-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
article has not abstract
Published in the journal: . PLoS Pathog 9(10): e32767. doi:10.1371/journal.ppat.1003674
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003674Summary
article has not abstract
Introduction
Genetic exchange occurs via horizontal gene transfer in bacteria and archea or sexual reproduction in fungal and parasitic eukaryotic microbes. Sexual reproduction is universal, or nearly so, in eukaryotes. Until recently, most eukaryotic microbial pathogens were thought to be clonal and asexual due to the absence of a compatible partner or the lack of morphological or population genetic evidence for sexual reproduction [1]. However, many of these eukaryotic pathogens have been found recently to have extant cryptic sexual cycles (Figure 1). Sex enables microbial pathogens to reshuffle their genomes, increase genetic diversity, purge deleterious mutations, and produce infectious propagules.
Fig. 1. Modes of unisexual reproduction. 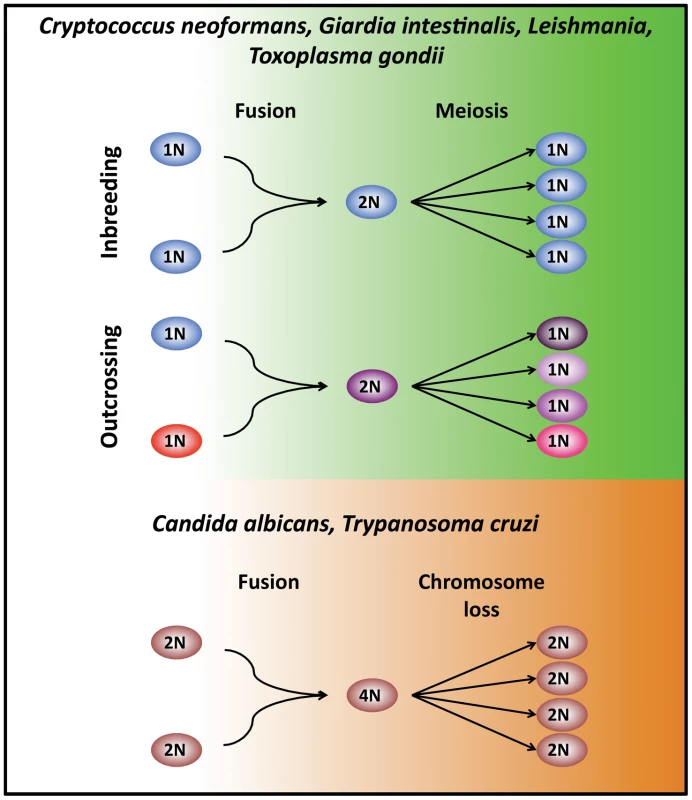
In haploid eukaryotes, cells (1N) of the same mating type can fuse or undergo endoreplication to generate a diploid intermediate (2N). DNA replication without cell division follows and meiosis produces four recombinant progeny (1N). Unisexual reproduction can also occur in 1) a clonal population, promoting inbreeding, or 2) between cells of the same mating type but of distinct genetic lineages to enable outcrossing. A similar unisexual cycle is observed in Candida and possibly also Trypanosoma, where diploid cells (2N) fuse to generate a tetraploid intermediate (4N). The resulting tetraploid cells (4N) return to the diploid state (2N) through either parasexual chromosome loss that occurs stochastically independent of meiosis (C. albicans, and possibly T. cruzi) or sexually via meiosis (T. brucei). Unisexual Reproduction in Fungi
Sexual reproduction involving cells of opposite mating types or sexes comes with costs. Locating a compatible partner and undergoing mating and meiosis requires time and energy. In addition, sexual reproduction introduces genetic diversity, but in so doing rearranges well-adapted genomic configurations. In contrast, sexual reproduction involving cells of only one type (unisexual reproduction), via either mother-daughter cell-cell fusion or endoreplication, lowers the barrier to locating a compatible mating partner. Unisexual reproduction ameliorates the cost of losing a well-adapted phenotype in a particular niche while introducing more limited genetic diversity that may enhance the fitness of progeny in response to environmental changes, including drug treatments.
