-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Novel Mouse Model of Egg-Induced Immunopathology
Schistosoma haematobium is the etiologic agent for urogenital schistosomiasis, a major source of morbidity and mortality for more than 112 million people worldwide. Infection with S. haematobium results in a variety of immunopathologic sequelae caused by parasite oviposition within the urinary tract, which drives inflammation, hematuria, fibrosis, bladder dysfunction, and increased susceptibility to urothelial carcinoma. While humans readily develop urogenital schistosomiasis, the lack of an experimentally-tractable model has greatly impaired our understanding of the mechanisms that underlie this important disease. We have developed an improved mouse model of S. haematobium urinary tract infection that recapitulates several aspects of human urogenital schistosomiasis. Following microinjection of purified S. haematobium eggs into the bladder wall, mice consistently develop macrophage-rich granulomata that persist for at least 3 months and pass eggs in their urine. Importantly, egg-injected mice also develop urinary tract fibrosis, bladder dysfunction, and various urothelial changes morphologically reminiscent of human urogenital schistosomiasis. As expected, S. haematobium egg-induced immune responses in the immediate microenvironment, draining lymph nodes, and systemic circulation are associated with a Type 2-dominant inflammatory response, characterized by high levels of interleukin-4, eosinophils, and IgE. Taken together, our novel mouse model may help facilitate a better understanding of the unique pathophysiological mechanisms of epithelial dysfunction, tissue fibrosis, and oncogenesis associated with urogenital schistosomiasis.
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002605
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002605Summary
Schistosoma haematobium is the etiologic agent for urogenital schistosomiasis, a major source of morbidity and mortality for more than 112 million people worldwide. Infection with S. haematobium results in a variety of immunopathologic sequelae caused by parasite oviposition within the urinary tract, which drives inflammation, hematuria, fibrosis, bladder dysfunction, and increased susceptibility to urothelial carcinoma. While humans readily develop urogenital schistosomiasis, the lack of an experimentally-tractable model has greatly impaired our understanding of the mechanisms that underlie this important disease. We have developed an improved mouse model of S. haematobium urinary tract infection that recapitulates several aspects of human urogenital schistosomiasis. Following microinjection of purified S. haematobium eggs into the bladder wall, mice consistently develop macrophage-rich granulomata that persist for at least 3 months and pass eggs in their urine. Importantly, egg-injected mice also develop urinary tract fibrosis, bladder dysfunction, and various urothelial changes morphologically reminiscent of human urogenital schistosomiasis. As expected, S. haematobium egg-induced immune responses in the immediate microenvironment, draining lymph nodes, and systemic circulation are associated with a Type 2-dominant inflammatory response, characterized by high levels of interleukin-4, eosinophils, and IgE. Taken together, our novel mouse model may help facilitate a better understanding of the unique pathophysiological mechanisms of epithelial dysfunction, tissue fibrosis, and oncogenesis associated with urogenital schistosomiasis.
Introduction
Schistosomal infections plague more than 240 million people worldwide. The most prevalent anthropophilic schistosome species globally, Schistosoma haematobium, accounts for nearly half of that number, primarily in sub-Saharan Africa and the Middle East [1]. S. haematobium infects humans through direct skin penetration by aquatic cercariae that emerge from Bulinus truncatus, the intermediate snail host. After entering the human host, the parasite rapidly migrates into the circulation as a schistosomulae, matures, and subsequently lodges in the venous plexus of the bladder where male-female worm pairs mate and produce eggs for years to decades. While in rare cases ectopic S. haematobium oviposition causes pathology outside of the urogenital tract, the vast majority of infections result in urogenital schistosomiasis. Although the symptoms are varied, the bulk of the morbidity and mortality of urogenital schistosomiasis can be ultimately attributed to the host immune response against Schistosoma eggs deposited within the walls of the urinary tract. This inflammation leads to: 1) compromise of urothelial integrity promoting urinary tract infections [2]–[7], hematuria, and protein-wasting [2]; 2) urothelial changes leading to carcinogenesis [8], [9]; and 3) urinary tract fibrosis causing bladder dysfunction, obstruction, infection, and renal failure [10], [11]. In fact, the annual death toll of 150,000 due to urogenital schistosomiasis-induced obstructive renal failure makes S. haematobium one of the most lethal worms worldwide [12].
Despite the global burden of urogenital schistosomiasis, there remains little known about the basic mechanisms underlying the pathophysiology of this disease [13]. This is primarily due to the lack of an experimentally tractable animal model. Indeed, the majority of research in schistosomiasis has focused on S. mansoni infections in mice, wherein the entire life cycle can be recapitulated. In contrast, the development of a mouse model of urogenital schistosomiasis, long pursued by investigators in the field, has historically failed due to the inability of S. haematobium cercariae to efficiently mature and migrate to the bladder venous plexus in the mouse [14], [15]. Thus, S. haematobium research is largely limited to primate [16] and non-murine rodent models [17], [18]. Primate models, while capable of faithful recapitulation of urogenital schistosomiasis, are prohibitively expensive and difficult to manipulate. Extant non-murine rodent models (e.g. hamster), in contrast, develop clinical outcomes which can differ dramatically from the human disease. These models also suffer from a paucity of species-specific tools.
Herein we report the development of a robust, highly manipulable mouse model of urogenital schistosomiasis achieved by the microinjection of viable S. haematobium eggs directly into the bladder wall. This model faithfully and reproducibly recapitulates some of the salient features of the human disease including inflammatory cell activation and infiltration, urinary tract granuloma formation and fibrosis, urinary dysfunction, systemic Type 2 immune activation, and egg excretion in urine. To our knowledge, this is the first experimentally tractable mouse model of urogenital schistosomiasis. Moreover, we provide direct evidence that egg deposition alone is sufficient to reproduce several important aspects of urogenital schistosomiasis, even in the absence of the other life stages of this important human pathogen.
Results
Intramural S. haematobium egg injection induces reproducible granuloma formation and maturation
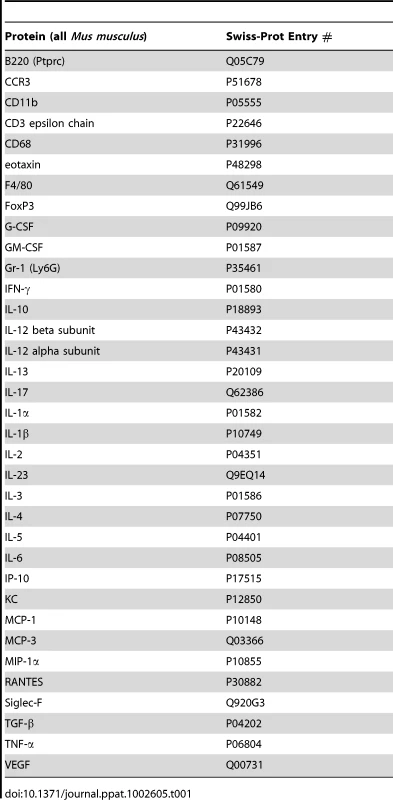
Although the morbidity associated with chronic S. haematobium infection is considered to result from egg deposition into the bladder wall, it is currently unclear whether oviposition alone, in the absence of adult worms, is necessary and sufficient for the bladder pathology associated with urogenital schistosomiasis. To address this issue, we directly microinjected viable S. haematobium eggs into the anterior bladder walls of female BALB/c mice. Serial transabdominal micro-ultrasonography paired with histologic verification demonstrated the development of injection site granulomata over time (Figure 1A–H and Video S1). The initial injection site response resolved entirely by day 4 in animals injected with egg-free control vehicle (Figure 1E); whereas egg-injected animals demonstrated a persistent mixed inflammatory infiltrate (Figure 1F) with a hypoechoic appearance on micro-ultrasonography (i.e., low density, grey mural nodules, Figure 1B). Over the following 4 weeks, the egg-associated mixed inflammatory infiltrate expanded and organized into a well-defined, egg-centered granuloma surrounded by peripheral eosinophils and neutrophils and containing distinct lymphoid follicles (Figure 1G). Robust granulomata were still present 99 days after egg injection (Figure 1H). These organized, dense lesions were correspondingly hyperechogenic on micro-ultrasonography (bright nodules, Figure 1D). Granuloma formation was neither sex - nor strain-specific, since male and C57BL/6 and C3H/He mice also developed granulomata after egg injection (unpublished data). The granulomatous character of the egg-associated lesions was confirmed by immunohistochemistry for CD68 (Figure 1I–K), which demonstrated complete encapsulation of the eggs by CD68-positive epithelioid cells (i.e., syncytial macrophages, Figure 1K). Similar to human disease, granuloma development is accompanied by eosinophiluria [19]–[24] and hematuria [25] (Figure S1). Additionally, by post-injection day 4 the urothelium demonstrated pronounced egg-dependent hyperplasia and squamous metaplasia that persisted throughout the experimental time course (Figure 1L–O and data not shown). These changes were present predominantly in the urothelium overlying the egg granuloma (Figure 1F–H), suggesting a highly localized microenvironmental effect. Importantly, these urothelial features closely parallel those observed in urogenital schistosomiasis [26].
