-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Biology and Taxonomy of Head and Body Lice—Implications for Louse-Borne Disease Prevention
article has not abstract
Published in the journal: . PLoS Pathog 9(11): e32767. doi:10.1371/journal.ppat.1003724
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003724Summary
article has not abstract
Sucking lice (Phthiraptera: Anoplura) are obligate blood-feeding ectoparasites of placental mammals including humans. Worldwide, more than 550 species have been described and many are specific to a particular host species of mammal [1]. Three taxa uniquely parasitize humans: the head louse, body louse, and crab (pubic) louse. The body louse, in particular, has epidemiological importance because it is a vector of the causative agents of three important human diseases: epidemic typhus, trench fever, and louse-borne relapsing fever. Since the advent of antibiotics and more effective body louse control measures in the 1940s, these diseases have markedly diminished in incidence. However, due to 1) increasing pediculicide resistance in human lice, 2) reemergence of body louse populations in some geographic areas and demographic groups, 3) persistent head louse infestations, and 4) recent detection of body louse-borne pathogens in head lice, lice and louse-borne diseases are an emerging problem worldwide. This mini-review is focused on human body and head lice including their biological relationship to each other and its epidemiological relevance, the status and treatment of human louse-borne diseases, and current approaches to prevention and control of human louse infestations.
Biological, Genetic, and Taxonomic Relationships between Head Lice and Body Lice
For over a century, scientists have argued about the exact taxonomic and biological relationships between human head lice and body lice and, in particular, whether they represent a single species with two ecotypes or two distinct species [2], [3]. The two-species argument considers the body louse to be Pediculus humanus and the head louse to be Pediculus capitis (Table 1) (Figure 1A, B, C, D). The single-species argument treats the body louse as Pediculus humanus humanus and the head louse as Pediculus humanus capitis. Further, although the name Pediculus humanus corporis has been used frequently in the medical literature for the body louse, it is an invalid name according to the rules of the International Commission on Zoological Nomenclature. Whether head and body lice represent distinct species, different subspecies (or strains, phylotypes, or