-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Deletion of the α-(1,3)-Glucan Synthase Genes Induces a Restructuring of the Conidial Cell Wall Responsible for the Avirulence of
α-(1,3)-Glucan is a major component of the cell wall of Aspergillus fumigatus, an opportunistic human fungal pathogen. There are three genes (AGS1, AGS2 and AGS3) controlling the biosynthesis of α-(1,3)-glucan in this fungal species. Deletion of all the three AGS genes resulted in a triple mutant that was devoid of α-(1,3)-glucan in its cell wall; however, its growth and germination was identical to that of the parental strain in vitro. In the experimental murine aspergillosis model, this mutant was less pathogenic than the parental strain. The AGS deletion resulted in an extensive structural modification of the conidial cell wall, especially conidial surface where the rodlet layer was covered by an amorphous glycoprotein matrix. This surface modification was responsible for viability reduction of conidia in vivo, which explains decrease in the virulence of triple agsΔ mutant.
Published in the journal: . PLoS Pathog 9(11): e32767. doi:10.1371/journal.ppat.1003716
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003716Summary
α-(1,3)-Glucan is a major component of the cell wall of Aspergillus fumigatus, an opportunistic human fungal pathogen. There are three genes (AGS1, AGS2 and AGS3) controlling the biosynthesis of α-(1,3)-glucan in this fungal species. Deletion of all the three AGS genes resulted in a triple mutant that was devoid of α-(1,3)-glucan in its cell wall; however, its growth and germination was identical to that of the parental strain in vitro. In the experimental murine aspergillosis model, this mutant was less pathogenic than the parental strain. The AGS deletion resulted in an extensive structural modification of the conidial cell wall, especially conidial surface where the rodlet layer was covered by an amorphous glycoprotein matrix. This surface modification was responsible for viability reduction of conidia in vivo, which explains decrease in the virulence of triple agsΔ mutant.
Introduction
α-(1,3)-Glucan is a major cell wall component of most ascomycetous and basidiomycetous fungi, including the human pathogens that establish their disease upon inhalation of their infective morphotypes (e.g., Paracoccidioides brasilisensis, Histoplasma capsulatum, Blastomyces dermatitidis, Cryptococcus neoformans Aspergillus fumigatus). The role of this polysaccharide during infection has been demonstrated and the mechanisms of its involvement in establishing virulence have been forwarded [1], [2]. In C. neoformans, α-(1,3)-glucan anchors the capsule, a well known virulence factor of this fungus, to the yeast cell wall and has been shown to be indirectly associated with virulence since a mutant devoid of α - (1,3)-glucan did not have any capsule and, most importantly, was unable to grow at 37°C [2]. In the yeast H. capsulatum, α-(1,3)-glucan was suggested to be essential for virulence because it masked immunogenic molecules: in the α-(1,3)-glucan synthase mutant, β-(1,3)-glucan that is recognized by Dectin-1, is exposed at the surface of the cell wall, whereas in the parental strain yeast cells, β-(1,3)-glucan is covered by α-(1,3)-glucan, preventing Dectin1-dependent immune response [1].
In A. fumigatus, α-(1,3)-glucan accounts for 40% and 19% of the mycelial and conidial cell wall polysaccharides, respectively [3]. It is a major adhesive involved in the aggregation of germinating conidia and in biofilm formation [4], [5]. Moreover, it has been shown in experimental murine aspergillosis models that α-(1,3)-glucan has a prominent immunological function conferring a long-term survival [6]. This immune protection was associated with a reduced neutrophil recruitment in the lungs and reduced inflammatory pathology [6]. α-(1,3)-glucan, like conidia, confers a Th1/Treg protection and concomitant Th2 inhibition. These in vivo data were confirmed by in vitro experiments where dendritic cells pulsed with α-(1,3)-glucan induced Il12p70 production, a classical Th1 promoting cytokine [6]. However, the physiological role of α-(1,3)-glucan could not be further investigated in absence of the mutants devoid of α-(1,3)-glucan. In A. fumigatus, this polysaccharide is synthesized by three α-(1,3)-glucan synthases (Agsp) [3], [7]. A triple deletion of the AGS1, AGS2 and AGS3 genes was recently generated in our lab that resulted in an A. fumigatus mutant lacking α-(1,3)-glucan in the cell wall. In contrast to other fungal pathogens, this triple AGS A. fumigatus deletion mutant did not show a distinct growth phenotype in vitro [8].
In the present study, three independently constructed triple ags1Δags2Δags3Δ (agsΔ) mutants devoid of α-(1,3)-glucan were used to investigate the role of α-(1,3)-glucan in A. fumigatus infection. As shown here, the virulence of these A. fumigatus triple agsΔ mutants was extremely attenuated in both immunocompetent and immunocompromised murine models of experimental aspergillosis tested. The defect in virulence correlated with a lack of vegetative fungal dissemination in the lungs, associated with a highly reduced inflammation following conidial inoculation. Analysis of the conidia of the triple mutants showed that the lack of virulence of the mutants in vivo was associated to major changes occurring on the cell wall, especially on the surface of the resting and swollen conidia, which resulted in an increased killing by phagocytes.
Results
The ags1Δags2Δags3Δ (agsΔ) mutants are less virulent than the parental strain in murine model of aspergillosis
In the immunocompetent mice after four days of infection, the number of CFUs of the agsΔ mutants per lung was much lower than the CFUs per lung of the parental ku80 strain (Fig. 1A; Fig. S1A). The reduced fungal burden of agsΔ was correlated to an absence of inflammation whereas a huge inflammatory response was observed with the parental strain (Fig. 1B, Fig. S1B). This was confirmed by the broncho-alveolar lavage (BAL) analysis, which showed a higher PMN recruitment after infection with ku80 conidia compared with agsΔ (Fig. 1C, Fig. S1C). The reduced growth and inflammation in agsΔ infections was associated with an increase in the expression of the gene coding for the anti-inflammatory IL10 and a decreased expression of the gene coding for the pro-inflammatory TNFα in the lungs (Fig. 1D, Fig. S1D). In contrast, ku80 infection was characterized by higher and lower expressions of TNFα and IL10, respectively.
Fig. 1. Immunocompetent mice infected with resting conidia of agsΔ_5T and parental (ku80) strains. 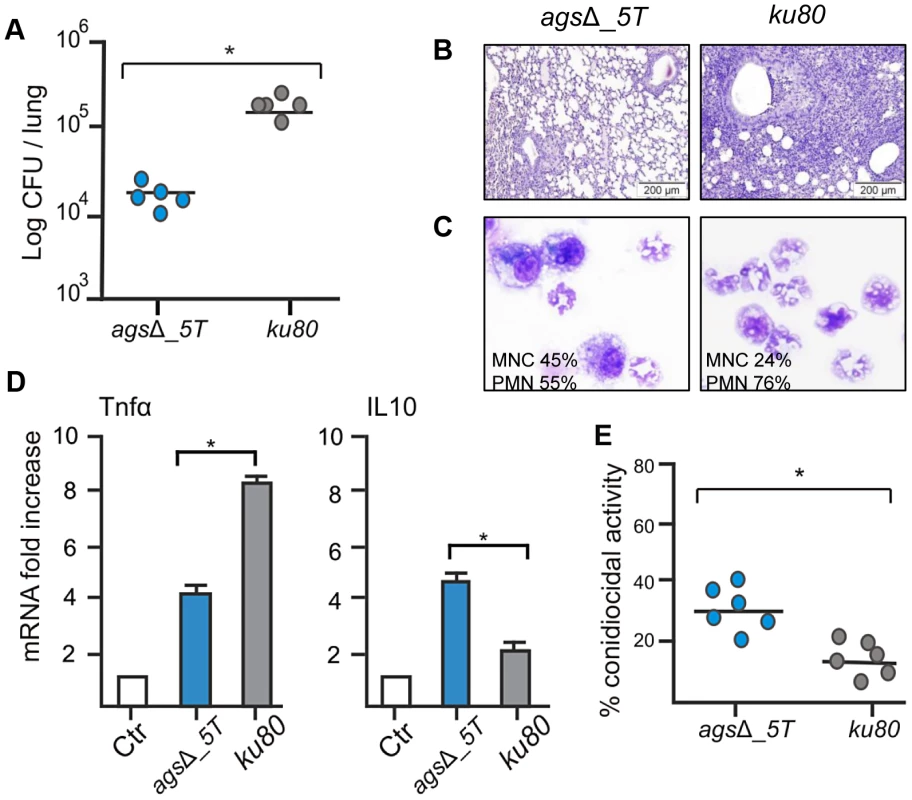
Observations and analysis on mice were done four days post-infection. (A) Fungal load was expressed as log10 CFU/lung. (B) Lung histology (periodic acid-Schiff-staining). Note the polymorphonuclear cells and mononuclear infiltrates surrounding the bronchi in ku80 infected lung. (C) After infection, percentages of monocytes and polymorphonuclear cells found in the lungs alveolar lavage (BAL). (D) Relative expression of TNFα and IL10 assessed by real time RT-PCR on lung total RNA from naïve and infected mice. (E) Conidiocidal activity by purified macrophages from uninfected mice expressed in percentage of CFU inhibition after 2 h incubation of the conidia with macrophages. Data are representative of at least three independent experiments. Ctl, naïve mice; *, P<0.05. The increased susceptibility of the agsΔ mutants was confirmed in vitro with murine alveolar macrophages isolated from BAL. After phagocytosis by the isolated macrophages, the killing of the agsΔ conidia was much higher than the parental strain. The resting conidia of agsΔ mutants were killed twice more than the parental strain after 2 h incubation with the macrophages (Fig. 1E). Further, after 6 h of incubation, the killing of the mutant reached 60–80% whereas a maximum of 30% of the parental strain conidia were killed at this time point (data not shown). Similar difference in the killing ratio between the mutant and parental strains was obtained when the conidia were pre-germinated (swollen conidia; after 6½ h incubation of the conidia in RPMI medium, at 37°C), suggesting that both resting and swollen conidia of the agsΔ mutants were more susceptible to conidial killing than the parental strain. This twofold increased killing susceptibility of the agsΔ mutants compared to parental strain did not change in the germinating morphotypes.
In the experimental model of aspergillosis using immunocompromised mice, the virulence of the agsΔ mutants was also significantly reduced. In a cyclophosphamide model of immuno-suppression, infection with the ku80 strain resulted in the mortality of all the mice within 4 days with a high inflammatory response, large foci of pneumonia and exudative bronchiolitis with destruction of bronchi and alveoli, whereas 60 to 80% mice infected by the agsΔ mutants survived and did not develop any inflammatory response (Fig. 2A–C, Fig. S2). Similar results were obtained when mice were immunocompromised by the injection of the RB6-8C5 MAb, which depletes circulating PMNs. Inhalation of the ku80 conidia resulted in an extensive pulmonary fungal invasion with high inflammation (Fig. 2D–E). In contrast, in the RB6-8C5 MAb-treated mice lungs, only resting and swollen agsΔ conidia were observed and their incapability to grow vegetatively culminated in low inflammation (Fig. 2D–E). These results showed that the reduced virulence of the agsΔ mutant was due to a defect in their conidial survival or vegetative growth in the lung of the infected mice.
