-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
Aspergillus spp.
are ubiquitous in the environment, and even though individuals are regularly exposed to fungal spores clinical invasive disease is a rare manifestation. In contrast, individuals with weakened immune systems develop severe disease, such as invasive pulmonary aspergillosis (IPA). IPA is associated with extremely poor prognoses and unacceptably high mortality rates. Knowledge gained from understanding how immunocompetent mammals control Aspergillus challenge will help develop new immunomodulatory strategies aimed at improving patient outcomes. It is well known that neutrophils and monocytes are crucial immune cells that act to limit fungal growth. Our work demonstrates a central role for the cytokine IL-1α in orchestrating the optimal recruitment of neutrophils and monocytes, whereas IL-1β and the inflammasome are more important in activation of anti-fungal activity of the monocytes. Moreover, our studies indicate that CCR2+ monocytes are required for optimal production of IL-1α in the lungs of A. fumigatus challenged mice. Thus, our data highlight a crucial role of the IL-1 cytokine in mediating anti-fungal immunity which might be harnessed to treat clinical cases of IPA.
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004625
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004625Summary
Aspergillus spp.
are ubiquitous in the environment, and even though individuals are regularly exposed to fungal spores clinical invasive disease is a rare manifestation. In contrast, individuals with weakened immune systems develop severe disease, such as invasive pulmonary aspergillosis (IPA). IPA is associated with extremely poor prognoses and unacceptably high mortality rates. Knowledge gained from understanding how immunocompetent mammals control Aspergillus challenge will help develop new immunomodulatory strategies aimed at improving patient outcomes. It is well known that neutrophils and monocytes are crucial immune cells that act to limit fungal growth. Our work demonstrates a central role for the cytokine IL-1α in orchestrating the optimal recruitment of neutrophils and monocytes, whereas IL-1β and the inflammasome are more important in activation of anti-fungal activity of the monocytes. Moreover, our studies indicate that CCR2+ monocytes are required for optimal production of IL-1α in the lungs of A. fumigatus challenged mice. Thus, our data highlight a crucial role of the IL-1 cytokine in mediating anti-fungal immunity which might be harnessed to treat clinical cases of IPA.Introduction
The mold Aspergillus fumigatus is one of the leading causes of invasive fungal infections. It is the causative agent of severe pulmonary infections such as invasive pulmonary aspergillosis (IPA), a disease of high morbidity and mortality which affects immunocompromised individuals [1]. IPA has been a disease of growing concern over recent decades due to an increase in the immunocompromised population, specifically caused by advances in immunosuppressive drugs and organ transplantation methods as well as chemotherapy treatments in cancer patients [2]. In addition, there is increasing evidence that IPA can sporadically develop in certain immunocompetent populations [3]. Currently there are no available vaccines for A. fumigatus and anti-fungal drugs have a modest rate of success in limiting high mortality rates typically due to late diagnosis of IPA [1,2,4]. Moreover, the recent emergence of drug resistance has further limited treatment options in certain clinical cases and geographic areas [5,6,7].
The concentration of Aspergillus conidia in air samples ranges from 0.2–15 conidia/m3 and on a daily basis an individual can inhale hundreds of conidia [8]. In most immunocompetent individuals the conidia are typically removed from the body by physical barriers encountered within the respiratory tract. However, if the conidia escape this primary immune barrier and enter the lung, they will be removed by alveolar macrophages and other resident leukocytes, such as CCR2+ monocytes. Conversely, in an individual lacking a sufficient immune response, Aspergillus conidia are able to swell, germinate, and form hyphae, invading pulmonary tissue with the potential to disseminate systemically [9,10]. Our understanding of the inflammatory pathways necessary for an immunocompetent individual to maintain control of A. fumigatus while constantly being exposed to conidia is an ongoing area of investigation.
Control of A. fumigatus growth in the lung during invasive infection is highly dependent on rapid recruitment and activation of innate immune cells, including neutrophils [11], inflammatory monocytes [10,12], NKT cells [13], and plasmacytoid dendritic cells [14]. The importance of appropriate activation of leukocytes in the control of A. fumigatus is highlighted by patients and mice with chronic granulomatous disease or lacking NADPH oxidase subunits, being highly susceptible to developing IPA after A. fumigatus challenge [15,16,17]. Furthermore, patients who become neutropenic after chemotherapy for a bone marrow transplant are at a higher risk for developing IPA [18,19,20,21]. In the murine model of A. fumigatus infection, CXCR2 and its ligands are important signaling components for neutrophil recruitment [22,23,24]. In the absence of CXCR2 signaling during pulmonary A. fumigatus infection, there is a decrease in neutrophil recruitment along with a higher fungal burden and increased mortality rate, similar to a neutropenic model [23]. Additionally, a role for CCR2 signaling has been shown to be necessary to promote recruitment and differentiation of inflammatory monocytes from the bone marrow into CD11b+ dendritic cells upon A. fumigatus infection [10,12]. However, the exact sequence of events necessary for the expression of chemotactic molecules for optimal leukocyte recruitment has not been well elucidated.
In addition, it has been shown that polymorphisms in the Interleukin (IL)-1 gene cluster may be important in determining the susceptibility or resistance to IPA in humans [25,26]. The IL-1 gene cluster codes for two pro-inflammatory cytokines, IL-1α and IL-1β, as well as the IL-1 receptor antagonist (IL-1Ra) [27]. All three of these IL-1 family members bind to the IL-1 receptor, type I (IL-1RI). IL-1α and IL-1β enhance the immune response while IL-1Ra competitively binds to IL-1RI, thereby preventing the binding of IL-1α and IL-1β [27]. Although IL-1α and IL-1β are both pro-inflammatory cytokines within the same IL-1 cytokine family, they differ in their maturation processes. IL-1α can be released as pro-IL-1α or mature IL-1α after calpain cleavage. In either form it can actively bind to IL-1RI and mediate downstream signaling [27,28]. Conversely, IL-1β is first produced as inactive pro-IL-1β which must be cleaved by a caspase-1 containing inflammasome to yield the mature biologically active cytokine [29]. After fungal exposure, IL-1β production has been linked to activation of the NLRP3 inflammasome [30,31,32,33,34,35,36,37]. Mice lacking the NLRP3 inflammasome are highly susceptible to disseminated candidiasis [30,36,37]. However, the role of inflammasome activation by A. fumigatus in vivo is unknown.
The role of IL-1α in regulating the pulmonary inflammatory response after infectious challenge is much less understood and is an active area of research. Importantly, several studies have shown that IL-1α and IL-1β can have non-redundant roles in infection and inflammation. Specifically, it has been demonstrated that an increase of IL-1α correlated with early neutrophil recruitment, while IL-1β correlated with macrophage recruitment during later time points in a model of sterile inflammation [38,39]. During pulmonary Legionella pneumophila infection IL-1α is essential for early neutrophil responses [40]. In a systemic candidiasis model, IL-1α and IL-1β played non-redundant roles in anti-fungal immunity by enhancing anti-fungal activity of leukocytes and recruitment of neutrophils, respectively [41]. However, the role(s) of IL-1 cytokines after challenge with the mold A. fumigatus remains to be fully defined.
Here, we delineate the differential roles of IL-1α and IL-1β after in vivo challenge with A. fumigatus and further define the sequence of events required for leukocyte recruitment after A. fumigatus challenge. Specifically, we observed, unlike the diseases caused by the yeast Candida albicans [30,36,37], that the inflammasome is not essential for preventing severe invasive pulmonary aspergillosis, but does participate in initiating the full anti-fungal activity of leukocytes. In stark contrast, IL-1α signaling through IL-1RI is crucial for the control of pulmonary A. fumigatus infection through optimal leukocyte recruitment, which correlated with CXCL1 expression. CCR2+ monocytes regulated the early expression of IL-1α and CXCL1, and promoted early neutrophil accumulation in the airways. Treatment of Il1r1-deficient mice with a chemokine known to enhance neutrophil recruitment enhanced immunity against pulmonary A. fumigatus infection. Thus, our studies define the specific sequence of events regulated by both IL-1α and IL-1β necessary for control of A. fumigatus growth and lung damage within the respiratory tract.
Results
Differential temporal expression kinetics of IL-1 cytokine family members after Aspergillus fumigatus challenge
To examine the early pulmonary inflammatory milieu induced after A. fumigatus challenge, bronchoalveolar lavage fluid (BALF) was collected 6, 12, 24, and 48 h after intratracheal (i.t.) instillation of ∼5×107 conidia of the CEA10 strain of A. fumigatus. Of note, both the inflammasome-dependent cytokines IL-1β (Fig. 1A) and IL-18 (Fig. 1B) were expressed within approximately 6 h after A. fumigatus challenge. In contrast, IL-1α (Fig. 1C) and IL-1Ra (Fig. 1D) were expressed in a linearly increasing manner during the first 48 h. Thus, A. fumigatus challenge results in temporally distinct expression of IL-1 cytokine family members.
Fig. 1. C57BL/6 mice show differential expression of IL-1α and IL-1ß after A. fumigatus infection. 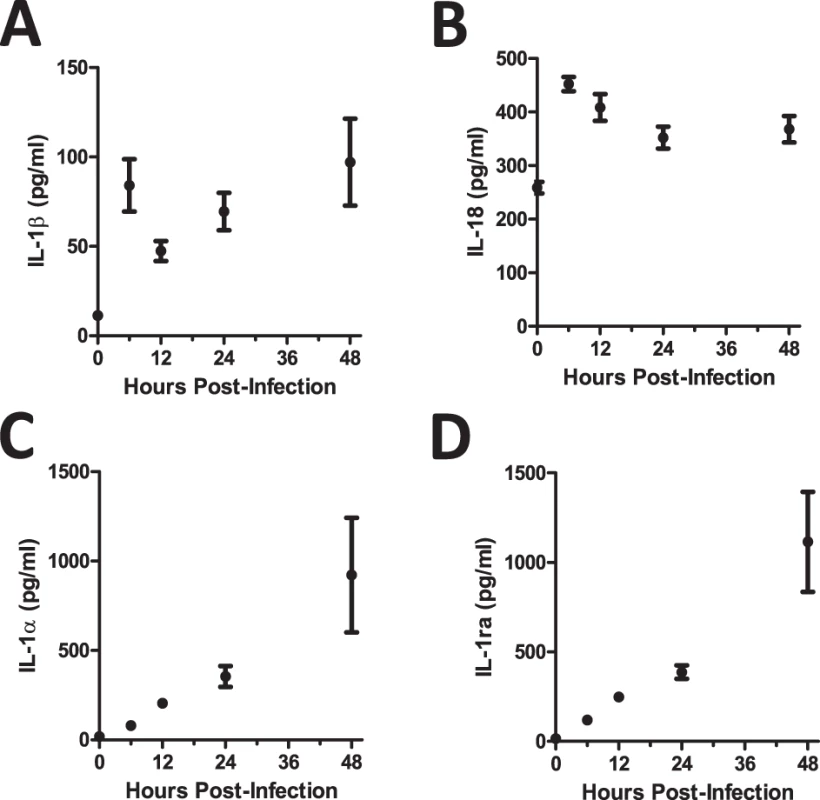
Mice were infected i.t. with 5×107 CEA10 conidia and at indicated time-points, mice were euthanized, bronchoalveolar lavage fluid (BALF) collected, and lung tissue homogenized. IL-1β (A), IL-18 (B), IL-1α (C), and IL-1Ra (D) levels in lung homogenate (IL-1α) and BALF (IL-1β, IL-18, and IL-1Ra) were measured using ProcartaPlex Mouse Cytokine & Chemokine 36-plex Immunoassay or ELISA. Data are representative of four mice per time point and two independent experiments. Each dot represents the mean ± one SEM. Il1r1-deficient mice are highly susceptible to pulmonary Aspergillus fumigatus infection
In one cohort of human patients it has been shown that a complex polymorphism in the Il1a, Il1b, and Il1rn genes, which was associated with decreased IL-1 dependent inflammatory events, resulted in increased risk for the development of IPA [25]. Because of this prior clinical observation plus our finding that both IL-1α and IL-1β are produced in the lungs after A. fumigatus challenge (Fig. 1), we questioned whether IL-1RI signaling was critical in the clearance of A. fumigatus from the lung. To globally test the role of IL-1 signaling in limiting A. fumigatus growth in the respiratory tract of mice, we challenged C57BL/6 and Il1r1-deficient mice with ∼5×107 conidia of A. fumigatus CEA10 delivered via the i.t. route. Subsequently, control of A. fumigatus in the respiratory tract was assessed by histological analysis at 24, 48, and 72 h after instillation. Strikingly, Grocott-Gomori methenamine silver (GMS) staining of lung tissue from Il1r1-deficient mice revealed the presence of a significant fraction of germinating A. fumigatus conidia at 48 h that was not observed in C57BL/6 mice (Fig. 2A). When the presence of germinating A. fumigatus conidia was quantified over the first 72 h, C57BL/6 mice displayed minimal germination that was ∼4% at 48 h before resolving (Fig. 2B); in contrast, Il1r1-deficient mice displayed a significant impairment in controlling A. fumigatus germination within 24 h (Fig. 2B). By 48 h, the majority of fungal conidia in Il1r1-deficient mice were germinated (Fig. 2B). High levels of germination were observed in the majority of Il1r1-deficient mice and this was associated with significant mortality in those mice (Fig. 2C). To strengthen our conclusion that IL-1RI signaling was crucial for controlling A. fumigatus germination in the lungs rather than a development issue in the Il1r1-deficient mice, we treated C57BL/6 mice intraperitoneally (i.p.) with 200 μg of hIL1ra, which antagonizes IL-1α and IL-1β, or placebo every 24 h starting one day prior to challenging mice with ∼5×107 conidia of A. fumigatus. Lung tissue from hIL1ra-treated C57BL/6 mice revealed the presence of a significant fraction of germinating A. fumigatus conidia at 48 h, which was not observed in placebo treated C57BL/6 mice (S1 Fig.). Taken together, these results strongly support the conclusion that IL-1RI signaling is critical for prevention of A. fumigatus strain CEA10 pulmonary proliferation and host damage.
