-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
article has not abstract
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004539
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004539Summary
article has not abstract
Biological fluids, such as blood and mucosal secretions, continuously flow within the human body and form prominent barriers to pathogen colonization and invasion of host cells [1, 2]. Consequently, pathogens have evolved sophisticated molecular strategies to overcome the mechanical shear forces associated with resisting the flow of biological fluids; in particular, membrane anchored biomolecular complexes enable controlled deceleration, tight adhesion and, in the case of intracellular pathogens, penetration through the host cell membrane (Fig. 1A) [3–7]. The architecture of these biomolecular complexes have been the subject of numerous structural and biophysical investigations, which have yielded high resolution molecular blueprints of the host pathogen interface. However, our understanding of precisely how these biomolecular complexes function is somewhat limited by the technical challenges associated with measuring or modelling the effects of shear flow and related forces on protein complexes in the context of biological membranes [8]; recent studies have revealed the development and application of advanced technologies, such as optical tweezers, for studying the roles that mechanical forces play during pathogen attachment, but the stresses associated with subsequent membrane penetration events remain elusive [3, 7, 9]. To highlight the potential for correlating structural and biophysical data with the extent of dynamic shear stress experienced during diverse host cell invasion processes, we review here several structurally characterized invasion complexes from protozoan, bacterial, and viral pathogens.
Fig. 1. Comparison of structurally characterized invasion mechanisms employed by protozoan, bacterial and viral pathogens. 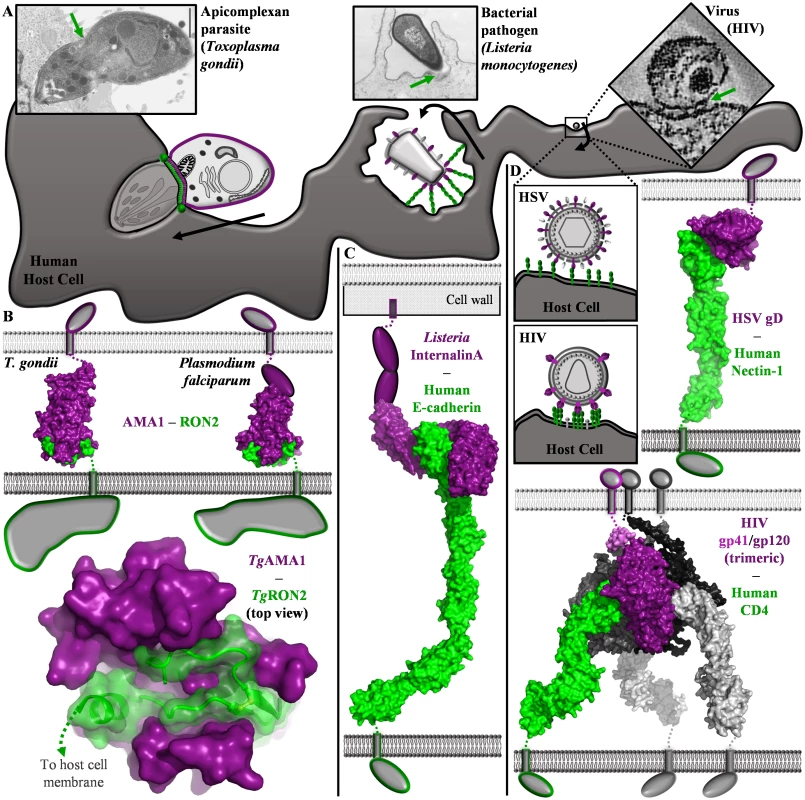
(A) Schematic of invasion mechanisms utilized by T. gondii, L. monocytogenes, and two enveloped viruses. Electron micrographs: T. gondii (reprinted from [19, 40] with permission from Macmillan Publishers Ltd and Elsevier, respectively), L. monocytogenes (originally published in [41], reproduced under CC-BY-SA-3.0-us), HIV (reprinted from [42] with permission from Elsevier). Black arrows indicate movement by the pathogen (left) or the host cell (middle), or membrane fusion (right). Green arrows indicate the parasite-host cell interface. (B-D) Thin rectangles, single pass transmembrane domain; top membrane (light grey), pathogen plasma membrane; bottom membrane (dark grey), host plasma membrane; intracellular domains shown as grey shapes (B) Top—apicomplexan parasite-derived AMA1 and RON2 complexes generate a tight link between parasite and host cell (Left: TgAMA1 (Domains I-II-III, purple surface) TgRON2 synthetic peptide (green surface), PDB ID 2Y8T; Right: PfAMA1 (Domains I-II, purple surface; Domain III, purple oval)-PfRON2 synthetic peptide (green surface), PDB ID 3ZWZ). Bottom—apical view of the TgAMA1 (solid purple surface)-TgRON2 (green cartoon and semi-transparent surface) complex. (C) L. monocytogenes Internalin A (cap, leucine rich repeat, and Ig-like inter-repeat domains shown as purple surface; C-terminal sequence shown as purple ovals) is anchored in the cell wall and grasps E-cadherin (green surface) in an extended pathogen-host cell link (composite of PDB IDs 1O6S and 3Q2V). (D) Top—HSV gD (purple surface) recognizes human nectin-1 (green surface) (PDB ID 3U82). Bottom—HIV gp41 (violet, dark grey surfaces)-gp120 (purple, dark grey surfaces) trimers coordinate human CD4 (green, light grey surfaces) on T cells (composite of PDB IDs 3LQA, 4NCO and 1WIP). Apicomplexan Parasites Provide Both Ligand and Receptor to Form a High Affinity Complex Capable of Supporting Active Invasion of Host Cells
