-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
Rice tungro disease is caused by synergistic interaction of an RNA picorna-like virus Rice tungro spherical virus (RTSV) and a DNA pararetrovirus Rice tungro bacilliform virus (RTBV). It is spread by insects owing to an RTSV-encoded transmission factor. RTBV has evolved a ribosome shunt mechanism to initiate translation of its pregenomic RNA having a long and highly structured leader. We found that a long leader of RTSV genomic RNA remarkably resembles the RTBV leader: both contain several short ORFs (sORFs) and potentially fold into a large stem-loop structure with the first sORF terminating in front of the stem basal helix. Using translation assays in rice protoplasts and wheat germ extracts, we show that, like in RTBV, both initiation and proper termination of the first sORF translation in front of the stem are required for shunt-mediated translation of a reporter ORF placed downstream of the RTSV leader. The base pairing that forms the basal helix is required for shunting, but its sequence can be varied. Shunt efficiency in RTSV is lower than in RTBV. But in addition to shunting the RTSV leader sequence allows relatively efficient linear ribosome migration, which also contributes to translation initiation downstream of the leader. We conclude that RTSV and RTBV have developed a similar, sORF-dependent shunt mechanism possibly to adapt to the host translation system and/or coordinate their life cycles. Given that sORF-dependent shunting also operates in a pararetrovirus Cauliflower mosaic virus and likely in other pararetroviruses that possess a conserved shunt configuration in their leaders it is tempting to propose that RTSV may have acquired shunt cis-elements from RTBV during their co-existence.
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002568
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002568Summary
Rice tungro disease is caused by synergistic interaction of an RNA picorna-like virus Rice tungro spherical virus (RTSV) and a DNA pararetrovirus Rice tungro bacilliform virus (RTBV). It is spread by insects owing to an RTSV-encoded transmission factor. RTBV has evolved a ribosome shunt mechanism to initiate translation of its pregenomic RNA having a long and highly structured leader. We found that a long leader of RTSV genomic RNA remarkably resembles the RTBV leader: both contain several short ORFs (sORFs) and potentially fold into a large stem-loop structure with the first sORF terminating in front of the stem basal helix. Using translation assays in rice protoplasts and wheat germ extracts, we show that, like in RTBV, both initiation and proper termination of the first sORF translation in front of the stem are required for shunt-mediated translation of a reporter ORF placed downstream of the RTSV leader. The base pairing that forms the basal helix is required for shunting, but its sequence can be varied. Shunt efficiency in RTSV is lower than in RTBV. But in addition to shunting the RTSV leader sequence allows relatively efficient linear ribosome migration, which also contributes to translation initiation downstream of the leader. We conclude that RTSV and RTBV have developed a similar, sORF-dependent shunt mechanism possibly to adapt to the host translation system and/or coordinate their life cycles. Given that sORF-dependent shunting also operates in a pararetrovirus Cauliflower mosaic virus and likely in other pararetroviruses that possess a conserved shunt configuration in their leaders it is tempting to propose that RTSV may have acquired shunt cis-elements from RTBV during their co-existence.
Introduction
Rice tungro disease is a significant constraint for rice cultivation in South and Southeast Asia. It is caused by a synergistic interaction of two viruses, Rice tungro bacilliform virus (RTBV) and Rice tungro spherical virus (RTSV). Individually these viruses exhibit rather mild symptoms: RTSV causes mild or indistinct symptoms, whereas RTBV infection causes yellowing and reddening of the leaves and results in stunted growth. The RTBV symptoms are accentuated in plants co-infected with RTBV and RTSV. Moreover, RTBV on its own cannot be transmitted from plant to plant, but it can do so with the help of RTSV that encodes an insect transmission factor [1]. This suggests that the two viruses have co-evolved into a unique disease complex, in which partners may have developed not only specialized but also shared mechanisms enabling the complex to establish systemic infection and to accumulate in the same plant tissues in order to be co-transmitted. Indeed, both RTBV and RTSV are phloem-restricted. It can be further suggested that during converging evolution the two viruses may have exchanged or independently developed certain cis-acting elements and sequence motifs to adapt to the host cell machinery and to synchronize their life cycles. Our study provides initial evidence for this hypothesis.
RTSV belongs to genus Waikavirus in the family Secoviridae of picorna-like viruses [2]. Its single-stranded, polyadenylated genomic RNA of 12.4 kb contains one large ORF encoding a viral polyprotein [3]. The polyprotein ORF is preceded with an unusually long leader sequence (514-nt in the type species NC_001632) which has several short ORFs (sORFs) and a high propensity to form stable secondary structure (see below): both features are known to inhibit 5′ end-dependent, scanning-mediated translation initiation on eukaryotic ribosomes [4]. Thus, translation of RTSV genomic RNA may involve either internal ribosome entry or 5′ end-dependent ribosome shunting. An internal initiation mechanism operates in animal picornaviruses that possess long and highly-structured leaders [5] and it is therefore an attractive possibility that plant picorna-like viruses have also evolved an internal ribosome entry site (IRES) to initiate translation. However, so far there is little evidence that viruses of the family Secoviridae use internal initiation of translation and the IRES elements identified in short leaders of two distinct viruses from the family Potyviridae do not resemble each other and those of animal picornaviruses [6]. Instead, compelling evidence indicates that plant pararetroviruses have evolved a ribosome shunt mechanism, which combines features of 5′ end-dependent scanning and internal initiation, to translate their pregenomic RNAs that all possess long and highly structured leaders [7]–[10].
RTBV is the only member of genus Tungrovirus in the family Caulimoviridae of pararetroviruses [11]. Its circular double-stranded DNA genome of 8 kbp is transcribed by Pol II into a pregenomic RNA (pgRNA) of more-than-genome length as a poly(A) signal located 195 bp downstream of the transcription start site is recognized efficiently only at its second encounter. The pgRNA is a polycistronic mRNA for three consecutive overlapping ORFs (I, II and III) that are translated by a leaky scanning mechanism [12]. This mechanism operates efficiently owing to the lack of additional AUGs within about 1 kb region between the start codons of ORFs I and III, the feature also conserved in a closely-related badnaviruses (genus Badnavirus of the Caulimoviridae) which have similar organization of ORFs I–III [13]. Unlike badnaviruses, RTBV has an additional ORF, ORF IV, located downstream of ORF III. This ORF is translated from a spliced version of pgRNA, in which the first sORF of the pgRNA leader is fused to ORF IV [14].
