-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Adaptive Prediction As a Strategy in Microbial Infections
article has not abstract
Published in the journal: . PLoS Pathog 10(10): e32767. doi:10.1371/journal.ppat.1004356
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004356Summary
article has not abstract
Microorganisms need to sense and respond to constantly changing microenvironments, and adapt their transcriptome, proteome, and metabolism accordingly to survive [1]. However, microbes sometimes react in a way which does not make immediate biological sense in light of the current environment—for example, by up-regulating an iron acquisition system in times of metal abundance. The reason for this seemingly nonsensical behavior can lie in the microbe's ability to predict a coming change in conditions by cues from the current environment. If the microbe (pre-)adapts accordingly, it will increase its fitness and chances of survival under subsequent selection pressures—a concept known as adaptive prediction (Figure 1) [2].
Fig. 1. The basis of adaptive prediction. 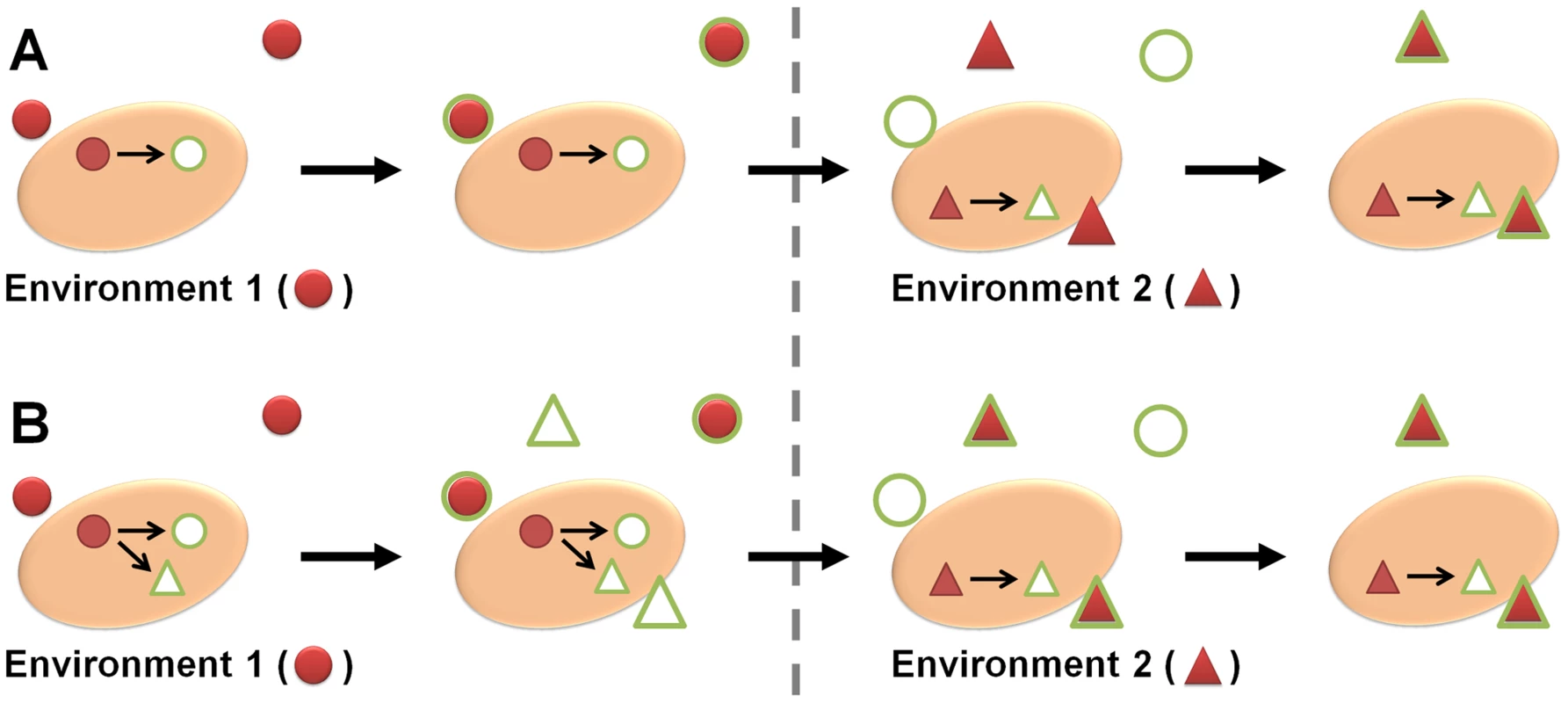
(A) The conditions in environment 1 (red circles) activate a distinct response (green circles) in a microbe. After changing to environment 2, altering, e.g., the expression pattern to respond to the new conditions (triangles) requires time, during which the microbe is not well adapted. (B) If the sequential temporal order of the two environments from (A) is kept over many generations, a new signal pathway can form. Now, the conditions in environment 1 induce responses to both the first and the second environment. When changing to environment 2, the microbe is hence already pre-adapted. In metazoans with complex neural network architecture, the capacity to anticipate changes in the environment is understandable. It can be achieved in a single multicellular organism, e.g., by classical conditioning. In unicellular organisms, however, this type of learning normally requires generations of selection pressure to connect one predictor to a coming condition.
Why Is Adaptive Prediction Relevant for Human Pathogens?
The human host is, to a certain extent, a highly predictable environment. In its different niches, pH values, ion concentrations, temperature, and many other factors are normally kept within small ranges. Transiting from one niche to another usually follows a predetermined pattern—entering the host from the environment is associated with an increase in temperature; in the gastrointestinal (GI) tract, the neutral gut will follow the strongly acidic stomach; invasion into tissue and entering the bloodstream will likely lead to engulfment by immune cells, followed by oxidative stress and starvation for micronutrients such as iron or zinc; and passaging through the gut means decreasing oxygen and glucose levels. These cues can be used by commensals and potential pathogens to optimize their fitness by predicting the next stage in host–microbe interaction.