Apicomplexan parasites such as Toxoplasma gondii (the etiological agent of toxoplasmosis and one of the most successful parasites on the planet) and Plasmodium falciparum (the etiological agent of malaria) actively invade host cells using a highly orchestrated process. Initially, parasites engage host cells through a series of reversible attachments that likely benefit from avidity enhanced clustering of ligands and receptors that effectively bring the parasite and host cell into close proximity [4]. A defining feature of the subsequent tight connection between parasite and host cell is a constricted ring of adhesion formed between the parasite and host cell membranes, termed the moving junction (MJ). Using its own motor complex, the parasite actively propels itself through the MJ ring leading to its encapsulation inside a nascent vacuole within the host cell [10]. Intriguingly, this circumferential ring appears to be formed primarily by parasite proteins. Apicomplexans first secrete their own receptor, rhoptry neck protein 2—RON2, into the host cell, which is then engaged by their own surface ligand, apical membrane antigen 1—AMA1 [11–14]. Thus, reminiscent of the self-reliant enteropathogenic Escherichia coli intimin-Tir system used to promote colonization of host cell surfaces [15] but unique among characterized eukaryotic invasion mechanisms, the apicomplexans provide both ligand and receptor to promote host cell invasion. The importance of this mechanism to ensuring successful invasion of the host cell is highlighted both by T. gondii’s ability to activate compensatory pairs of AMA1 and RON2 paralogs when the primary AMA1-RON2 complex is compromised [16, 17], and by recent vaccine trials showing that the preformed AMA1-RON2 complex provides significantly more protection than immunization with AMA1 or RON2 alone [18].
Visualization of the MJ using electron microscopy [19] and immunofluorescence reveals that T. gondii squeezes through the highly constricted MJ ring (Fig. 1A, left) in a process that is likely to result in substantial mechanical shear forces. Based on structural and biophysical studies, a model was proposed where intimate association between AMA1 and RON2 is sufficiently strong to offset the mechanical stresses of the parasite passing through the constricted MJ ring. This model was largely based on the co-crystal structures of AMA1 and a short region near the C-terminus of RON2 from T. gondii and P. falciparum (Protein Data Bank (PDB) IDs 2Y8T and 3ZWZ) (Fig. 1B, top) [20, 21], which revealed that a portion of the C-terminal ectodomain of RON2 integrates deeply into a hydrophobic groove on the apical surface of AMA1 (Buried Surface Area (BSA) [22] of 3,765 Å2 for T. gondii and 3,154 Å2 for P. falciparum) (Fig. 1B, bottom). Intriguingly, the large BSA of the AMA1-RON2 complex is enabled by substantial conformational change in AMA1 and results in one of the most extensive interfaces of a binary ligand-receptor interaction bridging a pathogen and host cell. Furthermore, binding studies have revealed that the AMA1 has a very high affinity for RON2, with a dissociation constant (Kd) for TgAMA1-TgRON2 of approximately 15 nM, consistent with the extensive interface [20, 21]. While no evidence of higher order assemblies or avidity enhanced interactions have emerged, the electron dense nature of the MJ ring suggests that AMA1-RON2 complexes are clustered in vivo. Additionally, the intracellular C-terminal domain of AMA1 plays a key role in signaling during parasite invasion [16, 23], and there is the possibility that conformational changes in AMA1 [20, 21, 24, 25] or its clustering into the circumferential MJ ring may be responsible for engaging the parasite motor or signalling productive invasion. Ultimately, elucidating a comprehensive functional profile of the important AMA1-RON2 invasion complex will require its characterization in the context of biological membranes.