Fig. 1. Bladder wall injection of S. haematobium eggs results in synchronous granuloma formation. 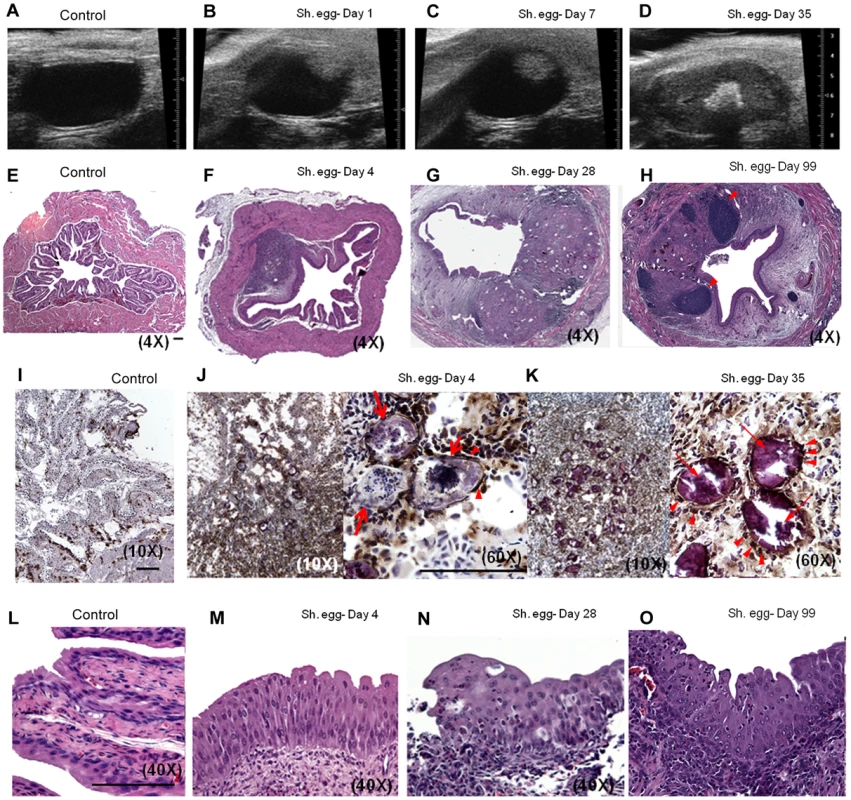
Intramural injection of S. haematobium eggs results in rapid and localized injection site response followed by progressive expansion and consolidation over several weeks (serial micro-ultrasonography of a single representative animal, A–D; histology, E–H). Lymphoid follicles in H are marked with arrowheads. Scale bars for panels E, I, J, and L (lower right hand corner for each) are 100 microns long. Immunohistochemical characterization demonstrates central macrophage granuloma formation around injected eggs (arrows) with peripheral accumulation of other inflammatory cells (I–K, anti-CD68 brown). Epithelioid cells (activated macrophages) are indicated with arrowheads. Egg injection also induces early and sustained urothelial hyperplasia with reactive nuclear changes (L–O). The observed pathology was not likely confounded by surgical complications. In more than 100 consecutive injections performed by four independent surgeons, no bladder perforation, extravesical egg deposition, or significant post-injection sequelae were observed. Micro-ultrasonographic and histologic analysis confirmed reproducible egg delivery to the same submucosal tissue plane. Moreover, 20% of mice shed eggs in their urine within one week of egg injection, which recapitulates egg shedding in infected humans (data not shown).
Intramural S. haematobium egg injection induces bladder fibrosis and urinary dysfunction
The host response to S. haematobium eggs in the human urogenital tract involves a fibroproliferative response which is thought to drive ureterovesical obstruction and bladder dysfunction, two major sources of morbidity associated with infection [27]. To determine whether our approach resulted in the development of bladder fibrosis, we evaluated egg-injected bladders through Masson's Trichrome staining and total extractable collagen assays. By day 7, loose immature collagen was observed within nascent granuloma (Figure 2A). At day 28 post-injection, dense mature collagen was found throughout the granuloma with variable extension into the surrounding bladder tissue (Figure 2B). Control vehicle-injected bladders demonstrated little or no collagen staining (data not shown). In addition, total bladder soluble collagen content was markedly increased 3–5 weeks post-egg injection (Figure 2C). Finally, S. haematobium egg-injected mice exhibited increased voiding frequency relative to control animals (Figure 2D), which is consistent with reports of urinary frequency observed in parasitized humans [28], [29].
Fig. 2. S. haematobium egg-injected bladders develop fibrosis and urinary dysfunction. 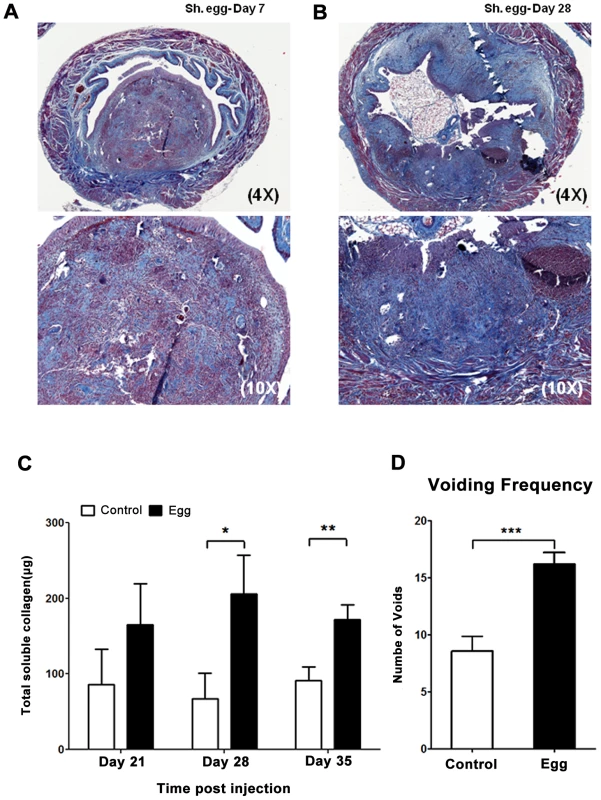
Egg-injected bladders demonstrate histologically-apparent fibrosis within granulomata beginning at post-injection day 7 (A, Masson's trichrome stain, collagen stains blue). Later time points demonstrate increased collagen staining area and intensity (B). Total bladder soluble collagen content correlates with histologic evaluation (C). Egg-injected mice demonstrate increased urinary frequency (1 week post injection, D). Egg-injected bladders accumulate an eosinophil - and neutrophil-dominated mixed inflammatory infiltrate
The defined and synchronous nature of our egg injection model allowed us to investigate the initial innate immune response to S. haematobium egg deposition. Importantly, there was an immediate, egg-independent upregulation of many cytokines in response to the injection itself; however, this rapidly resolved over time (Figures 3 and S2). Given that the foremost histologic hallmark of human parasitic infection is eosinophil infiltration, it was expected that eotaxin, a potent eosinophil chemoattractant, was significantly upregulated relative to control-injected bladders (Figure 3A).