ecotypes) inhabiting different habitats, or a single species is more than a taxonomic issue. This is because all well-investigated outbreaks of louse-transmitted diseases in humans, including many that have shaped our history, have involved pathogen transmission by the body louse, not by the head louse [3]. The recent sequencing and annotation of the small 108 Mb genome of P. humanus humanus, the chromosome and plasmid of its symbiotic bacterium, “Candidatus Riesia pediculicola” [4], [5], and the mitochondria of all three human louse taxa [6] have allowed reevaluation of this argument with potentially important epidemiological ramifications. The sensitivity of lice to sulfamethoxazole-trimethoprim is thought to reflect its lethality for Riesia, which lice depend upon for B vitamin synthesis [5], [7]. Differences between head and body lice in the complex developmental interactions that maintain Riesia between generations have been described [8], but whether these differences occur in all louse populations is unknown.
Fig. 1. Adult body louse and head lice. 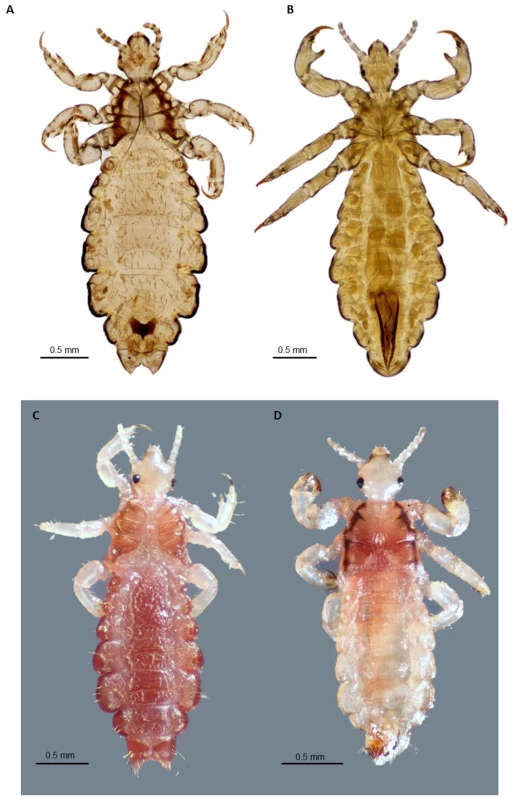
A. Ventral view of slide-mounted female head louse; B. Ventral view of slide-mounted male body louse; C. Dorsal view of ethanol-preserved female head louse; D. Dorsal view of ethanol-preserved male head louse. All photographs were taken using a Visionary Digital K2/SC long-distance microscope (Infinity Photo-Optical Company, Boulder, CO), courtesy of Lorenza Beati. Tab. 1. Selected morphological and biological differences between human head and body lice. 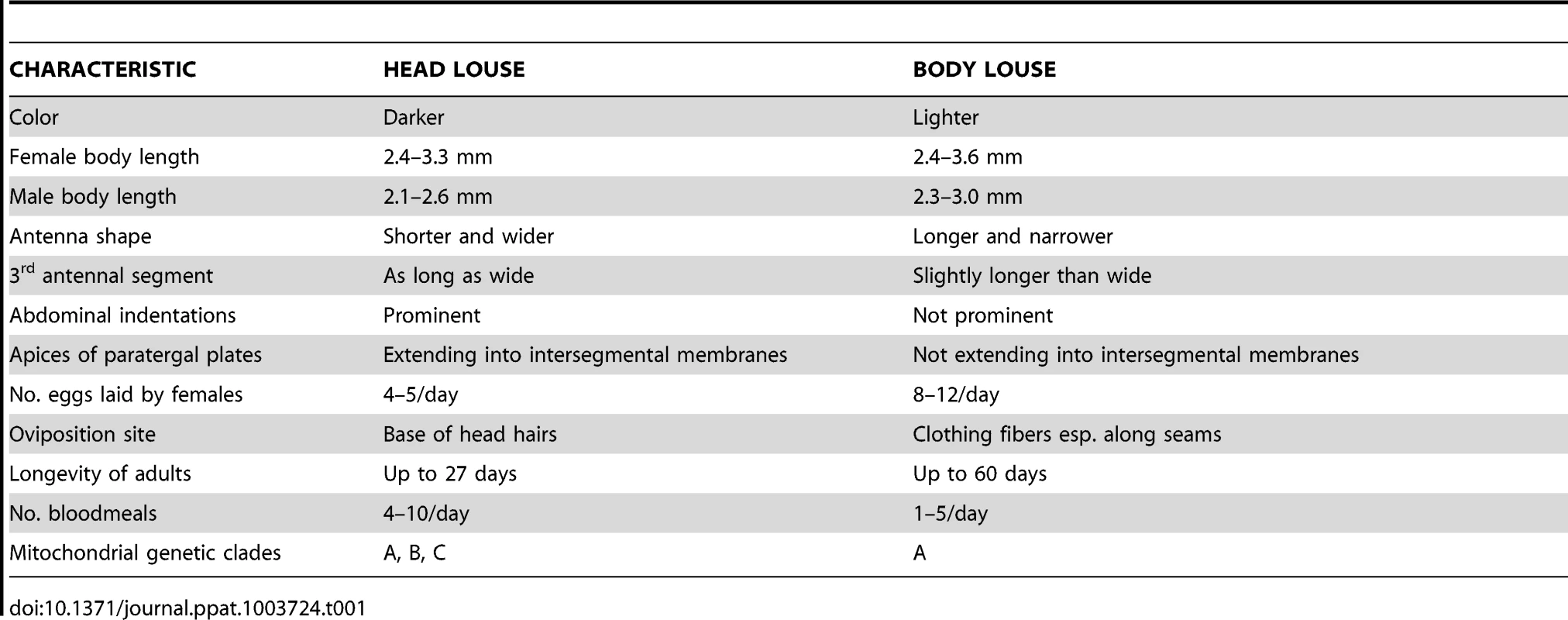
Because of somewhat effective treatment options and increased societal standards for clothing and body hygiene, body lice are currently quite rare in most developed countries [9]. However, they persist or have reemerged in some parts of the world and can also be common in homeless populations in both developed and developing nations [9], [10]. The number of homeless persons has increased significantly in recent decades, and the medical welfare of these people can be difficult to monitor for various reasons [10]. Since homeless persons may not have a change of clothing or be able to adequately delouse their clothes, their garb provides nourishing and unique environments needed for deposition and maintenance of body louse eggs [9], [10]. Explosive increases in populations of body lice have been reported in crowded refugee camps, especially in Africa [9]. Conversely, head lice are common and distributed worldwide with reported infestation prevalences up to 61% [11].