A Bacterial Strategy to Induce Receptor Mediated Internalization into Host Cells
In contrast to the protozoan apicomplexans, attachment of bacteria to host cells is predominately mediated by extended fimbria or pili, and interactions with large extracellular matrix proteins such as fibronectin. However, Listeria monocytogenes, a highly virulent food-borne bacterium, is a facultative intracellular pathogen that induces its own internalization through specific receptor mediated interactions (Fig. 1A, middle) [26]. Specifically, Internalin (Inl) A is covalently attached to the cell wall of L. monocytogenes and extends out with a curved solenoid domain that grasps E-cadherin on the host cell in a calcium dependent interaction (PDB ID 1O6S; BSA of 2,630 Å2; Kd of 8 μM in the presence of calcium) [27]. Despite the relatively low affinity of the InlA-E-cadherin interaction, a clustering of receptor-ligand complexes is predicted to mediate macroscopic adhesion [27]. Moreover, the transmembrane adherens junction protein E-cadherin displays a modular architecture with five immunoglobulin-like domains extending away from the host cell membrane that likely function as a flexible connector between the pathogen and host cell (Fig. 1A, middle and 1C). While the InlA interaction with E-cadherin facilitates crossing the intestinal wall, which is the first barrier encountered by L. monocytogenes, InlB works in conjunction with InlA by binding the Met tyrosine kinase to affect cell signalling and mediate host cell invasion to a limited extent in the intestinal tract and primarily in deeper tissues (PDB ID 2UZX; BSA of 2,848 Å2; Kd of approximately 25 nM) [28, 29]. Importantly, exploitation of the human E-cadherin and Met dependent adhesion and signalling system with Inls A and B has enabled L. monocytogenes to induce its own uptake in a range of nonphagocytic cells [26].
High resolution molecular blueprints of the InlA and InlB complexes have yielded substantial insight into the invasion mechanisms of L. monocytogenes. By characterizing these proteins in a membrane environment, it will be intriguing to determine precisely how these modular receptors (e.g., E-cadherin) and ligands (e.g., InlA) regulate the distance between the L. monocytogenes cell wall and host cells membrane, and establish what role this plays in ensuring efficient invasion of host cells. These studies may also help to define how the forces associated with induced phagocytosis compare to those experienced by pathogens invading through alternate mechanisms such as apicomplexan parasites squeezing through the AMA1-RON2 MJ ring, and how low affinity complexes enable macroscopic adhesion.
Multimeric and Multifunctional Complexes Employed in Viral Host Cell Invasion
The mechanisms employed by viruses to resist shear flow and engage host cells vary substantially, but a common requirement for their genetic material to be successfully internalized by host cells is specific receptor-mediated attachment (Fig. 1A, right). As examples of the diversity in viral adhesion strategies, we will focus here on the multifunctional protein strategy of herpes simplex virus (HSV) and the multimeric protein complex strategy of human immune deficiency virus (HIV).
