-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
The type III interferon (IFNλ) receptor IL-28R is abundantly expressed in the respiratory tract and has been shown essential for host defense against some viral pathogens, however no data are available concerning its role in the innate immune response to bacterial pathogens. Staphylococcus aureus and Pseudomonas aeruginosa induced significant production of IFNλ in the lung, and clearance of these bacteria from the lung was significantly increased in IL-28R null mice compared to controls. Improved bacterial clearance correlated with reduced lung pathology and a reduced ratio of pro - vs anti-inflammatory cytokines in the airway. In human epithelial cells IFNλ inhibited miR-21 via STAT3 resulting in upregulation of PDCD4, a protein known to promote inflammatory signaling. In vivo 18 hours following infection with either pathogen, miR-21 was significantly reduced and PDCD4 increased in the lungs of wild type compared to IL-28R null mice. Infection of PDCD4 null mice with USA300 resulted in improved clearance, reduced pathology, and reduced inflammatory cytokine production. These data suggest that during bacterial pneumonia IFNλ promotes inflammation by inhibiting miR-21 regulation of PDCD4.
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003682
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003682Summary
The type III interferon (IFNλ) receptor IL-28R is abundantly expressed in the respiratory tract and has been shown essential for host defense against some viral pathogens, however no data are available concerning its role in the innate immune response to bacterial pathogens. Staphylococcus aureus and Pseudomonas aeruginosa induced significant production of IFNλ in the lung, and clearance of these bacteria from the lung was significantly increased in IL-28R null mice compared to controls. Improved bacterial clearance correlated with reduced lung pathology and a reduced ratio of pro - vs anti-inflammatory cytokines in the airway. In human epithelial cells IFNλ inhibited miR-21 via STAT3 resulting in upregulation of PDCD4, a protein known to promote inflammatory signaling. In vivo 18 hours following infection with either pathogen, miR-21 was significantly reduced and PDCD4 increased in the lungs of wild type compared to IL-28R null mice. Infection of PDCD4 null mice with USA300 resulted in improved clearance, reduced pathology, and reduced inflammatory cytokine production. These data suggest that during bacterial pneumonia IFNλ promotes inflammation by inhibiting miR-21 regulation of PDCD4.
Introduction
The interferon (IFN) family is composed of three subgroups (types I, II, and III IFN), and through their distinct receptors, IFNs signal through STAT transcription factors to upregulate expression of over 300 IFN dependent genes. In the lung bacterial pathogen associated molecular patterns (PAMPs) can be internalized and thus gain access to the intracellular receptors involved in type I IFN signaling [1]–[3]. Activation of type I IFN signaling can be either protective or detrimental to the host depending on the specific pathogen [4]–[9]. Type I IFNs promote the pathogenesis of Staphylococcus aureus pulmonary infection through upregulation of CXCR3 chemokines and T-cell recruitment while improving eradication of Pseudomonas aeruginosa by reducing inflammasome signaling [1], [10]–[16].
Interferons induce expression of downstream genes through distinct receptors. Type I IFN signals through the ubiquitously expressed interferon-α/β receptor (IFNAR), while the type III IFN (IFNλ) family, composed of IL-28A/B and IL-29, signals through the more cell specific receptor complex of IL-10R2 and IL-28R [17]–[21]. Following activation of either IFN pathway, an autocrine signaling network mediates the cellular response, primarily through JAK/STAT signaling and the induction of IFN dependent gene expression. It would seem that the two IFNs activate redundant downstream signaling pathways. However altered signaling kinetics and the limited distribution of IL-28R, restricted primarily to mucosal tissues, suggest distinct roles for type I and type III IFN depending on the infection site [22]–[28]. For example in the lung type III IFN is the primary IFN produced by respiratory epithelial cells in response to viral stimulation and is required for clearance of influenza from the airway [29]–[32]. Several of the immunological effects of IFNλ such as upregulation of MHC I and II, induction of NF-κB dependent cytokine production and effects on DC maturation and differentiation, could be highly relevant to the pathogenesis of bacterial infection [33]–[36].
Interferon signaling is linked to the regulation of micro RNAs, small non-coding RNA inhibitors of mRNA translation capable of directly influencing innate immune signaling [4]–[9]. In tumor cells, miR-21 targets the tumor suppressor programmed cell death protein 4 (PDCD4) promoting tumor growth and contributing to the inflammatory tumor microenvironment [1], [10]–[16]. PDCD4 represses translation of cellular mRNA through its binding to eukaryotic initiation factor 4A (eIF4A) [17]–[21]. Phosphorylation of PDCD4 by the ribosomal S6 kinase (p70S6K) results in release of eIF4A from PDCD4, ubiquitin dependent degredation of PDCD4, and enhanced mRNA translation [22]–[28]. Inflammatory cytokine production in response to toll like receptor (TLR) 2 or 4 activation by decorin or lipopolysaccharide (LPS) is significantly influenced by expression of miR-21 and PDCD4 [29]–[32]. Therefore the pro-inflammatory contribution of PDCD4 to host signaling during bacterial pneumonia could significantly contribute to lung pathology [33]–[36].
In the experiments detailed in this report, we examined the importance of type III IFNs in the innate immune response to two major airway pathogens, USA300 MRSA and P. aeruginosa, currently the most common causes of ventilator associated pneumonia [37], [38]. We found that IFNλ is induced in the course of bacterial airway infection and results in downregulation of the microRNA miR-21, sustained expression of PDCD4, and increased proinflammatory cytokine production. Mice lacking the IFNλ receptor, IL-28R, or PDCD4 had significantly improved clearance of both airway pathogens and less pulmonary pathology associated with reduced levels of inflammatory cytokines.
Results
IL-28R−/− mice are more resistant to S. aureus respiratory infection
In vitro studies were performed to establish the kinetics of IFNλ induction in response to the extracellular bacterial pathogen S. aureus. A significant increase in IFNλ transcript (p < 0.001) was observed in wild type (WT) bone marrow derived dendritic cells (BMDCs) following 4 hours of stimulation with heat killed USA300, which returned to baseline by 8 hours (Fig. 1A). Similar induction was observed when BMDCs were stimulated with live bacteria (MOI 100, 4 hours), and induction was significantly reduced (p = 0.0062) in IL-28R−/− BMDCs (Fig. S1A).