Despite more than 12 years of concerted effort by many investigators to define genetic markers that clearly differentiate head and body louse ecotypes as species or subspecies, this goal has remained elusive [2], [3], [12]–[14]. Most of the data from both mitochondrial and nuclear genes using phylogenetic and population genetic methods fail to clearly separate body and head lice, [2], [12]–[14] indicating that they are conspecific. Although several mitochondrial genes (cytb, COI, and ND4) appear to separate head lice into three clades (A, B, C) and place body lice only in clade A, nuclear genes do not define the same clades, possibly because of modern recombination between different lineages of head lice [2], [12]–[14]. Although these clades exhibit some geographic differences (A is found worldwide, B is found in North America, Central America, Europe, and Australia, and C in Nepal, Ethiopia, and Senegal), it is possible these associations may fail with more extensive sampling of clades B and C [3], [15]. Moreover, in both head and body lice, the 37 mitochondrial genes are located on 18 minicircles, each containing one to three genes, and the intergenic regions are variable in each louse likely as a result of numerous recombination events that make those regions unsuitable for genetic analysis [6]. The ability of head and body lice to interbreed, the common movement of each over both head hair and clothing, and the existence of different color variants of body lice suggest that there has been substantial opportunity for generation of novel genetic variants of P. humanus [3], [16], [17]. Indeed, using more robust microsatellite and multispacer typing methods, the two studies that attempted to define genetic differences between head and body louse populations coinfesting the same persons in Nepal or France emphasized opposite conclusions regarding the genetic relationships between the two populations [17], [18]. The louse samples in both studies were small but both clearly demonstrated that individual humans as well as different individuals from the same site can have lice with different genotypes. Indeed, the extensive movement of lice over the human body in populations with both head and body pediculosis makes collections from each louse ecotype rather difficult. An expanded use of microsatellites derived from the genome sequence of body lice was used to differentiate lice from 11 sites in four different geographic regions into geographic clusters [19]. The hope that the global movements of people and their lice have not completely obscured the evolution of lice and the origins of human populations was further enhanced by development of a qPCR assay that distinguished head and body lice from 13 countries based on the PHUM540560 gene, which was expressed differently in transcriptome studies of head and body lice [20], [21]. Although head lice cause medical and psychological problems in their own right, the hypothesis that body lice have emerged repeatedly from head louse populations compounds their potential epidemiological importance [2], [14].
Louse-Borne Pathogens
The incidence of louse-borne diseases has decreased in humans since the widespread availability of effective antibiotics and pediculicides. Louse-borne relapsing/recurrent fever (RF), caused by infection with Borrelia recurrentis, has persisted especially in parts of Africa, and it has the potential to infect travelers returning to Europe and North America from endemic regions [22]. Borrelia recurrentis has been detected recently in 23% of head lice in Ethiopia, but whether head lice serve as a vector is unknown [23]. Although the close genetic relationship between Borrelia duttonii and B. recurrentis has made their laboratory differentiation by qPCR difficult [24], the speculation that acquisition of B. duttonii by body lice could quickly give rise to new strains of B. recurrentis is uncertain considering the massive loss of protein coding capacity, plasmids, and plasmid rearrangements of the latter [25], [26].
Some other widespread pathogenic bacteria that can be transmitted to humans by other routes, such as Salmonella typhi and Serratia marcescens, have been detected in human body lice, and Acinetobacter baumannii in both head and body lice with the assumption that lice can probably also transmit these agents to humans [27], [28]. There are also experimental and natural observations that human lice are not refractory to Yersinia pestis, the causative agent of plague, and that they may be supplementary vectors of this agent [29].