Fig. 3. S. haematobium egg-injected bladders demonstrate a local Type 2 immune response. 
Egg-injected bladders feature elevated levels of the Type 2-associated cytokines IL-4, IL-13, and eotaxin (A) without significant changes in TH1- or TH17-associated cytokines (e.g. IFN-γ [B] and IL-17 [C], respectively). Cytokines and chemokines associated with innate immune activation (e.g. KC, TNF-α, MIP-1α, MCP-3, [D]) are persistently elevated relative to control. The immunosuppressive cytokines IL-10 and TGF-β were not clearly differentially regulated in egg- versus control-injected mice (E). Consistent with the upregulation of eotaxin, we detected a large number of eosinophils (Siglec-F+ CCR3+) that rapidly infiltrated the injection site and persisted, whereas egg-free control vehicle injections did not produce a significant response (Figure 4A, Figure S1). In addition, there was a marked infiltration of neutrophils (CD11b+Gr-1+) into the injected bladder wall with kinetics that were similar to eosinophils (Figure 4B). This is consistent with the egg-injected bladder upregulation of neutrophil-associated chemokines such as KC (CXCL1) [30] and MIP-1α (CCL3) [31] (Figure 3). While B-cells (B220+) also accumulated at the injection site over time (Figure 4C), we noted a paucity of T cells (CD3+), though moderately elevated relative to egg-free controls (Figure 4D). These data are consistent with histologic observations (Figure 1F–H), and suggest that development of egg granulomata in our model features B cell and chemokine-driven innate immune cell infiltration with a relative dearth of T cells.
Fig. 4. S. haematobium egg-injected bladders accumulate a mixed inflammatory infiltrate dominated by eosinophils and neutrophils. 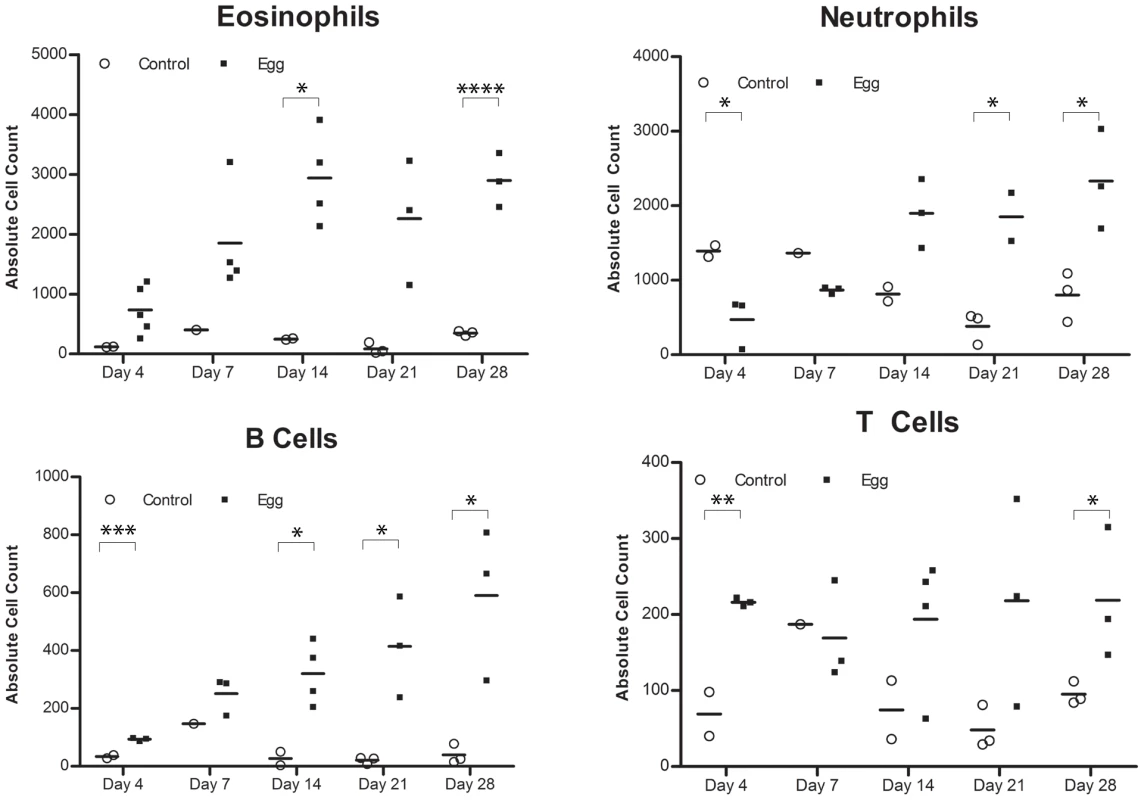
Congruent with histologic evaluation, serial flow cytometric analyses of single cell suspensions made from egg-injected bladders demonstrate progressive accumulation of (A) eosinophils (SiglecF+CCR3+), (B) neutrophils (CD11b+Gr-1+), (C) B cells (B220+), and—to a lesser extent— (D) T cells (CD3+). Egg-injected bladders demonstrate a distinctly Type 2-biased microenvironment
S. mansoni and S. japonicum eggs elicit a dominant Type 2 immune response within mouse hosts. Despite the paucity of T cells in our model, we sought to determine whether S. haematobium eggs elicited the production of the canonical Type 2 cytokines IL-4 and IL-13. The Luminex multiplexed liquid microbead platform was used to assay total cytokine expression within egg-injected bladders at early time points that corresponded to the initial immune response and nascent granuloma development (Figure 3 and Figure S2). IL-4 and IL-13 were upregulated by day 4 and remained elevated throughout all time points examined (Figure 3A). IL-5 levels were also markedly increased, which is consistent with the role of this Type 2-associated cytokine in eosinophil differentiation, activation, and recruitment [32] (Figure S2). In contrast, TH1 - and TH17-associated cytokines such as IFN-γ and IL-17 remained unaffected by the egg-induced inflammatory response (Figure 3B–C). Consistent with the marked neutrophil and macrophage infiltration of the egg-injected bladder wall (Figures 1 and 3), the innate immunity-associated cytokines TNF-α, KC, MCP-3, and MIP-1α demonstrated early and sustained increases relative to controls (Figure 3A and 3D). Interestingly, egg injection had no effect on IL-10 and TGF-β—immunosuppressive cytokines associated with regulation of tissue fibrosis in other diseases (Figure 3E).
S. haematobium egg injection induces Type 2 responses in draining lymph nodes
Despite the relative paucity of T cells within the egg-injected bladder (Figure 4D), the strikingly Type 2 cytokine-biased microenvironment (Figure 3) supported a hypothesis that T helper 2 cells were likely to be involved in the immune response to S. haematobium eggs. Indeed, pelvic lymph nodes draining egg-injected bladders demonstrated marked upregulation of the TH2-associated cytokine IL-4 throughout the experimental time course, while expression of the TH1-associated cytokines IFN-γ and IL-12 was moderately altered due to the injection procedure (Figure 5). Interestingly, at later time points the Treg-associated marker FoxP3 was markedly suppressed relative to controls while expression of the immunosuppressive cytokines IL-10 and TGF-β was unchanged. Expression of IL-17 was not detected.