Fig. 2. Il1r1-deficient mice are highly susceptible to Aspergillus fumigatus infection. 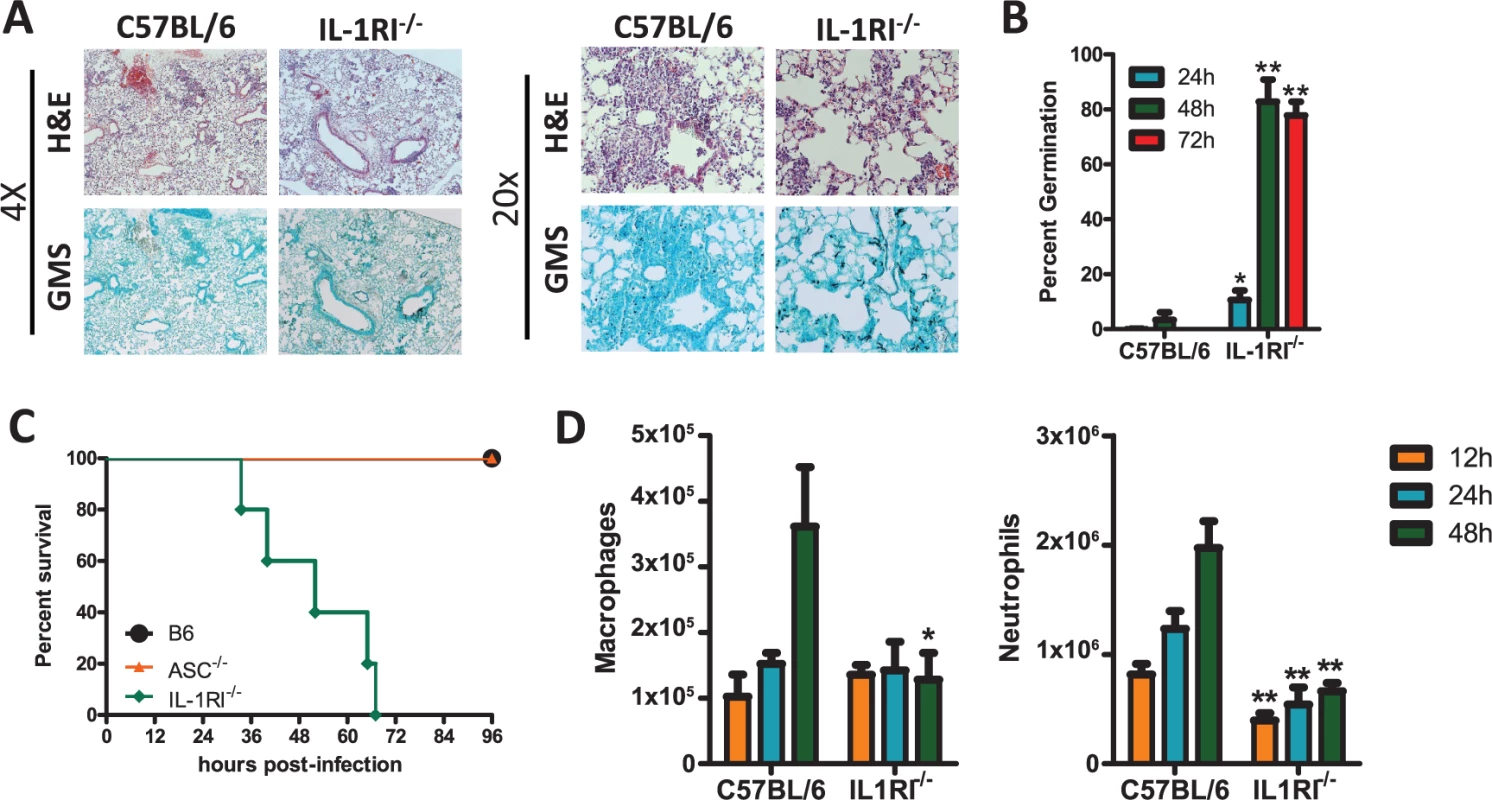
Age-matched C57BL/6 or Il1r1-deficient mice were infected i.t. with 5×107 CEA10 conidia and at indicated time-points, mice were euthanized, BALF collected, and lungs saved for histological analysis. (A) Formalin-fixed lungs were paraffin embedded, sectioned, and stained with H&E (top) or GMS (bottom) for analysis by microscopy. Representative lung sections from C57BL/6 and Il1r1-deficient mice infected with CEA10 for 48 h are shown using either the 4× (left) or 20× (right) objectives. (B) A. fumigatus germination rates were assessed over the first 72 h of infection by microscopically counting both the number of conidia and number of germlings in GMS-stained section. (C) Survival of C57BL/6, Pycard−/−, and Il1r1−/− mice challenged i.t. with 1.5×107 A. fumigatus (CEA10) conidia was then monitored for survival over the first 96 h (Mantel-Cox log-rank test, p = 0.0002). Data are representative of 2 independent experiments at each time point consisting of at least 5 mice per group. (D) Total macrophage (left panel) and neutrophil (right panel) recruitment in the BALF was measured at 12, 24, and 48 h post-infection. Data are representative of at least 2 independent experiments at each time point consisting of 3–5 mice per group. Bar graphs show the group mean ± one SEM. Statistically significant differences were determined using Student’s t-test (*p < 0.05; **p < 0.01). Neutrophils and macrophages are widely acknowledged to be critical effector cells for clearing A. fumigatus from the lungs [42]. Assessing cellular recruitment via differential microscopic counting of cytospins stained with Diff-Quik from the bronchoalveolar lavage fluid at 12, 24, and 48 h post-challenge demonstrated a significant impairment in neutrophil recruitment at each time point analyzed, while macrophage recruitment was similar between C57BL/6 and Il1r1-deficient mice at early time points after A. fumigatus challenge, but were decreased by 48 h (Fig. 2D). When inflammatory infiltrates within the BALF and lung parenchyma were assessed at 12, 24, and 36 h by flow cytometry a similar decrease in neutrophils in both compartments was observed in the Il1r1-deficient mice (S2A-S2B Fig.), while CD11b+ macrophages (S2C Fig.), CD11c+ alveolar macrophages (S2D Fig.), and CD103+ dendritic cells (S2E Fig.) were found at similar levels as observed in C57BL/6 mice. We next questioned whether leukocyte recruitment was diminished in Myd88-deficient mice because it is the key signaling adapter for IL-1RI, as well as TLRs [27,43], and Myd88-deficient mice have an impaired ability to control pulmonary A. fumigatus growth [44]. Indeed, Myd88-deficient mice exhibited defective neutrophil recruitment 12 and 24 h after A. fumigatus instillation, but normal macrophage recruitment at these early time points (S3 Fig.). Thus, mice deficient in Il1r1 and Myd88 are highly impaired in their ability to clear A. fumigatus from the lungs, which correlates with defects in early neutrophil recruitment to the lungs.
Pycard-deficient mice are only mildly susceptible to pulmonary Aspergillus fumigatus exposure
It is well documented that IL-1β secretion requires the function of the inflammasome [29] and that both the inflammasome and IL-1β are important in limiting systemic fungal infections [30,33,35,36,37,41,45]. Recent in vitro studies have shown that the NLRP3-ASC-Capase1 inflammasome can be triggered by A. fumigatus [31], but the in vivo relevance of this triggering during A. fumigatus infection remains unknown. Multiple inflammasome complexes exist, but ASC (Pycard) is a central adapter protein needed for maturation of IL-1β and IL-18 [29]. Thus, to determine the role of the inflammasome after A. fumigatus challenge, we challenged C57BL/6 and Pycard-deficient mice with ∼5×107 conidia of A. fumigatus CEA10 i.t.; subsequently, control of A. fumigatus in the respiratory tract was assessed by histological analysis at 24, 48, and 72 h after instillation. GMS staining of lung tissue from Pycard-deficient mice revealed the presence of germinating A. fumigatus conidia at elevated frequencies compared to C57BL/6 mice at 48 h (Fig. 3A-B), but this phenotype was less severe than what was observed in Il1r1-deficient mice and did not result in murine mortality (Fig. 2). When the presence of germinating A. fumigatus conidia was quantified over the first 72 h, C57BL/6 mice display minimal germination that was ∼3% at 48 h (Fig. 3B). Pycard-deficient mice displayed normal control of A. fumigatus germination at 24 h. However, by 48 h impaired control of A. fumigatus germination (∼22%) was observed, but these mice were ultimately able to resolve the A. fumigatus challenge (Fig. 3B). Because, neutrophils and macrophages are widely acknowledged to be critical effector cells for clearing A. fumigatus from the lungs [42] and were diminished in the absence of IL-1RI signaling (Fig. 2C), we next assessed inflammatory cell recruitment in BALF via differential microscopic counting of cytospins stained with Diff-Quik from the bronchoalveolar lavage fluid at 12, 24, and 48 h after instillation. Interestingly, C57BL/6 and Pycard-deficient mice demonstrated equivalent neutrophil and macrophage recruitment at each time point analyzed (Fig. 3C). Moreover, when the inflammatory infiltrates within the BALF and lung parenchyma were assessed at 12, 24, and 36 h by flow cytometry the number of neutrophils in the BALF and lung parenchyma, CD11b+ macrophages, CD11c+ alveolar macrophages, and CD103+ dendritic cells in the lung parenchyma were found at similar levels in C57BL/6 and Pycard-deficient mice (S2 Fig.). When we examined the expression of IL-1β in the BALF of Pycard-deficient mice, no expression of IL-1β at 12 h was observed while significant levels were detected in C57BL/6 mice (Fig. 3D); however, when IL-1α was examined in the lung parenchyma we observed equivalent levels of cytokine expression (Fig. 3E), suggesting that IL-1α signaling could still be activated in the Pycard-deficient mice.