Fig. 1. Induction of IFNλ by S. aureus USA300 inhibits bacterial clearance. 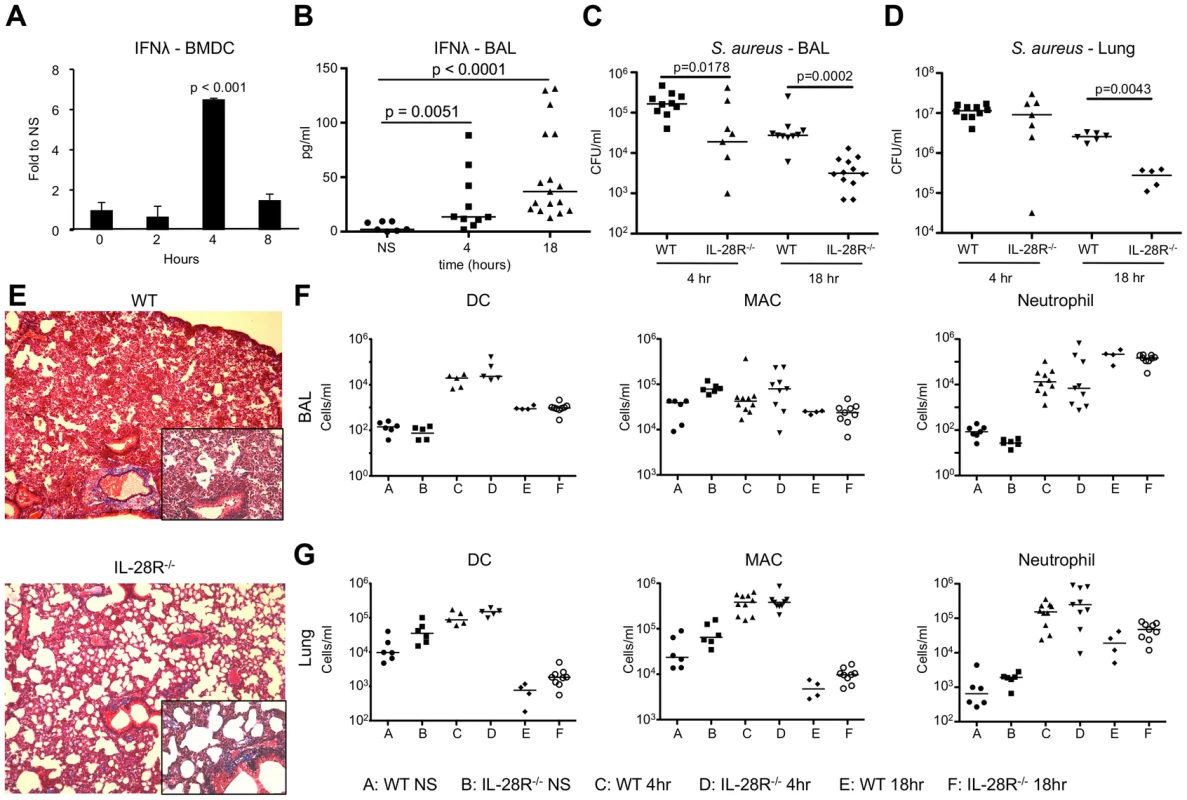
(A) qRT-PCR analysis of IFNλ mRNA in BMDCs stimulated with heat killed USA300, µ ± SD. (B) ELISA analysis of IFNλ in BAL of WT mice following 4 and 18 hours of infection with USA300. (C) Numbers (CFU) of USA300 recovered from BAL of WT and IL-28R−/− mice following 4 and 18 hours of infection. (D) Numbers (CFU) of USA300 recovered from lung tissue of WT and IL-28R−/− mice following 4 and 18 hours of infection. (E) Trichrome stained lungs from WT and IL-28R−/− mice following and 18 hour USA300 infection (original magnification 100x, insert 400x). (F) Numbers of dendritic cells (DC), macrophages (MAC), and neutrophils in BAL of WT and IL-28R−/− in unstimulated (NS) mice or following 4 or 18 hours of infection. (G) Numbers of dendritic cells (DC), macrophages (MAC), and neutrophils in lung tissue of WT and IL-28R−/− in unstimulated (NS) mice or following 4 or 18 hours of infection. Data are representative of at least 2 independent experiments. To evaluate the role of type III IFN in MRSA pneumonia we infected WT C57BL/6 and IL-28R−/− mice intranasally with 1×107 CFU USA300. IFNλ mRNA levels increased significantly by 4 hours in the lung of WT mice (p = 0.0047) (Fig. S1B), and protein levels were significantly increased in the airway of WT mice by 4 (p = 0.0051) and 18 (p < 0.0001) hours post infection (Fig. 1B). By 4 hours following intranasal infection the numbers of bacteria recovered from the airway were significantly reduced in IL-28R−/− mice compared with control (p = 0.0178) (Fig. 1C), a difference not observed in the lung tissue (Fig. 1D). By 18 hours significantly fewer bacteria were recovered from both the airway (p = 0.0002) and tissue (p = 0.0043) of knockout mice compared to control. Lung pathology was reduced in IL-28R−/− compared to WT controls as determined by trichrome stained lung sections (Fig. 1E). The numbers of dendritic cells, macrophages, or neutrophils recruited to the airway (Fig. 1F) or lung tissue (Fig. 1G) were not affected by lack of IL-28R, suggesting that differences in bacterial clearance and pathology were not due to increased numbers of phagocytotic cells.
Expression of inflammatory cytokines is enhanced by type III IFN
Type III IFN activates a family of over 300 genes through a Jak-Stat signaling cascade [39], [40]. We analyzed expression of cytokines in the bronchial alveolar lavage (BAL) of wild type and IL-28R−/− mice following 4 and 18 hour MRSA infection by ELISA. Significant differences in cytokine expression were not observed following a short (4 hour) infection suggesting that initial activation of cytokine production does not depend on signaling through IL-28R. By 18 hours significant reductions in KC (p < 0.0001), GM-CSF (p = 0.0009) and IL-1β (p = 0.0002) but not TNF or IL-10 were observed in IL-28R−/− as compared with WT mice (Fig. 2A). Levels of the interferon response gene MX1 were reduced in IL-28R−/ − mice compared to WT at the 18 hour time point, confirming the inhibition of interferon signaling (Fig. 2B). Installation of recombinant IFNλ did not significantly increase cytokine expression in the BAL of uninfected mice (Fig. S2). Therefore it appears that IFNλ does not directly induce cytokine production, but is more likely involved in the regulation of inflammatory cytokines during acute S. aureus infection.
Fig. 2. Cytokine production during USA300 infection contributes to pathology. 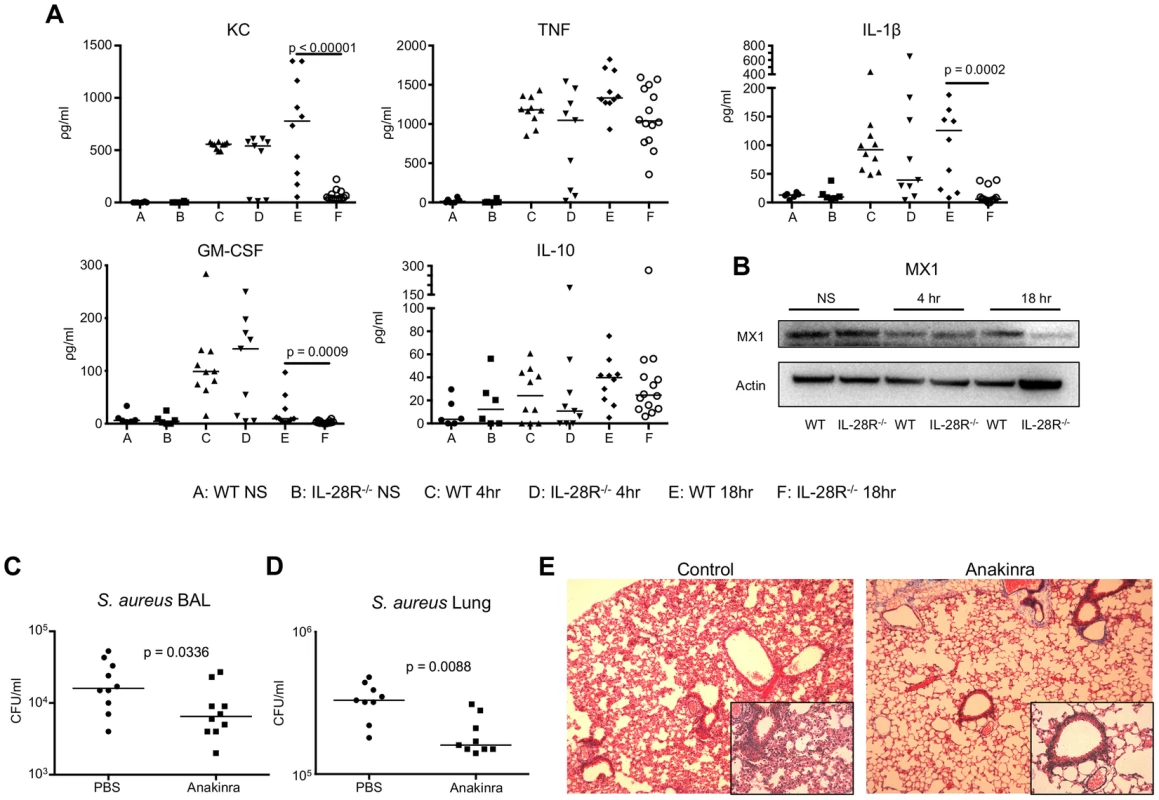
(A) ELISA analysis of individual cytokines in BAL of WT and IL-28R−/− mice. (B) Western blot analysis of MX1 expression in lungs of WT and IL-28R−/− mice following 4 and 18 hours of infection with USA300. (C) Numbers (CFU) of USA300 recovered from the BAL of from PBS control and Anakinra pre-treated mice following an 18 hour infection. (D) Numbers of USA300 (CFU) recovered from the lung of from PBS control and Anakinra pre-treated mice following an 18 hour infection. (E) Trichrome stained lungs from PBS control and Anakinra pre-treated mice following and 18 hour USA300 infection (original magnification 100x, insert 400x). Data are representative of at least 2 independent experiments. The pro-inflammatory cytokine IL-1β has shown to promote lung injury during P. aeruginosa pneumonia, and was significantly decreased in the airways of S. aureus infected IL-28R−/− mice [11]. To determine if decreased IL-1β was similarly associated with the improved outcome of the IL-28R−/− mice, we predicted that Anakinra, a synthetic IL-1R antagonist, would improve clearance of S. aureus from the lung and reduce lung pathology. Numbers of bacteria recovered 18 hours following infection with USA300 were significantly lower from the airway (p = 0.0336) (Fig. 2C) and lung (p = 0.0088) (Fig. 2D) of Anakinra treated compared to PBS pretreated controls. Lung pathology was reduced in Anakinra treated mice following an 18 hour infection compared to infected control mice (Fig. 2E). These data suggest that the reduced pathology observed in IL-28R null mice was due in part to reduction in the inflammatory cytokine IL-1β.
