-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Antibodies Trap Tissue Migrating Helminth Larvae and Prevent Tissue Damage by Driving IL-4Rα-Independent Alternative Differentiation of Macrophages
Approximately one-third of the world's population suffers from chronic helminth infections with no effective vaccines currently available. Antibodies and alternatively activated macrophages (AAM) form crucial components of protective immunity against challenge infections with intestinal helminths. However, the mechanisms by which antibodies target these large multi-cellular parasites remain obscure. Alternative activation of macrophages during helminth infection has been linked to signaling through the IL-4 receptor alpha chain (IL-4Rα), but the potential effects of antibodies on macrophage differentiation have not been explored. We demonstrate that helminth-specific antibodies induce the rapid trapping of tissue migrating helminth larvae and prevent tissue necrosis following challenge infection with the natural murine parasite Heligmosomoides polygyrus bakeri (Hp). Mice lacking antibodies (JH−/−) or activating Fc receptors (FcRγ−/−) harbored highly motile larvae, developed extensive tissue damage and accumulated less Arginase-1 expressing macrophages around the larvae. Moreover, Hp-specific antibodies induced FcRγ - and complement-dependent adherence of macrophages to larvae in vitro, resulting in complete larval immobilization. Antibodies together with helminth larvae reprogrammed macrophages to express wound-healing associated genes, including Arginase-1, and the Arginase-1 product L-ornithine directly impaired larval motility. Antibody-induced expression of Arginase-1 in vitro and in vivo occurred independently of IL-4Rα signaling. In summary, we present a novel IL-4Rα-independent mechanism of alternative macrophage activation that is antibody-dependent and which both mediates anti-helminth immunity and prevents tissue disruption caused by migrating larvae.
Published in the journal: . PLoS Pathog 9(11): e32767. doi:10.1371/journal.ppat.1003771
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003771Summary
Approximately one-third of the world's population suffers from chronic helminth infections with no effective vaccines currently available. Antibodies and alternatively activated macrophages (AAM) form crucial components of protective immunity against challenge infections with intestinal helminths. However, the mechanisms by which antibodies target these large multi-cellular parasites remain obscure. Alternative activation of macrophages during helminth infection has been linked to signaling through the IL-4 receptor alpha chain (IL-4Rα), but the potential effects of antibodies on macrophage differentiation have not been explored. We demonstrate that helminth-specific antibodies induce the rapid trapping of tissue migrating helminth larvae and prevent tissue necrosis following challenge infection with the natural murine parasite Heligmosomoides polygyrus bakeri (Hp). Mice lacking antibodies (JH−/−) or activating Fc receptors (FcRγ−/−) harbored highly motile larvae, developed extensive tissue damage and accumulated less Arginase-1 expressing macrophages around the larvae. Moreover, Hp-specific antibodies induced FcRγ - and complement-dependent adherence of macrophages to larvae in vitro, resulting in complete larval immobilization. Antibodies together with helminth larvae reprogrammed macrophages to express wound-healing associated genes, including Arginase-1, and the Arginase-1 product L-ornithine directly impaired larval motility. Antibody-induced expression of Arginase-1 in vitro and in vivo occurred independently of IL-4Rα signaling. In summary, we present a novel IL-4Rα-independent mechanism of alternative macrophage activation that is antibody-dependent and which both mediates anti-helminth immunity and prevents tissue disruption caused by migrating larvae.
Introduction
Intestinal helminths present a major global health burden, particularly in developing countries. Patients that are infected with nematodes such as Ascaris lumbricoides, Trichuris trichuria or Necator americanus often develop severe pathology and impaired responsiveness to vaccines [1] [2] [3] [4]. Approximately 2 billion people are infected with intestinal nematodes, with the most severe infections often found within school children [5] [6]. Although such infections can be treated by chemotherapy, worm burdens typically reach pretreatment levels within 6 months [7] [5]. Moreover, drug resistant helminths present a pressing problem for livestock [8], raising concerns about the long-term validity of chemotherapy amongst human populations [9] [10] [11] [6] [12] [13]. Unfortunately, no efficacious vaccines against intestinal nematodes are available to date, making an improved understanding of host immunity imperative.
Macrophages are highly plastic immune cells that can fulfill diverse tasks in immunity, metabolism and wound-healing depending on their tissue location and inflammatory context [14]. In the context of bacterial infection, the activation of classically activated macrophages by serum components such as antibodies and complement can enhance the phagocytosis and killing of bacterial or fungal pathogens [15]. By contrast, type 2 immune responses associated with helminth infection and allergies are characterized by a predominance of alternatively activated macrophages (AAM) that appear to play a role in anti-helminth immunity and wound repair through ill-defined mechanisms [16] [17].
Infection with the natural murine parasite Heligmosomoides polygyrus bakeri (Hp) is a common model used to study immunity against helminth infection [18]. Following primary (1°) infection with Hp, C57BL/6 mice develop a chronic infection [19]. In contrast, Hp fails to establish chronicity after challenge infection, largely due to the rapid development of a protective TH2 type granuloma around the tissue invasive larvae [20] [21] [22]. The highly concentrated accumulation of Arginase-1 (Arg1) expressing alternatively activated macrophages in inflammatory lesions is a hallmark of type 2 responses associated with allergy or helminth infection [23] [24] [25]. Recent reports have demonstrated an important role for type 2 cytokine driven alternative activation of macrophages in protective immunity against intestinal helminth infection [16] [26] [27]. Previous work [28] [29], including a study from our own group [30], additionally identified helminth-specific antibodies as essential components of immunity against Hp. Passive transfer of IgG or immune serum could also confer resistance to Hp, Ascaris suum and Strongyloides ratti [31] [32] [33], and antibody production has been found to correlate with protection in human helminth infection [34] [35] [36]. Whilst antibodies contribute to protective immunity against Hp and other nematodes such as Trichuris muris or whipworms such as Trichinella spiralis, which cause chronic infections [37] [38], they are not required for rapid expulsion of the hookworm Nippostrongylus braziliensis [29]. However, the mechanism of antibody-mediated immunity against intestinal helminths has remained obscure.
In the current study we investigated the mechanisms of antibody-mediated immunity following challenge Hp infection and demonstrate that antibodies function to trap helminth larvae and to prevent parasite-induced tissue damage. Using newly developed tools for image analysis, we show that antibody-activated macrophages upregulate Arg1 and immobilize infective and tissue dwelling larvae. Intriguingly, the reprogramming of macrophages by helminth-specific antibodies did not require IL-4Rα signaling, indicating that antibody activation of macrophages during helminth infection represents a novel pathway of alternative macrophage differentiation. In summary we have shown that antibodies in the presence of helminth antigens can elicit a novel subtype of IL-4Rα independent alternatively activated macrophages, which we refer to as helminth-antibody activated macrophages (HAAM).
Results
Mice deficient in antibodies or activating antibody receptors exhibit impaired early immunity to H. polygyrus bakeri challenge infections
Our previous work on antibody mediated protective immunity against Hp has largely been focused on late time points (day 14–20) after challenge infection, when adult worms can be found in the lumen of the small intestine [30]. However, protective immunity is initiated as early as day four of challenge Hp infection [16] [29], when larvae have first invaded the intestinal mucosa. Thus, in order to investigate a potential early effect of antibodies in the memory response to Hp, we analysed the numbers of larvae that had invaded the small intestine of infected antibody-deficient JH−/− mice (for accession numbers of gene name abbreviations used in this manuscript see Table 1). JH−/− mice carry a deletion of the joining fragment of the immunoglobulin heavy chain locus and are devoid of mature B-cells and antibodies [39]. As shown in Fig. 1A, and in line with previous work [29], JH−/− harbored significantly higher numbers of L4 on day 4 after challenge infection. As previous work indicated a small but significant contribution of cellular activation via activating Fc receptors on the numbers of adult worms [30], we additionally assessed numbers of larvae in challenge infected mice lacking activating Fc receptors (FcRγ−/−). Interestingly, these mice also exhibited clearly increased larval burdens (Fig. 1A).
Fig. 1. Antibody and FcRgamma-chain deficient mice show increased larval burdens, larger granulomas, a stronger tendency to develop necrosis and higher in-tissue motility as compared to wildtype mice. 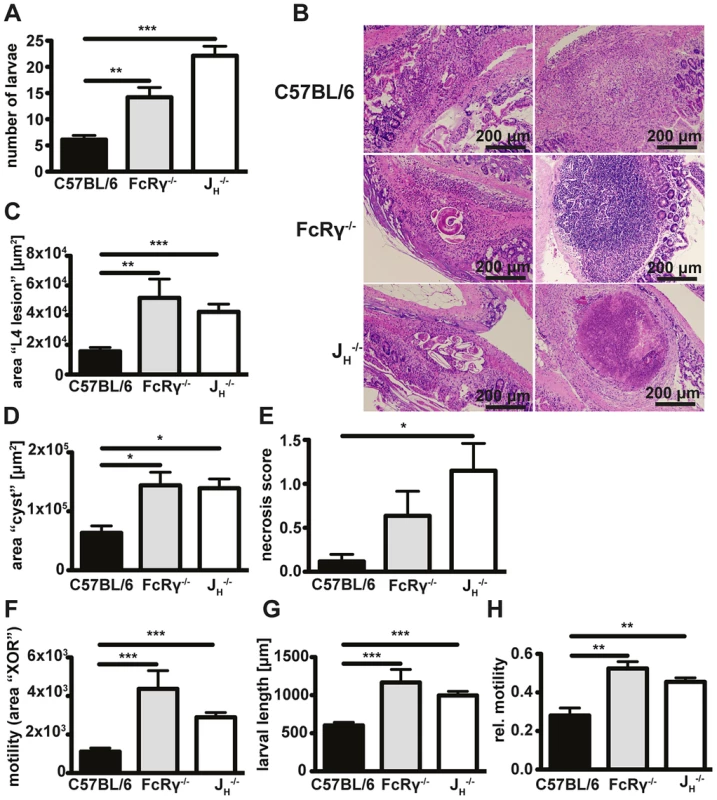
Mice were challenge infected with 200 infective L3 larvae after drug cured 1° infection and sacrificed on day 4 p.i.; (A) Numbers of tissue invasive larvae were analysed with a modified Baermann apparatus. (B) Paraffin sections of the duodenum of infected mice were hematoxylin and eosin (H&E) stained, (C/D) granuloma areas were quantified in light microscopy images of the largest cross-section of serial sections with larvae (C) or without larvae (D). (E) Necrosis in H&E-stained sections was scored by a blinded pathologist. (F–H) Absolute (F) and relative (H) in-tissue motility or larval length (G) was assessed by light microscopy combined with our Fiji macro. Pooled data of two independent experiments with 5 mice per group are shown as mean + SEM (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney test). Tab. 1. Accession numbers for abbreviations of gene name used in the text. 