Sensing and Making Sense—The Example of Escherichia coli and Other Enteric Bacteria
A good example for adaptive prediction comes from the gut bacterium Escherichia coli. In this microbe, an increase in temperature elicits a transcriptional response typical for low oxygen levels [3]. This makes biological sense, as the increase in temperature can indicate the bacterium's arrival in the gut, where oxygen will soon become limiting. Interestingly, this predictive function can be disrupted if temperature and oxygen levels are dissociated over evolutionary timescales. In a laboratory microevolution experiment with a reversed temperature–oxygen relationship (i.e., high temperature is followed by high oxygen), Tagkopoulos et al. obtained E. coli strains where the predictive quality of temperature for oxygen was largely lost [3]. Similarly, maltose utilization genes are activated in E. coli upon exposure to lactose, reflecting the sequential abundance of these sugars in the gut [2]. Again, disruption of this sequence over hundreds of generations was able to abolish this adaptive prediction in vitro [2]. These two examples show how strongly an evolved adaptive prediction response can impact microbial fitness.
As many pathogens are gut-associated, similar patterns can be found in pathogenic enteric bacteria. The enterohemorrhagic E. coli (EHEC) serotype O157:H7, for example, can use the presence of bile as a signal to induce transcription of iron acquisition genes, independent of actual iron levels [4]. This can be useful in the iron-sequestering environment of the small intestine, where bile abounds. On the other hand, pathogenicity-island encoded genes that are specifically expressed at later stages of the intestinal passage by EHECs were found to be repressed by bile in the upper part of the small intestine [4]. Many other enteric bacteria, like Salmonella, Shigella, and Vibrio spp. also use bile as a signal to regulate virulence programs, which are biologically unlinked to bile salts but are advantageous at later stages in their mammalian hosts (reviewed in [5]). Vibrio cholerae is also known to induce genes late in its infection cycle that are of no immediate use in the host. These genes, for example those involved in chitin binding and degradation, should benefit the bacteria only after they are released into the aquatic environment where crustaceans provide ample chitin [6]—although it is tempting to speculate that chitin degradation may play an additional role in competition with resident fungi in the gut. In summary, sensing certain host-specific factors can herald changing conditions, and pathogens can use these signals in their (pre-)adaptation to the host or for transition from the host.