Fig. 5. S. haematobium egg-injected mice demonstrate Type 2 immune responses in draining lymph nodes. 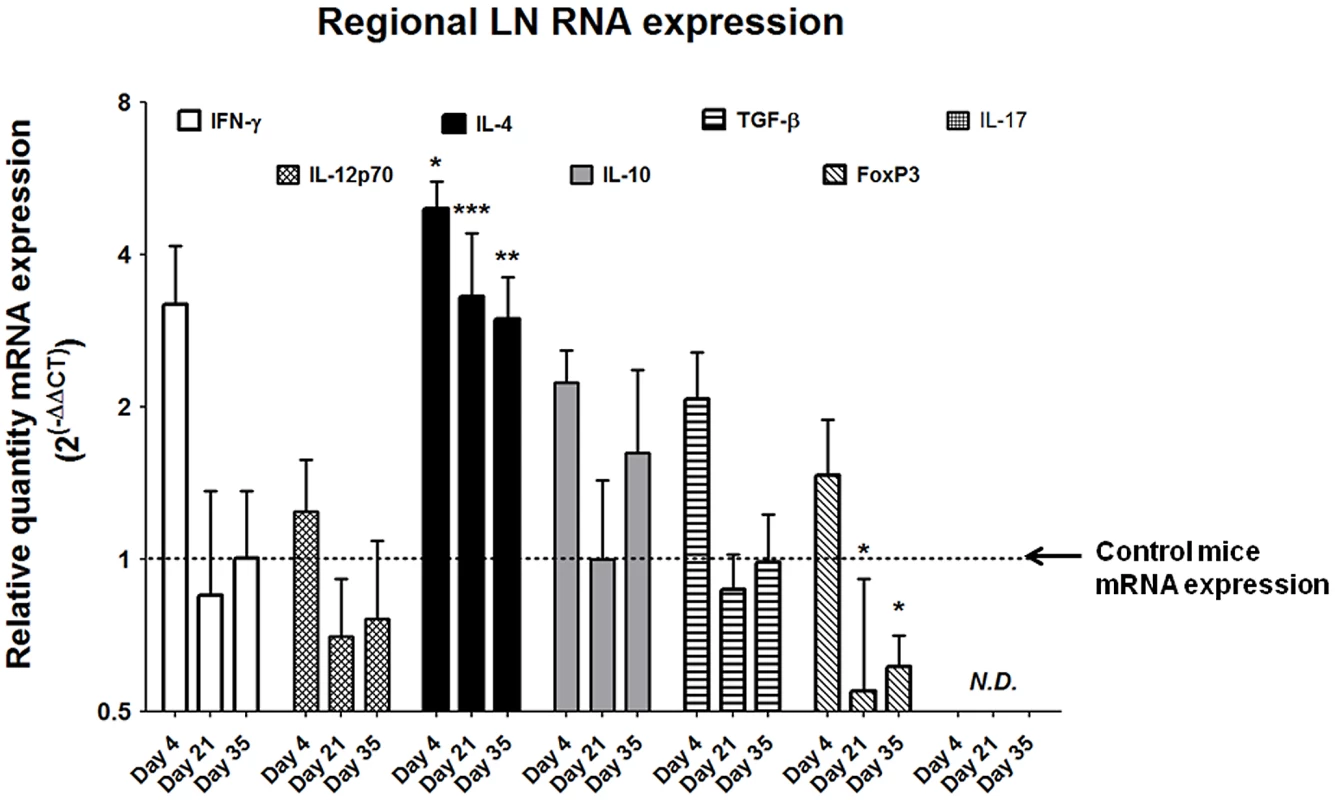
Pelvic lymph nodes from egg-injected mice demonstrate persistently elevated expression of Type 2-associated cytokines (e.g. IL-4) without corresponding increases in the TH1-associated cytokines IFN-γ and IL-12. The TReg-associated marker FoxP3 is suppressed late in the experimental time course, while the immunosuppressive cytokines IL-10 and TGF-β remain unchanged. IL-17 was not detected (N.D.). Egg-injected mice display systemic Type 2-skewed cytokine responses and IgE production
In our model, egg-injected mice demonstrated a reproducible, systemic Type 2-biased immune response similar to that observed in human infection. Serial multiplex serum cytokine profiling demonstrated persistently elevated levels of the Type 2-associated cytokine IL-5, while the TH1 - and TH17-associated cytokines IFN-γ and IL-17, respectively, evinced no such increase (Figure 6A). Congruent with chronic inflammation, the innate immunity-associated cytokine IL-1α was also persistently elevated (Figure 6A). Interestingly, serum levels of VEGF were increased in egg-injected mice, which may have promoted aberrant vasculogenesis and hematuria analogous to human disease [33]. Finally, serum levels of IgE, a quintessential Type 2-associated antibody isotype, were increased beginning at 14 days post egg injection relative to controls, and remain elevated through day 28 post-injection (Figure 6B).
Fig. 6. S. haematobium egg-injected mice display systemic Type 2 cytokine and immunoglobulin responses. 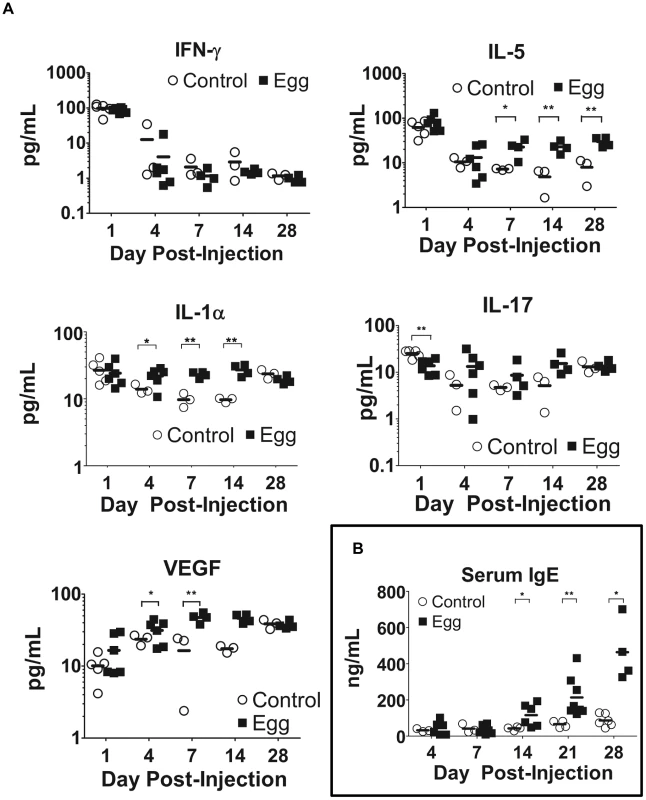
Egg-injected mice demonstrate elevated serum levels of Type 2-associated cytokines (e.g. IL-5) with decreased or unchanged serum levels of TH1- and TH17-associated cytokines (e.g. IFN-γ and IL-17, respectively, [A]). The Type 2 bias in systemic cytokine expression parallels increased IgE production (B). Discussion
S. haematobium infection, i.e. urogenital schistosomiasis, lacks a reliable mouse model despite being the most prevalent form of schistosomiasis and one of the deadliest worm infections worldwide. To address the lack of experimentally amenable tools for investigation of this medically important pathogen, we have developed an improved mouse model of urogenital schistosomiasis. Microinjection of viable S. haematobium eggs into the submucosa of the bladder wall elicits pathology similar to certain aspects of the human disease, including inflammatory cell infiltration, granuloma formation, urinary tract fibrosis and dysfunction, and systemic Type 2 immune activation. The focal deposition of eggs and resulting composite granulomata observed in this model recapitulates certain aspects of the immunopathology observed in human disease [34]–[36]. The persistent granulomatous inflammation in our model (at least 99 days after egg injection) parallels the chronicity of human infection. Moreover, the microinjection method features several advantages, including the induction of anatomically precise, synchronous, and reproducible pathology. Although microinjection of the bladder wall of a 20 gram mouse may appear daunting, proper magnification, clean egg preparations, sharp injection needles, and careful surgical technique render it feasible [37]. This is in contrast to existing mouse models of percutaneous or intravenous S. haematobium infection, which are prone to ectopic oviposition, variant kinetics, and unreliable disease burden [14], [15].