Fig. 3. Pycard-deficient mice are mildly susceptible to A. fumigatus infection. 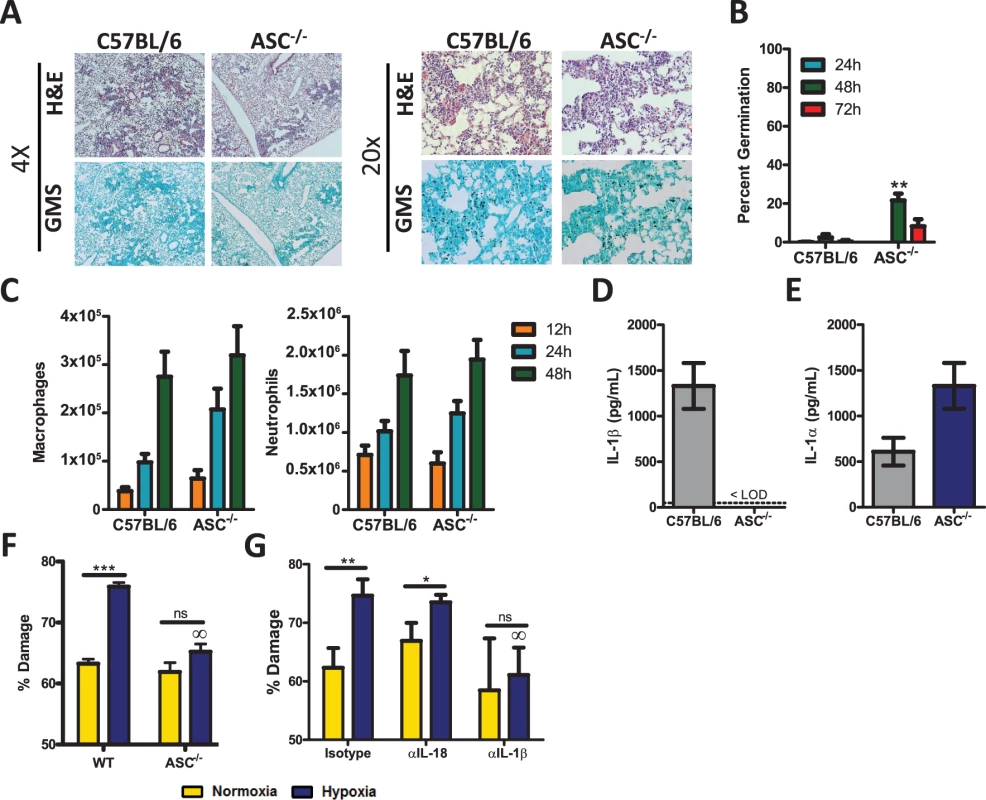
Age-matched C57BL/6 or Pycard-deficient mice were infected i.t. with 5×107 CEA10 conidia and at indicated time-points mice were euthanized, BALF collected, and lungs saved for histological analysis. (A) Formalin-fixed lungs were paraffin embedded, sectioned, and stained with H&E (top) or GMS (bottom) for analysis by microscopy. Representative lung sections from C57BL/6 and Pycard-deficient mice infected with CEA10 for 48 h are shown using either the 4× (left) or 20× (right) objectives. (B) A. fumigatus germination rates were assessed over the first 72 h of infection by microscopically counting both the number of conidia and number of germlings in GMS-stained section. (C) Total macrophage (left panel) and neutrophil (right panel) recruitment in the BALF was measured at 12, 24, and 48 h post-infection. (D) IL-1β levels in the bronchoalveolar lavage fluid (BALF) and (E) IL-1α levels in the lung parenchyma were assessed from C57BL/6 and Pycard-deficient mice infected i.t. 12 h prior with 5×107 CEA10 conidia. IL-1α and IL-1β levels in BALF were measured by ELISA. (B-E) All data are representative of at least 2 independent experiments at each time point consisting of 3–5 mice per group. Bar graphs show the group mean ± one SEM. Statistically significant differences were determined using Student’s t-test (*p < 0.05; **p < 0.01). LOD = limit of detection. (F-G) The anti-fungal activity of bone marrow-derived macrophages (BMDM) were assessed in vitro using the previously described XTT assay [46]. (E) BMDMs were obtained from C57BL/6 (WT) or Pycard-deficient (ASC) mice. An XTT assay was performed using BMDM from each mouse strain in both normoxic and hypoxic conditions. (B) BMDMs from C57BL/6 mice were obtained and incubated with isotype control antibody, IL-18 neutralizing antibody or IL-1ß neutralizing antibody. These BMDMs were then used in an XTT assay in both normoxic and hypoxic conditions. (E-F) Data are representative of four biological replicates in each group. Bar graphs show the group mean ± one SEM. Statistically significant differences were determined using an one-way ANOVA with Bonferroni’s post-test (∞p < 0.05 compared to C57BL/6 cells under the same conditions). Since Pycard-deficient mice did not demonstrate impaired leukocyte recruitment after A. fumigatus challenge, we next sought to quantitate the anti-fungal activity of macrophages from C57BL/6 and Pycard-deficient mice. Hyphal damage induced by macrophages was assessed using the XTT hyphal damage assay, which measures fungal cell metabolic activity as an indirect measure of fungal viability [46]. C57BL/6 and Pycard-deficient bone marrow-derived macrophages induced similar hyphal damage when co-cultured with A. fumigatus under normoxic conditions (Fig. 3F, yellow bars). Interestingly, a previous report demonstrated enhanced anti-fungal activity of leukocytes against fungal hyphae under hypoxic conditions [46], which occurs within the lungs after A. fumigatus challenge and at sites of microbial infection [47,48]. Intriguingly, time-points when hypoxia is observed also coincides with the recruitment of inflammatory monocytes to the site of infection [12]. Thus, we sought to test the contribution of the inflammasome to the anti-fungal response of macrophages under hypoxic conditions. Similar to the previous findings [46], C57BL/6 bone marrow-derived macrophages displayed significantly enhanced anti-fungal activity when cultured in hypoxia (Fig. 3F). In contrast, Pycard-deficient macrophages induced less hyphal damage when co-cultured with A. fumigatus under hypoxic conditions (Fig. 3F, blue bars). Since activation of the inflammasome triggers the release of both IL-1β and IL-18 we next sought to assess which inflammasome-dependent cytokine was responsible for increasing the anti-fungal activity of macrophages in hypoxia. C57BL/6 macrophages were treated with an isotype control antibody, anti-IL1β antibody, or anti-IL18 antibody during the co-culture with A. fumigatus germlings. Subsequently, fungal damage was again assessed by an XTT assay. Macrophages treated with an isotype control antibody display increased anti-fungal activity in hypoxia. This increased anti-fungal activity was lost in the presence of a blocking anti-IL1β antibody, but not a blocking anti-IL18 antibody (Fig. 3G). Collectively, these data demonstrate that mice deficient in Pycard are mildly impaired in their ability to clear A. fumigatus from the lungs, which correlated with in vitro defects in the anti-hyphal activity induced by IL-1β in hypoxia, rather than inflammatory cell recruitment to the lungs.
Anti-IL-1α treatment impairs pulmonary recruitment of leukocytes, enhancing the susceptibility of mice to pulmonary Aspergillus fumigatus infection
As Il1r1-deficient mice were much less able to control A. fumigatus germination than Pycard-deficient mice (Fig. 2 & 3) and Pycard-deficient mice still produced IL-1α in the lungs (Fig. 3D), we next sought to understand the role IL-1α played in the clearance of A. fumigatus from the lung. To determine the role of IL-1α after A. fumigatus challenge, we treated C57BL/6 mice i.p. with 40 μg of goat IgG or anti-IL1α 24 h prior to and 24 h after challenging mice with ∼5×107 conidia of A. fumigatus. Control of A. fumigatus in the respiratory tract was assessed by histological analysis at 48 h after instillation. GMS staining of lung tissue from anti-IL1α treated C57BL/6 mice revealed the presence of germinating A. fumigatus conidia at significantly higher frequencies than seen in goat IgG treated C57BL/6 mice (Fig. 4A & B). As leukocyte recruitment to the lungs was significantly impaired in Il1r1-deficient, but not Pycard-deficient, mice following A. fumigatus challenge, we next assessed inflammatory cell recruitment to the BALF via differential microscopic counting of cytospins stained with Diff-Quik from the bronchoalveolar lavage fluid at 24 and 48 h post-challenge. Interestingly, anti-IL1α treated C57BL/6 mice demonstrated reduced neutrophil recruitment at 24 h post-A. fumigatus challenge (Fig. 4C). Additionally, treatment of Pycard-deficient mice with anti-IL1α significantly enhanced the susceptibility of those mice to A. fumigatus challenge, mirroring what was found in Il1r1-deficient mice (S4 Fig.). Thus, blocking IL-1α in mice significantly impairs early neutrophil recruitment to the lungs early after A. fumigatus challenge resulting in impaired control of A. fumigatus germination in the lungs.
Fig. 4. C57BL/6 mice treated with IL-1α neutralizing antibody were more susceptible to Aspergillus fumigatus infection. 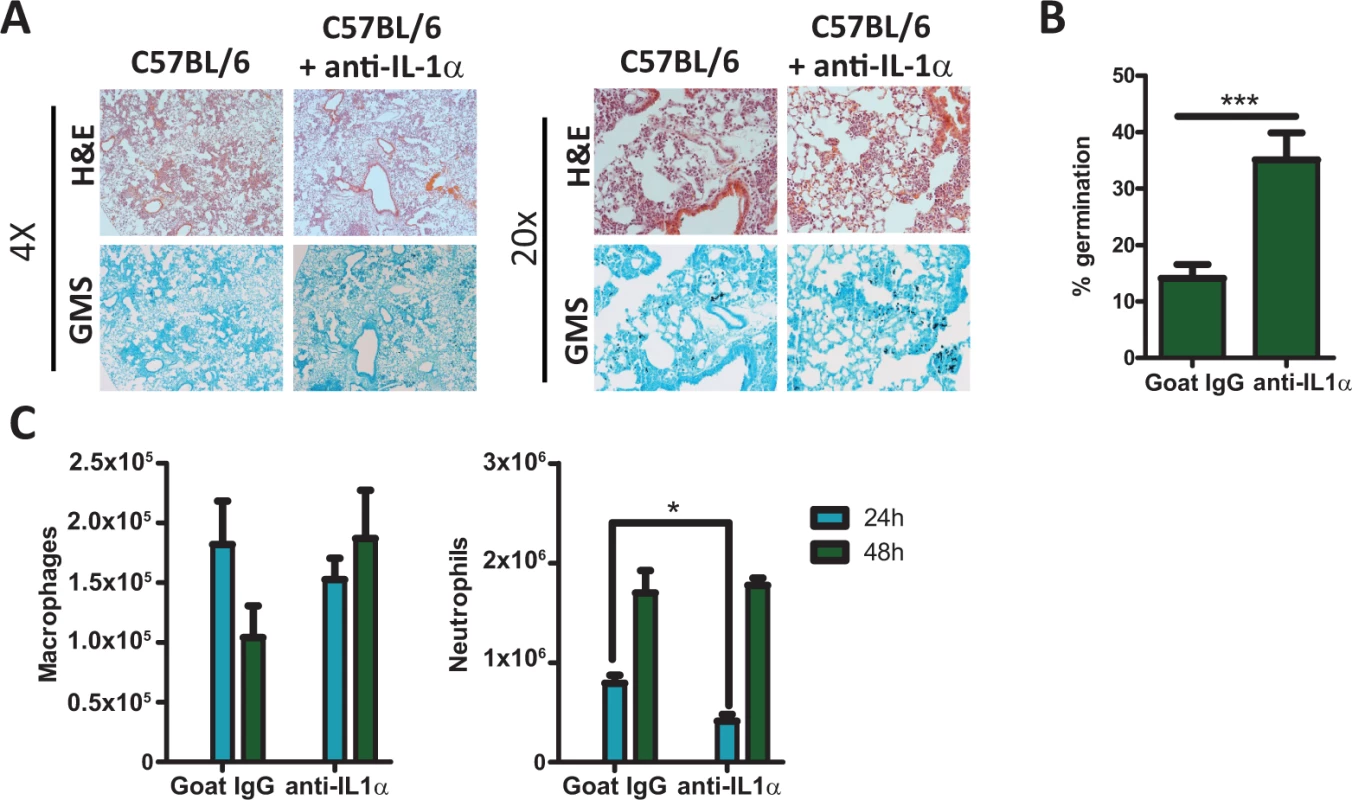
C57BL/6 mice treated with isotype control antibody or IL-1α neutralizing antibody were infected i.t. with 5×107 CEA10 conidia. At the indicated time points mice were euthanized, BALF collected and lungs saved for histological analysis. (A) Formalin-fixed lungs were paraffin embedded, sectioned and stained with H&E (top) or GMS (bottom) for analysis by microscopy. Representative lung sections from C57BL/6 mice treated with isotype control antibody (left) or with anti-IL-1α antibody (right) and infected with CEA10 for 48 h are shown using either the 4× (left) or 20× (right) objectives. (B) A. fumigatus germination rates at 48 h after challenge was determined by microscopically counting both the number of conidia and number of germlings in GMS-stained section. (C) Total macrophage (left panel) and neutrophil (right panel) recruitment in the BALF was measured at 24 and 48 h post-infection via cytospins. Data are representative of two independent experiments consisting of 4–5 mice per group. Bar graphs show the group mean ± one SEM. Statistically significant differences were determined using a Student’s t-test (*p < 0.05, ***p < 0.001). IL-1α signaling enhances the expression of CXCL1
As both Il1r1-deficient mice and anti-IL1α treated C57BL/6 mice displayed significantly decreased cellular infiltration into the BALF (Fig. 2D and 4C), we next sought to understand the roles that IL-1α, IL-1RI, and the inflammasome play in setting up the inflammatory milieu within the lungs. Thus, we challenged four cohorts of mice, C57BL/6 treated with 40 μg of goat IgG, C57BL/6 treated with 40 μg of anti-IL1α, Il1r1-deficient, and Pycard-deficient, with ∼5×107 conidia of A. fumigatus. Twenty-four hours after challenge, the inflammatory milieu in the lung parenchyma was assessed by a 12-plex multiplex cytokine assay. Anti-IL1α treatment, rather than Pycard-deficiency, largely mirrored the inflammatory cytokine defects found in the Il1r1-deficient mice (Fig. 5) fitting with the biological outcomes of A. fumigatus challenge in those mice. Specifically, TNFα, CCL3, and CCL4 expression was not diminished in the absence of IL-1α, IL-1RI, or ASC (Fig. 5). Interestingly, CXCL1 and G-CSF expression were significantly reduced in Il1r1-deficient mice. CXCL1 expression was almost entirely dependent on IL-1α signaling (Fig. 5), while G-CSF expression trend to being dependent on both IL-1α and ASC in an additive manner (Fig. 5). A similar trend, as observed with CXCL1, was seen with IL-6 and CCL2, but it did not reach significance (Fig. 5). Thus, blocking IL-1α in mice significantly decreased the abundance of CXCL1 in the lungs, which correlates with the decreased neutrophil recruitment in Il1r1-deficient mice.