Antibody and FcRgamma-deficient mice exhibit larger granulomas, a tendency to develop necrosis and greater larval motility
In addition to the increased numbers of larvae, we observed that JH−/− and FcRγ−/− mice exhibited larger granulomas at day 4 post challenge Hp infection. Assessment of hematoxylin and eosin (H&E) stained tissue sections revealed that granulomas in all sets of mice were characterized by epitheloid macrophages and eosinophils and often contained cuticle remains or intact larvae (Fig. 1B). Quantification of the inflamed area for lesions containing intact larvae (Fig. 1B, left column), or for lesions without intact larvae (Fig. 1B, right column), showed that JH−/− and FcRγ−/− mice had indeed developed more extensive granulomas around intact larvae (Fig. 1C) and larger “cysts” without visible larvae (Fig. 1D). Finally, pathological scoring of H&E stained granuloma sections showed an increased tendency to develop necrosis in both JH−/− and FcRγ−/− mice (Fig. 1E).
We rationalized that a failure to efficiently trap larvae in the granuloma might contribute to the observed increase in tissue necrosis. As the assessment of larval numbers by a modified Baermann apparatus only gives an indirect measure of larval motility [16], we quantified in-tissue motility directly by microscopy. Our analysis of time-lapse movies (Text S1, Fig. S1) showed that in-tissue motility was increased in the absence of antibodies or activating antibody receptors (Movie S1, Fig. 1F). During our analysis, we also noted pronounced differences in the size of larvae between wildtype, FcRγ−/− and JH−/− mice (Fig. 1G). We therefore calculated the difference in the larval position after 60s relative to the total larval area. In FcRγ−/− and JH−/− mice, the movement of the larvae was still observed to be greater than that seen for wildtype mice even when the movement was normalized to larval size (Fig. 1H).
Cellular compositions of granulomas are similar in wildtype and antibody-deficient mice
Due to the apparent involvement of cellular activation via Fc receptors in antibody-mediated protection against Hp (Fig. 1) we performed a flow cytometric analysis of granuloma cell populations, with a focus on macrophages and granulocytes as major FcR expressing cells. As the absence of antibodies resulted in a defective immune response already at day 4 post challenge infection, we compared granuloma cell populations from challenge infected JH−/− and wildtype mice by flow cytometry using the gating strategy described in Fig. S2A. No significant differences in the cellular infiltrate were noted between the two strains, with the exception of basophils that were absent from the granuloma of antibody-deficient mice (Fig. S2B). The absence of basophils observed in JH−/− mice was in keeping with our previous work, showing an important role for antibodies in basophil expansion during Hp infection [40]. However, basophils represent a minor cell population in the granuloma and basophil depletion during challenge infection only had a minor impact on protective immunity [40]. Thus, we concluded that antibody-FcRγ-chain-mediated protection is likely to involve other cell types.
Granuloma macrophages express high levels of antibody receptors and efficiently bind IgG1 and IgG3
The similarities in the cell recruitment between wildtype and antibody deficient mice suggested that antibodies might differentially activate resident or recruited cells at the site of infection. As our previous data indicated a predominant role of IgG [30] we analysed surface IgG on macrophages (F4/80high, Ly6G−), eosinophils (SSChigh, SiglecF+) and neutrophils (Ly6G+ Gr1+) by flow cytometry. Our analysis showed that macrophages, which were the most abundant cell type in day 4 granulomas, displayed the highest surface levels of IgG, with eosinophils exhibiting moderate binding and neutrophils very little binding (Fig. 2A, left panel). We then confirmed the different IgG binding capacities of macrophages and eosinophils in vitro with bone marrow derived cells incubated with Hp immune serum (Fig. 2A, right panel). Further characterization of granuloma macrophages by flow cytometry showed high surface expression of activating FcγRs (CD64 and CD16) as well as CD11b, which is involved in complement-mediated immune complex binding [41] (Figs. 2B, S2C). In addition, macrophages displayed low levels of IgE but considerable levels of IgG1 and IgG3 on their surface (Fig. 2C).
Fig. 2. Macrophages are the major IgGhigh population in the granuloma following challenge Hp infection and express high levels of antibody and complement receptors. 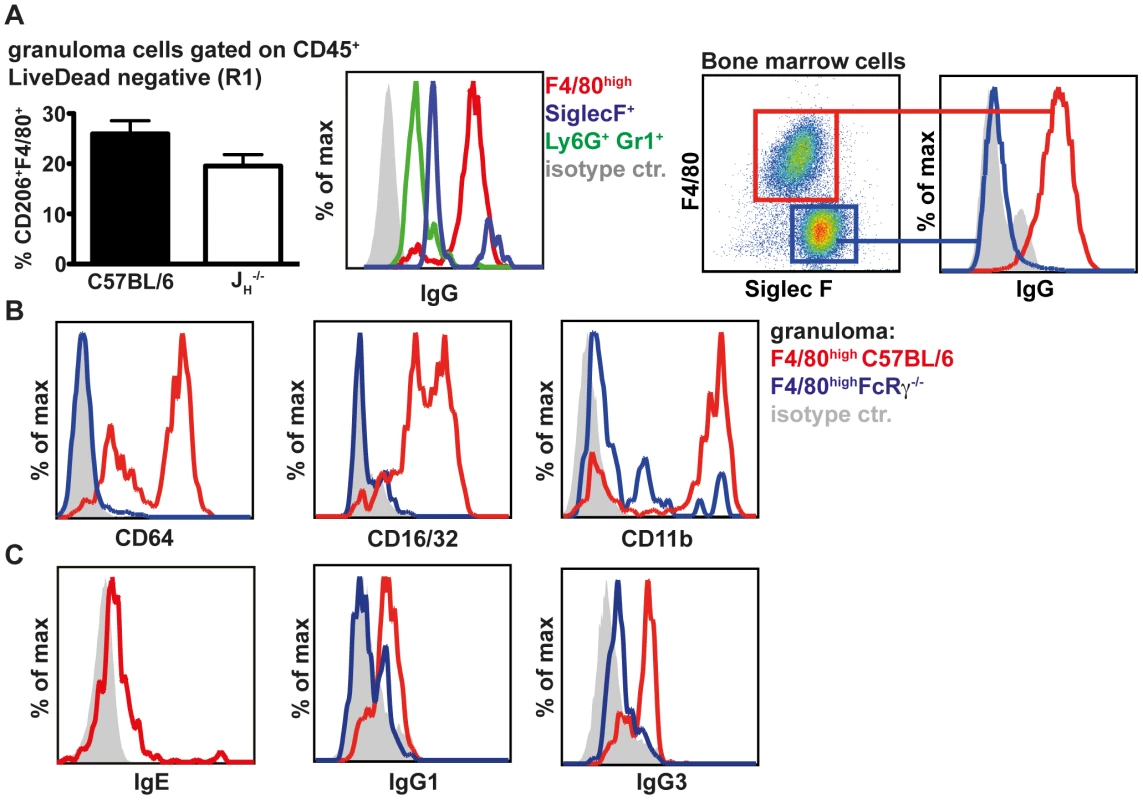
(A) 25–30 granuloma were excised from the small intestine of challenge Hp infected mice on day 4 p.i. and isolated granuloma cells (left panels) or immune serum activated bone marrow derived cells (right panels) were stained for cell surface markers and IgG. (B) Expression of CD64, CD16/32 and CD11b on granuloma macrophages from C57BL/6 or FcRγ−/− mice; (C) Levels of IgE, IgG1 and IgG3 on granuloma macrophages from C57BL/6 or FcRγ−/− mice; Representative FACS plots from two independent experiments with 4–5 mice per group are shown. When comparing granuloma macrophages in challenge Hp infected wildtype and FcRγ−/− mice, we observed that FcRγ−/− mice displayed reduced levels of surface IgG1 and IgG3 (Figs. 2C, S2C). Interestingly CD11b levels were also reduced on granuloma macrophages of FcRγ−/− mice (Fig. S2C). Thus, in addition to the engagement of activating Fc receptors, helminth-antigen-antibody immune complexes might bind to CD11b in a complement dependent manner [42] [43] [44].
Antibodies act to upregulate Arginase-1 expression by macrophages in vitro and in vivo
We next investigated potential differences in the activation of macrophages in the presence or absence of specific antibodies following challenge infection with Hp. As RNA samples from granulomas were not of sufficient quality for microarray analysis, we performed in vitro experiments with bone marrow-derived macrophages (BMMac) that we incubated with Hp immune serum (1∶50, v∶v) and infective Hp L3 larvae (500 larvae/106 cells). Gene expression was compared using microarray analysis of macrophages incubated with larvae alone or with larvae in combination with immune serum. The microarray analysis identified a total of 216 genes that were differentially expressed (up - or down - regulated more than 1.5 fold). Among the ten most downregulated genes, we identified several TH1 associated genes such as interleukin 12b (IL12b), interferon regulatory factor 1 (Irf1) and interferon inducible GTPase 1 (Iigp1). However for our further experiments, we focused on the ten genes that were found to be upregulated more than two-fold by the combination of immune serum and larvae. Of note, these included several factors involved in tissue repair processes such as angiogenesis, cell proliferation, and remodeling as well as granulocyte recruitment and activation, or type 2 immunity (Table 2). Subsequent qPCR analysis confirmed the significant up-regulation of CXCL3, CXCL2, IL-33, Jag1 and Arg1 (Figs. S3, 3A). However, Arg1 was the only gene that was upregulated to a lesser extent in macrophages from FcRγ−/− mice. Moreover, Arg1 was moderately upregulated when BMMac were stimulated with larvae and purified 2° IgG (100 µg/ml), which could be greatly enhanced, when naïve serum was added together with 2° IgG and larvae (Fig. S3J). Hence, helminth-specific IgG can reprogram macrophages and this effect is amplified by the presence of complement components.