While the herpes virus, HSV, has multiple surface proteins required for efficient adhesion and subsequent membrane fusion, the major surface adhesin is glycoprotein D (gD), a protein capable of forming higher order homo - and heteromultimeric complexes and coordinating three different classes of receptors: the primary receptor nectin-1 (Kd of 17 nM), and secondary receptors herpes virus entry mediator (HVEM) and 3-O-sulfonated heparan sulfate [30, 31]. The complex of HSV gD with two or three of the Ig-like domains of nectin-1 (PDB IDs 3SKU and 3U82, respectively) reveals an average BSA of 1,766 Å2 between gD and the N-terminal domain of nectin-1 (Fig. 1D, top) [30, 32]. Intriguingly, the structure of gD in complex with HVEM revealed binding in a region that is distinct from, but overlaps on, the nectin-1 binding site, and also a possible binding site for sulfonated heparan sulfate ligands (PDB ID 1JMA) [33]. From these costructures in combination with the structure of apo gD, it has been speculated that conformational changes in gD induced by receptor binding provide a signal that initiates fusion between the viral and host cell membranes [30, 32, 34].
In contrast, the surface of the AIDS virus, HIV, is dominated by cleavage products of envelope glycoprotein 160 (gp160), which is proteolytically processed into two fragments: membrane bound gp41 and noncovalently associated gp120. Trimers of gp41-gp120 heterodimers are anchored in the viral membrane, and gp120 recognizes CD4 primarily on human T cells. Based on structural studies of gp120 with the terminal immunoglobulin domain of CD4, each gp120-CD4 complex buries 1,950 Å2 of surface area, indicating an intimate association consistent with the measured Kd of 5 nM [35, 36]. Interestingly, the thermodynamics of the gp120-CD4 interaction suggest that a large conformational reorganization in gp120 is required for coordination of CD4 [36]. Since CD4 is comprised of four immunoglobulin-like domains, the gp41-gp120-CD4 link generates an extended link between the viral and host cell membranes (Fig. 1C, bottom). Not surprisingly, a subsequent, closer interaction between gp120 and a chemokine receptor (e.g. CXCR4 or CCR5) is required to bring the membranes into sufficiently close apposition to enable viral fusion with the host cell. The transmembrane nature of both CD4 and chemokine receptors is exploited by HIV, as binding of gp120 induces signalling events that prepare the host cell for viral entry [37].
Despite the structural and biophysical insights gained into these and other viral invasion strategies, it remains unclear precisely how the affinity, avidity, and conformational changes of these membrane anchored biomolecular complexes regulate the subsequent steps leading to membrane fusion and viral entry.
Conclusions and Implications for Future Studies
The structural study of biomolecular complexes that drive pathogen internalization into host cells offers targeted strategies for therapeutic development and enhances our basic understanding of molecular pathogenesis. In particular, the molecular assemblies reviewed here have contributed to the development of new drug targets [38, 39] and provided insight into novel strategies to enhance vaccine efficiency against global diseases such as AIDS and malaria [18, 36]. Furthermore, our comparison of just four protein complexes that allow pathogens to resist shear forces prevalent throughout the human body reveals an array of strategies enabled by an exceptionally diverse set of molecular interactions. However, attempting to correlate the high resolution structural data to precise roles in host cell invasion reveals many critical unanswered questions: Is there a direct relationship between the affinity, avidity, or BSA of a host-pathogen receptor-ligand complex and the shear stress that must be overcome to enable invasion? What role does shear flow play in functionally activating ligands and receptors? In situations of pathogen ligand binding sites overlapping the binding site for the native host cell ligand (e.g., HSV gD/nectin-1 versus nectin-1/nectin-1), have high affinity interactions evolved at least partially to overcome competition with the host complexes? In the case of ligand and receptor originating from the pathogen (e.g., apicomplexan AMA1-RON2), are high affinity interactions the result of optimized force resistance since no competition with a native host ligand occurs? Do mechanical forces play a role in how protein-protein complex formation at the host-pathogen interface is related to transmitting forward (i.e., into the host cell) and/or reverse (i.e., into the pathogen) signals that are important for successful invasion events? What roles do conformational change of pathogen ligands (e.g., apicomplexan AMA1, HSV gD, HIV gp120) play in regulating specificity, signal transduction, and immune evasion and do these conformational changes differ in the context of a membrane? Overall, our understanding of the intricate roles of biomolecular complexes linking pathogen and host cell membranes is greatly enhanced by the in vitro high resolution biophysical characterization of the isolated complexes such as those presented here. However, a detailed and comprehensive description of molecular invasion strategies will continue to rely on the development and application of innovative and multidisciplinary approaches to ensure data are interpreted in the context of biologically relevant environments.