Translation of RTBV pgRNA is initiated by ribosome shunting that overcomes the obstacles of a 700-nt leader sequence with multiple sORFs and a stable stem-loop structure [8]. This mechanism operates efficiently in rice protoplasts and involves (i) 5′ end-dependent ribosome scanning until the first sORF is encountered, (ii) translation of this sORF and its termination just in front of the stem basal helix, the formation of which is crucial for efficient shunting, (iii) ribosome shunting over the structured region, and (iv) resumption of scanning at the shunt landing site, where a fraction of the shunting ribosomes (about 10%) also initiates translation at the AUU start codon of ORF I [8], [15] (Figure 1A). The RTBV shunt strikingly resembles the shunt mechanism evolved by Cauliflower mosaic virus (CaMV) from genus Caulimovirus of plant pararetroviruses [7], [15]. Notably, in both cases, initiation and proper termination of the first sORF translation (but not an encoded peptide) are essential for shunting. Furthermore, the RTBV shunt elements including the sORF, the stem base section and the shunt landing sequence could functionally replace the corresponding elements in the CaMV genome in driving efficient polycistronic translation of CaMV pgRNA and in supporting infection of the chimeric virus in CaMV-host plants [16].
Fig. 1. Conserved shunt configurations in the RTBV and RTSV leader sequences. 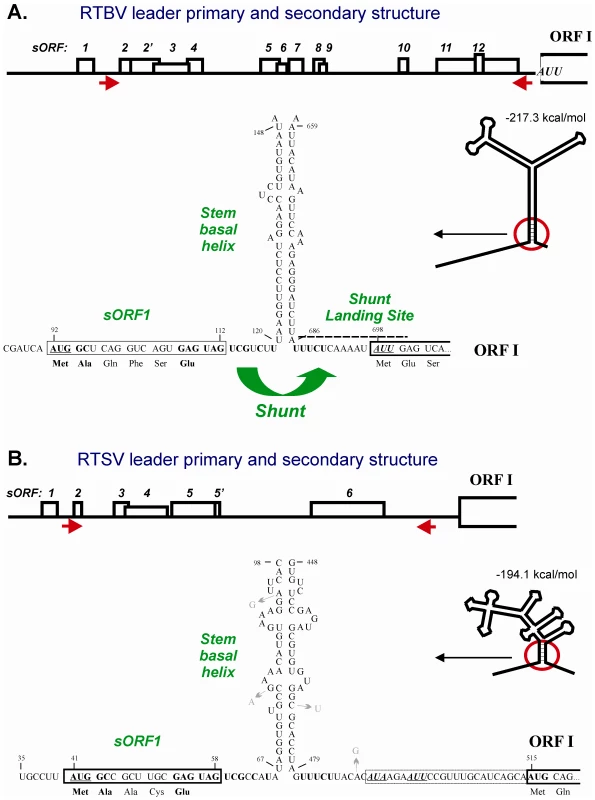
The primary and secondary structures of RTBV (A) and RTSV (B) leaders preceding the first large viral ORF (ORF I) are shown schematically. Short ORFs within the leaders are indicated by boxes, with internal AUGs indicated by vertical lines. Arrows under the leader line define the ascending and descending arms that form the base section of the large stem-loop structure. The stem-loop structures are predicted by the MFold program (Wisconsin GCG package) at 25°C and schematically drawn below the leader primary structures. The 5′- and 3′-sequences flanking the main structure are shown in open conformation. The stable structural element at the stem base (stem basal helix) and adjacent regions, are enlarged and their sequences shown. The nucleotide numbering is from the RNA 5′-end. The 5′-proximal short ORF (sORF1) is boxed. The sORF1 AUG and the non-AUG start codons in the shunt landing site are underlined. The identical nucleotide stretches/motifs in the shunt take-off and landing sites are highlighted in bold. Nucleotide substitutions that occur in five isolates of RTSV are indicated with arrows. The shunt configuration comprising an sORF terminating in front of the stable secondary structure has been identified in the pgRNA leader of most plant pararetroviruses [9], suggesting its evolutionary conservation within this family. Whether or not a shunt mechanism was also evolved in other families of plant viruses remained unknown so far. It is worth mentioning that an sORF-dependent shunt mechanism of the CaMV/RTBV-type has also evolved in a human spumavirus [17] and a human gene [18]. Here we provide evidence that sORF-dependent ribosome shunting operates in RTSV.
Results/Discussion
Identification of a conserved shunt configuration in the RTSV leader
Our computer-aided comparison of the 697-nt RTBV and the 514-nt RTSV leader sequences revealed remarkable similarities, suggesting that RTSV has co-evolved ribosome shunting (Figure 1):
-
Both leaders are unusually long, they contain several sORFs with a total number of 13 and 7 AUG codons, respectively, and can potentially fold into a stable stem-loop structure (deltaG = −217 and −194 kcal/mol, respectively). Although the RTSV structure is more branched, its bottom section is rich in GC base-pairs which would ensure the stability: indeed this section is present in all optimal and suboptimal structures predicted by MFOLD, whereas the upper part of the structure can potentially assume several different conformations (Figure S1, A and B). The primary sequences of the ascending and descending arms do not exhibit any apparent similarities between the two viruses. In CaMV, stability of the stem base but not primary sequences involved in its formation is an important parameter that determines shunt efficiency [15], [19]–[21]. Furthermore, in rice protoplasts the RTBV stem base section could be functionally replaced with the corresponding CaMV section composed of distinct primary sequences [15]. Moreover, a fully artificial stem structure placed downstream of a sORF could drive ribosome shunting in vitro [22]. According to our current shunt model, as compared to scanning ribosomes, the shunting ribosomes released after sORF translation have a reduced capacity to melt secondary structure and are therefore forced to resume scanning downstream of the structure [4], [10].