Trench Fever
Bartonella quintana is a bacterium that causes trench fever in humans. It is transmitted by the body louse and possibly by the head louse [3], [9], [30]. Infected lice excrete B. quintana onto the skin while feeding, and the bacteria are either scratched into the skin or rubbed into mucous membranes. Historically, trench fever was described in troops in World War I, and again in World War II, but now it is emerging as a problem in urban homeless populations [3]. B. quintana has been documented in the homeless and associated body lice from France, the United States, the Netherlands, Ethiopia, Japan, Russia, and Mexico and in refugees, prisoners, and rural populations in Burundi, Rwanda, Zimbabwe, and Peru [30]. B. quintana has been found in head lice from homeless people without concurrent body lice infestation [31] as well as in head lice and body lice of different genotypes in Ethiopia [32]. Humans were thought to be the sole reservoir for B. quintana, but recently macaque monkeys and their lice, Pedicinus obtusus, have also been implicated [33], [34].
Epidemic (Louse-Borne) Typhus
Rickettsia prowazekii is associated with louse and human populations in parts of Africa, South America, and Asia [3]. There is no current circulation of this agent between body lice and humans evident in developed countries of Europe or the Americas. Outbreaks of primary louse-borne epidemic typhus still occur infrequently in Africa. Only sporadic cases of flying squirrel – and tick-associated cases occur in North America as well as rare cases of recrudescent Brill-Zinsser Disease worldwide. Head lice can transmit R. prowazekii under laboratory conditions (to naive rhesus macaques and rabbits), and it has been argued that this louse could also be involved in the transmission or maintenance of this pathogen in nature [35], although it has not been detected yet in head lice in nature. Various populations of head lice infesting school children worldwide have tested negative for R. prowazekii and/or B. quintana despite the presence of both pathogens in body lice from adults in these areas [30]. This potentially indicates the lack of pathogen transmission in pediatric populations or less than critical burdens of these pathogens in head lice.
Controlling Head and Body Lice Infestations and Related Diseases
At present, there are no commercial vaccines against louse-borne diseases of humans. Therefore, louse-borne disease suppression has typically involved elimination and control of lice and, secondarily, treatment of infected patients with doxycycline [9], [10], [22]. Single-dose oral administration of doxycycline is most effective in controlling epidemic typhus when permethrin dusting of clothing for louse control is not possible. Body louse infestation is typically associated with poor body and clothing hygiene and crowding, which enables close person-to-person contact that facilitates the spread of lice [10]. However, head louse infestations, especially in developed countries, generally have little to do with hygiene, the socio-economic status, or race of the individual, and most frequently affect children between three and 11 years old [11], [36]–[38].
Body louse infestation is diagnosed by finding eggs and crawling lice in the seams and button holes of clothing, and therefore can be controlled by laundering and heat treatment of clothing and wearing permethrin-impregnated clothing [3], [9], [10], [22], [23]. Head lice are frequently viewed as posing no substantial health risk to infested persons but constitute a social embarrassment to parents and children. However, head lice and microbial factors, which can commonly contribute to persistent manifestations including pruritus, head scratching, anemia, and even more severe symptoms, need further investigation. An astonishingly large number of insecticides (Table 2), herbal remedies, occlusive agents, and head lice repellents have been developed to augment physical (combing, vacuuming, heat) methods of louse and nit removal [36]–[38]. However, while pediculicide resistance to over-the-counter treatments, particularly to permethrin and other pyrthethroid derivatives, as well as to other highly efficacious treatments, which may require a prescription, is thought to be widespread, bioassays are difficult to standardize [39] and the correlation of results of genetic assays to ex vivo assays remains problematic [40], [41]. Ivermectin [42] and spinosad lotions appear to be the most promising new treatments, while new molecular approaches to assessments of resistance [20], [40], [43], [44] are making it easier to survey head louse populations for decreasing responsiveness to specific therapies. The challenges that the biology of lice pose for development of customer-friendly, safe, rapid, and effective chemical treatments for killing both live mobile lice and unhatched live eggs are daunting. Unfortunately, herbal remedies and mechanical means do not have the same requirements for measurement of efficacy. However, creative use of nanoparticle and silicone formulations as well as development of safe and effective means to kill head lice in situ offer some expectation that these difficulties will be surmounted [36]–[38].
