-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships with Pneumococcal Colonization
Regulatory T cells (Treg) diminish immune responses to microbial infection, which may contribute to preventing inflammation-related local tissue damage and autoimmunity but may also contribute to chronicity of infection. Nasopharyngeal carriage of pneumococcus is common in young children and can persist for long periods but it is unknown whether the presence of Treg in the nasopharynx contributes to this persistence. We have investigated the numbers and activities of Foxp3+Treg in adenoidal tissues and their association with pneumococcal carriage in children. Expression of Treg cell-related markers including Foxp3, CD25, CD39, CD127 and CLTA4 were analysed by flow-cytometry in adenoidal mononuclear cells (MNC) and PBMC from children. Unfractionated MNC or Treg-depleted MNC were stimulated with a pneumococcal whole cell antigen (WCA) and T cell proliferation measured. Cytokine production by MNC was measured using a cytometric bead array. Higher numbers of CD25highFoxp3high Treg expressing higher CD39 and CTLA4 were found in adenoidal MNC than in PBMC. Children with pneumococcus positive nasopharyngeal cultures had higher proportions of Treg and expressed higher levels of CD39 and CTLA-4 than those who were culture negative (−). WCA induced adenoidal Treg proliferation which produce IL10 but not IL17, and CD4 T cell proliferation in Treg-depleted MNC was greater in pneumococcal culture positive than negative children. Significant numbers of Treg with an effector/memory phenotype which possess a potent inhibitory effect, exist in adenoidal tissue. The association of pneumococcal carriage with an increased frequency of adenoidal Treg suggests that Treg in nasal-associated lymphoid tissue (NALT) may contribute to the persistence of pneumococcus in children. Further studies to determine what component and mechanisms are involved in the promotion of Treg in NALT may lead to novel therapeutic or vaccination strategy against upper respiratory infection.
Published in the journal: . PLoS Pathog 7(8): e32767. doi:10.1371/journal.ppat.1002175
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002175Summary
Regulatory T cells (Treg) diminish immune responses to microbial infection, which may contribute to preventing inflammation-related local tissue damage and autoimmunity but may also contribute to chronicity of infection. Nasopharyngeal carriage of pneumococcus is common in young children and can persist for long periods but it is unknown whether the presence of Treg in the nasopharynx contributes to this persistence. We have investigated the numbers and activities of Foxp3+Treg in adenoidal tissues and their association with pneumococcal carriage in children. Expression of Treg cell-related markers including Foxp3, CD25, CD39, CD127 and CLTA4 were analysed by flow-cytometry in adenoidal mononuclear cells (MNC) and PBMC from children. Unfractionated MNC or Treg-depleted MNC were stimulated with a pneumococcal whole cell antigen (WCA) and T cell proliferation measured. Cytokine production by MNC was measured using a cytometric bead array. Higher numbers of CD25highFoxp3high Treg expressing higher CD39 and CTLA4 were found in adenoidal MNC than in PBMC. Children with pneumococcus positive nasopharyngeal cultures had higher proportions of Treg and expressed higher levels of CD39 and CTLA-4 than those who were culture negative (−). WCA induced adenoidal Treg proliferation which produce IL10 but not IL17, and CD4 T cell proliferation in Treg-depleted MNC was greater in pneumococcal culture positive than negative children. Significant numbers of Treg with an effector/memory phenotype which possess a potent inhibitory effect, exist in adenoidal tissue. The association of pneumococcal carriage with an increased frequency of adenoidal Treg suggests that Treg in nasal-associated lymphoid tissue (NALT) may contribute to the persistence of pneumococcus in children. Further studies to determine what component and mechanisms are involved in the promotion of Treg in NALT may lead to novel therapeutic or vaccination strategy against upper respiratory infection.
Introduction
Regulatory T cells (Treg) play a key role in the control of various aspects of the immune response including maintenance of immune tolerance and prevention of autoimmunity [1]. Progress has been made in recent years in the characterization of regulatory cells, including Foxp3+ Treg. Until recently, the expression of the transcription factor Foxp3+ on CD4 T cells was believed to indicate thymus-derived natural Treg. However, there is mounting evidence that Foxp3+ Treg also develop extrathymically, i.e. adaptive Treg [2]. Studies in vitro show conversion of naïve T CD4+CD25 − T cells into Foxp3+ Treg through TCR ligation in the presence of TGF-β [3].
Up until now, intracellular expression of Foxp3 is still considered the most specific single marker of Treg, although a combination of phenotypic expression of CD4+CD25+CD127low has also been established as a useful marker for natural Treg [4], [5]. Some phenotypic markers such as CD39 and CTLA-4 have been found to be associated with the activity of Treg [6]–[9]. In particular, CD39 expression on Treg has been found to be correlated with the inhibitory potency of Treg, and in humans it is considered to be a marker of effector/memory Tregs [10].
Recently, a growing number of studies suggest that Treg play an important role in the control of immunity to microbial pathogens including bacteria, viruses and parasites [11]. The repertoire of antigen specificities of Treg is considered to be broad, recognizing both self and non-self antigens. It has been suggested that Treg can be activated and expanded against a wide range of different pathogens in vivo. Such pathogen-specific Treg may prevent the infection-induced immunopathology but may prolong pathogen persistence by inhibiting protective immunity favoring chronicity of infections [12]. For example, Treg may contribute to the immunopathogenesis of chronic infections including Human Immunodeficiency virus (HIV), Hepatitis C virus (HCV) and Tuberculosis (TB) [13]–[15]. Mucosal Treg have been shown to be important in the modulation of gastrointestinal tract inflammation such as that related to Helicobacter pylori and HIV infection [12], [16]. However, data on mucosal Treg in the respiratory tract infections in humans are limited.
Streptococcus pneumoniae (pneumococcus) is a leading cause of bacterial pneumonia, meningitis and septicemia, and kills millions of people each year worldwide, especially children. Nasopharyngeal colonization with pneumococcus is common in young children, as are mucosal pneumococcal infections such as otitis media and pneumonia. By the age of three years, most children develop natural T - and B-cell specific immune responses to several pneumococcal protein antigens [17], [18] presumably due to previous colonization. These responses may protect against pneumococcal carriage either by preventing acquisition or hastening clearance or both and are induced in “nasal-associated lymphoid tissue”(NALT) [19], [20]. Nevertheless, pneumococcal nasopharyngeal carriage may be prolonged and may recur throughout life. Therefore we hypothesized that significant Treg activity in the nasopharynx may contribute to the persistence of pneumococcus and perhaps, recurrent infection in some cases, in children in the face of demonstrable mucosal specific immunity.
Establishment of chronic intracellular infections such as HIV and tuberculosis is associated with attenuated cell-mediated immunity [21], [22]. Pneumococcus is classically considered to be an extracellular bacterium against which antibody responses play a primary role in protection. However, recent studies in mice suggest that CD4+T cell-mediated immunity to pneumococcal protein antigens may play a major role in either preventing or reducing the duration of pneumococcal mucosal colonization [23]–[25]. Our own results also suggest that naturally developed CD4 T cell-mediated immunity to pneumolysin, an intracellular pneumococcal antigen released on autolysis, may be protective against pneumococcal carriage in children [18].
No previous data are available on the role of Treg in the regulation of CD4 T cell immunity to pneumococcus or other upper respiratory tract-colonising bacteria in humans. Understanding the regulation of naturally-acquired mucosal immunity should help inform the design of novel vaccination strategies against pneumococcal colonization and/or infection. In this study, we investigated both the association between numbers and activities of Treg in adenoids and nasopharyngeal carriage of pneumococcus in children, and the effects of Treg on CD4+ T cell responses induced by pneumococcus in vitro.
Materials and Methods
Patients and samples
Adenoidal tissues and peripheral blood samples were obtained from children (aged 3–6 years) undergoing adenoidectomy. Patients who received antibiotics or systemic steroids within 3 weeks of surgery or who had any known immunodeficiency were excluded from the study. A nasopharyngeal (NP) swab was taken on the day that the operation was performed which was stored and cultured for pneumococcus as described previously [18].
Ethics statement
The study was approved by the local Research Ethics Committees (Liverpool Paediatric Research Ethics Committee and South Bristol local research ethics committee) and written informed consent was obtained from parents or carers in all cases.
Cell separation and culture
Adenoidal mononuclear cells (MNC) and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation (GE Healthcare). Depletion of CD25+ cells was performed using MACS magnetic microbeads separation (Miltenyi). To ensure purity, CD25+ cell-depleted cells were passed through a second column and purity was confirmed by CD4/CD25/CD127 and Foxp3 staining (both <1% positive in CD4 T cells by flow-cytometry). Adenoidal MNC, Treg-depleted MNC or PBMC were cultured in RPMI medium (containing penicillin, streptomycin and glutamine) with or without the addition of an ethanol-killed unencapsulated pneumococcal whole cell antigen (WCA) at 106 or 107 colony-forming unit (CFU) equivalents [26] for up to 7 days. The WCA was derived from strain Rx1AL-, a capsule - and autolysin-negative mutant described previously [26]. Briefly, the organism was grown to mid-log phase in Todd-Hewitt Broth supplemented with yeast extract, killed by the addition of 70% ethanol, after which the cell pellet was harvested and resuspended. A pneumolysin-negative WCA was made in the same fashion, using an Rx1AL - strain in which the pneumolysin gene was replaced by the Janus cassette, as previously described [27]. In some experiment, WCA was treated with proteinase K (200 ug/ml) for 1 hour at 37°C followed by heating (98°C, 30 min) before cell stimulation.
In some experiments, Treg were purified using magnetic microbeads (MACS, Miltenyi) following manufacturer's instructions. Briefly, CD4+ T cells were purified from adenoidal MNC using negative selection followed by positive selection of CD25+ cells. To ensure CD25high T cell separation, the amount of anti-CD25 antibody-labelled microbead was titrated, and the optimal quantity was used. Also, to ensure purity, cells were passed through magnetic columns twice for each separation. Purity (>96%) of isolated Treg was confirmed by flow-cytometry following Foxp3 staining.
Flow-cytometry
Multi-color flow-cytometry was performed to analyze the phenotypic expressions of different cell subsets. Cells were stained with fluorescence-labeled mouse anti-human antibodies to CD4, CD25 and CD127, CD39, CD69, CD45RO following standard procedures and analyzed on a FACSCalibur (BD Bioscience). Intracellular expressions of Foxp3 and CTLA-4 (CD152) were analysed by flow-cytometry following cell permeabilization (eBioscience).
Carboxyfluorescein diacetate 5,6 succinimidyl ester (CFSE)(Molecular Probes) was used to label adenoidal MNC or PBMC before cell culture, allowing for tracking of cell division after stimulation as described previously [18], [28]. Percentage of proliferative T cell subpopulations including Foxp3+ subsets was analyzed by CFSE and T cell marker staining followed by flow-cytometry.
Measurement of cytokine production
Following cell culture and stimulation by antigens, cell culture supernatants were collected and analysed for production of IL2, IL4, IL5, IL10, IFNγ and TNFα using a cytometric bead array (BD Bioscience), and for IL17 using ELISA (R&D Systems) following manufacturers' instructions. Intracellular cytokine staining was performed in some experiments to determine the cellular sources of cytokine production after stimulation by WCA as described previously [29]. Briefly, adenoidal MNC were co-cultured with WCA for 6 hours and together with brefeldin A (eBioscience) in the last 4 hours. Cells were stained with fluorescence-labeled anti-human CD4, followed by fixation, permeabilization and staining with fluorescence-labeled Foxp3, anti-IL10 or anti-IL17 (BD Biosience), and were then analyzed by flow cytometry.
Statistical analysis
Two-group comparisons were analyzed using Student's t test, and multiple group comparisons by ANOVA. Correlation was analysed by Pearson's correlation. Analysis was performed using SPSS software (SPSS version 16, SPSS Inc).
Results
Numbers of Treg and their expression of activation markers in adenoidal tissue
The numbers of Treg (as percentage of CD4 T cells) in both PBMC and adenoidal MNC were counted by staining for intracellular Foxp3 and/or CD4, CD25 and CD127. The use of CD4+CD25+ CD127low as a phenotype for Treg correlated well with intracellular Foxp3 expression, both measured by flow-cytometry, in both PBMC and adenoidal MNC (r = 0.91 and 0.90 respectively, n = 12, p<0.01). The proportion of Foxp3+Treg in CD4+ T cells was higher in adenoidal MNC than PBMC (Table 1) and the percentages of adenoidal Treg expressing CD45RO or CD69 were also higher, in both cases, than in PBMC (both p<0.01, Table 1). Similarly, levels of CD39 and intracellular CTLA-4 expression were higher in adenoidal than in PBMC Treg (Fig. 1A–C).
Fig. 1. Expression of CD39 and CTLA4 by Foxp3+ Treg cells. 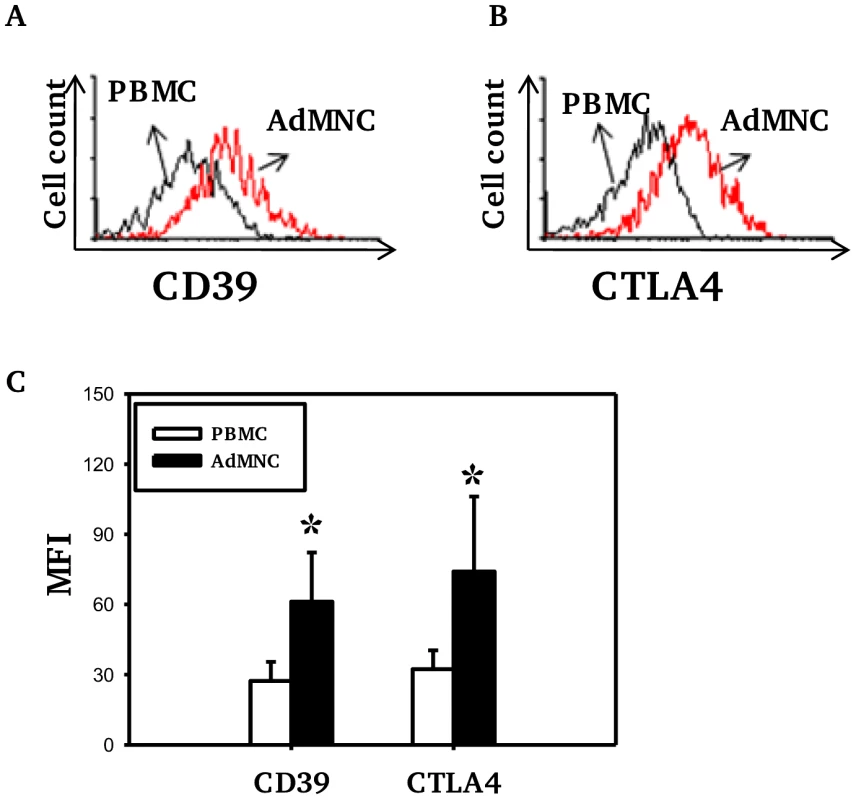
Fluorescence histograms showing CD39 (A) and CTLA4 (B) expression by Foxp3+ Treg cells in PBMC (black) and adenoidal MNC (AdMNC) (red) from one representative of 20 replicate experiments summarized in panel C. (*p<0.01 compared to PBMC). Tab. 1. Proportion of Treg in CD4+T cells and percentages of Treg expressing CD45RO and CD69 in PBMC and adenoidal MNC. 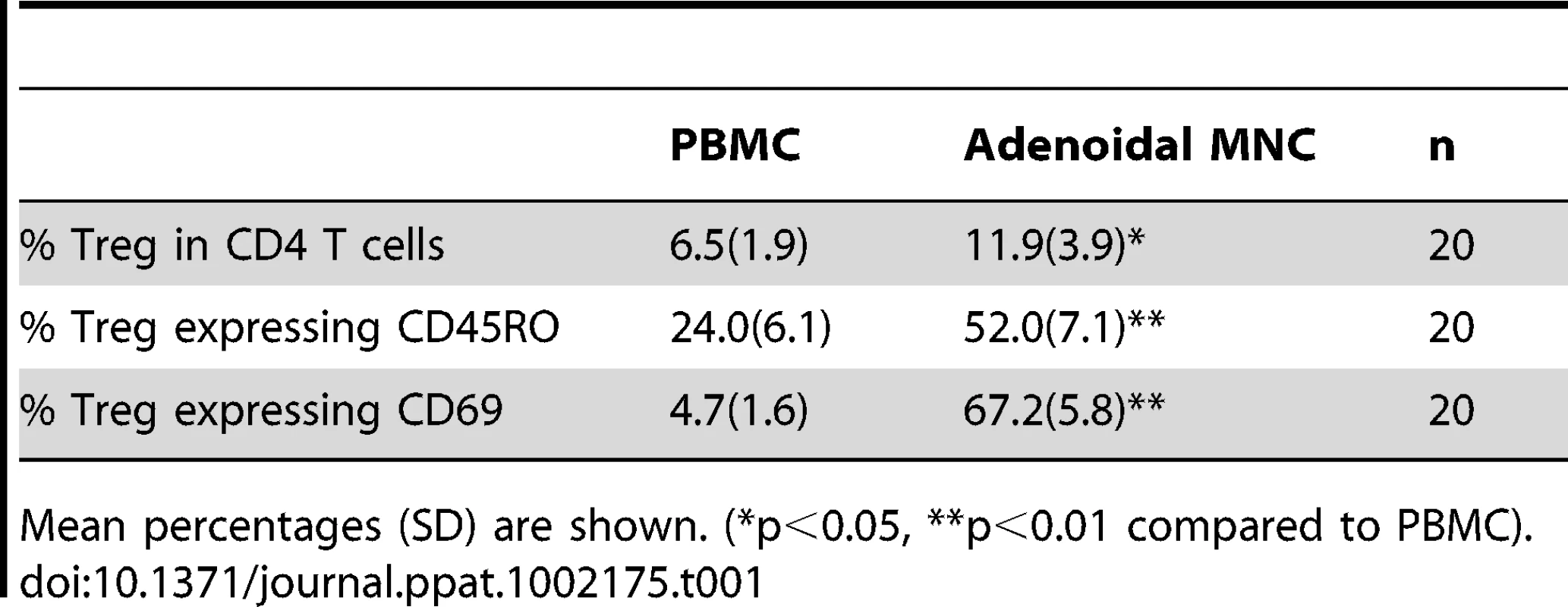
Mean percentages (SD) are shown. (*p<0.05, **p<0.01 compared to PBMC). Higher levels of CD25 expression were observed in adenoidal Treg than in PBMC when paired samples from each individual were analyzed (data not shown, p<0.01). Co-staining for CD25 and Foxp3 showed significant numbers of Treg with a CD25highFoxp3high phenotype in adenoidal MNC which were lacking in PBMC (Fig. 2A+B, region 3 (R3)). This phenotype of Treg exhibited higher levels of expression of both CD39 and CTLA-4 than CD25intermediateFoxp3intermediate cells (Fig. 2C). In comparison with R3, R4 region (CD25intermediateFoxp3low) which represents activated CD4+ T cells, showed higher CTLA-4 but lower CD39 levels of expression (Fig. 2C).
Fig. 2. Expression of CD25, Foxp3, CD39 and CTLA4 by CD4 T cell subsets. 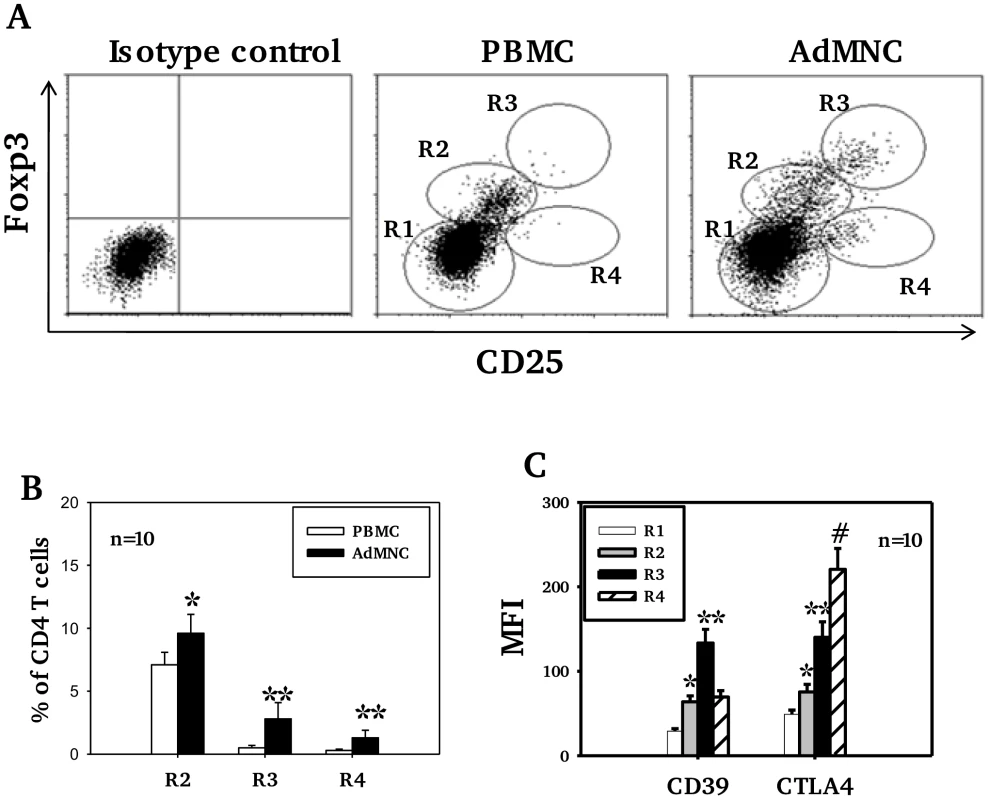
In Fig A, R1 represents CD25− Foxp3− (non-Treg) CD4 T cells, defined as those with fluorescence comparable to isotype control staining. R2, R3 and R4 regions represent 3 distinctive cell populations shown in adenoidal MNC (ADMNC) with fluorescence above the top of fluorescence of isotype staining for Foxp3 and/or CD25. R3 represents the cell population with the highest fluorescence for both staining, defined as CD25highFoxp3high Treg. R2 represents those cells with intermediate fluorescence (between R1 and R3) designated as CD25intermediateFoxp3intermediate Treg. R4 represents the CD25+Foxp3low cell population defined as activated CD4 T cells. One of 10 representative samples is shown in A. Fig B shows percentage of Foxp3+ Treg in PBMC and adenoidal MNC (*p<0.05, **p<0.01 compared to PBMC), and Fig C shows their expression of CD39 and CTLA4 (*p<0.01 compared to R1, **p<0.01 compared to R2 and R4, #p<0.01 compared to R3). Adenoidal Treg in children with pneumococcal colonization
Children who had positive nasopharyngeal cultures for pneumococcus (+) had higher proportions of Treg (Foxp3+ and/or CD4/CD25/CD127low) among CD4+T cells in adenoidal MNC than those who were culture negative (−) (Fig. 3A, p<0.05) but no such difference in Treg numbers was seen in PBMC (Fig. 3A, p>0.05). The adenoidal Treg from culture-positive children also expressed higher levels of CD39 and intracellular CTLA-4, again with no such differences seen in PBMC (Fig. 3B and C).
Fig. 3. Association of pneumococcal carriage and Treg cells in adenoidal MNC. 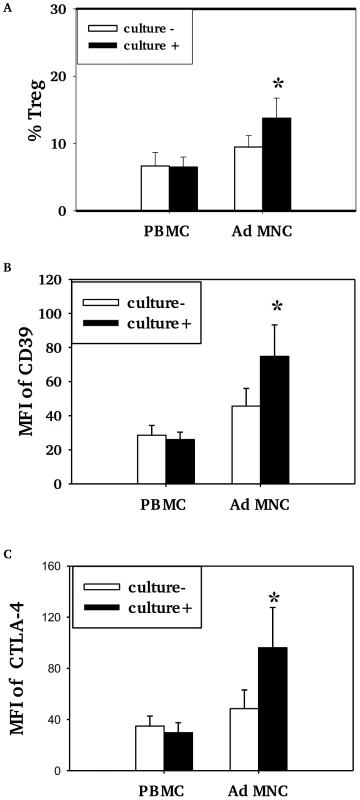
Percentages of CD4+Tcells which were Foxp3+ Treg (%Treg) (A) and their expression of CD39 (B) and intracellular CTLA4 (C) in PBMC and adenoidal MNC (AdMNC) in children who were culture positive (+) and negative (−) for pneumococcus. MFI – median fluorescence intensity. (*p<0.05 compared to culture negative children.) Culture+, n = 8; culture−, n = 12. Pneumococcal WCA stimulation induces proliferation of Treg
Pneumococcal WCA stimulation of adenoidal MNC induces a dose-dependent increase in the numbers of Foxp3+ Treg compared to unstimulated control cells (Fig. 4A, p<0.01). Pre-treatment of pneumococcal WCA with proteinase K followed by heating significantly reduced this effect (Fig. 4A), suggesting that WCA-induced increase in Treg is protein-dependent. The mean % increase in the Treg (of CD4 T cells) after WCA stimulation is significantly higher in adenoidal MNC from pneumococcal culture+ children than from culture − children (Fig. 4B, p<0.01). A small increase was also observed in PBMC after stimulation with WCA (data not shown). Measurement of CD4+T cell proliferation using CFSE confirmed that stimulation with WCA induces both proliferation of Foxp3+ Treg and Foxp3 − effector CD4+T cells in adenoidal MNC (Fig. 4C).
Fig. 4. Pneumococcal WCA stimulation induces proliferation of Treg. 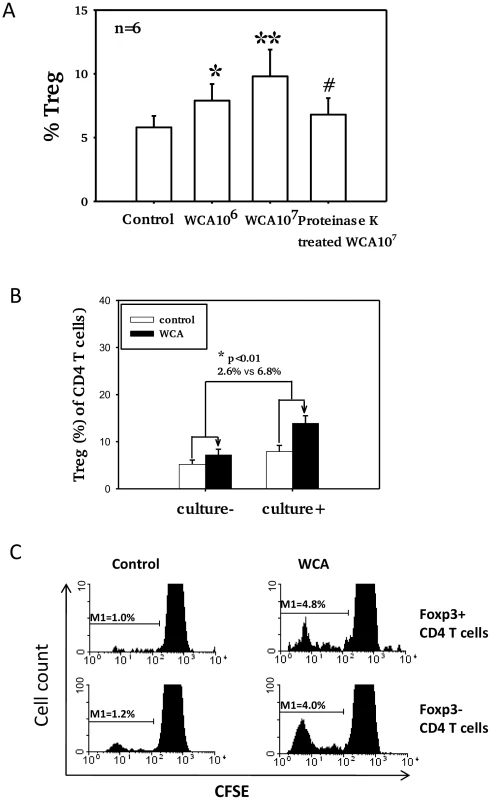
A). Proportions of Foxp3+ Treg in adenoidal CD4+T cells after pneumococcal WCA stimulation at 106 and 107 (CFU equivalents) for 5 days. *p<0.0 1, **p<0.001 compared to control. #p<0.01 compared to WCA 107 CFU equivalents. #p<0.01 compared to WCA 107 CFU equivalents B). Mean % increase in Treg (of CD4+ T cells) after WCA stimulation (WCA 107 CFU equivalents) in adenoidal MNC from pneumococcal culture+ (n = 6) and culture− children (n = 6)(p<0.01). C). CFSE staining shows proliferation of both Foxp3+ (Treg) and Foxp3− CD4+ T cells in adenoidal MNC following WCA stimulation. Result of one representative experiment of 6 replicates is shown. Adenoidal Treg exhibit strong inhibition of CD4+ T cell responses to WCA
To assess inhibition of T cell proliferation by adenoidal Treg, CD25+ cell-depleted adenoidal MNC were analyzed for CD4+ T cell proliferation following stimulation with pneumococcal WCA. WCA induced significantly higher CD4+ T cell proliferation in CD25+ cell-depleted MNC in those children who were pneumococcal culture positive than in those who were culture negative (Fig. 5a). The WCA-induced CD4+ T cell proliferation in CD25+cell-depleted MNC was suppressed when purified Treg (CD4+CD25+) were added back to the CD25+ cell-depleted MNC (Fig. 5B, p<0.01).
Fig. 5. Adenoidal Treg exhibit strong inhibition of CD4+ T cell responses to WCA. 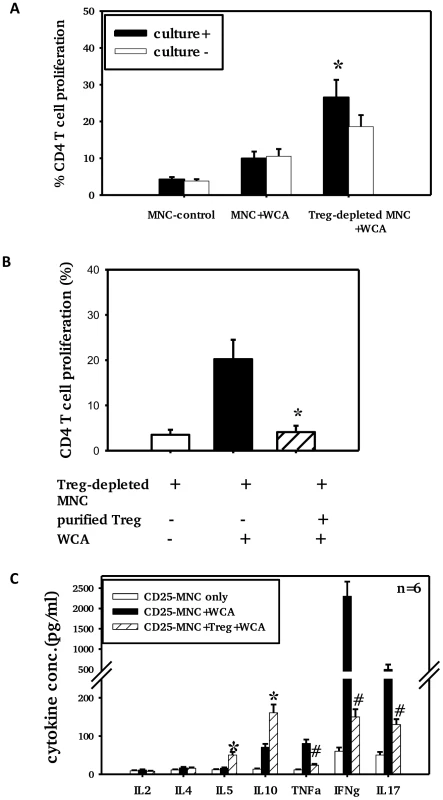
CD4+ T cell proliferation (A+B) and cytokine production (C) in the presence or absence of Treg after stimulation with pneumococcal WCA. A). % CD4+ T cell proliferation in adenoidal MNC and Treg-depleted MNC from pneumococcal culture+ (n = 6) and culture− children (n = 6) (*p<0.01 compared to culture− group). B). CD4+ T cell proliferation induced by WCA in Treg-depleted MNC suppressed by the repletion of purified Treg (*p<0.01 compared to Treg-depleted MNC+WCA, n = 4. C). *p<0.01, #p<0.01 compared to Treg-depleted MNC+WCA. Depletion of Treg from adenoidal MNC resulted in a significant decrease in IL10 and IL5 but an increase in IL17, IFNγ and TNFα concentrations in the cell culture supernatant after WCA stimulation (data not shown). The increase in concentrations of IL17, IFNγ and TNFα in Treg-depleted adenoidal MNC following WCA stimulation was inhibited when purified Treg were reintroduced, which also restored the IL10 and IL5 production (Fig. 5C). Intracellular cytokine staining shows that Foxp3+ Treg in adenoidal CD4+ T cells secrete IL10 but not IL17 following stimulation by WCA (Fig. 6).
Fig. 6. Cytokine expression by Foxp3+ Treg in adenoidal cells. 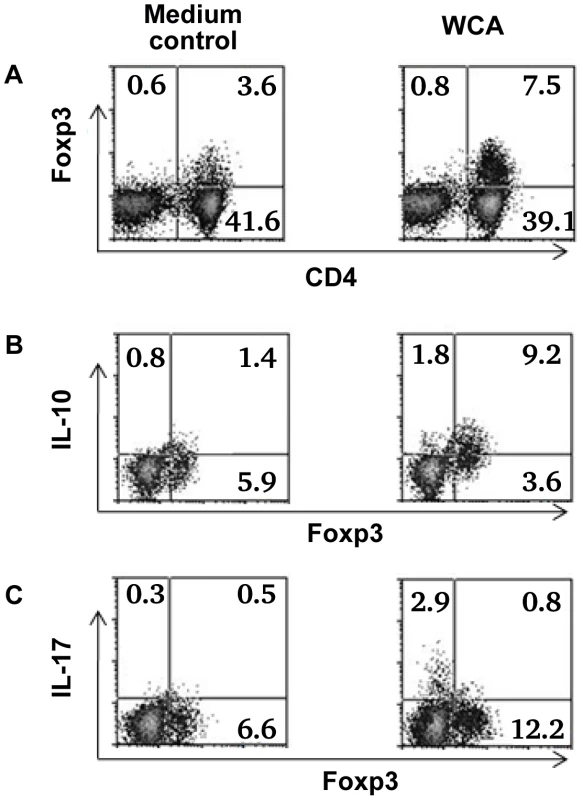
Percentage of Foxp3+ Treg in adenoidal MNC after stimulation with WCA compared with unstimulated medium control (A). Intracellular cytokine staining shows IL-10 (B) and IL-17 (C) production by Foxp3+ Treg and Foxp3− CD4+ T cells after WCA stimulation compared with unstimulated control. Only CD4+ T cell gate is shown in B and C. Result of one representative experiment of four replicates is shown. Discussion
Recent studies have suggested that human Treg are functionally and phenotypically diverse. Foxp3+ Treg can be divided into naïve and effector/memory phenotypes according to the expression of CD45RA or RO, CD69 and CD25 [30]. Naïve Treg are characterized by their expression of CD45RA and low levels of Foxp3, and effector/memory Treg by expression of CD45RO and Foxp3high [30], [31]. Although data in humans are lacking, animal studies suggest that CD45RA+Foxp3low Treg are thymus-derived natural Treg [30], [31] which is supported by the finding that almost all Foxp3+ CD4 T cells found in human cord blood are CD45RA+Foxp3low T cells [32], [33]. In this study, we found that the majority of Treg in PBMC in children express CD45RA (i.e. not expressing CD45RO), low levels of Foxp3, and do not express CD69 (Table 1). Thus these Treg are likely to be thymus-derived naïve Treg. In contrast, over 50% Treg in adenoidal tissues express CD45RO and CD69, and among them, a significant proportion expressing Foxp3highCD25high. Together with the high levels of CTLA-4 and CD39 expression (Fig. 1), these Foxp3high CD25highTreg are likely to be of the effector/memory phenotype with potent suppressive properties [30], [34].
CD4+ T cells with the highest expression of CD25 were found to have the strongest suppressive activity, and thus CD25high was used as a marker of Treg [34], However, in humans, levels of CD25 expression tend to show a continuous distribution and there is no consensus as to where the boundary lies between CD25high and CD25low to intermediate expression. In this study, with a combination of staining of Foxp3, CD4 and CD25, we demonstrate that Foxp3+ cells can be divided into two populations, Foxp3highCD25high and Foxp3intermediateCD25intermediate (Fig. 1A, region 3 and 2 respectively). Significant numbers of the Foxp3+ Treg in adenoids are of the former phenotype and express high levels of CD39, CTLA-4 and CD69. These results suggest that in human NALT there is a pool of effector/memory phenotype of Treg with potent suppressive function. These CD69+ Foxp3highCD25high effector Treg differ phenotypically from the activated (non-Treg) CD4+ T cells which express intermediate to high levels of CD25 but low levels of Foxp3 and marked levels of CTLA-4 (Fig. 2A and C, region 4).
We demonstrate here that the proportion of Foxp3+ Treg, especially those of effector/memory phenotype, in adenoidal tissues is significantly higher than in peripheral blood. High numbers of Treg in NALT tissues could be induced by local colonization with microbes or antigens, as there is mounting evidence suggesting that the induction of Foxp3+ Treg occurs in peripheral tissues in humans. HIV, TB and Leishmania infections promote pathogen-specific Treg in the local inflammatory site including lymphoid tissues [35]–[38]. It has been postulated that the presence of microbial pathogens in peripheral tissues could lead to the accumulation of activated Treg (both natural and inducible) that help maintain host immune homeostasis [2], [35], [39].
In this study, we show that the proportion of adenoidal CD4+T cells that are Treg in adenoids, but not in peripheral blood, was significantly higher in children who were culture positive for pneumococcus in their nasopharynges than in those who were culture negative. This is the first report showing evidence of such an association for any extracellular pathogen in the human nasopharynx. This suggests that pneumococcal colonization in the nasopharynx may contribute to the induction and/or promotion of adaptive Treg in adenoids, and that these Treg may contribute to the delayed clearance or persistence of pneumococcal carriage in children. We also show that in vitro stimulation with a pneumococcal whole cell antigen (WCA) can induce an increase in the numbers of Treg in adenoidal MNC, and the increase is significantly higher in those children who are culture positive for pneumococcus. This would be consistent with the hypothesis that local colonization with pneumococcus promotes antigen-specific Treg in vivo in local lymphoid tissues adjacent to the site of colonization, which proliferate upon pneumococcal WCA stimulation.
To determine whether these Treg in adenoidal tissues are functional and induce antigen-specific inhibition of T cell responses, we compared the WCA-induced effector CD4 T cell proliferation in the presence or absence of Treg. We show that depletion of adenoidal Treg leads to marked increase in WCA-induced CD4+ T cell proliferation and proinflammatory cytokines including IL17, TNFα and IFNγ and replacement of the Treg abolished such effects. We also show that the increase in CD4+ T cell proliferation was significantly higher in children who were culture positive for pneumococcus than those who were culture negative. These results are consistent with the hypothesis that the adenoidal Treg are potent inhibitors and have antigen-specific inhibitory effects on CD4 T cell responses.
Recent data in mice suggest that Th17 cells which secrete IL17 may play a critical role in protection against nasopharyngeal carriage of pneumococcus through promoting neutrophil-mediated phagocytic killing [40]. It is now well recognized that Treg and Th17 are two T cell subsets with opposing actions and interplay in the regulation of inflammation and autoimmunity [41]. This is supported by our results here that adenoidal Treg secrete the inhibitory cytokine IL10 but not IL17 (Fig. 6). The results in this study suggest a potent inhibitory effect on Th17 cells by adenoidal Treg which would be consistent with a role of Treg in the persistence of pneumococcal carriage in children.
It is unclear what component of pneumococcus may contribute to the promotion of Treg in adenoids. Proteinase treatment of WCA reduced the inhibitory effect on WCA-induced CD4+ T cell proliferation, suggesting that pneumococcal protein(s) may contribute to the accumulation of adenoidal Treg. No difference was shown in the increased frequency of Treg induced by the wild-type WCA and an isogenic pneumolysin-negative WCA (data not shown), which suggests pneumolysin may not contribute significantly in this respect.
It has been reported previously that Treg numbers at the mucosal site, tonsil and lymph node, were highly increased in untreated patients with HIV infection, whereas in contrast, the Treg numbers in peripheral blood of these patients were not increased compared to healthy controls [12], [35], [42]. Taken together, these results support the hypothesis that chronic infection or persistent antigen promotes the expansion and activation of antigen-specific Treg in the local tissues [43].
In conclusion, significant numbers of Treg exist in adenoids of children with an effector/memory phenotype which possess potent inhibitory effect on CD4 T cell proliferation. The association of pneumococcal carriage in the nasopharynx with an increased frequency of Treg in adenoids suggests that local colonization with pneumococcus promotes pathogen-specific Treg of the effector/memory phenotype which may contribute to the delayed clearance or persistence of pneumococcus in children. Further studies to determine which pneumococcal components and what mechanisms are involved in the promotion of antigen-specific Treg in the nasopharynx may lead to novel therapeutic or vaccination strategies against upper respiratory colonization and/or infection.
Zdroje
1. SakaguchiSSakaguchiS 2004 Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22 531 562
2. BelkaidYBelkaidY 2008 Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol 38 918 921
3. ChenWJinWHardegenNLeiKJLiL 2003 Conversion of peripheral CD4+CD25 − naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198 1875 1886
4. SeddikiNSantner-NananBMartinsonJZaundersJSassonS 2006 Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203 1693 1700
5. LiuWPutnamALXu-YuZSzotGLLeeMR 2006 CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203 1701 1711
6. FletcherJMLonerganRCostelloeLKinsellaKMoranB 2009 CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 183 7602 7610
7. IzcueACoombesJLPowrieFIzcueACoombesJL 2006 Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev 212 256 271
8. ZhengYManzottiCNBurkeFDussablyLQureshiO 2008 Acquisition of suppressive function by activated human CD4+ CD25 − T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol 181 1683 1691
9. WingKOnishiYPrieto-MartinPYamaguchiTMiyaraM 2008 CTLA-4 control over Foxp3+ regulatory T cell function. Science 322 271 275
10. BorsellinoGKleinewietfeldMDi MitriDSternjakADiamantiniA 2007 Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110 1225 1232
11. RouseBTSarangiPPSuvasSRouseBTSarangiPP 2006 Regulatory T cells in virus infections. Immunol Rev 212 272 286
12. EppleHJLoddenkemperCKunkelDTrogerHMaulJ 2006 Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 108 3072 3078
13. KinterALHennesseyMBellAKernSLinY 2004 CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med 200 331 343
14. BolacchiFSinistroACiapriniCDeminFCapozziM 2006 Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin & Exp Immunol 144 188 196
15. Guyot-RevolVInnesJAHackforthSHinksTLalvaniA 2006 Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 173 803 810
16. RaghavanSHolmgrenJRaghavanSHolmgrenJ 2005 CD4+CD25+ suppressor T cells regulate pathogen induced inflammation and disease. FEMS Immunol & Med Microbiol 44 121 127
17. ZhangQBernatonieneJBagradeLPollardAJMitchellTJ 2006 Serum and mucosal antibody responses to pneumococcal protein antigens in children: relationships with carriage status. Eur J Immunol 36 46 57
18. ZhangQBernatonieneJBagradeLClarkeEPatonJC 2007 Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis 195 1194 1202
19. BrandtzaegP 2003 Immunology of tonsils and adenoids: everything the ENT surgeon needs to know. Int J Pediatr Otorhinolaryngol 67 Suppl 1 S69 S76
20. KiyonoHFukuyamaSKiyonoHFukuyamaS 2004 NALT - versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol 4 699 710
21. McMichaelAJRowland-JonesS 2001 Cellular immune responses to HIV. Nature 410 980 987
22. CooperAMCooperAM 2009 Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27 393 422
23. MalleyRTrzcinskiKSrivastavaAThompsonCMAndersonPW 2005 CD4 T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA 102 4848 4853
24. McCoolTLWeiserJN 2004 Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun 72 5807 5813
25. KadiogluACowardWColstonMJHewittCRAndrewPW 2004 CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun 72 2689 2697
26. MalleyRLipsitchMStackASaladinoRFleisherG 2001 Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun 69 4870 4873
27. SungCKLiHClaverysJPMorrisonDASungCK 2001 An rpsL cassette, janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl & Environ Microbiol 67 5190 5196
28. ZhangQBagradeLClarkeEPatonJCNunezDA 2010 Bacterial lipoproteins differentially regulate human primary and memory CD4+ T and B cell responses to pneumococcal protein antigens through Toll-like receptor 2. J Infect Dis 201 1753 1763
29. ZhangQBernatonieneJBagradeLPatonJCMitchellTJ 2006 Regulation of mucosal antibody production to pneumococcal protein antigens by T-cell derived IFNg and IL-10 in children. Infect Immun 74 4735 4743
30. SakaguchiSMiyaraMCostantinoCMHaflerDASakaguchiS 2010 FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10 490 500
31. ValmoriDMerloASouleimanianNEHesdorfferCSAyyoubM 2005 A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 115 1953 1962
32. MiyaraMYoshiokaYKitohAShimaTWingK 2009 Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30 899 911
33. FritzschingBOberleNPaulyEGeffersRBuerJ 2006 Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood 108 3371 3378
34. Baecher-AllanCBrownJAFreemanGJHaflerDABaecher-AllanC 2001 CD4+CD25high regulatory cells in human peripheral blood. J Immunol 167 1245 1253
35. AnderssonJBoassoANilssonJZhangRShireNJ 2005 The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 174 3143 3147
36. ShafianiSTucker-HeardGKariyoneATakatsuKUrdahlKB 2010 Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med 207 1409 1420
37. NilssonJBoassoAVelillaPAZhangRVaccariM 2006 HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108 3808 3817
38. BelkaidYPiccirilloCAMendezSShevachEMSacksDL 2002 CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 502 507
39. MaizelsRMMaizelsRM 2005 Infections and allergy - helminths, hygiene and host immune regulation. Curr Opin Immunol 17 656 661
40. LuYJGrossJBogaertDFinnABagradeL 2008 Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathogens 4 e1000159
41. MaiJWangHYangXFMaiJWangH 2010 Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci 15 986 1006
42. KinterAMcNallyJRigginLJacksonRRobyG 2007 Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA 104 3390 3395
43. SakaguchiSSakaguchiS 2003 Regulatory T cells: mediating compromises between host and parasite. Nat Immunol 4 10 11
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated OligosaccharidesČlánek A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes inČlánek Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Phenotypic Screens, Chemical Genomics, and Antimalarial Lead Discovery
- Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships with Pneumococcal Colonization
- Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated Oligosaccharides
- Absence of Cross-Presenting Cells in the Salivary Gland and Viral Immune Evasion Confine Cytomegalovirus Immune Control to Effector CD4 T Cells
- Transcriptomic Analysis of Host Immune and Cell Death Responses Associated with the Influenza A Virus PB1-F2 Protein
- A Quorum Sensing Regulated Small Volatile Molecule Reduces Acute Virulence and Promotes Chronic Infection Phenotypes
- Autocrine Regulation of Pulmonary Inflammation by Effector T-Cell Derived IL-10 during Infection with Respiratory Syncytial Virus
- A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes in
- Association of Human TLR1 and TLR6 Deficiency with Altered Immune Responses to BCG Vaccination in South African Infants
- Histo-Blood Group Antigens Act as Attachment Factors of Rabbit Hemorrhagic Disease Virus Infection in a Virus Strain-Dependent Manner
- MrkH, a Novel c-di-GMP-Dependent Transcriptional Activator, Controls Biofilm Formation by Regulating Type 3 Fimbriae Expression
- Beta-HPV 5 and 8 E6 Promote p300 Degradation by Blocking AKT/p300 Association
- Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
- Transportin 3 Promotes a Nuclear Maturation Step Required for Efficient HIV-1 Integration
- Coordination of KSHV Latent and Lytic Gene Control by CTCF-Cohesin Mediated Chromosome Conformation
- A Novel Persistence Associated EBV miRNA Expression Profile Is Disrupted in Neoplasia
- The Plant Pathogen pv. Is Genetically Monomorphic and under Strong Selection to Evade Tomato Immunity
- IL-10 Blocks the Development of Resistance to Re-Infection with
- Anti-Apoptotic Machinery Protects the Necrotrophic Fungus from Host-Induced Apoptotic-Like Cell Death during Plant Infection
- Crystal Structure of PrgI-SipD: Insight into a Secretion Competent State of the Type Three Secretion System Needle Tip and its Interaction with Host Ligands
- Evades Immune Recognition of Flagellin in Both Mammals and Plants
- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Provides Insights into the Evolution of the Salmonellae
- B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein B of Human Cytomegalovirus which Are Target of Neutralizing Antibodies
- Thy1 Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes
- The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point?
- Dendritic Cell Status Modulates the Outcome of HIV-Related B Cell Disease Progression
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Activation of HIV Transcription by the Viral Tat Protein Requires a Demethylation Step Mediated by Lysine-specific Demethylase 1 (LSD1/KDM1)
- Unique Evolution of the UPR Pathway with a Novel bZIP Transcription Factor, Hxl1, for Controlling Pathogenicity of
- Disruption of PML Nuclear Bodies Is Mediated by ORF61 SUMO-Interacting Motifs and Required for Varicella-Zoster Virus Pathogenesis in Skin
- Flagellar Motility Is Not Directly Required to Maintain Attachment to Surfaces
- Viral Infection Induces Expression of Novel Phased MicroRNAs from Conserved Cellular MicroRNA Precursors
- Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4 T Cells and Is Reverted by CD8 Cell Depletion
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
- The Steroid Catabolic Pathway of the Intracellular Pathogen Is Important for Pathogenesis and a Target for Vaccine Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