Zdroje
1. Hansson GC (2012) Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol 15 : 57–62. doi: 10.1016/j.mib.2011.11.002
2. Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75 : 519–560. 7624393
3. Moriarty TJ, Shi M, Lin YP, Ebady R, Zhou H, et al. (2012) Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol 86 : 1116–1131. doi: 10.1111/mmi.12045 23095033
4. Carruthers V, Boothroyd JC (2007) Pulling together: an integrated model of Toxoplasma cell invasion. Current Opinion in Microbiology 10 : 83–89. doi: 10.1016/j.mib.2006.06.017 16837236
5. Nauman EA, Ott CM, Sander E, Tucker DL, Pierson D, et al. (2007) Novel quantitative biosystem for modeling physiological fluid shear stress on cells. Appl Environ Microbiol 73 : 699–705. doi: 10.1128/AEM.02428-06 17142365
6. Harvey KL, Gilson PR, Crabb BS (2012) A model for the progression of receptor-ligand interactions during erythrocyte invasion by Plasmodium falciparum. Int J Parasitol 42 : 567–573. doi: 10.1016/j.ijpara.2012.02.011 22710063
7. Harker KS, Jivan E, McWhorter FY, Liu WF, Lodoen MB (2014) Shear forces enhance Toxoplasma gondii tachyzoite motility on vascular endothelium. MBio 5: e01111–01113. doi: 10.1128/mBio.01111-13 24692639
8. Dasgupta S, Auth T, Gov NS, Satchwell TJ, Hanssen E, et al. (2014) Membrane-wrapping contributions to malaria parasite invasion of the human erythrocyte. Biophys J 107 : 43–54. doi: 10.1016/j.bpj.2014.05.024 24988340
9. Crick AJ, Theron M, Tiffert T, Lew VL, Cicuta P, et al. (2014) Quantitation of malaria parasite-erythrocyte cell-cell interactions using optical tweezers. Biophys J 107 : 846–853. doi: 10.1016/j.bpj.2014.07.010 25140419
10. Dobrowolski JM, Sibley LD (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84 : 933–939. doi: 10.1016/S0092-8674(00)81071-5 8601316
11. Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M (2009) Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5: e1000309. doi: 10.1371/journal.ppat.1000309 19247437
12. Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, et al. (2011) Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A 108 : 13275–13280. doi: 10.1073/pnas.1110303108 21788485
13. Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, et al. (2011) The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog 7: e1001276. doi: 10.1371/journal.ppat.1001276 21347343
14. Tyler JS, Boothroyd JC (2011) The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog 7: e1001282. doi: 10.1371/journal.ppat.1001282 21347354
15. Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, et al. (1997) Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91 : 511–520. doi: 10.1016/S0092-8674(00)80437-7 9390560
16. Lamarque MH, Roques M, Kong-Hap M, Tonkin ML, Rugarabamu G, et al. (2014) Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun 5 : 4098. doi: 10.1038/ncomms5098 24934579
17. Harvey KL, Yap A, Gilson PR, Cowman AF, Crabb BS (2014) Insights and controversies into the role of the key Apicomplexan invasion ligand, Apical Membrane Antigen 1. Int J Parasitol. doi: 10.1016/j.ijpara.2014.08.001 25157917
18. Srinivasan P, Ekanem E, Diouf A, Tonkin ML, Miura K, et al. (2014) Immunization with a functional protein complex required for erythrocyte invasion protects against lethal malaria. Proc Natl Acad Sci U S A 111 : 10311–10316. doi: 10.1073/pnas.1409928111 24958881
19. Dubremetz JF (1998) Host cell invasion by Toxoplasma gondii. Trends Microbiol 6 : 27–30. doi: 10.1016/S0966-842X(97)01165-7 9481821
20. Tonkin ML, Roques M, Lamarque MH, Pugniere M, Douguet D, et al. (2011) Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333 : 463–467. doi: 10.1126/science.1204988 21778402
21. Vulliez-Le Normand B, Tonkin ML, Lamarque MH, Langer S, Hoos S, et al. (2012) Structural and functional insights into the malaria parasite moving junction complex. PLoS Pathog 8: e1002755. doi: 10.1371/journal.ppat.1002755 22737069
22. Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372 : 774–797. doi: 10.1016/j.jmb.2007.05.022 17681537
23. Leykauf K, Treeck M, Gilson PR, Nebl T, Braulke T, et al. (2010) Protein kinase a dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog 6: e1000941. doi: 10.1371/journal.ppat.1000941 20532217
24. Ge X, MacRaild CA, Devine SM, Debono CO, Wang G, et al. (2014) Ligand-Induced Conformational Change of Plasmodium falciparum AMA1 Detected Using (19)F NMR. J Med Chem 57 : 6419–6427. doi: 10.1021/jm500390g 25068708
25. Poukchanski A, Fritz HM, Tonkin ML, Treeck M, Boulanger MJ, et al. (2013) Toxoplasma gondii Sporozoites Invade Host Cells Using Two Novel Paralogues of RON2 and AMA1. PLoS One 8: e70637. doi: 10.1371/journal.pone.0070637 23940612
26. Hamon M, Bierne H, Cossart P (2006) Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4 : 423–434. doi: 10.1038/nrmicro1413 16710323
27. Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, et al. (2002) Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 111 : 825–836. doi: 10.1016/S0092-8674(02)01136-4 12526809
28. Niemann HH, Jager V, Butler PJ, van den Heuvel J, Schmidt S, et al. (2007) Structure of the human receptor tyrosine kinase met in complex with the Listeria invasion protein InlB. Cell 130 : 235–246. doi: 10.1016/j.cell.2007.05.037 17662939
29. Machner MP, Frese S, Schubert WD, Orian-Rousseau V, Gherardi E, et al. (2003) Aromatic amino acids at the surface of InlB are essential for host cell invasion by Listeria monocytogenes. Mol Microbiol 48 : 1525–1536. doi: 10.1046/j.1365-2958.2003.03532.x 12791136
30. Zhang N, Yan J, Lu G, Guo Z, Fan Z, et al. (2011) Binding of herpes simplex virus glycoprotein D to nectin-1 exploits host cell adhesion. Nat Commun 2 : 577. doi: 10.1038/ncomms1571 22146396
31. Handler CG, Eisenberg RJ, Cohen GH (1996) Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol 70 : 6067–6070. 8709230
32. Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, et al. (2011) Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7: e1002277. doi: 10.1371/journal.ppat.1002277 21980294
33. Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, et al. (2001) Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8 : 169–179. doi: 10.1016/S1097-2765(01)00298-2 11511370
34. Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, et al. (2005) Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24 : 4144–4153. doi: 10.1038/sj.emboj.7600875 16292345
35. Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, et al. (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393 : 648–659. doi: 10.1038/31405 9641677
36. Myszka DG, Sweet RW, Hensley P, Brigham-Burke M, Kwong PD, et al. (2000) Energetics of the HIV gp120-CD4 binding reaction. Proc Natl Acad Sci U S A 97 : 9026–9031. doi: 10.1073/pnas.97.16.9026 10922058
37. Abbas W, Herbein G (2014) Plasma membrane signaling in HIV-1 infection. Biochim Biophys Acta 1838 : 1132–1142. doi: 10.1016/j.bbamem.2013.06.020 23806647
38. Srinivasan P, Yasgar A, Luci DK, Beatty WL, Hu X, et al. (2013) Disrupting malaria parasite AMA1-RON2 interaction with a small molecule prevents erythrocyte invasion. Nat Commun 4 : 2261. doi: 10.1038/ncomms3261 23907321
39. Vermeire K, Schols D (2005) Anti-HIV agents targeting the interaction of gp120 with the cellular CD4 receptor. Expert Opin Investig Drugs 14 : 1199–1212. doi: 10.1517/13543784.14.10.1199 16185162
40. Boothroyd JC, Dubremetz JF (2008) Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol 6 : 79–88. doi: 10.1038/nrmicro1800 18059289
41. Tilney LG, Portnoy DA (1989) Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109 : 1597–1608. doi: 10.1083/jcb.109.4.1597 2507553
42. Earl LA, Lifson JD, Subramaniam S (2013) Catching HIV ‘in the act’ with 3D electron microscopy. Trends Microbiol 21 : 397–404. doi: 10.1016/j.tim.2013.06.004 23850373
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