-
Both leaders have a very similar 5′-proximal sORF (sORF1). Firstly, sORF1 terminates at a short distance (7 and 8 nts, respectively) upstream of the stem base and a nucleotide context of the stop codon is identical (GAG UAG UCG). In CaMV and RTBV, the sORF1 stop codon is a take-off site for shunting ribosomes and proper termination of sORF1 translation in front of the stem base followed by peptide release is required for efficient shunting [4]. Secondly, the start codon of sORF1 is in a moderate initiation context in both RTBV and RTSV (UCA AUG GCU and CUU AUG GCC, respectively; the contexts deviate from a strong plant context because they lack A at position −3 relative to the first nucleotide of the start codon, but still have G at position +4) and it is positioned at a similar distance from the downstream secondary structure. Thirdly, the sORF1-encoded peptides are 6 and 5 amino acid long, respectively, and have identical termini: methionine and alanine at the N-terminus and glutamic acid at the C-terminus. Since the sORF1 amino acid composition generally does not affect shunt efficiency in vitro and in planta [15], [16], [20], [21], [23], the identity of terminal amino acids might reflect the importance of the nucleotide contexts surrounding the start and stop codon. The size of RTBV sORF1 may have become longer following the acquisition of ORF IV (a unique ORF, absent in closely-related badnaviruses) due to subsequent accommodation of an inefficient splice donor site within the sORF1 sequence in order to translate the sORF1-ORF IV fusion protein from spliced pgRNA [14].
-
In both leaders, the sequence downstream of the stem base (the landing site for shunting ribosomes in RTBV and CaMV) is UA-rich, which would ensure a low index of secondary structure. Unstructured nature of the shunt landing site is likely required for efficient resumption of scanning by shunting ribosomes. Furthermore, like in RTBV and CaMV, a presumptive shunt landing site in the RTSV leader contains a non-AUG start codon (AUA), which is located at a similar distance from the stem and followed by an additional in-frame non-AUG (AUU). Interestingly, both non-AUGs are in frame with the downstream AUG start codon of the polyprotein ORF, though unlike the RTBV AUU, the RTSV AUA and AUU codons are in suboptimal contexts. By analogy with RTBV, it is likely that one or both of these codons are inefficiently recognized by shunting ribosomes to initiate translation of N-terminally extended polyprotein. This hypothesis is further supported by an in vitro study of the CaMV shunt, in which two non-AUG codons located within the landing site were shown to initiate translation, albeit much less efficiently than the downstream AUG [23].
-
In both leaders, an identical stretch of pyrimidines (UUUCU) is located just downstream of the stem basal helix. By analogy with animal picornaviruses which contain a pyrimidine tract in their IRES elements just upstream of the initiation codon [5], it can be suggested that initiation at the non-AUG codon by shunting ribosomes might be facilitated by the UUUCU motif.
Thus, all the cis-acting elements known to drive ribosome shunting in RTBV are also present in RTSV, strongly supporting the idea that RTSV could have co-evolved an sORF-dependent shunt mechanism. Moreover, the identity of certain sequence motifs within these elements raises a possibility of their horizontal transfer from one virus to another during co-evolution. Alternatively, these motifs could have co-evolved independently through adaptation to the rice translational machinery.
Translation downstream of the RTSV leader is initiated by ribosome shunting
To test the hypothesis that translation of RTSV genomic RNA is initiated by an sORF1-dependent ribosome shunting, we used well-established translation assays based on rice protoplasts and wheat germ extracts, in which translation of a reporter ORF encoding chloramphenicol acetyl transferase (CAT) placed downstream of the RTSV leader sequence or its mutant versions was monitored. We followed the same experimental settings and protocols as those used previously in a comparative study of molecular mechanisms of the RTBV and CaMV shunting [15].
In rice protoplasts, both RTSV and RTBV leaders drove relatively efficient translation of the reporter ORF, although the RTBV leader allowed a 1.6-fold higher initiation rate. Confirming our previous results, knock out (KO) mutations of the start (AUG to UAG) or stop (UAG to UAC) codon of RTBV sORF1 drastically reduced translation (Figure 2). The same KO mutations of the RTSV sORF1 start or stop codons resulted in a significant decrease in downstream translation, albeit less dramatic than in the case of RTBV. This indicates that translation initiation downstream of the RTSV leader is sORF1-dependent, which is not consistent with internal ribosome entry at the 3′ end of the leader. Interestingly, the stop codon KO had a more pronounced effect by reducing the translation rate to 28%, whereas the start codon KO reduced translation only to 56% of the wild type level. This suggests that the RTSV leader lacking the first sORF AUG allows a relatively efficient linear ribosome migration towards the 3′ end, i.e. by leaky scanning through the remaining five AUGs and/or translation at some of the remaining five sORFs followed by reinitiation event(s). In CaMV, such a linear ribosome migration along the leader sequence has been investigated by mutating nine AUGs individually and in combinations and found to be 5 times less efficient than ribosome shunting in plant protoplasts [20] and wheat germ extracts [24]. In the case of RTBV, linear ribosome migration is even less efficient, likely because of a larger number of the intervening AUGs (twelve) and sORFs (eleven) (Figure 1). The KO of stop codon should not affect the initiation step of sORF1 translation but should result in termination of this translation event downstream of the shunt take-off site, which would diminish shunting but would not affect linear ribosome migration following sORF1 translation.
