-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
article has not abstract
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003603
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003603Summary
article has not abstract
Antivirulence Therapy as an Alternative to Antibiotics
Antibiotics are still critically important as a first line therapy for the treatment of various bacterial infections in the clinic. In addition to their use in human medicine, these compounds have also been used for decades in animal production, for both growth promotion and veterinary purposes [1], [2]. Because of the development and spread of antibiotic resistance, there is a growing awareness that antibiotics should be used with more care [3], and as a consequence, the development of alternative methods to control pathogenic bacteria in animal production will be important to ensure good productivity in the future.
Infection of both terrestrial and aquatic animals by bacterial pathogens requires the production of different virulence factors, i.e. gene products that allow the pathogenic bacteria to enter and damage the host. Major virulence factors include gene products involved in motility, adhesion, host tissue degradation, iron acquisition, secretion of toxins, and protection from host defense [4]. As virulence factors are required for infection, preventing pathogens from producing them constitutes an interesting alternative strategy for disease control, a strategy that has been termed antivirulence therapy [5]. Antivirulence therapy is based on a thorough understanding of the mechanisms by which bacterial pathogens cause disease. In this respect, studies aimed at understanding how bacteria cause disease have identified (and will probably continue to do so) targets for therapeutics with completely novel modes of action. Inhibitors of specific virulence factors, such as secretion systems, have been reported in literature [6]. However, considerably more research effort is being directed towards interference with regulatory mechanisms that control the expression of (multiple) virulence factors, such as bacterial cell-to-cell communication (quorum sensing) and host-pathogen signalling (Fig. 1). The following paragraphs will focus on interference with these mechanisms as a novel strategy to control animal pathogens, using Escherichia coli and Salmonella spp. as examples of pathogens for terrestrial animals, and Aeromonas spp. and Vibrio spp. as examples of aquatic pathogens.
Fig. 1. Simplified schematic representation of virulence regulatory systems based on detection of signal molecules in animal pathogenic bacteria. 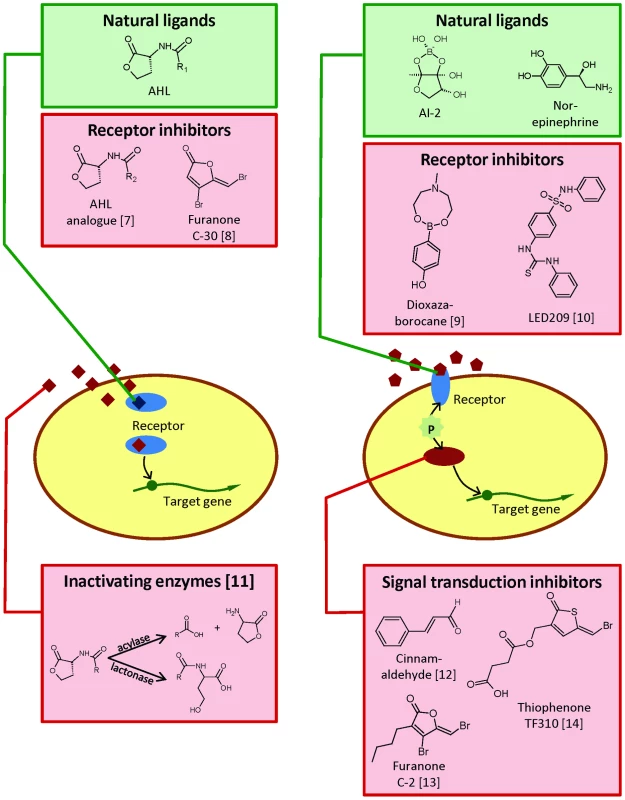
These include (left) quorum sensing based on acylhomoserine lactones (AHL) and (right) quorum sensing in vibrios and catecholamine stress hormone sensing. For each type of system, examples of natural ligands, receptor inhibitors, and other inhibiting agents are shown. Dioxazaborocane is an inhibitor of AI-2 sensing in V. harveyi and LED209 is an inhibitor of catecholamine sensing in E. coli. The signal transduction inhibitors are inhibitors of quorum sensing signal transduction in vibrios. Interfering with Bacterial Cell-to-Cell Communication in Animal Pathogens