Fig. 5. IL-1α signaling enhances expression of leukocyte recruiting chemokines. 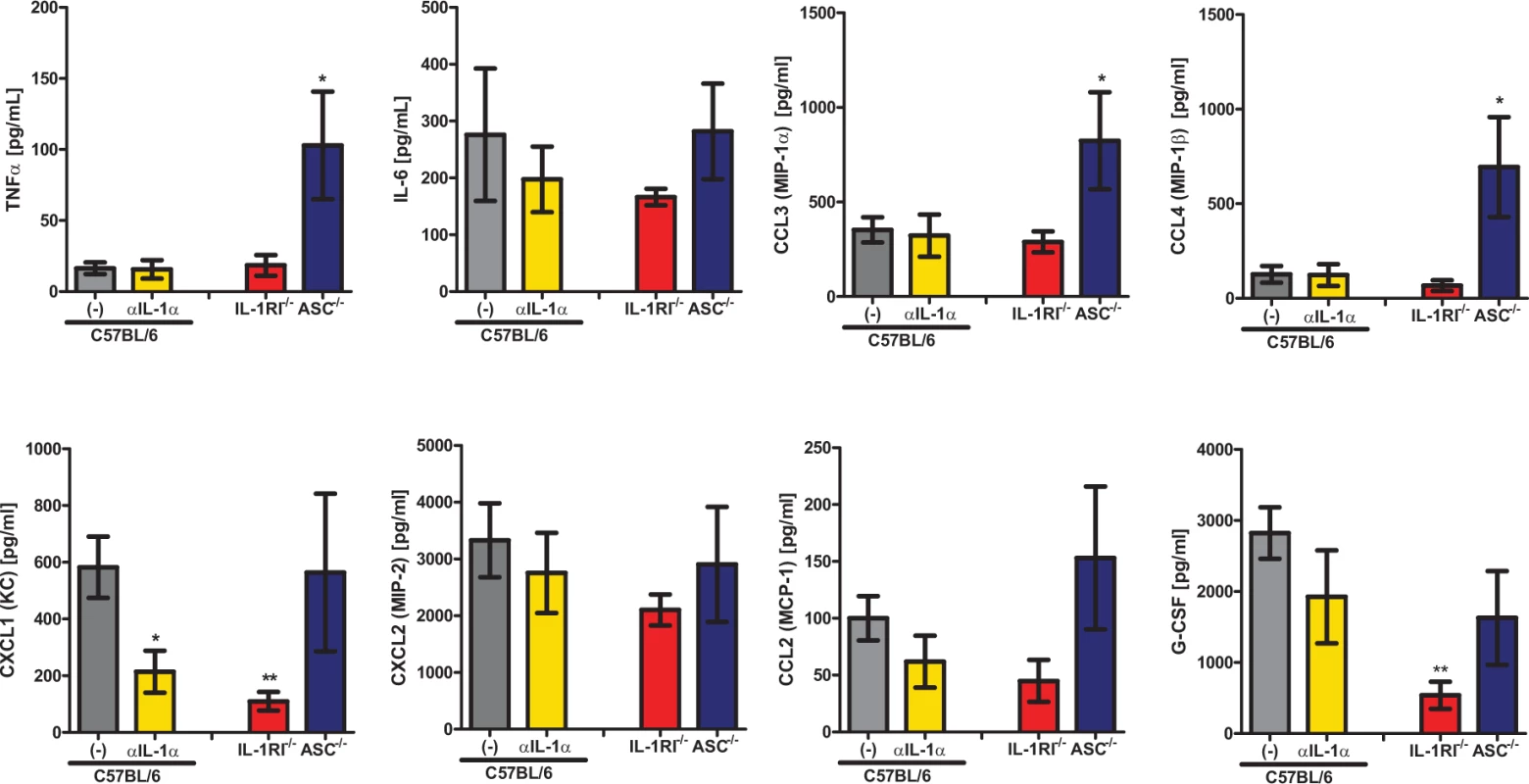
C57BL/6 mice treated with either isotype control antibody or IL-1α neutralizing antibody, Il1r1-deficient and Pycard-deficient mice were infected with 5×107 CEA10 conidia and at 24 hours post-infection, mice were euthanized, BALF collected, and lung tissue homogenized. Cytokine and chemokine levels in the lung homogenates were measured using 12-plex multiplex Luminex assay, similar trends were observed in BALF. Data are representative of two independent experiments consisting of 4–5 mice per group. Bar graphs show the group mean ± one SEM. Statistically significant differences were determined using a Kruskal-Wallis one-way ANOVA with Dunn’s post-test (*p < 0.05, **p < 0.01). Absence of CCR2+ monocytes results in decreased IL-1α, CXCL1, and neutrophil recruitment
As IL1α was necessary for optimal CXCL1 expression and neutrophil infiltration into the BALF (Fig. 4C & 5), we next sought to identify potential cellular sources of IL-1α in response to pulmonary challenge with A. fumigatus. Within the lung of a naïve mouse several potential sources of IL-1α exist including: non-hematopoietic cells (epithelial and endothelial cells), alveolar macrophages in the airway spaces, and CCR2+ monocytes within the lung parenchyma. During pulmonary Mycobacterium tuberculosis infection two distinct populations of myeloid cells co-express IL-1α and IL-1β: inflammatory monocytes which are CD11b+ CD11c− Ly6c+ and monocytic dendritic cells which are CD11b+ CD11c+ [49]. In response to pulmonary A. fumigatus challenge, CCR2+ inflammatory monocytes are rapidly recruited to the lung and give rise to monocyte-derived dendritic cells that play essential roles in innate defense against invasive aspergillosis [12]. Interestingly, both CCR2+ inflammatory monocytes and monocyte-derived dendritic cells show increased transcription of the Il1a gene at 48 h post-A.fumigatus challenge [12], but whether lung-resident CCR2+ monocytes could contribute to IL-1α production at early times after infection was not explored.
Thus, we challenged either C57BL/6 or CCR2-depleter mice [10,50], which had been treated one day prior with 250 ng of diphtheria toxin, with ∼5×107 conidia of A. fumigatus. To confirm depletion of the CCR2+ monocytes we quantified CCR2+ inflammatory monocytes (identified as CD45+CD11b+Ly6C+Ly6G−) in the BALF and lung parenchyma 8 h after A. fumigatus challenge by flow cytometry. We found that diphtheria toxin had no effect on CCR2+ monocytes in control animals, while CCR2-depleter mice treated with DT had no detectable Ly6C+ inflammatory monocytes, in the BALF or lung parenchyma as expected (Fig. 6A) [12]. We found that IL-1α protein levels were significantly decreased when CCR2+ monocytes were absent (Fig. 6B) consistent with the idea that lung-resident CCR2+ inflammatory monocytes are important for producing and/or inducing expression of this cytokine in the lung at early times after infection. Since blocking IL-1α in mice significantly decreased the expression of CXCL1 in the lungs (Fig. 5), we next asked whether CXCL1 protein levels were diminished in the lung parenchyma of the CCR2-depleter mice. We found that CXCL1 protein levels were also significantly decreased in the absence of CCR2+ monocytes (Fig. 6C). Thus, CCR2+ monocytes are important regulators of the early expression of IL-1α and CXCL1. Consistent with the importance of these factors in promoting early neutrophil recruitment (Fig. 2 and 4), diminished IL-1α and CXCL1 levels in CCR2-depleter mice correlated with diminished recruitment of neutrophils to the airways 8 h after A. fumigatus challenge (Fig. 6D). Thus, CCR2+ monocytes are important regulators of the early expression of IL-1α and CXCL1, which are required for optimal recruitment of neutrophils at early times after A. fumigatus challenge.
