-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Anti-Apoptotic Machinery Protects the Necrotrophic Fungus from Host-Induced Apoptotic-Like Cell Death during Plant Infection
Necrotrophic fungi are unable to occupy living plant cells. How such pathogens survive first contact with living host tissue and initiate infection is therefore unclear. Here, we show that the necrotrophic grey mold fungus Botrytis cinerea undergoes massive apoptotic-like programmed cell death (PCD) following germination on the host plant. Manipulation of an anti-apoptotic gene BcBIR1 modified fungal response to PCD-inducing conditions. As a consequence, strains with reduced sensitivity to PCD were hyper virulent, while strains in which PCD was over-stimulated showed reduced pathogenicity. Similarly, reduced levels of PCD in the fungus were recorded following infection of Arabidopsis mutants that show enhanced susceptibility to B. cinerea. When considered together, these results suggest that Botrytis PCD machinery is targeted by plant defense molecules, and that the fungal anti-apoptotic machinery is essential for overcoming this host-induced PCD and hence, for establishment of infection. As such, fungal PCD machinery represents a novel target for fungicides and antifungal drugs.
Published in the journal: . PLoS Pathog 7(8): e32767. doi:10.1371/journal.ppat.1002185
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002185Summary
Necrotrophic fungi are unable to occupy living plant cells. How such pathogens survive first contact with living host tissue and initiate infection is therefore unclear. Here, we show that the necrotrophic grey mold fungus Botrytis cinerea undergoes massive apoptotic-like programmed cell death (PCD) following germination on the host plant. Manipulation of an anti-apoptotic gene BcBIR1 modified fungal response to PCD-inducing conditions. As a consequence, strains with reduced sensitivity to PCD were hyper virulent, while strains in which PCD was over-stimulated showed reduced pathogenicity. Similarly, reduced levels of PCD in the fungus were recorded following infection of Arabidopsis mutants that show enhanced susceptibility to B. cinerea. When considered together, these results suggest that Botrytis PCD machinery is targeted by plant defense molecules, and that the fungal anti-apoptotic machinery is essential for overcoming this host-induced PCD and hence, for establishment of infection. As such, fungal PCD machinery represents a novel target for fungicides and antifungal drugs.
Introduction
Plant pathogenic fungi have evolved two prominent infection strategies: biotrophic pathogens proliferate within the living plant tissue deriving nutrition from living plant cells, and necrotrophic pathogens that do not occupy living plant cells and instead, kill host cells before tissue colonization. In order to infect plants, biotrophic pathogens suppress plant defenses [1], a strategy that is commonly used by pathogens to overcome host immunity [2], [3]. In contrast, necrotrophic pathogens secrete enzymes and toxins that kill the host tissue ahead of pathogen invasion, thus avoiding direct contact with defense molecules in living plant cells. It is unclear how this group of pathogens overcomes the host defenses during the early stages of infection, when the fungus is in contact with living host cells.
The grey mold fungus Botrytis cinerea has become an important model for the study of interactions between plants and necrotrophic pathogens [4]. B. cinerea infects over 200 cultivated plant species and causes significant economic damage to crops worldwide. Host-specific resistance is ineffective against B. cinerea and, conversely, the fungus utilizes the hypersensitive resistance response (HR) and the associated programmed cell death of the host to advance infection [5], [6]. The plant defense responses activated by B. cinerea are regulated in several ways, including the jasmonate and ethylene signaling pathways, as well as by additional signaling cascades that have not yet been identified [7], [8], [9]. Collectively, these defense responses can slow B. cinerea infection, but they do not completely block disease development [10], [11], [12].
Production of anti-microbial secondary metabolites represents an important component of plant defense responses against pathogens [13], [14]. In case of B. cinerea, the most significant secondary metabolites are glucosinolate, indolic, and phenylpropanoid compounds [15], [16]. Specifically, the indolic phytoalexin camalexin has been shown to play important role in defense of A. thaliana against a range of pathogens, including B. cinerea [10], [11], [15]. Production of camalexin is induced in plants following infection with B. cinerea, and A. thaliana mutant plants affected in camalexin biosynthesis show variable levels of enhanced sensitivity to pathogens [17]. The final step of camalexin production is catalyzed by a cytochrome P-450 monooxygenase encoded by the locus PAD3 [18]; pad3 mutant plants do not produce camalexin and are hypersensitive to a wide range of pathogens including B. cinerea. It has been proposed that PAD3 is required for basal resistance to B. cinerea strains sensitive to camalexin [7], [15]. Although it has been shown that camalexin is toxic to a wide range of fungi, the mode of action remained unclear.
At least some plant antimicrobial compounds have the potential to induce apoptotic-like programmed cell death (PCD) in fungi [19], [20], [21]. Because necrotrophic fungi are unable to evade the plant defenses, we reasoned that plant defense molecules might target the fungal PCD machinery as a way to induce cell death in necrotrophic pathogens. Here we show that B. cinerea is exposed to massive, plant-induced PCD during the early phase of infection. Mutant plants that are affected in defense responses and are hypersensitive to B. cinerea, induce reduced levels of PCD in the fungus. We also show that camalexin induces PCD in B. cinerea and that it is important for the full scale host-driven PCD during the early stages of infection. The fungal anti PCD machinery attenuates camalexin and host-driven PCD and is therefore necessary for infection.
Results
Generation of apoptosis-attenuated fungal strains
To investigate the importance of PCD in Botrytis pathogenesis, we first generated B. cinerea strains showing both enhanced and impaired levels of PCD. In order to generate strains showing altered PCD, we isolated BcBIR1 (BC1G_14521.1), a B. cinerea homolog of the Saccharomyces cerevisiae BIR1 gene (GenBank P47134) (Figure S1). Bir1 is a component of the chromosomal passenger complex, which regulates chromosome segregation, an activity that is mediated by the carboxy-terminal domain of the protein and essential for yeast survival [22]. Bir1p also has anti-apoptotic activity, which is mediated by the two BIR domains at the N' terminal part of the protein and is not necessary for the cell cycle regulating activities of the protein [23]. The B. cinerea BcBir1 protein also contains two BIR domains at the N' part of the protein, it shares 24% of amino acid sequence identity with Bir1, but it is considerably shorter than Bir1 (601 amino acids and 954 amino acids, respectively).
Transgenic strains were first produced showing high level expression of the entire BcBIR1 gene or an N' terminal region of the protein containing the two BIR domains (Figure S2). Complete deletion of BcBIR1 was not possible because the gene is essential, similar to the S. cerevisiae BIR1 gene. We therefore used a partial knockout (heterokaryon) strain (Δbcbir1) in which the expression of BcBIR1 was reduced, but not completely eliminated.
PCD in BcBIR1 transgenic strains
Next, we tested the ability of these B. cinerea strains to undergo PCD. When treated with apoptosis-inducing agents such as H2O2, or under physiological conditions that induce PCD such as ageing or in stationary phase [19], [24]. BcBIR1 over-expression strains (expressing either the entire protein or only the N' part) retained higher growth rates and showed reduced PCD markers (chromatin condensation, DNA strand breaks, ROS accumulation), whereas Δbcbir1 mutant strains exhibited reduced growth rates and showed enhanced PCD compared with the isogenic wild type strain (Figures 1, S3). These results show that BcBir1 has anti-apoptotic activity, which is mediated by the BIR domains at the N' terminal part of the protein, similar to the S. cerevisiae homologue Bir1p.