Quorum sensing, or bacterial cell-to-cell communication, is a mechanism of gene regulation in which bacteria coordinate the expression of certain genes in response to the presence of small signal molecules. This regulatory mechanism has been shown to control virulence gene expression in many different pathogens, and a wide range of molecules (both of natural and synthetic origin) able to interfere with quorum sensing systems have been reported (for a recent review see [15]). Quorum sensing has been documented to be required for full virulence of Aeromonas spp. and vibrios towards different aquatic hosts, including fish and crustaceans [16]–[18]; moreover, different quorum sensing-disrupting agents have been proven effective in controlling disease. Effective compounds include cinnamaldehyde, brominated furanones and brominated thiophenones [14], antagonistic acylhomoserine lactones [7], and signal molecule-degrading enzymes [11]. Virulence-related phenotypes (including motility and adhesion) of E. coli and Salmonella spp. have also been reported to be controlled by quorum sensing molecules [19], [20], and the signal molecule indole has been shown to affect killing of the nematode C. elegans [21]. However, to the best of my knowledge, no reports have been published thus far mentioning the successful use of inhibitors of these types of bacterial cell-to-cell communication to protect terrestrial farmed animals from disease caused by these pathogens. The evaluation of these kind of compounds in terrestrial animals should be rather straightforward, as many inhibitors have been isolated and/or synthesised [15]. Although the peptide quorum sensing systems of Gram-positive bacteria thus far have received much less attention than acylhomoserine lactone systems in Gram-negative bacteria, some inhibitors of these systems have been documented as well (e.g. cyclic peptide inhibitors of quorum sensing in staphylococci [22]), and these kind of compounds might also prove effective in controlling animal diseases caused by Gram-positive pathogens.
Interfering with Host-Pathogen Signalling in Animal Pathogens
In addition to bacterial signals, E. coli and Salmonella spp. can also sense and respond to host cues such as the catecholamine stress hormones adrenaline and noradrenaline. These hormones are an integral part of the acute “fight or flight” stress response in animals and are conserved among vertebrates and invertebrates. Catecholamines can facilitate the removal of iron from host iron-binding proteins, thereby making it available to the bacteria and increasing their growth under iron-limited conditions [23]. In addition to their growth-stimulatory effect, catecholamines also increase virulence gene expression of pathogenic bacteria. In different pathogenic E. coli strains, the compounds have been reported to affect the production of virulence-related phenotypes such as motility and type III secretion [24], Shiga toxin expression [25], and expression of pilus and fimbrial adhesins [26]. In Salmonella spp., they have been reported to affect motility [27], hemolysin production [28], type III secretion [10], and intestinal colonization in chicks, pigs, and calves [29], [30]. Different bacterial adrenergic sensors have recently been described (with the best-described one being QseC), showing different susceptibilities to blocking with eukaryotic α - and β - adrenergic receptors, respectively [31], [32]. An inhibitor of bacterial catecholamine sensing, LED209, has also been described [10]. It needs to be noted that (at least in Salmonella spp.) different research groups have reported conflicting effects of catecholamines, which may reflect differences in host species, bacterial strains, routes of infection, and nature of mutations [23], [31], [32]. Interestingly, vibrios and Aeromonas spp. also respond to catecholamines, and QseC homologues have been reported in these bacteria as well [33].