(Re-)interpreting Old Cues—The Candida albicans Example
Candida albicans is a fungal pathogen that can transit from a commensal state in the gut to an aggressive pathogen that invades tissue and disseminates via the bloodstream. Tissue invasion is linked to a specific morphology change, the yeast-to-hypha transition (Figure 2). The hyphal program is triggered by multiple stimuli, including contact with epithelial cells and body temperature [7]. Part of this program is the expression of the multipurpose, hypha-associated cell wall protein, Als3, which enables the fungus to attach to and invade host cells and use the intracellular host iron storage protein ferritin as an iron source after invasion [8]. Other hypha-associated factors are the Sap4-6 proteases, which can degrade host proteins during invasion, and the cell surface localized superoxide dismutase Sod5, which can be used to detoxify reactive oxygen species likely to be produced by attracted immune effector cells when tissue is damaged. Therefore, by triggering hyphal morphogenesis, C. albicans produces factors that are required during or after tissue invasion even before the actual invasion process is initiated [7].
Fig. 2. Candida albicans as an example for adaptive prediction of pathogens in the host. 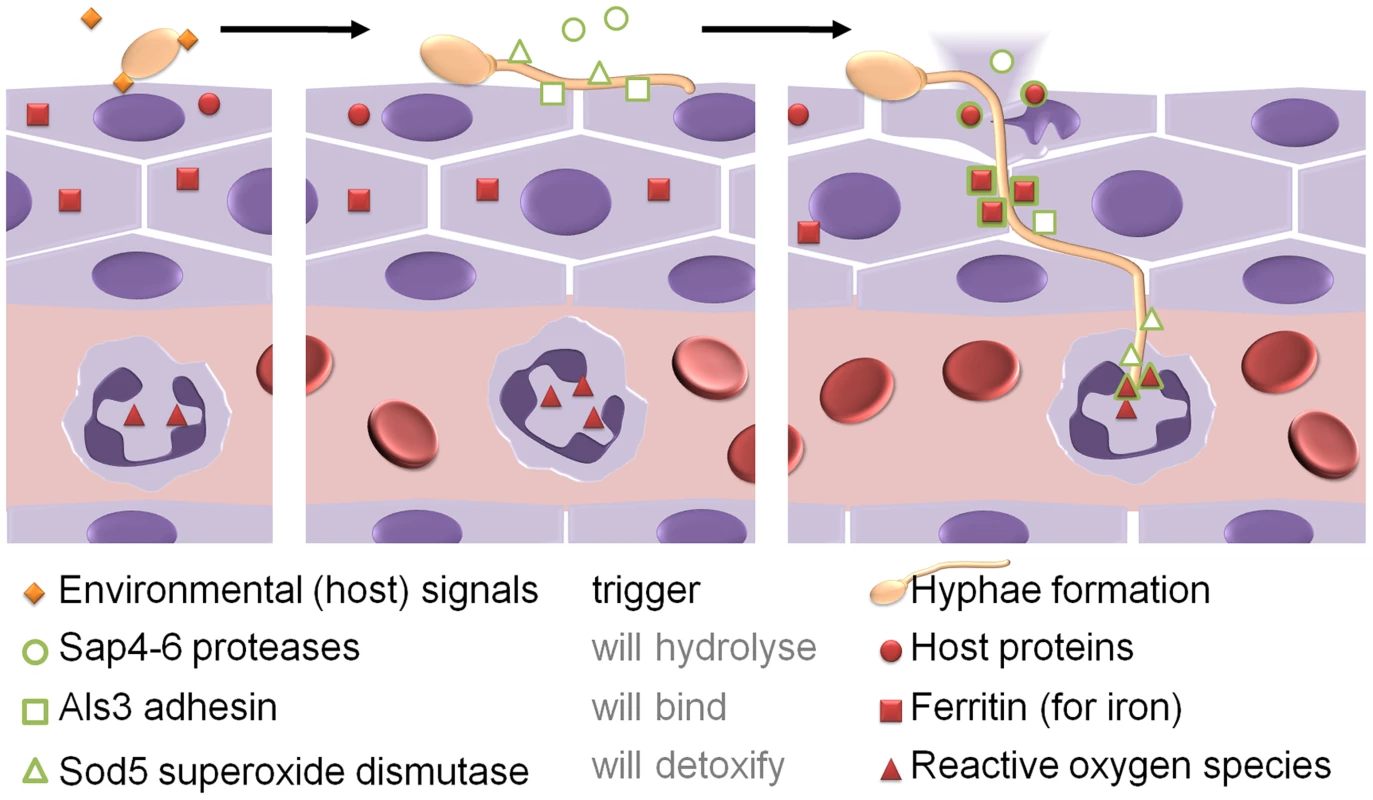
When attaching to epithelial cells, environmental signals trigger hyphae formation. The hyphae start to express a set of proteins which are not apparently beneficial for the fungus in its current situation (green symbols). Only when penetrating into the host tissue and during encounters with host immune cells like neutrophils, the stresses (red symbols) occur under which these proteins give C. albicans an advantage in survival and growth (see text for more details). In the blood, C. albicans seems to interpret the presence of (even low) amounts of glucose as a cue for potential encounters with immune cells. While the related baker's yeast, Saccharomyces cerevisiae, down-regulates most stress-response genes in the presence of glucose, C. albicans up-regulates oxidative and