Type III IFN regulates PDCD4 expression in epithelial cells
PDCD4 has been shown to promote inflammatory cytokine production in response to LPS, and reduction of PDCD4 expression in human epithelial cell monolayers (16HBE) significantly reduced IL-8 induction (p = 0.0103) in response to USA300 (Fig. 3A) [31]. Since PDCD4 expression is regulated by the micro RNA miR-21, we tested whether IFNλ promotes inflammatory signaling by inhibiting miR-21 expression. In vitro, miR-21 expression in 16HBEs was reduced following 1 hour treatment with recombinant IFNλ (Fig. 3B), which correlated with an increase in PDCD4 protein levels (Fig. 3C). Reduction of miR-21 (Fig. 3D) and increased PDCD4 expression (Fig. 3E) following IFNλ treatment was dependent on STAT3 phosphorylation.
Fig. 3. IFNλ regulation of miR-21 and PDCD4. 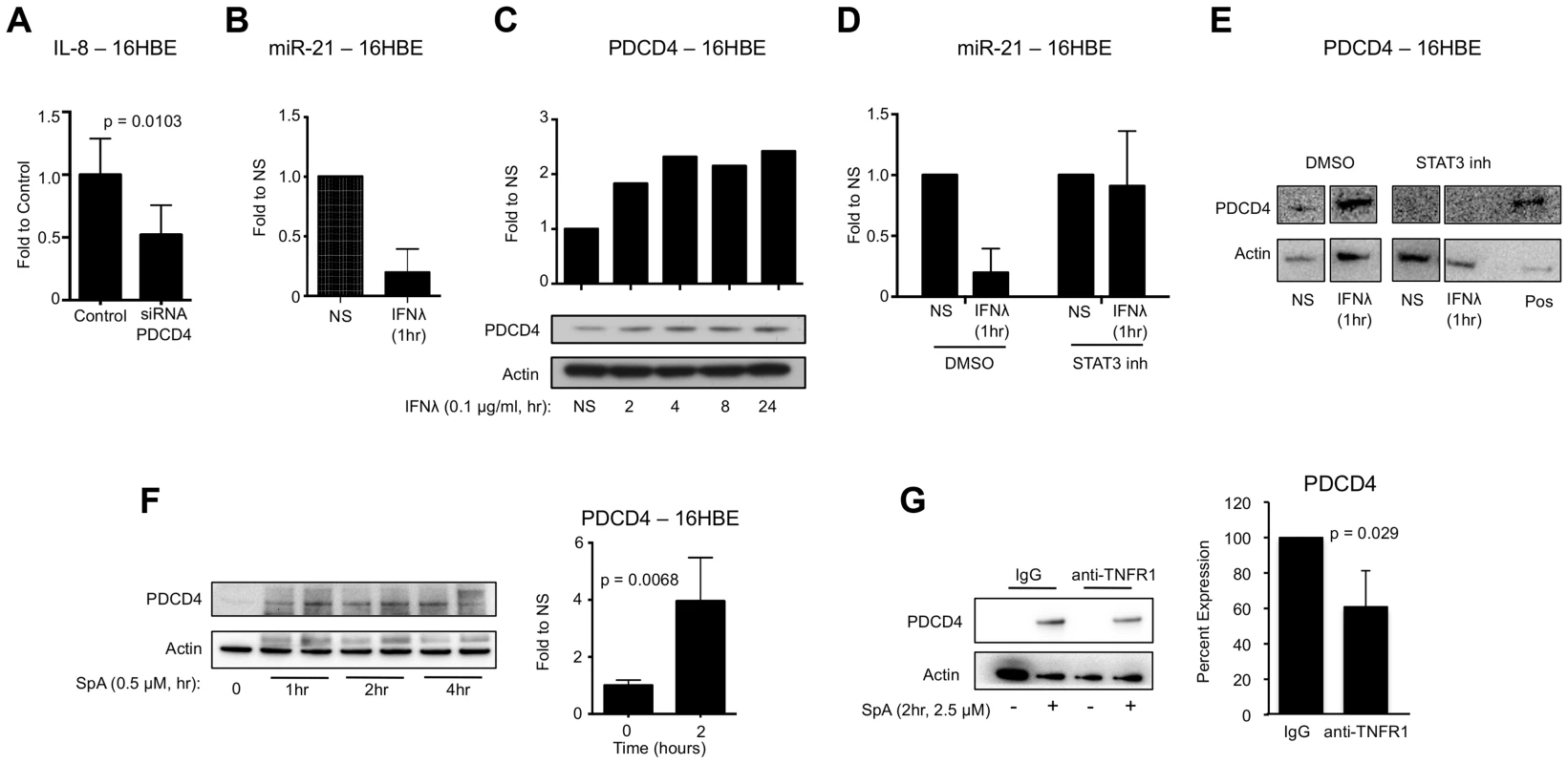
(A) qRT-PCR analysis of IL-8 in 16HBE cells treated with control or PDCD4 siRNA and infected with USA300, µ ± SD. (B) qRT-PCR analysis of miR-21 in 16HBE cells treated with recombinant IFNλ for 1 hour, µ ± SD. (C) Western blot analysis of PDCD4 in 16HBE cells treated with recombinant IFNλ. (D) qRT-PCR analysis of miR-21 in 16HBE cells pretreated with STAT3 inhibitor or DMSO treated with recombinant IFNλ for 1 hour, µ ± SD. (E) Western blot analysis of PDCD4 in 16HBE cells pretreated with STAT3 inhibitor or DMSO treated with recombinant IFNλ for 1 hour. Lysate from 16HBE cells treated with DMSO and IFNλ was used as a positive control (pos). (F) Western blot analysis of PDCD4 in human epithelial cells (16HBE) following stimulation with S. aureus protein A (SpA), µ ± SD. (G) Western blot analysis of PDCD4 in 16HBE cells treated with anti-TNFR1 or control IgG and SPA for 2 hours, µ ± SD. Data are representative of at least 2 independent experiments. S. aureus SpA induced PDCD4 expression
In response to acute LPS exposure macrophages upregulate PDCD4 expression [31]. S. aureus, as a Gram positive pathogen, can induce inflammatory signaling in epithelial cells through interaction of its major surface component protein A (SpA) and host receptor TNFR1 [13], [41]–[43]. We hypothesized that SpA-TNFR1 interaction would upregulate PDCD4 expression and promote production of inflammatory cytokines. 16HBE epithelial cells were exposed to S. aureus protein A (SpA) and increases in the expression of PDCD4 (p = 0.0068) were observed (Fig. 3F). To confirm the relationship between TNFR1 and PDCD4, we pre-treated 16HBEs with antibody to the extracellular domain of TNFR1 and demonstrated significantly reduced induction of PDCD4 by SpA compared to IgG pretreatment (p = 0.029) (Fig. 3G). These data demonstrate that SpA, like LPS, is able to induce expression of PDCD4 in host cells.
