phytomonas genomes [32]. While the function of these surface proteins in either the plant or insect host have yet to be established, it has been shown that the expression of these proteins in Phytomonas is responsive to culture conditions [75]. For example, incubation in media-containing high levels of protein causes expression of GP63 to decrease [75]. Expression also correlates with growth rate, and therefore it may play a role in allowing the Phytomonas parasites to adapt to different environments [72].
In addition to proteins, trypanosomatids express a rich and varied array of glycolipids on their cell surface. Leishmania cells, for example, are coated in a layer of lipophosphoglycan (LPG) that helps to protect the parasite from host pressures such as complement-mediated lysis in mammals [76]. While LPG has yet to be isolated from Phytomonas, the complete enzyme complement for the synthesis of LPG is present in the Phytomonas genomes [32] and thus LPG is likely to be present at the cell surface. Moreover, these genes may also function in the biosynthesis of other classes of structurally related glycolipids known as glycoinositol phospholipids (GIPLs). Interestingly, it has been shown that the major glycolipids of the Phytomonas cell surface are GIPLs [67]. Four different GIPLs have been characterised from a Phytomonas sp. isolated from Euphorbia characias and collectively these GIPLs are present at ≥ 5 ×106 copies per cell [67]. The function of these highly abundant surface molecules is as yet unknown; however, one hypothesis is that the dense negative charge they give to the cell surface may provide some protection to the Phytomonas parasites in the plant host [67]. Specifically, we propose that in Phytomonas the dense negative charge may help repel negatively charged hydroxide ions that are produced by the plant host as part of the oxidative burst; a rapid, transient production of reactive oxygen species that is one of the first responses in a plant’s defence strategy [77]. It may be that the GIPLs function together with other cell surface glycolipids and glycoproteins in this role, for example in Leishmania there is evidence to show that LPG provides some protection against reactive oxygen species [78,79]. The specific composition of GIPLs in Phytomonas may also be important for adaptation to different plant hosts. For instance, in one Phytomonas strain, it was found that the GIPLs lack galactose residues that are common in Leishmania GIPLs [67]. As plants express a diverse array of lectins with affinities for different hexose moieties, it may be that lectins from some plant species will effectively agglutinate individual Phytomonas species while others will not [67]. For example, the latex of the host plant (Euphorbia characias) from which the Phytomonas strain above was isolated, contains a galactose-specific lectin [80] that may agglutinate any parasite expressing galactose on its cell surface [67]. Though this hypothesis was not tested in the plant host, a galactose-specific lectin from a different lactiferous plant (Ricinus communis RCA1) resulted in effective agglutination of the same species of Phytomonas [81]. Further investigation will reveal whether the composition of GPILs play a role in plant host specificity.
Intriguingly, immunogenic similarities between P. serpens and T. cruzi are enough that oral exposure to P. serpens has been observed to attenuate the symptoms of Chagas disease in C57BL/6 mice [82]. It is also interesting to note that while naturally occurring Phytomonas infections in mammals have not been described, inoculation of mice with infected latex has been reported to produce sustained infections [83]. Following inoculation, it was noted that the spleen was increased in volume and that blood smears from this organ contained amastigote-like forms of the parasites. It may be that some species of Phytomonas are capable of infecting mammals but that their host range is governed by the feeding habits of their invertebrate host. However, caution should be exercised here as the parasites used were identified solely on the basis of their presence in plant latex, and this may have occurred through infection from other opportunistic trypanosomatids. Furthermore, there have yet to be reports of animal infections with Phytomonas in wild populations. Taken together, this suggests that if animal infections do exist, either they do not persist or retransmission does not occur.
One particularly poorly understood aspect of Phytomonas biology is whether they secrete protein effectors to modulate the host plant immune response, an adaptation commonly found in other plant parasites [40]. A genome-wide analysis of the predicted Phytomonas EM1 and HART1 secretomes was undertaken in the hope that it would lead to the identification of potential pathogenicity effectors in Phytomonas parasites. This resulted in the identification of several aspartyl proteases in both Phytomonas strains that were absent from the genomes of Leishmania and Trypanosoma [32]. These proteases may play a role in host–parasite interactions as phloem sap often contains aspartyl protease inhibitors, and aspartyl proteases are known to be secreted by plant pathogenic fungi such as Botrytis cinerea [41] and have been implicated in the suppression of systemic acquired resistance [40]. However, the role of these inhibitors has so far mainly been investigated in the context of phloem-feeding herbivores [42], and there is some doubt whether these proteases are involved in plant–fungal interactions as protease deficient strains of B. cinerea are not attenuated in virulence [41].
Comparative surface proteomics between Phytomonas, Leishmania, and Trypanosoma may yield further insight into the molecular reasons for these immunogenic similarities. Moreover, it may help to elucidate the evolution of these disparate parasite cell surfaces and identify factors that distinguish plant-adapted from mammalian-adapted trypanosomatids and facilitate colonisation of different plant environments.