Fig. 3. Arg1 is induced by immune serum and Hp larvae in vitro and Arg1 expressing macrophages are less abundant in the granulomas from antibody and FcRgamma-chain deficient mice. 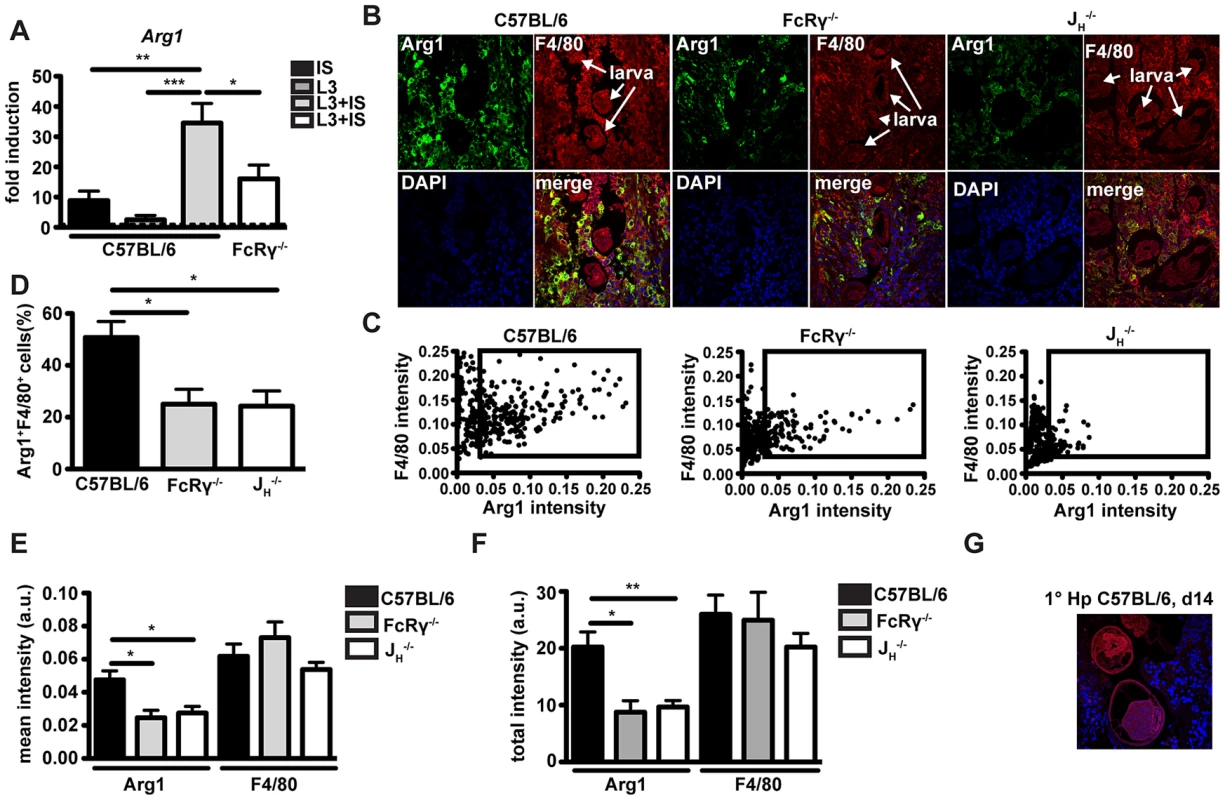
(A) Arg1 expression in BMMac from C57BL/6 or FcRγ−/− mice cultured in the presence or absence of larvae and/or immune serum was quantified by qPCR. (B) Paraffin sections of the upper duodenum of challenge Hp (day 4 p.i.) infected C57BL/6, FcRγ−/− or JH−/− mice were immune-fluorescently stained for Arg1 (green), F4/80 (red) and nuclei (blue), representative pictures are shown. (C–F) Intensities of Arg1 and F4/80 were quantified using Fiji and Cell Profiler software. (C) Scatter plots of intensities for F4/80 and Arg1 for all detected cells in the region of interest (ROI) (D) Frequency of Arg1+ F4/80+ (intensity >0.035) cells in the ROI, (E) Mean intensity of Arg1 and F4/80 staining in all detected cells in the ROI (F) Total intensity of Arg1 and F4/80 staining per ROI; Pooled data from two independent experiments with 3–6 mice per group are shown as mean + SEM (*p<0.05, **p<0.01); (G) Arg1+ macrophages are absent from the granuloma of 1° Hp infected mice (day 14 p.i.), representative picture. Tab. 2. Fold induction and function of the 10 most upregulated genes in macrophages co-cultured with immune serum in combination with larvae as compared to larvae alone. 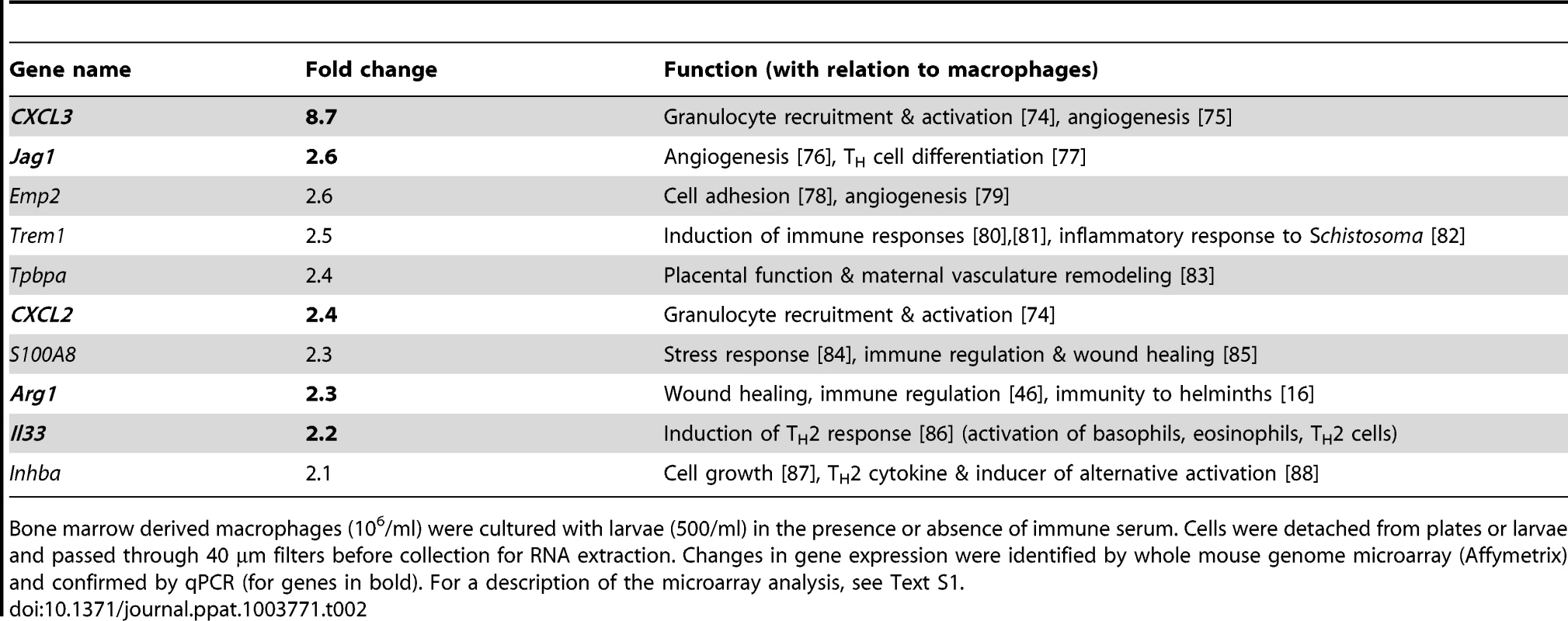
Bone marrow derived macrophages (106/ml) were cultured with larvae (500/ml) in the presence or absence of immune serum. Cells were detached from plates or larvae and passed through 40 µm filters before collection for RNA extraction. Changes in gene expression were identified by whole mouse genome microarray (Affymetrix) and confirmed by qPCR (for genes in bold). For a description of the microarray analysis, see Text S1. We next investigated the impact of antibodies on Arg1 protein expression by macrophages in vivo. Arg1 expression in granuloma sections from wildtype, JH−/− or FcRγ−/− mice was analysed by immunofluorescence and confocal microscopy (Fig. 3B). In keeping with our gene expression data, granulomas of antibody-deficient mice harbored significantly lower numbers of Arg1high macrophages (Fig. 3B). Quantification of the intensities of the Arg1 and F4/80 staining for each cell resulted in scatter plots, which clearly showed that most F4/80+ cells expressed Arg1 in the granuloma of C57BL/6 but not of FcRγ−/− or JH−/− mice (Fig. 3C). Furthermore, significant differences were noted not only for the frequency of Arg1high macrophages (Fig. 3D) but also for the mean Arg1 intensity in all detected cells (Fig. 3E) and the total Arg1 intensities in the region of interest (Fig. 3F). Of note, we could not detect any Arg1 expressing cells in the granuloma of 1° Hp infected C57BL/6 mice (Fig. 3G), indicating that effector mechanisms that arise only following challenge infection are required for Arg1 upregulation.