Current, non-mouse animal models for urogenital schistosomiasis mostly rely on non-human primates [16] and hamsters [17], [18]. Non-human primate models, while capable of high fidelity recapitulation of human disease, are costly and difficult to use. In hamsters transdermally infected with S. haematobium cercariae, schistosomula reach the lung by 3 days post-infection, followed by pairing of worms at approximately day 28–29. Oviposition in hamster tissues, primarily lung, liver, intestine, spleen, kidney, and uterus, begins to occur between weeks 7–11 [38]–[42]. Clustered egg deposition (often >20 eggs) results in giant, composite granulomata in the hamster liver. In comparison, S. mansoni infections of hamsters result in single egg-based liver granulomata containing more eosinophils, fewer polymorphonuclear leukocytes and histiocytes [43], [44]. Rates of hamster bladder involvement after exposure to S. haematobium cercaria are low and inconsistent, ranging from 0 to approximately 60%. Even when bladder oviposition occurs, egg burdens tend to be much lighter than that found in other organs, and less than two-thirds of hamsters with bladder eggs feature urothelial hyperplasia or squamous metaplasia [45]. Thus, hamsters develop clinical pathology which can differ dramatically from human disease, restricting their biological relevance. Hamsters also feature fewer species-specific reagents than mice. Hence, our improved mouse model of urogenital schistosomiasis may prove to be a useful alternative to existing animal models of this disease.
In the course of characterizing our model we noted that significant Type 2 inflammation occurs after egg injection, but this is not accompanied by large numbers of granuloma-associated T cells. Instead, we noted a rapid and pronounced chemokine response ensued following egg inoculation. The rapidity of the eotaxin response may indicate that eosinophils were recruited in the absence of adaptive immunity, perhaps by the secretion of eotaxin from urothelial, endothelial, smooth muscle, or other resident cells that were likely in direct contact with the inoculum. Certainly, urothelial cells can serve as a rapid source of chemokines and other cytokines in response to exposure to microbial antigens [46]. While the precise mechanism by which eosinophils are initially recruited to the sites of Schistosoma infection is not known, their later recruitment and accumulation is driven by a local, robust Type 2-biased immune response [47]. Indeed many of the aspects of parasitic morbidity, including those associated with urogenital schistosomiasis, are driven by this immune program. In humans, the parasite microenvironment has been well-characterized in its later, chronic stages; however, the early development and etiologic determinants of this immunologic milieu are poorly understood, most especially in urogenital schistosomiasis. The few T cells present in our model's bladder granulomata may be amplifying and organizing the local immune response, given the development of lymphoid follicles late after egg injection (Figure 1H). Despite the paucity of bladder-infiltrating T cells, the observed increase in IL-4 gene expression in draining lymph nodes (Figure 5) argues in favor of regional activation of TH2 cells. Alternatively, it is possible that basophils stimulated by S. haematobium egg-derived IL-4 inducing principle from S. mansoni eggs [48] (IPSE, originally known as S. mansoni chemokine binding protein [smCKBP] [49]) subsequently transit through lymph nodes and secrete IL-4 in these sites [50]. Other potential sources of IL-4 include mast cells and natural killer T (NKT) cells, both of which have been reported to localize to the lymph nodes [51], [52]. The latter cellular subset has been specifically implicated in anti-schistosomal immune responses [53]. Regardless of the source of IL-4, IgE titers increased beginning two weeks after egg injection (Figure 6B), providing consistent evidence for IL-4-dependent B cell isotype switching [54].
Another important observation was the lack of differential regulation of Th1 and Th17 cytokines (Figure 3). Immunologic aspects of natural S. mansoni infections of mice feature early Th0 or Th1 responses [55]–[57], with certain inbred mouse strains also exhibiting a propensity for Th17-associated activity [58]. We speculate that the differences between our model and this body of work are due in part to the synchronous granuloma nature of our approach, which does not include the cercarial-, schistosomular-, and worm-triggered immune response. It is also possible that the bladder immune microenvironment differs from other sites of schistosomal infection, namely the lung, liver, and intestinal tract. Further refinements to our model, and combination of our model with natural infection models, will be necessary to dissect out these important questions.
Besides differential cytokine expression, the systemic upregulation of the growth factor VEGF in response to egg injection was particularly striking (Figure 6A). Hematuria is a hallmark of urogenital schistosomiasis, and by definition results when bladder blood vessels and the urothelium break open and communicate with the bladder lumen. We theorize that VEGF triggers disorganized vasculogenesis and results in friable, easily disrupted bladder neovasculature. Interestingly, cervicovaginal lesions associated with urogenital schistosomiasis exhibit increased amounts of sprouting blood vessels and granulation tissue, indicating a possible role for VEGF and/or other vasculogenic influences [59].
The successful mimicry of several pathophysiologic facets of urogenital schistosomiasis by direct egg microinjection suggests that egg deposition alone may be sufficient to recapitulate some of the salient aspects of human disease. We have preliminary evidence that soluble S. haematobium egg antigens alone are also capable of generating bladder inflammation (manuscript in preparation). Use of genetically modified eggs in our model will further define the molecular basis of bladder immunopathology [60]. The observed long-term inflammation does not appear to be caused by bacterial or endotoxin contamination of injection solutions, since: 1) solutions are sterile; 2) endotoxin levels are <0.06 EU/dose; and 3) injection of eggs or control vehicle does not result in more TNF production than injection with low endotoxin saline (data not shown).
The availability of an improved animal model of urogenital schistosomiasis is of importance to multiple avenues of study. Firstly, the ability to monitor and manipulate the disease in a host (Mus musculus) for which numerous species-specific tools are available enables experimental approaches which were previously inaccessible. Secondly, the reliable reproduction of systemic and urogenital stigmata in our model may allow identification and evaluation of novel diagnostic and therapeutic strategies. Our use of micro-ultrasound is, to our knowledge, the first reported application of this technology for in vivo imaging of experimental schistosomiasis. We have successfully employed mouse-specific mass spectrometry and microarray analyses to profile S. haematobium-induced host protein and gene expression signatures, respectively (manuscripts in preparation). Egg-specific biomarker studies are ongoing. These approaches have only been possible through use of a mouse model featuring precisely controlled and reproducible S. haematobium egg-induced immunopathology. Our model may alleviate the bottleneck on urogenital schistosomiasis research imposed by the scarcity and heterogeneity of infected human samples, particularly bladder and lymphoid tissue.
When combined with other routes of egg injection or transdermal infection with cercariae, our model of schistosome egg-induced, Type 2-associated fibrosis is capable of synchronous fibrosis in multiple anatomic sites including the bladder, liver, and subcutis within the same animal (unpublished data). Cheever et al. have demonstrated that sensitization of mice by adult S. mansoni worm antigens enhances the egg-specific, lung immune response [61]. Our model is amenable to testing analogous questions using S. haematobium and the bladder. Through this strategy fibrosis in different organ systems may be compared to identify shared and organ-specific mechanisms and potential therapeutic targets. Prior work by others has definitively demonstrated that schistosome egg-induced lung, liver, and intestinal granuloma development is greatly schistosome species-dependent, with differences among S. haematobium, S. japonicum, S. mekongi, and S. mansoni [62], [63]. In addition, others have reported that hepatic - and lung-associated, S. mansoni egg granulomata develop in a highly organ-specific fashion [64]. S. japonicum granulomata also evolve in a tissue-specific manner in the liver, lung, and intestinal tract [65]. These reports highlight the critical need to develop in vivo models which properly match schistosome species with their tropism for specific host organs.
Additionally, our model of urogenital schistosomiasis presents a unique opportunity to study schistosomiasis-associated carcinogenesis [3], [9], and potentially inflammatory carcinogenesis in general (reviewed by Kuraishy et al. [66]). Urothelial carcinoma associated with S. haematobium infection arises in a Type 2-biased inflammatory environment. Approaches combining egg-induced Type 2 immunopathology and inducible models of bladder carcinogenesis represent new methods to study the role of inflammatory bias in carcinogenesis.