Fig. 6. CCR2+ monocyte regulate early IL-1α and CXCL1 expression. 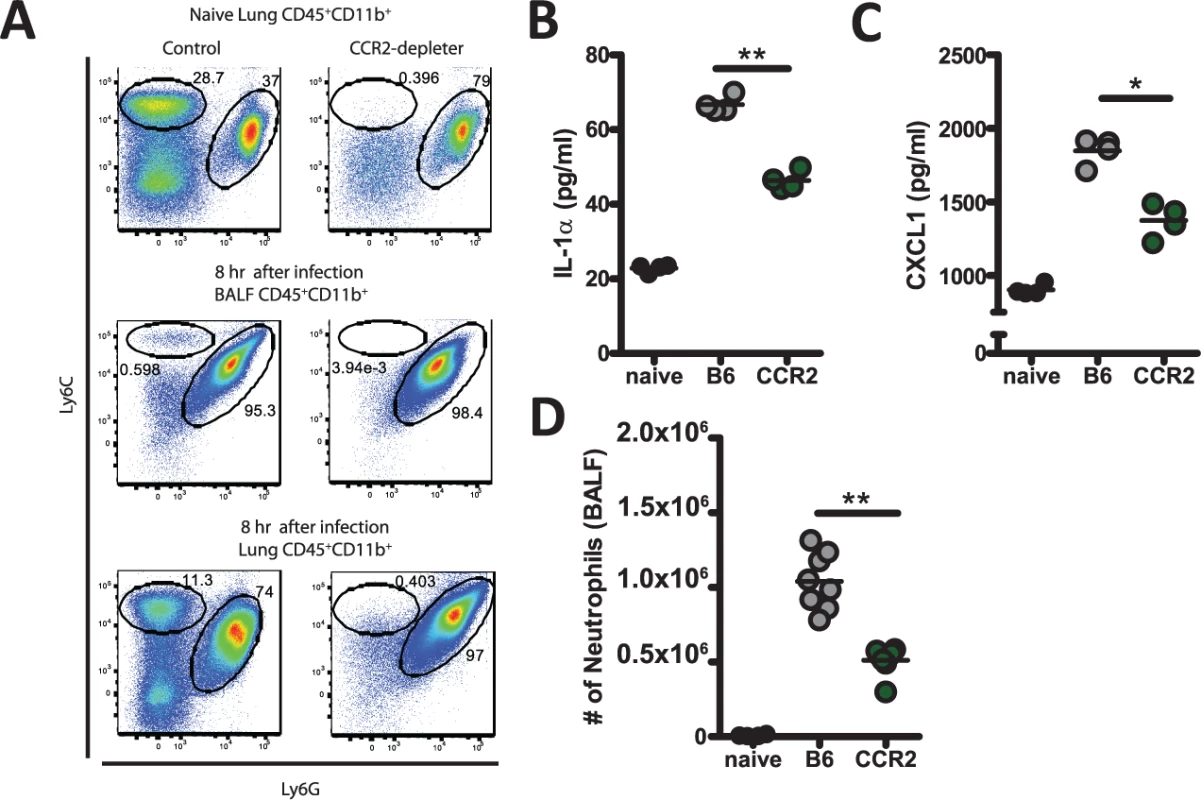
C57BL/6 or CCR2-depleter mice were treated i.p. with 250 ng of DT 24 h prior to challenge with 5×107 Af293 conidia. (A) Naïve C57BL/6 or CCR2-depleter mice or C57BL/6 or CCR2-depleter mice challenged eight hours prior were euthanized and the BALF and lung tissue collected for flow cytometric analysis to assess depletion of target cells by DT. Plots are gated on CD45+ CD11b+ cells and show Ly6c and Ly6g staining, which identify the CCR2+ monocytes and neutrophils, respectively. (B) IL-1α and (C) CXCL1 protein levels in the lung parenchyma at 8 h post-challenge with 5×107 conidia of A. fumigatus strain Af293 were measured using ELISA assays. Bar graphs show the group means ± one SEM. (D) Eight hours post-challenge with 5×107 conidia of A. fumigatus strain Af293, neutrophils in the BALF were enumerated. Data are representative (B-C) or pooled (D) from two independent experiments consisting of 4 mice per group. Each symbol represents an individual mouse and the line represents the group mean. Statistically significant differences were determined using a one-way ANOVA with Bonferroni’s post-test compared C57BL/6 mice (*p < 0.05, **p < 0.01). CXCL1 supplementation of Il1r1-deficient mice enhances neutrophil recruitment and resistance to pulmonary Aspergillus fumigatus challenge
As both Il1r1-deficient mice and anti-IL1α treated mice displayed significantly decreased cellular infiltration into the BALF (Fig. 2D and 4C) that correlated with decreased abundance of CXCL1 (Fig. 5), we next sought to test whether immunotherapy which enhances neutrophil accumulation in the lungs, such as CXCL1 supplementation, could enhance control of A. fumigatus growth in the Il1r1-deficient mice. We challenged either C57BL/6 or Il1r1-deficient mice with ∼5×107 conidia of A. fumigatus. Three hours after challenge mice were treated i.t. with either PBS or 0.5 μg CXCL1. As expected, Il1r1-deficient mice displayed a significant impairment in controlling A. fumigatus germination at 48 h when compared with C57BL/6 mice (Fig. 7A-B). Provision of CXCL1 to Il1r1-deficient mice could partially rescue control of A. fumigatus germination in the lungs, while no enhancement in control of fungal growth was observed in the CXCL1 treated C57BL/6 mice (Fig. 7A-B). Furthermore, provision of CXCL1 i.t. rescued the impairment of anti-IL1α treated C57BL/6 mice in controlling A. fumigatus infection (S5A Fig.). As expected, twenty-four hours after challenge the recruitment of neutrophils, but not macrophages, to the BALF in Il1r1-deficient mice was enhanced by the provision of CXCL1 (Fig. 7C). Additionally, neutrophil recruitment to the BALF in anti-IL1α treated C57BL/6 mice was significantly enhanced (S5B Fig.). While CXCL1 provision enhanced neutrophil accumulation in the airways of Il1r1-deficient mice, we also sought to test whether the anti-hyphal activity of neutrophils was altered in the absence of IL-1RI signaling but exogenous addition of CXCL1. Hyphal damage induced by neutrophils isolated from the bone marrow of respective mouse genotypes was assessed using the XTT hyphal damage assay [46]. C57BL/6 bone marrow neutrophils induced robust hyphal damage when co-cultured with A. fumigatus, which was not further enhanced by treated with 50 nM of CXCL1 (Fig. 7D). Interestingly, Il1r1-deficient bone marrow neutrophils induced significantly less damage to A. fumigatus hyphae than was observed with C57BL/6 bone marrow neutrophils (Fig. 7D). In contrast to the treatment of C57BL/6 bone marrow neutrophils, treatment of Il1r1-deficient bone marrow neutrophils with 50 nM of CXCL1 significantly enhanced the anti-hyphal activity of those cells (Fig. 7D). When cell death and endothelial/epithelial cell leakage were assessed in vivo by lactate dehydrogenase (LDH) and albumin measurement, respectively, in the BALF both markers were increased in the absence of IL-1RI (Fig. 7E-F). CXCL1 supplementation reduced both markers, but albumin levels were more dramatically reduced than LDH levels (71% versus 32%, respectively) (Fig. 7E-F). Thus, provision of CXCL1 could significantly enhance neutrophil recruitment to the lungs in the absence of IL-1α signaling and enhanced the in vitro anti-hyphal activity of Il1r1-deficient bone marrow neutrophils, which together ultimately resulted in a partial repair of the A. fumigatus control mechanisms in the Il1r1-deficent lungs.
Fig. 7. Treatment of Il1r1-deficient mice with CXCL1 partially increases resistance to Aspergillus fumigatus infection. 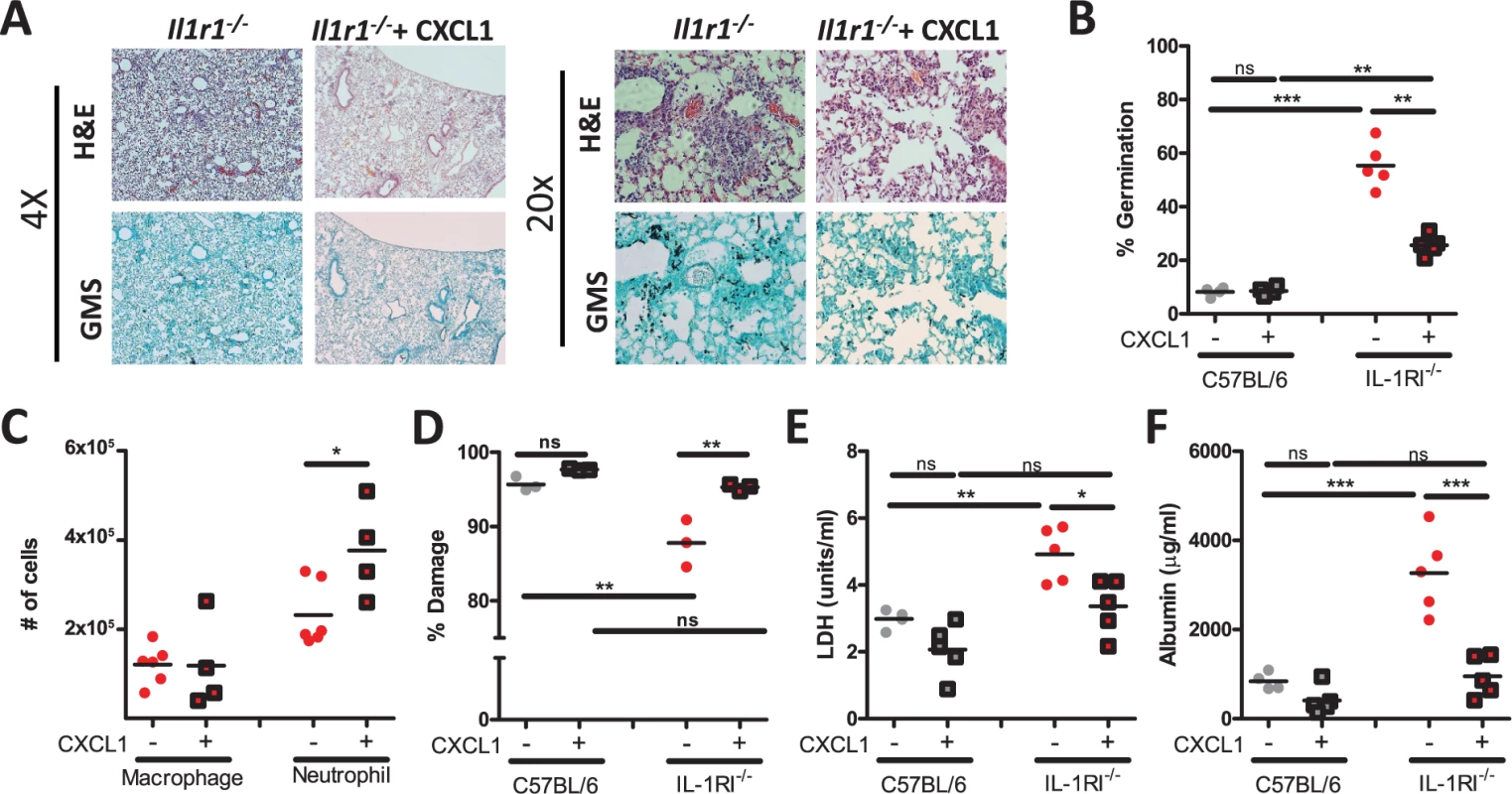
C57BL/6 mice and Il1r1-deficient mice were challenged i.t. with 5×107 CEA10 conidia. Three hours post-challenge mice were given 0.5 μg CXCL1 i.t. or PBS alone. Twenty-four hours post-infection, mice were euthanized, BALF collected, and lungs saved for histological analysis. (A) Formalin-fixed lungs were paraffin embedded, sectioned and stained with H&E (top) or GMS (bottom) for analysis by microscopy. Representative lung sections from Il1r1-deficient mice challenged with CEA10 for 48 h and treated with either PBS or CXCL1 are shown using either the 4× (left) or 20× (right) objectives. (B) A. fumigatus germination rates were assessed at 48 h of infection by microscopically counting both the number of conidia and number of germlings in GMS-stained section. Number of conidia and number of germlings were counted for each GMS-stained section to quantify the percent germination. (C) Macrophage and neutrophil recruitment in Il1r1-deficient mice 24 h post-challenge infected with A. fumigatus treated with PBS or CXCL1 given i.t. was determined via cytospins.(D) Bone marrow neutrophils from C57BL/6 and Il1r1-deficient mice were incubated with CEA10 germlings in vitro at a 10:1 ratio in normoxia for 2 h. The XTT assay was used to determine percent fungal damage. (E) Lung damage and (F) leakage were assessed by measuring LDH and albumin, respectively. Data is representative of at least two independent experiments consisting of three to five mice per group, except for the bone marrow neutrophil anti-hyphal XTT assay which is a single experiment which consisted of pooled bone marrow neutrophils from three mice done in triplicate. Each symbol represents an individual mouse or replicate and the line represents the group mean. Statistically significant differences were determined using a one-way ANOVA with Bonferroni’s post-test (*p < 0.05, **p < 0.01, ***p < 0.001, ns = not significant). Discussion
In this study, we uncover an essential function for IL-1RI in preventing fungal proliferation and host damage in murine lungs. We have demonstrated a novel dichotomy for the IL-1 cytokines in regulating the innate immune response induced by A. fumigatus. Specifically, IL-1α is required for initiating the correct inflammatory signals necessary for optimal leukocyte recruitment, while the inflammasome and IL-1β was necessary for optimal anti-fungal activity against fungal hyphae. We have elucidated that IL-1α plays the dominant role in activating IL-1RI signaling which results in amplified CXCL1 expression, which correlated with optimal leukocyte recruitment to the respiratory tract. CCR2+ monocytes were important cells in regulating the early production of IL-1α, CXCL1, and neutrophil recruitment. Taken together, our data demonstrate that signaling through IL-1RI by both IL-1α and IL-1β was necessary for optimal control of A. fumigatus pulmonary challenge to prevent IPA development.