Fig. 2. Cyclophosphamide immunosuppressed mice and anti-Ly6G treated neutropenic mice infected with resting conidia of agsΔ_5T and parental (ku80) strains. 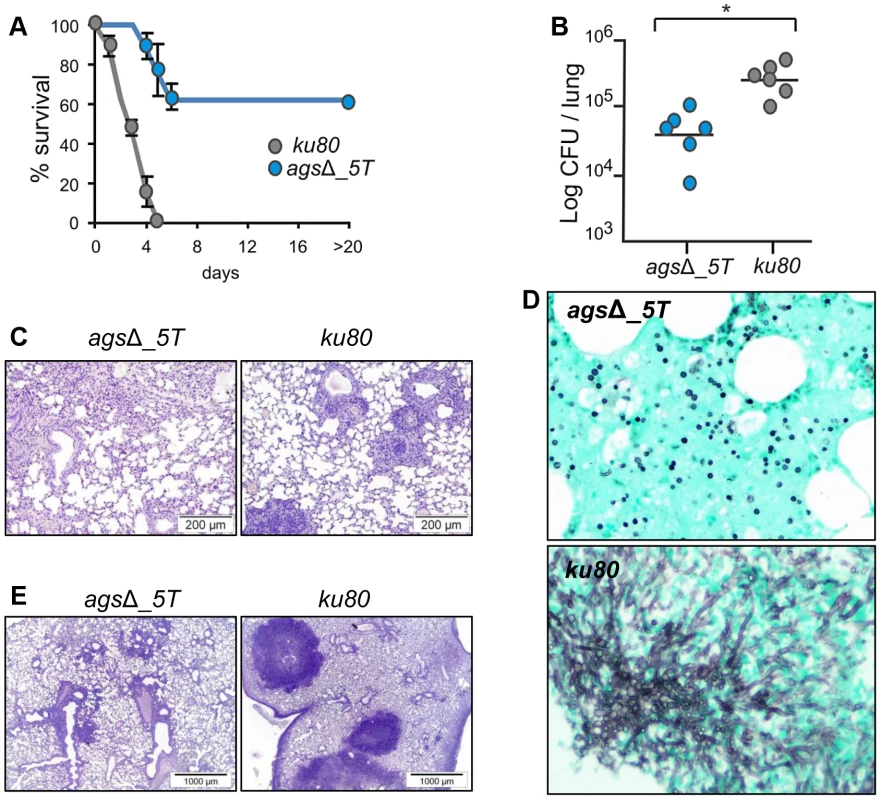
(A–C) Cyclophosphamide immunosuppressed mice; (D–E) anti-Ly6G treated neutropenic mice; (A) Survival (%) and (B) fungal growth estimated as CFUs in lung. (C and E) lung histology (periodic acid-Schiff-staining). Note the polymorphonuclear cells and mononuclear infiltrates surrounding the bronchi in ku80 infected lung. (D) Histological appearance of lungs of anti-Ly6G neutropenic mice infected with conidia of agsΔ_5T and ku80 (Gomori's methanamine silver-staining). Note the absence of mycelial development of agsΔ_5T conidia in neutropenic mice. Data are representative of at least three independent experiments. *: p<0.05. Susceptibility of the agsΔ and parental strain conidia to antifungal molecules is similar
To investigate the mechanisms responsible for the in vivo growth defect, the germination of agsΔ mutant conidia was tested in vitro under stress conditions mimicking the in vivo environment, such as, in the presence of reactive oxidants (ROS), cationic peptides, hypoxia and depletion of iron. The agsΔ mutants showed similar growth rates as their parental strain in the presence of Menadione, hydrogen peroxide and Luperox®101 with minimum inhibitory concentrations (MIC) of 30 µM, 10 mM and 2 µM, respectively (data not shown) irrespective of the pH of the medium (pH 7 or 4). The killing of resting conidia after 2–6 h of incubation with macrophages purified from uninfected p47phox−/− mice (depleted in ROS production) were similar to the killing by purified macrophages from uninfected wild type mice (C57BL6 H-2b) (Fig. 2B, data not shown for 6 h and Fig. 3). These results suggested that the agsΔ mutant conidia were not more susceptible than the parental strain conidia to reactive oxidants in vitro as well as in vivo. Interestingly, these results also suggested that in our experimental models, conidia from both mutant and parental strains were efficiently killed by ROS-independent mechanisms. Moreover, the absence of iron or the presence of a hypoxic environment did not modify the survival and conidial germination of agsΔ mutants compared to their parental strain (data not shown). In vitro, the agsΔ conidia germinated like parental strain conidia in culture medium without supplementation with iron as well as under hypoxic conditions (<1% (v/v) O2 and 9–13% (v/v) CO2). The agsΔ mutants were not more susceptible than the parental strain to cationic peptides. At doses of 230, 100, 40 and 230 µg/ml of Cathelicidin LL-37, α HNP2 and β hBD2 defensins and lactoferrin, respectively, no germination differences were seen between parental and mutant strains (data not shown). Similarly, both mutant and parental strain conidial killing was comparable with 0.05% SDS (data not shown). In addition, no increase in the intracellular labeling of the agsΔ mutant conidia was seen after incubation with Calcofluor White or FITC (data not shown). These results suggested that the agsΔ conidia were not more permeable to extracellular toxic molecules than the parental strain. Testing of these different inhibitors in combination (such as H2O2 or SDS, with Lactoferrin or LL-37) did not result in a differential sensitivity between the parental and mutant strains (data not shown).
Fig. 3. Conidiocidal activity of macrophages isolated from uninfected p47phox−/− mice against resting conidia of agsΔ_5T and parental (ku80) strains. 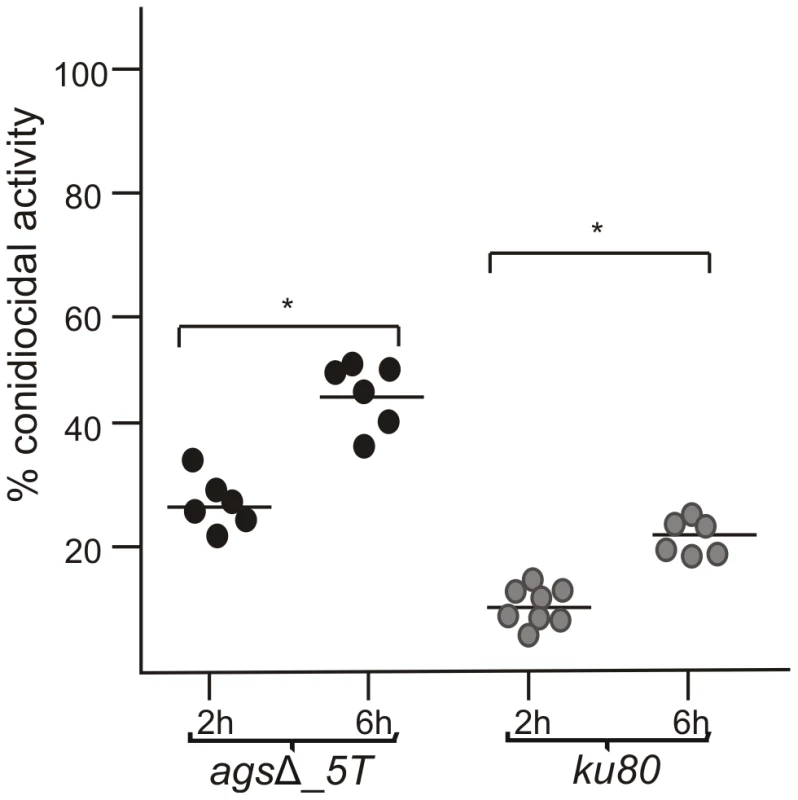
Conidiocidal activity is expressed in percentage of CFU inhibition after 2 and 6 h incubation of the conidia with macrophages. Data are representative from at least three independent experiments. *, P<0.05. These results suggested that, in vitro, the triple agsΔ mutants were not more susceptible to environmental stresses and antifungal molecules compared to the parental strain. To further investigate the differences in virulence between the mutant and parental strains in vivo, we hypothesize that the killing of the agsΔ mutant conidia could be due to the induction of an early and strong host immune response towards the mutant conidial morphotypes.
The resting conidia of the agsΔ mutants are immediately recognized by the innate immune system because the surface rodlet layer is masked by a layer of glycoproteins
Resting conidia of the agsΔ mutant were more efficiently phagocytosed by mouse alveolar macrophages than that of the parental ku80 strain. After 1 h incubation, an average of 3.4 and 1.4 conidia of agsΔ mutants and ku80 were engulfed per macrophage, respectively (Fig. 4, Fig. S3). This result suggested that the agsΔ mutant and parental strain conidial surfaces are different. To investigate such structural modifications, conidial surfaces were imaged by atomic force microscopy (AFM). In contrast to the ku80 conidia that are covered with a crystalline-like array of rodlets [9], the agsΔ mutant conidial surface was amorphous without any organized structure (Fig. 5A). The presence of an amorphous material covering the surface of the agsΔ conidia was further confirmed by TEM (Fig. 5B).