osmotic stress responses when encountering glucose levels similar to the bloodstream [9]. While not necessary for growth on glucose, these adaptations would allow better survival of attacks by blood-borne phagocytic cells after leaving the glucose-poor gut. Accordingly, the signaling networks leading from glucose to stress response differ significantly between the two species, allowing C. albicans to reinterpret glucose as a pre-indicator of possible future dangers [9].
In a similar vein, C. albicans responds to neutral or alkaline pH by expressing genes involved in iron and zinc uptake via an alkaline-induced transcription factor, Rim101 [10], [11]. As these metals are generally less soluble at high pH, this connection makes biological sense and may help in a timely response, even before the full effect of metal limitation is felt by the cell. Thus, common environmental cues like presence of carbon sources or pH changes can obtain a new, additional meaning and allow the pathogen to predict conditions in different host niches. To this end, established signaling pathways for these conditions can be rewired to novel outputs and thus allow an adaptive prediction response.
Weighing Costs and Risks—The Plasmodium Example
Predicting the future environment comes with a risk. A “false positive” prediction—in which the pathogen falsely predicts a future environment that it will not encounter in reality—will leave the pathogen in a state less adapted to the current environment, with all the associated fitness costs. A “false negative” prediction (in which the pathogen does not interpret the signal correctly to prepare for a future change) will lead to a severe loss in fitness in the coming environment [12]. Because of this trade-off, any (costly) adaptation must rely on robust and reliable signals before a population of cells commits to a new phenotype. Alternatives exist in the form of stochastic switching and phenotypic heterogeneity, in which only a random subpopulation expresses a certain trait [13]. This strategy is more common in unpredictable and fluctuating environments [14].