Fig. 2. Translation downstream of the RTSV and RTBV leaders is regulated by the first sORF. 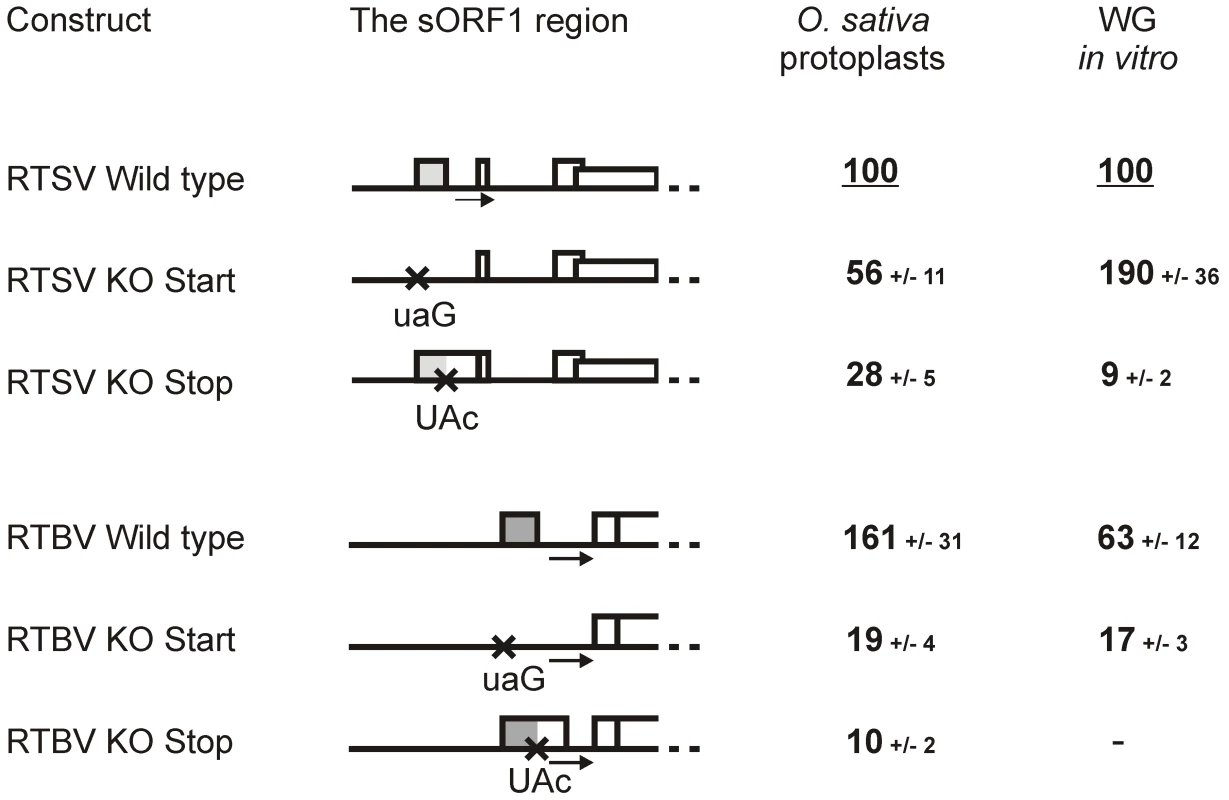
Relative values of CAT expression downstream of the wild type and mutated versions (‘KO start’ and ‘KO stop”) of the RTSV (top panel) and RTBV (bottom panel) leaders in the two translation systems are given. Expression from the wild type RTSV construct in O. sativa (rice) protoplasts and in the wheat germ (WG) in vitro system is set to 100%. The sORF1 region of the leaders in each construct is shown schematically; point mutations are indicated with crosses and sORFs with boxes. To further verify that sORF1-dependent translation downstream of the RTSV leader is initiated by ribosome shunting and evaluate a contribution of linear ribosome migration, we used a 40-nt Kozak-stem (KS) sequence which forms a perfect, compact stem-loop structure and blocks linear migration of scanning ribosomes [25]. In the case of RTBV and CaMV, insertion of KS in the leader region upstream of the first sORF abolished downstream translation, whereas its insertion within the leader region which is bypassed by shunting ribosomes had no dramatic effect on downstream translation [7], [8], [15], [20]. Likewise, insertion of KS at the 5′-end of the wild-type RTSV leader or its mutant versions with the sORF1 start or stop codon KO mutation nearly abolished downstream translation (Figure 3). This indicates that translation initiation in RTSV is 5′ end-dependent, thus ruling out internal initiation. Insertion of KS in the middle of the wild type RTSV leader did not abolish downstream translation, although the initiation rate was reduced to 42%. With KS inserted in the middle of the RTSV leader, KO mutation of either start or stop codon of sORF1 abolished downstream translation (Figure 3).
Fig. 3. Translation downstream of the RTSV leader is initiated by shunting but not internal initiation. 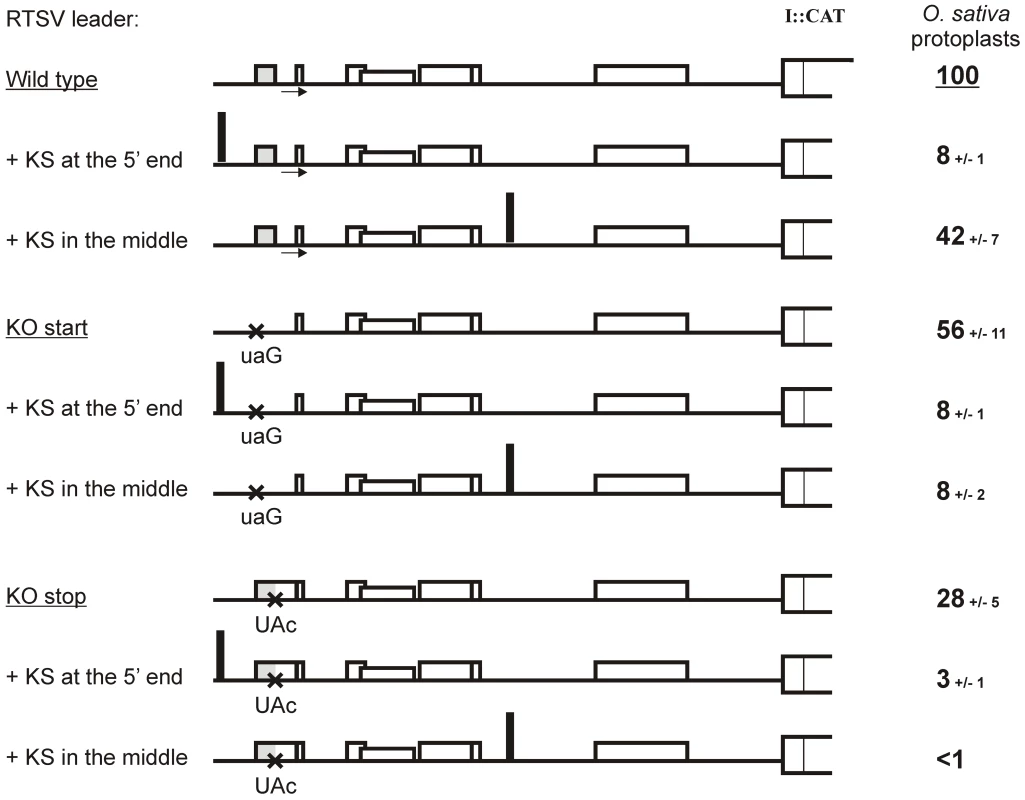
Relative values of CAT expression downstream of the wild type (“Wild type”) and sORF1-mutated versions (“KO start” and “KO stop”) of the RTSV leader carrying the KS at the 5′ end or in the middle region in O. sativa (rice) protoplasts are given. CAT expression from the wild-type leader construct in the absence of KS is set to 100%. For each construct, the RTSV leader preceding the polyprotein ORF (ORF I) fused to the CAT reporter ORF is depicted as thick line: the sORFs are indicated by boxes, point mutations shown with crosses, KS insertions indicated with thick vertical lines. Taken together, we conclude that almost half of the ribosomes entering at the 5′ end of the RTSV leader and initiating translation of sORF1 are able to shunt over the structure and re-initiate translation at the 3′-end of the leader. Notably, like in CaMV and RTBV, this mechanism depends on proper termination of sORF1 translation in front of the structured region. Extension of RTSV sORF1 by the stop codon KO mutation should lead to termination at the in-frame stop codon located 10 triplets downstream, i. e. within the ascending arm of the structure. This would melt the stem basal helix and bring the terminating ribosome away from the take-off and landing sites.