Fig. 4. Phagocytosis activity by isolated macrophages from uninfected mice against resting conidia of agsΔ_5T and parental (ku80) strains. 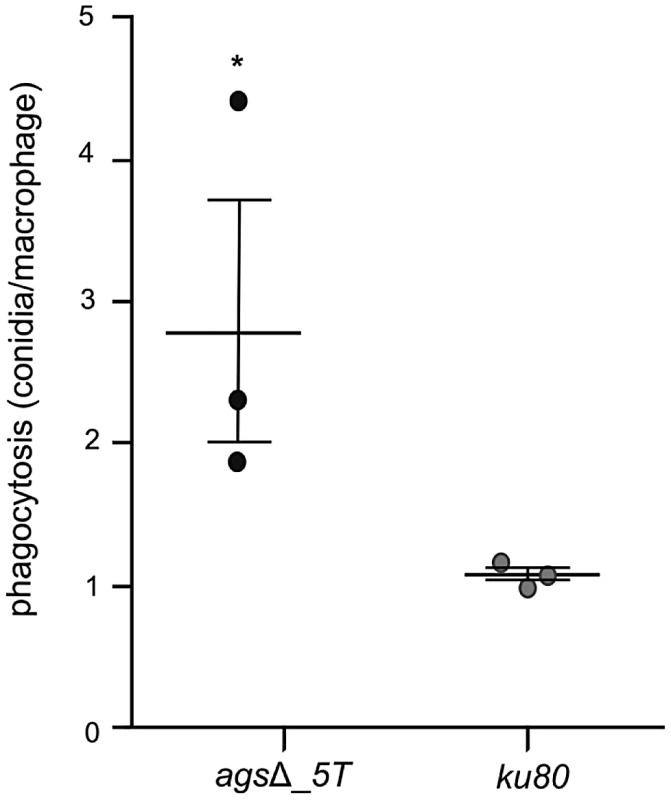
Index of phagocytosis is expressed in number of conidia per alveolar macrophage after 1*, P<0.05. Fig. 5. Surface analysis of resting conidia of agsΔ_5T mutant and parental (ku80) strains. 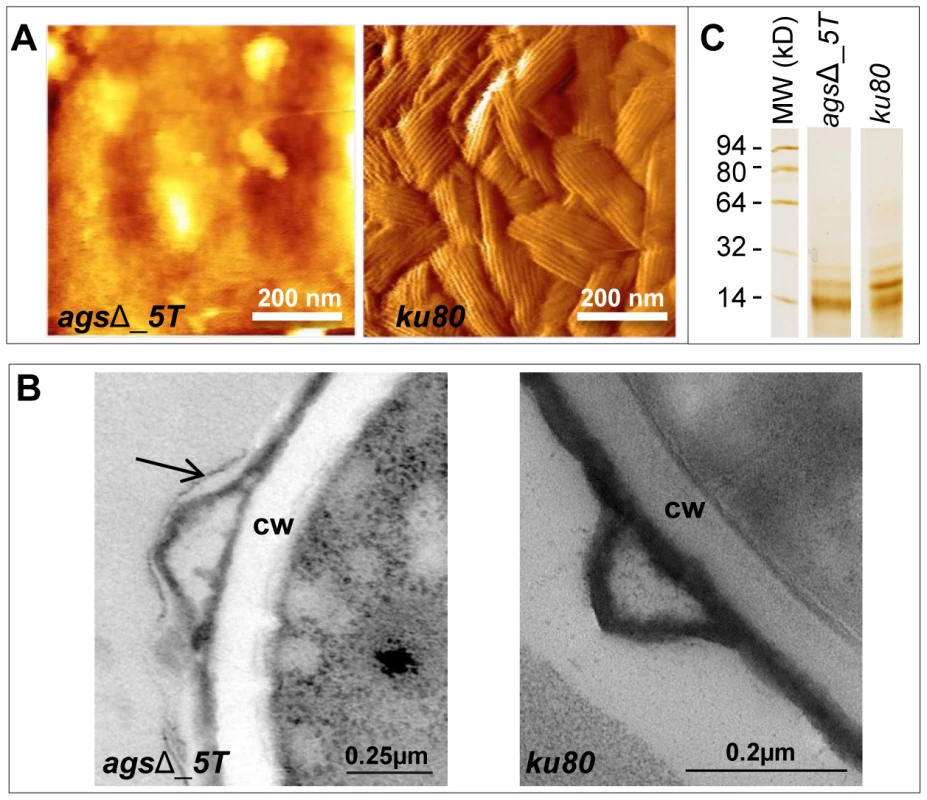
(A): height images (z-range = 1 µm; recorded in water with silicon nitride tips). Atomic Force Microscopy (AFM) images showing the amorphous surface without the rodlet layer on the triple agsΔ_5T mutant conidia whereas the rodlet are observed on the parental strain conidial surface. (B): TEM observations. Note the presence of an extracellular material on the surface of the agsΔ_5T conidia (arrow); CW: cell wall. (C): SDS-PAGE (15% gel) of Hydrofluoric acid (HF) extracts of rodlets from resting conidia showing the two bands, 16 kDa and 14.5 kDa of RodAp classically seen from HF treatment of the conidia [10]. Data are representative of at least three independent experiments. To investigate if the rodlet layer is still present on the agsΔ mutant conidial surface but masked by this amorphous material, ku80 and agsΔ resting conidia were treated with hydrofluoric acid (HF) to extract the rodlet protein. Similar amount of the hydrophobic RodA protein, which constitutes the rodlet layer, could be extracted from the agsΔ and parental strain conidia (26.7±4.9 µg and 26.5±3.0 µg per 109 conidia, respectively). Figure 5C shows that the two bands, 16 kDa and 14.5 kDa of RodAp classically seen from HF treatment of the conidia [10] were present in the SDS-PAGE profiles of agsΔ and ku80 resting conidial HF-extracts. These data confirmed AFM and TEM observations that on the agsΔ mutant conidial surface the rodlets were present but hidden by an amorphous material.
Because of the presence of this amorphous material covering the hydrophobic rodlets, we asked whether the observed surface changes correlated with differences in conidial adhesive properties. To understand this, we mapped and quantified the nanoscale adhesion properties of ku80 and agsΔ mutant conidia by AFM using bare Si3N4 tip. Figure 6 (and Fig. S4) showed that the presence of this unorganized material on the agsΔ mutant conidial surface was associated with a dramatic reduction in their conidial surface adhesive properties. For the parental strain, force-distance curves recorded across the cell surface revealed large adhesion forces, with a magnitude of 0.6±0.039 nN as shown by the adhesion force histogram (Fig. 6A–C). In contrast, structural changes in agsΔ conidia caused profound modifications of the cell surface physico-chemical properties (Fig. 6D–F, Fig. S4). Force-distance curves showed the absence of adhesion forces over the entire surface of the mutant conidia. This decrease in the agsΔ conidial adhesion capacities indicated a modification of the cell surface hydrophobicity that could have influenced conidial phagocytosis.
Fig. 6. Imaging and adhesive properties of A. fumigatus resting conidia of the parental strain and agsΔ_5T mutant. 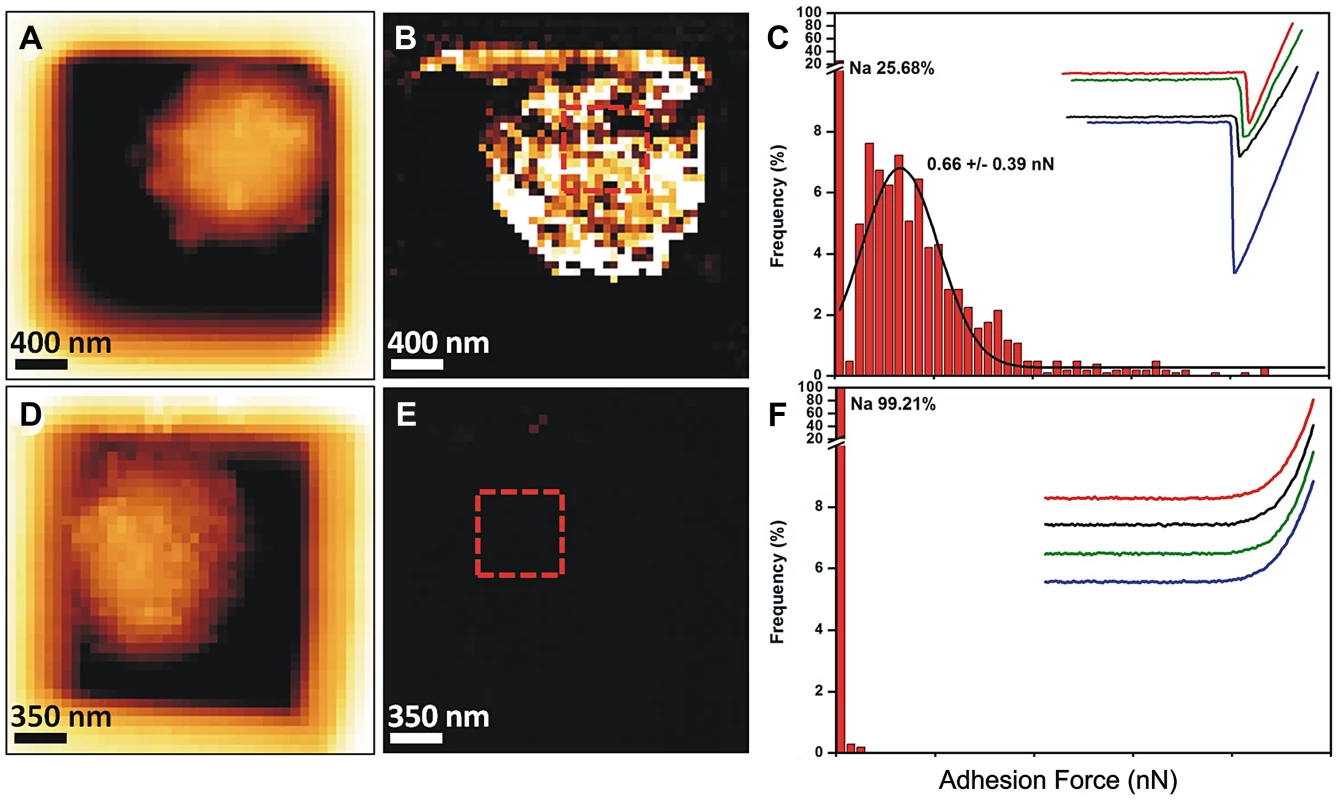
Structural changes of agsΔ_5T correlate with a loss of cell surface adhesive properties. (A–C) parental strain; (D–F) agsΔ_5T mutant; (A, D) height images (z-range = 1 µm; recorded in water with silicon nitride tips); (B, E) adhesion force maps (z-range: 5 nN) corresponding to the height image; (C, F) Representative force-distance curves and adhesion force histograms (n = 1024) recorded on the surface of parental strain (C) and agsΔ_5T (F). Further, chemical nature of the amorphous layer present on the agsΔ mutant conidial surface was investigated. It was not composed of polysaccharides since the labeling of β-(1,3)-glucan with the β-(1,3)-glucan receptor GNBP3, chitin with WGA, galactomannan (GM) with an anti-GM monoclonal antibody and galactosaminogalactan (GAG) with an anti-GAG monoclonal antibody were negative (data not shown). In contrast, a strong labeling of the resting agsΔ conidium with ConA was observed suggesting that the surface layer was rich in glyco-conjugates (Fig. 7).