Like all experimental models, our approach has limitations. The injection procedure itself induces non-specific upregulation of a number of cytokines at day 1 post-injection (Figures 4, 6, S2, and S3). Use of uninfected hamster tissue homogenates as a control “vehicle” injection only partially mitigates this confounding issue. Accordingly, the cytokine expression observed at day 1 post-injection is likely the result of both parasite-specific and non-specific stimuli. While local immune responses to eggs are directly responsible for much of the observed pathology in S. haematobium infection, this activity is part of a larger, systemic immune response elicited in response to multiple life stages of the schistosome. In human disease, S. haematobium infection proceeds through cercarial skin invasion, systemic schistosomular circulation and maturation, adult worm mating within the bladder venous plexus, and egg deposition/excretion [67]. This complex natural history provides potential exposure to a broad range of antigens; however, the relative brevity of cercarial persistence and schistosomular circulation (hours to days [68]) and the relative lack of antigenicity of adult worms [69] may limit their contribution to long-term immunopathology. The unexcreted schistosome egg, in contrast, may persist for many years in host tissues and is a well-established, potent immunogen (e.g., soluble egg antigen or SEA [69]). Indeed, the systemic and granulomatous immune response to urogenital schistosomiasis is primarily driven by egg-associated antigens [70], [71]. Our model has methodologic similarities to synchronous granuloma formation induced by bolus injection of eggs into the mouse tail, cecal, or portal vein [64], [72], [73]. Like other synchronous granuloma models, our approach by definition is unsuitable for the study of the cercarial, schistosomular, and worm stages of S. haematobium.
In summary, we report the development of an improved mouse model of urogenital schistosomiasis. The ease and robustness of this model make it attractive for potential application to the elucidation of disease mechanisms, discovery of novel diagnostic biomarkers, and evaluation of candidate therapeutics. Additionally, this model is a prospective platform for the study of basic mechanisms of disease such as epithelial dysfunction, fibrosis, and inflammatory carcinogenesis.
Materials and Methods
Ethics statement
All animal work has been conducted according to relevant U.S. and international guidelines. Specifically, all experimental procedures were carried out in accordance with the Administrative Panel on Laboratory Animal Care (APLAC) protocol and the institutional guidelines set by the Veterinary Service Center at Stanford University (Animal Welfare Assurance A3213-01 and USDA License 93-4R-00). Stanford APLAC and institutional guidelines are in compliance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. The Stanford APLAC approved the animal protocol associated with the work described in this publication.
Mice
7 to 8 week-old female BALB/c mice were purchased from Jackson Laboratories. All experimental procedures were carried out in accordance with the APLAC protocol and the institutional guidelines set by the Veterinary Service Center at Stanford University.
S. haematobium egg isolation
S. haematobium-infected LVG hamsters were obtained from the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center of the National Institutes of Health. The hamsters were sacrificed at the point of maximal liver and intestinal Schistosoma egg levels (18 weeks post-infection [74]), at which time livers and intestines were minced, homogenized in a Waring blender, resuspended in 1.2% NaCl containing antibiotic-antimycotic solution (100 units Penicillin, 100 µg/mL Streptomycin and 0.25 µg/mL Amphotericin B, Sigma-Aldrich), passed through a series of stainless steel sieves with sequentially decreasing pore sizes (450 µm, 180 µm, and 100 µm), and finally retained on a 45 µm sieve. Control injections were performed using similarly prepared liver and intestine lysates from age-matched, uninfected LVG hamsters (Charles River Laboratories).
S. haematobium egg injection
7 to 8 week-old female BALB/c mice were anesthetized with isoflurane, a midline lower abdominal incision was made, and the bladder exteriorized. Freshly prepared S. haematobium eggs (3,000 eggs in 50 µl of phosphate-buffered saline, experimental group) or uninfected hamster liver and intestinal extract (in 50 µl of phosphate-buffered saline, control group) was injected submucosally into the anterior aspect of the bladder dome [37]. Abdominal incisions were then closed with 4-0 Vicryl suture, and the surgical site was treated once with topical antibiotic ointment.
Micro-ultrasonography
At various time points after bladder wall injection, mice were anesthetized using vaporized isoflurane and their abdominal walls were depilated. Transabdominal images of the bladder were then obtained using a VisualSonics Vevo 770 high-resolution ultrasound micro-imaging system with an RMV 704 scanhead [40 MHz] (Small Animal Imaging Facility, Stanford Center for Innovation in In-Vivo Imaging).
Bladder histopathologic analysis and collagen measurement
Mice were sacrificed at serial time points 4 to 99 days after bladder wall injection, and bladders processed for routine histology. Morphologic and morphometric analyses were conducted on H&E - and Masson's Trichrome-stained sections. Total collagen content was determined from fresh-frozen (−70°C) bladder homogenates using the Sircol Soluble Collagen Assay Kit (Biocolor, Carrickfergus, United Kingdom) according to the manufacturer's instructions. Collagen concentrations were determined using standard curve analysis. Statistical comparisons were conducted using Student's t-test.
IgE ELISA
ELISA measurement of serum IgE was performed using manufacturer's instructions (Bethyl Laboratories Mouse IgE ELISA Quantitation Kit). In brief, coating antibody was aliquoted into and allowed to bind to microtiter plate wells. Excess antibody was washed away. Next, blocking solution was added to the wells, allowed to bind, and excess was washed away. IgE standards and experimental samples were added to wells, incubated for an hour at ambient temperature, and washed. HRP detection antibody was added to each well, incubated, and washed. TMB substrate solution was then added to each well, developed for 15 minutes at ambient temperature, and the reactions stopped using Stop Solution. Absorbance of each well was then read on a plate reader at 450 nm.
Macrophage-specific immunohistochemistry
Five µm sections from OCT-embedded frozen bladders were fixed in 10% buffered formalin phosphate, blocked with 10% horse serum, and incubated overnight at 4°C with a mouse-specific anti-CD68 antibody (BioLegend, San Diego, CA). Sections were then processed and developed using an anti-rat IgG staining kit (Biocare Medical, Concord, CA) according to the manufacturer's instructions and counterstained with hematoxylin.
Analysis of bladder-associated leukocytes
Freshly-excised bladders from egg-injected mice were minced and incubated with agitation in 0.5% heat-inactivated FBS (Thermo Scientific Hyclone, IL), 20 mM HEPES pH 7, 0.057 Kunitz U/ml DNase I (Sigma-Aldrich), and 1 mg/ml collagenase B (Roche) in RPMI 1640 medium for 1 hr at 37°C [75]. The tissue was then passed through a 70 µm nylon cell strainer to remove undigested tissue and macrocellular debris. After erythrocyte lysis (8.02 mg/ml NH4Cl, 0.84 mg/ml NaHCO3, and 0.37 mg/ml EDTA in distilled water), 106 cells were treated with mouse anti-CD16/CD32 (clone 2.4G2, BioLegend, San Diego, CA) for 10 min and stained with mouse anti–CD3-PE/Cy7 (clone 145-2C11, BioLegend), anti–CD45 (B220)-FITC (clone RA3-6B2, BioLegend), anti-F4/80-FITC (clone BM8, eBioscience, San Diego, CA), anti–CD11b-PE (clone M1/70, BioLegend), anti–Ly-6G(Gr-1)-PECy7 (clone RB6-8C5, eBioscience), anti-CCR3-FITC (clone RB6-8C5, R&D System, Minneapolis, MN), and/or anti-Siglec-F-PE (clone E50-2440, BD Pharmingen, San Diego, CA) for 30 minutes at 4°C. Cells were analyzed using a BD LSRII flow cytometer and BD FACSDiva software. Data were analyzed using FlowJo v7.2.4 (Tree Star, Ashland, OR). Assayed proteins are listed in Table 1.
Cytokine analysis
Rapidly-excised bladders were placed immediately on ice, minced in RNAlater solution (Qiagen), and stored at −80°C. For protein analysis, 50 mg of tissue was sonicated to homogeneity in 1 ml of ice-cold tissue extraction reagent (Biosource, San Diego, CA) supplemented with 1 mM phenylmethanesulfonyl fluoride. Clarified bladder extracts and serum samples were assayed using a mouse 26-plex cytokine kit (Affymetrix, Santa Clara, CA) according to the manufacturer's instructions. Samples were read using a Luminex 200 (Luminex, Austin, TX) with a lower cut off of 100 beads per sample (Human Immune Monitoring Core, Stanford University). Assayed proteins are listed in Table 1.