To test if the stem basal helix structure is required for RTSV shunting, twelve point mutations were introduced either in its 5′-proximal or 5′-distal arms, which would disrupt secondary structure, and the compensatory mutations in both arms, which would restore stable secondary structure (Figure 4). The basal helix mutants with and without the KS sequence in the middle of the RTSV leader were constructed. Transient expression of the resulting constructs in rice protoplasts showed that disruption of the basal helix drastically reduced translation downstream of the RTSV leader, whereas restoration of the helix structure by the compensatory mutations almost fully restored downstream translation (Figure 4). We conclude that integrity of stable secondary structure but not primary sequences involved in formation of the stem basal helix is essential for ribosome shunting in RTSV.
Fig. 4. Integrity of the stem base secondary structure is essential for RTSV shunting. 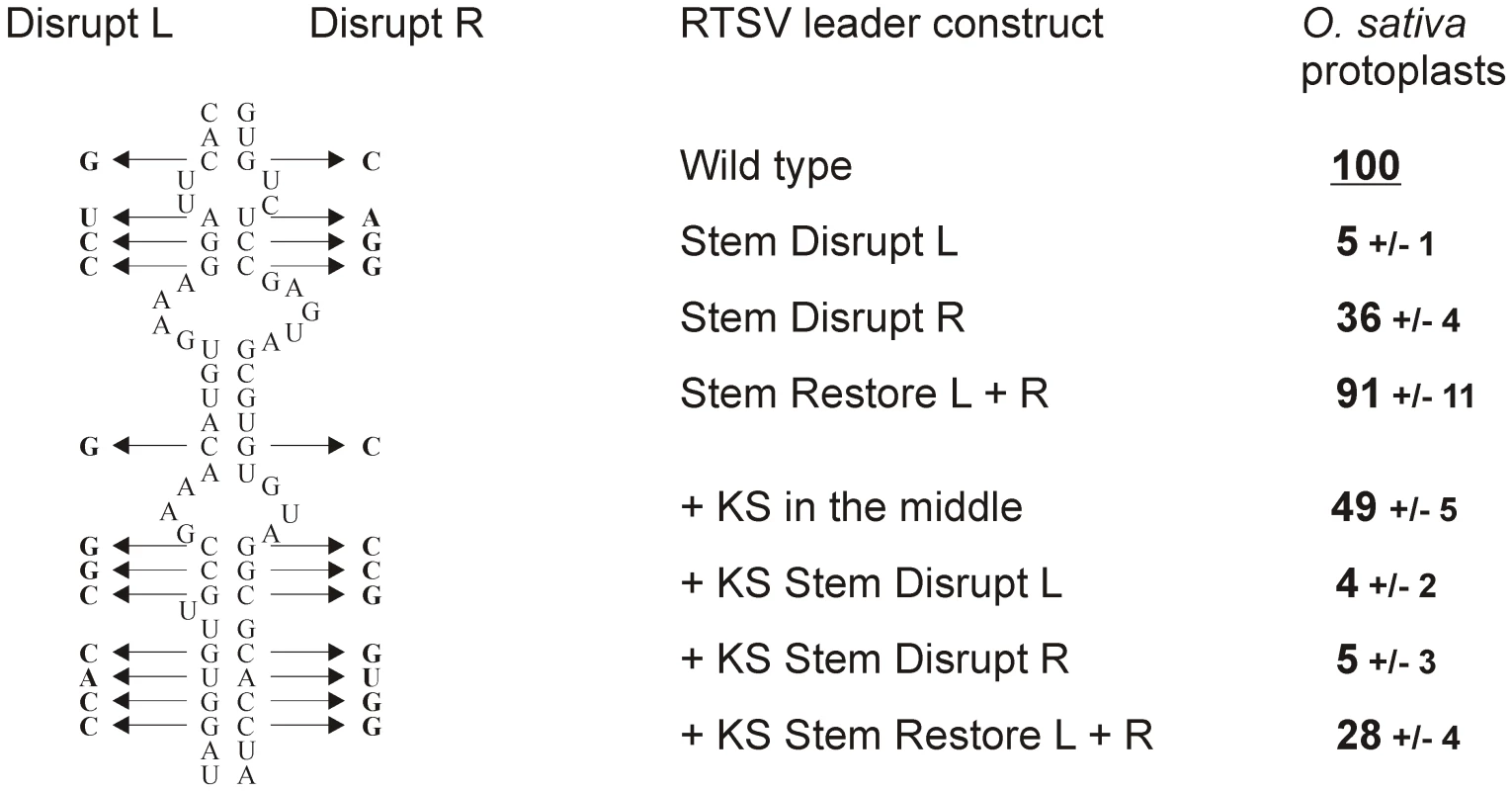
Twelve point mutations in either ascending (Disrupt L) or descending (Disrupt R) arm of the RTSV stem base secondary structure are shown on the left side. A combination of these mutations (Restore L+R) restores stable secondary structure. On the right side, relative values of CAT expression downstream of the wild type (“Wild type”) and the stem base-mutated versions (“Stem Disrupt L”, “Stem Disrupt R” and “Stem Restore L+R”) of the RTSV leader [or its variant with the Kozak stem (KS) sequence in the middle part] in O. sativa (rice) protoplasts are given. CAT expression from the wild-type leader construct is set to 100%. Interestingly, in the absence of KS, the mutations in the 5′-proximal arm nearly abolished translation (5% of the wild type level), whereas the mutations in the 5′-distal arm reduced translation to 36% of the wild type level. The latter mutations in the presence of KS nearly abolished downstream translation (5% of the wild type level) (Figure 4). This suggests that, besides shunting, linear ribosome migration following translation of sORF1 is also abolished by the mutations in the primary sequence just downstream of sORF1. By contrast the mutations of the 5′-distal arm sequence located far away of sORF1 do not appear to affect linear ribosome migration, which would account for relatively high translation efficiency in this case, comparable to the translation efficiency of the RTSV constructs lacking sORF1. Notably, the negative effect of the 5′-proximal arm mutations is also evident when the RTSV basal helix is restored by compensatory mutations in the 5′-distal arm.