IL-1RI signaling was essential in resisting pulmonary A. fumigatus challenge in our studies, as demonstrated by Il1r1-deficient mice being unable to resist fungal growth resulting in significant mortality in those animals. This finding is consistent with results reported by Pearlman and colleagues who also found that Il1r1 was needed to prevent the development of A. fumigatus induced keratitis [51]. Moreover, van de Veerdonk and colleagues have recently shown that a polysaccharide fungal virulence factor, galactosaminogalactan (GAG), from A. fumigatus induces the expression of IL-1Ra, which antagonizes IL-1 signaling resulting in enhanced susceptibility to IPA [52]. Gresnigt et al demonstrated that GAG pretreatment of BALB/c mice resulted in more fungal growth associated with impaired neutrophil recruitment, which was completely dependent on IL-1Ra expression [52]. However, the importance of GAG induction of IL-1Ra during a live pulmonary A. fumigatus infection remains unknown. GAG expression might actually be reduced at specific infection sites during in vivo A. fumigatus challenge because hypoxia, which occurs after A. fumigatus challenge [47] has been observed to reduce GAG production [46]. In general, the temporal and spatial dynamics of fungal cell wall PAMPs in vivo during an active infection is not fully understood and likely complicated by the heterogeneous nature of the lung and infection site microenvironments.
Downstream of IL-1RI the proximal signaling adapter to propagate IL-1 signaling is MyD88. Similar to our observation with Il1r1-deficient mice, Marr and colleagues [44] and Hohl and colleagues (Jhingran A. et al, in press) have found the Myd88-deficient mice are more susceptible to A. fumigatus challenge. Additionally, impaired control of pulmonary histoplasmosis and disseminated candidiasis was observed in Il1r1-deficient and Il1a/Il1b-doubly deficient mice, respectively [41,45]. While our studies and the studies just discussed strongly support a role for IL-1RI signaling in limiting IPA, and other invasive fungal infections, Romani and colleagues have observed that Il1r1-deficient mice were more resistant to pulmonary A. fumigatus challenge [53]. The difference with our study is potentially due to the A. fumigatus strain studied, as Romani and colleagues have shown that different A. fumigatus strains have diverse abilities to induce pathology and immune responses [54]. Importantly, the infection models studied are significantly different, with Romani and colleagues utilizing a cyclophosphamide-induced immunosuppression model with Aspergillus conidia delivered on 3 consecutive days intranasally [53], while our studies used immunocompetent mice and a single dose of Aspergillus conidia given intratracheally. Additionally Bellocchio et al. [53], reported that histological analyses in the Il1r1-deficient mice revealed “numerous fungal elements in the relative absence of signs of inflammatory pathology” which is consistent with the results we report here in our experimental model. Murine mortality in infectious disease models can result from direct pathogen mediated damage or immunopathogenesis, and it is unclear, in this regard, how our models differ. What appears to be clear, however, is that in the absence of IL-1RI signaling, Aspergillus proliferation increases in vivo. Taken together, all these findings demonstrate that the IL-1 signaling pathway is likely central for resistance to fungal diseases, but their role during immunosuppression and frequency of fungal exposure/quantity may differ, warranting further exploration of the IL-1 cytokine family in each clinically relevant model of IPA.
In further support of our observation, the protective role of IL-1 cytokines in anti-fungal immunity uncovered in our study using the murine model of A. fumigatus infection is likely to be operational in humans as indicated by genetic linkage studies. First, individuals with SNPs in the IL-1 gene cluster, which are associated with decreased IL-1 dependent inflammatory events, were at increased risk for the development of IPA [25,26]. Second, polymorphisms in the CIAS1 gene play a central role regulating inflammasome activity and IL-1β production, which can alter the risk of a subset of patients to developing recurrent vulvovaginal candidiasis [55]. Third, macrophages from patients with chronic cavitary pulmonary aspergillosis (CCPA) had prolonged expression of Il1a and Il1b after A. fumigatus treatment when compared to healthy controls and SNPs in the Il1b and Il1rn loci are associated with susceptibility to developing CCPA [56]. Thus, targeting the IL-1 cytokine pathways in humans could be important in managing fungal infections.
In previous papers exploring the role of MyD88 during A. fumigatus [44,51] it was shown that fungal growth was not controlled, but the mechanism impaired in the absence of IL-1RI and MyD88 signaling remains an open question. In this study and a parallel study by Jhingran et al (in press) it was demonstrated that MyD88 and IL1RI mediated signals are necessary for optimal leukocyte recruitment after pulmonary A. fumigatus challenge, which is needed for preventing the development of IPA. Analogously, in the A. fumigatus keratitis model both Myd88 - and Il1r1-deficient mice demonstrated reduced cellular infiltrate early after inoculation [51]. However, why the lack of IL-1RI or MyD88 signaling results in decreased cellular infiltrates was an open question. Interestingly, we found a decrease in the expression of the chemokine CXCL1 in Il1r1-deficient mice, which others have also observed in challenged Myd88-deficient mice [44] (Jhingran A. et al, in press). CXCL1, together with CXCL2 and CXCL5, are ligands for CXCR2 and are key chemoattractants for recruitment of neutrophils. Administration of a blocking anti-CXCR2 antibody or genetic ablation of Cxcr2 has been shown to exacerbate mortality and delay neutrophil recruitment following pulmonary A. fumigatus challenge [23,24]. Moreover, transient over-expression of CXCL1 in CC10-expressing lung epithelial cells resulted in significantly enhanced leukocyte accumulation and reduced fungal burden [22]. Correspondingly, when we treated Il1r1-deficient mice with recombinant murine CXCL1 we observed significantly enhanced neutrophil accumulation. In addition, Il1r1-deficient bone marrow neutrophils displayed decreased anti-hyphal activity in vitro, which was restored by treatment with CXCL1. These data demonstrate that both IL-1RI and CXCL1 signaling is critical in not only enhancing neutrophil recruitment to the airways in the Il1r1-deficient mice, but also in inducing the optimal anti-hyphal state of the recruited neutrophils. While the mechanism behind CXCL1 mediated anti-hyphal activity in our model is unknown, neutrophils from Cxcl1-deficient mice have an impaired reactive oxygen response in a polymicrobial sepsis model [57]. Moreover, Cxcl1-deficient neutrophils stimulated with Klebsiella pneumoniae had reduced expression of p67phox and p47phox and reduced production of myeloperoxidase, nitric oxide, and hydrogen peroxide, which results in decreased killing of Klebsiella pneumoniae by the neutrophils [58]. Finally, IL-8 has been shown to be important for priming the human neutrophils reactive oxygen burst [59]. Thus, our data supports a model where IL-1RI signaling is critical for optimal neutrophil recruitment and activation of their anti-hyphal activity in part through the regulation of CXCL1 abundance. Further support for this conclusion comes from a recent analysis of mice with a myeloid deficiency of the transcriptional regulator HIF1α. Loss of myeloid HIF1α results in severe susceptibility to the same strain of A. fumigatus utilized here in part through reduction in neutrophil recruitment. Importantly, loss of HIF1α resulted in decreased IL1-α and CXCL1 levels after A. fumigatus challenge similar to what we observed in our studies [60]. Interestingly, other inflammatory pathways are also temporally regulating neutrophil recruitment after A. fumigatus challenge, because Card9-deficient mice had a late defect in neutrophil recruitment that was associated with a more global diminution of the inflammatory milieu [61]. In addition to the early defect in neutrophil recruitment Il1r1-deficient mice also had decreased macrophage recruitment to the airways by 48 h post-inoculation. The reason for this is unknown at this time, but it is known that G-CSF deficient mice have monocyte defects and our cytokine analysis demonstrated that Il1r1-deficient mice had significantly lower level of G-CSF in the airways [62]. Thus, further studies exploring the regulation of multiple neutrophil chemotactic pathways, such as CXCR2-, CCR1-, IL17-, leukotriene-, and complement-dependent pathways, and monocyte chemotactic pathways, such as G-CSFR—and CCR2-dependent pathways, are needed after pulmonary fungal challenge.
IL1RI, together with IL1RAcP, is the high-affinity receptor for both IL-1α and IL-1β [27]. The maturation and secretion of IL-1α and IL-1β is known to be regulated by distinct proteolytic pathways dependent on calpain and caspase-1, respectively [27,63]. Numerous fungal pathogens have been shown to activate the inflammasome resulting in the production of IL-1β [30,31,32,33,34,35,36,37]. Importantly for our studies, others have demonstrated that the NLRP3-ASC-Caspase1 inflammasome could be activated by A. fumigatus [31], but the in vivo relevance of that finding was unknown. Control of C. albicans infection, which also activated the NLRP3-ASC inflammasome, was highly dependent on NLRP3 and IL-1β [30,36,37,41]. In sharp contrast, our current results indicate that the inflammasome only plays a modest role in the control of pulmonary A. fumigatus growth. In our experiments, neutrophil recruitment in mice lacking the inflammasome was completely normal, which is in contrast to C. albicans infection where mice deficient in IL-1β displayed a significant reduction in neutrophil recruitment [41]. Furthermore, antibody blockade of IL-1β during pulmonary Histoplasma capsulatum infection resulted in decreased survival associated with decreased recruitment of Gr-1+ cells early and CD4+ cells late to the lungs of challenged animals [45]. Thus, we were surprised to observe such a dominant role for IL-1α in regulating early leukocyte recruitment following pulmonary A. fumigatus challenge, which correlates with its regulation of the chemokine CXCL1. In support of this finding, during sterile inflammation the importance of IL-1α in regulating neutrophil recruitment is unquestionable [38,39]. It has also been demonstrated that IL-1α plays a critical role during murine L. pneumophila infection, initiating neutrophil recruitment and the inflammatory response early after infection [40]. Others have previously shown that IL-1RI and MyD88 expression within a radioresistant population of cells was essential for optimal expression of CXCL1 and CXCL2 during L. pneumophila infection [64]. Interestingly, in their parallel study Hohl and colleagues found that IL-1RI/MyD88 signaling in a radioresistant cell population was necessary for optimal CXCL1 expression and neutrophil recruitment early after pulmonary A. fumigatus challenge (Jhingran A. et al, in press).
Because of the early importance of IL-1 cytokines in regulating the pulmonary anti-fungal immune response, non-hematopoietic cells (epithelial or endothelial cells) or lung-resident myeloid cells could represent potential sources of IL-1α and IL-1β after A. fumigatus challenge. During pulmonary Mycobacterium tuberculosis infection two distinct populations of myeloid cells co-express IL-1α and IL-1β: inflammatory monocytes which are CD11b+ CD11c− Ly6c+ and monocytic dendritic cells which are CD11b+ CD11c+ [49]. After pulmonary A. fumigatus challenge both inflammatory monocytes and monocytic dendritic cells are found in the lung parenchyma and both show increased transcription of the Il1a gene [12]. Here our data demonstrates that CCR2+ monocytes are at least one of the important cell types regulating the early expression of IL-1α and CXCL1, as well as neutrophil recruitment at 8 hpi. However, in the absence of CCR2+ monocytes there is still a significant amount of IL-1α and CXCL1 produced in the lungs after A. fumigatus challenge, thus there are likely multiple sources of IL-1α that can regulate early pulmonary neutrophil accumulation. Moreover, by 48 h after infection CXCL1 levels and neutrophil recruitment to the lung is unaffected in CCR2-depleter mice [12], thus suggesting that distinct mechanisms of neutrophil recruitment are operational at various times after infection. This is supported by observation that Myd88-deficient and Card9-deficient mice have early or late defects in neutrophil recruitment, respectively (Jhingran A. et al, in press and [61]).