Fig. 7. ConA-FITC labeling of agsΔ_5T mutant and parental strain (ku80) resting conidia. 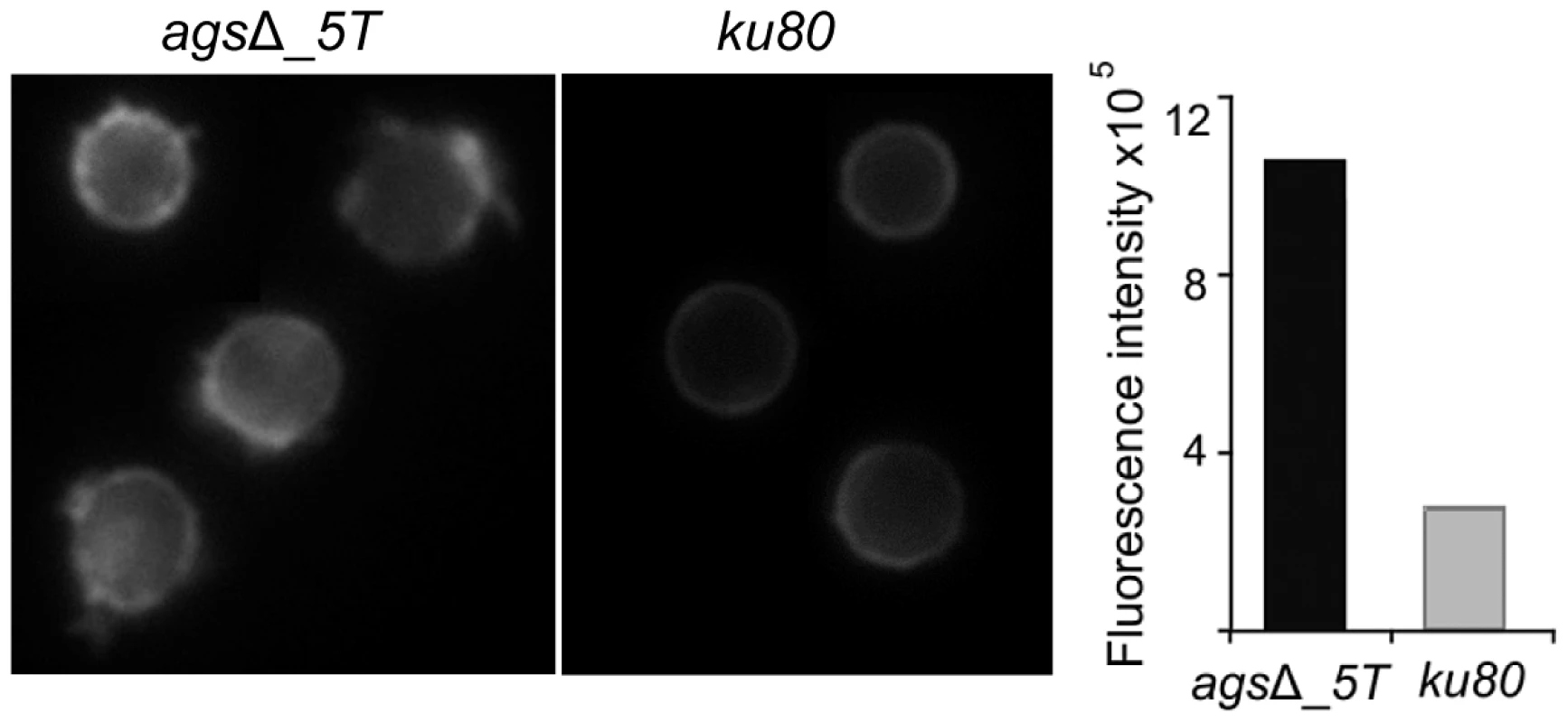
Note the increase in the ConA labeling on the agsΔ_5T mutant conidial surface. Histograms represent the calculated fluorescence intensity of the corresponding images, expressed in Einstein per seconds. To extract these amorphous surface materials, agsΔ resting conidia were incubated in 0.5 M NaCl for 2 h and the extracted materials were positive for protein assay. As shown in the Figure 8 (and Fig. S5), incubation with NaCl did not release any proteins from the parental ku80 strain whereas the extracts from agsΔ mutant conidia contained 160 µg proteins per 1010 conidia. It was verified that the amorphous glycoprotein layer was removed after NaCl treatment because ConA labeling on the conidia after NaCl treatment was negative (data not shown). Further, extracted protein mixture was subjected to proteomic analysis. Thirty-four proteins were identified and in-silico analysis of these proteins by SigPred (http://www.cbs.dtu.dk/services/SignalP/) and CADRE (http://www.cadre-genomes.org.uk/Aspergillus_fumigatus/) revealed that all of them had a signal peptide except Sod1 (AFUA_5G09240, [11]) (Table 1, Table S1). Most of these proteins were hydrolases and the most abundant protein was a putative β-(1,4)-glucan hydrolase (AFUA_7G06140). Other glycosylhydrolases were hexosidases or N-acetylhexosaminidases (AFUA_1G05770; AFUA_1G14560, AFUA_1G10790, AFUA_8G05020, AFUA_6G10730). A unique aspartic phosphatase was identified that was different from the one previously identified as a major mycelial cell wall protein [12]. Three peptidases (AFUA_2G03510, AFUA_4G03490, AFUA_8G04120) and the two aspartic proteases, Pep1p and Pep2p (AFUA_5G13300, AFUA_3G11400), known to be associated with the conidial cell wall were found [13]. Two well known allergens of A. fumigatus were also detected (Aspf1 (AFUA_5G02330) and Aspf13 (AFUA_2G12630) [14]). Other protein such as oxidoreductases and enzymes of sugar metabolism (pyruvate dehydrogenase kinase AFUA_2G11900 and isopropylmalate dehydrogenase AFUA_1G15780) were present in lower amount as they were identified only once or twice in the proteomic survey. Interestingly, Sod1p and RodAp (AFUA_5G09580), known to be highly expressed in resting conidia [11], were also found in this NaCl extract. A similar SDS-PAGE profile was obtained when urea/thiourea buffer was used to extract agsΔ conidial surface material, indicating that the proteins recovered were not depending on the extraction buffer (data not shown). The fact that many proteins were present above the surface rodlet layer suggested that in contrast to the parental strain, the lack of α1,3 glucan has led to a different cell wall retainment of these glycoproteins in the agsΔ mutant conidia.
Fig. 8. NaCl extracted proteins from the surface of agsΔ_5T resting conidia. 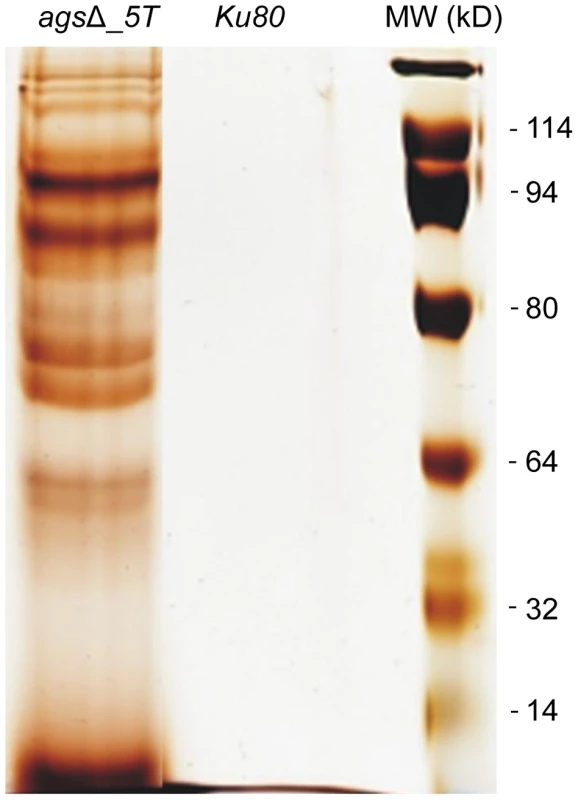
SDS-PAGE (10% gel) of proteins extracted after 2 h incubation of agsΔ_5T and ku80 resting conidia in 0.5 M NaCl. Tab. 1. Proteins identified in the NaCl extract of agsΔ_5T and agsΔ_n8 conidia. 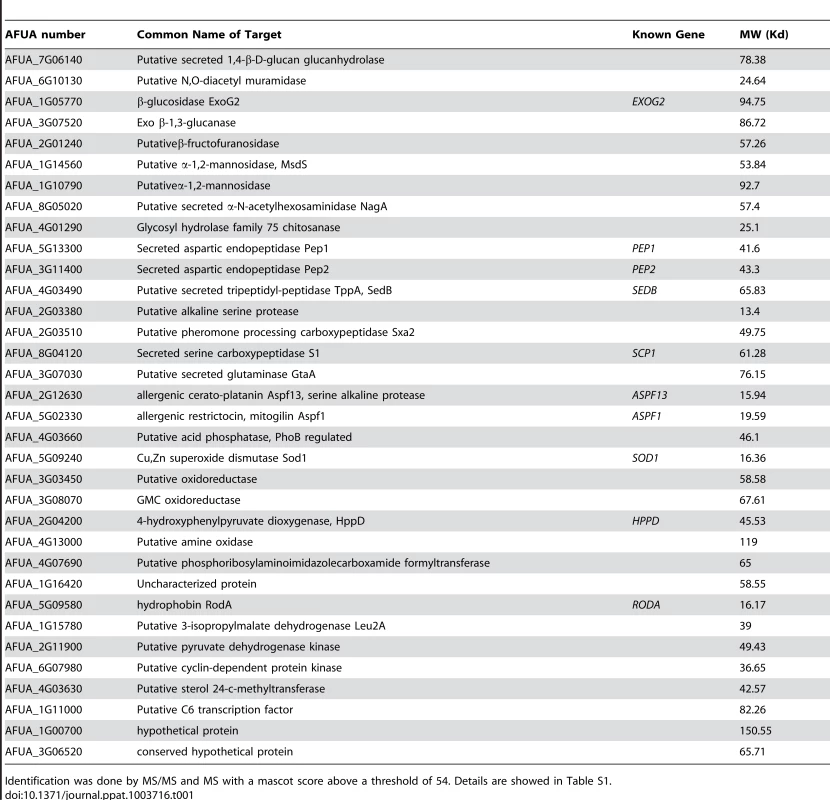
Identification was done by MS/MS and MS with a mascot score above a threshold of 54. Details are showed in Table S1. In vitro analysis of the cytokines produced during the first 5 h of incubation with alveolar macrophages showed that high amounts of pro-inflammatory TNFα cytokine were produced upon interaction with agsΔ mutant conidia whereas no TNFα was produced when the parental strain was incubated with macrophages under the same incubation conditions (Fig. 9A, Fig. S6A). Stimulation of the macrophages with the agsΔ conidial NaCl extract also induced TNFα expression (Fig. 9B; Fig. S6B). These results suggested that the surface glycoprotein layer on the resting agsΔ conidia was responsible for the induction of pro-inflammatory cytokine production immediately after conidial phagocytosis.
Fig. 9. TNFα production or expression by macrophages (isolated from uninfected immunocompetent mice) upon interaction with resting conidia of parental (ku80) and agsΔ_5T strains or agsΔ_5T conidial NaCl extract (3.2 µg proteins) respectively. 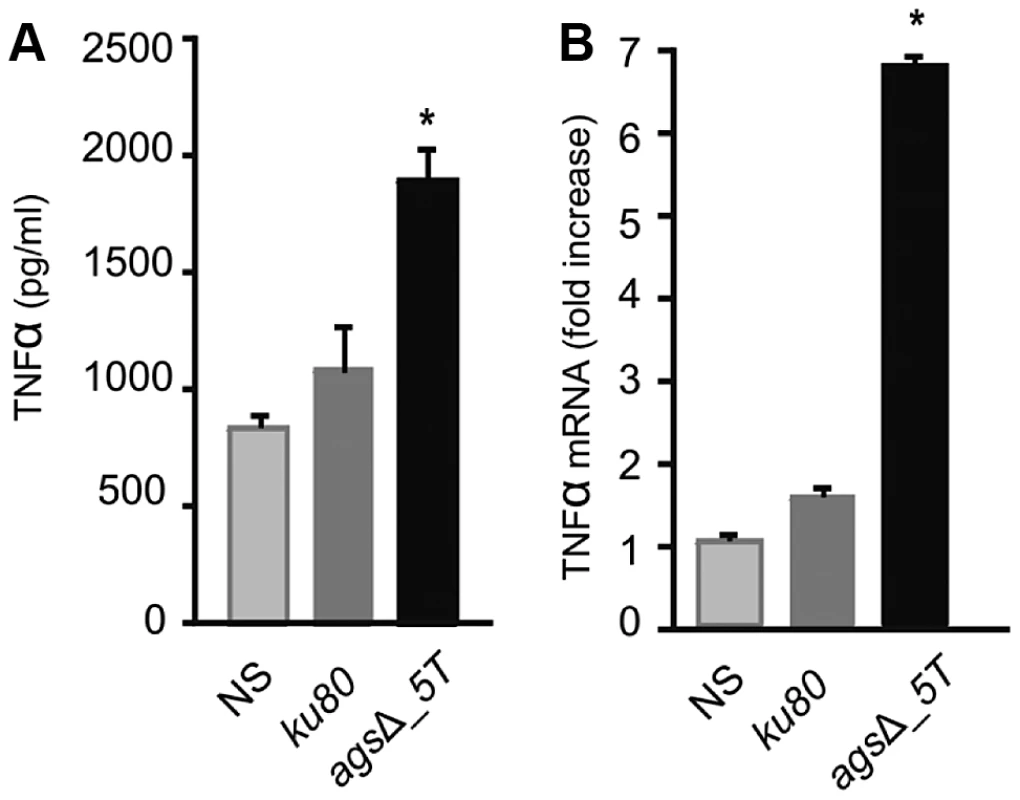
(A) TNFα was quantified after 5 h incubation of the conidia with macrophages; (B) Relative expression of TNFα assessed by real time RT-PCR in total RNA from macrophages after 5 h incubation of the agsΔ_5T conidial NaCl extract with macrophages. NaCl supernatant from ku80 resting conidia incubated for 2 h in 0.5M NaCl was used as a control. NS: Non-stimulated. *, P<0.05. Thus, the deletion of the AGS genes resulted in an unexpected modification of the mutant conidial surface with the emergence of an amorphous layer on the resting conidial surface over the rodlet layer, which altered biophysical properties, consequently affecting conidial interaction with the host immune system.