Immune serum from challenge-infected mice induces the adherence of macrophages to Hp larvae in vitro
In our in vitro co-cultures of bone marrow derived macrophages (BMMac) and larvae, we observed that immune serum from challenge Hp infected C57BL/6 mice (collected at day 4 p.i.) induced the adherence of macrophages to larvae (Fig. 4A). By contrast, serum from 1° Hp infected C57BL/6 or challenge Hp infected JH−/− mice showed an attenuated capacity to induce macrophage adherence (Fig. 4A). Immune serum-induced adherence could be confirmed using macrophages from the peritoneal cavity of naïve mice (Fig. S4A), or using BMMac of a different genetic background (Balbc) (Fig. S4B). As IgG+ eosinophils were also present in the granuloma, we additionally tested the ability of these cells to adhere to larvae. Bone marrow-derived eosinophils activated with immune serum hardly adhered to larvae, and addition of these cells to macrophage cultures decreased rather than increased macrophage adherence (Fig. S4B). These data indicate that Hp-specific antibodies predominantly act on macrophages to promote cellular adherence to larvae.
Fig. 4. Antibodies from challenge Hp infected wildtype mice induce adherence of bone marrow derived macrophages to Hp larvae in vitro, causing larval immobilization dependent on FcRgamma-chain and complement component 3; Larval infectivity and viability is not affected by adhering macrophages. 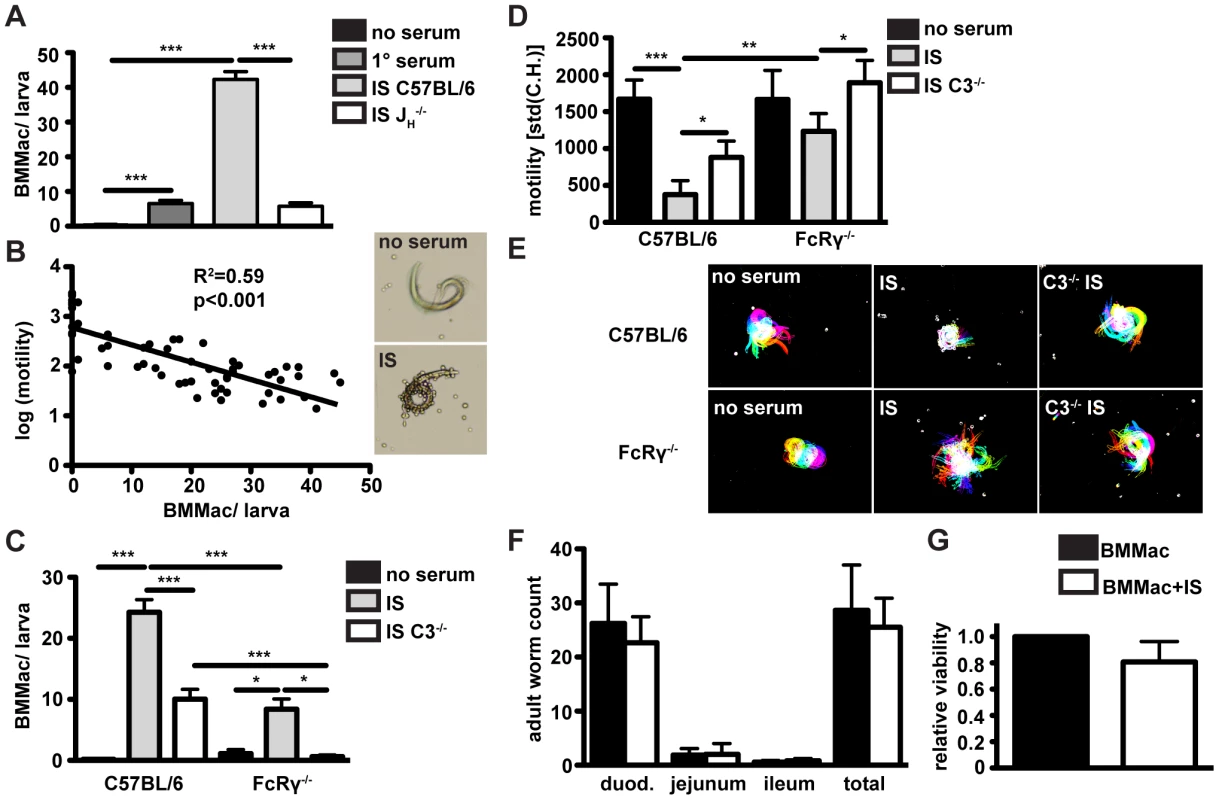
(A) BMMac from C57BL/6 mice were co-cultured with larvae in the presence or absence of serum from 1° or challenge Hp infected C57BL/6 or JH−/− mice, adherent macrophages per larva were counted in light microscopy images. (B) Correlation of the number of adhering BMMac and larval motility, (C/D) C57BL/6 or FcRγ−/− BMMac were cultured with larvae in the presence or absence of immune serum from C57BL/6 or FcRγ*C3−/− mice. Adherent macrophages per larva were counted in light microscopy images (C) and larval motility was quantified by Fiji (D). (E) Representative time lapse pictures, (F) Larval infectivity was analysed by infecting C57BL/6 wildtype mice with 100 larvae recovered from BMMac co-cultures in the presence or absence of immune serum. Adult worms in the three parts of the small intestine were counted on day 10 p.i.. (G) The larval viability relative to control (cultured without BMMac) after co-culture with or without immune serum was quantified by ATP-assay. Pooled data from three independent experiments with bone marrow from 2–3 mice or with 5 mice per group are shown as mean + SEM (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney test). To rule out a possible contribution of IL-4 from immune serum we determined the role of this cytokine in macrophage adherence. Addition of IL-4 to macrophage larvae co-cultures did not alter immune serum-induced macrophage adherence and larval immobilization (Fig. S5A) or larval fitness (Fig. S5B), and levels of IL-4 or IL-13 present in the immune serum or macrophage-larvae co-culture supernatants were all below the ELISA detection limit (Figs. S5C, D).
Macrophages immobilize Hp larvae in an antibody-, FcγR - and complement-dependent manner
We also noted that larvae covered in helminth-antibody activated macrophages exhibited a striking reduction of larval motility. To analyze this in a quantitative manner we developed a method to analyze larval motility in time-lapse movies. This analysis showed a clear correlation between the number of adherent macrophages per larvae and the reduction in larval motility, measured as the difference in the larval shape between consecutive frames of time-lapse movies (Fig. 4B) (detailed description Fig. S1B–D). We next explored the role of activating FcRs and complement in immune serum-induced macrophage adherence to Hp larvae. When co-cultured with larvae in the presence of immune serum, FcRγ−/− macrophages adhered less efficiently to larvae as compared to wildtype macrophages (Fig. 4C). Using BMMac from mice lacking all IgG receptors (FcγRI/II/III/IV−/−), we confirmed the important role of IgG for efficient trapping of larvae (Fig. S4E). As expected, FcγRI/II/III/IV−/− displayed no detectable surface IgG, CD16/32 (FcγRII/III) or CD64 (FcγRI) and both FcγRI/II/III/IV−/− and C57BL/6 BMMac did not show IgM binding (Fig. S4F). To rule out a possible role of IgE for larval trapping, we studied macrophage adherence and larval immobilization, using immune serum from challenge infected IgE−/− mice. IgE-deficient immune serum potently induced BMMac adherence as well as larval immobilization (Figs. S4G, H).
As for FcRγ−/− mice, our previous work had shown a small but significant contribution of complement to reducing adult worm numbers following challenge Hp infection [30]. Thus to determine a possible contribution of complement in larval immobilization we used complement component 3 (C3)-deficient immune serum in our macrophage assays. C3-deficient immune serum had a clearly reduced ability to promote macrophage adherence to wildtype macrophages and adherence of macrophages from FcRγ−/− mice was completely abolished (Fig. 4C). Moreover, FcRγ−/− macrophages had a lower capacity to trap larvae in the presence of wildtype immune serum, and failed completely to immobilize larvae when incubated with C3-deficient immune serum (Figs. 4D/E) (Movie S2). These data indicate that immune serum acts to promote macrophage adherence and larval immobilization in a manner involving the contribution of activating FcRs and complement.
Of note, immune serum also triggered adherence of BMMac to tissue dwelling L4 stage larvae, which we recovered from the small intestine of infected mice. Although L4 larvae were less motile than infective L3 larvae, immune-serum induced macrophage adherence also had a negative effect on their motility in vitro (Movie S3).
In order to study a potential effect of immune serum-induced macrophage adherence on larval fitness, we next infected mice with larvae recovered from immune serum-supplemented macrophage co-cultures. Adult worm counts on day 10 p.i. indicated that larvae cultured with BMMac and immune serum for 24h were still as infective as larvae cultured with macrophages alone (Fig. 4F). As another measure of larval fitness, we quantified ATP levels in larval homogenates following their recovery from macrophage co-cultures. Again we could not detect any significant difference in the viability after culture with BMMac alone, or with a combination of immune serum and BMMac (Fig. 4G). Taken together these data suggest that helminth-antibody-dependent macrophage activation promotes their adherence to result in an impact on larval motility but not on larval viability.
Absence of Arginase-1 reduces immune serum-induced larval trapping
Our previous data demonstrated that antibody-FcγR interactions upregulated Arg1 gene expression in macrophages co-cultured with larvae in vitro, and were necessary for Arg1 protein expression by granuloma macrophages in vivo. Moreover Arg1 has previously been reported to contribute to protective immunity against Hp [16]. We therefore investigated a possible contribution of this enzyme to macrophage adherence and larval immobilization. We performed in vitro co-cultures of bone marrow-derived macrophages from Arg1f/fTie2-Cre [45] [46] or Tie2-Cre [47] mice with Hp larvae in the presence of immune serum. The Tie2-Cre deleter was originally reported to result in Cre recombinase mediated gene ablation in endothelial cells [47], but was later discovered to show robust (80–90%) recombination also in hematopoietic cells [48]. We confirmed Arg1f/f and Tie2-Cre transgene expression and partial deletion of Arg1 in Arg1f/fTie2-Cre mice (Figs. S5H–J). We could not observe any difference in the capacity of Arg1f/fTie2-Cre and wildtype Arg1Tie2-Cre macrophages to adhere to larvae (Fig. 5A). However, Arg1f/fTie2-Cre macrophages had a reduced ability to immobilize larvae after incubation with immune serum (Figs. 5B, C) (Movie S4). To further confirm the role of Arg1 in antibody - mediated larval trapping, we added the Arg1 inhibitor S-(2-boronoethyl)-L-cysteine (BEC) to co-cultures of BMMac and larvae in the presence of immune serum. Addition of BEC in vitro resulted in reduced macrophage adherence and clearly impaired larval immobilization (Figs. 5, E).