Fig. 1. BcBIR1 encodes a protein with anti-apoptotic activity. 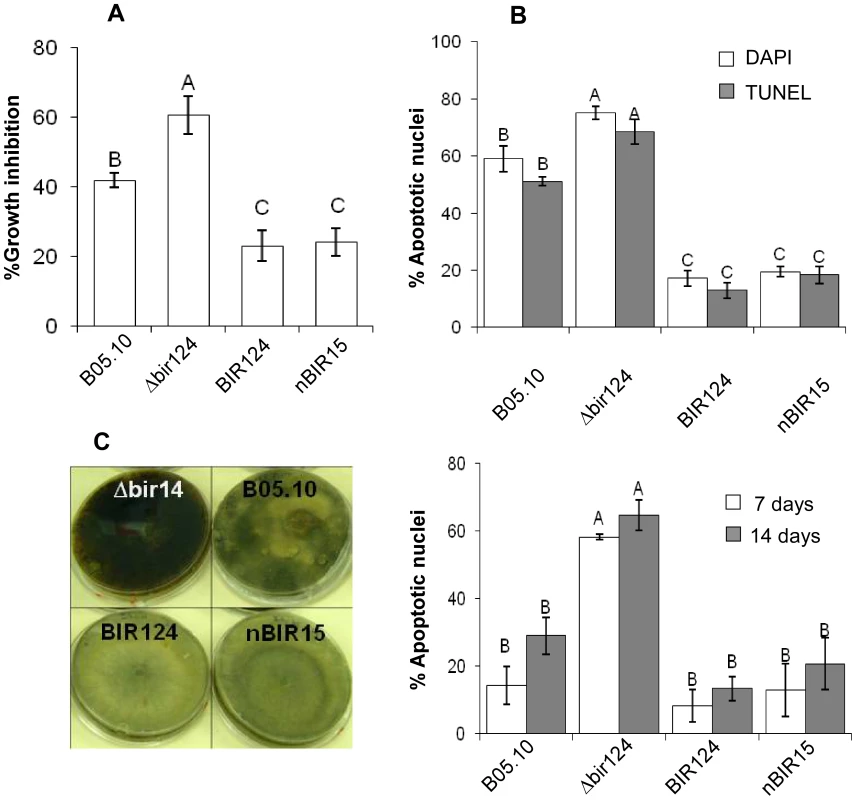
(A, B) PCD induced by H2O2 (see also Figure S3 for additional conditions). (A) Fungi were grown in PDB for 24 h, 8 mM H2O2 was added and relative biomass was determined after 48 h by measuring the OD of the cultures. Percent of growth inhibition was calculated by dividing the OD of treated and untreated cultures. Data shown represent mean ±SEM for four independent experiments performed in triplicates. (B) Accumulation of apoptotic markers. Chromatin condensation (DAPI) and DNA strand breaks (TUNEL) were recorded 4 h after treatment with H2O2. Data shown represent mean ±SEM for triplicate samples of 200 nuclei per sample (n = 3). Percentage of apoptotic nuclei in untreated cultures were less than 0.5% for WT, BcBir124 and nBir15, and lower than 7% (DAPI) and 4% (TUNEL) for Δbir14. (C) Ageing. Cultures were maintained under normal growth conditions, spores were collected after 7 and 14 days, stained with DAPI, and percent of apoptotic nuclei was determined. Data shown represent mean ±SEM for triplicate samples of 100 spores per sample (n = 4). Columns and lines not connected with the same letter are statistically different (p<0.05) as determined by one-way ANOVA (p<0.001) followed by a post-hoc Tukey HSD analysis. Pictures show increased accumulation of black pigments, which indicates senescence of the cultures in the Δbcbir1 mutant. The strains were cultured on PDA plates as described above. Pictures were taken after 14 days of culturing. The BcBIR1 mutant strains are affected in pathogenicity
To test the effect of BcBIR1 on pathogenicity, we inoculated beans leaves and measured disease symptoms. The BcBIR1 over-expression strains (in which PCD was attenuated) caused increased disease symptoms, while Δbcbir1 mutant strains (in which PCD was enhanced) caused restricted lesions (Figure 2A). Similar results were observed on Arabidopsis thaliana (Figure 2B) and on a number of additional plant species that are host for B. cinerea, such as peas, tomato, tobacco and basil (data not shown). Thus, PCD and pathogenicity in BcBIR1 transgenic strains are modified such that enhanced or reduced PCD is associated with reduced or enhanced pathogenicity, respectively. These results support the possibility that the fungus undergoes apoptotic-like PCD during infection, which might have an impact on disease establishment.
Fig. 2. BcBIR1 mutant strains are affected in pathogenicity. 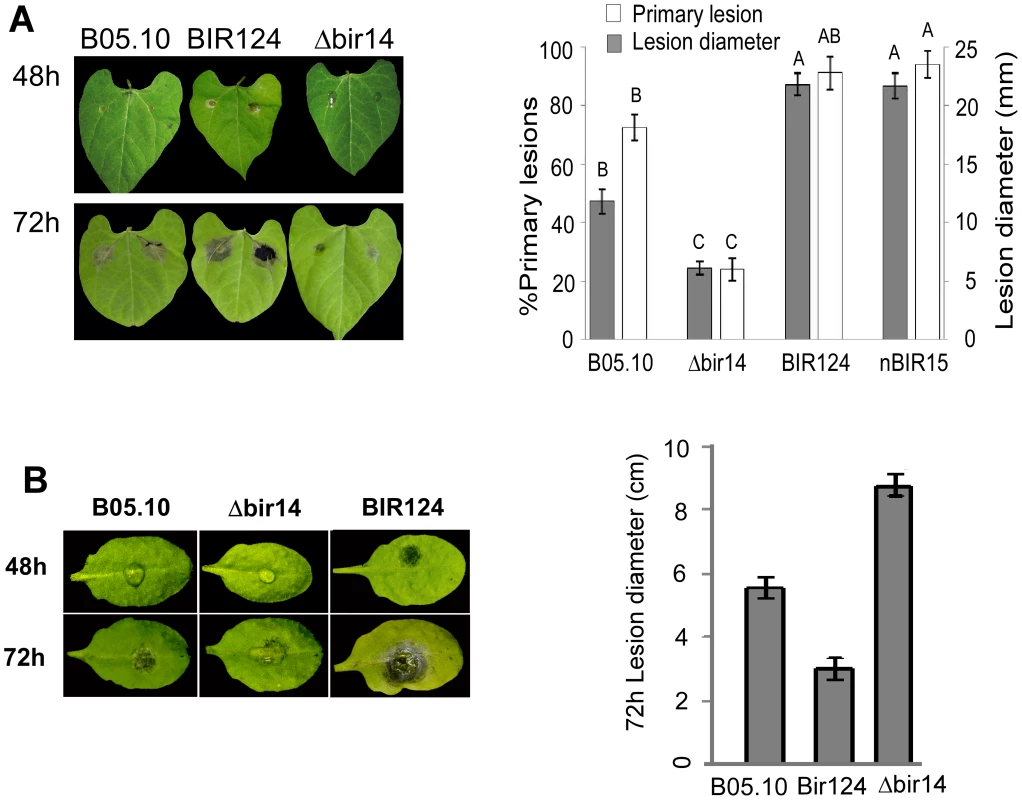
Leaves of P. vulgaris (A) and A. thaliana (B) were inoculated with B. cinerea B05.10 wild type strain, Δbir14 mutant strain and BIR124 and nBIR15 strains that over-express the entire BcBIR1 or the N' part of the protein, respectively. Percent of primary lesions on beans (P. vulgaris) leaves was recorded 48 h PI by counting the number of droplets that produced lesions. Size of expending lesions (on beans and Arabidopsis) was recorded 72 h PI. Data represent mean ±SEM of 32 droplets from four different plants per each fungal genotype (n = 4). Columns not connected with the same letter are statistically different (p<0.05) as determined by one-way ANOVA (p<0.001) followed by a post-hoc Tukey HSD analysis. Use of H1-GFP tagged strains to follow fungal infection
To test whether the fungus undergoes apoptotic-like PCD during infection, we generated a transgenic B. cinerea strain (H1-GFP), in which nuclei were tagged with GFP, and followed changes in the nuclear GFP signal in fungal hyphae during progression of plant infection. Cytoplasmic localization of the histone H1 was shown to play an important role during apoptosis in mammals [25], [26]. Furthermore, the disappearance of the nuclear signal in H1-GFP tagged strains has previously been used to indicate autophagic programmed cell death in Magnaporthe oryzae during appressorium formation [27], and apoptotic-like programmed cell death in Colletotrichum gloeosporioides following treatment with apoptosis-inducing agents [28].
First, we tested the effect of H2O2 on the GFP signal in the B. cinerea H1-GFP strain and compared it with accumulation of apoptotic markers, DNA strand breaks (determined by TUNEL) and chromatin condensation and fragmentation (determined by DAPI staining). During the first hours after treatment with H2O2, the nuclear GFP signal faded out and it could no longer be detected 12 h after treatment (Figure S4A). At this time point, as well as at 6 h post treatment, green fluorescence could be visualized in the entire hyphae, which might be attributed to dislocation of the GFP-tagged histone to cytoplasm during apoptosis, as was shown for mammalian cells [25], [26]. Alternatively, the green fluorescence in damaged hyphae at this stage might also result from disintegration of the nuclei, or due to auto-fluorescence of destroyed hyphae. DAPI staining showed enhanced staining of nuclei 6 and 12 h post H2O2 treatment, which is indicative of chromatin condensation (Figure S4A). Since it is impossible to distinguish between the TUNEL and GFP signals (in both cases nuclei fluoresce in green), we performed TUNEL assay on B. cinerea H1-GFP hyphae 12 h after treatment with H2O2, when the nuclear H1-GFP signal completely disappeared (Figure S4B). At this time point there was intense TUNEL staining, further demonstrating the correlation between loss of the nuclear GFP signal in the H1-GFP strain and progression of PCD. These analyses confirmed that loss of the nuclear GFP signal in the H1-GFP strain is correlated with early stages of apoptotic-like cell death.
We then used the H1-GFP strain to determine the fate of the fungal cells during plant infection. A. thaliana Col-0 plants were inoculated with conidia of the H1-GFP strain and GFP signal in fungal hyphae was monitored using live cell imaging during the first 72 h of plant infection (PI). The nuclear GFP signal was retained for the first 24 h PI, but during the next 24 h (24–48 h PI) it dissociated; some GFP fluorescence was first observed in entire hyphae (as was also seen following H2O2 treatment) and later faded out, consistent with progression of PCD within proliferating hyphae (Figure 3A). In accordance with results of the H2O2 treatment, significant reduction in the number of DAPI stained nuclei, which represents a final stage of cell death, lagged behind the disappearance of the H1-GFP signal and could only be observed at 48 h PI (Figure 3A, 48 h, Figure S4D).
Fig. 3. Disease symptoms are negatively correlated with PCD in Botrytis. 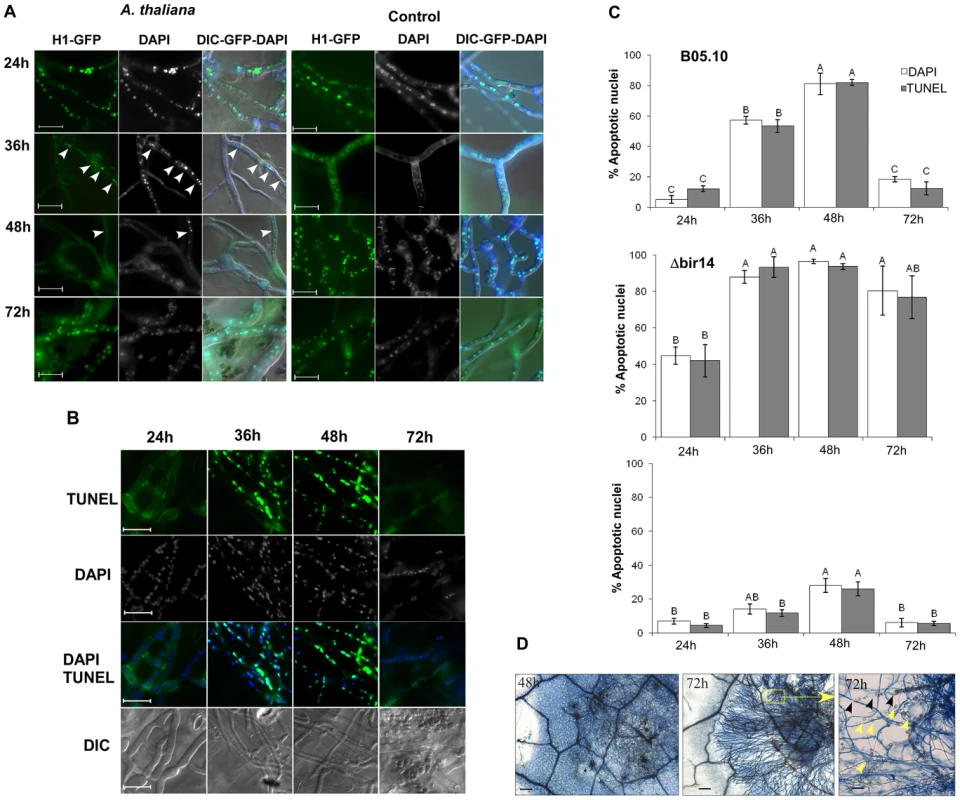
(A) Plants were inoculated with the B. cinerea H1-GFP. Images show gradual decrease in the number of fluorescent nuclei between 24–48 h, and then recovery of the signal at 72 h. Very few nuclei are visible at 48 h (arrowhead). Control panels (right) show hyphae that were developed following spore germination on PDA medium. Abundant nuclei signal are observed at all times. (B) TUNEL and DAPI staining of B. cinerea on infected leave of A. thaliana. Leaves of Col-0 plants were inoculated with B. cinerea B05.10 wild type strain and TUNEL assay was performed to determine PCD in the developing hyphae. The green fluoresce of nuclei in these images indicate positive TUNEL staining of nuclei, i.e., apoptotic cells. Bar = 20 µm. (C) Levels of PCD during infection of A. thaliana Col-0 wild type plants with the B. cinerea BcBIR1 mutant strains. Col-0 plants were inoculated with B05.10 wild type, Δbir14 mutant and BIR24 over expression strains. PCD was recorded by DNA strand break (TUNEL assay) and chromatin condensation (DAPI staining). Data represent mean ±SEM for triplicate samples of 200 nuclei per sample (n = 3). The effect of fungal genotype and infection phase on PCD level was determined using two-way ANOVA. Columns not connected with the same letter are statistically different (p<0.05) as determined by two-way ANOVA (p<0.001) followed by a post-hoc Tukey HSD analysis. (D) Typan blue staining of Botrytis-infected Arabidopsis leaves. Leaves of A. thaliana Col-0 plants were inoculated with a spore suspension of B. cinerea B05.10. Leaves were stained with trypan blue 48 h and 72 h PI. Blue staining of plant cells surrounding the fungal mycelium indicates dead plant cells. The right panel shows a higher magnification of the inset from the middle panel (both from 72 h PI). Degradation of the leaf veins (black arrowheads) and accumulation of vesicles that are produced from remnants of plant cells (yellow arrowheads) show complete maceration of the tissue in the center of the lesion. Bars in first two panels (48 h and 72 h) = 100 µm, bar in last panel (inset 72 h) = 20 µm. Very few GFP-labeled nuclei could be detected at the 48 h time point in isolated fungal cells within the mass of mycelium in the necrotic zone (Figure 3A, 48 h arrowhead). However, despite massive cell death at 48 h, the fungus quickly recovered as indicated by reappearance of the nuclear signal at 72 h PI. Similar results were obtained on additional host plants, such as beans and peas (Figure S4C). This sequence of events only occurred on plants; when germinated on a glass slide, a stable nuclear signal was retained at all time points (Figure 3A, control). Thus, following germination and establishment of contact with the host, nuclear signal in H1-GFP strain starts to disappear between 24–36 h PI, and almost no nuclei can be detected by 48 h PI. Since disappearance of the H1-GFP signal is correlated with accumulation of apoptotic markers, these results suggest that the majority of fungal cells are exposed to plant-induced PCD during the early infection phase, when the fungus is in direct contact with living plant tissue.
We observed a similar pattern (though on a different time scale) of disappearance and reappearance of the H1-GFP nuclear signal during infection of corn plants by the necrotrophic fungus Cochliobolus heterostrophus (Figure S5, Cc-Zm). In contrast, in the hemibiotrophic pathogen C. gloeosporioides the H1-GFP signal was stable throughout the infection process on pea leaves (Figure S5, Cg-Ps). Significantly, inoculation of pea leaves with B. cinerea produced similar results to inoculation of beans (Figure S4C), indicating that the differences in H1-GFP signal did not result from different host responses and are therefore specific to the fungus. Further, similar to C. gloeosporioides, the nuclear signal was stable throughout infection also in M. oryzae, a hemibiotrophic fungus that infects rice and barley (Figure S5, Mo-Os). Thus, retention or loss of the H1-GFP signal is not a universal phenomenon of fungal pathogenesis, but rather it is specific to B. cinerea, and possibly to other necrotrophic pathogens.
PCD is induced in Botrytis during the early infection phase
To verify that the results obtained with the H1-GFP strain are indicative of PCD, we measured DNA strand breaks (TUNEL positive nuclei) and chromatin condensation (condensed nuclei following DAPI staining) during infection of A. thaliana plants by the wild type B05.10 strain. In accordance with the results obtained with the H1-GFP marker, the level of PCD markers increased between 24 h and 48 h PI, and then reduced to near baseline levels at 72 h (Figure 3B–C). These results support the results obtained with the H1-GFP strain and confirm that massive PCD occurs during the early infection phase, but that the fungus manages to recover upon transition to the second phase.
Modified PCD in BcBIR1 mutant strains during plant infection
Infection of A. thaliana wild type plants with BcBIR1 over-expression strains led to larger necrotic lesions whereas the Δbcbir1 mutant strain caused attenuated disease symptoms (Figure 2B). If PCD was a limiting factor in disease development, intensified and reduced disease symptoms are expected to be paralleled by reduced or increased levels of PCD. In support of this rational, the amount of PCD in the BcBIR1 over-expression and Δbcbir1 mutant strains on infected Col-0 plants was reduced or enhanced, respectively (Figure 3C). These results further support the role of BcBIR1-mediated anti-apoptotic response as a mechanism to cope with plant-induced PCD during the direct contact of the fungus with living host cells.
Attenuated fungal PCD on Botrytis-hypersensitive plants
To further investigate whether fungal PCD was induced by host defenses, we used a collection of A. thaliana mutant plants that represent mutations in a range of defense responses, all of which are hypersensitive to B. cinerea; ein2 (ethylene signaling), bik1 (receptor-mediated basal defense), wrky33 (transcriptional regulation/MPK4-mediated signaling pathway) and pad3 (camalexin production). When the A. thaliana mutant plants that are hyper-sensitive to B. cinerea were inoculated with the H1-GFP strain, the nuclear GFP signal was retained also at 48h, and enhanced disease symptoms developed (Figure 4), confirming that the amount of fungal PCD negatively correlates with plant susceptibility to the fungus.
Fig. 4. Lower PCD levels and enhanced pathogenicity of B. cinerea on A. thaliana immune-deficient plants. 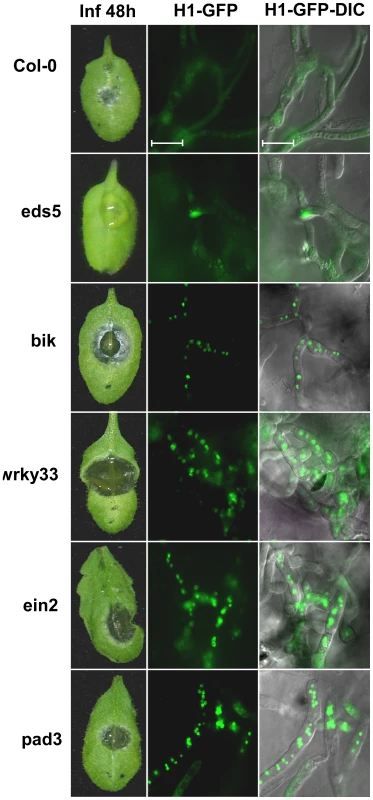
Leaves of all the plant lines were inoculated with spore suspension of the H1-GFP strain. Symptoms and GFP signals were recorded 48 h PI. Col-0 – wild type, eds5 – a mutant in SA signaling with slightly reduced sensitivity to B. cinerea (both the Col-0 and eds5 are used a controls), bik1, wrky33, ein2, pad3 are mutant lines with defects in the immune response (see text for details) and are hyper sensitive to B. cinerea. Enhanced pathogenicity on these mutants is paralleled by retention of intact nuclei (H1-GFP images). Induced fungal PCD by camalexin
A. thaliana pad3 plants do not produce camalexin, the principal phytoalexin in A. thaliana [29]. Because B. cinerea undergoes reduced PCD on pad3 mutant plants, it is possible that camalexin might be involved in the induction of fungal PCD. To determine whether camalexin induces PCD in B. cinerea, we treated mycelium with purified camalexin and measured growth and levels of PCD. Consistent with previous reports [10], 20% growth inhibition of the wild type strain was induced by exposure to camalexin at 15 µg/ml, which was accompanied by appearance of apoptotic markers (Figure 5A). The BcBIR1 over expression and mutant strains showed reduced or enhanced sensitivity to camalexin respectively, along with reduced or enhanced PCD. We conclude that camalexin induces PCD in B. cinerea.
Fig. 5. Camalexin induces PCD in B. cinerea and limits lesion spreading. 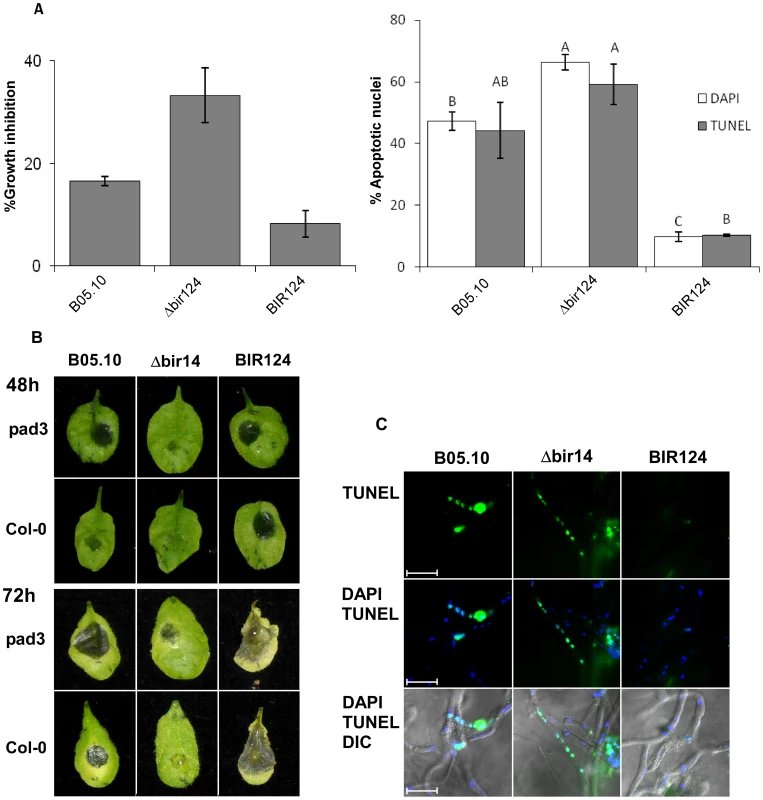
(A) Growth inhibition and induction of PCD in B. cinerea by camalexin. Fungi were grown in PDB for 24 h and then camalexin was added at 15 µg/ml. A. Effects on growth (left graph) and PCD (right graph) were recorded after 48 h and 4 h, respectively. Growth assays were performed in triplicates. Apoptotic nuclei counts were determined by counting 200 nuclei per sample. Data represent mean ±SEM (n = 3). Columns not connected with the same letter are statistically different (p<0.05) as determined by one-way ANOVA (p<0.001) followed by a post-hoc Tukey HSD analysis. (B) Infection of camalexin-deficient pad3 mutant and Col-0 wild type plants by B. cinerea. Plants were inoculated with spore suspensions of B. cinerea B05.10, Δbir14 mutant and BIR24 over expression strains. (C) PCD was detected by TUNEL assay 48 h PI. Note that in this case the green nuclear signal denotes apoptotic nuclei. Bar = 20 µm. When inoculated with Δbcbir1 mutant strain, pad3 plants developed clear necroses 72 h PI, unlike wild type Col-0 plants in which the initial lesions that were formed following inoculation with Δbcbir1 did not develop into spreading disease lesions (Figure 5B). Direct assays confirmed that in comparison to Col-0, on pad3 mutant plants the wild type and Δbcbir1 fungal strains showed reduced levels of PCD, consistent with intensified disease symptoms (Figures 5C, S6) Notably, the BcBIR1 over-expression strains showed little difference in plant infection and PCD on pad3 plants compared with Col-0 plants, possibly due to the reduced sensitivity of these strains to camalexin-induced PCD and thus reduced PCD and enhanced pathogenicity on the wild type plants.
Discussion
In this report we have shown that in the broad host range necrotrophic fungus B. cinerea, and possibly in additional necrotrophic fungi, massive PCD of fungal cells is induced by the plant defense response during the early phase of plant infection. An anti-apoptotic machinery is essential for protection of the fungus during this stage, and hence for establishment of initial infection.
A major element in the B. cinerea anti-apoptotic machinery is the protein BcBir1. Unlike most other fungal homologues of animal apoptotic genes that have been analyzed, which usually have only partial effect on PCD, our results show that BcBir1 plays major role in PCD, and that it is essential for function of the anti-apoptotic machinery. This allowed us to generate a set of transgenic strains with attenuated PCD through manipulation of BcBIR1. We used these strains together with a strain expressing the H1-GFP nuclear marker, to measure changes in fungal PCD during plant infection and determine the role of PCD, particularly the anti-apoptotic machinery of the fungus, in disease development.
Rapid PCD of plant cells around the site of pathogen invasion is the main component of the HR resistance response, which is effective against specific biotrophic pathogens. In contrast, the HR response is rather ineffective against necrotrophic pathogens, which induce spreading cell death in infected plants, partly by manipulation of the plant's natural PCD response [1]. We have shown that in the broad host range fungus B. cinerea, rapid apoptotic-like PCD of fungal cells at the primary infection site is induced by the plant defense. The anti-apoptotic machinery in the fungus blocks host-induced PCD between 24–48 h PI, thereby preserving a small number of viable fungal cells within the necrotic zone. According to our model (Figure 6), surviving fungal cells within the destroyed plant tissue are sufficient to give rise to new mycelium, which initiates spreading lesions in the second phase of infection. Consistent with this model and in agreement with previous reports [11], staining of infected leaves with trypan blue (which stains dead cells) showed that fungal mycelium was restricted to areas of destroyed tissue, which were surrounded by a ring of dead plant cells that expanded as the lesion progressed (Figure 3D). Simulations on PDA plates show that a single viable cell is sufficient to give rise to a micro-colony composed of loosely packed, but elongated (1–2 cm) hyphae (not shown). Accordingly, fungal cells that remain viable at 48 h within the necrotic zone, and thus are protected from the host defense, are potentially capable of producing abundant hyphae by 72 h. Furthermore, the plant tissue within the necrotic region is massively degraded at 72 h (Figure 3D). Thus, inside the necrotic spot the fungus is protected from the host defense and has ample supply of nutrients, conditions which are supportive of intensive growth during the second infection phase. Spreading lesion is induced by fungal activity in the periphery, while development of new mycelium is restricted to areas of dead tissue (Figure 3D, 72 h, Figure 6).
Fig. 6. A model showing the role of PCD in early events of B. cinerea infection. 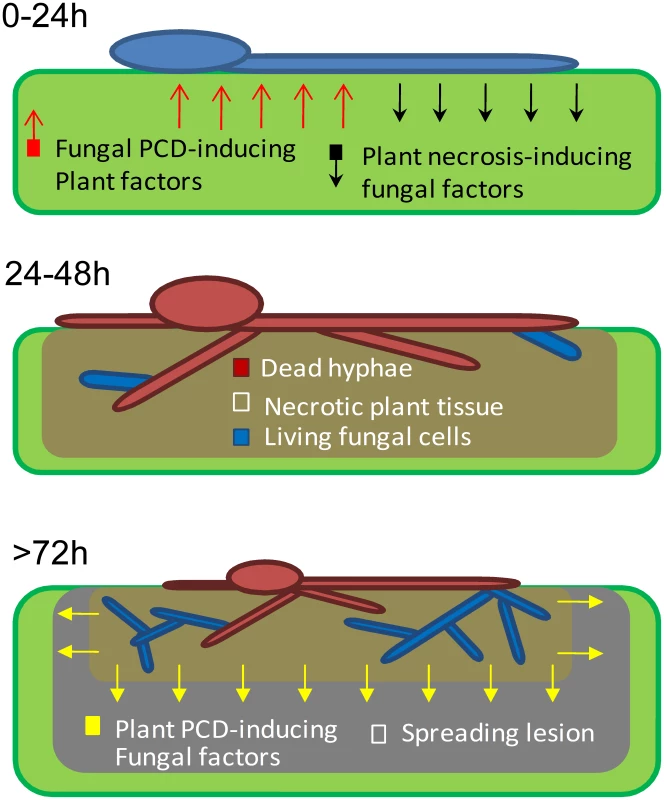
0–24: Following landing on the host, conidia germinate and start secreting necrosis-inducing factors. 24–48: Anti-fungal plant metabolites trigger PCD in developing hyphae, which kill most of the fungal cells. Anti-apoptotic machinery (mediated by BcBir1) prevents complete elimination of the fungus at this stage. >72: Viable fungal cells retained within the necrotized plant tissue are protected from the host toxic molecules. These cells give rise to new hyphae which secrete molecules that induce PCD in surrounding plant tissue and promote lesion spreading. A similar trend of H-1GFP signal disappearance and reappearance was observed in the corn necrotrophic pathogen C. heterostrophus, but not in two hemibiotrophic pathogens, M. oryzae and C. gloeosporioides, in which the nuclear signal was stable at all stages of infection. These results suggest that the phenomenon discovered in B. cinerea, namely, massive cell death followed by recovery of the fungus, might be a common strategy in necrotrophic pathogens; because pathogens in this class are unable to evade the host defense, and since host defense molecules target and trigger fungal PCD, the fungal anti-apoptotic machinery serves as a virulence mechanism by protecting the fungus from plant-induced PCD. Following the establishment of a protected infection zone, the fungus induces PCD in surrounding host tissues to promote disease spreading [4], [6], [30].
Plant and animal pathogens have evolved common features including conserved systems for deploying virulence proteins and convergent pathogenic strategies [2], [31], [32]. Increasing evidence suggest structural and functional similarities in microbial-host interaction mechanisms of plants, insects and vertebrates [3], [33], [34]. Particularly, a few studies have shown that PCD of eukaryotic pathogens of insect and mammals occur during host invasion [35], [36], [37]. Thus, anti-apoptotic activity of the pathogen might be important for host invasion in various other systems including human pathogens. Accordingly, metabolites that target the fungal PCD machinery might be valuable in developing novel antifungal drugs and in engineering plant resistance against economically significant fungal pathogens.
Materials and Methods
Fungal strains, growth conditions, and DNA analysis
B. cinerea wild type strain B05.10 and H1-GFP transgenic strain were used throughout this study. The H1-GFP strain carries the eGFP gene fused to the C-terminus of the histone E11-encoding gene hH1 from Neurospora crassa under control of B. cinerea actin promoter. The strain displays fluorescent nuclei and is hygromycin resistant. In addition we used transgenic strains of Cochliobolus heterostrophus c4 that express an hH1-eGFP gene fusion under control of B. cinerea actin promoter (this work), and C. gloeosporioides f. sp. aeschynomene strain 3.1.3 [28] and M. oryzae strain Guy11 [27] that express an hH1-RFP gene fusions, both under control of N. crassa CCG1 promoter. B. cinerea strains were routinely cultured on potato dextrose agar (PDA, Acumedia) and maintained at 21°C under fluorescent light. Conidia were obtained from 7–10 – days old cultures. Mycelium was produced from cultures that were grown in potato dextrose broth (PDB, Acumedia) in 250 ml Erlenmeyer-flasks, with agitation at 180 rpm. Growth assays were conducted in 24-wells plates.
Gel electrophoresis, restriction enzyme digestion, PCR and DNA gel blots hybridizations were all carried out using standard procedures [38]. DNA-mediated transformation of B. cinerea was carried out as previously described [39], [40]. DNA and RNA extraction, and cDNA synthesis were performed as previously described [41].
Oligonucleotides and construction of plasmids DNA
The B. cinerea B05.10 genome sequence at BROAD (http://www.broad.mit.edu) was used to design primers for amplification of BcBIR1 ORF and flanking sequences (Table S1). BcBIR1 5′ and 3′ flanking sequences (800 bp each) were amplified by PCR using primers 3/4 (5′) and 5/6 (3′). The two fragments were cloned into pUC57R/T (Fermentas), excised with NotI/SmaI (5′ flank) and MluI/SacI (3′ flank), and cloned into pOliHP to produce pΔbcbir1 gene replacement vector. Over expression vectors were constructed with BcBIR1 cDNA. A full length cDNA clone (1803 bp) was amplified by PCR using primers 1/2. B. cinerea ActA promoter (Bc_08198.1; 1,500 bp) and β-glucanase terminator (BC1T_00642; 500 bp) were amplified from genomic DNA using primers 14/15 and 11/12, respectively. The three PCR fragments were cloned into the corresponding restriction sites of pUC57R/T, the cassette was excised with HindIII and SpeI restriction enzymes and sub-cloned into pBluescript KS+ to produce the KS-BcBIR1 plasmid. The Hygromycin resistance cassette from pOliHP vector was digested with NotI and SacI and sub-cloned into KS-BcBIR1 to produce the BcBIR1 over-expression vector pKSHAIG. For over-expression of the N' part, nucleotides 0 - 891 of BcBIR1 containing both Bir domains were amplified with primers 1/13 and cloned into PacI/AscI sites in pKSHAIG in place of the BcBIR1 ORF to produce the BcBIR1 N' over expression vector pKSHANIG.
Plant infections
Infection assays of beans (Phaseolus vulgaris cv. French bean, genotype N9059) plants with B. cinerea were performed according to [39]. Infection levels were estimated by counting primary lesions 24–48 h PI (early phase) and measurement of lesions' diameter 72 or 96 h PI (late phase). Inoculation of A. thaliana plants with B. cinerea was performed in a similar way by placing a 6 µl droplet of spore suspension on each leaf. Wild type Columbia (Col-0) plants were routinely used. We also used the A. thaliana mutant plants ein2 [42], bik1 [12], wrky33 [9], and pad3 [17], all of which are affected in defense response genes that confer increased sensitivity to B. cinerea. In addition, the eds-5 mutant, which has slightly reduced levels of sensitivity to B. cinerea was used as control. Pea (Pisum sativum, cv. white sugar) plants were cultivated and inoculated with C. gloeosporioides or B.cinerea, rice plants (Oryza sativa cv CO-39) were cultivated and inoculated with M. oryzae, and corn (Zea maize cv Jubilee) plants were cultivated and inoculated with C. heterostrophus as previously described [27], [43], [44]. Trypan blue staining was used to detect plant cell death following fungal infection [45]. Infected leaves were placed in lactophenol-trypan blue stain solution (1 mL of lactic acid, 1 mL of glycerol, 10 g of phenol, 10 mg of trypan blue, dissolved in 10 mL of distilled water) and boiled for 1 min. The stained leaves were decolorized in chloral hydrate (2.5 g of chloral hydrate dissolved in 1 mL of distilled water) for 30 min and then mounted on microscope slides and observed by bright filed microscope.
Apoptosis assays and staining procedures
Apoptotic-like cell death was determined by measurement of accumulation of reactive oxygen species (ROS), chromatin condensation and amount of DNA-strand breaks, criteria that are commonly used to determine apoptotic PCD in fungi [24], [46]. All assays were performed in triplicates. Isolates were grown overnight before application of PCD-inducing compounds. Growth response was determined by measurement of the optical density using a synergy HT plate reader (Biotek). Intracellular ROS levels were detected by staining with Dihydro-rhodamine 123 (DHR123) (Sigma). Tissue was incubated for 3 h in PDB medium with 4 µg/ml DHR123 with agitation. Following incubation, samples were washed twice with ddH2O and then observed under fluorescent microscope using a rhodamine filter. Relative ROS levels were determined by measurement of fluorescence intensity divided by the relative biomass (both measurements were performed with a Biotek synergy HT plate reader). Chromatin condensation was detected following nuclear staining. Nuclei were stained with DAPI as previously described [43] or by incubation for 10 min with 15 µg/ml Hoechst 33342 (Sigma). Samples were visualized by fluorescent microscope using a DAPI filter. DNA strand breaks were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the In Situ Cell Death Detection kit (Roche Applied Science, Indianapolis, IN), as previously described [43]. Briefly, mycelia and infected leaves were fixed with 3.7% formaldehyde, digested with lysing enzyme (sigma), rinsed twice with PBS, incubated with 50 µl TUNEL reaction mixture for 70 min at 37°C with agitation and then rinsed 3 times with PBS. Samples were examined using a GFP filter. The percent of condensed and TUNEL positive nuclei was calculated by counting 200 cells/nuclei per sample.
Microscopy
Epifluorescence and light microscopy were performed with a Zeiss Axio imager M1 microscope. Differential interference microscopy (DIC) was used for bright field images. The following filters were used for examination of fluorescent samples: DAPI filter (excitation 340–390 nm, emission 420–470 nm), rhodamine filter (excitation 540–552 nm, emission 575–640 nm), GFP filter (excitation 450–490 nm, emission 500–550 nm). Images were captured with a Zeiss AxioCam MRm camera and analyzed using Axiovision Rel 4.5 software package. Further processing and pixel intensity measurements were performed using the ImageJ 1.42q software (http//rsb.info.nih.gov/ij/).
Statistical analysis
Statistical tests were applied using JMP 7.0.2 (SAS Institute, Cary, NC). Data were analyzed by analysis of variance (one-way or two-way ANOVA), followed by post hoc Tukey test for comparisons among genotypes. In all graphs, results represent the mean value of 3–5 independent experiments ± SEM. Columns marked with the same letters do not differ from each other at the significance level of P<0.05 (α = 5%) according Tukey HSD test for post-ANOVA analysis (p<0.001).
Supporting Information
Zdroje
1. GlazebrookJ 2005 Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205 227
2. ButtnerDBonasU 2003 Common infection strategies of plant and animal pathogenic bacteria. Cur Opin Plant Biol 6 312 319
3. SextonACHowlettBJ 2006 Parallels in fungal pathogenesis on plant and animal hosts. Eukar Cell 5 1941 1949
4. van KanJAL 2006 Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11 247 253
5. El OirdiMBouarabK 2007 Plant signalling components EDS1 and SGT1 enhance disease caused by the necrotrophic pathogen Botrytis cinerea. New Phytol 175 131 139
6. GovrinEMLevineA 2000 The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 751 757
7. FerrariSGallettiRDenouxCDe LorenzoGAusubelFM 2007 Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144 367 379
8. RoweHCKliebensteinDJ 2008 Complex genetics control natural variation in Arabidopsis thaliana resistance to Botrytis cinerea. Genetics 180 2237 2250
9. ZhengZQamarSAChenZMengisteT 2006 Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48 592 605
10. FerrariSPlotnikovaJMDe LorenzoGAusubelFM 2003 Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35 193 205
11. RoweHCWalleyJWCorwinJChanEKFDeheshK 2010 Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog 6 e1000861
12. VeronesePNakagamiHBluhmBAbuQamarSChenX 2006 The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18 257 273
13. BednarekPPislewska-BednarekMSvatosASchneiderBDoubskyJ 2009 A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323 101 106
14. ClayNKAdioAMDenouxCJanderGAusubelFM 2009 Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323 95 101
15. KliebensteinDJRoweHCDenbyKJ 2005 Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44 25 36
16. ŁaźniewskaJMacioszekVLawrenceCKononowiczA 2010 Fight to the death: Arabidopsis thaliana defense response to fungal necrotrophic pathogens. Acta Physiol Plant 32 1 10
17. GlazebrookJAusubelFM 1994 Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci U S A 91 8955 8959
18. ZhouNTootleTLGlazebrookJ 1999 Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11 2419 2428
19. FinkelshteinAShlezingerNBunisOSharonA 2011 Botrytis cinerea BcNma is involved in apoptotic cell death but not in stress adaptation. Fung Genet Biol 48 621 630
20. ItoS-iIharaTTamuraHTanakaSIkedaT 2007 [alpha]-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal pathogen Fusarium oxysporum. FEBS Lett 581 3217 3222
21. SharonAFinkelshteinA 2009 Programmed cell death in fungus–plant interactions. EsserKDeisingH The Mycota 2 ed Heidelberg Springer 221 236
22. UrenAGBeilharzTO'ConnellMJBuggSJvan DrielR 1999 Role for yeast inhibitor of apoptosis (IAP)-like proteins in cell division. Proc Natl Acad Sci U S A 96 10170 10175
23. WalterDWissingSMadeoFFahrenkrogB 2006 The inhibitor-of-apoptosis protein Bir1p protects against apoptosis in Saccaromyces cerevisiae and is a substrate for the yeast homologue of Omi/HtrA2. J Cell Sci 119 1843 1851
24. SharonAFinkelsteinAShlezingerNHatamI 2009 Fungal apoptosis: function, genes and gene function. FEMS Microbiol Rev 33 833 854
25. EscaffitFVauteOChevillard-BrietMSeguiBTakamiY 2007 Cleavage and cytoplasmic relocalization of histone deacetylase 3 are important for apoptosis progression. Mol Cell Biol 27 554 567
26. KonishiAShimizuSHirotaJTakaoTFanY 2003 Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell 114 673 688
27. Veneault-FourreyCBarooahMEganMWakleyGTalbotNJ 2006 Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science 312 580 583
28. NesherIBarhoomSSharonA 2008 Cell cycle and cell death are not necessary for appressorium formation and plant infection in the fungal plant pathogen Colletotrichum gloeosporioides. BMC Biol 6 9
29. GlawischnigE 2007 Camalexin. Phytochemistry 68 401 406
30. DickmanMBParkYKOltersdorfTLiWClementeT 2001 Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci U S A 98 6957 6962
31. NurnbergerTBrunnerF 2002 Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol 5 318 324
32. Van GijsegemFGeninSBoucherC 1993 Conservation of secretion pathways for pathogenicity determinants of plant and animal bacteria. Tren Microbiol 1 175 180
33. BakerBZambryskiPStaskawiczBDinesh-KumarSP 1997 Signaling in plant-microbe interactions. Science 276 726 733
34. BergeyDRHoweGARyanCA 1996 Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci U S A 93 12053 12058
35. Al-OlayanEMWilliamsGTHurdH 2002 Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int J Parasitol 32 1133 1143
36. Fernandez-ArenasECabezonVBermejoCArroyoJNombelaC 2007 Integrated proteomics and genomics strategies bring new insight into Candida albicans response upon macrophage interaction. Mol Cel Prot 6 460 478
37. Jaso-FriedmannLLearyJHEvansDL 2000 Role of nonspecific cytotoxic cells in the induction of programmed cell death of pathogenic protozoans: participation of the fas ligand-fas receptor system. Exper Parasitol 96 75 88
38. SambrookJFritschEFManiatisT 1989 Molecular cloning: a laboratory manual. Cold spring harbor, NY Laboratory Press
39. SiewersVSmedsgaardJTudzynskiP 2004 The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 70 3868 3876
40. TurgeonBGGarberRCYoderOC 1985 Transformation of the fungal maize pathogen Cochliobolus heterostrophus using the Aspergillus nidulans amd gene. Mol Gen Genet 201 450 453
41. ChagueVDanitL-VSiewersVGronoverCSTudzynskiP 2006 Ethylene sensing and gene activation in Botrytis cinerea: a missing link in ethylene regulation of fungus-plant interactions? Mol Plant-Microbe Interact 19 33 42
42. GuzmanPEckerJR 1990 Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513 523
43. BarhoomSSharonA 2007 Bcl-2 proteins link programmed cell death with growth and morphogenetic adaptations in the fungal plant pathogen Colletotrichum gloeosporioides. Fungal Genet Biol 44 32 43
44. LevSSharonAHadarRMaHHorwitzBA 1999 A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen-activated protein kinase homologs in foliar pathogens. Proc Natl Acad Sci U S A 96 13542 13547
45. KeoghRCDeverallBJMcLeodS 1980 Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans British Mycol Soc 74 329 333
46. SemighiniCPHarrisSD 2009 Methods to detect apoptotic-like cell death in filamentous fungi. SharonA Molecular and Cell Biology Methods for Fungi Humana Press USA
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated OligosaccharidesČlánek A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes inČlánek Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Phenotypic Screens, Chemical Genomics, and Antimalarial Lead Discovery
- Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships with Pneumococcal Colonization
- Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated Oligosaccharides
- Absence of Cross-Presenting Cells in the Salivary Gland and Viral Immune Evasion Confine Cytomegalovirus Immune Control to Effector CD4 T Cells
- Transcriptomic Analysis of Host Immune and Cell Death Responses Associated with the Influenza A Virus PB1-F2 Protein
- A Quorum Sensing Regulated Small Volatile Molecule Reduces Acute Virulence and Promotes Chronic Infection Phenotypes
- Autocrine Regulation of Pulmonary Inflammation by Effector T-Cell Derived IL-10 during Infection with Respiratory Syncytial Virus
- A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes in
- Association of Human TLR1 and TLR6 Deficiency with Altered Immune Responses to BCG Vaccination in South African Infants
- Histo-Blood Group Antigens Act as Attachment Factors of Rabbit Hemorrhagic Disease Virus Infection in a Virus Strain-Dependent Manner
- MrkH, a Novel c-di-GMP-Dependent Transcriptional Activator, Controls Biofilm Formation by Regulating Type 3 Fimbriae Expression
- Beta-HPV 5 and 8 E6 Promote p300 Degradation by Blocking AKT/p300 Association
- Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
- Transportin 3 Promotes a Nuclear Maturation Step Required for Efficient HIV-1 Integration
- Coordination of KSHV Latent and Lytic Gene Control by CTCF-Cohesin Mediated Chromosome Conformation
- A Novel Persistence Associated EBV miRNA Expression Profile Is Disrupted in Neoplasia
- The Plant Pathogen pv. Is Genetically Monomorphic and under Strong Selection to Evade Tomato Immunity
- IL-10 Blocks the Development of Resistance to Re-Infection with
- Anti-Apoptotic Machinery Protects the Necrotrophic Fungus from Host-Induced Apoptotic-Like Cell Death during Plant Infection
- Crystal Structure of PrgI-SipD: Insight into a Secretion Competent State of the Type Three Secretion System Needle Tip and its Interaction with Host Ligands
- Evades Immune Recognition of Flagellin in Both Mammals and Plants
- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Provides Insights into the Evolution of the Salmonellae
- B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein B of Human Cytomegalovirus which Are Target of Neutralizing Antibodies
- Thy1 Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes
- The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point?
- Dendritic Cell Status Modulates the Outcome of HIV-Related B Cell Disease Progression
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Activation of HIV Transcription by the Viral Tat Protein Requires a Demethylation Step Mediated by Lysine-specific Demethylase 1 (LSD1/KDM1)
- Unique Evolution of the UPR Pathway with a Novel bZIP Transcription Factor, Hxl1, for Controlling Pathogenicity of
- Disruption of PML Nuclear Bodies Is Mediated by ORF61 SUMO-Interacting Motifs and Required for Varicella-Zoster Virus Pathogenesis in Skin
- Flagellar Motility Is Not Directly Required to Maintain Attachment to Surfaces
- Viral Infection Induces Expression of Novel Phased MicroRNAs from Conserved Cellular MicroRNA Precursors
- Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4 T Cells and Is Reverted by CD8 Cell Depletion
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
- The Steroid Catabolic Pathway of the Intracellular Pathogen Is Important for Pathogenesis and a Target for Vaccine Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