Polysaccharide PAMPS are exposed on the surface of the swollen conidia of the triple agsΔ mutants
Increased cytokine production seen in the macrophages over a 5 h-time period could also come from changes occurring at the surface of germinating conidia since it has been shown previously that conidia starts germinating intracellularly in the macrophage lysosome after the first 2 h of phagocytosis [15]. In addition, Figure 2 shows that agsΔ conidia undergo swelling in the infected lungs before being killed. The structural changes of the early germ tubes resulting from the AGS deletion were investigated by cytochemistry. The swollen conidia of the triple agsΔ mutants presented an increased labeling by WGA compared to the parental strain (Fig. 10A and data not shown). In addition, swollen agsΔ conidia were positive with the β-(1,3)-glucan receptor GNBP3, whereas both resting and swollen conidia of the parent strain were negative (Fig. 10B and data not shown). In contrast, there were no differences in the immunolabeling of the swollen conidia of parental and agsΔ mutants with anti-GAG and anti-GM monoclonal antibodies (Fig. S7). These results suggest that the absence of α-(1,3)-glucan that normally hides β-(1,3)-glucan and chitin, exposes these PAMPs at the surface of the swollen agsΔ conidia. These results were also in agreement with the chemical analysis of the cell wall: the mycelium cell wall of the agsΔ contained 1.7 and 2 times more chitin and β-(1,3)-glucan, respectively, than the cell wall of the parental strain [8].
Fig. 10. Labeling of the surfaces of agsΔ_5T and parental strain swollen conidia by WGA and the β(1,3)-glucan receptor GNBP3. 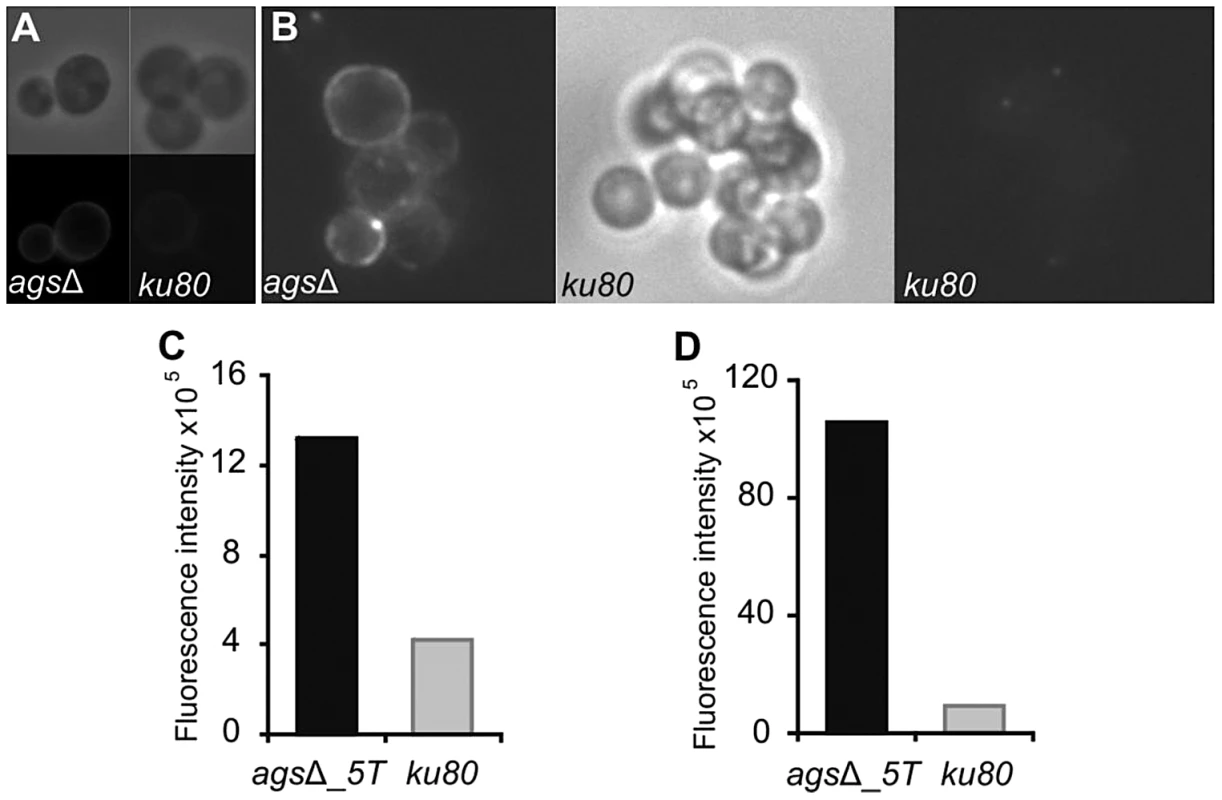
The surfaces of the swollen conidia were labeled by WGA-FITC (A) and GNBP3 (B) as described in material and methods. (C, D) Histograms represented the calculated fluorescence intensity of the corresponding images (A, B respectively), expressed in Einstein per seconds. Figure 11 represents a model to explain the sequential immune events upon inhalation of the agsΔ mutant and parental strain conidia and their differential impact/in vivo fate based on our in vitro assays as well as in vivo experiments using murine aspergillosis models. The presence of glycoproteins hiding the rodlet layer increases the phagocytic rate and promotes an immediate host immunological response towards the triple agsΔ mutants during phagocytosis. Once the mutant conidium is internalized, the conidial swelling results in an increased exposure of PAMPs on the swollen agsΔ conidial surface. Such surface modifications further boosts pre-existing host defense induced by the resting agsΔ conidia. In contrast, the resting conidium of the parental strain are not recognized by the phagocytes and do not display major PAMPs on the surface of the conidium during the intracellular swelling. Since agsΔ conidia did not seem more sensitive to host antifungal molecules compared to the parental strain, we hypothesize that differences in the killing in the later growth stages resulted from an early and enhanced host response induced by the modified surface of the resting agsΔ conidia. This early stimulation will be responsible for the killing of the germinating agsΔ conidia. On the contrary, in the partially immunosuppressed experimental murine models, limited and delayed killing of the parental strain conidia enables their further vegetative growth.
Fig. 11. Working model explaining sequential and differential immune events upon inhalation of the agsΔ mutant and the parental (ku80) strain conidia. 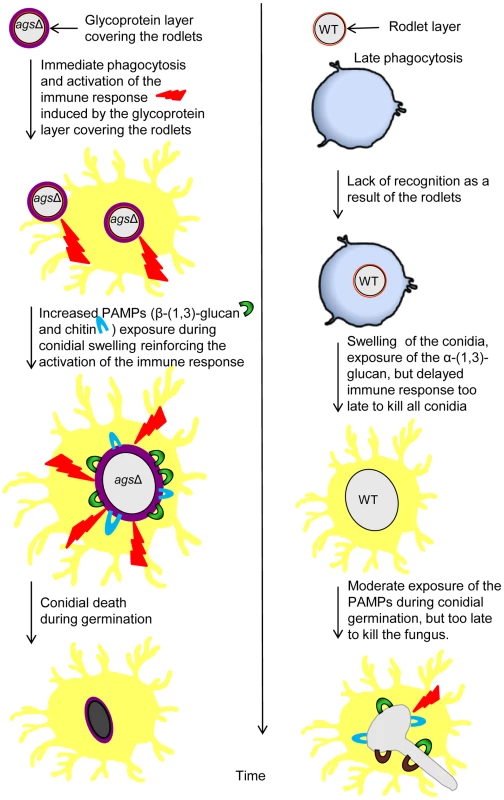
The presence of the glycoprotein layer on the triple agsΔ mutant conidial surface hides the rodlet layer. Increased exposure of PAMPs (WGA and ConA positive molecules and β-(1,3)-glucans) during vegetative growth in the triple agsΔ mutant modifies the host immunological response. This facilitates phagocytosis and killing of the triple agsΔ mutant and stimulates pro-inflammatory immune responses. Discussion
In this study we showed that the agsΔ mutants displayed a reduced virulence associated with an inhibition of germination in vivo and a reduction of the inflammatory response after 24 h infection (decreased TNFα and increased IL10 expressions and reduced recruitment of PMNs). The low level of TNFα seen with the triple agsΔ mutants fits with the lack of recruitment of neutrophils seen with this mutant after 24 h infection. However, during our in vitro experiments with macrophages incubated during 5 h with agsΔ or ku80 conidia, we observed the induction of pro-inflammatory cytokines. This indicated that the lack of inflammation seen at later stages of infection in mice was due to the inhibition of vegetative growth of the agsΔ mutants rather than a failure to stimulate inflammation. This was in agreement with the fact that agsΔ conidia were killed before their hyphal development.
The primary phenotype of the resting conidia of the agsΔ mutants was the absence of visible rodlet layer on the conidial surface. Even though the rodlets were present in the mutant conidia, their masking by a (glyco-)protein layer restored the immune sensing that is usually silenced when the rodlets are present on the surface of the wild type conidia [10], [16]. The agsΔ conidia were covered by proteins, which are usually secreted during vegetative growth. Most hydrolases found in the additional amorphous surface layer of the resting agsΔ conidia were usually identified during mycelial growth in a protein-based medium [14], [17]. How these proteins are able to cross the conidial cell wall remains an open question. Their presence on the surface is certainly due to the modifications of the cell wall integrity resulting from the three AGS deletions. Interestingly, in three independent HF extractions, the amount of 14.5 kDa RodAp was slightly higher than the 16 kDa RodAp (20–23% 16 kDa RodA in agsΔ mutants compared to 40–50% in the parental strain; Fig. 5C) suggesting that the rodlet structure of the mutant was less organized than the rodlet of the parental strain, which putatively modified the ionic strength of the hydrophobin layer in the agsΔ mutants [18]. Such structural modifications may affect the adherence of the hydrophilic glycoproteins to rodlets through electrostatic binding, since these proteins were easily extracted by salt. How these glycoproteins reached the surface of the cell wall is still not understood. This should not be related to changes in cell wall permeability since the agsΔ mutants were not more permeable to FITC or drugs that affect viability such as ROS, cationic peptides or Calcofluor White than the parental strain (data not shown). Alternatively, the hydrolases, because of their enzymatic activity, may harm the cell wall structure itself and this would help the proteins to cross the cell wall barrier. The stimulation of the expression of TNFα after incubation with macrophages (isolated from naive mice BAL) with agsΔ mutant conidial NaCl extract showed that these proteins located on the conidial surface were sensed first by the immune system and were able to induce an immediate immune response towards agsΔ conidia. It was previously shown that some of these surface proteins are recognized by T cells and can induce a Th1 protective response [6]. In particular, the secreted aspartic protease Pep1 that has been found in NaCl extract from agsΔ conidia conferred protection against infection, associated with a reduced neutrophil recruitment in BAL and a reduced inflammatory pathology in the lung. Hiding of the rodlet layer by an amorphous glycoprotein layer that stimulates the host response is not exclusively specific to the agsΔ deletion, since a similar conidial phenotype was observed on chitin synthase mutants [19], [20]. Similarly, in B. dermatitidis, the absence of α-(1,3)-glucan at the surface of the yeast increased the expression of W1-1 adhesin/antigen that were bound to phagocytic cells and suppressed the generation of the pro-inflammatory cytokine TNFα [21], [22].
