-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
article has not abstract
Published in the journal: . PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1001241
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1001241Summary
article has not abstract
Measles remains an important cause of child mortality, although the numbers of measles-related deaths has decreased during the last decade [1] through childhood immunisation programmes and follow-up measles vaccine campaigns. In 2005, the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) launched a global plan to further reduce measles mortality in the years 2006–2010 [2]. Despite these joint efforts, an increased number of large and deadly outbreaks of measles on the African continent were reported, with the most severe outbreaks in Chad, Nigeria, and Zimbabwe. The current increase in measles cases has been attributed to a failure in maintaining high measles vaccine coverage [3]. There are obviously several factors of medical and social relevance to take into consideration when trying to explain the increased measles outbreaks in Africa. In this article, our focus is to highlight the possibility of a co-existing link between the measles outbreaks and pathological features of HIV-1 infection in mothers and children, as the measles outbreaks occurred in countries with a high HIV-1 prevalence.
Passively acquired maternal antibodies protect infants against measles until the time of measles vaccination, which in most developing countries is administered at 9 months of age. In 1992, an increased risk of measles before 9 months of age was reported in children born to mothers with HIV-1 [4], which was suggested to be due to lower levels of passively acquired antibodies at birth [5]. In a recent study, the level of measles antibodies were followed from 6 weeks of age until 11 months in HIV-1-infected, HIV-1-exposed non-infected (born to mothers with HIV-1 but not HIV-1 positive), and HIV-1-seronegative children [6]. By 6 months of age, 91% and 83% of HIV-1-infected and HIV-1-exposed non-infected children had measles antibody levels of <50 mIU/mL (cut-off value for specific immune response); 42% of HIV-1-negative children, on the other hand, retained high antibody levels at 6 months. These findings confirm the previous observation [5] of low titres of maternal antibodies being transferred to infants of mothers with HIV-1. Children born to mothers with HIV-1 have a higher risk of contracting early measles independently of whether they are themselves HIV-1 infected [5]. In a study from Zambia, co-infection with HIV-1 and measles in children was shown to more than double the risk of death in measles during hospitalisation [7]. Deaths due to measles infection occurred in 12.2% of the children with HIV-1 (median age 12 months) as compared to 4.3% of non-HIV-1-infected children (14 months). Since the control of measles and HIV-1 relay on efficient CD8 T cell responses, the increased morbidity observed in children with HIV-1 upon measles infection can be related to the shift in cytokine profile from Th1 to Th2 occurring in these young individuals and impairing T cell responses to both pathogens [8]. A Th1 to Th2 shift during the course of chronic HIV-1 infection is associated with progression to AIDS [9], and measles virus infection also suppresses the ability of T cells to produce IL-12, thus hampering T cell responses [10].
To reduce the risk of contracting measles in areas with high HIV-1 prevalence, WHO recommended that infants receive two doses of measles vaccine, at 6 and 9 months [11]. This regimen was evaluated in Zambia [12] and results published in 2008 showed that 59% of children with HIV-1 were measles antibody positive after the first vaccine dose; this number increased to 64% after the second dose. Among HIV-1-exposed non-infected children, 68% and 94% were seropositive after the first and second immunisation, respectively, and similar figures were shown for control children (62% and 92%). To further pinpoint the B cell impairments leading to low antibody levels after measles vaccination in children with HIV-1, Nair [13] characterised early antibody responses to measles following vaccination at 9 months of age. Interestingly, HIV-1 infection impaired IgG responses after vaccination as well as the development of high avidity measles antibodies. In a study from Kenya, antibody titres to measles were evaluated 2 to 5 years after measles immunisation received during the first year of life [14]. Several years after immunisation, only 33% of the children with HIV-1 maintained measles IgG antibodies, indicating impairment in the establishment and the maintenance of serological memory responses.
Which, then, could be the mechanism accounting for the decreased amount of measles antibodies circulating in mothers with HIV-1 and poor response to measles vaccination in children with HIV-1? The rapid IgM immune response towards measles occurring to a great extent in the splenic marginal zone B cells is an important first-line defence upon natural infection and after immunisation. In healthy children, the splenic marginal zone is not fully developed until 2 years of age [15], and this observation explains the low responses observed upon vaccination in young children. During HIV-1 infection, the structure of lymphoid tissue is altered, leading to follicular hyperplasia and likely to impairment of marginal zone responses [16].
We suggest that the decline of resting memory B cells reported by us [17]–[19] and others [20] that occurs during HIV-1 infection may be an important pathogenic mechanism linked to the low level of measles-specific antibodies found in mothers with HIV-1 and their children (Figure 1). Memory B cells are responsible for mounting and maintaining an adequate serological response to antigens previously encountered in life through natural infection or vaccination. The decline in B cells carrying immunological memory correlated to loss of antibody titres to measles, tetanus, and pneumococcal antigens [17], [18]. Interestingly, in turn, the decline of serum measles antibodies correlated to a decreased number of measles-specific memory B cells in blood. The antibody levels to pneumococcal antigens were dramatically reduced already from primary HIV-1 infection [17].