We have established previously that, in wheat germ extracts supporting efficient ribosome shunting driven by the CaMV shunt elements [23], [24], the RTBV shunting is about 7 times less efficient [15]. This is entirely due to incompatibility of the RTBV landing sequence, because the wheat germ translation machinery prefers A-rich rather than U-rich sequences and perhaps other unknown cis-elements present in the CaMV landing site but absent in the RTBV one [15]. Similar to RTBV, translation downstream of the RTSV leader was also relatively inefficient in wheat germ, although the RTSV leader allowed a 1.6-fold higher initiation rate (Figure 2). The KO mutation of sORF1 start codon increased downstream translation 1.9-fold. This is unlike RTBV, in which the sORF1 start codon KO reduced downstream translation about 4-fold (Figure 2). As discussed above, the RTSV leader allows much more efficient linear ribosome migration downstream of sORF1 than the RTBV leader, which explains a positive effect of the RTSV sORF1 start codon removal in the wheat system where shunt efficiency is diminished. KO mutation of the RTSV sORF1 stop codon abolished downstream translation in the wheat system (Figure 2). This shows that most of translation downstream of the RTSV leader depends on proper termination of sORF1 translation.
Taken together, we demonstrate here that translation initiation of RTSV genomic RNA is controlled by its long leader and mediated largely by sORF1 - and stem basal helix-dependent ribosome shunting. Further research is needed to characterize this mechanism in more detail. But given the striking similarity of all the shunt elements in RTSV and RTBV and especially the identity of certain sequence motifs in the shunt take-off and landing sites, it is very likely that both RTSV and RTBV use a similar shunt mechanism.
Conservation of the shunt cis-elements in RTSV isolates
Our comparison of five isolates of RTSV (NC_001632 and AM234048, AM234049, U71440, and AB064963) showed that the leader sequence is remarkably conserved with only 35 polymorphic positions including 33 single nucleotide substitutions and 1-nt and 2-nt insertions/deletions (not shown). Only four substitutions occur in the shunt elements – one in the shunt landing sequence between the pyrimidine stretch and the non-AUG codon and three in the stem basal helix primary sequence (but not in the secondary structure) (Figure 1B). Notably, in some regions downstream of the leader, RTSV sequences have a much higher polymorphism than the leader itself (not shown).
We conclude that the shunt elements are well preserved in all RTSV isolates, indicating their biological importance for the virus. When an infectious clone of RTSV becomes available it will be important to test the role of sORF1 and other cis-elements identified in this study for viral infectivity. Previously, it has been shown that sORF-dependent ribosome shunting is essential for infectivity of CaMV [21] and that the RTBV shunt elements can functionally substitute for the corresponding CaMV elements in systemic infection with a chimeric virus [16].
RTSV may have acquired ribosome shunting after its encounter with RTBV
Maize chlorotic dwarf virus (MCDV), the second recognized member of genus Waikavirus [2], also possesses a long leader (434-nt in the type species NC_003626) with several sORFs (a total of 6 AUGs in NC_003626) and stable secondary structure (−154 kcal/mole in NC_003626; Figure S2). However, this leader sequence is highly polymorphic in three known isolates (less than 40% nucleotide identity) (data not shown). This suggests that a translation initiation mechanism may not be conserved. Interestingly, in all three isolates the first sORF in the MCDV leader is preserved in length (5 codons) but not nucleotide content. However, it terminates 145 nts upstream of the main structure in NC_003626 (Figure S2), which is not compatible with ribosome shunting. Furthermore, owing to high polymorphism, the shape, stability and position of the main structure are not preserved in MCDV isolates and the number and configuration of sORFs is also variable (data not shown). This again argues against shunting as the initiation mechanism. Nevertheless, the preservation of the first sORF suggests its importance in controlling translation initiation on MCDV genomic RNA which may occur via linear ribosome migration following translation of the first sORF. In support of this hypothesis, our above results for the RTSV leader indicate that in addition to ribosome shunting, sORF1-dependent linear ribosome migration also contributes to translation initiation downstream of the leader. It can therefore be proposed that in waikaviruses a linear ribosome migration-dependent mechanism has evolved earlier than shunting and that the ribosome shunt is a so-far unique acquisition by RTSV following its encounter with RTBV in a disease complex. However we cannot exclude an independent evolution of ribosome shunting in RTSV in the process of adaptation of the virus to the host plant translational machinery.