RNA purification, cDNA synthesis, and real-time PCR
Regional lymph nodes were harvested and placed in RNAlater solution (Ambion, Austin, TX), and stored overnight at 4°C, then at −80°C for long-term storage. RNA was isolated and purified using RNAqueous -Micro kits (Ambion, Austin, TX) according to the manufacturer's instructions. The concentration of RNA was determined by Quant-iT RNA assay kit (Invitrogen, Eugene, OR) with the Qubit fluorimeter. The ribosomal RNA band integrity of each RNA sample was run on an Agilent Bioanalyzer using an RNA 6000 Nano Labchip. RNA samples with RNA Integrity Numbers (RIN) of 6 or higher were used for cDNA synthesis and real-time PCR arrays. cDNA synthesis was performed using the RT2 First Strand cDNA Kit (SABiosciences, Frederick, MD).
Real-time PCR was performed in the Mx3005p thermal cycler (Stratagene) using an RT2 custom PCR array (SABiosciences) with RT2 SYBR Green qPCR Master Mixes (SABiosciences). Cycle thresholds (Ct) were calculated for each reaction. Using the comparative Ct method relative gene expression was calculated as 2(−ΔΔCt), where ΔCt = Ct (gene of interest) - ΔCt (normalizer = β-actin). ΔΔCt was calculated as ΔCt (egg-injected) - ΔCt (calibrator). Data are expressed as mean ± SD. P values are ΔCt of egg - versus control-injected mice. *, P<0.05; **, P<0.01; ***, P<0.005. Proteins corresponding to assayed genes are listed in Table 1.
Voided spot on paper analysis
Voided spot on paper analysis was performed as previously described [76], [77]. In brief, mice underwent bladder wall injection with either eggs or control vehicle. One week later, mice were housed singly and acclimated for one hour in cages lined with filter paper laid underneath a wire floor bottom. Animals were given ad libitum access to food and water-soaked sponges placed on wire cage covers. After 8 hours, each piece of filter paper was photographed under ultraviolet light to localize voided urine spots. Total spots were counted for each mouse and the average number of voids was compared between the egg - and vehicle-injected mice using two-tailed T-tests. *, P<0.05; **, P<0.01; ***, P<0.005.
Statistical analysis
Unpaired t tests with Welch's correction were used for comparisons between control - and egg-injected groups, and data were expressed as mean ± standard deviation. P<0.05 was considered statistically significant.
Supporting Information
Zdroje
1. MedinaDCFindleySEDoumbiaS 2008 State-space forecasting of Schistosoma haematobium time-series in Niono, Mali. PLoS Negl Trop Dis 2 e276
2. WilkinsHAGollPMarshallTFMooreP 1979 The significance of proteinuria and haematuria in Schistosoma haematobium infection. Trans R Soc Trop Med Hyg 73 74 80
3. HicksRMIsmailMMWaltersCLBeechamPTRabieMF 1982 Association of bacteriuria and urinary nitrosamine formation with Schistosoma haematobium infection in the Qalyub area of Egypt. Trans R Soc Trop Med Hyg 76 519 527
4. KassimOOStekMJr 1983 Bacteriuria and hematuria in infections due to Schistosoma haematobium. J Infect Dis 147 960
5. NmorsiOPKwanduUNEbiaguanyeLM 2007 Schistosoma haematobium and urinary tract pathogens co-infections in a rural community of Edo State, Nigeria. J Commun Dis 39 85 90
6. UnekeCJUgwuoruCDNgwuBAOgbuOAgalaCU 2006 Public health implication of bacteriuria and antibiotic susceptibility of bacteria isolates in schistosoma haematobium-infected school pupils in Southeast Nigeria. World Health Popul 8 66 76
7. WilkinsHA 1977 Schistosoma haematobium in a Gambian community. III. The prevalence of bacteriuria and of hypertension. Ann Trop Med Parasitol 71 179 186
8. GelfandMWeinbergRWCastleWM 1967 Relation between carcinoma of the bladder and infestation with Schistosoma haematobium. Lancet 1 1249 1251
9. BrandKG 1979 Schistosomiasis–cancer: etiological considerations. A review. Acta Trop 36 203 214
10. von LichtenbergF 1975 Schistosomiasis as a worldwide problem: pathology. J Toxicol Environ Health 1 175 184
11. Abdel-WahabMFEsmatGRamzyIFouadRAbdel-RahmanM 1992 Schistosoma haematobium infection in Egyptian schoolchildren: demonstration of both hepatic and urinary tract morbidity by ultrasonography. Trans R Soc Trop Med Hyg 86 406 409
12. van der WerfMJde VlasSJBrookerSLoomanCWNagelkerkeNJ 2003 Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86 125 139
13. RollinsonD 2009 A wake up call for urinary schistosomiasis: reconciling research effort with public health importance. Parasitology 136 1593 1610
14. LokerES 1983 A comparative study of the life-histories of mammalian schistosomes. Parasitology 87 Pt 2 343 369
15. RheinbergCEMoneHCaffreyCRImbert-EstabletDJourdaneJ 1998 Schistosoma haematobium, S. intercalatum, S. japonicum, S. mansoni, and S. rodhaini in mice: relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol Res 84 338 342
16. OrdanPGoatlyKD 1966 Experimental schistosomiasis in primates in Tanzania. I. A preliminary note on the susceptibility of Cercopithecus aethiops centralis to infection with Schistosoma haematobium and Schistosoma mansoni. Ann Trop Med Parasitol 60 3 9
17. KuntzREMalakatisGM 1955 Susceptibility studies in schistosomiasis. IV. Susceptibility of wild mammals to infection by Schistosoma haematobium in Egypt, with emphasis on rodents. J Parasitol 41 467 475
18. VuongPNBayssade-DufourCAlbaretJLFarhatiK 1996 Histopathological observations in new and classic models of experimental Schistosoma haematobium infections. Trop Med Int Health 1 348 358
19. EltoumIAGhalibHWSualaimanSKordofaniAMustafaMD 1989 Significance of eosinophiluria in urinary schistosomiasis. A study using Hansel's stain and electron microscopy. Am J Clin Pathol 92 329 338
20. EltoumIASuliamanSMIsmailBMIsmailAIAliMM 1992 Evaluation of eosinophiluria in the diagnosis of schistosomiasis hematobium: a field-based study. Am J Trop Med Hyg 46 732 736
21. IssaRMShalabyMA 1999 Eosinophilia as a diagnostic value in patients suffering from schistosomiasis haematobium comparing to eosinophiluria and egg count in the urine. J Egypt Soc Parasitol 29 431 449
22. ReimertCMMshindaHMHatzCFKombeYNkulilaT 2000 Quantitative assessment of eosinophiluria in Schistosoma haematobium infections: a new marker of infection and bladder morbidity. Am J Trop Med Hyg 62 19 28
23. ReimertCMOumaJHMwanjeMTMagakPPoulsenLK 1993 Indirect assessment of eosinophiluria in urinary schistosomiasis using eosinophil cationic protein (ECP) and eosinophil protein X (EPX). Acta Trop 54 1 12
24. TischendorfFWBrattigNWBurchardGDKubicaTKreuzpaintnerG 1999 Eosinophils, eosinophil cationic protein and eosinophil-derived neurotoxin in serum and urine of patients with onchocerciasis coinfected with intestinal nematodes and in urinary schistosomiasis. Acta Trop 72 157 173
25. MahmoodA 1966 Blood loss caused by helminthic infections. Trans R Soc Trop Med Hyg 60 766 769
26. SabeIMangoudAMElalfyYElsayedMShaabanW 2008 New concept of schistosomiasis lesions of urinary bladder versus development of bladder cancer. J Egypt Soc Parasitol 38 85 102
27. GirgesMR 1966 The syndrome of bladder-neck obstruction and ureteric fibrosis in Schistosoma haematobium infection. J Trop Med Hyg 69 187 188
28. LavenJSVleugelsMPDofferhoffASBloembergenP 1998 Schistosomiasis haematobium as a cause of vulvar hypertrophy. Eur J Obstet Gynecol Reprod Biol 79 213 216
29. NdambaJNyazemaNMakazaNAndersonCKaonderaKC 1994 Traditional herbal remedies used for the treatment of urinary schistosomiasis in Zimbabwe. J Ethnopharmacol 42 125 132
30. MoserBClark-LewisIZwahlenRBaggioliniM 1990 Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med 171 1797 1802
31. WolpeSDDavatelisGSherryBBeutlerBHesseDG 1988 Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med 167 570 581
32. CampbellHDTuckerWQHortYMartinsonMEMayoG 1987 Molecular cloning, nucleotide sequence, and expression of the gene encoding human eosinophil differentiation factor (interleukin 5). Proc Natl Acad Sci U S A 84 6629 6633
33. ShariatiFPerez-ArellanoJLCarranzaCLopez-AbanJVicenteB 2011 Evaluation of the role of angiogenic factors in the pathogenesis of schistosomiasis. Exp Parasitol 128 44 49
34. CheeverAWKamelIAElwiAMMosimannJEDannerR 1977 Schistosoma mansoni and S. haematobium infections in Egypt. II. Quantitative parasitological findings at necropsy. Am J Trop Med Hyg 26 702 716
35. CheeverAWKamelIAElwiAMMosimannJEDannerR 1978 Schistosoma mansoni and S. haematobium infections in Egypt. III. Extrahepatic pathology. Am J Trop Med Hyg 27 55 75
36. GhoneimIRabetsJMawhorterS 2011 Campbell-Walsh urology. WeinAJKavoussiLRCampbellMF 10th ed Philadelphia, PA Elsevier Saunders4 v. (xxxvii, 3753, 3795 p.)