Tab. 2. Therapeutic options for the treatment of pediculosis. 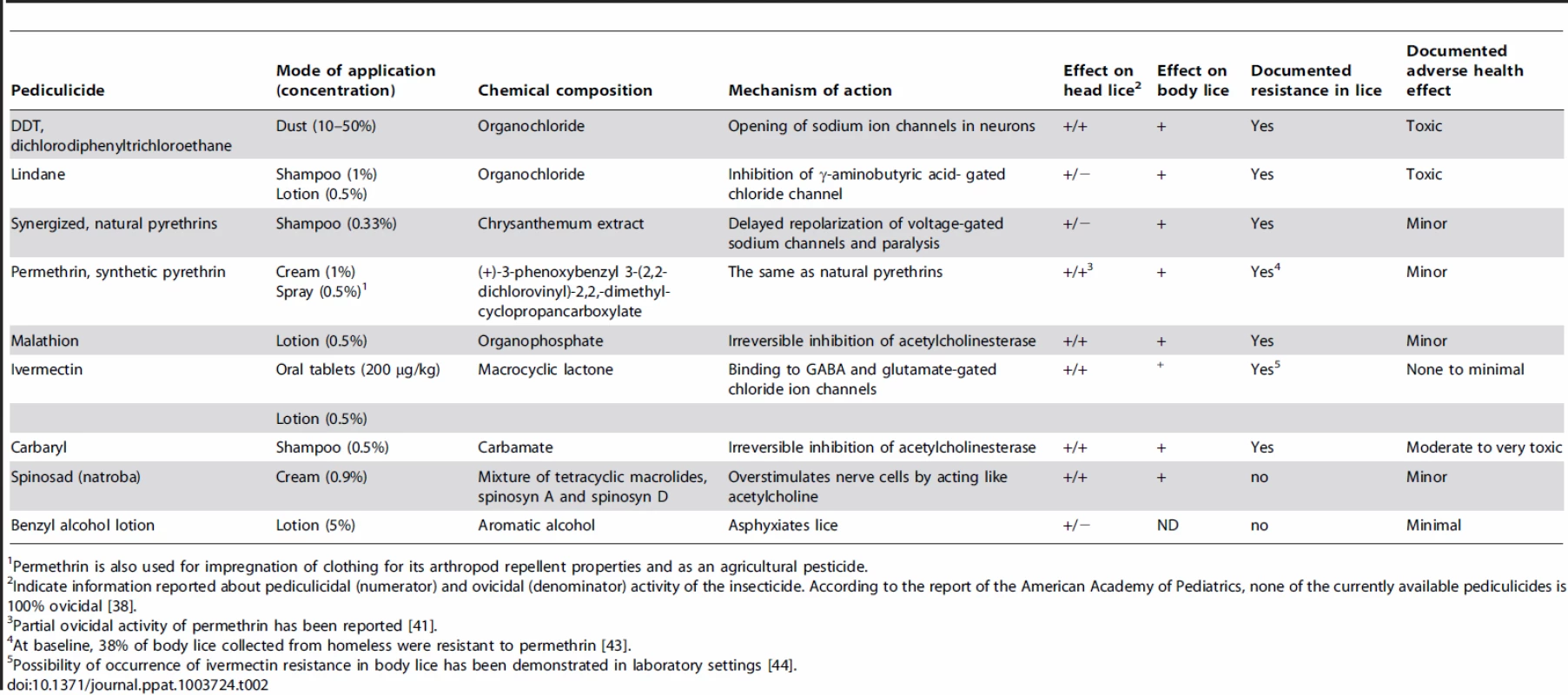
Permethrin is also used for impregnation of clothing for its arthropod repellent properties and as an agricultural pesticide. Concluding Remarks
In the 21st century, the prevalence of human louse infestation is still very high worldwide. New molecular tools have been developed and applied to head and body louse ecotypes and to the bacterial agents they transmit. Surprising and novel insights into the evolution of lice, their bacterial disease agents, and the epidemiology of louse-borne diseases have stimulated a renewal of interest in these arthropods. These discoveries may in turn provide new tools for improved understanding and control of these ancient and highly personal scourges of humans.
Zdroje
1. DurdenLA, MusserGG (1994) The sucking lice (Insecta: Anoplura) of the world: a taxonomic checklist with records of mammalian hosts and geographical distributions. Bull Am Mus Nat Hist 218 : 1–90.
2. LightJE, ToupsMA, ReedDL (2008) What's in a name: the taxonomic status of human head and body lice. Mol Phylogenet Evol 47 : 1203–1216.