Phytomonas Metabolism
Analysis of the genes encoding metabolic proteins in the Phytomonas genome sequences revealed a cohort of enzymes consistent with life in a plant environment. Both HART1 and EM1 genomes encode glucoamylase, alpha-glucosidase, and alpha, alpha-trehalose phosphorylase (acquired through horizontal gene transfer) genes, allowing them to utilise plant carbohydrates [32]. Interestingly, only the phloem restricted pathogen, Phytomonas HART1, encodes invertase genes for degradation of sucrose [32]. This finding is consistent with the diverse metabolic profiles of fruit [84] and latex [85] compared to the overwhelming abundance of sucrose in the phloem. In addition, the Phytomonas genomes contain few tandemly duplicated genes in comparison to Trypanosoma and Leishmania species where the genes encoding metabolic enzymes are frequently found as tandem duplicates [32].
Another remarkable feature of Phytomonas parasites is the loss of genes encoding the cytochrome c oxidase subunits I–III (COI, COII, COIII), and cytochrome b (CyB) of the bc1 complex [32,86,87]. This loss appears to have occurred at the base of the Phytomonas clade [31]. One aspect of Phytomonas biology that may have facilitated the loss of the cytochromes is the feeding behaviour of the insect host. Phytophagous insects feed exclusively on carbohydrate rich plant sap and therefore there may be no requirement for a metabolic shift from a carbohydrate to an amino acid energy substrate [32]. In the case of the former, adenosine triphosphate (ATP) production can occur at the level of substrate phosphorylation [88], but in the case of the latter, the mitochondrial respiratory chain is required for the complete oxidation of the substrate [36]. The absence of a requirement to use amino acids as the main source of energy for Phytomonas in the insect host may have contributed to the loss of the mitochondrial cytochromes and the sole production of ATP via glycolysis in glycosomes [32,86,87,89,90]. In this regard, it will be interesting to see whether these metabolic differences manifest changes in the enzyme content of glycosomes in Phytomonas in comparison to other trypanosomatids.
An essential cofactor for the mitochondrial cytochromes is heme, an iron molecule contained by a porphyrin ring, which functions in redox reactions and electron transport [91]. Interrogation of the available Phytomonas genomes and the culture of P. serpens in a heme-free medium suggests that they are the only aerobic eukaryotes with the ability to survive in the absence of this molecule [31]. However, the relationship between these plant trypanosomatids and heme is not straightforward, as P. serpens cells grown in the presence of heme did contain small amounts of the compound, indicating that they are able to scavenge heme from the medium [31]. To further complicate this curious aspect of Phytomonas biology, the coding sequence for the enzyme ferrocheltase (involved in heme synthesis) is present in P. serpens, and sequences encoding two heme-containing proteins are found in all three available Phytomonas genomes [31].
In addition to the alpha, alpha-trehalose phosphorylase mentioned above, there is also at least one other instance of horizontal gene transfer that conferred novel metabolic capabilities to Phytomonas. The transferred gene encodes a zinc-dependant alcohol dehydrogenase [92,93], which in combination with malate dehydrogenase enables Phytomonas species to produce lactate [32,94]. It is unknown whether the production of lactate may produce a selective advantage for Phytomonas within the plant or insect host.
Discussion and Future Perspectives
Phytomonas are an apparently ubiquitous and diverse group of plant parasites exhibiting both pathogenic and endophytic lifestyles. From their initial discovery in 1909 to the publication of the first Phytomonas genomes over 100 years later, much progress has been made in their classification, taxonomy, and aspects of cell biology.
The apparent abundance of endophytes in the genus Phytomonas may be representative of the extant diversity, or may be the result of a sampling bias. The first plant trypanosomatids were first discovered in the latex of a Mediterranean spurge, and as a result the bulk of subsequent sampling was conducted in the plant family Euphorbiaceae [9,16]. The apparent lack of disease causing Phytomonas may simply be due to the fact that Euphorbia-infecting Phytomonas are generally asymptomatic. Thus, increased sampling needs to be completed before broad conclusions about the diversity and pathogenicity in this genus are made. In this context, it is important to bear in mind that the breadth of the host range (both plant and insect) for any individual Phytomonas species is unknown, and many aspects of the life cycle are poorly understood. While different cell types and life cycle stages have been described in the literature [45], molecular markers for these stages have yet to be identified, and it is unknown whether cells differentiate to adapt to plant and insect hosts and to what extent both host stages are necessary to complete the life cycle.
The wide morphological diversity exhibited by this genus of plant-infecting flagellates is still poorly understood in an adaptive context. In other trypanosomatids, changes in cell morphology represent different life cycle stages, and cell shape, and form is the product of the different selection pressures associated with the discrete host environments colonised [44]. Further studies concerning Phytomonas cell morphology during the cell cycle could address the question as to whether the diverse morphology seen in infections is simply a product of the cell division cycle or is adaptive and bears implications for virulence.