PDCD4 expression inversely correlates with clearance of USA300
In vivo, miR-21 expression in WT and IL-28R null mice was not different prior to infection. Expression of miR-21 increased by greater than 10-fold in both WT and IL-28R null mice following four hour infection with USA300 (Fig. 4A). By 18 hours following the initial infection miR-21 levels in WT mice returned to uninfected levels, while levels in IL-28R null mice remained significantly higher (p = 0.002). PDCD4 mRNA levels following 18 hour infection inversely correlated with miR-21 expression, and were significantly lower in IL-28R null mice compared to WT (p = 0.045) (Fig. 4B). PDCD4 protein expression at the 4 hour time point was reduced in both WT and IL-28R−/− mice compared to unstimulated levels, and reduced further at the 18 hour time point (Fig. 4C). PDCD4 protein expression tended to be lower at the 18 hour time point in IL-28R null mice than WT, correlating with elevated levels of miR-21. Thus it appears that following the initial pro-inflammatory innate immune response to S. aureus in WT mice, IFNλ signaling suppresses expression of miR-21 maintaining PDCD4 levels. Loss of IL-28R resulted in sustained miR-21 levels and a decrease in PDCD4 gene expression by 18 hours of S. aureus infection.
Fig. 4. IFNλ regulates PDCD4 in vivo. (A) qRT-PCR analysis of miR-21 in the lungs of WT and IL-28R−/− mice following infection with USA300, µ ± SD. 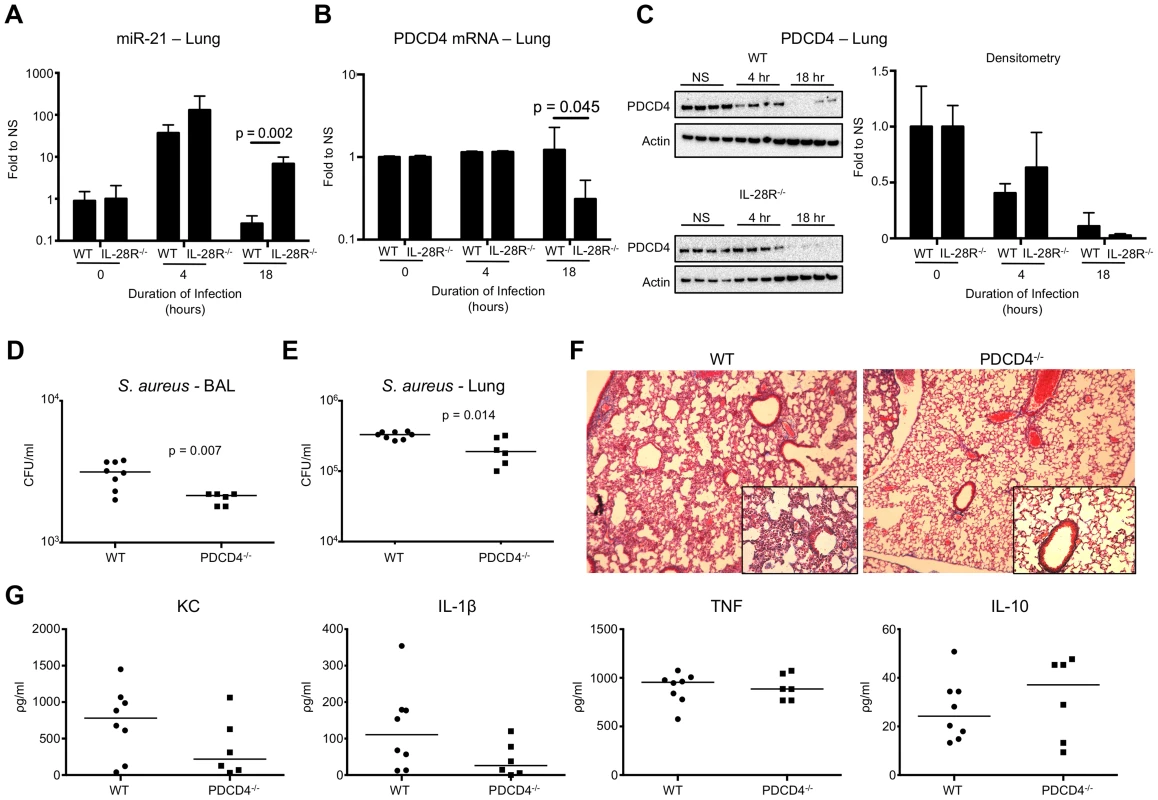
(B) qRT-PCR analysis of PDCD4 mRNA in the lungs of WT and IL-28R−/− mice following infection with USA300, µ ± SD. (C) Western blot analysis of PDCD4 in the lungs of WT and IL-28R−/− mice following infection with USA300, µ ± SD. (D) Numbers (CFU) of USA300 recovered from BAL of WT and PDCD4−/− mice following 18 hours of infection. (E) Numbers (CFU) of USA300 recovered from lung tissue of WT and PDCD4−/− mice following 18 hours of infection. (F) Trichrome stained lungs from WT and PDCD4−/− mice following and 18 hour USA300 infection (original magnification 100x, insert 400x). (G) ELISA analysis of individual cytokines in BAL of WT and PDCD4−/− mice. Data are representative of at least 2 independent experiments. To confirm that PDCD4 expression negatively influences clearance of USA300 from the airway we infected WT and PDCD4−/− mice with 107 CFU USA300 for 18 hours. Significantly fewer organisms were recovered from both the airway (p = 0.007) (Fig. 4D) and lung tissue (p = 0.014) (Fig. 4E) of PDCD4−/− mice compared to WT. Bacterial clearance correlated with reduced lung pathology (Fig. 4F) but not immune cell recruitment into the lung (Fig. S3). Expression of the inflammatory cytokines KC and IL-1β, not TNF, were reduced and expression of anti-inflammatory IL-10 was increased in PDCD4−/− mice compared to WT. Although differences in cytokines were not statistically significant, the trends were similar to those observed in IL-28R−/− compared to WT suggesting a link between type III IFN and PDCD4.
Type III IFN promotes Gram negative pneumonia
Due to previous reports demonstrating improved survival of PDCD4−/− mice during LPS challenge, we hypothesized that IL-28R−/− mice would clear the Gram negative pathogen P. aeruginosa PAK more rapidly than WT mice [31]. In vitro, P. aeruginosa PAK stimulation (MOI 10, 4 hours) of BMDCs induced increased mRNA levels of IFNλ compared to unstimulated cells (Fig. S1C). As shown for MRSA in Figure S1A, loss of IL-28R resulted in reduced IFNλ mRNA levels following P. aeruginosa stimulation.
WT and IL-28R null mice were intranasally infected with 107 CFU PAK and bacterial clearance from the airway and lung tissue monitored at 4 and 18 hours of infection. In vivo, IFNλ mRNA levels (p = 0.0031) (Fig. S1D) and protein expression (p = 0.005) (Fig. 5A) were significantly increased in WT mice by 4 hours post infection, but were not different than baseline at the 18 hour time point. Significantly fewer bacteria were recovered from BAL (p = 0.0003) (Fig. 5B) and lung tissue (p = 0.0023) (Fig. 5C) of IL-28R−/− at the 18 hour time point compared to WT, and no differences were observed at the 4 hour time point. Bacterial clearance was not altered in mice lacking the type I IFN receptor IFNAR (Fig. 5D). Pathology in the lung was reduced in knockout mice following PAK infection (p = 0.0010) (Fig. 5E). Similar to S. aureus infection, there were no differences in the numbers of immune cells recruited to the BAL or lung (Figs. 5F and 5G).