The exposure of polysaccharide PAMPs on the surface of germinating conidia consecutively to triple AGS deletions also plays a role in stimulating the host innate immune response and inducing the production of antifungal molecules by the innate immune cells. The exposure of β-(1,3)-glucan at the surface of germinating agsΔ conidia will favor a Dectin-1-mediated host response [23]. Similarly, increased β-(1,3)-glucan exposure due to caspofungin treatment stimulated the host defense reaction against A. fumigatus [24], [25]. In addition, the positive binding of WGA and ConA also suggested that other receptors such as the mannose or/and chitin/N-acetylglucosamine, which are known to stimulate an antifungal response, can also be involved in this modified immune response [26]. Similar to the situation with the agsΔ mutants, it was shown that the lack of α-(1,3)-glucan in H. capsulatum also led to the unmasking of PAMPs [1]. The protective role of α-(1,3)-glucan has been also shown in B. dermatitidis and P. brasiliensis where the absence of α-(1,3)-glucan at the surface of the yeast and/or its replacement by β-(1,3)-glucan stimulated the host defense reaction [21], [27]. Recently, the masking of chitin by α-(1,3)-glucan has been shown to be essential for the virulence of the plant pathogen Magnaporthe grisea [28].
The molecules responsible for the killing of the agsΔ conidia remain unknown. However, it is clear that ROS were not responsible for the differences in killing between the agsΔ mutants and the parental strain conidia since the agsΔ mutants did not display a higher sensitivity to ROS in vitro and the killing of agsΔ conidia was similar in p47phox−/− mice compared to C57BL/6 (Fig. 3). Although a link between increased oxidative response and enhanced damage to A. fumigatus has been repeatedly demonstrated in the past [29], [30], recent studies, especially with chronic granulomatous disease (CGD) patients, have shown that NADPH-independent mechanisms can contribute to Aspergillus killing as much as ROS [31], [32]. Among possible mechanisms of NADPH-independent activity, D'Angelo et al. [33] have suggested that defensins and cathelicidins, known for their role in host defense, could be responsible for A. fumigatus killing in CGD mice. This seems however not the case for the agsΔ mutants as our in vitro studies indicated that the agsΔ mutants did not show a higher susceptibility to cathelicidin LL-37 or HNP2 and hBD2 defensins. Modification of the conidial surface may also lead to an increased binding of Surfactant Proteins A and D, Mannose Binding Lectin C or Penthraxin 3 that are known to be associated to an increased phagocytosis and an activation of the complement pathway known to play a major role in the killing of A. fumigatus [21], [34], [35], [36], [37]. Based on our data, it remains impossible to infer the killing of the agsΔ mutant conidia to currently known antifungal immune defense mechanisms. It can also be postulated that the killing may be due to an early burst of unknown toxic molecules or that the killing is the result of several antifungal molecules acting synergistically [38]. Our cell wall analysis suggested also that the cell wall architecture is perturbed in the inner as well as in the outer layer and that this perturbation may result in modifications of the cell wall permeability to specific antifungal molecules [8]. These could be responsible for an increased susceptibility of the agsΔ mutant to the host defense molecules.
The story of A. fumigatus α-(1,3)-glucan remains a two-sided coin. In the wild type strain, α-(1,3)-glucan induces an anti-A. fumigatus response as the injection of this polysaccharide into mice was immunoprotective and obviously responsible for the production of a Th1 response that is directed against A. fumigatus [6]. It could be expected that their removal favors the virulence of the mutant. In reality, the opposite happens due to the reorganization of the cell wall of the resting and germinating conidia upon triple AGS deletions. The presence of glycoproteins hiding the rodlet layer and the exposure of PAMPs in the germinating conidia modified the immunological response of the host, which increased phagocytosis and killing of the agsΔ mutants, and induced pro-inflammatory cytokine production. It is the structural modification of the entire cell wall consecutive to the AGS deletions that is responsible for an early stimulation of the host defense reactions. Interestingly, these structural modifications did not modify the survival of the fungus in vitro but are essential for the in vivo survival. The difference in the surface composition of the resting and swollen conidia of the agsΔ mutants led to an immediate sensing of the immunogenic molecules resulting in an early response of the phagocyte towards the agsΔ conidia. The deleterious effect of a delayed immune response on the microbial virulence is well known.
The α-(1,3)-glucan study tells us that the deletion of one cell wall gene does not lead only to the disappearance of the product of the encoded gene but results in a complete restructuration of the fungal cell wall. This has been shown with the deletion of the AGS genes in this study but also with other cell wall genes or consecutively to the use of antifungals acting on the cell wall in several fungal species [39]. Such structural and chemical modifications in the cell wall will have an obvious impact on the immune response of the host towards the corresponding mutant. Our study also suggests, any interpretation stating that the immune response towards a cell wall mutant is only due to the lack of the product of the deleted gene should be considered with care [40], [41].
Materials and Methods
Strains and culture conditions
All strains were grown in 2% (w/v) malt agar slants and 1 week-old conidia were recovered from the slants by vortexing with 0.05% (v/v) Tween 20 aqueous solution. Swollen conidia and germ tubes were produced after 5 h and 10 h, respectively, after incubation at 37°C in Brian's medium (Brian) [42]
The A. fumigatus parental strain AkuBku80ΔpyrG (ku80, [43]) and three agsΔ mutant strains independently obtained: ags1Δags2Δags3Δ_5T (agsΔ_5T) obtained previously [8] and two new ones, ags1Δags2Δags3Δn8and ags1Δags2Δags3Δ_n6.2 (agsΔ_n8 and agsΔ_n6.2), were used in this study. Since it had been impossible to complement agsΔ mutant for reasons explained previously [8], two new triple agsΔ mutants were constructed independently using the strategy described previously to exclude the possibility that undesired mutations had occurred during the deletion process. The lack of α-(1,3)-glucan in the cell wall of mutant strains was confirmed by both chemical and immunolabeling assays (Fig. S8). Chemical analysis of the cell wall was performed as previously described [44]. For immunolabeling assays, 5–10 h germinated conidia were labeled using the MOPC 104E monoclonal antibody, which binds specifically to α-(1,3)-glucan [45] (Beauvais A. Institut Pasteur, Paris, France, unpublished results). Paraformaldehyde (PFA) fixed swollen and germinating conidia were permeabilized prior to immunolabeling as previously described [46]. MOPC 104E (Sigma) and control mouse IgM (Sigma) were used at a dilution of 1∶25 and the goat antimouse IgG-TRITC (H+L, Sigma) was used as the secondary antibody at a dilution of 1∶50. The three triple mutants used in this study germinated, sporulated and conidiated like the parental strain in vitro (data not shown, [8]).
Analysis of the conidial surface
Conidial surface was analyzed by Atomic Force Microscopy (AFM). The sample immobilization is achieved by assembling the living conidia within the patterns of microstructured, functionalized poly-dimethylsiloxane (PDMS, Sylgard 184) stamps using convective/capillary deposition [47]. Images and force measurements were performed in deionised water, respectively in contact mode and in Quantitative Imaging (QI) mode and Force Volume (FV) mode. For both experiments we used bare MLCT AUWH cantilever (nominal spring constant 0.01 N/m) (Bruker). Single cells were first localized and imaged and then switched over to QI and FV modes to record adhesion force maps. AFM Nanowizard II and III (JPK Instruments, Berlin, Germany) were used to capture the images. The cantilevers spring constants were measured by the thermal noise method [48] ranging from 0.0160 to 0.0190 N/m. Force curves were analyzed in order to determine the adhesion force between the conidia and the AFM tip. These adhesions were plotted as bright pixels, brighter colors indicating larger adhesion values. For each strain, images that were obtained for at least three conidia from independent cultures and analyzed with different tips, were representative of the entire conidial population inside each mutant and parental strain. The results acquired on the spores were analyzed on JPK Data Processing software.
The rodlet layer was extracted from the spore surface by incubating 109 dry conidia with 48% (v/v) hydrofluoric acid (HF) for 72 h at 4°C. The contents were centrifuged (10,000 rpm, 10 min) and the supernatant obtained was dried under N2. The dried material was reconstituted in H2O and an aliquot was subjected to 15% (w/v) SDS-PAGE analysis and visualized by silver nitrate staining. Bands were quantified using Image lab software (BioRad).
To analyze the components present on the surface, conidia were incubated in 0.5 M NaCl solution for 2 h at room temperature at a ratio of 1010 conidia per ml. The NaCl supernatant was recovered after centrifugation and directly subjected to 10% SDS-PAGE (w/v). The protein concentrations in the extracts were determined by the Coomassie brilliant blue method [49], using BioRad kit and BSA as the standard. Proteomic analysis of the NaCl extract was carried out as described previously with slight modifications [50]. A total amount of 50–100 µg protein was loaded onto IPG strips (11 cm, pH 3–7; GE Healthcare Life Sciences) by in-gel rehydration. After equilibration of the IPG strips, SDS-gel electrophoresis was carried out using Criterion AnykD TGX gels (Bio-RAD). Proteins were visualised by colloidal Coomassie staining [51]. After scanning, gel images were analysed with the software Delta 2D 4.3. (Decodon). Protein spots were excised and analysed by mass spectrometry using an ultrafleXtreme MALDI-TOF/TOF device (Bruker Daltonics).
Fluorescence microscopy
Resting and swollen conidia were PFA-fixed (2.5% (v/v) PFA in PBS) for one night at 4°C, washed three times with 0.1 M NH4Cl in PBS, once with PBS and then incubated with different antibodies or lectins as described previously [52].
Galactosaminogalactan (GAG) was labeled with a monoclonal mouse antibody as described previously [53] (20 µg/ml) and a mock monoclonal antibody was used as a control. The secondary goat anti-mouse IgG-TRITC (Sigma) antibody was used at a dilution of 1∶200.
Galactomannan was labeled with a rat anti-Galactofuranose (Galf) monoclonal antibody (EBA2, diluted 1∶1000, a kind gift of M. Tabouret from BioRad, Steenvorde [54]). Control Rat monoclonal antibody of the same isotype and the secondary goat anti-rat FITC (Sigma-Aldrich) antibody were used at a dilution of 1∶1000 and 1∶500, respectively.
β-(1,3)-glucan was labeled with the N-terminal β-(1,3)-glucan binding domain of Drosophila pattern recognition receptor, GNBP3 (homologous to Mammalian Dectin 1) at a concentration of 3 µg/ml and a polyclonal mouse antiserum against GNBP3 at 1∶200 dilution (kind gifts from A. Roussel, CNRS, Orleans and D. Ferrandon, CNRS, Strasbourg, France [55]). Goat anti-mouse IgG FITC 1∶200 diluted (Sigma) was used as secondary antibodies.
The glucosamine moiety of chitin/chitosan and mannose/glucose moieties of glycoproteins and glucans were labeled respectively with WGA-FITC and ConA-FITC (Sigma) at 0.1 mg/ml concentrations upon incubating the conidia for 15 min at lab temperature.