3. VeracxA, RaoultD (2012) Biology and genetics of human head and body lice. Trends Parasitol 28 : 563–571.
4. PittendrighBR, ClarkJM, JohnstonJS, LeeSH, Romero-SeversonJ, et al. (2006) Sequencing of a new target genome: the Pediculus humanus humanus (Phthiraptera: Pediculidae) genome project. J Med Entomol 43 : 1103–1111.
5. KirknessEF, HaasBJ, SunW, BraigHR, PerottiMA, et al. (2010) Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc Natl Acad Sci U S A 107 : 12168–12173.
6. ShaoR, ZhuXQ, BarkerSC, HerdK (2012) Evolution of extensively fragmented mitochondrial genomes in the lice of humans. Genome Biol Evol 4 : 1088–1101.
7. HipolitoRB, MallorcaFB, Zuniga-MacaraigZO, ApolinarioPC, Wheeler-ShermanJ (2001) Head lice infestation: single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics 107: e30.
8. PerottiMA, AllenJM, ReedDL, BraigHR (2007) Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J 21 : 1058–1066.
9. BrouquiP (2011) Arthropod-borne diseases associated with political and social disorder. Annu Rev Entomol 56 : 357–374.
10. BadiagaS, RaoultD, BrouquiP (2008) Preventing and controlling emerging and reemerging transmissible diseases in the homeless. Emerg Inf Dis 14 : 1353–1359.
11. FalagasME, MathaiouDK, RafailidisPI, PanosG, PappasG (2008) Worldwide prevalence of head lice. Emerg Inf Dis 14 : 1493–1494.
12. LightJE, AllenJM, LongLM, CarterTE, BarrowL, et al. (2008) Geographic distributions and origins of human head lice (Pediculus humanus capitis) based on mitochondrial data. J Parasitol 94 : 1275–1281.
13. LeoNP, BarkerSC (2005) Unravelling the evolution of the head lice and body lice of humans. Parasitol Res 98 : 44–47.
14. LiW, OrtizG, FournierP-E, GimenezG, ReedDL, et al. (2010) Genotyping of human lice suggests multiple emergences of body lice from local head louse populations. PLoS Negl Trop Dis 4: e641 doi:10.1371/journal.pntd.0000641
15. BoutellisA, VeracxA, AbrahãoJ, RaoultD (2013) Amazonian head lice-specific genotypes are putatively pre-Columbian. Am J Trop Med Hyg 88 : 1180–1184.
16. VeracxA, BoutellisA, MerhejV, DiattaG, RaoultD (2012) Evidence for an African cluster of human head and body lice with variable colors and interbreeding of lice between continents. PLoS ONE 7: e37804 doi:10.1371/journal.pone.0037804
17. LeoNP, HughesJM, YangX, PoudelSK, BrogdonWG, et al. (2005) The head and body lice of humans are genetically distinct (Insecta: Phthiraptera, Pediculidae): evidence from double infestations. Heredity 95 : 34–40.
18. VeracxA, RivetR, McCoyKD, BrouquiP, RaoultD (2012) Evidence that head and body lice on homeless persons have the same genotype. PLoS ONE 7: e45903 doi:10.1371/journal.pone.0045903
19. AscunceMS, ToupsMA, KassuG, FaneJ, SchollK, et al. (2013) Nuclear genetic diversity in human lice (Pediculus humanus) reveals continental differences and high inbreeding among worldwide populations. PLoS ONE 8: e57619 doi:10.1371/journal.pone.0057619
20. OldsBP, CoatesBS, SteeleLD, SunW, AgunbiadeTA, et al. (2012) Comparison of the transcriptional profiles of head and body lice. Insect Mol Biol 21 : 257–268.
21. DraliR, BoutellisA, RaoultD, RolainJM, BrouquiP (2013) Distinguishing body lice from head lice by multiplex real-time PCR analysis of the Phum_PHUM540560 gene. PLoS ONE 8: e58088 doi:10.1371/journal.pone.0058088
22. Durden LA, Lloyd JE (2009) Lice (Phthiraptera). In: Mullen GR, Durden LA, editors. Medical and veterinary entomology. 2nd edition. Amsterdam: Elsevier. pp. 59–82.