In our experiments the inflammasome and IL-1β appear to regulate the anti-fungal activity of macrophages against hyphae, especially under hypoxic conditions. This enhancement of anti-fungal activity in hypoxic microenvironments is physiologically and clinically important because hypoxia can be generated within the lungs of mice with IPA [47], which is coincident with inflammatory monocyte arrival to the lungs [12]. Understanding how hypoxia can enhance the anti-fungal activity of macrophage in an inflammasome and IL-1β dependent manner will be important in understanding how macrophages limit fungal growth. Interestingly, a recent paper from Torres et al demonstrated that acidosis, which can be driven by hypoxia, resulted in increased IL-1β production in response to P. aeruginosa challenge [65]. Perhaps somewhat surprisingly, in the absence of MyD88 anti-fungal activity against A. fumigatus conidia remains intact (Jhingran A. et al, in press). It has been shown in other fungal pathogens that IL-1β treatment of human peripheral blood leukocytes enhances their anti-fungal activity against Paracoccidiodies brasiliensis [66,67]. Additionally, Il1r1 - and Nlrp3-deficient macrophages have impaired antifungal activity against P. brasiliensis [34]. In contrast, during disseminated candidiasis Il1a-deficiency was associated with decreased anti-fungal activity of leukocytes [41]. Thus, studies designed to understand the differential dependencies of the IL-1 cytokines in regulating leukocyte recruitment and anti-fungal activity during a range of fungal diseases and morphological forms are needed.
In addition to understanding the cellular source of IL-1α and IL-1β, understanding the inflammatory pathways leading to expression of IL-1α and IL-1β are essential to our understanding of resistance to IPA. In the absence of dectin-1 signaling there is decreased expression of both IL-1α and IL-1β [68,69]. The loss of HIF1α in the LysM-expressing cells also resulted in decreased IL-1α levels after A. fumigatus challenge [60], which can be regulated by dectin-1 agonists such as β-glucan [70]. Pulmonary A. fumigatus infection results in significant tissue damage and cell death, but the exact type of cell death is not known. Moreover, the phenotype of cell death will be shaped by the hypoxic microenvironment found during IPA. The type of cellular death occurring in vivo during A. fumigatus will have important immunological impacts shaping the early IL-1α and IL-1β response because necrotic cell death favors IL-1α release while pyroptosis favors IL-1β release [71]. Interestingly, C. albicans mutants with defects in inducing pyroptosis also demonstrated defects in inducing IL-1β secretion, but IL-1α release was not examined [72]. Additionally, understanding how deficiencies in PRR signaling alters the overall inflammatory response will be crucial as patients with SNPs in PRRs are known to have elevated risks for developing IPA [73]. Our data demonstrate that in the Pycard-deficient mice there are elevated levels of TNFα, CCL3, and CCL4. One explanation for observing elevated levels of TNFα, CCL3, and CCL4 could be the range or degree that PRRs are being engaged in the Pycard-deficient mice and/or temporal and spatial dynamics of fungal cell wall PAMP engagement with PRRs in vivo that are not fully understood and further complicated by a PRR known to be engaged during infection now being absent.
It is well defined that prolonged corticosteroid treatment increases susceptibility of hosts to IPA [74]. Interestingly, dexamethasone induces the expression and release of IL-1RII [75]. IL-1RII is known to limit the activity of IL-1 cytokines and/or sequester IL-1α protein in the cytosol, preventing the cleavage of IL-1α by calpain [63]. Dexamethasone has also been shown to impair IL-1α and IL-1β secretion from human mast cells in response to Pseudomonas aeruginosa stimulation [76]. Moreover, dexamethasone treatment of bronchoalveolar macrophages prior to treatment with A. fumigatus conidia significantly impaired their release of IL-1α [77,78]. Because we have uncovered such a prominent role for IL-1α in controlling pulmonary A. fumigatus challenge, future studies exploring the cleavage status of IL-1α and expression of IL-1RII in clinically relevant models are critical.
Finally, the importance of appropriate activation of leukocytes in the control of A. fumigatus is highlighted by patients with chronic granulomatous disease being highly susceptible to A. fumigatus [15,16,17]. Interestingly, CGD patients or mice are typically in a hyperinflammatory state, which is linked to inflammasome activity and IL-1β expression [79,80]. Further, blockade of IL-1 cytokines in p47phox-deficient mice through treatment with hIL1ra results in improved control of A. fumigatus [79]. Together, these studies demonstrate that further exploration of the positive and negative regulators of IL-1 signaling during invasive fungal infections is needed. Moreover, it is critical that we continue to explore the regulation of the inflammatory response induced in each of the different subpopulations of hosts susceptible to developing invasive fungal infections in order to develop patient specific novel immunotherapeutic approaches that could complement treatment with anti-fungal agents.
Materials and Methods
Mice
C57BL/6J mice were bred in-house. Pycard (ASC)-deficient and Myd88-deficient mice were originally provided by Dr. Vishiva Dixit (Genentech) and Dr. Mark Jutila (Montana State University), respectively. Il1r1-deficient (Stock #003245) and C57BL/6 (Stock #000664) mice were originally purchased from Jackson Laboratories. Mouse strains were then bred in-house. The CCR2-depleter (CCR2-DTR) strain was generated on the C57BL/6 background as previously described [10,50]. Control animals for CCR2+ monocyte depletion experiments were sex—and age-matched, non-transgenic littermates. All mice were 8–10 weeks of age at the time of infection. All animal experiments were approved by the Montana State University or Rutgers University Institutional Animal Care and Use Committee.
Preparation of Aspergillus fumigatus conidia
A. fumigatus strain CEA10 or Af293 was grown on glucose minimal media (GMM) agar plates for 3 days or Sabouraud dextrose agar (SDA) for 7–10 days at 37°C, respectively. Conidia were harvested by adding 0.01% Tween 80 to plates and gently scraping conidia from the plates using a cell scraper. Conidia were then filtered through sterile Miracloth, were washed and resuspended in phosphate buffered saline (PBS), and counted on a hemacytometer.
Aspergillus fumigatus challenge pulmonary model
Mice were challenged with A. fumigatus conidia by the i.t. route. Mice were anesthetized with 2.5% 2,2,2-tribromoethanol or a Ketamine/Xylazine solution given i.p.; subsequently, mice were challenged i.t. with ∼5 × 107 A. fumigatus conidia in a volume of 100 μl. At the indicated time after A. fumigatus challenge, mice were euthanized using a lethal overdose of pentobarbital. Bronchoalveolar lavage fluid (BALF) was collected by washing the lungs with 2 ml of PBS containing 0.05M EDTA. BALF was clarified by centrifugation and stored at −20°C until analysis. BAL cells were resuspended in 200 μl of PBS and total BAL cells were determined by hemacytometer count. BAL cells were subsequently spun onto glass slides using a Cytospin4 cytocentrifuge (Thermo Scientific) and stained with Diff-Quik stain set (Siemens) for differential counting. For histological analysis lungs were filled with and stored in 10% buffered formalin phosphate for at least 24 hours. Lungs were then embedded in paraffin and sectioned into 5-micron sections. Sections were stained with H&E and GMS using standard histological techniques to assess lung inflammatory infiltrates and fungal germination, respectively. For cytokine analysis lungs were homogenized in 2 ml of PBS. After clarification, lung homogenates were stored at −20°C until analysis.
Neutralizing antibodies and chemokine reconstitution
For IL-1α neutralization studies, normal goat IgG control and anti-mIL-1α neutralizing antibody were purchased from R&D systems. IgG control or anti-mIL-1α neutralizing antibody were administered i.p. at 40 μg per mouse. Administration of neutralizing antibody was given every other day, beginning the day prior to A. fumigatus challenge. For CXCL1 reconstitution studies, recombinant murine CXCL1 was purchased from PeproTech. CXCL1 was administered i.t. at 0.1–0.5 μg per mouse and was given 3 hours after A. fumigatus challenge. For the hIL1ra studies, recombinant hIL1ra and the appropriate placebo (Amgen) were kindly provided by Dr. Charles A. Dinarello. The hIL1ra and placebo were administered i.p. at 200 mg per mouse given at −24, 0, and +24 h relative to A. fumigatus challenge.
CCR2+ cell depletion strategy
For depletions of CCR2+ cells, CCR2-DTR mice and control littermates received 250 ng of diphtheria toxin i.p. one day prior to infection. Diphtheria toxin was purchased from List Biological Laboratories (Campbell, CA) and reconstituted at 1 mg/ml in PBS. Aliquots were stored at −80°C. The specificity and efficiency of depletion in the lung was confirmed by flow cytometry.
Quantification of lung damage and leakage
To assess lung damage, bronchoalveolar lavage fluid was analyzed by measuring lactate dehydrogenase levels using a CytoTox 96 Cytotoxicity Assay (Promega) following the manufacturer’s instructions. To assess vascular/pulmonary leakage, bronchoalveolar lavage fluid was analyzed using an Albumin BCG Reagent Set (Eagle Diagnostics). A standard curve was made by diluting the calibrator in PBS. Then 100 μl of sample or standard was transferred to a 96 well flat-bottomed plate, mixed with 100 μl of BCG reagent, let sit at RT for 5 min and then read on a plate reader at 630 nm.
Lung cell isolation and flow cytometric analysis
After collection of the BAL fluid, lung samples were minced in RPMI containing 100 units/ml of collagenase (Gibco) at 37°C for 60 minutes, followed by disruption through a 40-μm filter. After which, red blood cells were lysed using a Tris ammonium chloride solution. Staining of ∼107 cells was performed in 200 μl of PBS containing 2% bovine serum and 2 mM EDTA. For analysis of leukocytes, antibody staining was both conducted at 4°C for 30 minutes. Phenotypic analysis of leukocytes was conducted using a panel of cell surface markers: CD11b, CD11c, Ly6g, Ly6c, 7/4, CD19, and I-A/I-E, as previously described [10]. All antibodies used for analysis were purchased from Biolegend, BD Biosciences, eBioscience or Novus Biologicals. After staining, cells were washed and fixed with 1% paraformaldehyde in PBS. Fluorescent intensities were measured using an LSR (BD Biosciences) and data were analyzed using FlowJo software (Tree Star).
Assay for cytokine, chemokine, and soluble receptor secretion
Bronchoalveolar lavage fluid and lung homogenates from C57BL/6 mice challenged with A. fumigatus for 6, 12, 24, and 48 h were initially analyzed for cytokines and chemokines using ProcartaPlex Mouse Cytokine & Chemokine 36-plex (Affymetrix-eBioscience). IL-1Ra levels were determined by ELISA (R&D Systems). Plates were read using a BioPlex 200 (Bio-Rad) or a SpectraMax Paradigm plate reader (Molecular Devices).
Growth of bone marrow-derived macrophages (BMDM)
Femurs and tibias from 8–10 week old mice were obtained and centrifuged to collect bone marrow. Cells were resuspended in media containing RPMI 1640, 2 mM L-glutamic acid, 50 mg/l gentamycin, 100 U/ml penicillin/streptomycin, 30% L929 cell supernatant, 20% FBS and 0.0004% 2-ME. On day 3 fresh medium was added to the cultures. Cells were incubated for a total of 6 days at 37°C and 5% CO2.
Isolation of bone marrow neutrophils
Bone marrow neutrophils were isolated from femurs and tibias from 8–12 week old C57BL/6 and Il1r1-deficient mice as previously described [60]. Briefly, single cell suspensions of bone marrow in HBSS containing 0.1% FBS and 1% glucose were resuspended in 3 ml of 45% Percoll (GE Healthcare). A discontinuous Percoll gradient was set-up consisting of (top to bottom) 3 ml 45%, 2 ml 50%, 2 ml 55%, 2 ml 62%, and 3 ml 81%. Gradients were then centrifuged for 30 min at 1600 × g in a Sorvall Legend Mach 1.6R benchtop centrifuge. Bone marrow neutrophils were collected from the 62%/81% border and washed with HBSS before counting and viability assessment.
In vitro fungal damage assay
An XTT assay was used to measure fungal metabolic activity as previously described [46]. Bone marrow derived macrophages and CEA10 germlings were incubated together in normoxic or hypoxic conditions at a 10 : 1 (effector:target) ratio for 5 hours. Bone marrow neutrophils and CEA10 germlings were incubated together in normoxic conditions at a 10 : 1 (effector:target) ratio for 2 hours with or without 50 nM CXCL1 [81]. Following incubation, macrophages or neutrophils were lysed and the remaining fungi were incubated with 0.4 mg/ml XTT and 0.05 mg/ml coenzyme Q for 1 h and the optical density (OD) subsequently measured on a spectrophotometer at a wavelength of 450 nm. The percent fungal damage was defined by the equation: (1-[A450 of fungi with cells—A450 of cells alone] / [A450 of fungi alone]) * 100.