The release of the first Phytomonas genomes has already begun to reveal clues into their biology and pathogenicity. For example, the expansion of a gene family of putative secretable aspartyl proteases in the pathogenic isolate is reminiscent of similar phenomena in plant pathogenic fungi [32]. It will be interesting to see whether the publication of these novel genome resources will help elucidate mechanisms of pathogenicity in Phytomonas and resolve whether disease symptoms in host plants arise as a result of a parasite mediated degradation of plant tissues or as an indirect by-product of depletion of plant assimilates. In either case, it is likely that surface proteins play a role in the adaptation of Phytomonas to their plant hosts because of the involvement of such proteins in environmental sensing and parasite–host interactions. Moreover, there is abundant precedent in Leishmania and Trypanosoma that parasite surface proteins play a crucial role in mediating parasite–host interactions and in evading host immune responses. Thus, it is likely that characterisation of a Phytomonas surface-localised and-secreted proteome may yield insights into both disease biology and the evolution of parasite surfaces in the trypanosomatids. This may also provide an avenue for the identification of the factors necessary for the invasion of plant tissue and the development of systemic infection.
Taken together, the emergence of genome resources will provide a new foundation for discovery in this enigmatic and globally-distributed group of plant parasites. Moreover, it will provide new insight into the evolution of the trypanosomatids and how these intriguing parasites now inhabit a host range that spans from crocodiles to coconuts.
Supporting Information
Zdroje
1. Stuart K, Brun R, Croft S, Fairlamb A, Gürtler RE, et al. (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest 118 : 1301–1310. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2276762&tool=pmcentrez&rendertype=abstract. Accessed 4 April 2014. doi: 10.1172/JCI33945 18382742
2. MacGregor P, Szöőr B, Savill NJ, Matthews KR (2012) Trypanosomal immune evasion, chronicity and transmission: an elegant balancing act. Nat Rev Microbiol 10 : 431–438. http://www.ncbi.nlm.nih.gov/pubmed/22543519. Accessed 5 March 2013. doi: 10.1038/nrmicro2779 22543519
3. Ferguson MA (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci 112 : 2799–2809. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=11&SID=R16dDneN8f2mlAjL72b&page=1&doc=1. Accessed 18 June 2013. 10444375
4. Benne R, Van den Burg J, Brakenhoff JP, Sloof P, Van Boom JH, et al. (1986) Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46 : 819–826. http://www.ncbi.nlm.nih.gov/pubmed/3019552. Accessed 4 April 2014. 3019552
5. Siegel T, Gunasekera K (2011) Gene expression in Trypanosoma brucei: lessons from high-throughput RNA sequencing. Trends Parasitol 27 : 434–441. http://www.sciencedirect.com/science/article/pii/S1471492211000997. Accessed 1 August 2014. doi: 10.1016/j.pt.2011.05.006 21737348
6. Sutton RE, Boothroyd JC (1986) Evidence for trans splicing in trypanosomes. Cell 47 : 527–535. http://www.ncbi.nlm.nih.gov/pubmed/3022935. Accessed 31 July 2014. 3022935
7. Akiyoshi B, Gull K (2014) Discovery of unconventional kinetochores in kinetoplastids. Cell 156 : 1247–1258. http://www.ncbi.nlm.nih.gov/pubmed/24582333. Accessed 20 March 2014. doi: 10.1016/j.cell.2014.01.049 24582333
8. Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, et al. (2006) Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440 : 224–227. http://www.ncbi.nlm.nih.gov/pubmed/16525475. Accessed 10 March 2013.
9. Lafont A (1909) Sur la présence d’un Leptomonas, parasite de la classe des Flagelles dans le lates de l’Euphorbia pilulifera. Comptes Rendus des Seances la Soc Biol 66 : 1011–1013.
10. Donovan C (1909) Kala-azar in Madras, especially with regard to its connecxion with the dog and the bug (Chonnorrhinus). The Lanclet 177 : 1195–1196.
11. Franca C (1920) La flagellose des Euphorbes II. Ann l’instutut Pasteur 34 : 432–465. doi: 10.1167/iovs.14-15247 25190660
12. Bensaude M (1925) Flagellates in plants: a review of foreign literature. Phytopathology 15 : 273–281.
13. Aragão H de B (1931) Untersuchungen über Phytomonas françai. Mem Inst Oswaldo Cruz 25 : 299–306. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02761931000400001&lng=en&nrm=iso&tlng=pt. Accessed 23 April 2014.
14. Parthasarathy M V, VAN Slobbe WG, Soudant C (1976) Trypanosomatid flagellate in the Phloem of diseased coconut palms. Science 192 : 1346–1348. http://www.ncbi.nlm.nih.gov/pubmed/17739841. Accessed 14 July 2014. 17739841
15. Di Lucca AGT, Trinidad Chipana EF, Talledo Albújar MJ, Dávila Peralta W, Montoya Piedra YC, et al. (2013) Slow wilt: another form of Marchitez in oil palm associated with trypanosomatids in Peru. Trop Plant Pathol 38 : 522–533. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1982-56762013000600008&lng=en&nrm=iso&tlng=en. Accessed 22 April 2014.