Fig. 5. P. aeruginosa induction of IFNλ signaling inhibits bacterial clearance. 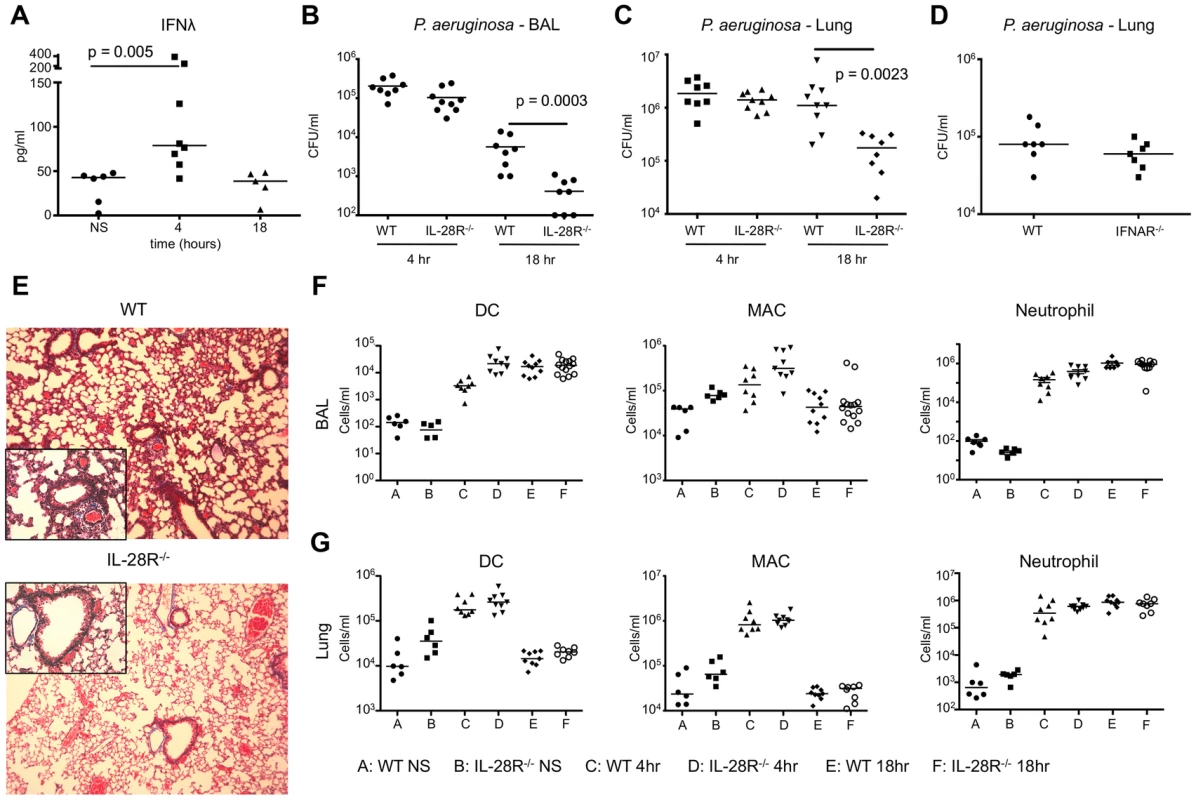
(A) ELISA analysis of IFNλ in BAL of WT mice following 4 and 18 hours of infection with PAK. (B) Numbers (CFU) of PAK recovered from BAL of WT and IL-28R−/− mice following 4 and 18 hours of infection. (C) Numbers (CFU) of PAK recovered from lung tissue of WT and IL-28R−/− mice following 4 and 18 hours of infection. (D) Numbers of PAK recovered from lung tissue of WT and IFNAR−/− mice following 18 hours of infection. (E) Trichrome stained lungs from WT and IL-28R−/− mice following and 18 hour PAK infection (original magnification 100x, insert 400x). (F) Numbers of dendritic cells (DC), macrophages (MAC), and neutrophils in BAL of unstimulated (NS) WT and IL-28R−/− mice or following 4 or 18 hours of infection. (G) Numbers of dendritic cells (DC), macrophages (MAC), and neutrophils in lung tissue of unstimulated (NS) WT and IL-28R−/− mice or following 4 or 18 hours of infection. Data are representative of at least 2 independent experiments. BAL fluid was analyzed for the presence of pro - and anti-inflammatory cytokines to determine if IFNλ signaling increased expression of pro-inflammatory cytokines during PAK infection. Significant reductions in expression of pro-inflammatory cytokines KC (p = 0.0141) and TNF (p = 0.0006) were observed in BAL of IL-28R null mice at 18 hours compared to WT (Fig. 6A). The anti-inflammatory IL-10 was significantly increased (p = 0.0134) in the IL-28R−/− mice as compared to controls at this time point. No differences in IL-1β production were observed. MX1 levels were lower in IL-28R null mice at 18 hours compared to WT (Fig. 6B).
Fig. 6. P. aeruginosa induced cytokine, miR-21, and PDCD4 expression in WT and IL-28R−/− mice. 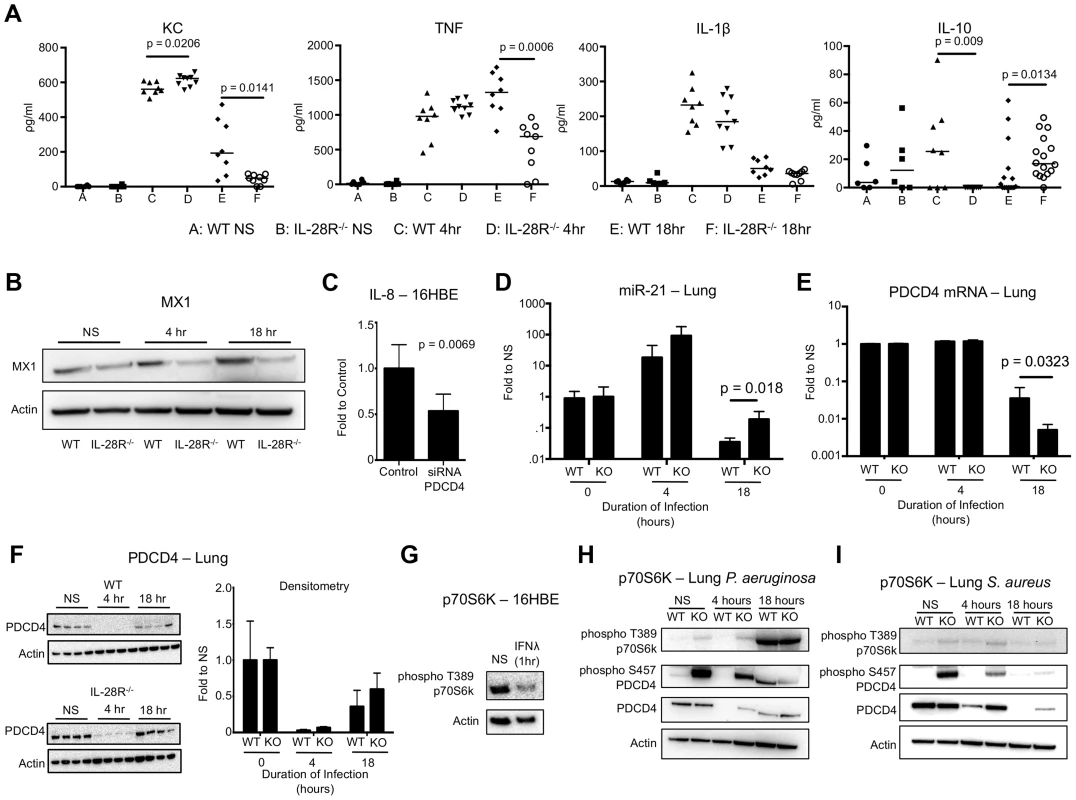
(A) ELISA analysis of individual cytokines in BAL of WT and IL-28R−/− mice. (B) Western blot analysis of MX1 expression in lungs of WT and IL-28R−/− mice following 4 and 18 hours of infection with PAK. (C) qRT-PCR analysis of IL-8 in 16HBE cells treated with control or PDCD4 siRNA and infected with PAK, µ ± SD. (D) qRT-PCR analysis of miR-21 in the lungs of WT and IL-28R−/− mice following infection with PAK, µ ± SD. (E) qRT-PCR analysis of PDCD4 mRNA in the lungs of WT and IL-28R−/− mice following infection with PAK, µ ± SD. (F) Western blot analysis of PDCD4 in the lungs of WT and IL-28R−/− mice following infection with PAK, µ ± SD. (G) Western blot analysis of phosphorylation of p70S6K in 16HBE cells treated with recombinant IFNλ. (H) Western blot analysis of phosphorylation of p70S6K and PDCD4 in the lungs of WT and IL-28R−/− mice following infection with PAK. (I) Western blot analysis of phosphorylation of p70S6K and PDCD4 in the lungs of WT and IL-28R−/− mice following infection with USA300. Data are representative of at least 2 independent experiments. We then determined if the reduced pro-inflammatory cytokine expression in IL-28R−/− mice infected with PAK was also due to alterations in miR-21 and PDCD4. In vitro, PDCD4 siRNA significantly reduced IL-8 expression (p = 0.0069) in 16HBE in response to PAK (Fig. 6C). MiR-21 expression in lung tissue of IL-28R null mice was increased (p = 0.0122) compared to WT at 18 hours, demonstrating that loss of IFNλ signaling resulted in increased expression of this miRNA during PAK infection (Fig. 6D). PDCD4 mRNA levels were significantly decreased in IL-28R null mice compared to WT correlating with the changes in miR-21 level (p = 0.0323) (Fig. 6E). PDCD4 protein in the lung was almost undetectable at the 4 hour time point. PDCD4 levels increased slightly by 18 hours although no differences were observed between knockout and WT mice (Fig. 6F).