Fig. 5. Arg1 is needed for to efficient larval trapping in vitro and in vivo and L-ornithine or polyamines but not urea can reduce larval motility in vitro. 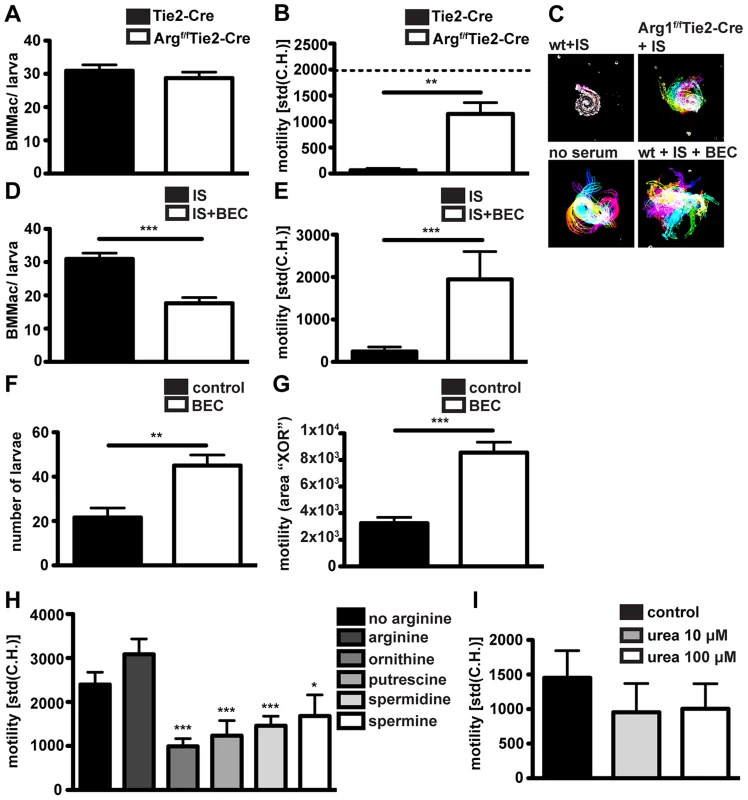
(A/B) BMMac from Tie2-Cre or Argf/fTie2-Cre mice were co-cultured with larvae in the presence or absence of immune serum from C57BL/6 mice and adherence (A) and motility (B) were assessed by light microscopy. Dashed line in panel B depicts motility of larvae in the presence of BMMac without addition of immune serum. (C) Representative time lapse pictures; (D/E) Adherence and motility of C57BL/6 BMMac in the presence of the Arg1 inhibitor BEC (1 µM); (F/G) C57BL/6 mice were treated with BEC (0.2%) during challenge infection and larval motility (F) and numbers (G) were analysed; (H/I) Larvae were cultured in L-arginine free medium with or without supplementation with L-arginine (570 µM) +/− L-ornithine, putrescine, spermidine or spermine (each 100 µM) (H) or urea (I) and motility was quantified. Pooled data from two independent experiments (3–6 mice per group) are shown as mean + SEM (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney test). Finally, we tested the relevance of Arg1 in larval trapping in vivo, by treating challenge-infected C57BL/6 mice with BEC (0.2%, oral gavage, once daily). In keeping with previous findings [16], BEC treatment resulted in higher larval burdens (Fig. 5F). Moreover, larvae in BEC treated mice showed higher in-tissue motility (Fig. 5G, Movie S5).
Arg1 catalyzes the breakdown of L-Arginine into urea and L-ornithine, and BMMac produced considerable amounts of urea when activated with immune serum and Hp larvae (Fig. S5F). We first investigated a potential effect of L-arginine depletion by culturing larvae in L-arginine-free medium. However, we did not observe significant changes in the larval motility in the absence of L-arginine (Fig. 5H). As larvae are surrounded by large numbers of Arg1 expressing macrophages in challenge Hp infected mice, trapped larvae are likely to be exposed to high levels of Arg1 products. To replicate this process in vitro we incubated larvae with L-ornithine or polyamines (putrescine, spermidine and spermine) or urea. While urea did not significantly affect larval motility (even at 100 µM) (Fig. 5I), L-ornithine and polyamines clearly impaired larval movement (Fig. 5H, Movie S6). These data implicate L-arginine metabolism to polyamines as a possible mechanism by which Arg1 contributes to protective immunity against Hp.
Antibodies can induce Arg1 independently of IL-4Rα signaling
IL-4 has long been recognized as a major factor responsible for the upregulation of Arg1 [49]. Thus, we analysed Arg1 expression in BMMac after culture with larvae and immune serum in the presence of IL-4. IL-4 alone clearly induced Arg1 to similar levels as observed for immune serum (Fig. 6A), and the combination of IL-4 plus immune serum resulted in a slightly stronger Arg1 induction, both on the mRNA and enzymatic activity level (Figs. 6A, B).
Fig. 6. Antibodies can induce Arg1 and larval trapping by macrophages independently of IL-4Ralpha signaling. 
(A) Arg1 expression in BMMac treated with larvae and immune serum and/or IL-4 or was quantified by qPCR and calculated relative to untreated control (B) Urea production in macrophages (treated as in (A)) was quantified after performing an Arg1 activity assay. (C) Sections of the small intestine from challenge-infected C57BL/6 or IL-4Ralpha−/− mice, treated with immune or naïve serum were stained for Arg1 (green) and F4/80 (red) and counterstained with DAPI (blue). Representative pictures from two independent experiments with 3–4 mice per group are shown. (D) Frequencies of Arg1+F4/80+ cells, mean intensities or total intensities of Arg1 and F4/80 were quantified using Fiji and Cell Profiler software. Dashed line depicts background fluorescence (E). Arg1 expression in wildtype or IL-4Ralpha−/− BMMac treated with larvae and immune serum was quantified by qPCR. (F/G) BMMac adherence (F) and larval motility (G) were analysed after co-culture with larvae in the presence or absence of immune serum. (H/I) Adherence (H) and motility (I) after treatment with larvae and purified 2° IgG −/+ naïve serum (NS). Dashed line depicts motility of larvae with BMMac alone. Pooled data from two independent experiments with 3–4 mice per group are shown as mean + SEM (*p<0.05, **p<0.01, ***p<0.001, Mann-Whitney test). Arg1 expression during helminth infection is normally associated with an AAM phenotype dependent upon IL-4Rα signaling [49] [16] [50]. Hence, we further investigated the role of IL-4Rα signaling on antibody-induced upregulation of Arg1 following challenge Hp infection. As IL-4Rα−/− mice exhibit a strongly impaired antibody response during primary and challenge infections with Hp [40], we treated challenge-infected mice with immune serum (collected at day 4 p.i.) from immune C57BL/6 mice. As shown in Fig. 6C, IL-4Rα−/− mice showed reduced numbers of F4/80+ macrophages surrounding tissue dwelling larvae. However, treatment with immune serum did result in an increase in Arg1+ macrophages within the granuloma of IL-4Rα−/− mice, indicating that Hp-specific antibodies can induce Arg1 expression in vivo independently of IL-4Rα signaling (Figs. 6C, D). To confirm that the ability of antibodies to trigger Arg1 expression in macrophages occurs independently of IL-4Rα signaling, we performed co-cultures of IL-4Rα−/− BMMac with Hp larvae. As shown in Fig. 6E, Arg1 was potently upregulated in IL-4Rα−/− BMMac in response to immune serum and larvae. Taken together these data indicate that IL-4 and antibodies can upregulate expression of Arg1 independently, but when present together will act in an additive manner.
Lastly, we investigated a potential role of IL-4Rα and other effector mechanisms that may contribute to immune serum-triggered larval trapping by macrophages. We observed that IL-4Rα−/− BMMac displayed a significantly reduced capacity to adhere (Fig. 6F), but were still able to immobilize larvae (Fig. 6G). Furthermore, IgG antibodies purified from challenge immune serum promoted weak adherence and moderate larval immobilization, which could be greatly enhanced by naïve serum (Figs. 6H, I). These data suggest that helminth specific antibodies together with complement components can trigger larval trapping even in the absence of other effector molecules that may be present in challenge immune serum.
Discussion
Numerous studies have documented a role for antibodies in providing protective immunity against helminths [34] [35] [36] [51] [37] [28] [30] [29], yet the mechanisms by which antibodies act against these large multicellular parasites have remained elusive. Our current work demonstrates a novel effector function of antibodies in activating macrophages to modulate the expression of genes normally associated with the alternatively activated phenotype. Antibody-mediated macrophage activation also triggered macrophage adherence to helminth larvae and resulted in a potent suppression of larval motility.
Interestingly, activating Fc receptors and complement C3 component acted together to activate macrophages and to limit larval motility in vitro. Crosstalk between the complement cascade and activating Fc receptors is well known to occur in autoimmunity [52]. We have previously shown that genetic ablation of either FcRγ-chain or the complement component C3 led to a significant but minor defect in the emergence of adult worms following challenge Hp infection between days 14–28. Our current data suggest that these two pathways act in a redundant and additive manner to promote antibody-induced larval trapping and to provide immunity against challenge Hp infections. The rapid activation of cells via Fc receptors is likely to be of particular importance during the early response (day 4) against the tissue dwelling larvae, as mice genetically deficient for FcRγ or B cells exhibited similar increases in larval motility and tissue necrosis within intestinal granulomas. The tissue dwelling stage of helminth larvae has been suggested to be a primary target of antibody-mediated protective immunity, which may be advantageous due to the immune-suppressive capacities of adult worms [53]. Our data further suggest that the antibody-mediated activation of granuloma macrophages mainly involves IgG1 and/or IgG3 isotypes, which may form immune complexes with helminth antigens or directly bind to the larval surface. As essential components of the larval cuticle are shared between different helminths, future work should delineate the specificities of protective antibody isotypes in order to identify potential vaccine targets.