The causative agents of malaria, Plasmodium spp., normally replicate asexually inside the bloodstream of their host. However, at every replication cycle, a portion of the parasites develops into gametocytes instead. For these sexual stages, the mammalian host is a dead end, as the gametocytes cannot replicate asexually anymore. However, after a mosquito bite, only gametocytes can enter this new, suitable host to differentiate and mate [15]. Thus, in every replication cycle, there is a trade-off between investing resources into forming the sexual stage for propagation between hosts and asexual reproduction within a host. Interestingly, the rate of conversion to the sexual stage varies between Plasmodium species, and antimalarial treatment, as well as an increase in young reticulocytes, increases the number of sexual gametocytes [16]. The malaria parasites use these indicators as signs of imminent host death or clearance of infection. In a “terminal investment,” the sexual between-host transmission strategy is then followed. Similarly, in a freshly infected naive host, investment in sexual forms is possible since the associated fitness costs are low. In contrast, in the presence of low levels of stress, for example caused by parasites of different genotypes competing for the same host resources, fitness costs for not replicating asexually are high, and asexual reproduction, hence, dominates (discussed in [17]). Overall, environmental cues allow the pathogen to weigh the risks for committing to a pre-adapted phenotype.
Adaptive Prediction and Coordinated Regulation
Adaptive prediction seems, in many aspects, similar to the concept of coordinate regulation, in which several genes, often including virulence factors, are controlled by a common regulatory system in response to an environmental trigger [18]. Conceptually, however, coordinate regulation responds to environmental factors that are linked by their simultaneous occurrence rather than their temporal succession. A good example is the iron-starvation–induced expression of the siderophore synthesis machinery, siderophore binding proteins, and cytolytic toxins in many bacteria. In that process, iron starvation indicates a host environment or activities by the host, and a coordinated transcriptional regulation allows immediate destruction of host cells, binding, and finally, uptake of iron in response. In a sense, signal and bacterial adaptation responses are spatially linked, as they occur in the immediate environment of the microbe. In contrast, in adaptive prediction, signal and responses are temporally linked.
It may prove difficult, however, to draw a precise dividing line between the two concepts, as many intermediate forms likely exist. Furthermore, a coordinated regulation could feasibly evolve into an adaptive prediction system. Coordinately regulated genes come under control of one or a few transcription factors or regulatory pathways. If an independent signal (nearly) always predictively precedes the coordinated expression, these few signal pathways (or the single pathway) can easily evolve to accept this signal for a “pre-emptive” response [3]. Adding a predictive to the existing immediate trigger, hence, allows a complex and fully coordinated response to take place in anticipation of a new environment. This way, coordinated regulation could make the appearance of adaptive prediction evolutionary more likely.
On the other hand, the expression of many genes can come with a higher fitness cost. Mathematical models show that this kind of adaptive prediction is more likely to occur in environments where stresses (rather than future improvement in growth conditions) are able to be predicted well and may be even modified to include a partial response (for details, see [12]).
Prevalence and Possible Medical Applications of Adaptive Prediction
How prevalent is this phenomenon in pathogens? It seems likely that adaptive prediction processes are more common than is currently appreciated. In the laboratory, microbes are rarely exposed to two or more consecutive environments that reflect the natural progression through habitats. Unusual (i.e., predictive) transcriptional responses occur, but without a biological explanation these may not be followed up when investigating the microbe's response to a specific environment. Especially in environmental microbes, which are not known to be generally associated with animal hosts, a host-adaptive response to certain environmental stresses may indicate potential for pathogenicity. Such adaptations would likely be different to commensal organisms, and may result from transient but repeated exposure to animal hosts.
In simulations, predictive behavior of genetic networks appears fast and frequently [3]. In directed evolution experiments, yeast can acquire the ability to predict one stress from the presence of another remarkably quickly [19]. Finally, without the need for evolutionary processes spanning generations, associative learning is considered feasible in individual single cell organisms [20] and even in simple chemical networks [21]. While still mostly hypothetical, this would allow microbes to expand beyond evolutionarily acquired adaptive prediction into responses shaped by individual cell life histories.
It therefore seems highly likely that many pathogens can switch to a (currently) non-adaptive phenotype when external cues indicate a coming change in environment. Using these signals to “trick” a pathogen into a phenotypic conversion may be exploited to render microbes maladapted to their current surroundings. As an avenue for future treatment options, adaptive prediction responses may therefore deserve deeper consideration.