Fig. 1. Maintenance and formation of measles-specific antibodies and memory B cells in mother and child. 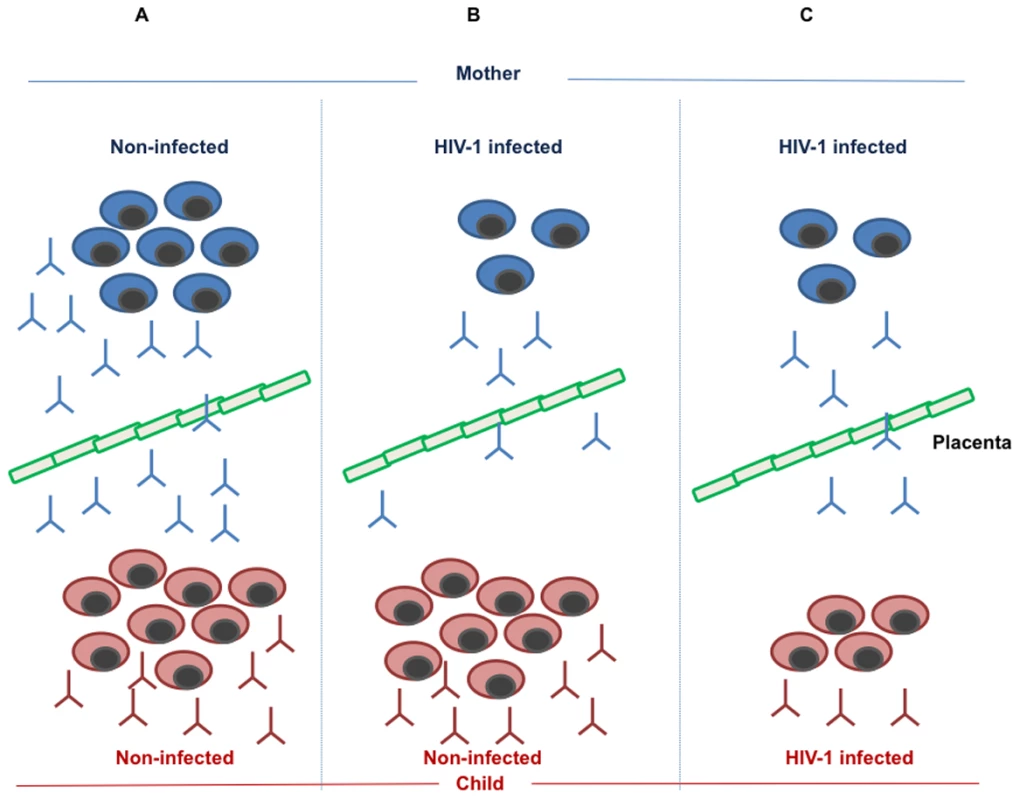
(A) In the non-infected mother, a normal number of memory B cells produce a protective level of measles specific antibodies, which are transmitted to the child via the placental barrier. In addition, the child will respond to measles vaccination by producing memory B cells and specific antibodies. (B) The HIV-1-infected mother loses a large number of memory B cells as a result of pathogenic mechanisms linked to HIV-1 infection; this phenomenon leads to a reduced amount of measles-specific antibodies in the mother and a low level of transmitted antibodies to the child. The HIV-1-exposed, non-infected child, is, however, competent to respond to measles vaccination by generating protective levels of measles antibodies and measles-specific memory B cells. (C) As a consequence of HIV-1 infection, both the HIV-1-infected mother and child lose measles-specific memory B cells formed upon measles natural infection or vaccination. This leads to a low, non-protective level of measles antibodies in the mother, a low level of antibodies transmitted through the placental barrier to the child, and a low, non-protective level of measles-specific antibodies produced from the infected child upon vaccination. Our studies on the loss of memory B cells strongly suggest that this pathogenic mechanism may be causing a reduced level of protective anti-measles antibodies in mothers with HIV-1; it is also very likely that the levels of measles antibodies in breast milk of mothers with HIV-1 may be reduced in comparison to that of healthy women. The loss of memory B cells was positively correlated to the number of CD4+ T cells, a pivotal hallmark of immune deficiency during HIV-1 infection [17]. It is likely that an increased number of CD4+ T cells following highly active antiretroviral therapy (HAART) may lead to a new generation of measles-specific memory B cells through repeated antigenic exposure in countries with high measles prevalence.
Active HIV-1 replication correlates to the lack of development of an adequate serological memory as measured by the poor response to measles vaccination occurring in children with HIV-1 [21]. We showed that HAART treatment administered early after birth led to control of HIV-1 replication and also preserved the evolution of the memory B cell compartment and the likelihood of response to childhood vaccines. In children treated after 1 year of age, a decline in memory B cells was observed, accompanied by a modest response to measles vaccination. When matched with the measles vaccination studies conducted in developing countries, our findings indicate that a low level of protective measles antibodies in children with HIV-1, resulting from the impaired incapacity to mount serological memory, may represent the cause of measles outbreaks in countries with high levels of HIV-1 infection.
It is encouraging that measles catch-up vaccination programmes have been shown to reduce measles morbidity and mortality in southern Africa, although children born to mothers with HIV-1 remained highly susceptible to measles infection and its lethal consequences [22]. In conclusion, the recommended vaccination schedule to eradicate measles may be inadequate in countries with a high proportion of adults and children with HIV-1. According to the findings presented in this article, we propose that HAART should be administered to children and adults with HIV-1 prior to measles vaccination since HAART improves the capacity to establish long-term serological memory and maintain memory B cell responses in individuals with HIV-1.
Zdroje
1. World Health Organization (WHO)
2011
Measles surveillance data.
Available: http://www.who.int/immunization_monitoring/diseases/measles_monthlydata/en/index.html. Accessed 13 January 2011
2. WHO
2010
WHO/UNICEF joint statement - global plan for reducing measles mortality 2006–2010.
Available: http://www.who.int/immunization/documents/WHO_IVB_05.11/en/index.html. Accessed 13 January 2011
3. SiegfriedN
WiysongeCH
PienaarD
2010
Too little, too late; measles epidemic in South Africa.
Lancet
376
160
160
4. EmbreeJE
DattaP
StackiwW
SeklaL
BraddickM
1992
Increased risk of early measles in infants of human immunodeficiency virus type 1-seropositive mothers.
J Infect Dis
165
262
267
5. de Moraes-PintoMI
AlmeidaAC
KenjG
FilgueirasTE
TobiasW
1996
Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection.
J Infect Dis
173
1077
1084
6. ScottS
MossWJ
CousensS
BeelerJA
AudetSA
2007
The influence of HIV-1 exposure and infection on levels of passively acquired antibodies to measles virus in Zambian infants.
Clin Infect Dis
45
1417
1424
7. MossWJ
FisherC
ScottS
MonzeM
RyonJJ
2008
HIV type 1 infection is a risk factor for mortality in hospitalized Zambian children with measles.
Clin Infect Dis
46
523
527
8. TrinchieriG
1997
Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12 , IFN-gamma).
Curr Opin Immunol
9
17
23
9. ClericiM
ShearerGM
1993
A TH1–TH2 switch is a critical step in the etiology of HIV infection.
Immunol Today
14
107
111
10. KarpCL
WysockaM
WahlLM
AhearnJM
CuomoPJ
SherryB
TrinchieriG
GriffinDE
1996
Mechanism of suppression of cell-mediated immunity by measles virus.
Science
273
228
231
11. WHO
2004
Measles vaccines.
Wkly Epidemiol Rec
79
130
142
12. HelfandRF
WitteD
FowlkesA
GarciaP
YangC
2008
Evaluation of the immune response to a 2-dose measles vaccination schedule administered at 6 and 9 months of age to HIV-infected and HIV-uninfected children in Malawi.
J Infect Dis
198
1457
1465
13. NairN
MossWJ
ScottS
MugalaN
NdhlovuZM
2009
HIV-1 infection in Zambian children impairs the development and avidity maturation of measles virus-specific immunoglobulin G after vaccination and infection.
J Infect Dis
200
1031
1038
14. FarquharC
WamalwaD
SeligS
John-StewartG
MabukaJ
2009
Immune responses to measles and tetanus vaccines among Kenyan human immunodeficiency virus type 1 (HIV-1)-infected children pre - and post-highly active antiretroviral therapy and revaccination.
Pediatr Infect Dis J
28
295
299
15. ZandvoortA
TimensW
2002
The dual function of the splenic marginal zone: essential for initiation of anti-TI-2 responses but also vital in the general first-line defense against blood-borne antigens.
Clin Exp Immunol
130
4
11
16. CohenOJ
PantaleoG
LamGK
FauciAS
1997
Studies on lymphoid tissue from HIV-infected individuals: implications for the design of therapeutic strategies.
Springer Semin Immunopathol
18
305
322
17. TitanjiK
De MilitoA
CagigiA
ThorstenssonR
GrutzmeierS
2006
Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection.
Blood
108
1580
1587
18. De MilitoA
NilssonA
TitanjiK
ThorstenssonR
ReizensteinE
2004
Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection.
Blood
103
2180
2186
19. De MilitoA
MorchC
SonnerborgA
ChiodiF
2001
Loss of memory (CD27) B lymphocytes in HIV-1 infection.
AIDS
15
957
964
20. MoirSFA
2009
B cells in HIV infection and disease.
Nat Rev Immunol
9
235
245
21. PensierosoS
CagigiA
PalmaP
NilssonA
CapponiC
2009
Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children.
Proc Natl Acad Sci U S A
106
7939
7944
22. BiellikR
MademaS
TaoleA
KutsulukutaA
AlliesE
2002
First 5 years of measles elimination in southern Africa: 1996–2000.
Lancet
359
1564
1568
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance inČlánek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