Cryptococcus neoformans is a basidiomycetous pathogenic yeast found in both the environment and infected hosts. Cryptococcus has a bipolar mating system with two mating types, a and α [2], and a well-defined sexual cycle involving cells of opposite mating type that fuse and undergo a dimorphic yeast-hyphal transition. At the hyphal tips, basidia form where meiosis occurs followed by multiple rounds of mitosis, producing long spore chains [3], [4]. Spores are infectious propagules that are inhaled by the host [5], [6].
Although C. neoformans has a defined a-α sexual cycle, the α mating type predominates in environmental and clinical isolates, and in many niches the population is exclusively α, dramatically limiting opportunities for a-α sexual reproduction [7]. Recent studies have shown that C. neoformans undergoes unisexual reproduction that involves an alternative dimorphic transition to hyphal growth, production of basidia, meiosis, and sporulation [5], [6], [8]. This commonly occurs with α strains but has also been reported to occur with some strains of the a mating type [3], [9], [10]. The pathway is controlled by multiple genetic loci as a quantitative trait, of which the MATα locus allele provides the major contribution [10]. Diploidization occurs during unisexual reproduction: either early to produce a diploid yeast that then produces a diploid monokaryotic hyphae, or late in which a haploid yeast produces a haploid monokaryotic hyphae in which karyogamy is delayed until the basidia form (similar to a-α sexual reproduction) [10], [11].
Meiotic recombination occurs at a similar frequency in spores produced by either opposite sexual or unisexual reproduction [8]. Moreover, the key meiotic genes SPO11 and DMC1 (which induce and repair DNA DSBs that provoke meiotic recombination) are both dispensable for hyphal and basidia development, but critical for sporulation during unisexual reproduction: the dmc1 and spo11 mutants produce fewer spores, often in only two instead of four chains, and their germination frequency is severely impaired resulting in only 2% or 3.7% the wild-type level of viable spore production in these meiotic mutants [8], [12]. Thus, unisexual reproduction is a complete sexual cycle involving meiotic production of spores.
While the unisexual cycle has been directly observed only under laboratory conditions, mounting population genetic evidence supports that unisexual cycles occur in nature in both clonal and genetically divergent populations leading to recombination in exclusively α populations and also resulting in diploid intermediates or products with αADα or αAAα genotypes [13]–[19]. Moreover, recent studies have shown that unisexual reproduction between genetically identical cells generates phenotypic and genotypic diversity, including SNPs, chromosomal translocations, and aneuploidy, which may enhance competitive fitness in different environments [20]. Interestingly, population genetic analyses also implicate unisexual reproduction in the origins of the strains responsible for the outbreak caused by the sibling species Cryptococcus gattii on Vancouver Island and in the Pacific Northwest. Unisex may also be producing the spores causing the outbreak given that the lineages responsible are all of the α mating type [15].
These studies illustrate how unisexual reproduction may 1) admix genetic diversity to facilitate adaptive selection and 2) serve as a mutagen to generate genetic diversity de novo in otherwise clonal populations. In the latter case, the intermediate diploid state could also serve as a capacitor for evolution, allowing the accumulation of multiple recessive mutations whose combination may be advantageous when released into the haploid state, as has been observed in A. nidulans [21].
Candida albicans, the most common human fungal pathogen, was until recently thought to be strictly asexual. An extant heterothallic parasexual cycle has been defined involving diploid a/a and α/α cells that undergo cell-cell fusion and nuclear fusion, yielding a tetraploid intermediate [22]–[24]. Surprisingly, meiosis has not yet been observed in C. albicans, and the tetraploid returns to the diploid state through stochastic parasexual chromosome loss, which generates considerable aneuploidy, as well as infrequent Spo11-dependent recombination [25]. Unexpectedly, a/a cells can express both the a and α pheromone genes in response to nutrient limitation, which led to the discovery of a unisexual cycle in C. albicans [26]. Mutation of Bar1, a protease that cleaves α-factor to regulate autocrine and paracrine signaling, enables an a-a unisexual cycle [27]. This homothallic parasexual cycle is also induced in ménage à trois matings in which a third mating type partner in limited abundance donates pheromone to promote a-a or α-α mating [27]. Remarkably, pheromones from other species such as Candida dubliniensis or Candida parapsilosis can induce unisexual reproduction of C. albicans [28].