Among other viruses of the family Secoviridae, Parsnip yellow fleck virus (PYFV), the only recognized member of genus Sequivirus, is most closely related to RTSV and MCDV [2]. Unlike RTSV and MCDV, this virus has a shorter leader sequence (278 nts) that does not contain sORFs and cannot fold into stable secondary structure as predicted by MFOLD (data not shown). This suggests a linear scanning-dependent mechanism of translation initiation in PYFV. We cannot rule out, however, that PYFV (and MCDV) may use an internal initiation mechanism similar to that of potyviruses [5].
It is thought that the ribosome shunt mechanism in plant pararetroviruses has evolved in order to protect the viral coat protein-binding, secondary structure element located within the leader [26] – an RNA packaging signal – from being melted by linearly-migrating scanning ribosome [16]. A mechanism of packaging in RTSV is unknown: but conservation of the shunt mechanism between RTBV and RTBV raises a possibility that a packaging element may reside within the structured region of the RTSV leader.
Materials and Methods
Plasmid constructs
The RTBV leader constructs “Wild type” and “KO start” have been described earlier [15]. The RTSV leader construct “Wild type” (Figure 1) is a derivative of the corresponding RTBV construct, in which the RTSV genomic RNA sequence from position +1 till position +535 (i.e. the leader sequence followed with a 21-nt segment of the polyprotein ORF) was inserted between the CaMV 35S promoter and the CAT reporter ORF in place of the RTBV leader (as a PCR-amplified RTSV fragment flanked with Cla I and Xho I and cloned into the corresponding sites of the vector). Note that this construct contains the natural polyprotein ORF start codon in a strong initiation context followed by 6 codons of this ORF and the CAT ORF fused to these 7 codons lacks its own ATG. In the RTBV constructs the CAT ORF begins with its own ATG in a strong context, which is in frame with the upstream AUU initiation codon located in the RTBV shunt landing site [15]. Point mutations of the RTSV sORF 1 start (ATG to taG) or stop (TAG to TAc) codons were introduced by PCR-based mutagenesis, yielding constructs ‘KO start’ and ‘KO stop’, respectively. The Kozak-stem (KS) sequence was introduced at the 5′ end of RTSV leader by cloning of a pre-annealed, self-complementary oligonucleotide CGGGGGCGCGTGGTGGCGGCTGCAGCCGCCACCACGCGCCCC (the self-complementary KS sequence is underlined) into the Cla I site of the RTSV plasmids “Wild type”, “KO start” and “KO stop”. The KS sequence shown above was also introduced in the middle of the RTSV leader sequence (in place of a guanosine at position 255) by a PCR ligation method, similar to that which we described previously [15]. Note that this insertion does not disrupt the RTSV secondary structure except that one of its branches is extended by KS (Figure S1C).
The RTSV leader constructs “Stem Disrupt L”, “Stem Disrupt R” and “Stem Restore L+R” were obtained using 353 bp and 200 bp synthetic DNA fragments of the RTSV wild type construct that contain sequences from Cla I to EcoRV and from EcoRV to Xho I, respectively, each with 12 point mutations shown in Figure 4. These fragments were introduced into the RTSV wild type construct individually or in combination by a two fragment ligation method. Same mutations were also introduced in the above-described construct carrying the KS sequence in the middle part of the RTSV leader: in this case 262 bp and 330 bp synthetic DNA fragments of the RTSV+KS construct were used, which contain sequences from Cla I to Pst I (located in the KS sequence) and from Pst I to Xho I, respectively, each with the 12 point mutations.
For the in vitro translation experiments, the T7 promoter was introduced just upstream of the RTSV full-length leader and their variants with the sORF1 mutations by subclonning the Cla I-Sph I fragment from the RTSV plasmids “Wild type”, “KO start” and “KO stop” in place of the corresponding fragment of the T7 promoter-RTBV leader-CAT ORF plasmid described previously [15].
Transient expression in rice protoplasts
Protoplasts from suspension culture of O. sativa were prepared and transfected with plasmid DNA by a polyethylene glycol method as described previously [8], [15]. Briefly, 0.6×106 protoplasts were transfected with 10 µg CAT-expressing plasmid and 2 µg β-glucuronidase (GUS)-expressing plasmid or 5 µg green fluorescent protein (GFP)-expressing plasmid. The GUS or GFP plasmid served as an internal control of transfection efficiency. Following incubation for 19–24 hrs at 27°C in the dark, protoplasts were harvested, protein extracts prepared and assayed for CAT and GUS (or GFP) accumulation, as described previously [20]. Relative GUS activities were taken for normalization of the CAT expression levels given in Figure 2 and Figure 3, while relative GFP activities were taken for normalization of the CAT expression levels given in Figure 4. For each construct, the values given are the means of at least three experiments in independent batches of protoplasts. Deviations from the mean values generally did not exceed 20%. The levels of CAT mRNA accumulation were measured by quantitative RT-PCR with CAT ORF-specific primers using previously-described protocols for total RNA preparation, cDNA synthesis and real time PCR [27] and found to be comparable for all the RTSV constructs (data not shown).
In vitro transcription and translation
The in vitro experiments were performed as described in detail earlier [15]. Briefly, the T7-promoter plasmids were linearized by Sph I and transcribed in the presence of the cap analog 7mGpppG (in 6-fold molar excess over GTP) by incubation with T7 RNA polymerase (Biofinex). The integrity of the synthesized transcripts was evaluated on a 6% denaturing polyacrylamide gel. Equimolar amounts of capped transcripts (0.5 pmol) were translated for 1 hour at 27°C in a wheat germ extract. Accumulation of CAT protein in translation mixture was measured in duplicate by CAT ELISA (Roche) as recommended by the manufacturer. For each construct, in vitro translation was performed at least three times with freshly prepared capped RNA, yielding similar results.
Prediction of RNA secondary structure
Secondary structures at 25°C were predicted using the MFOLD program (Wisconsin Package, version 6.0; Genetics Computer Group, Madison, WI, USA). The most optimal and suboptimal secondary structures of the 515 nt RTBV leader sequence are shown in Figure S1. Folding of the RTSV leader sequence extended by either the natural RTSV coding sequence or the CAT reporter ORF sequence (present in the RTSV constructs tested here in the translational assays) did not affect the formation of the base section present in both optimal and suboptimal conformations (data not shown). Notably a free energy of the most optimal leader structure in RTSV (deltaG = −194.1 kcal/mol) is much more negative than that of fully randomized sequences of the same length (deltaG = ca. −100 kcal/mol; [28]).