Susceptibility to oxidative stress conditions, Lactoferrin, Cathelicidin LL-37, HNP2 and hBD2 defensins, absence of iron and hypoxia
Stress conditions induced by Menadione (0 to 30 µM) and 2,5-Bis(tert-butylperoxy)-2,5-dimethylhexane (Luperox®101) (0 to 2 mM) were tested on both parental and mutant A. fumigatus strains grown on agar-RPMI (RPMI 1640, Sigma without glutamine) supplemented with 1% agar (Difco), 0.3 g/1 L-glutamine and 0.1 M MOPS or MES (to obtain a pH of 7 or 4, respectively) at 37°C for 24–48 h.
Stress conditions induced by Lactoferrin 0.45–231 µg/ml (Sigma) or Cathelicidin LL-37 0.45–231 µg/ml (Sigma), SDS (0.006–0.2%; Merck) and H2O2 (0.003–0.1%; Fluka) were tested on A. fumigatus strains grown on Brian medium without supplementation with iron or RPMI-glutamine-MOPS medium (described above) [38]. Combinations of 0.05% SDS or 0.012% H2O2 and Lactoferrin or Cathelicidin LL37 at concentrations of 231 µg/ml were tested in the same media, as described in Clavaud et al [38].
Stress condition induced by HNP2 (100 µg/ml; Sigma) and hBD2 (25 µg/ml; Sigma) defensins were also tested by incubating 106 conidia/ml with the defensins for 10–16 h at 37°C in RPMI-glutamine-MOPS medium.
The growth of A. fumigatus strains was tested in Brian medium without supplementation with iron at 37°C and under hypoxia conditions using AnaeroGen sachet (Oxoid), which reduces the oxygen level in a jar to below 1% that results to a CO2 level between 9–13%.
Transmission electron microscopy (TEM)
Aliquots (20 µl) of concentrated conidia were placed onto a Formvar-coated nickel or gold mesh grids, which were then placed between the flat sides of two B-type brass planchets (Ted Pella Inc., Redding, CA). The grids were used as spacer creating a thin layer of cells that allows higher yields of well-frozen cells. The samples were immediately frozen with liquid nitrogen under high pressure (2,100 bar) using a Bal-Tec HPM 010 high pressure freezing machine (Bal-Tec Products, Middlebury, CT, USA). Following cryofixation, the samples were freeze-substituted at −85°C in 1% glutaraldehyde (Electron Microscopy Sciences, Washington, PA, USA) and 1% tannic acid in acetone for 72 h. After, the samples were rinsed thoroughly with three changes of fresh acetone at −85°C for a total of 45 min. Cells were infiltrated with 1% OsO4 in acetone for 1 h at −85°C before being slowly warmed to room temperature over 5 h. The cells were then rinsed in acetone and slowly infiltrated with and polymerized in Spurr's resin. Embedded cells were cut into serial 70 nm thick sections with an Ultracut R Microtome (Leica, Vienna, Austria) and collected on Formvar-coated copper slot grids. Sections were post-stained with 2% uranyl acetate in 50% ethanol for 5 min followed by 5 min with Sato's lead citrate [56]. The grids were carbon-coated and viewed at 80 kV using a JEOL 1200EX transmission electron microscope (JEOL USA, Inc., Pleasanton, CA, USA).
Analysis of agsΔ mutant virulence
Female 8 - to 10-week-old inbred C57BL6 (H-2b) mice were obtained from Charles River Breeding Laboratories (Calco, Italy). Experiments were performed according to the Italian Approved Animal Welfare Assurance A-3143-01. Breeding pairs of homozygous p47phox−/−mice, raised on C57BL6 background, were purchased from Harlan Laboratories and bred under specific-pathogen free conditions at the breeding facilities of the University of Perugia, Perugia, Italy [33]. Infections were performed on one model of immunocompetent mice and in two different models of invasive pulmonary aspergillosis as previously described [6]. In the first immunosuppressed model, mice were subjected to intra-peritoneal administration of cyclophosphamide (150 mg/kg body weight) one day before infection as described previously [6]. In the second immunosuppressed model, mice were treated with anti-Ly6G monoclonal antibody (clone RB6-8C5 MAb; eBienscience; 100 µg/mouse) administered intra-peritoneally one day before infection. Rat anti-E. coli β-galactosidase (clone GLL 113) was used as a control IgG. Treatment with the anti-Ly6G MAb is known to selectively deplete mature neutrophils, eosinophils and dendritic cells [57] and at 24 h after administration, the number of circulating neutrophils dropped to 20±12/mm3 compared to 1120±227/mm3 in controls, and the treated mice continued to be low for circulating neutrophils counts up to 5-days. Mice were monitored for survival and fungal growth (determined as colony forming unit (CFU) per organ) four days post-infection as described previously [58]. All mice underwent necropsy for histopathological observation of fungal burden in the lungs four days post-infection. For histology, sections (3–4 µm) of paraffin-embedded lungs were stained following periodic acid-Schiff (PAS) protocol. Collection of the bronchoalveolar lavage (BAL) fluid and the morphometry [% monocytes (MNC) or polymorphonuclear (PMN) cells] was performed after four days infection as previously described [6]. Total and differential cell counts were performed after staining BAL smears with May-Grünwald Giemsa reagents (Sigma) before analysis. At least 200 cells per cytospin preparation were counted and the absolute number of each cell type was calculated. Cytospin preparations were observed using a BX51 microscope (Olympus, Milan, Italy). Histology images were captured using a high-resolution DP71 camera (Olympus).
For phagocytosis and conidiocidal activity, alveolar macrophages from uninfected mice were isolated from BAL as described [15]. For phagocytosis, macrophages were incubated at 37°C with unopsonized FITC (Sigma) labeled conidia [59] at an effector to conidial ratio of 5∶1, for 1 h in RPMI medium in micro-chambers (Ibitreat). Unbound conidia were removed by washing with RPMI and cells were fixed with 3% (v/v) PFA for 1 h in PBS. After fixation, the cells were incubated with a rabbit polyclonal anti-FITC antibody (Invitrogen) diluted 1∶2000 and a secondary rabbit antibody conjugated to Alexafluor 568 (dilution, 1∶2000) (Invitrogen). This last procedure labels only cell surface-associated conidia and the ingested conidia remained unlabeled. The number of ingested conidia per macrophage was determined on 200 macrophages. For conidiocidal activity, macrophages isolated from uninfected C57BL6 (H-2b) and p47phox−/− mice were incubated at 37°C with unopsonized resting or swollen conidia (6½ h in RPMI at 37°C), at an effector to fungal cell ratio of 1∶10, for 2–6 hours in an ELISA plate wells. After removing the supernatant, Triton X100 (1%) was added to the wells and incubated at 37°C for 10 min to lyse the macrophages and to collect phagocytized conidia. The percentage of phagocytized conidia capable of further germination was determined by spotting phagocytized conidia (at suitable dilution) on a nutritive agar medium and counting those conidia capable of forming germ tube among spotted conidial population. We verified that the use of Triton X100 to lyse macrophage did not affect conidial germination as the percentage of germinations were similar (97±1%) for the agsΔ_5T, agsΔ_n6.2, agsΔ_n8 mutants and the parental strain with or without Triton-treatment. The differences in the germination of the conidia from the stock solution used for macrophage conidicidal activity study permitted the calculation of conidiocidal activity.
For cytokine quantification, total RNA was extracted from lungs of immunocompetent mice four days post-infection, or from macrophages isolated from BAL fluid of uninfected mice and incubating with agsΔ NaCl extracts containing 3.2 µg proteins, for 5 h. The cytokines expressed and productions were quantified by Real-time PCR and ELISA, respectively as described previously [6].
Statistical significance was analyzed by one - or two-way ANOVA or paired t-test with Prism software (GraphPad software, San Diego, CA) and p-values≤0.05 were considered to be significant. Data were representative of at least two independent experiments or pooled from three to five experiments. The in vivo groups consisted of six mice/group and experiments were repeated at least three times. Macrophage experiments were done three times with three different batches of macrophages and conidia.
All experiments were performed using the agsΔ_5T(Figs. 1–10, Table 1, Table S1). Virulence and proteomic analyses were performed also using agsΔ_n8 (Figs. S1, S2, S3, S4, S5, S6, S7, S8, Table 1 and Table S1). Major phenotypes and virulence data were verified with agsΔ_n6.2 (Figs. S1, S5, S6, S7, S8).
Ethics statement
Mouse experiments were performed according to the Italian Approved Animal Welfare Assurance 245/2011-B. Legislative decree 157/2008-B regarding the animal license was obtained by the Italian Ministry of Health lasting for three years (2008–2011). Infections were performed under avertin anesthesia and all efforts were made to minimize suffering.
Supporting Information
Zdroje
1. RappleyeCA, EissenbergLG, GoldmanWE (2007) Histoplasma capsulatum alpha-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. 2007 104 : 1366–1370.
2. ReeseAJ, YonedaA, BregerJA, BeauvaisA, LiuH, et al. (2007) Loss of cell wall alpha(1–3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol 63 : 1385–1398.
3. MaubonD, ParkS, TanguyM, HuerreM, SchmittC, et al. (2006) AGS3, an alpha(1–3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet Biol 43 : 366–375.
4. BeauvaisA, SchmidtC, GuadagniniS, RouxP, PerretE, et al. (2007) An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol 9 : 1588–1600.
5. FontaineT, BeauvaisA, LoussertC, ThevenardB, FulgsangCC, et al. (2010) Cell wall alpha1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet Biol 47 : 707–712.
6. BozzaSC, Cecile (2009) GiovanniniGloria (2009) FontaineThierry (2009) BeauvaisAnne (2009) SarfatiJacqueline (2009) et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids and polysaccharides and the impact on Th immunity and vaccination. The Journal of Immunology 183 : 2407–2414.
7. BeauvaisA, MaubonD, ParkS, MorelleW, TanguyM, et al. (2005) Two alpha(1–3) glucan synthases with different functions in Aspergillus fumigatus. Appl Environ Microbiol 71 : 1531–1538.
8. HenryC, LatgeJP, BeauvaisA (2012) alpha1,3 glucans are dispensable in Aspergillus fumigatus. Eukaryot Cell 11 : 26–29.
9. DagueE, DelcorteA, LatgeJP, DufreneYF (2008) Combined use of atomic force microscopy, X-ray photoelectron spectroscopy, and secondary ion mass spectrometry for cell surface analysis. Langmuir 24 : 2955–2959.
10. AimaniandaV, BayryJ, BozzaS, KniemeyerO, PerruccioK, et al. (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460 : 1117–1121.
11. LambouK, LamarreC, BeauR, DufourN, LatgeJP (2010) Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol Microbiol 75 : 910–923.
12. MorelleW, BernardM, DebeaupuisJP, BuitragoM, TabouretM, et al. (2005) Galactomannoproteins of Aspergillus fumigatus. Eukaryot Cell 4 : 1308–1316.
13. MonodM, CapocciaS, LechenneB, ZauggC, HoldomM, et al. (2002) Secreted proteases from pathogenic fungi. Int J Med Microbiol 292 : 405–419.
14. SinghB, OellerichM, KumarR, KumarM, BhadoriaDP, et al. (2010) Immuno-reactive molecules identified from the secreted proteome of Aspergillus fumigatus. J Proteome Res 9 : 5517–5529.
15. PhilippeB, Ibrahim-GranetO, PrevostMC, Gougerot-PocidaloMA, Sanchez PerezM, et al. (2003) Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun 71 : 3034–3042.