Zdroje
1. PerkinsTJ, SwainPS (2009) Strategies for cellular decision-making. Mol Syst Biol 5 : 326.
2. MitchellA, RomanoGH, GroismanB, YonaA, DekelE, et al. (2009) Adaptive prediction of environmental changes by microorganisms. Nature 460 : 220–224.
3. TagkopoulosI, LiuYC, TavazoieS (2008) Predictive behavior within microbial genetic networks. Science 320 : 1313–1317.
4. HamnerS, McInnerneyK, WilliamsonK, FranklinMJ, FordTE (2013) Bile salts affect expression of Escherichia coli O157:H7 genes for virulence and iron acquisition, and promote growth under iron limiting conditions. PLoS ONE 8: e74647.
5. GunnJS (2000) Mechanisms of bacterial resistance and response to bile. Microbes Infect 2 : 907–913.
6. SchildS, TamayoR, NelsonEJ, QadriF, CalderwoodSB, et al. (2007) Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe 2 : 264–277.
7. JacobsenID, WilsonD, WächtlerB, BrunkeS, NaglikJR, et al. (2012) Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10 : 85–93.
8. AlmeidaRS, BrunkeS, AlbrechtA, ThewesS, LaueM, et al. (2008) The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog 4: e1000217.
9. RodakiA, BohovychIM, EnjalbertB, YoungT, OddsFC, et al. (2009) Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Biol Cell 20 : 4845–4855.
10. BensenES, MartinSJ, LiM, BermanJ, DavisDA (2004) Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol 54 : 1335–1351.
11. CitiuloF, JacobsenID, MiramonP, SchildL, BrunkeS, et al. (2012) Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog 8: e1002777.
12. MitchellA, PilpelY (2011) A mathematical model for adaptive prediction of environmental changes by microorganisms. Proc Natl Acad Sci U S A 108 : 7271–7276.
13. AverySV (2006) Microbial cell individuality and the underlying sources of heterogeneity. Nat Rev Microbiol 4 : 577–587.
14. AcarM, MettetalJT, van OudenaardenA (2008) Stochastic switching as a survival strategy in fluctuating environments. Nat Genet 40 : 471–475.
15. BatonLA, Ranford-CartwrightLC (2005) Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol 21 : 573–580.
16. PeateyCL, Skinner-AdamsTS, DixonMW, McCarthyJS, GardinerDL, et al. (2009) Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis 200 : 1518–1521.
17. CarterLM, KafsackBF, LlinasM, MideoN, PollittLC, et al. (2013) Stress and sex in malaria parasites: Why does commitment vary? Evol Med Public Health 2013 : 135–147.
18. MillerJF, MekalanosJJ, FalkowS (1989) Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243 : 916–922.
19. DharR, SagesserR, WeikertC, WagnerA (2013) Yeast adapts to a changing stressful environment by evolving cross-protection and anticipatory gene regulation. Mol Biol Evol 30 : 573–588.
20. SorekM, BalabanNQ, LoewensteinY (2013) Stochasticity, bistability and the wisdom of crowds: a model for associative learning in genetic regulatory networks. PLoS Comput Biol 9: e1003179.