Interestingly, although immune serum efficiently activated macrophages to immobilize larvae in vitro, it did not impact on larval viability. Given that the destruction of a large extracellular parasite such as Hp is likely to result in severe inflammation, the trapping and starvation of invasive larvae might be more beneficial for the host. In line with this view we observed that type 2-associated antibody production functioned to limit tissue necrosis and to induce tissue repair genes. Thus, type 2 immunity may act to limit larval migration through tissues, leading to a halt in the parasitic lifecycle and additionally serving to prevent excessive tissue damage. However we cannot rule out the possibility that other immune-mediated mechanisms also contribute to the killing of helminth larvae in vivo.
Of particular interest, arginase was required for the ability of helminth-antibody activated macrophages to inhibit larval motility as well as for efficient larval trapping in vivo. Arg1 expressing AAM are strongly associated with helminth infection [16] [54] [46] [55], and are typically regarded as controlling inflammation and tissue repair [46] [55] rather than as protective immune cells [56]. However, in keeping with our data Anthony et al. [16], previously reported a protective role for Arg1 expressing AAM in Hp infection. Our work expands on these findings to indicate that antibodies are essential for the robust Arg1 expression by macrophages following Hp infection. We also show that the Arg1 product L-ornithine and the polyamine metabolites putrescine, spermidine and spermine have a direct negative effect on larval motility, suggesting that excessive polyamines can impact on larval metabolism in a manner that interferes with motility. The finding that L-ornithine immobilizes larvae at concentrations that are below usual serum levels of this metabolite (around 50 µM) is surprising especially as granuloma may be expected to be a “leaky” environment. However, under homeostatic conditions, cell-free L-ornithine levels in the intestine might be relatively low due to the co-expression of Arginase 2 and L-ornithine metabolizing enzymes in enterocytes [57]. Thus, Arg1 activity might serve not only to control aberrant inflammation and fibrosis during parasite infection [46], but to also create a metabolically unfavorable environment for parasites. Future studies will be required to delineate the exact mechanism(s) by which polyamines affect larval motility.
Of note, IL-4Rα−/− mice failed to accumulate the large numbers of macrophages that were observed in the granuloma of wildtype mice, indicating that IL-4Rα signaling is necessary for the recruitment and/or expansion of macrophages at the site of infection [16] [58]. Thus, even if macrophages could immobilize larvae in the absence of IL-4Rα signaling in vitro, IL-4Rα signaling might be essential for the accumulation of macrophages in vivo, which can then be activated by antibodies to immobilize tissue invasive larvae. This suggests that in vivo, antibodies and IL-4 may work together to elicit a potent activation of Arg1 expressing macrophages, which mediate protection. This might be due to the convergence of IL-4Rα and FcRγ-chain signaling at the level of downstream signaling events such as Spleen tyrosine (Syk) or PI3 kinases [59] [60] [61]. In a lung model of Schistosoma infection, Arg1 expression in the granuloma was found to be tightly concentrated around the egg despite abundant AAM and type 2 cytokines (IL-4 and IL-13) in the overall granuloma environment [25]. Thus, it is tempting to speculate that antibodies directed against helminth eggs or larvae can promote local Arg1 expression in areas of high antigen availability.
Previous publications have indicated that macrophages activated by type 2 cytokines express high levels of Arg1 [16] [49] [27], whilst macrophages activated by immune complexes and TLR ligands in vitro express high levels of IL-10 and low levels of Arg1 [62]. In our hands, antibodies together with helminth larvae elicited potent Arg1 expression even in the absence of IL-4Rα signaling. Interestingly, immune complexes were previously shown to induce a robust FcR - and complement-mediated activation of C/EBPbeta, and C/EBPbeta has previously been associated with Arg1 induction in response to bacterial infection [63] [45], indicating it may also play a role in our model. Importantly the prominent expression of Arg1 by helminth-antibody activated macrophages did not correlate with other markers typical of AAM, such as Relmα. Thus the helminth-antibody activated macrophages described in this study seem to represent a novel “regulatory” macrophage type, which expresses Arg1 and wound healing genes in the absence of Relmα. The exact role of these helminth-antibody activated macrophages in tissue repair during the resolution phase of intestinal helminth infection would be of great interest for future studies.
In addition to the “canonical” pathway of Arg1 induction via the IL-4/IL-13/Stat6 pathway [64], Arg1 expression can be elicited via MyD88/IL-6/Stat3 and adenosine dependent mechanisms [65] [66]. Here, we have identified a novel pathway, which leads to the induction of Arg1 in the context of an adaptive type 2 response and which involves antibody-helminth interactions. The existence of multiple pathways for the induction of Arg1 may serve to achieve redundancy in an essential anti-inflammatory effector mechanism functional in many settings of infection, inflammation and wound healing.
In summary, we have demonstrated that antibodies mediate the activation of macrophages resulting in Arg1 expression and immobilization of tissue invasive helminth larvae. Macrophages often form a large component of the inflammatory infiltrate following allergen challenge or helminth infection, and our data indicate that antibodies form a previously unrecognized component of macrophage regulation during type 2 immune responses.
Materials and Methods
All animal experiments were approved by the office Affaires vétérinaires (1066 Epalinges, Canton Vaud, Sitzerland) with the authorization Number 2238 according to the guidelines set by the service de la consummation et des affaires vétérinaires federal (Canton Vaud, Switzerland).
Mice
C57BL/6, BALB/c, JH−/−, FcRγ-chain−/− [67], C3−/− [68], IgE−/− [69] and IL-4Rα−/− [70] were bred and maintained under specific pathogen free conditions at the Ecole Polytechnique Fédérale (EPFL) de Lausanne, Switzerland. FcγRI/II/III/IV−/− mice were bred and maintained at Leiden University, Netherlands and Arg1fl/flTie2-Cre [45] and Tie2-Cre [47] mice were bred and maintained at New Jersey Medical School, USA. The following primers were used for genotyping of Arg1fl/flTie2-Cre mice: wildtype Arg1/Arg1fl/fl: 5′-TGCGAGTTCATGACTAAGGTT-3′ (forward), 5′-AAAGCTCAGGTGAATCGG-3′(reverse); Tie2-Cre: 5′-CGCATAACCAGTGAAACAGCATTGC-3′ (forward) 5′-CCCTGTGCTCAGACAGAAATGAGA-3′ (reverse).
Infection, parasitology and collection of immune serum
C57BL/6 mice were infected with 200 Hp L3 larvae by oral gavage. Worms were cleared by treatment with two courses of Cobantril (Interdelta - Givisiez, Fribourg, Switzerland) 28 days after primary infection. 14 days later mice were challenge-infected with 200 larvae. BEC (Cayman Chemicals, Ann Arbor, MI) was administered (0.1 ml, 0.2%) once daily from the first day of infection by oral gavage. Mice were sacrificed on day 4 post infection and immune serum was collected from the inferior vena cava. Adult worms were counted under a stereomicroscope on longitudinally opened small intestines of 1° or challenge-infected mice. Tissue dwelling L4 larvae were counted after recovery with a modified Baermann apparatus [16].
Histology, immunofluorescence stainings and confocal microscopy
Serial paraffin sections were stained with hematoxylin and eosin. Granulomas were identified by light microscopy and serial sections were used for pathological scoring (details see Text S1) or immunofluorescence staining for Arginase-1 and F4/80. Stained tissue sections were imaged with an inverted point scanning confocal microscope (Zeiss LSM 710) with a Plan-Apochromat (63×/1.4 NA or 40×/1.3 NA) objective and pixel intensities were analysed using a custom built CellProfiler pipeline (for details see Text S1).
Flow cytometry
In vitro cultured cells or cells isolated from granuloma were stained with fluorescently labeled monoclonal antibodies (see Text S1) and acquired on a BD LSRII flow cytometer (BD, Franklin Lakes, NJ).
Luminescent Cell Viability Assay for assessment of larval viability
Larvae were recovered from co-cultures with macrophages with or without immune serum, washed thoroughly with enzyme free cell dissociation solution (Milipore, Billerica, MA) to remove all adherent cells, counted and homogenized in a cell/tissue homogenizer in RPMI1640 by using 0.1 mm Zirconia/Silica beads (BioSpec Products, Inc.). ATP levels in larval homogenates were analysed by CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI).
Culture and treatment of murine bone marrow cells and peritoneal macrophages
Peritoneal macrophages were isolated by plating peritoneal wash cells on petri dishes overnight and by removing non-adherent cells. Bone marrow was flushed from the femur and tibia of wildtype or transgenic mice and passed through cell strainers (70 µm). Cells (106/ml) were cultured in M-CSF (L929) supplemented medium (RPMI, 10% FCS, penicillin/streptomycin, β-mercaptoethanol) for 7 days as described previously [71]. On day 7, macrophages were harvested and stimulated for 24 h as indicated with L3 larvae (500 larvae/106 cells),(10 ng/ml) (Peprotech, Rocky Hill, NJ), or immune sera (1∶50 v/v). For differentiation into eosinophils, bone marrow cells were cultured as described [72] in the presence of Flt3 and SCF (Peprotech, Rocky Hill, NJ) for 4 days and in the presence of IL-5 (Peprotech, Rocky Hill, NJ) for subsequent 8 days. For RNA extraction, cells were re-suspended in Trizol and kept at −80°C.
Adherence of macrophages to larvae was determined by manual counting of bright field microscopy pictures taken with an Olympus AX70 microscope (UPLAN 10×/0.3 NA objective).