PDCD4 function can be regulated by p70S6K dependent phosphorylation at serine 475, which inhibits its interaction with AP-1 and eukaryotic translation initiation factor 4A (eIF4A) transcription factors and leads to ubiquitin dependent degradation [22], [33], [44]-[46]. In vitro stimulation of 16HBE monolayers with recombinant IFNλ for 1 hour reduced phosphorylation of p70S6K, suggesting that type III IFN inhibits this regulatory pathway (Fig. 6G). In vivo we observed phosphorylation of PDCD4 in uninfected IL-28R−/− mice compared to WT control. In both WT and knockout mice phosphorylation of p70S6K increased following 18 hour PAK infection (Fig. 6H), not observed during MRSA infection (Fig. 6I). Phosphorylation of PDCD4 in PAK infected WT mice also increased following 18 hours, correlating with phosphorylation of p70S6K. These data suggest that modifications to PDCD4, mediated by both miR-21 and p70S6K, regulate its ability to influence cytokine production during P. aeruginosa pneumonia.
Discussion
Much like the other members of the IFN family, IFNλ is required for host defense against viral pathogens and is capable of inhibiting tumor growth [23]–[28], [30], [47], [48]. There is clearly a major role for IFNλ in the successful clearance of the hepatitis C virus consistent with expression of IL-28R on hepatocytes [49], [50]. Type III IFN has also been linked to killing of the intracellular bacterial pathogen Listeria monocytogenes, correlating with reduced colonization of the spleen and liver [51]. Fitting with the abundance of IL-28R in the respiratory tract, we found a major role for IFNλ signaling in the pathogenesis of both S. aureus and P. aeruginosa pneumonia [21], [28].
In contrast to the beneficial effects of IFNλ in the eradication of viral pathogens, our data suggest that IFNλ promotes a prolonged inflammatory cytokine response to bacterial pathogens that detracts from the efficiency of bacterial clearance and promotes pathology. Mice lacking the type III IFN receptor demonstrated significantly improved clearance of S. aureus and P. aeruginosa coincident with a reduced duration of the host inflammatory response. Particularly in the pathogenesis of pneumonia, the balance between pro and anti-inflammatory signaling is critical. While the mechanism through which the inflammatory milieu affects bacterial clearance is unclear, our data clearly demonstrate that reducing levels of inflammatory cytokines through modulation of IFNλ or inhibition of IL-1R improves clearance of S. aureus and P. aeruginosa [11]. Once a sufficient number of phagocytes are recruited to the airways to deal with the pathogens, excessive cytokine production, particularly toxic cytokines such as IL-1β, elicits tissue damage and impairs normal host defenses.
A major effector of IFNλ signaling is PDCD4, which appears to be regulated by multiple pathways in response to infection. A confirmed target of miR-21, PDCD4 influences production of pro-inflammatory signaling through its interaction with AP-1, NF-κB, and eIF4A, and has been linked to the inflammatory microenvironment surrounding tumor cells [10], [12], [29], [31], [33], [52]. Similar to previous findings with LPS, S. aureus SpA, through its interaction with TNFR1 upregulates PDCD4 in epithelial cells, promoting pro-inflammatory signaling while inhibiting anti-inflammatory cytokine production. It is likely that additional S. aureus pathogen associated molecular patterns (PAMPs) such as the lipoproteins that activate TLR2 also participate.
Following ligation of IL-28R by IFNλ, STAT3 suppresses expression of miR-21 resulting in increased expression of PDCD4. MiRNAs like miR-21 comprise a regulatory system which makes subtle changes to mRNA expression levels, including the genes involved in Toll-like receptor signaling, and therefore are capable of having significant affects on innate immunity [7]–[9], [31], [53]. MiR-21, specifically, is induced by NF-κB signaling and has been implicated in the regulation of TLR2 mediated signaling in both skin and lung inflammation [33], [54]–[57]. We similarly observe rapid increases in miR-21 during the initial 4 hour infection with either S. aureus or P. aeruginosa, and sustained expression of miR-21 in IL-28R−/− mice was associated with reduced levels of inflammatory cytokines in the airway. As predicted by our in vitro studies, in mice expressing IL-28R reduced levels of miR-21 at 18 hours post infection correlated with prolonged PDCD4 mRNA expression. Mice lacking PDCD4 were better able to clear USA300 from their lungs, similar to previous findings demonstrating PDCD4 null mice are resistant to lethal LPS challenge [31]. Therefore mice lacking IL-28R are less susceptible to S. aureus and P. aeruginosa induced lung damage due in part to reduced PDCD4 and a shortened inflammatory response.
A second pathway acting through mTOR and p70S6K regulates PDCD4 function by phosphorylating PDCD4, inhibiting its interaction with AP-1 and eIF4A and promoting its degradation [17], [19], [22], [29]. Our data suggest that type III IFN has an inhibitory affect on p70S6K and PDCD4 phosphorylation. In vitro IFNλ treatment of epithelial cells reduced p70S6K phosphorylation. In vivo, PDCD4 was strongly phosphorylated in uninfected IL-28R−/− mice while phosphorylation was not observed in uninfected control mice. Rapid turnover of PDCD4 due to phosphorylation dependent degradation, or an inability of PDCD4 to bind eIF4A in IL-18R null mice could further limit its affects on signaling.
Increases in p70S6K phosphorylation during bacterial infection were IL-28R independent and were only observed 18 hours following infection with P. aeruginosa. The ability to activate different regulatory pathways such as p70S6K signaling could contribute to the differences in cytokine induction observed during S. aureus and P. aeruginosa infections. For example levels of TNF and IL-10 were significantly different between WT and IL-28R−/− mice during PAK not USA300 infections, whereas IL-1β was significantly different during a S. aureus infection. Thus IFNλ and PDCD4 are conserved elements in the response to airway pathogens which clearly have other differential effects in inducing innate immunity.
The selective expression of IL-28R, substantially more abundant on murine and human epithelial cells than on immune cells as previously shown and demonstrated by the inability of macrophages to respond to IFNλ (Fig. S4), may also be important in the specific cytokine responses evoked by the different pathogens [28]. Epithelial cells are not the primary producers of IL-1β in response to PAK, therefore IL-1β levels in the airways of P. aeruginosa infected WT and IL-28R−/− mice were not different [11], [58]. It is unclear which cells in the lung produce IL-1β in response to S. aureus. Our data suggest that IL-1β producing cells express IL-28R, as cytokine levels were significant reduced in IL-28R null mice. While we do not propose that IL-1β is the sole cytokine responsible for lung damage in response to S. aureus, inhibition of this signaling pathway in WT mice does improve clearance of S. aureus and limits the extent of tissue damage. We conclude that while the specific cytokines modulated by type III IFN are different in the context of specific bacterial infections, the overall affect of inhibiting IL-28R dependent signaling is a reduction of lung inflammation and improved bacterial clearance.
The type III IFN pathway is recognized as an important host defense pathway for the eradication of viral infection and tumor growth and the distribution of the receptor at mucosal sites makes it an active participant in the innate immune response to bacterial pathogens as well. While differences in the expression of PAMPs and their receptors, mechanisms of p70S6K and PDCD4 phosphorylation, and ultimately levels of cytokine expression are evident in the models of S. aureus and P. aeruginosa pneumonia, IFNλ functions as a central regulator of airway inflammation in both types of bacterial pneumonia. Given the pathology associated with excess cytokine induction in the airways, targeting specific components of the type III IFN/PDCD4 regulatory pathway could be a potential therapy for limiting lung damage in the setting of acute airway infection.
Materials and Methods
Ethics statement
Animal work in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Animal Welfare Act and US federal law. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University (protocol number AAAC3059).
Bacterial strains
S. aureus strain LAC USA300 and P. aeruginosa strain PAK were grown on Luria-Bertani agar at 37°C. For infection, LB broth was inoculated with single colonies and grown overnight at 37°C, diluted 1∶100 in the morning and grown to OD 1.000 (S. aureus) or 0.500 (PAK). S. aureus cultures grown to OD 1.000 were incubated at 55°C to inactivate the bacteria for heat killed experiments.
Models of infection
Seven week old C57BL/6 WT, IL-28R−/−, IFNAR−/−, and PDCD4−/− mice were intranasally inoculated with 50 µl S. aureus or P. aeruginosa (1*107 CFU/mouse) as previously described [2], [14]. WT and IFNAR−/− mice were previously described, IL-28R−/− mice were provided by Bristol Myers Squibb, and PDCD4−/− mice were from Jackson Laboratories [13]. Control mice received 50 µl of PBS. To determine the affect of IFNλ on cytokine production, mice were intranasally treated with 1 µg recombinant mIL-28 (PBL Interferon Source) in 50 µl PBS or PBS control. The role of IL-1β was determined in mice pretreated i.p. with Anakinra (10 mg/kg, Amgen) for four days and were infected on the fourth day as described previously [59]. Control mice were treated with PBS also given i.p. BAL fluid was harvested 18 hours after infection and used to quantify immune cell populations, cytokine expression, and bacteria CFU. Histology was performed by Columbia University Molecular Pathology core on tissues fixed in 4% paraformaldehyde.
Cell culture
Bone marrow - derived dendritic cells (BMDCs) were cultured from wild-type and knockout mice as described previously [3]. Human airway epithelial cells (16HBE) were grown as previously described [60]. Human monocytic THP-1 cells were grown in RPMI 1640 medium with 10% fetal bovine serum with penicillin (100 units/ml) and streptomycin (100 µg/ml) and treated with 100 nM phorbol 12-myristate 13-acetate (PMA) to induce terminal differentiation for IFN stimulation studies. On-target control or PDCD4 siRNA (Thermo Scientific) was transfected into 16HBE monolayers using Fugene (Promega) according to the manufacturers instructions. Cells were stimulated with 5 µM S. aureus protein A (Calbiochem) or 0.1 µg/ml human recombinant IL-28 or IFNβ (PBL Interferon). In select experiments antibody to the extracellular domain of TNFR1 or control IgG (4 µg/ml) (Santa Cruz Biotechnology Inc.) or STAT3 inhibitor V (50 µM) (Calbiochem) or DMSO control were applied to 16HBEs 1 hour prior to SpA.
FACS analysis
Enumeration of neutrophil (MHCII−Ly6+), macrophages (CD11c+MHCIIlow), and DC (CD11c+MHCII+) populations was performed as previously described [1].
ELISA and immunoblotting
BAL was analyzed for cytokine and chemokine content by ELISA (R&Dbiosystems, PBL Interferon Source, or eBioscience) according to the manufacture’s instructions. Anti-phospho-p70S6K, anti-phospho-PDCD4, anti-PDCD4 (Rockland Immunochemicals), anti-MX1 (Santa Cruz Biotechnology Inc.) and anti-β-actin (Sigma) antibodies followed by secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology Inc.) were used to measure expression in human epithelial cells and mouse lung. Protein separation, transfer and immunnoblotting were performed as described [13]. Densitometry was done using Image J (NIH).
mRNA and miRNA analysis
Total RNA was isolated using mirVana miRNA Isolation Kit (Life Technologies) according to the manufacturers instructions. For mRNA analysis cDNA was generated using the High Capacity cDNA reverse Transcriptase Kit (Applied Biosystems). Quantitative real-time RT-PCR (QRT-PCR) was performed using Power SYBR Green PCR Master Mix in a Step One Plus Thermal Cycler (Applied Biosystems). Mouse primers for PDCD4 were 5′ – ATGGATATAGAAAATGAGCAGAC -3′ and 5′ – CCAGATCTGGACCGCCTATC -3′ and actin were 5′ – CCTTTGAAAAGAAATTTGTCC – 3′ and 5′ – AGAAACCAGAACTGAAACTGG – 3′. Human primers for IL-8 were 5′-TACTCCAAACCTTTCCAACCC-3′ and 5′ - AACTTCTCCACAACCCTCTG-3′ and actin were 5′ - GTGGGCCGCTCTAGGCACCA-3′ and 5′-CGGTTGGCCTTAGGGTTCAGGGGGG - 3′. IL-8 and PDCD4 expression was normalized to actin. For miRNA analysis cDNA was generated and qRT-PCR was run using NCode miRNA First-Strand cDNA Synthesis and qRT-PCR Kit (Life Technologies) according to the manufacturers instructions. A universal reverse primer for small RNAs was supplied with the NCode kit. The forward primer for miR-21 was 5′-TAGCTTATCAGACTGATGTTGA-3′ and ΔCt values were normalized to the small non-coding RNA U6 5′-GGGCAGGAAGAGGGCCTAT-3′.
Statistics
Significance of data was determined using a nonparametric Mann-Whitney test. For experiments with greater than one comparison we used a nonparametric Kruskal-Wallis test followed by post-hoc Dunn’s test to correct for multiple comparisons. Statistics were performed with GraphPad Prism software with significance defined as p<0.05. Cytokine, bacterial counts, and immune cell number data are presented as individual points with a bar representing the median value.
Supporting Information
Zdroje
1. ParkerD, CohenTS, AlhedeM, HarfenistBS, MartinFJ, et al. (2012) Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 46 : 6–13 doi:10.1165/rcmb.2011-0080OC
2. ParkerD, PrinceA (2012) Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J Immunol 189 : 4040–4046 doi:10.4049/jimmunol.1201055
3. ParkerD, MartinFJ, SoongG, HarfenistBS, AguilarJL, et al. (2011) Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2: e00016–11 doi:10.1128/mBio.00016-11
4. DavidM (2010) Interferons and microRNAs. Journal of Interferon & Cytokine Research 30 : 825–828 doi:10.1089/jir.2010.0080
5. TrinchieriG (2010) Type I interferon: friend or foe? Journal of Experimental Medicine 207 : 2053–2063 doi:10.1084/jem.20101664
6. HeL, HannonGJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5 : 522–531 doi:10.1038/nrg1379
7. MaX, Becker BuscagliaLE, BarkerJR, LiY (2011) MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3 : 159–166 doi:10.1093/jmcb/mjr007
8. O'NeillLA, SheedyFJ, McCoyCE (2011) MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol 11 : 163–175 doi:10.1038/nri2957
9. QuinnSR, O'NeillLA (2011) A trio of microRNAs that control Toll-like receptor signalling. International Immunology 23 : 421–425 doi:10.1093/intimm/dxr034
10. YasudaM, SchmidT, RübsamenD, ColburnNH, IrieK, et al. (2010) Downregulation of programmed cell death 4 by inflammatory conditions contributes to the generation of the tumor promoting microenvironment. Mol Carcinog 49 : 837–848 doi:10.1002/mc.20660
11. CohenTS, PrinceAS (2013) Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest 123 : 1630–1637 doi:10.1172/JCI66142DS1
12. FrankelLB, ChristoffersenNR, JacobsenA, LindowM, KroghA, et al. (2008) Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283 : 1026–1033 doi:10.1074/jbc.M707224200
13. MartinFJ, GómezMI, WetzelDM, MemmiG, O'SeaghdhaM, et al. (2009) Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest. 119 : 1931–1939.
14. MartinFJ, ParkerD, HarfenistBS, SoongG, PrinceA (2011) Participation of CD11c(+) leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun 79 : 1898–1904 doi:10.1128/IAI.01299-10
15. CarriganSO, JunkinsR, YangYJ, MacneilA, RichardsonC, et al. (2010) IFN regulatory factor 3 contributes to the host response during Pseudomonas aeruginosa lung infection in mice. J Immunol 185 : 3602–3609 doi:10.4049/jimmunol.0903429
16. PowerMR, LiB, YamamotoM, AkiraS, LinT-J (2007) A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J Immunol 178 : 3170–3176.