MFOLD prediction of RNA secondary structure has proven to be reliable. For example, an MFOLD-predicted, large stem-loop structure of the 612-nt CaMV leader has been largely confirmed in vitro using chemical and enzymatic methods, though alternative conformations were also revealed in that study [29].
Supporting Information
Zdroje
1. HullR 1996 Molecular biology of rice tungro viruses. Annu Rev Phytopathol 34 275 297
2. SanfaçonHWellinkJLe GallOKarasevAvan der VlugtR 2009 Secoviridae: a proposed family of plant viruses within the order Picornavirales that combines the families Sequiviridae and Comoviridae, the unassigned genera Cheravirus and Sadwavirus, and the proposed genus Torradovirus. Arch Virol 154 899 907
3. ShenPKaniewskaMSmithCBeachyRN 1993 Nucleotide sequence and genomic organization of rice tungro spherical virus. Virology 193 621 30
4. Thiébeauld de la CrouéeOPoogginMMRyabovaLA 2007 Alternative translation strategies in plant viruses. Plant Viruses 1 20 The Global Science Books
5. BelshamGJ 2009 Divergent picornavirus IRES elements. Virus Res 139 183 92
6. KnellerELRakotondrafaraAMMillerWA 2006 Cap-independent translation of plant viral RNAs. Virus Res 119 63 75
7. FüttererJKiss-LaszloZHohnT 1993 Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell 73 789 802
8. FüttererJPotrykusIBaoYLiLBurnsTM 1996 Position-dependent ATT initiation during plant pararetrovirus rice tungro bacilliform virus translation. J Virol 70 2999 3010
9. PoogginMMFüttererJSkryabinKGHohnT 1999 A short open reading frame terminating in front of a stable hairpin is the conserved feature in pregenomic RNA leaders of plant pararetroviruses. J Gen Virol 80 2217 2228
10. RyabovaLAPoogginMMHohnT 2002 Viral strategies of translation initiation: ribosomal shunt and reinitiation. Prog Nucleic Acid Res Mol Biol 72 1 39
11. HullR 2007 Caulimoviridae (Plant Pararetroviruses). eLS John Wiley & Sons Ltd, Chichester http://www.els.net [doi: 10.1002/9780470015902.a0000746.pub2]
12. FüttererJRothnieHMHohnTPotrykusI 1997 Rice tungro bacilliform virus open reading frames II and III are translated from polycistronic pregenomic RNA by leaky scanning. J Virol 71 7984 9
13. PoogginMMRyabovaLAHohnT 2002 Translation strategies in members of the family Caulimoviridae. KhanJADijkstraJ Plant viruses as molecular pathogens New York Haworth Press Inc 317 338
14. FüttererJPotrykusIValles BrauMPDasguptaIHullR 1994 Splicing in a plant pararetrovirus. Virology 198 663 670
15. PoogginMMRyabovaLAHeXFuttererJHohnT 2006 Mechanism of ribosome shunting in Rice tungro bacilliform pararetrovirus. RNA 12 841 850
16. PoogginMMFüttererJHohnT 2008 Cross-species functionality of pararetroviral elements driving ribosome shunting. PLoS One 3 e1650
17. SchepetilnikovMSchottGKatsarouKThiébeauldOKellerM 2009 Molecular dissection of the prototype foamy virus (PFV) RNA 5′-UTR identifies essential elements of a ribosomal shunt. Nucleic Acids Res 37 5838 5847
18. SherrillKWLloydRE 2008 Translation of cIAP2 mRNA is mediated exclusively by a stress-modulated ribosome shunt. Mol Cell Biol 28 2011 22
19. DominguezDIRyabovaLAPoogginMMSchmidt-PuchtaWFüttererJ 1998 Ribosome shunting in cauliflower mosaic virus. Identification of an essential and sufficient structural element. J Biol Chem 273 3669 78
20. PoogginMMHohnTFüttererJ 2000 Role of a short open reading frame in ribosome shunt on the cauliflower mosaic virus RNA leader. J Biol Chem 275 17288 17296
21. PoogginMMFüttererJSkryabinKGHohnT 2001 Ribosome shunt is essential for infectivity of cauliflower mosaic virus. Proc Natl Acad Sci U S A 98 886 891
22. Hemmings-MieszczakMHohnT 1999 A stable hairpin preceded by a short open reading frame promotes nonlinear ribosome migration on a synthetic mRNA leader. RNA 5 1149 57
23. RyabovaLAHohnT 2000 Ribosome shunting in the cauliflower mosaic virus 35S RNA leader is a special case of reinitiation of translation functioning in plant and animal systems. Genes Dev 14 817 29
24. RyabovaLAPoogginMDominguezDHohnT 2000 Continuous and Discontinuous Ribosome Scanning on the Cauliflower Mosaic Virus 35 S RNA Leader Is Controlled by Short Open Reading Frames J Biol Chem 275 37278 37284
25. KozakM 1986 Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A 83 2850 4
26. Guerra-PerazaOde TapiaMHohnTHemmings-MieszczakM 2000 Interaction of the cauliflower mosaic virus coat protein with the pregenomic RNA leader. J Virol 74 2067 2072
27. BlevinsTRajeswaranRAreggerMBorahBKSchepetilnikovM 2011 Massive production of small RNAs from a non-coding region of Cauliflower mosaic virus in plant defense and viral counter-defense. Nucleic Acids Res 39 5003 5014
28. SeffensWDigbyD 1999 mRNAs have greater negative folding free energies than shuffled or codon choice randomized sequences. Nucleic Acids Res 27 1578 84
29. Hemmings-MieszczakMStegerGHohnT 1997 Alternative structures of the cauliflower mosaic virus 35 S RNA leader: implications for viral expression and replication. J Mol Biol 267 1075 88
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