Advantages of this Strategy
When compared to the use of antibiotics, a major advantage of antivirulence therapy is that there will be less interference with non-target organisms (i.e. the commensal microbiota), as it specifically targets virulence gene expression or virulence gene regulation; in the latter case there might be some interference with regulatory mechanisms in non-target organisms. Moreover, because such a strategy will pose selective pressure only under conditions in which the virulence genes are required, the tendency towards resistance development and spread will probably also be lower (though not absent) [34]. It should be noted, however, that some of the resistance mechanisms that bacteria have acquired during exposure to antibiotics can also render them resistant to antivirulence agents. This was recently demonstrated in Pseudomonas aeruginosa, in which clinical isolates showing an increased expression of a multidrug efflux pump were also resistant to a quorum sensing-disrupting brominated furanone [35]. A major advantage of targeting the regulatory mechanisms described above is that agents can be used that do not need to enter the cells to exert their activity (e.g., signal molecule-degrading enzymes or compounds that interfere with cell surface receptors). Consequently, pre-existing nonspecific resistance mechanisms (e.g. multidrug efflux pumps and decreased cell membrane permeability) will not alter the effectiveness of such agents.
Conclusion
It is of significant interest to further develop antivirulence therapy as a novel biocontrol strategy for animal production. Further research is needed to document the impact of such a strategy in different host-pathogen settings and to continue the quest for novel antivirulence agents, i.e. inhibitors of either natural or synthetic origin, or microorganisms able to interfere with virulence (regulatory) mechanisms.
Zdroje
1. HeuerH, SchmittH, SmallaK (2011) Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol 14 : 236–243 doi: 10.1016/j.mib.2011.04.009
2. DefoirdtT, SorgeloosP, BossierP (2011) Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol 14 : 251–258 doi: 10.1016/j.mib.2011.03.004
3. CabelloFC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8 : 1137–1144.
4. DonnenbergMS (2000) Pathogenic strategies of enteric bacteria. Nature 406 : 768–774.
5. ClatworthyAE, PiersonE, HungDT (2007) Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3 : 541–548.
6. BaronC (2010) Antivirulence drugs to target bacterial secretion systems. Curr Opin Microbiol 13 : 100–105 doi: 10.1016/j.mib.2009.12.003
7. NatrahFMI, AlamMdI, HarzeviliAS, SorgeloosP, BossierP, et al. (2012) The impact of quorum sensing on the virulence of Aeromonas hydrophila and Aeromonas salmonicida towards burbot (Lota lota L.) larvae. Vet Microbiol 159 : 77–82 doi: 10.1016/j.vetmic.2012.03.014
8. ManefieldM, RasmussenTB, HentzerM, AndersenJB, SteinbergP, et al. (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148 : 1119–1127.
9. BrackmanG, Al QuntarAAA, EnkCD, KaralicI, NelisHJ, et al. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi. Bioorg Med Chem 21 : 660–667 doi: 10.1016/j.bmc.2012.11.055
10. RaskoDA, MoreiraCG, LiDR, ReadingNC, RitchieJM, et al. (2008) Targeting QseC signaling and virulence for antibiotic development. Science 321 : 1078–1080 doi: 10.1126/science.1160354
11. CaoYA, HeSX, ZhouZG, ZhangMC, MaoW, et al. (2012) Orally administered thermostable N-acyl homoserine lactonase from Bacillus sp strain AI96 attenuates Aeromonas hydrophila infection in zebrafish. Appl Environ Microbiol 78 : 1899–1908 doi: 10.1128/AEM.06139-11
12. BrackmanG, DefoirdtT, MiyamotoC, BossierP, Van CalenberghS, et al. (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8 : 149 doi: 10.1186/1471-2180-8-149
13. DefoirdtT, MiyamotoCM, WoodTK, MeighenEA, SorgeloosP, et al. (2007) The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein luxR. Environ Microbiol 9 : 2486–2495.