Similar to C. neoformans, where unisexual reproduction can introduce genomic changes in a clonal population [20], the aneuploidy-prone parasexual cycle also induces a range of novel genotypes in C. albicans that may provide selective advantages in novel environments. Although aneuploidy can be deleterious, in fungi aneuploidy can also confer drug resistance to antifungal therapy and promote pathogen survival in the host [29]–[31]. Moreover, parasexual reproduction in C. albicans is linked to a morphogenic change from white to opaque cells. Opaque cells are specialized in mating, while white cells are highly virulent. Although white cells are infertile, pheromone from rare opaque cells stimulates adhesion and biofilm formation of white cells [32]. Thus, roles of unisexual development may extend beyond mating and genetic diversity with the capacity to induce virulence attributes in hostile environments.
There are other pathogenic fungi once thought to be asexual that we now appreciate may be cryptically sexual or unisexual. Recent studies on the AIDS-associated pathogen Penicillium marneffei provide genetic evidence of a sexual cycle, and the presence of a clonal population with limited recombination rates suggests selfing may also occur in this fungus [33]. Unisexual reproduction is so far uncommon in the fungal kingdom, but many different species undergo other forms of homothallic sexual cycles. Some fungi harbor both mating type locus alleles or idiomorphs in their genome, which can be linked or unlinked, while others can switch mating type (Saccharomyces cerevisiae and Schizosaccharomyces pombe and related yeasts). Others have a single mating type locus and complete a sexual cycle in the absence of an opposite–mating type partner. Others species, such as N. africana, N. galapagosensis, N. dodgei, and N. lineolata, may undergo unisexual reproduction given that their populations appear to harbor one MAT locus idiomorph yet they are sexual [34]–[38]. Furthermore, the obligate intracellular microsporidian fungal pathogen Encephalitozoon cuniculi contains two HMG domains similar to the MAT locus of zygomycetes, although an extant sexual cycle has not been observed [39]. Recent studies have revealed low levels of heterozygosity in four E. cuniculi strains, indicative of a diploid nuclear state that may reflect a cryptic unisexual cycle in this “asexual” fungus [40].
Unisexual Reproduction in Parasites
Among a broader group of microbial pathogens, the protozoan parasites also harbor extant sexual cycles that in some cases are now known to be cryptic or unisexual. Although these pathogens exhibit interesting and unusual sexual cycles, essentially nothing is as yet known about how sexes or mating types are established.
Until recently the intestinal parasite Giardia intestinalis was thought to be asexual; however, population genetics studies revealed evidence of genetic exchange, and the genome harbors a suite of meiotic genes [41], [42]. This diplomonad parasite is binucleate, and the two nuclei of a single isolate can fuse to exchange genetic information, followed by homologous recombination that is possibly directed by meiotic gene homologs [43]. The cues that trigger cell-cell fusion in the population remain to be explored. However, high levels of genetic exchange in the population provide evidence that outcrossing is likely occurring.
In the pathogen Leishmania, which is highly clonal and was therefore thought to be asexual, recent studies have revealed that an extant sexual cycle occurs in the sand fly vector [44]–[46]. Both outcrossing and selfing have been observed in Plasmodium species, in which a single isolate can differentiate and produce fertile male and female gametes that undergo sexual reproduction. In Trypanosoma, diploids may fuse to create an intermediate tetraploid that may undergo random chromosome loss through a parasexual cycle similar to C. albicans [47]. In Toxoplasma gondii, sexual outcrossing and subsequent self-mating generated highly virulent T. gondii clones that were responsible for a toxoplasmosis outbreak in Brazil and other global locales [48]. Unisexual reproduction preserved a well-adapted genomic configuration and generated abundant spores fueling this outbreak.
Other Examples
Unisexual reproduction has emerged as an adaptive mechanism in eukaryotic microbes, but also extends beyond unicellular organisms. In plants, self-pollination is surprisingly common. Transitions from cross-pollination to self-pollination are frequently observed; for example, the model plant Arabidopsis thaliana reproduces almost exclusively through self-pollination (∼99%) yet retains the ability to outcross at a low frequency (∼1%). Such transitions are thought to enable provincial species with restricted niches to emerge as successfully dispersed cosmopolitan species. In Bdelloid rotifers, males, an extant sexual cycle, and meiosis all appear to be absent, and these organisms are only known to reproduce via parthenogenesis [49]. More than 80 unisexual species of fishes, insects, reptiles, and amphibians also reproduce via parthenogenesis. Facultative parthenogenesis, where sexual species resort to reproduction in the absence of a compatible partner, is more widespread than obligate parthenogenesis. In vertebrates and mammals, even facultative parthenogenesis is very rare due to genomic imprinting. However, some isolated female sharks have given birth to live young (all daughters) in the absence of a male partner [50]. Facultative selfing has been documented not only in sharks but also in komodo dragons [51], and some species of domesticated birds. Moreover, genetic manipulation of the imprinting mechanisms in female mice resulted in healthy, live offspring produced via parthenogenesis under laboratory conditions [52]. Both unisexual reproduction and parthenogenesis can mitigate some of the costs associated with sex, but a common consequence is a reduced level of genetic exchange or diversity.
Conclusions
We now appreciate that eukaryotic microbial pathogens, including fungi and parasites, are not clonal and asexual, but rather have extant sexual cycles that are cryptic, parasexual, or even unisexual. Two of the three most common systemic human fungal pathogens (Candida and Cryptococcus) have retained extant sexual and parasexual cycles involving both bisexual and unisexual reproduction, which may provide a broader range of adaptive evolutionary strategies. Similar paradigms have now emerged for several eukaryotic parasites, suggesting this may be a general mode of adaptation for microbial pathogens, enabling them to preserve well-adapted genotypes. Given that sex is ubiquitous throughout the eukaryotic tree of life and yet we are confronted with a panoply of diverse mechanisms via which mating type (or sex) is specified and mating partners (or gametes) are distinguished and recognized, one possibility is that unisexual reproduction was the original ancestral form of sexual reproduction to which mating types and sexes were added later. If so, the finding that extant unisexual reproduction occurs in both fungal and parasitic pathogens may reflect a return to a more ancestral mode of reproduction rather than the emergence of an entirely new process promoting genetic change and exchange.
Zdroje
1. TibayrencM, AyalaFJ (2012) Reproductive clonality of pathogens: a perspective on pathogenic viruses, bacteria, fungi, and parasitic protozoa. Proc Natl Acad Sci U S A 109: E3305–3313.
2. LengelerKB, FoxDS, FraserJA, AllenA, ForresterK, et al. (2002) Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot Cell 1 : 704–718.
3. HullCM, HeitmanJ (2002) Genetics of Cryptococcus neoformans. Annu Rev Genet 36 : 557–615.
4. Kwon-ChungKJ (1976) Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68 : 821–833.
5. GilesSS, DagenaisTR, BottsMR, KellerNP, HullCM (2009) Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun 77 : 3491–3500.
6. VelagapudiR, HsuehYP, Geunes-BoyerS, WrightJR, HeitmanJ (2009) Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 77 : 4345–4355.
7. Kwon-ChungKJ, BennettJE (1978) Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol 108 : 337–340.
8. LinX, HullCM, HeitmanJ (2005) Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434 : 1017–1021.
9. TscharkeRL, LazeraM, ChangYC, WickesBL, Kwon-ChungKJ (2003) Haploid fruiting in Cryptococcus neoformans is not mating type α-specific. Fungal Genet Biol 39 : 230–237.
10. LinX, HuangJC, MitchellTG, HeitmanJ (2006) Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet 2: e187 doi:10.1371/journal.pgen.0020187
11. LeeSC, HeitmanJ (2012) Function of Cryptococcus neoformans KAR7 (SEC66) in karyogamy during unisexual and opposite-sex mating. Eukaryot Cell 11 : 783–794.
12. FeretzakiM, HeitmanJ (2013) Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans. PLoS Genet 9: e1003688 doi:10.1371/journal.pgen.1003688
13. LinX, LitvintsevaAP, NielsenK, PatelS, FloydA, et al. (2007) αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet 3: e186 doi:10.1371/journal.pgen.0030186
14. LinX, PatelS, LitvintsevaAP, FloydA, MitchellTG, et al. (2009) Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog 5: e1000283 doi:10.1371/journal.ppat.1000283
15. FraserJA, GilesSS, WeninkEC, Geunes-BoyerSG, WrightJR, et al. (2005) Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437 : 1360–1364.
16. BuiT, LinX, MalikR, HeitmanJ, CarterD (2008) Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot Cell 7 : 1771–1780.
17. SaulN, KrockenbergerM, CarterD (2008) Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single eucalyptus tree hollows. Eukaryot Cell 7 : 727–734.
18. HiremathSS, ChowdharyA, KowshikT, RandhawaHS, SunS, et al. (2008) Long-distance dispersal and recombination in environmental populations of Cryptococcus neoformans var. grubii from India. Microbiology 154 : 1513–1524.
19. ChowdharyA, HiremathSS, SunS, KowshikT, RandhawaHS, et al. (2011) Genetic differentiation, recombination and clonal expansion in environmental populations of Cryptococcus gattii in India. Environ Microbiol 13 : 1875–1888.
20. NiM, FeretzakiM, LiW, Floyd-AveretteA, MieczkowskiP, et al. (2013) Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol 11: e1001653 doi:10.1371/journal.pbio.1001653
21. SchoustraSE, DebetsAJM, SlakhorstM, HoekstraRF (2007) Mitotic recombination accelerates adaptation in the fungus Aspergillus nidulans. PLoS Genet 3: e68 doi:10.1371/journal.pgen.0030068
22. HullCM, RaisnerRM, JohnsonAD (2000) Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289 : 307–310.
23. MageeBB, MageePT (2000) Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289 : 310–313.
24. MillerMG, JohnsonAD (2002) White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110 : 293–302.
25. ForcheA, AlbyK, SchaeferD, JohnsonAD, BermanJ, et al. (2008) The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol 6: e110 doi:10.1371/journal.pbio.0060110
26. BennettRJ, JohnsonAD (2006) The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans. Mol Microbiol 62 : 100–119.
27. AlbyK, SchaeferD, BennettRJ (2009) Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460 : 890–893.
28. AlbyK, BennettRJ (2011) Interspecies pheromone signaling promotes biofilm formation and same-sex mating in Candida albicans. Proc Natl Acad Sci U S A 108 : 2510–2515.
29. SelmeckiA, ForcheA, BermanJ (2006) Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313 : 367–370.
30. SelmeckiAM, DulmageK, CowenLE, AndersonJB, BermanJ (2009) Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet 5: e1000705 doi:10.1371/journal.pgen.1000705
31. SionovE, LeeH, ChangYC, Kwon-ChungKJ (2010) Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6: e1000848 doi:10.1371/journal.ppat.1000848
32. DanielsKJ, SrikanthaT, LockhartSR, PujolC, SollDR (2006) Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J 25 : 2240–2252.
33. HenkDA, Shahar-GolanR, DeviKR, BoyceKJ, ZhanN, et al. (2012) Clonality despite sex: the evolution of host-associated sexual neighborhoods in the pathogenic fungus Penicillium marneffei. PLoS Pathog 8: e1002851 doi:10.1371/journal.ppat.1002851
34. MahoneyDP, HuangLH, BackusMP (1969) New homothallic Neurosporas from tropical soils. Mycologia 61 : 264–272.
35. NygrenK, StrandbergR, WallbergA, NabholzB, GustafssonT, et al. (2011) A comprehensive phylogeny of Neurospora reveals a link between reproductive mode and molecular evolution in fungi. Mol Phylogenet Evol 59 : 649–663.
36. GlassNL, VollmerSJ, StabenC, GrotelueschenJ, MetzenbergRL, et al. (1988) DNAs of the two mating-type alleles of Neurospora crassa are highly dissimilar. Science 241 : 570–573.
37. GlassNL, SmithML (1994) Structure and function of a mating-type gene from the homothallic species Neurospora africana. Mol Gen Genet 244 : 401–409.
38. ArnaiseS, ZicklerD, GlassNL (1993) Heterologous expression of mating-type genes in filamentous fungi. Proc Natl Acad Sci U S A 90 : 6616–6620.
39. LeeSC, CorradiN, DoanS, DietrichFS, KeelingPJ, et al. (2010) Evolution of the sex-related locus and genomic features shared in microsporidia and fungi. PLoS ONE 5: e10539 doi:10.1371/journal.pone.0010539
40. SelmanM, SakB, KvacM, FarinelliL, WeissLM, et al. (2013) Extremely reduced levels of heterozygosity in the vertebrate pathogen Encephalitozoon cuniculi. Eukaryot Cell 12 : 496–502.
41. CooperMA, AdamRD, WorobeyM, SterlingCR (2007) Population genetics provides evidence for recombination in Giardia. Curr Biol 17 : 1984–1988.