23. BoutellisA, MediannikovO, BilchaKD, AliJ, CampeloD, et al. (2013) Borrelia recurrentis in head lice, Ethiopia. Emerg Infect Dis 19 : 796–798 doi:10.3201/eid1905.121480
24. ElbirH, HenryM, DiattaG, MediannikovO, SokhnaC, et al. (2013) Multiplex real-time PCR diagnostic of relapsing fevers in Africa. PLoS Negl Trop Dis 7: e2042 doi:10.1371/journal.pntd.0002042
25. ElbirH, GimenezG, SokhnaC, BilchaKD, AliJ, et al. (2012) Multispacer sequence typing relapsing fever borreliae in Africa. PLoS Negl Trop Dis 6: e1652 doi:10.1371/journal.pntd.0001652
26. LescotM, AudicS, RobertC, NguyenTT, BlancG, et al. (2008) The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet 4: e1000185 doi:10.1371/journal.pgen.1000185
27. La ScolaB, FournierP-E, BrouquiP, RaoultD (2001) Detection and culture of Bartonella quintana, Serratia marcescens, and Acinetobacter spp. from decontaminated human body lice. J Clin Microbiol 39 : 1707–1709.
28. BouvresseS, SocolovshiC, BerdjaneZ, DurandR, IzriA, et al. (2011) No evidence of Bartonella quintana but detection of Acinetobacter baumannii in head lice from elementary schoolchildren in Paris. Comp Immunol Microbiol Infect Dis 34 : 475–477.
29. HouhamdiL, LepidiH, DrancourtM, RaoultD (2006) Experimental model to evaluate the human body louse as a vector of plague. J Infect Dis 194 : 1589–1596.
30. FournierP-E, NdihokubwayoJB, GuidranJ, KellyP, RaoultD (2002) Human pathogens in body and head lice. Emerg Inf Dis 8 : 1515–1518.
31. BonillaDL, KabeyaH, HennJ, KramerV, KosoyMY (2009) Bartonella quintana in body and head lice collected from homeless persons, San Francisco, California, USA. Emerg Inf Dis 5 : 912–915.
32. AngelakisE, DiattaG, AbdissaA, TrapeJF, MediannikovO, et al. (2011) Altitude-dependent Bartonella quintana genotype C in head lice, Ethiopia. Emerg Infect Dis 17 : 2357–2359.
33. LiH, BaiJY, WangLY, ZengL, ShiYS, et al. (2013) Genetic diversity of Bartonella quintana in macaques suggests zoonotic origin of trench fever. Mol Ecol 22 : 2118–2127.
34. LiH, LiuW, ZhangGZ, SunZZ, BaiJY, et al. (2013) Transmission and maintenance cycle of Bartonella quintana among rhesus macaques, China. Emerg Infect Dis 19 : 297–300.
35. RobinsonD, LeoN, ProcivP, BarkerSC (2003) Potential role of head lice, Pediculus humanus capitis, as vectors of Rickettsia prowazekii. Parasitol Res 90 : 209–211.
36. FeldmeierH (2012) Pediculosis capitis: new insights into epidemiology, diagnosis and treatment. Eur J Clin Microbiol Infect Dis 31 : 2105–2110.
37. NutansonI, SteenCJ, SchwartzRA, JannigerCK (2008) Pediculus humanus capitis: an update. Acta Derrmatoven PVA 17 : 147–159.
38. FrankowskiBL, BocchiniJAJr (2010) Council on School Health and Committee on Infectious Diseases (2010) Head lice. Pediatrics 126 : 392–403.
39. BarkerSC, BurgessI, MeinkingTL, MumcuogluKY (2012) International guidelines for clinical trials with pediculicides. Int J Dermatol 51 : 853–858.
40. BouvresseS, BerdjaneZ, DurandR, BouscaillouJ, IzriA, et al. (2012) Permethrin and malathion resistance in head lice: results of ex vivo and molecular assays. J Am Acad Dermatol 67 : 1143–1150.
41. DurandR, BouvresseS, BerdjaneZ, IzriA, ChosidowO, et al. (2012) Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect 18 : 338–344.
42. ChosidowO, GiraudeauB, CottrellJ, IzriA, HofmannR, et al. (2010) Oral ivermectin versus malathion lotion for difficult-to-treat head lice. N Engl J Med 362 : 896–905.