16. Camargo EP (1999) Phytomonas and other trypanosomatid parasites of plants and fruit. Adv Parasitol 42 : 29–112. http://www.ncbi.nlm.nih.gov/pubmed/10050272. 10050272
17. Stahel G (1931) Zur Kenntis der Siebrohrenkrankheit (Phloemnekrose) des Kaffeebaumes in Surinam. I. Mikroskopische Untersuchungen und Infektionsversuche. Phytopathol Zeitschrift 4 : 65–82.
18. Vainstein MH, Roitman I (1986) Cultivation of Phytomonas francai Associated with Poor Development of Root System of Cassava. J Protozool: 511–513.
19. Kitajima EW (1986) Flagellate Protozoon Associated with Poor Development of the Root System of Cassava in the Espirito Santo State, Brazil. Phytopathology 76 : 638. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=Refine&qid=10&SID=S1UzkZyWX6xs8ccJMLV&page=1&doc=3. Accessed 10 April 2014.
20. Aragão HDB (1927) Sur un flagellé du latex de Manihot palmata, Phytomonas francai nsp. CR Soc Biol 97 : 1077–1080.
21. Teixeira MM, Campaner M, Camargo EP (1994) Detection of trypanosomatid Phytomonas parasitic in plants by polymerase chain reaction amplification of small subunit ribosomal DNA. Parasitol Res 80 : 512–516. http://www.ncbi.nlm.nih.gov/pubmed/7809002. 7809002
22. Serrano MG, Nunes LR, Campaner M, Buck GA, Camargo EP, et al. (1999) Trypanosomatidae: Phytomonas detection in plants and phytophagous insects by PCR amplification of a genus-specific sequence of the spliced leader gene. Exp Parasitol 91 : 268–279. http://www.sciencedirect.com/science/article/pii/S001448949894379X. Accessed 29 April 2014. 10072329
23. Bebber DP, Ramotowski MAT, Gurr SJ (2013) Crop pests and pathogens move polewards in a warming world. Nat Clim Chang 3 : 985–988. http://dx.doi.org/10.1038/nclimate1990. Accessed 23 May 2014.
24. Berrio C, Ocho A (1991) La Marchitez Sorpresiva de la Palma Aceitera en la Zona Sur del Lago de Maracaibo. Rev difusión Tecnol agrícola y Pesq del FONAIAP 38.
25. Magán R, Marín C, Salas JM, Barrera-Pérez M, Rosales MJ, et al. (2004) Cytotoxicity of three new triazolo-pyrimidine derivatives against the plant trypanosomatid: Phytomonas sp. isolated from Euphorbia characias. Mem Inst Oswaldo Cruz 99 : 651–656. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762004000600021&lng=en&nrm=iso&tlng=en. Accessed 26 January 2014. 15558180
26. Vainstein MH, Da Silva JBT, De Lima VMQG, Roitman I, De Souza W, et al. (1987) Electrophoretic Analysis of Isoenzymes in the Identification of Trypanosomatids of the Genus Phytomonas 1. J Protozool 34 : 442–444. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=1&SID=V2fImhT6osjM9vFHpym&page=1&doc=1. Accessed 14 April 2014.
27. Teixeira MMG, Takata CSA, Conchon I, Campaner M, Camargo EP (1997) Ribosomal and kDNA Markers Distinguish Two Subgroups of Herpetomonas among Old Species and New Trypanosomatids Isolated from Flies. J Parasitol 83 : 58. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=2&SID=X1jJ6r6FAiuwRfh4phT&page=1&doc=1. Accessed 5 May 2014. 9057697
28. Dollet M, Sturm NR, Campbell DA (2001) The spliced leader RNA gene array in phloem-restricted plant trypanosomatids (Phytomonas) partitions into two major groupings: epidemiological implications. Parasitology 122 : 289–297. http://www.ncbi.nlm.nih.gov/pubmed/11289065. 11289065
29. Sturm NR, Dollet M, Lukes J, Campbell DA (2007) Rational sub-division of plant trypanosomes (Phytomonas spp.) based on minicircle conserved region analysis. Infect Genet Evol 7 : 570–576. http://www.ncbi.nlm.nih.gov/pubmed/17499027. Accessed 29 April 2013. 17499027
30. Dollet M, Sturm NR, Campbell DA (2012) The internal transcribed spacer of ribosomal RNA genes in plant trypanosomes (Phytomonas spp.) resolves 10 groups. Infect Genet Evol 12 : 299–308. http://www.ncbi.nlm.nih.gov/pubmed/22155359. Accessed 29 April 2013. doi: 10.1016/j.meegid.2011.11.010 22155359
31. Koreny L, Lukes J, Al. E (2012) Aerobic kinetoplastid flagellate Phytomonas does not require heme for viability. PNAS 109 : 3808–3813. doi: 10.1073/pnas.1201089109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1201089109 22355128
32. Porcel BM, Denoeud F, Opperdoes F, Noel B, Madoui M-A, et al. (2014) The streamlined genome of Phytomonas spp. relative to human pathogenic kinetoplastids reveals a parasite tailored for plants. PLoS Genet 10: e1004007. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3916237&tool=pmcentrez&rendertype=abstract. Accessed 26 March 2014. doi: 10.1371/journal.pgen.1004007 24516393
33. Santos ALS, d’Avila-Levy CM, Dias F a, Ribeiro RO, Pereira FM, et al. (2006) Phytomonas serpens: cysteine peptidase inhibitors interfere with growth, ultrastructure and host adhesion. Int J Parasitol 36 : 47–56. http://www.ncbi.nlm.nih.gov/pubmed/16310789. Accessed 21 March 2013. 16310789
34. Kelly S, Ivens A, Manna PT, Gibson W, Field MC (2014) A draft genome for the African crocodilian trypanosome Trypanosoma grayi. Sci Data 1. http://www.nature.com/articles/sdata201424. Accessed 8 August 2014.
35. Ivens AC, Peacock CS, Worthey E a, Murphy L, Aggarwal G, et al. (2005) The genome of the kinetoplastid parasite, Leishmania major. Science 309 : 436–442. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1470643&tool=pmcentrez&rendertype=abstract. Accessed 3 June 2013. 16020728
36. Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, et al. (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309 : 416–422. http://www.ncbi.nlm.nih.gov/pubmed/16020726. Accessed 6 March 2013. 16020726
37. El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, et al. (2005) The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309 : 409–415. http://www.sciencemag.org/content/309/5733/409.short. Accessed 15 July 2014. 16020725
38. Votýpka J, Klepetková H, Jirků M, Kment P, Lukeš J (2012) Phylogenetic relationships of trypanosomatids parasitising true bugs (Insecta: Heteroptera) in sub-Saharan Africa. Int J Parasitol 42 : 489–500. http://www.ncbi.nlm.nih.gov/pubmed/22537738. Accessed 6 March 2013. doi: 10.1016/j.ijpara.2012.03.007 22537738
39. Maslov D a, Votýpka J, Yurchenko V, Lukeš J (2013) Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol 29 : 43–52. http://www.ncbi.nlm.nih.gov/pubmed/23246083. Accessed 23 March 2013. doi: 10.1016/j.pt.2012.11.001 23246083
40. Breitenbach HH, Wenig M, Wittek F, Jordá L, Maldonado-Alconada AM, et al. (2014) Contrasting Roles of the Apoplastic Aspartyl Protease APOPLASTIC, ENHANCED DISEASE SUSCEPTIBILITY1-DEPENDENT1 and LEGUME LECTIN-LIKE PROTEIN1 in Arabidopsis Systemic Acquired Resistance. Plant Physiol 165 : 791–809. http://www.plantphysiol.org/content/early/2014/04/22/pp.114.239665.short. Accessed 6 June 2014. 24755512
41. Ten Have A, Espino JJ, Dekkers E, Van Sluyter SC, Brito N, et al. (2010) The Botrytis cinerea aspartic proteinase family. Fungal Genet Biol 47 : 53–65. http://www.sciencedirect.com/science/article/pii/S1087184509001765. Accessed 5 May 2014. doi: 10.1016/j.fgb.2009.10.008 19853057
42. Kehr J (2006) Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J Exp Bot 57 : 767–774. http://jxb.oxfordjournals.org/content/57/4/767.full. Accessed 28 May 2014. 16495410
43. Hoare CA, Wallace FG (1966) Developmental Stages of Trypanosomatid Flagellates: a New Terminology. Nature 212 : 1385–1386. http://dx.doi.org/10.1038/2121385a0. Accessed 14 April 2014.
44. Wheeler RJ, Gluenz E, Gull K (2013) The limits on trypanosomatid morphological diversity. PLoS One 8: e79581. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0079581#s3. Accessed 3 April 2014. doi: 10.1371/journal.pone.0079581 24260255
45. Gibbs AJ (1957) Leptomonas-serpens nsp. parasitic in the digestive tract and salivary glands of Nezara-viridula (Pentatomidae) and in the sap of Solanum-lycopersicum (tomato) and other plants. Parasitology 47 : 297–303. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=5&SID=W2qYUfkCAoQXYhjPlT4&page=1&doc=1. Accessed 9 January 2014. 13504849
46. Wheeler RJ, Gluenz E, Gull K (2011) The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology. Mol Microbiol 79 : 647–662. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3166656&tool=pmcentrez&rendertype=abstract. Accessed 21 March 2014. doi: 10.1111/j.1365-2958.2010.07479.x 21255109
47. Lafont A (1910) Sur la présence d’un Leptomonas, parasite de la classe des Flagelles, dans le latex de trois Euphorbiacees. Ann l’instutut Pasteur, Paris 24 : 205–219. doi: 10.1167/iovs.14-15247 25190660
48. Franchini G (1922) Sur un flagelle nouveau du latex de deux Apocynees. Bull la Soc Pathol Exot 15 : 109–113.
49. Franchini G (1922) Sur un trypanosome du latex de deux especes d’Euphorbes. Bull la Soc Pathol Exot 15 : 18–23.
50. Lafont A (1911) Sur la transmission du Leptomonas Davidi des euphorbes par un hemiptere, Nysius euphorbiae. Comptes Rendus des Seances la Soc Biol 70 : 58–59.