37. FuCLApeloCATorresBThaiKHHsiehMH 2011 Mouse bladder wall injection. J Vis Exp doi:10.3791/2523
38. GhandourAM 1978 The development of Schistosoma haematobium in the hamster. Ann Trop Med Parasitol 72 219 225
39. BurdenCSUbelakerJE 1981 Schistosoma mansoni and Schistosoma haematobium: difference in development. Exp Parasitol 51 28 34
40. WrightCAKnowlesRJ 1972 Studies on Schistosoma haematobium in the laboratory. 3. Strains from Iran, Mauritius and Ghana. Trans R Soc Trop Med Hyg 66 108 118
41. WrightCABennettMS 1967 Studies on Schistosoma haematobium in the laboratory. I. A strain from Durban, Natal, South Africa. Trans R Soc Trop Med Hyg 61 221 227
42. WrightCABennettMS 1967 Studies on Schistosoma haematobium in the laboratory. II. A strain from South Arabia. Trans R Soc Trop Med Hyg 61 228 233
43. Von LichtenbergFEricksonDGSadunEH 1973 Comparative histopathology of schistosome granulomas in the hamster. Am J Pathol 72 149 178
44. HusseinMRAbu-DiefEEEl-HadyHAMahmoudSSSalahEM 2005 Quantitative comparison of infected Schistosomiasis mansoni and Haematobium: animal model analysis of the granuloma cell population. J Egypt Soc Parasitol 35 467 476
45. el-MorsiBSherifMel-RazikiES 1975 Experimental bilharzial squamous metaplasia of the urinary bladder in hamsters. Eur J Cancer 11 199 201
46. BillipsBKForrestalSGRycykMTJohnsonJRKlumppDJ 2007 Modulation of host innate immune response in the bladder by uropathogenic Escherichia coli. Infect Immun 75 5353 5360
47. CoffmanRLSeymourBWHudakSJacksonJRennickD 1989 Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245 308 310
48. SchrammGFalconeFHGronowAHaischKMamatU 2003 Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J Biol Chem 278 18384 18392
49. SmithPFallonREManganNEWalshCMSaraivaM 2005 Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med 202 1319 1325
50. SokolCLBartonGMFarrAGMedzhitovR 2008 A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 9 310 318
51. KetavarapuJMRodriguezARYuJJCongYMurthyAK 2008 Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc Natl Acad Sci U S A 105 9313 9318
52. LalouxVBeaudoinLRonetCLehuenA 2002 Phenotypic and functional differences between NKT cells colonizing splanchnic and peripheral lymph nodes. J Immunol 168 3251 3258
53. MallevaeyTFontaineJBreuilhLPagetCCastro-KellerA 2007 Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun 75 2171 2180
54. SnapperCMPaulWE 1987 Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236 944 947
55. ChikunguwoSMQuinnJJHarnDAStadeckerMJ 1993 The cell-mediated response to schistosomal antigens at the clonal level. III. Identification of soluble egg antigens recognized by cloned specific granulomagenic murine CD4+ Th1-type lymphocytes. J Immunol 150 1413 1421
56. VellaATPearceEJ 1992 CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol 148 2283 2290
57. ZhuYLukacsNWBorosDL 1994 Cloning of TH0 - and TH2-type helper lymphocytes from liver granulomas of Schistosoma mansoni-infected mice. Infect Immun 62 994 999
58. RutitzkyLILopes da RosaJRStadeckerMJ 2005 Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol 175 3920 3926
59. JourdanPMRoaldBPoggenseeGGundersenSGKjetlandEF 2011 Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis 5 e1170
60. RinaldiGOkatchaTIPopratiloffAAyukMASuttiprapaS 2011 Genetic manipulation of Schistosoma haematobium, the neglected schistosome. PLoS Negl Trop Dis 5 e1348
61. CheeverAWLewisFAWynnTA 1997 Schistosoma mansoni: unisexual infections sensitized mice for granuloma formation around intravenously injected eggs. Parasitol Res 83 57 59
62. WarrenKSDomingoEO 1970 Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg 19 292 304
63. ShimadaMKirinokiMShimizuKKato-HayashiNChigusaY 2010 Characteristics of granuloma formation and liver fibrosis in murine schistosomiasis mekongi: a morphological comparison between Schistosoma mekongi and S. japonicum infection. Parasitology 137 1781 1789
64. EdungbolaLDSchillerEL 1979 Histopathology of hepatic and pulmonary granulomata experimentally induced with eggs of Schistosoma mansoni. J Parasitol 65 253 261
65. HirataMTakushimaMKageMFukumaT 1993 Comparative analysis of hepatic, pulmonary, and intestinal granuloma formation around freshly laid Schistosoma japonicum eggs in mice. Parasitol Res 79 316 321
66. KuraishyAKarinMGrivennikovSI 2011 Tumor promotion via injury - and death-induced inflammation. Immunity 35 467 477
67. GryseelsBPolmanKClerinxJKestensL 2006 Human schistosomiasis. Lancet 368 1106 1118
68. BeaverPCJungRCCuppEW 1984 Clinical Parasitology Philadelphia Lea and Febiger
69. El RidiRIsmailSGaafarTEl DemellawyM 1997 Differential responsiveness of humans with early-stage schistosomiasis haematobium to Schistosoma haematobium soluble adult-worm and egg antigens. Parasitol Res 83 471 477
70. GaafarTHelmyMIsmailSAfifiAGuirguisN 1992 Identification of the Schistosoma haematobium soluble egg antigens inducing antibody production and/or T cell proliferation in humans. J Egypt Soc Parasitol 22 441 451
71. GaafarTIsmailSHelmyMAfifiAGuirguisN 1993 Identification of Schistosoma haematobium soluble egg antigens that elicit human granuloma formation in vitro. Parasitol Res 79 103 108
72. von LichtenbergF 1962 Host response to eggs of S. mansoni. I. Granuloma formation in the unsensitized laboratory mouse. Am J Pathol 41 711 731
73. HirataMTakushimaMKageMFukumaT 1991 Induction of experimental murine granuloma formation against Schistosoma japonicum eggs produced by in vitro ova deposition, in vitro tissue extraction, or lyophilization. Parasitol Res 77 315 319
74. BotrosSSHammamOAEl-LakkanyNMEl-DinSHEbeidFA 2008 Schistosoma haematobium (Egyptian strain): rate of development and effect of praziquantel treatment. J Parasitol 94 386 394
75. SivickKESchallerMASmithSNMobleyHL 2010 The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol 184 2065 2075
76. GomezP3rdGilESLovettMLRockwoodDNDi VizioD 2011 The effect of manipulation of silk scaffold fabrication parameters on matrix performance in a murine model of bladder augmentation. Biomaterials 32 7562 7570
77. SuginoYKanematsuAHayashiYHagaHYoshimuraN 2008 Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27 548 552
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání