-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
The protective immune response to intracellular parasites involves in most cases the differentiation of IFNγ-secreting CD4+ T helper (Th) 1 cells. Notch receptors regulate cell differentiation during development but their implication in the polarization of peripheral CD4+ T helper 1 cells is not well understood. Of the four Notch receptors, only Notch1 (N1) and Notch2 (N2) are expressed on activated CD4+ T cells. To investigate the role of Notch in Th1 cell differentiation following parasite infection, mice with T cell-specific gene ablation of N1, N2 or both (N1N2ΔCD4Cre) were infected with the protozoan parasite Leishmania major. N1N2ΔCD4Cre mice, on the C57BL/6 L. major-resistant genetic background, developed unhealing lesions and uncontrolled parasitemia. Susceptibility correlated with impaired secretion of IFNγ by draining lymph node CD4+ T cells and increased secretion of the IL-5 and IL-13 Th2 cytokines. Mice with single inactivation of N1 or N2 in their T cells were resistant to infection and developed a protective Th1 immune response, showing that CD4+ T cell expression of N1 or N2 is redundant in driving Th1 differentiation. Furthermore, we show that Notch signaling is required for the secretion of IFNγ by Th1 cells. This effect is independent of CSL/RBP-Jκ, the major effector of Notch receptors, since L. major-infected mice with a RBP-Jκ deletion in their T cells were able to develop IFNγ-secreting Th1 cells, kill parasites and heal their lesions. Collectively, we demonstrate here a crucial role for RBP-Jκ-independent Notch signaling in the differentiation of a functional Th1 immune response following L. major infection.
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002560
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002560Summary
The protective immune response to intracellular parasites involves in most cases the differentiation of IFNγ-secreting CD4+ T helper (Th) 1 cells. Notch receptors regulate cell differentiation during development but their implication in the polarization of peripheral CD4+ T helper 1 cells is not well understood. Of the four Notch receptors, only Notch1 (N1) and Notch2 (N2) are expressed on activated CD4+ T cells. To investigate the role of Notch in Th1 cell differentiation following parasite infection, mice with T cell-specific gene ablation of N1, N2 or both (N1N2ΔCD4Cre) were infected with the protozoan parasite Leishmania major. N1N2ΔCD4Cre mice, on the C57BL/6 L. major-resistant genetic background, developed unhealing lesions and uncontrolled parasitemia. Susceptibility correlated with impaired secretion of IFNγ by draining lymph node CD4+ T cells and increased secretion of the IL-5 and IL-13 Th2 cytokines. Mice with single inactivation of N1 or N2 in their T cells were resistant to infection and developed a protective Th1 immune response, showing that CD4+ T cell expression of N1 or N2 is redundant in driving Th1 differentiation. Furthermore, we show that Notch signaling is required for the secretion of IFNγ by Th1 cells. This effect is independent of CSL/RBP-Jκ, the major effector of Notch receptors, since L. major-infected mice with a RBP-Jκ deletion in their T cells were able to develop IFNγ-secreting Th1 cells, kill parasites and heal their lesions. Collectively, we demonstrate here a crucial role for RBP-Jκ-independent Notch signaling in the differentiation of a functional Th1 immune response following L. major infection.
Introduction
Following activation by pathogens, naïve CD4+ T cells can differentiate into several functionally distinct T helper (Th) subsets, defined by the cytokines they secrete. CD4+ Th1 cells secrete IFNγ as a signature cytokine and the transcription factor T-bet is essential for their differentiation. Although cytokines such as IL-12 contribute to Th1 cell differentiation, Th1 cells can be generated in the absence of cytokine signaling, demonstrating a role for other molecules in this process. Among these are Notch receptors and their ligands (Reviewed in [1], [2]). Notch signaling plays crucial roles in binary cell fate decisions in many developmental systems including the development and differentiation of immune cells. In mammals, there are four Notch receptors (Notch1-4) that are activated by five ligands (Jagged (Jag) 1, and 2, and Delta-like (Dll) 1, 3, and 4). Upon interaction with its ligand, the active intracellular domain of Notch (NICD) is released from the membrane by proteolytic cleavages and translocates into the nucleus. Once there, NICD can form a complex with recombination signal-binding protein-J (RBP-Jκ), converting it to an activator of transcription (canonical Notch signaling). Alternatively, NICD could interact with members of the NF-κB pathway (non-canonical Notch signaling) [3]. In the T cell lineage, the Notch1 receptor is essential for the development of αβ T cells [4], and Notch plays a poorly understood role in the differentiation of peripheral Th cell subsets (reviewed in [1], [5]).
The importance of Notch signaling during CD4+ Th1 differentiation and its correlated resolution of pathogen infection is currently unclear. Inhibitors of γ-secretase impairing Notch signaling prevented Th1 differentiation in vitro and in vivo, potentially through the blocking of T-bet expression [6]. Blocking of the Notch3 receptor using antisense N3 DNA also blocked Th1 differentiation in vitro [7]. In contrast, T cell-specific expression of dominant negative mastermind-like protein (MAML1), which is needed for RBP-Jκ-dependent Notch signaling, or T cell specific ablation of Notch1 or RBP-Jκ did not have an impact on Th1 differentiation in vitro [8] nor in vivo [9], [10].
The role of Notch ligands on dendritic cells instructing Th1 differentiation is also debated. Dll1 and/or Dll4 expression is upregulated in vitro on APCs encountering pathogens driving a CD4+ Th1 response [8], [11], [12]. Interaction of Notch with Dll1 promoted Th1 differentiation during Leishmania major infection [7]. Furthermore, Dll4 expression on DC was shown to induce Th1 cell differentiation in an IL-12-independent way [11]. On the contrary, Dll1, Jag1 and Jag2 were shown to be insufficient to instruct the differentiation of Th1 or Th2 CD4+ cells in absence of polarizing cytokines in vitro, suggesting that the induction of selective ligands by pathogens may not exert a direct influence on T helper differentiation [13], [14].
Altogether, these studies indicate a role of Notch in CD4+ Th1 differentiation, but it is not clear yet which member and how each member of this family contributes to this process during infection with pathogens. Most of the above studies investigated the role of Notch using total inhibition of Notch signaling, but the individual contribution and potential crosstalk of individual Notch receptors during infections with pathogens inducing CD4+ Th1 cells has not been investigated.
Here, mice carrying a T cell specific deletion of Notch1 (N1ΔCD4Cre), Notch2 (N2ΔCD4Cre) or both Notch1 and Notch2 (N1N2ΔCD4Cre) on a resistant C57BL/6 genetic background were infected with L. major to study the importance of Notch receptors in Th1 differentiation and resolution of the infection. We show that Notch signaling through either N1 and/or N2 induces the secretion of IFNγ by CD4+ Th1 cells. Moreover, using mice with T cell-specific ablation of RBP-Jκ (RBP-JΔCD4Cre), we show that Th1 differentiation is induced mainly by non-canonical (RBP-Jκ-independent) Notch signaling. Collectively, our data indicate that Notch signaling drives the differentiation of L. major-specific IFNγ-secreting Th1 cells required to mount an efficient immune response against this parasite.
Results
Notch affects the development of a protective L. major-specific Th1 cell response
To investigate Notch function in Th1 cell development we infected mice with L. major, a parasite promoting a predominant Th1 immune response in most strains of mice including C57BL/6 [15]. Of the four Notch receptors, only N1 and N2 are expressed in activated T cells [8], [16]. Thus, to investigate the effect of T cell ablation of these two receptors (N1N2ΔCD4Cre) on CD4+ Th1 differentiation and the consequent resolution of L. major infection, N1N2ΔCD4Cre and control N1N2lox/lox mice on the L. major resistant C57BL/6 genetic background were inoculated with the parasite. In contrast to N1N2lox/lox control mice that developed a small self-healing lesion, N1N2ΔCD4Cre were unable to heal their lesions (Figure 1A). In addition, L. major-infected N1N2ΔCD4Cre mice failed to control parasite load at the site of parasite inoculation (Figure 1B) and L. major disseminated to the lymph nodes and spleen (Figure 1C, D).
Fig. 1. N1N2ΔCD4Cre mice on the C57BL/6 L. major resistant background are susceptible to infection. 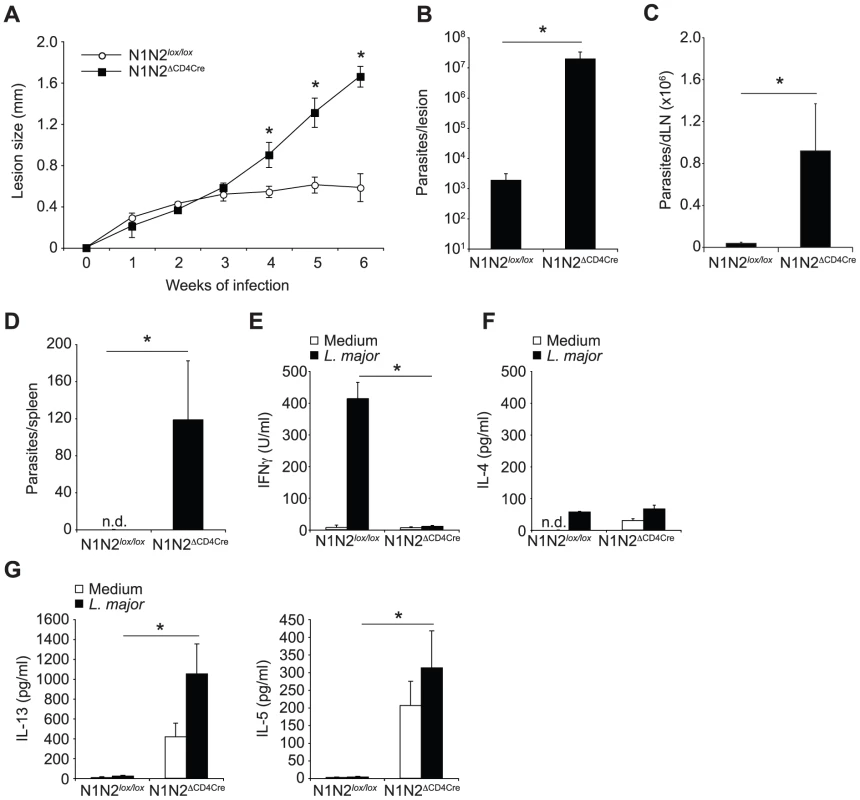
(A) N1N2ΔCD4Cre and control N1N2lox/lox mice were infected with 3×106 L. major promastigotes and lesion size measured weekly. Dots represent group mean of lesion size ± SEM. (B, C) Six weeks after infection, parasite load was assessed by LDA in footpads (B), dLN (C) and spleen (D). Histograms represent the mean number of parasite ± SEM (n≥3 mice per group). (E–G) IFNγ (E), IL-4 (F), IL-13 and IL-5 (G) secretion was quantified in supernatants of draining lymph node cells restimulated or not with UV-irradiated L. major 6 weeks after infection. Mean cytokine secretion ± SEM are given (n≥3 mice per group). Data are representative of at least 3 individual experiments. n.d. not-detectable. * p-value<0.05 versus control mice. The impact of the absence of Notch on T cells in the differentiation of CD4+ IFNγ-secreting Th1 cells was assessed six weeks after infection. N1N2ΔCD4Cre and control draining lymph node (dLN) cells were restimulated in vitro with UV-irradiated L. major and cytokine levels measured. Strikingly, secretion of IFNγ was abrogated in supernatants of N1N2ΔCD4Cre dLN cells, while high levels of this cytokine were measured in dLN from infected control mice (Figure 1E). Similarly low IL-4 levels were measured in each group (Figure 1F). IL-13 and IL-5 were found predominantly in N1N2ΔCD4Cre dLN cells (Figure 1G). The persistence of parasites in the dLN of L. major infected N1N2ΔCD4Cre but not N1N2lox/lox mice was sufficient to induce IL-13 and IL-5 secretion by T cells (Figure 1G), albeit at a lower level than that reached following stimulation with exogenous addition of L. major. These results show that Notch signaling contributes to the generation of IFNγ-secreting CD4+ T cells, which are essential in the control of parasite load and lesion size. The absence of Notch expression on T cells, while preventing IFNγ secretion, favored the development of IL-13 - and IL-5-secreting cells.
N1 or N2 expression on T cells is sufficient to drive Th1 cell differentiation
N1ΔCD4Cre mice are able to develop a protective Th1 response in response to L. major inoculation [9]. The inability of L. major-infected N1N2ΔCD4Cre mice to develop a protective Th1 immune response suggested that N2 could be the receptor involved in Th1 differentiation. To investigate this, N2ΔCD4Cre and N2lox/lox control mice were infected with L. major and evolution of lesion size and development of immune response were compared to that developing in N1ΔCD4Cre, and N1N2ΔCD4Cre infected mice. N2ΔCD4Cre mice were able to control their lesion size (Figure 2A) and parasitemia (Figure 2B) as well as N1ΔCD4Cre and control mice, unlike N1N2ΔCD4Cre mice. To analyze their immune response, cytokine secretion by dLN cells was analyzed. L. major-infected N2ΔCD4Cre dLN cells secreted similar levels of IFNγ than L. major-infected N1ΔCD4Cre and control mice. Low levels of IL-4, IL-5 and IL-13 were similarly measured in their dLN cells (Figure 2C).
Fig. 2. N1 and N2 alone can drive CD4+ Th1 differentiation. 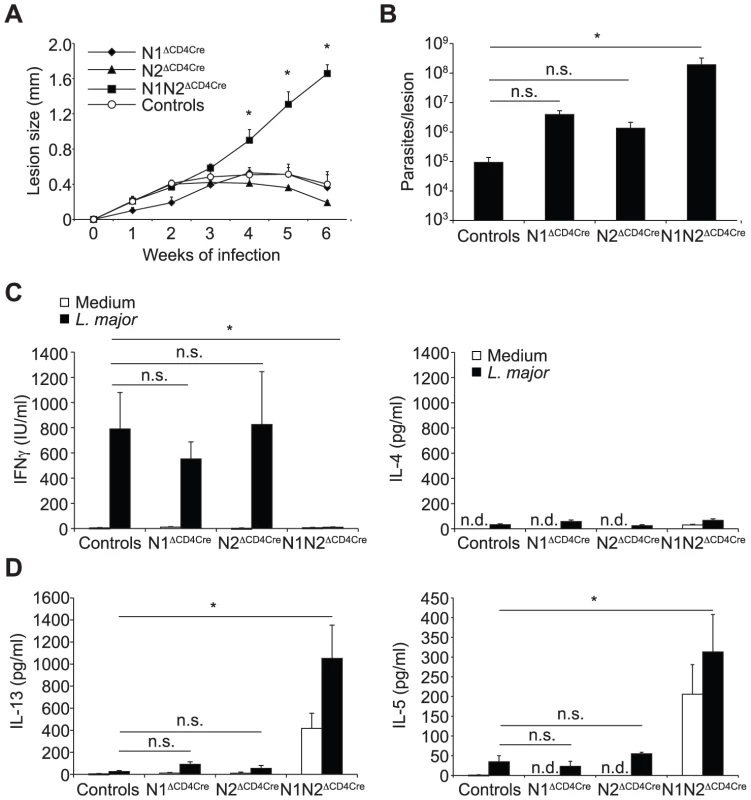
(A) N1ΔCD4Cre, N2ΔCD4Cre, N1N2ΔCD4Cre, and control mice were infected with 3×106 L. major promastigotes and lesion size measured weekly. Data are represented as the mean of lesion size ± SEM with n≥3 mice per group. (B) Parasite load in the lesion was assessed by LDA 6 weeks after infection. Mean parasite number is given ± SEM (n≥3 mice per group) (C, D) Six weeks after infection, IFNγ (C), IL-4 and IL-13 (D) secretion was assessed in supernatant of dLN cells restimulated or not with UV-irradiated L. major for 72 h. Histograms show the mean cytokine secretion ± SEM (n≥3 mice per group). n.d. not-detectable, n.s. not significant. * p-value<0.05 versus control mice. N1N2ΔCD4Cre mice are susceptible to L. major infection and fail to induce IFNγ secretion by CD4+ T cells, indicating that expression of either N1 or N2 is sufficient to induce CD4+ Th1 differentiation in N2ΔCD4Cre or N1ΔCD4cre mice, respectively. To investigate if following parasite inoculation, compensatory expression of one or all of the Notch receptors on T cells could occur, dLN cells of L. major infected N1ΔCD4Cre or N2ΔCD4Cre mice were stimulated in vitro with L. major, and Notch expression on their CD4+ T cells measured by FACS. N1 expression was significantly and similarly expressed in both N2ΔCD4Cre and N2lox/lox CD4+ T cells (Figure 3A). Low levels of N2 surface expression were induced following restimulation of control dLN T cells with L. major, however, a significantly higher induction of N2 was measured in L. major-activated N1ΔCD4cre CD4+ T cells (Figure 3B), suggesting that compensatory mechanisms allow increased N2 expression in absence of N1. No expression of N3 and N4 mRNA or proteins was detectable on T cells of all genotypes, in contrast to positive control cells (Figure 3C and data not shown). Altogether, these data reveal that signaling through either N1 or N2 is sufficient for the generation of functional Th1 cells following infection with L. major, and that in absence of N1 compensatory higher expression of N2 is measured on T cells.
Fig. 3. Increased N2 expression can compensate the absence of N1 on L. major stimulated CD4+ T cells. 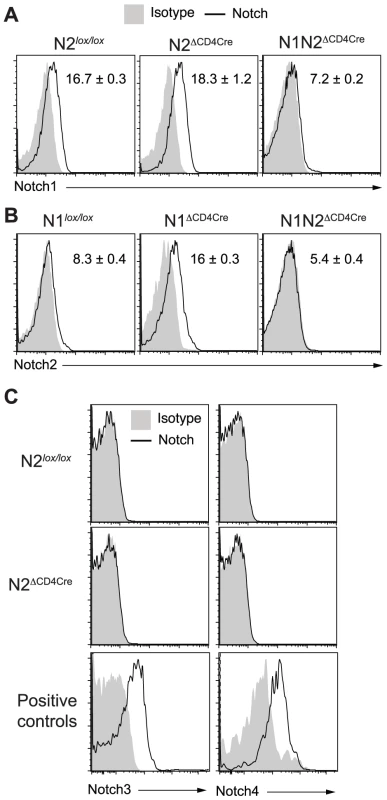
(A–C) Three weeks after L. major infection, dLN cells from the indicated mouse strains were isolated and restimulated for 16 h with UV-treated L. major. Notch1 (A), Notch2 (B), Notch3 and Notch4 (C) expression by CD4+ T cells was assessed by FACS. CD11c+CD8α+ splenic dendritic cells and CD4−CD8−CD25+ thymocytes were stained as positive controls for Notch3 and Notch4 respectively. Representative flow cytometry plots are shown. Numbers in plots represent mean fluorescence intensity MFI ± SEM of ≥3 mice per group. Data are representative of 3 independent experiments. Susceptibility to L. major infection in absence of Notch on T cells results mainly from lack of IFNγ secretion
Draining LN CD4+ T cells of L. major-infected N1N2ΔCD4Cre mice fail to secrete IFNγ but release high levels of IL-13. This cytokine has been associated with susceptibility to L. major infection, mostly by preventing the classical activation of macrophages by IFNγ [17]. To investigate if susceptibility of N1N2ΔCD4Cre mice resulted from a lack of IFNγ secretion and/or from the presence of high levels of IL-13, IL-13 was neutralized with an anti-IL-13 mAb after inoculation of L. major in N1N2ΔCD4Cre and N1N2lox/lox control mice. No effect was observed in lesion development and parasite control in IL-13-depleted N1N2ΔCD4Cre mice, that developed unhealing lesions similar to mice treated with PBS (Figure 4A). Similar low levels of IFNγ were measured in isolated CD4+ T cells of mice depleted or not of IL-13 (Figure 4B). The efficacy of the anti-IL-13 treatment was confirmed by measuring dLN levels of Fizz1 and Ym1 expression, two markers of alternative macrophage activation. The mRNA levels of both markers were decreased in dLN of anti-IL-13-treated N1N2ΔCD4Cre mice (Figure 4C). Collectively, these data demonstrate that the non-healing phenotype measured in N1N2ΔCD4Cre mice results primarily from the decreased IFNγ secretion by CD4+ T cells which does not allow activation of macrophage to kill the intracellular parasites. The high levels of IL-13 which induce alternative macrophage activation do not play a critical role in the failure of macrophages to kill the parasites, as in absence of IFNγ, macrophages are already not classically activated.
Fig. 4. Treatment with anti-IL-13 does not restore resistance of N1N2ΔCD4Cre mice to L. major. 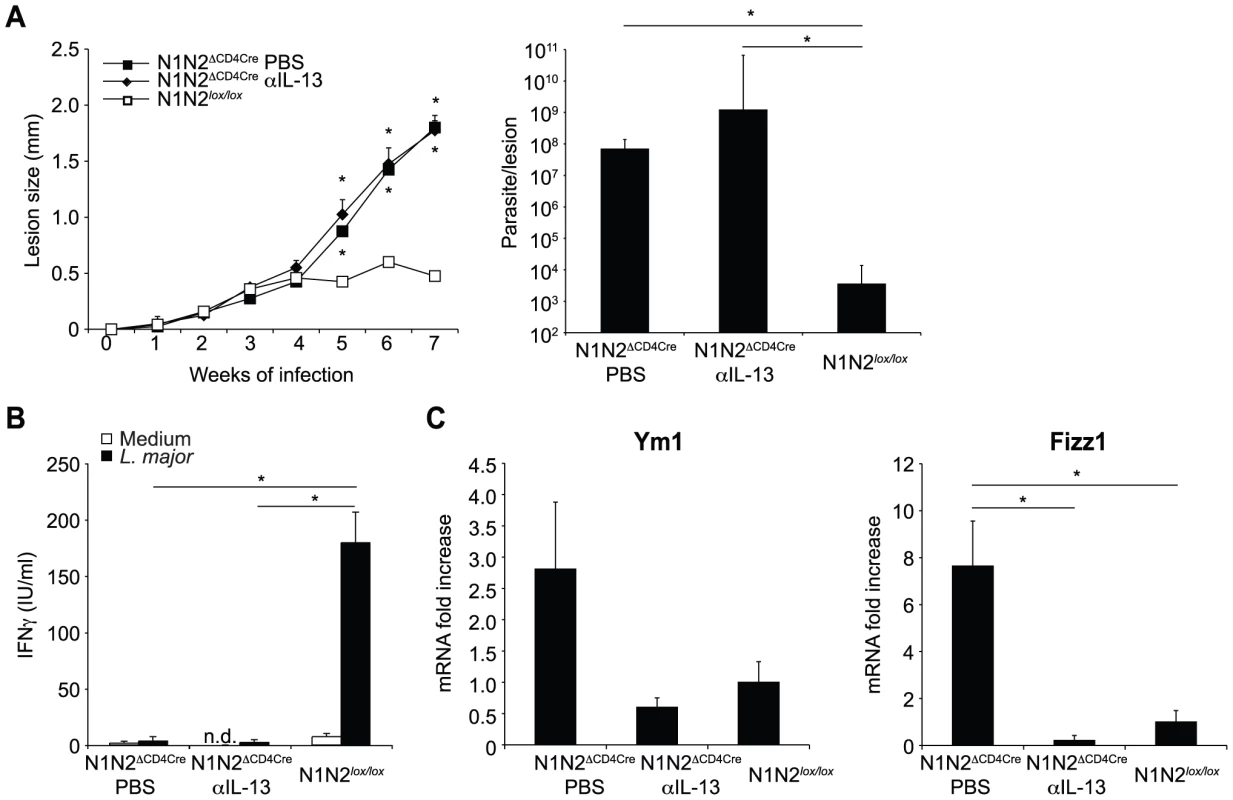
(A) N1N2ΔCD4Cre and control N1N2lox/lox mice were infected s.c. with 3×106 L. major promastigotes. At day 21 of infection, N1N2ΔCD4Cre mice were treated i.p. with either anti-IL-13 mAb or PBS as control. Treatment was repeated once a week until the end of the experiment, and lesion development was monitored. Group means of lesion size ± SEM (n≥3 mice per group) are represented. The parasite load at the site of infection was assessed by LDA 47 days post infection. Group means of parasite number are given ± SEM (n≥3 mice per group). (B) CD4+ T cells were isolated by MACS from dLN of L. major-infected mice 47 days post infection and restimulated with or without UV-treated L. major in presence of irradiated syngenic splenocytes. IFNγ level was measured in supernatant after 72 h of stimulation. Data are expressed as the group mean ± SEM of cytokine measurement of n≥3 draining lymph nodes (C) Ym1 and Fizz1 mRNA expression was analyzed in dLN cells by quantitative real-time PCR and normalized to HPRT mRNA expression. Results are represented as fold-increase in mRNA levels relative to levels measured in control mice ± SEM (n≥3 mice per group). Data are representative of three independent experiments. Similar results were obtained when anti-IL-13 was administrated 7 days post infection (data not shown). * p-value<0.05 versus control mice. Notch signaling prevents the release but not the transcription of IFNγ by CD4+ dLN cells
We then investigated if the impaired IFNγ secretion measured in N1N2ΔCD4Cre CD4+ T cells could result from defective in vitro proliferation. To this end, dLN cells of L. major-infected N1N2ΔCD4Cre and control mice were stained with CFSE and restimulated for 72 h with L. major. CD4+ T cells of both N1N2ΔCD4Cre and control proliferated in response to the parasite. N1N2ΔCD4Cre showed a slightly lower CD4+ T cell proliferation compared to that of N1N2lox/lox CD4+ T cells but the difference was not statistically significant (Figure 5A). Despite comparable proliferation, IFNγ was not secreted in response to L. major stimulation. However, high levels of intracellular IFNγ were measured by FACS in N1N2ΔCD4Cre CD4+ T cells restimulated with L. major for 72 h, in absence of PMA-ionomycin stimulation (Figure 5B). To further determine at which level the absence of N1 and N2 on T cells affects secretion of IFNγ in the dLN of L. major-infected mice, mRNA levels of IFNγ and T-bet, the major transcription factor of Th1 cells, were measured ex vivo on FACS sorted CD4+ T cells 3 weeks after infection. Reduced levels of secreted IFNγ did not result from impaired transcription of T-bet or IFNγ mRNA as demonstrated by higher levels of both T-bet and IFNγ mRNA measured in N1N2ΔCD4Cre CD4+ T cells compared to those measured in CD4+ T cells of control mice (Figure 5C). No defect in activation status or in CD4+ T cell number was measured in dLN cell N1N2ΔCD4Cre mice (Figure S1). IFNγ signaling is mediated by STAT1 phosphorylation and IFNγ was reported to signal to the majority of cells throughout the dLN during a Th1 response after T. gondii infection [18]. To further show that secretion of IFNγ is impaired in CD4+ T cells during infection, we measured STAT1 phosphorylation in dLN CD4+ T cells of L. major-infected N1N2ΔCD4Cre mice. STAT1 phosphorylation was detected in CD4+ T cells of control mice while only minimal STAT1 phosphorylation was measured in CD4+ T cells of N1N2ΔCD4Cre dLN cells (Figure 5D). These data confirm that in absence of N1 and N2 on T cells, IFNγ secretion by CD4+ T cells is impaired, thereby preventing IFNγ-induced STAT1 phosphorylation occurring in vivo. The impairment of IFNγ secretion is antigen-specific and not due to an intrinsic secretion default in N1N2ΔCD4Cre mice as revealed by the high levels of IFNγ detected by intracellular staining following TCR-independent T cell stimulation (PMA-ionomycin) ex vivo (Figure 5E). In addition, defective secretion could be overcome in vitro by antibody-mediated CD3 crosslinking stimulation (Figure S2A).
Fig. 5. N1N2ΔCD4Cre mice transcribe T-bet and IFNγ in dLN CD4+ T cells but do not secrete it. 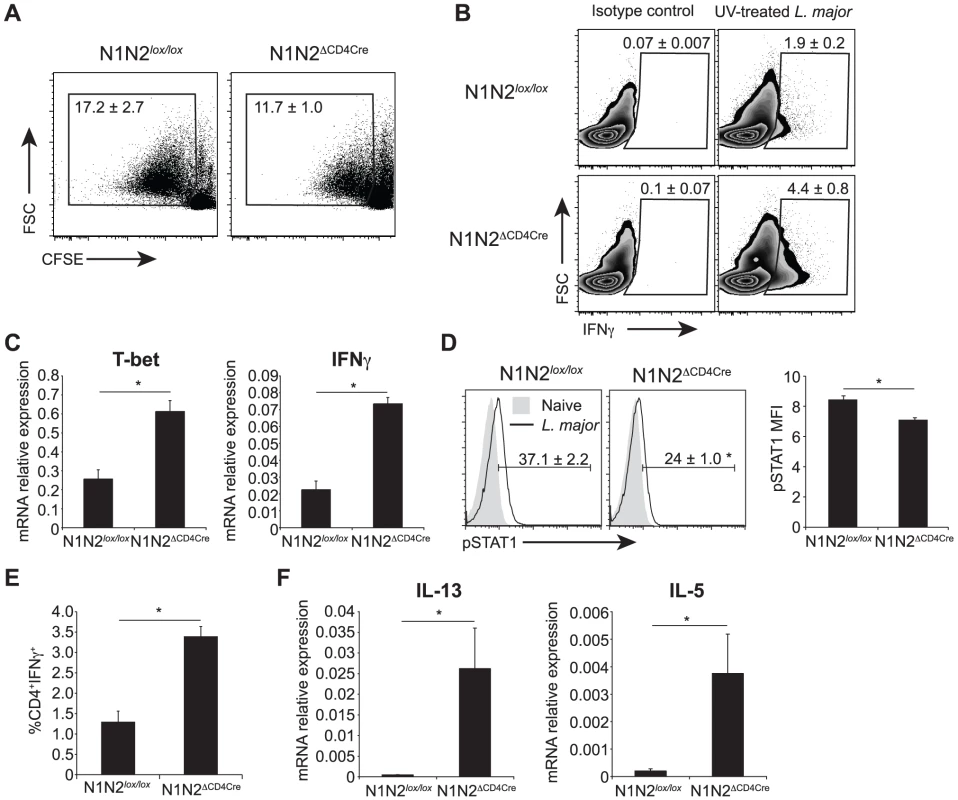
(A) Proliferation of CD4+ T cells was assessed by FACS. Draining LN cells of L. major-infected mice were isolated 6 weeks after infection, stained with CFSE and restimulated with UV-treated L. major for 72 h. Representative flow cytometry plots gated on CD4+ T cells are shown. Numbers in plots represent the mean percentage of proliferating cells ± SEM for 5 mice. (B) Intracellular levels of IFNγ were analysed by FACS in L. major-infected dLN cells restimulated for 72 h with UV-treated L. major. Representative flow cytometry plots are given. Numbers in plots represent the mean percentage of IFNγ+ cells within CD4+ T cells ± SEM for 5 mice. (C) Draining LN CD4+ T cells from N1N2ΔCD4Cre and N1N2lox/lox mice were sorted by FACS 21 days post L. major infection, T-bet and IFNγ mRNA levels were analyzed by quantitative RT-PCR. Data are represented as the mean ± SEM mRNA transcript levels normalized to HPRT mRNA levels (n≥3 mice per group). (D) Phosphorylation of STAT1 was assessed by FACS on dLN cells of N1N2ΔCD4Cre and N1N2lox/lox mice 3 weeks post infection. Naive mice were used as control. Representative flow cytometry plots gated on CD4+ T cells are shown. Numbers in quadrants indicate the mean frequency of pSTAT1+CD4+ T cells ± SEM. pSTAT1 mean fluorescence intensity MFI ± SEM is shown (n≥3 mice per group). (E) Draining LN cells of L. major-infected mice were restimulated ex vivo with PMA/ionomcyin for 4 h and level of intracellular IFNγ was assessed by FACS. The frequency of CD4+IFNγ+ T cells is given ± SEM for n≥3 mice per group. (F) mRNA expression of IL-13 and IL-5 was analyzed by quantitative real-time PCR in dLN cells isolated from N1N2ΔCD4Cre and N1N2lox/lox mice 6 weeks post L. major infection. Results are given as mean mRNA expression relative to HPRT ± SEM for n≥3 mice per group. Data are representative of 2–3 individual experiments. * p-value<0.05 versus control mice. In the same line, naïve N1N2ΔCD4Cre CD4+ T cells stimulated in vitro in the presence of standard Th1 polarizing conditions followed by stimulation with anti-CD3/CD28 for 48 hours were able to develop into IFNγ-secreting cells (Figure S2B). In contrast, the strong increase in IL-13 and IL-5 mRNA levels measured in dLN of L. major-infected mice (Figure 5F) correlated with the high secretion levels of these cytokines in L. major-stimulated CD4+ T cells. Altogether, these data reveal that following inoculation of L. major, absence of N1 and N2 on T cells prevents the release of IFNγ by CD4+ T cells, favoring the differentiation of IL-13 - and IL-5-secreting cells. This effect is obscured in vitro by exogenous addition of high amounts of cytokines and/or antigen-non specific activation of T cells.
Notch signaling driving CD4+ Th1 differentiation occurs in absence of the RBP-Jκ transcription factor
Mice with dominant negative MAML (DNMAML) protein preventing the canonical transcriptional activation by all four Notch receptors were previously reported to be able to control infection with L. major and to have normal levels of IFNγ in their dLN CD4+ T cells [10]. Different strains of L. major may induce distinct type of T helper immune response [19]. To further insure that the different outcomes on the differentiation of Th1 cells measured in theirs (L. major Friedlin) and the present studies (L. major LV39) were not due to differences in the L. major strains used, N1N2ΔCD4Cre mice were infected with two other L. major strains (Friedlin or IR175). N1N2ΔCD4Cre mice infected with these two L. major strains failed to develop an efficient Th1 response with decreased secretion of IFNγ and increased secretion of IL-13 and IL-5 by their dLN T cells and high intralesional parasite load (Figure S3). These data show that N1 and N2 are required for Th1 differentiation following infection with different strains of L. major.
Having ruled out a potential effect due to the strain of Leishmania used, the lack of effect of DNMAML on Th1 differentiation [10] suggested that Notch signaling may not drive Th1 cell differentiation through the NICD–MAML–RBP-Jκ transcriptional activation complex. To investigate if the requirement of Notch signaling for CD4+ Th1 differentiation and the associated resolution of the lesion could be RBP-Jκ-independent, we infected RBP-JκΔCD4Cre and RBP-Jκlox/lox control mice with L. major. No difference in lesion development (Figure 6A) nor parasite control was measured between RBP-JκΔCD4Cre and control mice (Figure 6B). Furthermore, the development of CD4+ IFNγ-secreting Th1 cells was normal, as revealed by high levels of IFNγ secretion by dLN T cells, and low levels of IL-13 and IL-5 (Figure 6C). These results demonstrate that RBP-Jκ-independent Notch signaling is required for CD4+ Th1 differentiation following L. major infection.
Fig. 6. The impact of Notch signaling on Th1 differentiation is RBP-Jκ-independent. 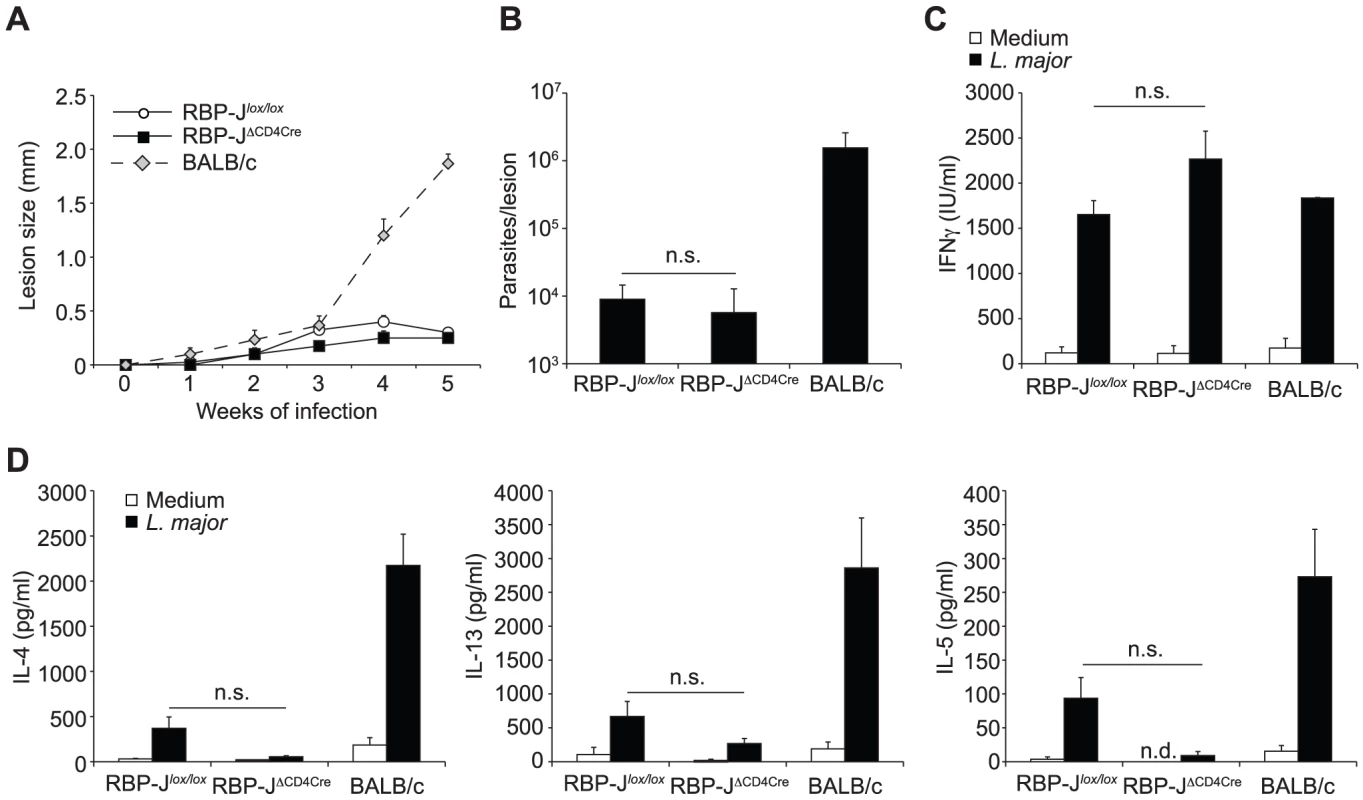
(A) RBP-JκΔCD4Cre and RBP-Jκlox/lox mice were infected s.c. with 3×106 L. major and lesion size measured weekly. Group mean of lesion size ± SEM for n≥3 mice per group is shown. (B) Parasite load in footpad was analyzed by LDA 5 weeks post infection. Data represent mean parasite number ± SEM for n≥3 mice per group. (C, D) IFNγ (C), IL-4, IL-13 and IL-5 (D) levels were measured in supernatant of dLN cells isolated from L. major-infected mice 5 weeks post infection and restimulated for 72 h with or without UV-treated L. major. Group mean of cytokine secretion ± SEM is given (n≥3 mice per group). n.s. not significant. * p-value<0.05 versus control mice. Discussion
The development of Th1 cells, through their secretion of IFNγ, contributes to a number of protective effects against many pathogens. Despite a growing understanding on the mechanisms leading to T helper differentiation these last years (reviewed in [20]), there are still unresolved issues including the identification of which receptor triggering leads to Th1 differentiation. The role of Notch in Th1 differentiation has been controversial, depending on the mode of activation/deactivation of Notch [1], [5]. Our data showing the crucial role of RBP-jκ-independent Notch signaling in the differentiation of IFNγ-secreting T cells help to reconcile discrepant results obtained using different loss or gain of function approaches that attributed or not a role for Notch signaling in Th1 differentiation.
Here, using mice with ablation of Notch in their T cells, we demonstrate that expression of either N1 or N2 on T cells is necessary and sufficient for the differentiation of IFNγ-secreting Th1 cells and the consequent control of infection. N1N2ΔCD4Cre mice infected with L. major failed to mount a protective Th1 immune response while mice with single deletion of N1 (this study and [9]) or N2 in their T cells developed a protective Th1 immune response. In control mice, N1 is the only receptor expressed at significant levels at the surface of anti-CD3 and L. major-activated CD4+ T cells. There appears to be functional redundancy of N1 and N2 in driving CD4+ T helper 1 differentiation. Expression of N2 is low in activated T cells of L. major-infected control mice, but compensatory increased N2 expression was measured in absence of N1 expression. This suggests that N1 is the main receptor involved in signaling leading to the induction of IFNγ secretion by T cells following infection with L. major, but that in absence of N1, higher levels of N2 can compensate its absence. Functional redundancy of N1 and N2 was previously suggested in N1IAS mice that had decreased but not abrogated IFNγ secretion [6], however the expression of Notch receptors was not assessed in that study. Of note, we show here that N2 is the only receptor that could functionally substitute for N1 in driving Th1 differentiation in vivo, and T cell expression of N3 or N4 were not detected in presence or absence of N1 and/or N2 in CD4+ T cells of L. major-infected mice. In addition, N1N2ΔCD4Cre mice do not control infection revealing that N3 and N4 are not functionally redundant in driving IFNγ secretion by CD4+ T cells. Overexpression of N3 intracellular domain (N3IC) in T cells was previously reported to induce IFNγ secretion in vitro following anti-CD3 activation, while overexpression of N1IC did not, suggesting that N3 could be involved in Th1 differentiation [7]. Together with our reported increased expression of N2 in absence of N1, these results show that individual Notch receptors may potentially drive IFNγ secretion by CD4+ T cells, but during L. major infection N1, and to a lesser extent N2 appear to be the only receptors involved in driving Th1 differentiation.
It was reported that N1 could regulate Th1 cell differentiation by interacting with CSL sequences present in the promoter of the Tbx21 gene which codes for T-bet, the master regulator of Th1 cell differentiation [6]. However, in another study, Notch was not found to reside at the Tbx21 promoter [21]. In addition, we show here that mice with specific ablation of RBP-Jκ in their T cells, unlike N1N2ΔCD4Cre mice, are able to mount a Th1 response and heal their lesion following infection with L. major. These results show that the Notch signaling playing a major role in the differentiation IFNγ-secreting cells following infection with L. major occurs in absence of a CSL/RBP-Jκ-transcription complex. In line with these data, it was previously reported that mice that conditionally expressed a dominant negative MAML protein (DNMAML) and thereby are deprived of RBP-Jκ-mediated transcriptional activation of all Notch receptors, were able to develop a protective Th1 immune response following L. major infection [10]. The present results show that Notch receptors are crucial to trigger secretion of IFNγ by CD4+ T cells in a CSL/RBP-Jκ-independent manner.
The nature of a CSL/RBP-Jκ-independent Notch pathway is complex and not yet defined [22]. It has been reported that Notch can associate with the nuclear factor κB (NF-κB) proteins p50 and p65. Furthermore, Notch1-NF-κB complexes could be immunoprecipitated from the Ifng promoter despite the lack of consensus binding sites for RBP-Jκ in the promoter of this gene [23]. This suggested that N1ICD could contribute to Th1 differentiation in a RBP-Jκ-independent way leading to the hypothesis of a connection between Notch, NF-κB and Th1 differentiation [1], [5]. Of note, NF-κB p50 is required for optimal Th1 development and L. major-infected NF-κB1 knockout mice show a susceptible phenotype associated with defective secretion of IFNγ [24]. However in that study, failure to secrete IFNγ was linked to a major defect in CD4+ T cell proliferation measured in vitro, while we did not detect such impairment of CD4+ T cell proliferation in Notch deficient CD4+ T cells. Thus Notch may interact with distinct transcription factors involved in the secretion of IFNγ by Th1 cells and further molecular studies will be needed to determine the nature of these factors as well as the molecular mechanisms involved in the RBP-Jκ-independent Notch signaling during Th1 differentiation.
Notch signaling is required for proper secretion of IFNγ by CD4+ Th1 cells following antigen-specific stimulation. Interestingly, increased expression of T-bet and IFNγ mRNA was measured in dLN CD4+ T cells of L. major-infected N1N2ΔCD4Cre mice revealing that Notch signaling does not prevent the differentiation of “competent” CD4+ Th1 cells [25], but appears to act downstream of it. The increase in T-bet and IFNγ mRNA measured in CD4+ N1N2ΔCD4Cre T cells suggests that intact Notch signaling regulates the extent transcription for these genes in vivo. Low levels of STAT1 phosphorylation in dLN CD4+ T cells confirmed that only very small amounts of IFNγ protein, maybe released by NK cells, are present in the dLN of L. major-infected N1N2ΔCD4Cre mice. In absence of IFNγ, mice on the resistant C57BL/6 genetic background develop a Th2 immune response, with high levels of IL-4, IL-5 and IL-13 cytokines [26]. Accordingly, impaired secretion of IFNγ by CD4+ T cells of L. major-infected N1N2ΔCD4Cre mice allowed the differentiation of IL-5 - and IL-13-secreting Th2 cells. However, no increased secretion of IL-4 was measured in CD4+ T cells of N1N2ΔCD4Cre L. major-infected mice, in line with the previously reported crucial importance of Notch in driving IL-4 secretion by CD4+ T cells [8], [21], [27]. Interestingly, absence of Notch did not impair the differentiation of other Th2 effector T cells, suggesting that following L. major infection, Notch is acting directly on the IL-4 promoter, as previously reported [8], and does not affect the differentiation of IL-13 - and IL-5-Th2 secreting cells.
Notch signaling is resulting from an interaction between Notch receptors and ligands on antigen presenting cells. Several ligands have been linked to Th1 differentiation in distinct experimental models of disease and Delta-like ligands have been linked to Th1 differentiation or impaired Th2 differentiation [8], [11], [12], [28], [29]. Dll1 stimulation was shown to trigger Th1 development following L. major infection, but it was not determined which Notch receptor was interacting with this ligand [7]. The present study shows that either N1 or N2 could be interacting with Dll1. Whether other Notch ligands are involved in Notch signaling during Leishmania infection remains to be investigated. Interestingly, it was reported recently that within the 6q27 gene cluster, the Dll1 gene was linked to susceptibility to visceral leishmaniasis, and reduced Dll1 expression was measured in VL patients in Sudan, Brazil, and Northen India [30]. Thus genetic regulation of one of the Notch ligand, such as the downregulation of Dll1 expression appears to have major consequences on susceptibility to VL. Together with the present study, it reveals that a proper regulation of the Notch signaling pathway during infection with Leishmania parasites is essential for the development of a protective response against these parasites.
Further understanding of the mechanisms by which Notch receptors regulate the differentiation of IFNγ-secreting Th1 cells as well as the ligands involved in this process should contribute to the development of new vaccines and immunotherapeutic targets towards Leishmania pathology, as well as in other infections requiring protective IFNγ-secreting CD4+ Th1 immune response.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the care and use of laboratory animals from the Department of security and Environment (DSE) from the state of Vaud, Switzerland. The protocol has been approved by the Ethics and Veterinary office regulations of the state of Vaud (SAV), Switzerland. Our laboratory has the administrative authorization numbers 1266-3, -4 and -5.
Mice
The following T cell specific gene-targeted mice were generated by crossing floxed Notch1 [31], floxed Notch2 [32], double floxed Notch1-Notch2 or floxed RBP-Jκ [33] mice, with mice carrying the CD4Cre transgene [34]. N1lox/lox, N2lox/lox, N1N2lox/lox and RBP-Jκlox/lox littermates were used as controls. All mice were on a C57BL/6 genetic background. T cell-specific deletion of Notch and RBP-Jκ was verified for each strain by PCR. All mice were bred and maintained under pathogen-free conditions in the animal facility at the CIIL, Epalinges, Switzerland.
Parasites and infections
Leishmania major LV39 (MRHO/Sv/59/P strain) was used. Mice were infected s.c. with 3×106 stationary phase L. major promastigotes in the footpad. Parasite load was assessed by limiting dilution analysis (LDA). Treatment with CNTO 134, a rat anti-mouse IL-13 mAb [35], a gift from Centocor, Inc, was initiated either six days or 21 days after infection, with injection of 500 µg i.p., once weekly in N1N2ΔCD4Cre mice, while a control group similarly infected was treated with control IgG or PBS. As no biological differences were observed between L. major-infected mice treated with control IgG or PBS, PBS was used as control vehicle for CNTO 134.
Lymphocyte culture and cytokine assays
Draining lymph node cells were cultured ± UV-irradiated L. major promastigotes or anti-CD3 (clone 145-2C11, eBioscience) for 72 h. CD4+ T cells were isolated by MACS (Miltenyi Biotec), and cultured in the presence of irradiated C57BL/6 splenocytes. For in vitro experiment, naïve CD4+CD62L+ T cells were isolated by MACS and cultured as previously described [9]. The cytokine content of the cell supernatant was measured by ELISAs. IFNγ with a limit of detection of 10 IU/ml. IL-4, IL-5 (OptEIA from BD Biosciences) and IL-13 (DuoSet from R&D Systems) cytokines were analyzed with commercial kits.
mRNA extraction and Real-Time PCR
Extraction of total RNA was performed as previously described [36]. Quantitative Real-Time PCRs were done using SYBR Green and a LightCycler system (Roche). Each cytokine mRNA was normalized to the relative hypoxanthine phosphoribosyltransferase (HPRT) endogenous mRNA expression, and represented as arbitrary units as described previously [36]. Primers used were previously described [36], [37], [38].
Flow cytometry
Draining lymph node cells were isolated 3 weeks after L. major infection. Phosphorylation of STAT1 at tyrosine 701 (pY701) was detected by intracellular staining using an Alexa Fluor 488 conjugated anti-Stat1, PhosFlow Fix Buffer I and Perm Buffer III (BD Biosciences) according to manufacturer's instructions. CD4-PE-Cy5 and CD44-APC (eBiosciences) were used to stain cell surface. To assess Notch receptor expression, dLN cells were isolated and restimulated with UV-irradiated L. major for 16 hours. Cells were stained with anti-N1, anti-N2 biotinylated mAbs [16], followed by Streptavidin-PE, -APC (eBiosciences), PE-conjugated anti-N3 and anti-N4 (Biolegends). CD4−CD8−CD25+ thymocytes were used as positive control for N3 staining, and splenic CD8α+CD11c+ dendritic cells were used as positive control for N4 staining. CD4+ T cells were gated using TCRβ-APC and CD4-FITC (eBiosciences) mAbs. Dead cells were excluded using 7AAD (BD Pharmingen). For T cell proliferation, dLN cells were isolated 6 weeks post L. major infection and stained with CFSE (Molecular Probes). Cells were then restimulated ± UV-irradiated L. major promastigotes for 72 h and analyzed by FACS. The following monoclonal Ab conjugates were used: CD4-PE-Cy5, CD8-APC, B220-Pe-TexasRed (eBioscience) and dead cells were excluded with DAPI. Intracellular IFNγ was analyzed in dLN cells isolated 6 weeks post infection and restimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) and BrefelinA (1 µg/ml) for 4 h. Cells were stained for surface marker with the following mAb conjugates: CD4-PE-Cy5, CD8-APC (eBiosciences). Intracellular IFNγ-PE was detected with an anti-IFNγ-PE (BD Pharmingen). All analyses were performed on a FACS Calibur or a LSR II (Becton Dickinson) flow cytometers and data processed with FlowJo (TreeStar).
Statistical analysis
Data were analyzed using the Student's t-test for unpaired data.
Supporting Information
Zdroje
1. RadtkeFFasnachtNMacdonaldHR 2010 Notch signaling in the immune system. Immunity 32 14 27
2. SandyARMaillardI 2009 Notch signaling in the hematopoietic system. Expert Opin Biol Ther 9 1383 1398
3. OsipoCGoldeTEOsborneBAMieleLA 2008 Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest 88 11 17
4. KopanRIlaganMX 2009 The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137 216 233
5. AmsenDAntovAFlavellRA 2009 The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol 9 116 124
6. MinterLMTurleyDMDasPShinHMJoshiI 2005 Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol 6 680 688
7. MaekawaYTsukumoSChibaSHiraiHHayashiY 2003 Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity 19 549 559
8. AmsenDBlanderJMLeeGRTanigakiKHonjoT 2004 Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117 515 526
9. Tacchini-CottierFAllenbachCOttenLARadtkeF 2004 Notch1 expression on T cells is not required for CD4+ T helper differentiation. Eur J Immunol 34 1588 1596
10. TuLFangTCArtisDShestovaOProssSE 2005 Notch signaling is an important regulator of type 2 immunity. J Exp Med 202 1037 1042
11. SkokosDNussenzweigMC 2007 CD8 − DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med 204 1525 1531
12. SunJKrawczykCJPearceEJ 2008 Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol 180 1655 1661
13. OngCTSedyJRMurphyKMKopanR 2008 Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS One 3 e2823
14. WorsleyAGLeibundGut-LandmannSSlackEPhngLKGerhardtH 2008 Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo. Eur J Immunol 38 1043 1049
15. SacksDNoben-TrauthN 2002 The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2 845 858
16. FioriniEMerckEWilsonAFerreroIJiangW 2009 Dynamic regulation of notch 1 and notch 2 surface expression during T cell development and activation revealed by novel monoclonal antibodies. J Immunol 183 7212 7222
17. MatthewsDJEmsonCLMcKenzieGJJolinHEBlackwellJM 2000 IL-13 is a susceptibility factor for Leishmania major infection. J Immunol 164 1458 1462
18. Perona-WrightGMohrsKMohrsM 2010 Sustained signaling by canonical helper T cell cytokines throughout the reactive lymph node. Nat Immunol 11 520 526
19. Revaz-BretonMRonetCIvesATorreYHMasinaS 2010 The MyD88 protein 88 pathway is differently involved in immune responses induced by distinct substrains of Leishmania major. Eur J Immunol 40 1697 1707
20. O'SheaJJPaulWE 2010 Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327 1098 1102
21. FangTCYashiro-OhtaniYDel BiancoCKnoblockDMBlacklowSC 2007 Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27 100 110
22. SanalkumarRDhaneshSBJamesJ 2010 Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol Life Sci 67 2957 2968
23. ShinHMMinterLMChoOHGottipatiSFauqAH 2006 Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J 25 129 138
24. ArtisDSpeirsKJoyceKGoldschmidtMCaamanoJ 2003 NF-kappa B1 is required for optimal CD4+ Th1 cell development and resistance to Leishmania major. J Immunol 170 1995 2003
25. MohrsKWakilAEKilleenNLocksleyRMMohrsM 2005 A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity 23 419 429
26. WangZEReinerSLZhengSDaltonDKLocksleyRM 1994 CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med 179 1367 1371
27. TanakaSTsukadaJSuzukiWHayashiKTanigakiK 2006 The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity 24 689 701
28. ElyamanWBradshawEMWangYOukkaMKivisakkP 2007 JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol 179 5990 5998
29. KrawczykCMSunJPearceEJ 2008 Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol 180 7931 7937
30. FakiolaMMillerENFadlMMohamedHSJamiesonSE 2011 Genetic and functional evidence implicating DLL1 as the gene that influences susceptibility to visceral leishmaniasis at chromosome 6q27. J Infect Dis 204 467 477
31. RadtkeFWilsonAStarkGBauerMvan MeerwijkJ 1999 Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10 547 558
32. BesseyriasVFioriniEStroblLJZimber-StroblUDumortierA 2007 Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med 204 331 343
33. TanigakiKHanHYamamotoNTashiroKIkegawaM 2002 Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 3 443 450
34. LeePPFitzpatrickDRBeardCJessupHKLeharS 2001 A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15 763 774
35. YangGLiLVolkAEmmellEPetleyT 2005 Therapeutic dosing with anti-interleukin-13 monoclonal antibody inhibits asthma progression in mice. J Pharmacol Exp Ther 313 8 15
36. OttenLATacchini-CottierFLohoffMAnnunziatoFCosmiL 2003 Deregulated MHC class II transactivator expression leads to a strong Th2 bias in CD4+ T lymphocytes. J Immunol 170 1150 1157
37. CharmoyMMegnekouRAllenbachCZweifelCPerezC 2007 Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol 82 288 299
38. MenziesFMHenriquezFLAlexanderJRobertsCW 2010 Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol 160 369 379
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