51. Gluenz E, Höög JL, Smith AE, Dawe HR, Shaw MK, et al. (2010) Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J 24 : 3117–3121. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2923350&tool=pmcentrez&rendertype=abstract. Accessed 20 May 2014. doi: 10.1096/fj.09-151381 20371625
52. Overath P, Haag J, Mameza MG, Lischke A (1999) Freshwater fish trypanosomes: definition of two types, host control by antibodies and lack of antigenic variation. Parasitology 119 : 591–601. http://journals.cambridge.org/abstract_S0031182099005089. Accessed 11 April 2014. 10633921
53. Siddall ME, Desser SS (1992) Alternative leech vectors for frog and turtle trypanosomes. J Parasitol 78 : 562–563. http://www.ncbi.nlm.nih.gov/pubmed/1597811. Accessed 11 April 2014. 1597811
54. Hamilton PB, Stevens JR, Gidley J, Holz P, Gibson WC (2005) A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae). Int J Parasitol 35 : 431–443. http://www.sciencedirect.com/science/article/pii/S0020751905000020. Accessed 8 April 2014. 15777919
55. Hamilton PB, Gibson WC, Stevens JR (2007) Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Mol Phylogenet Evol 44 : 15–25. http://www.sciencedirect.com/science/article/pii/S1055790307000905. Accessed 8 April 2014. 17513135
56. Alves E Silva TL, Vasconcellos LRC, Lopes AH, Souto-Padrón T (2013) The Immune Response of Hemocytes of the Insect Oncopeltus fasciatus against the Flagellate Phytomonas serpens. PLoS One 8: e72076. http://dx.plos.org/10.1371/journal.pone.0072076. Accessed 26 September 2013. doi: 10.1371/journal.pone.0072076 24015207
57. Camargo EP, Kastelein P, Roitman I (1990) Trypanosomatid parasites of plants (phytomonas). Parasitol Today 6 : 22–25. http://dx.doi.org/10.1016/0169-4758(90)90388-K. Accessed 17 June 2013. 15463252
58. Brazil RP, Fiorini JE, Silva PMFE (1990) Phytomonas sp., a trypanosomatid parasite of tomato, isolated from salivary glands of Phthia picta (Hemiptera: Coreidae) in southeast Brazil. Mem Inst Oswaldo Cruz 85 : 239–240. http://www.cabdirect.org/abstracts/19910881423.html;jsessionid=E654E3491B07A246AEFC3B74997D2B40?freeview=true. Accessed 8 June 2014.
59. Barry J (1998) VSG gene control and infectivity strategy of metacyclic stage Trypanosoma brucei. Mol Biochem Parasitol 91 : 93–105. http://www.sciencedirect.com/science/article/pii/S016668519700193X. Accessed 19 July 2014. 9574928
60. Späth GF, Beverley SM (2001) A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 99 : 97–103. http://www.ncbi.nlm.nih.gov/pubmed/11748963. Accessed 6 August 2014. 11748963
61. Späth GF, Epstein L, Leader B, Singer SM, Avila HA, et al. (2000) Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A 97 : 9258–9263. http://www.pnas.org/content/97/16/9258.short. Accessed 6 August 2014. 10908670
62. Jankevicius JV, Jankevfcius SI, Campaner M, Conchon I, Madea LA, et al. (1989) Life Cycle and Culturing of Phytomonas serpens (Gibbs), a Trypanosomatid Parasite of Tomatoes. J Protozool 36 : 265–271. http://doi.wiley.com/10.1111/j.1550-7408.1989.tb05361.x. Accessed 11 April 2014.