14. DefoirdtT, BennecheT, BrackmanG, CoenyeT, SorgeloosP, et al. (2012) A quorum sensing-disrupting brominated thiophenone with a promising therapeutic potential to treat luminescent vibriosis. PLOS One 7 (7) e41788 doi: 10.1371/journal.pone.0041788
15. KaliaVC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31 : 224–245 doi: 10.1016/j.biotechadv.2012.10.004
16. SchwenteitJ, GramL, NielsenKF, FridjonssonOH, BornscheuerUT, et al. (2011) Quorum sensing in Aeromonas salmonicida subsp. achromogenes and the effect of the autoinducer synthase AsaI on bacterial virulence. Vet Microbiol 147 : 389–397 doi: 10.1016/j.vetmic.2010.07.020
17. BjellandAM, SorumH, TegegneDA, Winter-LarsenHC, WillassenNP, et al. (2012) LitR of Vibrio salmonicida is a salinity-sensitive quorum-sensing regulator of phenotypes involved in host interaction and virulence. Infect Immun 80 : 1681–1689 doi: 10.1128/IAI.06038-11
18. DefoirdtT, SorgeloosP (2012) Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. ISME J 6 : 2314–2319 doi: 10.1038/ismej.2012.58
19. HanX, BaiH, LiuL, DongH, LiuR, et al. (2013) The luxS gene functions in the pathogenesis of avian pathogenic Escherichia coli. Microb Pathog 55 : 21–27 doi: 10.1016/j.micpath.2012.09.008
20. HirakawaH, InazumiY, MasakiT, HirataT, YamaguchiA (2005) Indole induces the expression of multidrug exporter genes in Escherichia coli.. Mol Microbiol 55 : 1113–1126.
21. AnyanfulA, Dolan-LivengoodJM, LewisT, ShethS, DezaliaMN, et al. (2005) Paralysis and killing of Caenorhabditis elegans by enteropathogenic Eschrichia coli requires the bacterial tryptophanase gene. Mol Microbiol 57 : 988–1007.
22. GeorgeEA, NovickRP, MuirTW (2008) Cyclic peptide inhibitors of staphylococcal virulence prepared by Fmoc-based thiolactone peptide synthesis. J Am Chem Soc 130 : 4914–4924 doi: 10.1021/ja711126e
23. LyteM, VulchanovaL, BrownDR (2011) Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 343 : 23–32 doi: 10.1007/s00441-010-1050-0
24. SperandioV, TorresAG, JarvisB, NataroJP, KaperJB (2003) Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA 100 : 8951–8956.
25. LyteM, ArulanandamBP, FrankCD (1996) Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J Lab Clin Med 128 : 392–398.
26. LyteM, EricksonAK, ArulanandamBP, FrankCD, et al. (1997) Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli. Biochem Biophys Res Commun 232 : 682–686.
27. BearsonBL, BearsonSMD (2008) The role of the QseC quorum-sensing sensor kinase in colonization and norepinephrine-enhanced motility of Salmonella enterica serovar Typhimurium. Microb Pathog 44 : 271–278.
28. KaravolosMH, BulmerDM, SpencerH, RampioniG, SchmalenI, et al. (2011) Salmonella Typhi sense host neuroendocrine stress hormones and release the toxin haemolysin E. EMBO Rep 12 : 252–258 doi: 10.1038/embor.2011.4
29. PullingerGD, CarnellSC, SharaffFF, van DiemenPM, DzivaF, et al. (2010) Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE. Infect Immun 78 : 372–380 doi: 10.1128/IAI.01203-09
30. MethnerU, RabschW, ReissbrodtR, WilliamsPH (2008) Effect of norepinephrine on colonisation and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int J Med Microbiol 298 : 429–439.
31. HughesDT, SperandioV (2008) Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6 : 111–120 doi: 10.1038/nrmicro1836
32. KaravolosMH, WinzerK, WilliamsP, KhanCMA (2013) Pathogen espionage: multiple bacterial adrenergic sensors eavesdrop on host communication systems. Mol Microbiol 87 : 455–465 doi: 10.1111/mmi.12110
33. DefoirdtT (2013) Virulence mechanisms of bacterial aquaculture pathogens and antivirulence therapy for aquaculture. Rev Aquaculture in press. doi: 10.1111/raq.12030
34. DefoirdtT, BoonN, BossierP (2010) Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathog 6 (7) e1000989 doi: 10.1371/journal.ppat.1000989
35. MaedaT, García-ContrerasR, PuM, ShengL, GarciaLR, et al. (2012) Quorum quenching quandary: resistance to antivirulence compounds. ISME J 6 : 493–501 doi: 10.1038/ismej.2011.12
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