43. DraliR, BenkouitenS, BadiagaS, BitamI, RolainJM, et al. (2012) Detection of a knockdown resistance mutation associated with permethrin resistance in the body louse Pediculus humanus corporis by use of melting curve analysis genotyping. J Clin Microbiol 50 : 2229–2233.
44. YoonKS, StrycharzJP, BaekJH, SunW, KimJH, et al. (2011) Brief exposures of human body lice to sublethal amounts of ivermectin over-transcribes detoxification genes involved in tolerance. Insect Mol Biol 20 : 687–699.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Baculoviruses: Sophisticated Pathogens of Insects
- How Do Viruses Avoid Inhibition by Endogenous Cellular MicroRNAs?
- The Regulation of Trypanosome Gene Expression by RNA-Binding Proteins
- DNA Damage Repair and Bacterial Pathogens
- Disease to Dirt: The Biology of Microbial Amyloids
- Fungal Immune Evasion in a Model Host–Pathogen Interaction: Versus Macrophages
- Infectious Prions Accumulate to High Levels in Non Proliferative C2C12 Myotubes
- The Biology and Taxonomy of Head and Body Lice—Implications for Louse-Borne Disease Prevention
- Antibodies Trap Tissue Migrating Helminth Larvae and Prevent Tissue Damage by Driving IL-4Rα-Independent Alternative Differentiation of Macrophages
- The Effects of Somatic Hypermutation on Neutralization and Binding in the PGT121 Family of Broadly Neutralizing HIV Antibodies
- Natural Selection Promotes Antigenic Evolvability
- Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung
- Mode of Parainfluenza Virus Transmission Determines the Dynamics of Primary Infection and Protection from Reinfection
- Type I and Type III Interferons Drive Redundant Amplification Loops to Induce a Transcriptional Signature in Influenza-Infected Airway Epithelia
- Unraveling a Three-Step Spatiotemporal Mechanism of Triggering of Receptor-Induced Nipah Virus Fusion and Cell Entry
- A Novel Membrane Sensor Controls the Localization and ArfGEF Activity of Bacterial RalF
- Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity
- Global Rescue of Defects in HIV-1 Envelope Glycoprotein Incorporation: Implications for Matrix Structure
- Turning Defense into Offense: Defensin Mimetics as Novel Antibiotics Targeting Lipid II
- The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells
- Brd4 Is Displaced from HPV Replication Factories as They Expand and Amplify Viral DNA
- A Viral Genome Landscape of RNA Polyadenylation from KSHV Latent to Lytic Infection
- The Cytotoxic Necrotizing Factor of (CNF) Enhances Inflammation and Yop Delivery during Infection by Activation of Rho GTPases
- The Inflammatory Kinase MAP4K4 Promotes Reactivation of Kaposi's Sarcoma Herpesvirus and Enhances the Invasiveness of Infected Endothelial Cells
- Conservative Sex and the Benefits of Transformation in
- Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior
- Colonization Resistance: Battle of the Bugs or Ménage à Trois with the Host?
- Intracellular Interferons in Fish: A Unique Means to Combat Viral Infection
- SPOC1-Mediated Antiviral Host Cell Response Is Antagonized Early in Human Adenovirus Type 5 Infection
- Involvement of the Cellular Phosphatase DUSP1 in Vaccinia Virus Infection
- Killer Bee Molecules: Antimicrobial Peptides as Effector Molecules to Target Sporogonic Stages of
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- A Role for Host Activation-Induced Cytidine Deaminase in Innate Immune Defense against KSHV
- Haploid Genetic Screens Identify an Essential Role for PLP2 in the Downregulation of Novel Plasma Membrane Targets by Viral E3 Ubiquitin Ligases
- A Small Molecule Glycosaminoglycan Mimetic Blocks Invasion of the Mosquito Midgut
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- Can Non-lytic CD8+ T Cells Drive HIV-1 Escape?
- Deletion of the α-(1,3)-Glucan Synthase Genes Induces a Restructuring of the Conidial Cell Wall Responsible for the Avirulence of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Baculoviruses: Sophisticated Pathogens of Insects
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- Natural Selection Promotes Antigenic Evolvability
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