63. Zakai HA, Chance ML, Bates PA (1998) In vitro stimulation of metacyclogenesis in Leishmania braziliensis, L. donovani, L. major and L. mexicana. Parasitology 116 (Pt 4 : 305–309. http://www.ncbi.nlm.nih.gov/pubmed/9585932. Accessed 6 August 2014. 9585932
64. Rogers ME, Bates PA (2007) Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog 3: e91. http://dx.plos.org/10.1371/journal.ppat.0030091. Accessed 24 January 2014. 17604451
65. Hogenhout SA, Oshima K, Ammar E-D, Kakizawa S, Kingdom HN, et al. (2008) Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9 : 403–423. http://www.ncbi.nlm.nih.gov/pubmed/18705857. Accessed 3 October 2013. doi: 10.1111/j.1364-3703.2008.00472.x 18705857
66. Ienne S, Freschi L, Vidotto VF, DE Souza TA, Purgatto E, et al. (2014) Auxin production by the plant trypanosomatid Phytomonas serpens and auxin homoeostasis in infected tomato fruits. Parasitology 141 : 1299–1310. Available: http://journals.cambridge.org/abstract_S0031182014000547. Accessed 31 July 2014. doi: 10.1017/S0031182014000547 24805281
67. Redman CA, Schneider P, Mehlert A, Ferguson MA (1995) The glycoinositol-phospholipids of Phytomonas. Biochem J 311 : 495–503. http://www.biochemj.org/bj/311/bj3110495.htm. Accessed 11 April 2014. 7487886
68. D’Avila-Levy CM, Santos LO, Marinho FA, Dias FA, Lopes AH, et al. (2006) Gp63-like molecules in Phytomonas serpens: possible role in the insect interaction. Curr Microbiol 52 : 439–444. http://www.ncbi.nlm.nih.gov/pubmed/16732452. Accessed 21 March 2013. 16732452
69. Manna PT, Kelly S, Field MC (2013) Adaptin evolution in kinetoplastids and emergence of the variant surface glycoprotein coat in African trypanosomatids. Mol Phylogenet Evol 67 : 123–128. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3650584&tool=pmcentrez&rendertype=abstract. Accessed 30 April 2013. doi: 10.1016/j.ympev.2013.01.002 23337175
70. Jaffe CL, Dwyer DM (2003) Extracellular release of the surface metalloprotease, gp63, from Leishmania and insect trypanosomatids. Parasitol Res 91 : 229–237. http://www.ncbi.nlm.nih.gov/pubmed/12923634. Accessed 16 January 2014. 12923634
71. Santos ALS, d’Avila-Levy CM, Elias CGR, Vermelho AB, Branquinha MH (2007) Phytomonas serpens: immunological similarities with the human trypanosomatid pathogens. Microbes Infect 9 : 915–921. 17556002
72. Elias CGR, Pereira FM, Dias FA, Silva TLA, Lopes AHCS, et al. (2008) Cysteine peptidases in the tomato trypanosomatid Phytomonas serpens: influence of growth conditions, similarities with cruzipain and secretion to the extracellular environment. Exp Parasitol 120 : 343–352. http://www.ncbi.nlm.nih.gov/pubmed/18793639. Accessed 21 March 2013. doi: 10.1016/j.exppara.2008.08.011 18793639
73. Beneteau J, Renard D, Marché L, Douville E, Lavenant L, et al. (2010) Binding properties of the N-acetylglucosamine and high-mannose N-glycan PP2-A1 phloem lectin in Arabidopsis. Plant Physiol 153 : 1345–1361. http://www.plantphysiol.org/content/153/3/1345.abstract. Accessed 28 May 2014. doi: 10.1104/pp.110.153882 20442276
74. Gaupels F, Ghirardo A (2013) The extrafascicular phloem is made for fighting. Front Plant Sci 4 : 187. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3678090&tool=pmcentrez&rendertype=abstract. Accessed 6 June 2014. doi: 10.3389/fpls.2013.00187 23781225
75. Elias CGR, Chagas MG, Souza-Gonçalves AL, Pascarelli BMO, d’Avila-Levy CM, et al. (2012) Differential expression of cruzipain - and gp63-like molecules in the phytoflagellate trypanosomatid Phytomonas serpens induced by exogenous proteins. Exp Parasitol 130 : 13–21. http://www.ncbi.nlm.nih.gov/pubmed/22033075. Accessed 21 March 2013. doi: 10.1016/j.exppara.2011.10.005 22033075
76. Späth GF, Garraway LA, Turco SJ, Beverley SM (2003) The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci U S A 100 : 9536–9541. http://www.pnas.org/content/100/16/9536. Accessed 23 May 2014. 12869694
77. Jones JDG, Dangl JL (2006) The plant immune system. Nature 444 : 323–329. http://dx.doi.org/10.1038/nature05286. Accessed 21 May 2013. 17108957
78. El-On J, Bradley DJ, Freeman JC (1980) Leishmania donovani: action of excreted factor on hydrolytic enzyme activity of macrophages from mice with genetically different resistance to infection. Exp Parasitol 49 : 167–174. http://www.ncbi.nlm.nih.gov/pubmed/6767620. Accessed 17 September 2014. 6767620
79. McNeely TB, Turco SJ (1990) Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol 144 : 2745–2750. http://www.ncbi.nlm.nih.gov/pubmed/2319134. Accessed 17 September 2014. 2319134
80. Barbieri L, Falasca A, Franceschi C, Licastro F, Rossi CA, et al. (1983) Purification and properties of two lectins from the latex of the euphorbiaceous plants Hura crepitans L. (sand-box tree) and Euphorbia characias L. (Mediterranean spurge). Biochem J 215 : 433–439. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1152420&tool=pmcentrez&rendertype=abstract. Accessed 5 May 2014. 6661180