21. McGregorS, VasasV, HusbandsP, FernandoC (2012) Evolution of associative learning in chemical networks. PLoS Comput Biol 8: e1002739.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance SystemČlánek Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer CellsČlánek APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse ModelČlánek Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Theory and Empiricism in Virulence Evolution
- -Related Fungi and Reptiles: A Fatal Attraction
- Adaptive Prediction As a Strategy in Microbial Infections
- Antimicrobials, Stress and Mutagenesis
- A Novel Function of Human Pumilio Proteins in Cytoplasmic Sensing of Viral Infection
- Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
- Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer
- Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance System
- mRNA Structural Constraints on EBNA1 Synthesis Impact on Antigen Presentation and Early Priming of CD8 T Cells
- Infection Causes Distinct Epigenetic DNA Methylation Changes in Host Macrophages
- Neutrophil Crawling in Capillaries; A Novel Immune Response to
- Live Attenuated Vaccine Protects against Pulmonary Challenge in Rats and Non-human Primates
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
- HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men
- Uses a Unique Ligand-Binding Mode for Trapping Opines and Acquiring A Competitive Advantage in the Niche Construction on Plant Host
- Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence
- Ethanol Stimulates WspR-Controlled Biofilm Formation as Part of a Cyclic Relationship Involving Phenazines
- Densovirus Is a Mutualistic Symbiont of a Global Crop Pest () and Protects against a Baculovirus and Bt Biopesticide
- Insights into Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria
- Mycobacterial Antigen Driven Activation of CD14CD16 Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
- Lipoprotein LprG Binds Lipoarabinomannan and Determines Its Cell Envelope Localization to Control Phagolysosomal Fusion
- Dampens the DNA Damage Response
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
- Detecting Differential Transmissibilities That Affect the Size of Self-Limited Outbreaks
- One Small Step for a Yeast - Microevolution within Macrophages Renders Hypervirulent Due to a Single Point Mutation
- Expression Profiling during Arabidopsis/Downy Mildew Interaction Reveals a Highly-Expressed Effector That Attenuates Responses to Salicylic Acid
- Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer Cells
- Interaction with Tsg101 Is Necessary for the Efficient Transport and Release of Nucleocapsids in Marburg Virus-Infected Cells
- The N-Terminus of Murine Leukaemia Virus p12 Protein Is Required for Mature Core Stability
- Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in
- Allele-Specific Induction of IL-1β Expression by C/EBPβ and PU.1 Contributes to Increased Tuberculosis Susceptibility
- Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly
- APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
- Structural Basis for the Recognition of Human Cytomegalovirus Glycoprotein B by a Neutralizing Human Antibody
- Systematic Analysis of ZnCys Transcription Factors Required for Development and Pathogenicity by High-Throughput Gene Knockout in the Rice Blast Fungus
- Epstein-Barr Virus Nuclear Antigen 3A Promotes Cellular Proliferation by Repression of the Cyclin-Dependent Kinase Inhibitor p21WAF1/CIP1
- The Host Protein Calprotectin Modulates the Type IV Secretion System via Zinc Sequestration
- Cyclophilin A Associates with Enterovirus-71 Virus Capsid and Plays an Essential Role in Viral Infection as an Uncoating Regulator
- A Novel Alpha Kinase EhAK1 Phosphorylates Actin and Regulates Phagocytosis in
- The pH-Responsive PacC Transcription Factor of Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis
- Sensing of Immature Particles Produced by Dengue Virus Infected Cells Induces an Antiviral Response by Plasmacytoid Dendritic Cells
- Co-opted Oxysterol-Binding ORP and VAP Proteins Channel Sterols to RNA Virus Replication Sites via Membrane Contact Sites
- Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity
- HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells
- Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera
- CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection with Rapid Turnover of Infected Cells
- A Sialic Acid Binding Site in a Human Picornavirus
- Contact Heterogeneity, Rather Than Transmission Efficiency, Limits the Emergence and Spread of Canine Influenza Virus
- Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of
- HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation
- Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence
- A Critical Role for IL-17RB Signaling in HTLV-1 Tax-Induced NF-κB Activation and T-Cell Transformation
- Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90
- Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
- Kaposi's Sarcoma-Associated Herpesvirus Induces Nrf2 during Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection
- A Relay Network of Extracellular Heme-Binding Proteins Drives Iron Acquisition from Hemoglobin
- Glutamate Secretion and Metabotropic Glutamate Receptor 1 Expression during Kaposi's Sarcoma-Associated Herpesvirus Infection Promotes Cell Proliferation
- Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and Anti-pathogen Activities
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