42. RameshMA, MalikSB, LogsdonJMJr (2005) A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol 15 : 185–191.
43. PoxleitnerMK, CarpenterML, MancusoJJ, WangCJ, DawsonSC, et al. (2008) Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 319 : 1530–1533.
44. AkopyantsNS, KimblinN, SecundinoN, PatrickR, PetersN, et al. (2009) Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science 324 : 265–268.
45. RougeronV, BanulsAL, CarmeB, SimonS, CouppieP, et al. (2011) Reproductive strategies and population structure in Leishmania: substantial amount of sex in Leishmania Viannia guyanensis. Mol Ecol 20 : 3116–3127.
46. InbarE, AkopyantsNS, CharmoyM, RomanoA, LawyerP, et al. (2013) The mating competence of geographically diverse Leishmania major strains in their natural and unnatural sand fly vectors. PLoS Genet 9: e1003672 doi:10.1371/journal.pgen.1003672
47. GauntMW, YeoM, FrameIA, StothardJR, CarrascoHJ, et al. (2003) Mechanism of genetic exchange in American trypanosomes. Nature 421 : 936–939.
48. WendteJM, MillerMA, LambournDM, MagargalSL, JessupDA, et al. (2010) Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii. PLoS Genet 6: e1001261 doi:10.1371/journal.pgen.1001261
49. Mark WelchDB, MeselsonM (2000) Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288 : 1211–1215.
50. FeldheimKA, ChapmanDD, SweetD, FitzpatrickS, ProdohlPA, et al. (2010) Shark virgin birth produces multiple, viable offspring. J Hered 101 : 374–377.
51. WattsPC, BuleyKR, SandersonS, BoardmanW, CiofiC, et al. (2006) Parthenogenesis in Komodo dragons. Nature 444 : 1021–1022.
52. KonoT, ObataY, WuQ, NiwaK, OnoY, et al. (2004) Birth of parthenogenetic mice that can develop to adulthood. Nature 428 : 860–864.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Are We There Yet? Recent Progress in the Molecular Diagnosis and Novel Antifungal Targeting of and Invasive Aspergillosis
- Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events
- Emergence of Azole-Resistant Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health
- Fungal Adenylyl Cyclase Acts As a Signal Sensor and Integrator and Plays a Central Role in Interaction with Bacteria
- Sensing of the Microbial Neighborhood by
- Antivirulence Therapy for Animal Production: Filling an Arsenal with Novel Weapons for Sustainable Disease Control
- The Cell Biology of : How to Teach Using Animations
- A Structure-Guided Mutation in the Major Capsid Protein Retargets BK Polyomavirus
- RNA Biology in Fungal Phytopathogens
- , , and the Human Mouth: A Sticky Situation
- The Gene Is Essential for Resistance to Human Serum in
- Unisexual Reproduction Drives Evolution of Eukaryotic Microbial Pathogens
- Bacterial Pathogens Activate a Common Inflammatory Pathway through IFNλ Regulation of PDCD4
- Bats and Viruses: Friend or Foe?
- Protein Trafficking through the Endosomal System Prepares Intracellular Parasites for a Home Invasion
- IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection
- B Cells Enhance Antigen-Specific CD4 T Cell Priming and Prevent Bacteria Dissemination following Genital Tract Infection
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Chemicals, Climate, and Control: Increasing the Effectiveness of Malaria Vector Control Tools by Considering Relevant Temperatures
- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
- Driven Enforced Viral Replication in Dendritic Cells Contributes to Break of Immunological Tolerance in Autoimmune Diabetes
- IL-4Rα-Associated Antigen Processing by B Cells Promotes Immunity in Infection
- A Gammaherpesvirus Uses Alternative Splicing to Regulate Its Tropism and Its Sensitivity to Neutralization
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Epigenetic Dominance of Prion Conformers
- MAIT Cells Detect and Efficiently Lyse Bacterially-Infected Epithelial Cells
- The Role of TcdB and TccC Subunits in Secretion of the Tcd Toxin Complex
- A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Vaccines: Strongly Sought but Not a Reality Just Yet
- MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb
- Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis
- Feeding Uninvited Guests: mTOR and AMPK Set the Table for Intracellular Pathogens
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