Statistical analysis
Statistical significance was determined by a Student’s t-test, one-way ANOVA using a Bonferroni post-test, or Kruskal-Wallis one-way ANOVA with Dunn’s post-test through the GraphPad Prism 5 software as outlines in the figure legends.
Supporting Information
Zdroje
1. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 : 165rv113. 23253612
2. Segal BH (2009) Aspergillosis. N Engl J Med 360 : 1870–1884.
3. Stevens DA, Melikian GL (2011) Aspergillosis in the ‘nonimmunocompromised’ host. Immunol Invest 40 : 751–766. 21985304
4. Hayes GE, Denning DW (2013) Frequency, diagnosis and management of fungal respiratory infections. Curr Opin Pulm Med 19 : 259–265. 23411576
5. Vermeulen E, Lagrou K, Verweij PE (2013) Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26 : 493–500. 24126719
6. van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, et al. (2011) Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17 : 1846–1854. doi: 10.3201/eid1710.110226 22000354
7. Verweij PE, Mellado E, Melchers WJ (2007) Multiple-triazole-resistant aspergillosis. N Engl J Med 356 : 1481–1483. 17409336
8. VandenBergh MF, Verweij PE, Voss A (1999) Epidemiology of nosocomial fungal infections: invasive aspergillosis and the environment. Diagn Microbiol Infect Dis 34 : 221–227. 10403102
9. Park SJ, Mehrad B (2009) Innate immunity to Aspergillus species. Clin Microbiol Rev 22 : 535–551.
10. Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, et al. (2009) Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6 : 470–481. doi: 10.1016/j.chom.2009.10.007 19917501
11. Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM (2009) Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 200 : 647–656. 19591573
12. Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, et al. (2014) Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog 10: e1003940. doi: 10.1371/journal.ppat.1003940 24586155
13. Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, et al. (2011) Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10 : 437–450. doi: 10.1016/j.chom.2011.09.011 22100160
14. Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, et al. (2011) A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 9 : 415–424. doi: 10.1016/j.chom.2011.04.007 21575912
15. Ben-Ari J, Wolach O, Gavrieli R, Wolach B (2012) Infections associated with chronic granulomatous disease: linking genetics to phenotypic expression. Expert Rev Anti Infect Ther 10 : 881–894. doi: 10.1586/eri.12.77 23030328
16. Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC (1997) Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 185 : 207–218. doi: 10.1586/eri.12.77 9016870
17. Pollock JD, Williams DA, Gifford MA, Li LL, Du X, et al. (1995) Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9 : 202–209. 7719350
18. Marr KA, Carter RA, Boeckh M, Martin P, Corey L (2002) Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100 : 4358–4366. 12393425
19. Wald A, Leisenring W, van Burik JA, Bowden RA (1997) Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 175 : 1459–1466. 9180187
20. Perfect JR, Cox GM, Lee JY, Kauffman CA, de Repentigny L, et al. (2001) The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis 33 : 1824–1833. 11692293
21. Torres HA, Rivero GA, Lewis RE, Hachem R, Raad II, et al. (2003) Aspergillosis caused by non-fumigatus Aspergillus species: risk factors and in vitro susceptibility compared with Aspergillus fumigatus. Diagn Microbiol Infect Dis 46 : 25–28. 12742315
22. Mehrad B, Wiekowski M, Morrison BE, Chen SC, Coronel EC, et al. (2002) Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am J Respir Crit Care Med 166 : 1263–1268. 12403697
23. Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, et al. (1999) CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J Immunol 163 : 6086–6094. 10570298
24. Bonnett CR, Cornish EJ, Harmsen AG, Burritt JB (2006) Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus Conidia. Infect Immun 74 : 6528–6539. 16920786
25. Sainz J, Perez E, Gomez-Lopera S, Jurado M (2008) IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol 28 : 473–485. doi: 10.1007/s10875-008-9197-0 18484169
26. Wojtowicz A, Gresnigt MS, Lecompte T, Bibert S, Manuel O, et al. (2014) IL1B and DEFB1 Polymorphisms Increase Susceptibility to Invasive Mold Infection After Solid Organ Transplantation. J Infect Dis.
27. Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39 : 1003–1018. doi: 10.1016/j.immuni.2013.11.010 24332029
28. Kim B, Lee Y, Kim E, Kwak A, Ryoo S, et al. (2013) The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol 4 : 391. doi: 10.3389/fimmu.2013.00391 24312098
29. Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13 : 397–411.
30. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, et al. (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459 : 433–436. doi: 10.1038/nature07965 19339971
31. Said-Sadier N, Padilla E, Langsley G, Ojcius DM (2010) Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One 5: e10008. doi: 10.1371/journal.pone.0010008 20368800
32. Mao L, Zhang L, Li H, Chen W, Wang H, et al. (2014) Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infect Immun 82 : 882–892. doi: 10.1128/IAI.01097-13 24478101
33. Lei G, Chen M, Li H, Niu JL, Wu S, et al. (2013) Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res 23 : 965–968. doi: 10.1038/cr.2013.49 23567555
34. Tavares AH, Magalhaes KG, Almeida RD, Correa R, Burgel PH, et al. (2013) NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis 7: e2595. doi: 10.1371/journal.pntd.0002595 24340123
35. Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, et al. (2011) A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7: e1002379. doi: 10.1371/journal.ppat.1002379 22174673
36. Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, et al. (2009) Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183 : 3578–3581. doi: 10.4049/jimmunol.0901323 19684085
37. Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, et al. (2009) An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5 : 487–497. doi: 10.1016/j.chom.2009.05.002 19454352
38. Chen CJ, Kono H, Golenbock D, Reed G, Akira S, et al. (2007) Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13 : 851–856. 17572686
39. Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, et al. (2011) IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 187 : 4835–4843. doi: 10.4049/jimmunol.1102048 21930960
40. Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE (2013) IL-1alpha signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J Immunol 190 : 6329–6339. doi: 10.4049/jimmunol.1300100 23686480
41. Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, et al. (2006) Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis 193 : 1419–1426. 16619190
42. Cramer RA, Rivera A, Hohl TM (2011) Immune responses against Aspergillus fumigatus: what have we learned? Curr Opin Infect Dis 24 : 315–322. doi: 10.1097/QCO.0b013e328348b159 21666456
43. Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34 : 637–650. doi: 10.1016/j.immuni.2011.05.006 21616434
44. Bretz C, Gersuk G, Knoblaugh S, Chaudhary N, Randolph-Habecker J, et al. (2008) MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect Immun 76 : 952–958. 18039832
45. Deepe GS Jr, McGuinness M (2006) Interleukin-1 and host control of pulmonary histoplasmosis. J Infect Dis 194 : 855–864. 16941354
46. Shepardson KM, Ngo LY, Aimanianda V, Latge JP, Barker BM, et al. (2013) Hypoxia enhances innate immune activation to Aspergillus fumigatus through cell wall modulation. Microbes Infect.
47. Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, et al. (2011) In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog 7: e1002145. doi: 10.1371/journal.ppat.1002145 21811407
48. Nizet V, Johnson RS (2009) Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9 : 609–617. doi: 10.1038/nri2607 19704417
49. Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, et al. (2013) Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35 : 1023–1034. doi: 10.1016/j.immuni.2011.12.002 22195750
50. Serbina NV, Hohl TM, Cherny M, Pamer EG (2009) Selective expansion of the monocytic lineage directed by bacterial infection. J Immunol 183 : 1900–1910. doi: 10.4049/jimmunol.0900612 19596996
51. Leal SM Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, et al. (2010) Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog 6: e1000976. doi: 10.1371/journal.ppat.1000976 20617171
52. Gresnigt MS, Bozza S, Becker KL, Joosten LA, Abdollahi-Roodsaz S, et al. (2014) A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog 10: e1003936. doi: 10.1371/journal.ppat.1003936 24603878
53. Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, et al. (2004) The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 172 : 3059–3069. 14978111
54. Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, et al. (2013) Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PLoS One 8: e56651. doi: 10.1371/journal.pone.0056651 23441211
55. Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, et al. (2009) Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol 200 : 303 e301–306. doi: 10.1016/j.ajog.2008.10.039 19254587
56. Smith NL, Hankinson J, Simpson A, Bowyer P, Denning DW (2013) A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis. Clin Microbiol Infect.
57. Jin L, Batra S, Douda DN, Palaniyar N, Jeyaseelan S (2014) CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J Immunol 193 : 3549–3558. doi: 10.4049/jimmunol.1401138 25172493
58. Batra S, Cai S, Balamayooran G, Jeyaseelan S (2012) Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol 188 : 3458–3468. doi: 10.4049/jimmunol.1101985 22379035
59. Swain SD, Rohn TT, Quinn MT (2002) Neutrophil priming in host defense: role of oxidants as priming agents. Antioxid Redox Signal 4 : 69–83. 11970845
60. Shepardson KM, Jhingran A, Caffrey A, Obar JJ, Suratt BT, et al. (2014) Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Aspergillus fumigatus Infection. PLoS Pathog 10: e1004378. doi: 10.1371/journal.ppat.1004378 25255025
61. Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, et al. (2012) Tracing Conidial Fate and Measuring Host Cell Antifungal Activity Using a Reporter of Microbial Viability in the Lung. Cell Rep.
62. Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, et al. (1994) Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84 : 1737–1746. 7521686
63. Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC (2013) Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity 38 : 285–295. doi: 10.1016/j.immuni.2013.01.008 23395675
64. LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A (2011) Nonhematopoietic cells are key players in innate control of bacterial airway infection. J Immunol 186 : 3130–3137. 21270399
65. Torres IM, Patankar YR, Shabaneh TB, Dolben E, Hogan DA, et al. (2014) Acidosis Potentiates the Host Pro-Inflammatory IL-1beta Response to Pseudomonas aeruginosa Infection. Infect Immun.
66. Kurita N, Oarada M, Brummer E (2005) Fungicidal activity of human peripheral blood leukocytes against Paracoccidioides brasiliensis yeast cells. Med Mycol 43 : 417–422. 16178370
67. Kurita N, Oarada M, Miyaji M, Ito E (2000) Effect of cytokines on antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 38 : 177–182. 10817235
68. Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, et al. (2009) Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182 : 4938–4946. doi: 10.4049/jimmunol.0804250 19342673
69. Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, et al. (2005) The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1: e42. 16344862
70. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, et al. (2014) mTOR - and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345 : 1250684. doi: 10.1126/science.1250684 25258083
71. England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D (2014) Release of IL-1alpha or IL-1beta depends on mechanism of cell death. J Biol Chem.
72. Wellington M, Koselny K, Krysan DJ (2013) Candida albicans Morphogenesis Is Not Required for Macrophage Interleukin 1beta Production. MBio 4. 23269828
73. Romani L (2011) Immunity to fungal infections. Nat Rev Immunol 11 : 275–288. 25404119
74. Lewis RE, Kontoyiannis DP (2009) Invasive aspergillosis in glucocorticoid-treated patients. Med Mycol 47 Suppl 1: S271–281. 18654923
75. Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, et al. (1993) Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science 261 : 472–475. 8332913
76. Lin TJ, Garduno R, Boudreau RT, Issekutz AC (2002) Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 alpha and beta. J Immunol 169 : 4522–4530. 12370389
77. Kamberi M, Brummer E, Stevens DA (2002) Regulation of bronchoalveolar macrophage proinflammatory cytokine production by dexamethasone and granulocyte-macrophage colony-stimulating factor after stimulation by Aspergillus conidia or lipopolysaccharide. Cytokine 19 : 14–20. 12200108
78. Brummer E, Kamberi M, Stevens DA (2003) Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J Infect Dis 187 : 705–709. 12599092
79. de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, et al. (2014) IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A 111 : 3526–3531. doi: 10.1073/pnas.1322831111 24550444
80. van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, et al. (2010) Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A 107 : 3030–3033. doi: 10.1073/pnas.0914795107 20133696
81. Klesney-Tait J, Keck K, Li X, Gilfillan S, Otero K, et al. (2013) Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest 123 : 138–149. doi: 10.1172/JCI64181 23241959
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