17. DennisMD, JeffersonLS, KimballSR (2012) Role of p70S6K1-mediated Phosphorylation of eIF4B and PDCD4 Proteins in the Regulation of Protein Synthesis. Journal of Biological Chemistry 287 : 42890–42899 doi:10.1074/jbc.M112.404822
18. KotenkoSV, GallagherG, BaurinVV, Lewis-AntesA, ShenM, et al. (2003) IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 4 : 69–77 doi:10.1038/ni875
19. LiwakU, ThakorN, JordanLE, RoyR, LewisSM, et al. (2012) Tumor Suppressor PDCD4 Represses Internal Ribosome Entry Site-Mediated Translation of Antiapoptotic Proteins and Is Regulated by S6 Kinase 2. Mol Cell Biol 32 : 1818–1829 doi:10.1128/MCB.06317-11
20. SheppardP, KindsvogelW, XuW, HendersonK, SchlutsmeyerS, et al. (2003) IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 4 : 63–68 doi:10.1038/ni873
21. de WeerdNA, NguyenT (2012) The interferons and their receptors--distribution and regulation. Immunology and Cell Biology 90 : 483–491 doi:10.1038/icb.2012.9
22. KroczynskaB, SharmaB, EklundEA, FishEN, PlataniasLC (2012) Regulatory effects of programmed cell death 4 (PDCD4) protein in interferon (IFN)-stimulated gene expression and generation of type I IFN responses. Mol Cell Biol 32 : 2809–2822 doi:10.1128/MCB.00310-12
23. MeagerA, VisvalingamK, DilgerP, BryanD, WadhwaM (2005) Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine 31 : 109–118 doi:10.1016/j.cyto.2005.04.003
24. MaherSG, SheikhF, ScarzelloAJ, Romero-WeaverAL, BakerDP, et al. (2008) IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther 7 : 1109–1115.
25. AnkN, IversenMB, BartholdyC, StaeheliP, HartmannR, et al. (2008) An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J Immunol 180 : 2474–2485.
26. ZhouZ, HammingOJ, AnkN, PaludanSR, NielsenAL, et al. (2007) Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol 81 : 7749–7758 doi:10.1128/JVI.02438-06
27. PulvererJE, RandU, LienenklausS, KugelD, ZietaraN, et al. (2010) Temporal and spatial resolution of type I and III interferon responses in vivo. J Virol 84 : 8626–8638 doi:10.1128/JVI.00303-10
28. SommereynsC, PaulS, StaeheliP, MichielsT (2008) IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4: e1000017 doi:10.1371/journal.ppat.1000017
29. MerlineR, MorethK, BeckmannJ, NastaseMV, Zeng-BrouwersJ, et al. (2011) Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Science Signaling 4: ra75–ra75 Available: http://stke.sciencemag.org/cgi/content/abstract/sigtrans4/199/ra75.
30. WangJ, Oberley-DeeganR, WangS, NikradM, FunkCJ, et al. (2009) Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol 182 : 1296–1304.
31. SheedyFJ, Palsson-McDermottE, HennessyEJ, MartinC, O'LearyJJ, et al. (2009) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11 : 141–147 doi:10.1038/ni.1828
32. JewellNA, ClineT, MertzSE, SmirnovSV, FlanoE, et al. (2010) Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol 84 : 11515–11522 doi:10.1128/JVI.01703-09
33. YangHS, JansenAP, NairR, ShibaharaK, VermaAK, et al. (2001) A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene 20 : 669–676 doi:10.1038/sj.onc.1204137
34. KoltsidaO, HausdingM, StavropoulosA, KochS, TzelepisG, et al. (2011) IL-28A (IFN-λ2) modulates lung DC function to promote Th1 immune skewing and suppress allergic airway disease. EMBO Mol Med 3 : 348–361 doi:10.1002/emmm.201100142
35. GallagherG, MegjugoracNJ, YuRY, EskdaleJ, GallagherGE, et al. (2010) The lambda interferons: guardians of the immune-epithelial interface and the T-helper 2 response. Journal of Interferon & Cytokine Research 30 : 603–615 doi:10.1089/jir.2010.0081
36. PekarekV, SrinivasS, EskdaleJ, GallagherG (2007) Interferon lambda-1 (IFN-λ1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun 8 : 177–180 doi:10.1038/sj.gene.6364372
37. ChastreJ, FagonJ-Y (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165 : 867–903.
38. Centers for Disease Control and Prevention (CDC) (2003) Methicillin-resistant Staphylococcus aureus infections in correctional facilities---Georgia, California, and Texas, 2001-2003. MMWR Morb Mortal Wkly Rep 52 : 992–996.
39. MarcelloT, GrakouiA, Barba-SpaethG, MachlinES, KotenkoSV, et al. (2006) Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 131 : 1887–1898 doi:10.1053/j.gastro.2006.09.052
40. DoyleSE, SchreckhiseH, Khuu-DuongK, HendersonK, RoslerR, et al. (2006) Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44 : 896–906 doi:10.1002/hep.21312
41. GómezMI, SeaghdhaMO, PrinceAS (2007) Staphylococcus aureus protein A activates TACE through EGFR-dependent signaling. EMBO J 26 : 701–709 doi:10.1038/sj.emboj.7601554
42. GómezMI, LeeA, ReddyB, MuirA, SoongG, et al. (2004) Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med 10 : 842–848 doi:10.1038/nm1079
43. GómezMI, O'SeaghdhaM, MagargeeM, FosterTJ, PrinceAS (2006) Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J Biol Chem 281 : 20190–20196.
44. PalamarchukA, EfanovA, MaximovV, AqeilanRI, CroceCM, et al. (2005) Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res 65 : 11282–11286 doi:10.1158/0008-5472.CAN-05-3469
45. WedekenL, SinghP, KlempnauerK-H (2011) Tumor suppressor protein Pdcd4 inhibits translation of p53 mRNA. Journal of Biological Chemistry 286 : 42855–42862 doi:10.1074/jbc.M111.269456
46. WedekenL, OhnheiserJ, HirschiB, WethkampN, KlempnauerK-H (2010) Association of tumor suppressor protein Pdcd4 with ribosomes is mediated by protein-protein and protein-RNA interactions. Genes & Cancer 1 : 293–301 doi:10.1177/1947601910364227
47. SatoA, OhtsukiM, HataM, KobayashiE, MurakamiT (2006) Antitumor activity of IFN-lambda in murine tumor models. J Immunol 176 : 7686–7694.
48. LasfarA, Lewis-AntesA, SmirnovSV, AnanthaS, AbushahbaW, et al. (2006) Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res 66 : 4468–4477 doi:10.1158/0008-5472.CAN-05-3653
49. DickensheetsH, SheikhF, ParkO, GaoB, DonnellyRP (2013) Interferon-lambda (IFN-λ) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol 93 : 377–385 doi:10.1189/jlb.0812395
50. GeD, FellayJ, ThompsonAJ, SimonJS, ShiannaKV, et al. (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461 : 399–401 doi:10.1038/nature08309
51. LebretonA, LakisicG, JobV, FritschL, ThamTN, et al. (2011) A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response. Science 331 : 1319–1321 doi:10.1126/science.1200120
52. SuzukiC, GarcesRG, EdmondsKA, HillerS, HybertsSG, et al. (2008) PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. PNAS 105 : 3274–3279 doi:10.1073/pnas.0712235105
53. LiuX, ZhanZ, XuL, MaF, LiD, et al. (2010) MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol 185 : 7244–7251 doi:10.4049/jimmunol.1001573
54. LiuPT, WheelwrightM, TelesR, KomisopoulouE, EdfeldtK, et al. (2012) MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat Med 18 : 267–273 doi:10.1038/nm.2584
55. CaseSR, MartinRJ, JiangD, MinorMN, ChuHW (2011) MicroRNA-21 inhibits toll-like receptor 2 agonist-induced lung inflammation in mice. Exp Lung Res 37 : 500–508 doi:10.3109/01902148.2011.596895
56. LuTX, HartnerJ, LimE-J, FabryV, MinglerMK, et al. (2011) MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol 187 : 3362–3373 doi:10.4049/jimmunol.1101235
57. RuanQ, WangT, KameswaranV, WeiQ, JohnsonDS, et al. (2011) The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic {beta} cell death. PNAS 108 : 12030–12035 doi:10.1073/pnas.1101450108/-/DCSupplemental/pnas.201101450SI.pdf
58. ParkerD, PrinceA (2013) Epithelial uptake of flagella initiates proinflammatory signaling. PLoS ONE 8: e59932 doi:10.1371/journal.pone.0059932.g005
59. MijaresLA, WangdiT, SokolC, HomerR, MedzhitovR, et al. (2011) Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186 : 7080–7088 doi:10.4049/jimmunol.1003687
60. ChunJ, PrinceA (2009) TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 5 : 47–58 doi:10.1016/j.chom.2008.11.009
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