81. Sanchez-Moreno M, Fernandez-Becerra C, Mascaro C, Rosales MJ, Dollet M, et al. (1995) Isolation, in vitro culture, ultrastructure study, and characterization by lectin-agglutination tests of Phytomonas isolated from tomatoes (Lycopersicon esculentum) and cherimoyas (Anona cherimolia) in southeastern Spain. Parasitol Res 81 : 575–581. http://link.springer.com/10.1007/BF00932024. Accessed 22 April 2014. 7479649
82. Da Silva R V, Malvezi AD, Augusto L da S, Kian D, Tatakihara VLH, et al. (2013) Oral exposure to Phytomonas serpens attenuates thrombocytopenia and leukopenia during acute infection with Trypanosoma cruzi. PLoS One 8: e68299. http://www.plosone.org/article/info:doi/10.1371/journal.pone.0068299;jsessionid=973A60CAB98EA0D186580F375A605096. Accessed 31 January 2014. doi: 10.1371/journal.pone.0068299 23844182
83. Franchini G (1922) Essais d’inoculation aux souris blanches du latex parasite de differentes especes d’euphorbes. Ann l’instutut Pasteur 36 : 873–881. doi: 10.1167/iovs.14-15247 25190660
84. Carrari F, Baxter C, Usadel B, Urbanczyk-Wochniak E, Zanor M - I, et al. (2006) Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol 142 : 1380–1396. http://www.plantphysiol.org/content/142/4/1380. Accessed 29 April 2014. 17071647
85. Oliveira AP, Silva LR, Andrade PB, Valentão P, Silva BM, et al. (2010) Further insight into the latex metabolite profile of Ficus carica. J Agric Food Chem 58 : 10855–10863. http://dx.doi.org/10.1021/jf1031185. Accessed 28 April 2014. doi: 10.1021/jf1031185 20923221
86. Maslov D, Nawathean P, Scheel J (1999) Partial kinetoplast-mitochondrial gene organization and expression in the respiratory deficient plant trypanosomatid Phytomonas serpens. Mol Biochem Parasitol 99 : 207–221. Available: http://www.sciencedirect.com/science/article/pii/S0166685199000286. Accessed 1 August 2014. 10340485
87. Nawathean P, Maslov DA (2000) The absence of genes for cytochrome c oxidase and reductase subunits in maxicircle kinetoplast DNA of the respiration-deficient plant trypanosomatid Phytomonas serpens. Curr Genet 38 : 95–103. doi: 10.1007/s002940000135 10975258
88. Bringaud F, Rivière L, Coustou V (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. http://www.sciencedirect.com/science/article/pii/S0166685106001150. Accessed 1 August 2014.
89. Chaumont F, Schanck A, Blum JJ, Opperdoes FR (1994) Aerobic and anaerobic glucose metabolism of Phytomonas sp. isolated from Euphorbia characias. Mol Biochem Parasitol 67 : 321–331. http://dx.doi.org/10.1016/0166-6851(94)00141-3. Accessed 17 June 2013. 7870136
90. Sanchez-Moreno M, Lasztity D, Coppens I, Opperdoes FR (1992) Characterization of carbohydrate metabolism and demonstration of glycosomes in a Phytomonas sp. isolated from Euphorbia characias. Mol Biochem Parasitol 54 : 185–199. http://www.sciencedirect.com/science/article/pii/016668519290111V. Accessed 1 August 2014. 1435859
91. Frankenberg N, Moser J, Jahn D (2003) Bacterial heme biosynthesis and its biotechnological application. Appl Microbiol Biotechnol 63 : 115–127. http://www.ncbi.nlm.nih.gov/pubmed/13680202. Accessed 13 July 2014. 13680202
92. Molinas SM, Altabe SG, Opperdoes FR, Rider MH, Michels PAM, et al. (2003) The multifunctional isopropyl alcohol dehydrogenase of Phytomonas sp. could be the result of a horizontal gene transfer from a bacterium to the trypanosomatid lineage. J Biol Chem 278 : 36169–36175. http://www.ncbi.nlm.nih.gov/pubmed/12853449. Accessed 1 August 2014. 12853449
93. Uttaro AD, Opperdoes FR (1997) Purification and characterisation of a novel iso-propanol dehydrogenase from Phytomonas sp. Mol Biochem Parasitol 85 : 213–219. http://www.ncbi.nlm.nih.gov/pubmed/9106194. Accessed 1 August 2014. 9106194
94. Uttaro AD, Opperdoes FR (1997) Characterisation of the two malate dehydrogenases from Phytomonas sp. Purification of the glycosomal isoenzyme. Mol Biochem Parasitol 89 : 51–59. http://www.ncbi.nlm.nih.gov/pubmed/9297700. Accessed 6 August 2014. 9297700
95. Aslett M, Aurrecoechea C, Berriman M, Brestelli J, Brunk BP, et al. (2010) TriTrypDB: a functional genomic resource for the Trypanosomatidae. Nucleic Acids Res 38: D457–62. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2808979&tool=pmcentrez&rendertype=abstract. Accessed 27 May 2014. doi: 10.1093/nar/gkp851 19843604
96. Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=403725&tool=pmcentrez&rendertype=abstract. Accessed 26 May 2014. 12952885
97. Collingridge PW, Kelly S (2012) MergeAlign: improving multiple sequence alignment performance by dynamic reconstruction of consensus multiple sequence alignments. BMC Bioinformatics 13 : 117. http://www.biomedcentral.com/1471-2105/13/117. Accessed 3 June 2014. doi: 10.1186/1471-2105-13-117 22646090
98. Price MN, Dehal PS, Arkin AP (2009) FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26 : 1641–1650. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2693737&tool=pmcentrez&rendertype=abstract. Accessed 27 May 2014. doi: 10.1093/molbev/msp077 19377059
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