Measurement of larval motility
Time-lapse series (60 s: 20 frames, 3 s intervals) of larvae in the small intestine ex vivo or in vitro were acquired in order to measure their motility with an Olympus AX70 microscope (UPLAN 10×/0.3 NA objective). Larval motility was quantified by using custom-made Fiji macros (for details see Text S1).
qPCR and microarray analysis
RNA was extracted with a Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA) and reverse transcribed using RevertAid cDNA synthesis reagents (Thermo Scientific, Waltham, MA) for qPCR analysis. QPCR was performed using SYBR Green I Master Mix (Eurogentec, Liege, Belgium) on an Applied Biosystems 7900HT System (for qPCR primer sequences see Table S1). Microarray analysis was performed using Affymetrix mouse arrays (Affymetrix, Santa Clara, CA, USA) (for details see Text S1).
ELISA
Concentrations of IL-4 or IL-13 in cell culture supernatants or mouse serum were quantified by using ELISA Ready-SET-Go! Kits (eBioscience, San Diego, CA).
Arginase-1 activity assay
Macrophage arginase-1 activity was determined according to previously published methods [73]. Briefly, adherent macrophages were lysed and conversion of L-arginine was quantified indirectly by measuring urea production with a QuantiChrom Urea Assay (BioAssay Systems, Hayward, CA).
Supporting Information
Zdroje
1. BeaverPC (1975) Biology of soil-transmitted helminths: the massive infection. Health Lab Sci 12 : 116–125.
2. CromptonDW (1988) The prevalence of Ascariasis. Parasitol Today (Regul Ed) 4 : 162–169.
3. StephensonLS, HollandCV, CooperES (2000) The public health significance of Trichuris trichiura. Parasitology 121 Suppl: S73–95.
4. CooperPJ, ChicoME, LosonskyG, SandovalC, EspinelI, et al. (2000) Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis 182 : 1199–1206 doi:10.1086/315837
5. BethonyJ, BrookerS, AlbonicoM, GeigerSM, LoukasA, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367 : 1521–1532 doi:10.1016/S0140-6736(06)68653-4
6. AlbonicoM, AllenH, ChitsuloL, EngelsD, GabrielliA-F, et al. (2008) Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis 2: e126 doi:10.1371/journal.pntd.0000126
7. AppletonCC, MosalaTI, LevinJ, OlsenA (2009) Geohelminth infection and re-infection after chemotherapy among slum-dwelling children in Durban, South Africa. Ann Trop Med Parasitol 103 : 249–261 doi:10.1179/136485909X398212
8. SangsterNC (1999) Anthelmintic resistance: past, present and future. Int J Parasitol 29 : 115–124 discussion 137–138.
9. GeertsS, GryseelsB (2000) Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev 13 : 207–222.
10. GeertsS, GryseelsB (2001) Anthelmintic resistance in human helminths: a review. Trop Med Int Health 6 : 915–921.
11. GalvaniAP (2005) Age-dependent epidemiological patterns and strain diversity in helminth parasites. J Parasitol 91 : 24–30 doi:10.1645/GE-191R1
12. Taylor-RobinsonDC, MaayanN, Soares-WeiserK, DoneganS, GarnerP (2012) Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev 11: CD000371 doi:10.1002/14651858.CD000371.pub5
13. KhieuV, SchärF, MartiH, SayasoneS, DuongS, et al. (2013) Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis 7: e2035 doi:10.1371/journal.pntd.0002035
14. WynnTA, ChawlaA, PollardJW (2013) Macrophage biology in development, homeostasis and disease. Nature 496 : 445–455 doi:10.1038/nature12034
15. AderemA, UnderhillDM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17 : 593–623 doi:10.1146/annurev.immunol.17.1.593
16. AnthonyRM, UrbanJFJr, AlemF, HamedHA, RozoCT, et al. (2006) Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 12 : 955–960 doi:10.1038/nm1451
17. GordonS (2003) Alternative activation of macrophages. Nat Rev Immunol 3 : 23–35 doi:10.1038/nri978
18. CamberisM, Le GrosG, UrbanJJr (2003) Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19: Unit 19.12 doi:10.1002/0471142735.im1912s55
19. RobinsonM, WahidF, BehnkeJM, GilbertFS (1989) Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): dose-dependent expulsion of adult worms. Parasitology 98(Pt 1): 115–124.
20. UrbanJFJr, KatonaIM, FinkelmanFD (1991) Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol 73 : 500–511.
21. UrbanJFJr, KatonaIM, PaulWE, FinkelmanFD (1991) Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA 88 : 5513–5517.
22. AnthonyRM, RutitzkyLI, UrbanJFJr, StadeckerMJ, GauseWC (2007) Protective immune mechanisms in helminth infection. Nat Rev Immunol 7 : 975–987 doi:10.1038/nri2199
23. ZimmermannN, KingNE, LaporteJ, YangM, MishraA, et al. (2003) Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest 111 : 1863–1874 doi:10.1172/JCI17912
24. HesseM, ModolellM, La FlammeAC, SchitoM, FuentesJM, et al. (2001) Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol 167 : 6533–6544.
25. BarronL, SmithAM, El KasmiKC, QuallsJE, HuangX, et al. (2013) Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS ONE 8: e61961 doi:10.1371/journal.pone.0061961
26. ZhaoA, UrbanJFJr, AnthonyRM, SunR, StiltzJ, et al. (2008) Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology 135 : 217–225.e1 doi:10.1053/j.gastro.2008.03.077
27. YangZ, GrinchukV, UrbanJFJr, BohlJ, SunR, et al. (2013) Macrophages as IL-25/IL-33-Responsive Cells Play an Important Role in the Induction of Type 2 Immunity. PLoS ONE 8: e59441 doi:10.1371/journal.pone.0059441
28. WojciechowskiW, HarrisDP, SpragueF, MousseauB, MakrisM, et al. (2009) Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 30 : 421–433 doi:10.1016/j.immuni.2009.01.006
29. LiuQ, KreiderT, BowdridgeS, LiuZ, SongY, et al. (2010) B cells have distinct roles in host protection against different nematode parasites. J Immunol 184 : 5213–5223 doi:10.4049/jimmunol.0902879
30. McCoyKD, StoelM, StettlerR, MerkyP, FinkK, et al. (2008) Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4 : 362–373 doi:10.1016/j.chom.2008.08.014
31. KhouryPB, StrombergBE, SoulsbyEJ (1977) Immune mechanisms to Ascaris suum in inbred guinea-pigs. I. Passive transfer of immunity by cells or serum. Immunology 32 : 405–411.
32. BehnkeJM, ParishHA (1979) Expulsion of Nematospiroides dubius from the intestine of mice treated with immune serum. Parasite Immunol 1 : 13–26.
33. MurrellKD (1981) Protective role of immunoglobulin G in immunity to Strongyloides ratti. J Parasitol 67 : 167–173.
34. WoolhouseME, TaylorP, MatanhireD, ChandiwanaSK (1991) Acquired immunity and epidemiology of Schistosoma haematobium. Nature 351 : 757–759 doi:10.1038/351757a0
35. HagelI, LynchNR, Di PriscoMC, RojasE, PérezM, et al. (1993) Ascaris reinfection of slum children: relation with the IgE response. Clin Exp Immunol 94 : 80–83.
36. AtmadjaAK, AtkinsonR, SartonoE, PartonoF, YazdanbakhshM, et al. (1995) Differential decline in filaria-specific IgG1, IgG4, and IgE antibodies in Brugia malayi-infected patients after diethylcarbamazine chemotherapy. J Infect Dis 172 : 1567–1572.
37. BlackwellNM, ElseKJ (2001) B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infect Immun 69 : 3860–3868 doi:10.1128/IAI.69.6.3860-3868.2001
38. GurishMF, BrycePJ, TaoH, KisselgofAB, ThorntonEM, et al. (2004) IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol 172 : 1139–1145.
39. ChenJ, TrounstineM, AltFW, YoungF, KuraharaC, et al. (1993) Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol 5 : 647–656.
40. HerbstT, EsserJ, PratiM, KulaginM, StettlerR, et al. (2012) Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA 109 : 14954–14959 doi:10.1073/pnas.1117584109
41. Sanchez-MadridF, NagyJA, RobbinsE, SimonP, SpringerTA (1983) A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med 158 : 1785–1803.
42. Díaz de StåhlT, DahlstromJ, CarrollMC, HeymanB (2003) A role for complement in feedback enhancement of antibody responses by IgG3. J Exp Med 197 : 1183–1190 doi:10.1084/jem.20022232
43. HazenbosWL, HeijnenIA, MeyerD, HofhuisFM, Renardel de LavaletteCR, et al. (1998) Murine IgG1 complexes trigger immune effector functions predominantly via Fc gamma RIII (CD16). J Immunol 161 : 3026–3032.
44. DingJW, ZhouT, ZengH, MaL, VerbeekJS, et al. (2008) Hyperacute rejection by anti-Gal IgG1, IgG2a, and IgG2b is dependent on complement and Fc-gamma receptors. J Immunol 180 : 261–268.
45. El KasmiKC, QuallsJE, PesceJT, SmithAM, ThompsonRW, et al. (2008) Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9 : 1399–1406 doi:10.1038/ni.1671
46. PesceJT, RamalingamTR, Mentink-KaneMM, WilsonMS, El KasmiKC, et al. (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371 doi:10.1371/journal.ppat.1000371
47. KisanukiYY, HammerRE, MiyazakiJ, WilliamsSC, RichardsonJA, et al. (2001) Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230 : 230–242 doi:10.1006/dbio.2000.0106
48. ConstienR, FordeA, LiliensiekB, GröneHJ, NawrothP, et al. (2001) Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30 : 36–44.
49. MunderM, EichmannK, ModolellM (1998) Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol 160 : 5347–5354.
50. HerbertDR, OrekovT, PerkinsC, RothenbergME, FinkelmanFD (2008) IL-4R alpha expression by bone marrow-derived cells is necessary and sufficient for host protection against acute schistosomiasis. J Immunol 180 : 4948–4955.
51. NyindoM, KariukiTM, MolaPW, FarahIO, ElsonL, et al. (1999) Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoni infection in baboons. Infect Immun 67 : 636–642.