16. BayryJ, AimaniandaV, GuijarroJI, SundeM, LatgeJP (2012) Hydrophobins–unique fungal proteins. PLoS Pathog 8: e1002700.
17. SriranganadaneD, WaridelP, SalaminK, ReichardU, GrouzmannE, et al. (2010) Aspergillus protein degradation pathways with different secreted protease sets at neutral and acidic pH. J Proteome Res 9 : 3511–3519.
18. WangZ, LienemannM, QiauM, LinderMB (2010) Mechanisms of protein adhesion on surface films of hydrophobin. Langmuir 26 : 8491–8496.
19. Jimenez-OrtigosaC, AimaniandaV, MuszkietaL, MouynaI, AlsteensD, et al. (2012) Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins. Antimicrob Agents Chemother 56 : 6121–6131.
20. AlsteensD, AimaniandaV, HegdeP, PireS, BeauR, et al. (2013) Unraveling the Nanoscale Surface Properties of Chitin Synthase Mutants of Aspergillus fumigatus and Their Biological Implications. Biophys J 105(2): 320–7.
21. HoganLH, KleinBS (1994) Altered expression of surface alpha-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect Immun 62 : 3543–3546.
22. RappleyeCA, GoldmanGH (2006) Defining virulence genes in the dimorphing fungi. Ann Rev Microbiol 60 : 281–303.
23. McDonaldJU, RosasM, BrownGD, JonesSA, TaylorPR (2012) Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS One 7: e45781.
24. HohlTM, FeldmesserM, PerlinDS, PamerEG (2008) Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis 198 : 176–185.
25. LamarisGA, LewisRE, ChamilosG, MayGS, SafdarA, et al. (2008) Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis 198 : 186–192.
26. LatgeJP (2010) Tasting the fungal cell wall. Cell Microbiol 12 : 863–872.
27. FeriottiC, LouresFV, Frank de AraujoE, da CostaTA, CalichVL (2013) Mannosyl-recognizing receptors induce an M1-like phenotype in macrophages of susceptible mice but an M2-like phenotype in mice resistant to a fungal infection. PLoS One 8: e54845.
28. FujikawaT, SakaguchiA, NishizawaY, KouzaiY, MinamiE, et al. (2012) Surface alpha-1,3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog 8: e1002882.
29. RoilidesE, Dimitriadou-GeorgiadouA, SeinT, KadiltsoglouI, WalshTJ (1998) Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun 66 : 5999–6003.
30. RoilidesE, UhligK, VenzonD, PizzoPA, WalshTJ (1993) Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun 61 : 1185–1193.
31. HenrietSS, HermansPW, VerweijPE, SimonettiE, HollandSM, et al. (2011) Human leukocytes kill Aspergillus nidulans by reactive oxygen species-independent mechanisms. Infect Immun 79 : 767–773.
32. ZaremberKA, SuguiJA, ChangYC, Kwon-ChungKJ, GallinJI (2007) Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 178 : 6367–6373.
33. D'AngeloC, De LucaA, ZelanteT, BonifaziP, MorettiS, et al. (2009) Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J Immunol 183 : 4609–4618.
34. KaurS, GuptaVK, ThielS, SarmaPU, MadanT (2007) Protective role of mannan-binding lectin in a murine model of invasive pulmonary aspergillosis. Clin Exp Immunol 148 : 382–389.
35. MoalliF, DoniA, DebanL, ZelanteT, ZagarellaS, et al. (2010) Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood 116 : 5170–5180.
36. SpethC, RambachG (2012) Complement Attack against Aspergillus and Corresponding Evasion Mechanisms. Interdiscip Perspect Infect Dis 2012 : 463794.
37. ZhangS, FanY, XiaYX, KeyhaniNO (2010) Sulfonylurea resistance as a new selectable marker for the entomopathogenic fungus Beauveria bassiana. Appl Microbiol Biotechnol 87 : 1151–1156.
38. ClavaudC, BeauvaisA, BarbinL, Munier-LehmannH, LatgeJP (2012) The composition of the culture medium influences the beta-1,3-glucan metabolism of Aspergillus fumigatus and the antifungal activity of inhibitors of beta-1,3-glucan synthesis. Antimicrob Agents Chemother 56 : 3428–3431.
39. ShethCC, HallR, LewisL, BrownAJ, OddsFC, et al. (2011) Glycosylation status of the C. albicans cell wall affects the efficiency of neutrophil phagocytosis and killing but not cytokine signaling. Med Mycol 49 : 513–524.
40. ShalerCR, HorvathC, LaiR, XingZ (2012) Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis. Clin Dev Immunol 2012 : 628293.
41. SvirshchevskayaEV, ShevchenkoMA, HuetD, FemeniaF, LatgeJP, et al. (2009) Susceptibility of mice to invasive aspergillosis correlates with delayed cell influx into the lungs. Int J Immunogenet 36 : 289–299.
42. BrianPW, DawkinsAW, GroveJF, HemmingHG, LoweD, et al. (1961) Phytotoxic compounds produced by Fusarium equiseti. J Exper Bot 12 : 1–12.
43. da Silva FerreiraME, KressMR, SavoldiM, GoldmanMH, HartlA, et al. (2006) The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell 5 : 207–211.
44. MouynaI, KniemeyerO, JankT, LoussertC, MelladoE, et al. (2010) Members of PMT family in Aspergillus fumigatus differentially affect growth, morphogenesis, and viability. Mol Microbiol 76 : 1205–1221.
45. KlimpelKR, GoldmanWE (1988) Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect Immun 56 : 2997–3000.
46. HarrisSD, MorrellJL, HamerJE (1994) Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136 : 517–532.
47. DagueE, JauvertE, LaplatineL, VialletB, ThibaultC, et al. (2011) Assembly of live micro-organisms on microstructured PDMS stamps by convective/capillary deposition for AFM bio-experiments. Nanotechnology 22 : 395102.
48. HutterS, HeinritziK, ReichE, EhretW (1993) [Effects of different methods of tooth resection in suckling piglets]. Tierarztl Prax 21 : 417–428.
49. BradfordM (1976) A rapide and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. . Anal Biochem 72 : 248–254.
50. KniemeyerO, LessingF, ScheibnerO, HertweckC, BrakhageAA (2006) Optimisation of a 2-D gel electrophoresis protocol for the human-pathogenic fungus Aspergillus fumigatus. Curr Genet 49 : 178–189.
51. CandianoG, BruschiM, MusanteL, SantucciL, GhiggeriGM, et al. (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25 : 1327–1333.
52. LamarreC, BeauR, BalloyV, FontaineT, Wong Sak HoiJ, et al. (2009) Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cell Microbiol 11 : 1612–1623.
53. FontaineT, DelangleA, SimenelC, CoddevilleB, van VlietSJ, et al. (2011) Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog 7: e 1002372.
54. StynenD, SarfatiJ, GorisA, PrevostMC, LesourdM, et al. (1992) Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun 60 : 2237–2245.
55. MishimaY, QuintinJ, AimaniandaV, KellenbergerC, CosteF, et al. (2009) The N-terminal domain of Drosophila Gram-negative binding protein 3 (GNBP3) defines a novel family of fungal pattern recognition receptors. J Biol Chem 284 : 28687–28697.
56. TakagiI, YamadaK, SatoT, HanaichiT, IwamotoT, et al. (1990) J Electron Microsc (Tokyo) 39 : 67–68.
57. BonifaziP, D'AngeloC, ZagarellaS, ZelanteT, BozzaS, et al. (2010) Intranasally delivered siRNA targeting P13K/Akt/mTOR inflammatory pathways protects from aspergillosis. Mucosal Immnunol 3 : 193–205.
58. BozzaS, PerruccioK, MontagnoliC, GazianoR, BellocchioS, et al. (2003) A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102 : 3807–3814.
59. SturtevantJ, LatgeJP (1992) Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J Infect Dis 166 : 580–586.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Baculoviruses: Sophisticated Pathogens of Insects
- How Do Viruses Avoid Inhibition by Endogenous Cellular MicroRNAs?
- The Regulation of Trypanosome Gene Expression by RNA-Binding Proteins
- DNA Damage Repair and Bacterial Pathogens
- Disease to Dirt: The Biology of Microbial Amyloids
- Fungal Immune Evasion in a Model Host–Pathogen Interaction: Versus Macrophages
- Infectious Prions Accumulate to High Levels in Non Proliferative C2C12 Myotubes
- The Biology and Taxonomy of Head and Body Lice—Implications for Louse-Borne Disease Prevention
- Antibodies Trap Tissue Migrating Helminth Larvae and Prevent Tissue Damage by Driving IL-4Rα-Independent Alternative Differentiation of Macrophages
- The Effects of Somatic Hypermutation on Neutralization and Binding in the PGT121 Family of Broadly Neutralizing HIV Antibodies
- Natural Selection Promotes Antigenic Evolvability
- Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung
- Mode of Parainfluenza Virus Transmission Determines the Dynamics of Primary Infection and Protection from Reinfection
- Type I and Type III Interferons Drive Redundant Amplification Loops to Induce a Transcriptional Signature in Influenza-Infected Airway Epithelia
- Unraveling a Three-Step Spatiotemporal Mechanism of Triggering of Receptor-Induced Nipah Virus Fusion and Cell Entry
- A Novel Membrane Sensor Controls the Localization and ArfGEF Activity of Bacterial RalF
- Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity
- Global Rescue of Defects in HIV-1 Envelope Glycoprotein Incorporation: Implications for Matrix Structure
- Turning Defense into Offense: Defensin Mimetics as Novel Antibiotics Targeting Lipid II
- The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells
- Brd4 Is Displaced from HPV Replication Factories as They Expand and Amplify Viral DNA
- A Viral Genome Landscape of RNA Polyadenylation from KSHV Latent to Lytic Infection
- The Cytotoxic Necrotizing Factor of (CNF) Enhances Inflammation and Yop Delivery during Infection by Activation of Rho GTPases
- The Inflammatory Kinase MAP4K4 Promotes Reactivation of Kaposi's Sarcoma Herpesvirus and Enhances the Invasiveness of Infected Endothelial Cells
- Conservative Sex and the Benefits of Transformation in
- Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior
- Colonization Resistance: Battle of the Bugs or Ménage à Trois with the Host?
- Intracellular Interferons in Fish: A Unique Means to Combat Viral Infection
- SPOC1-Mediated Antiviral Host Cell Response Is Antagonized Early in Human Adenovirus Type 5 Infection
- Involvement of the Cellular Phosphatase DUSP1 in Vaccinia Virus Infection
- Killer Bee Molecules: Antimicrobial Peptides as Effector Molecules to Target Sporogonic Stages of
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- A Role for Host Activation-Induced Cytidine Deaminase in Innate Immune Defense against KSHV
- Haploid Genetic Screens Identify an Essential Role for PLP2 in the Downregulation of Novel Plasma Membrane Targets by Viral E3 Ubiquitin Ligases
- A Small Molecule Glycosaminoglycan Mimetic Blocks Invasion of the Mosquito Midgut
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- Can Non-lytic CD8+ T Cells Drive HIV-1 Escape?
- Deletion of the α-(1,3)-Glucan Synthase Genes Induces a Restructuring of the Conidial Cell Wall Responsible for the Avirulence of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Baculoviruses: Sophisticated Pathogens of Insects
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- Natural Selection Promotes Antigenic Evolvability
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