52. SchmidtRE, GessnerJE (2005) Fc receptors and their interaction with complement in autoimmunity. Immunol Lett 100 : 56–67 doi:10.1016/j.imlet.2005.06.022
53. HarrisNL, PleassR, BehnkeJM (2013) Understanding the role of antibodies in murine infections with Heligmosomoides (polygyrus) bakeri: 35 years ago, now and 35 years ahead. Parasite Immunol doi:10.1111/pim.12057
54. MarslandBJ, KurrerM, ReissmannR, HarrisNL, KopfM (2008) Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur J Immunol 38 : 479–488 doi:10.1002/eji.200737827
55. ChenF, LiuZ, WuW, RozoC, BowdridgeS, et al. (2012) An essential role for T(H)2-type responses in limiting acute tissue damage during experimental helminth infection. Nature Medicine 18 : 260–6 Available: http://www.ncbi.nlm.nih.gov/pubmed/22245779. Accessed 19 January 2012.
56. MurrayPJ, WynnTA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11 : 723–737 doi:10.1038/nri3073
57. OzakiM, GotohT, NagasakiA, MiyanakaK, TakeyaM, et al. (1999) Expression of arginase II and related enzymes in the rat small intestine and kidney. J Biochem 125 : 586–593.
58. JenkinsSJ, RuckerlD, CookPC, JonesLH, FinkelmanFD, et al. (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332 : 1284–1288 doi:10.1126/science.1204351
59. IndikZK, ParkJG, HunterS, SchreiberAD (1995) The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86 : 4389–4399.
60. EnnaciriJ, GirardD (2009) IL-4R(alpha), a new member that associates with Syk kinase: implication in IL-4-induced human neutrophil functions. J Immunol 183 : 5261–5269 doi:10.4049/jimmunol.0900109
61. WeisserSB, McLarrenKW, VoglmaierN, van Netten-ThomasCJ, AntovA, et al. (2011) Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol 41 : 1742–1753 doi:10.1002/eji.201041105
62. EdwardsJP, ZhangX, FrauwirthKA, MosserDM (2006) Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80 : 1298–1307 doi:10.1189/jlb.0406249
63. YanC, ZhuM, StaigerJ, JohnsonPF, GaoH (2012) C5a-regulated CCAAT/enhancer-binding proteins β and δ are essential in Fcγ receptor-mediated inflammatory cytokine and chemokine production in macrophages. J Biol Chem 287 : 3217–3230 doi:10.1074/jbc.M111.280834
64. RutschmanR, LangR, HesseM, IhleJN, WynnTA, et al. (2001) Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J Immunol 166 : 2173–2177.
65. QuallsJE, NealeG, SmithAM, KooM-S, DeFreitasAA, et al. (2010) Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal 3: ra62 doi:10.1126/scisignal.2000955
66. CsókaB, SelmeczyZ, KoscsóB, NémethZH, PacherP, et al. (2012) Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 26 : 376–386 doi:10.1096/fj.11-190934
67. TakaiT, LiM, SylvestreD, ClynesR, RavetchJV (1994) FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76 : 519–529.
68. WesselsMR, ButkoP, MaM, WarrenHB, LageAL, et al. (1995) Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA 92 : 11490–11494.
69. OettgenHC, MartinTR, Wynshaw-BorisA, DengC, DrazenJM, et al. (1994) Active anaphylaxis in IgE-deficient mice. Nature 370 : 367–370 doi:10.1038/370367a0
70. MohrsM, LedermannB, KöhlerG, DorfmüllerA, GessnerA, et al. (1999) Differences between IL-4 - and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol 162 : 7302–7308.
71. HossA, ZwarthoffEC, ZawatzkyR (1989) Differential expression of interferon alpha and beta induced with Newcastle disease virus in mouse macrophage cultures. J Gen Virol 70(Pt 3): 575–589.
72. DyerKD, MoserJM, CzapigaM, SiegelSJ, PercopoCM, et al. (2008) Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol 181 : 4004–4009.
73. AllenJE, WynnTA (2011) Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog 7: e1002003 doi:10.1371/journal.ppat.1002003
74. GeiserT, DewaldB, EhrengruberMU, Clark-LewisI, BaggioliniM (1993) The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem 268 : 15419–15424.
75. WenteMN, KeaneMP, BurdickMD, FriessH, BüchlerMW, et al. (2006) Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett 241 : 221–227 doi:10.1016/j.canlet.2005.10.041
76. BeneditoR, RocaC, SörensenI, AdamsS, GosslerA, et al. (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137 : 1124–1135 doi:10.1016/j.cell.2009.03.025
77. BailisW, Yashiro-OhtaniY, FangTC, HattonRD, WeaverCT, et al. (2013) Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity 39 : 148–159 doi:10.1016/j.immuni.2013.07.006
78. WadehraM, IyerR, GoodglickL, BraunJ (2002) The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem 277 : 41094–41100 doi:10.1074/jbc.M206868200
79. GordonLK, KiyoharaM, FuM, BraunJ, DhawanP, et al. (2013) EMP2 regulates angiogenesis in endometrial cancer cells through induction of VEGF. Oncogene doi:10.1038/onc.2012.622
80. BouchonA, DietrichJ, ColonnaM (2000) Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164 : 4991–4995.
81. BleharskiJR, KiesslerV, BuonsantiC, SielingPA, StengerS, et al. (2003) A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol 170 : 3812–3818.
82. ChengP-C, LinC-N, ChenY-J, ChangF-S, TsaihongJC, et al. (2011) Triggering receptor expressed on myeloid cells (TREM)-1 participates in Schistosoma mansoni inflammatory responses. Parasite Immunol 33 : 276–286 doi:10.1111/j.1365-3024.2011.01284.x
83. HuD, CrossJC (2011) Ablation of Tpbpa-positive trophoblast precursors leads to defects in maternal spiral artery remodeling in the mouse placenta. Dev Biol 358 : 231–239 doi:10.1016/j.ydbio.2011.07.036
84. EhrchenJM, SunderkötterC, FoellD, VoglT, RothJ (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86 : 557–566 doi:10.1189/jlb.1008647
85. PasseyRJ, XuK, HumeDA, GeczyCL (1999) S100A8: emerging functions and regulation. J Leukoc Biol 66 : 549–556.
86. Wills-KarpM, RaniR, DiengerK, LewkowichI, FoxJG, et al. (2012) Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 209 : 607–622 doi:10.1084/jem.20110079
87. PhillipsDJ, WoodruffTK (2004) Inhibin: actions and signalling. Growth Factors 22 : 13–18.
88. OgawaK, FunabaM, ChenY, TsujimotoM (2006) Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol 177 : 6787–6794.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Baculoviruses: Sophisticated Pathogens of Insects
- How Do Viruses Avoid Inhibition by Endogenous Cellular MicroRNAs?
- The Regulation of Trypanosome Gene Expression by RNA-Binding Proteins
- DNA Damage Repair and Bacterial Pathogens
- Disease to Dirt: The Biology of Microbial Amyloids
- Fungal Immune Evasion in a Model Host–Pathogen Interaction: Versus Macrophages
- Infectious Prions Accumulate to High Levels in Non Proliferative C2C12 Myotubes
- The Biology and Taxonomy of Head and Body Lice—Implications for Louse-Borne Disease Prevention
- Antibodies Trap Tissue Migrating Helminth Larvae and Prevent Tissue Damage by Driving IL-4Rα-Independent Alternative Differentiation of Macrophages
- The Effects of Somatic Hypermutation on Neutralization and Binding in the PGT121 Family of Broadly Neutralizing HIV Antibodies
- Natural Selection Promotes Antigenic Evolvability
- Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung
- Mode of Parainfluenza Virus Transmission Determines the Dynamics of Primary Infection and Protection from Reinfection
- Type I and Type III Interferons Drive Redundant Amplification Loops to Induce a Transcriptional Signature in Influenza-Infected Airway Epithelia
- Unraveling a Three-Step Spatiotemporal Mechanism of Triggering of Receptor-Induced Nipah Virus Fusion and Cell Entry
- A Novel Membrane Sensor Controls the Localization and ArfGEF Activity of Bacterial RalF
- Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity
- Global Rescue of Defects in HIV-1 Envelope Glycoprotein Incorporation: Implications for Matrix Structure
- Turning Defense into Offense: Defensin Mimetics as Novel Antibiotics Targeting Lipid II
- The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells
- Brd4 Is Displaced from HPV Replication Factories as They Expand and Amplify Viral DNA
- A Viral Genome Landscape of RNA Polyadenylation from KSHV Latent to Lytic Infection
- The Cytotoxic Necrotizing Factor of (CNF) Enhances Inflammation and Yop Delivery during Infection by Activation of Rho GTPases
- The Inflammatory Kinase MAP4K4 Promotes Reactivation of Kaposi's Sarcoma Herpesvirus and Enhances the Invasiveness of Infected Endothelial Cells
- Conservative Sex and the Benefits of Transformation in
- Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior
- Colonization Resistance: Battle of the Bugs or Ménage à Trois with the Host?
- Intracellular Interferons in Fish: A Unique Means to Combat Viral Infection
- SPOC1-Mediated Antiviral Host Cell Response Is Antagonized Early in Human Adenovirus Type 5 Infection
- Involvement of the Cellular Phosphatase DUSP1 in Vaccinia Virus Infection
- Killer Bee Molecules: Antimicrobial Peptides as Effector Molecules to Target Sporogonic Stages of
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- A Role for Host Activation-Induced Cytidine Deaminase in Innate Immune Defense against KSHV
- Haploid Genetic Screens Identify an Essential Role for PLP2 in the Downregulation of Novel Plasma Membrane Targets by Viral E3 Ubiquitin Ligases
- A Small Molecule Glycosaminoglycan Mimetic Blocks Invasion of the Mosquito Midgut
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- Can Non-lytic CD8+ T Cells Drive HIV-1 Escape?
- Deletion of the α-(1,3)-Glucan Synthase Genes Induces a Restructuring of the Conidial Cell Wall Responsible for the Avirulence of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Baculoviruses: Sophisticated Pathogens of Insects
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- Natural Selection Promotes Antigenic Evolvability
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



