-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
The prevalence of antibiotic resistance genes in pathogenic bacteria is a major challenge to treating many infectious diseases. The spread of these genes is driven by the strong selection imposed by the use of antibacterial drugs. However, in the absence of drug selection, antibiotic resistance genes impose a fitness cost, which can be ameliorated by compensatory mutations. In Streptococcus pneumoniae, β-lactam resistance is caused by mutations in three penicillin-binding proteins, PBP1a, PBP2x, and PBP2b, all of which are implicated in cell wall synthesis and the cell division cycle. We found that the fitness cost and cell division defects conferred by pbp2b mutations (as determined by fitness competitive assays in vitro and in vivo and fluorescence microscopy) were fully compensated by the acquisition of pbp2x and pbp1a mutations, apparently by means of an increased stability and a consequent mislocalization of these protein mutants. Thus, these compensatory combinations of pbp mutant alleles resulted in an increase in the level and spectrum of β-lactam resistance. This report describes a direct correlation between antibiotic resistance increase and fitness cost compensation, both caused by the same gene mutations acquired by horizontal transfer. The clinical origin of the pbp mutations suggests that this intergenic compensatory process is involved in the persistence of β-lactam resistance among circulating strains. We propose that this compensatory mechanism is relevant for β-lactam resistance evolution in Streptococcus pneumoniae.
Published in the journal: . PLoS Pathog 7(2): e32767. doi:10.1371/journal.ppat.1002000
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002000Summary
The prevalence of antibiotic resistance genes in pathogenic bacteria is a major challenge to treating many infectious diseases. The spread of these genes is driven by the strong selection imposed by the use of antibacterial drugs. However, in the absence of drug selection, antibiotic resistance genes impose a fitness cost, which can be ameliorated by compensatory mutations. In Streptococcus pneumoniae, β-lactam resistance is caused by mutations in three penicillin-binding proteins, PBP1a, PBP2x, and PBP2b, all of which are implicated in cell wall synthesis and the cell division cycle. We found that the fitness cost and cell division defects conferred by pbp2b mutations (as determined by fitness competitive assays in vitro and in vivo and fluorescence microscopy) were fully compensated by the acquisition of pbp2x and pbp1a mutations, apparently by means of an increased stability and a consequent mislocalization of these protein mutants. Thus, these compensatory combinations of pbp mutant alleles resulted in an increase in the level and spectrum of β-lactam resistance. This report describes a direct correlation between antibiotic resistance increase and fitness cost compensation, both caused by the same gene mutations acquired by horizontal transfer. The clinical origin of the pbp mutations suggests that this intergenic compensatory process is involved in the persistence of β-lactam resistance among circulating strains. We propose that this compensatory mechanism is relevant for β-lactam resistance evolution in Streptococcus pneumoniae.
Introduction
Streptococcus pneumoniae is the causal agent of human infections such as otitis, pneumonia and meningitis, which particularly affect pediatric patients. Penicillin and other β-lactams (βLs) are the treatments of choice for any pneumococcal infection. However, β-lactam resistance has been growing steadily since the first clinical resistant isolate was reported in the 1960s, with its dissemination greatly threatening the clinical efficacy of these compounds [1]. βLs act by binding to the active sites of PBPs, which are involved in peptidoglycan synthesis, thus altering the normal cell wall formation and inducing cell death by lysis. Specifically, PBPs catalyze the last two stages of peptidoglycan biosynthesis, namely transglycosylation and transpeptidation. Its ability to uptake exogenous DNA by induction of competence allows S. pneumoniae to acquire antibiotic resistance mutations. β-lactam resistance results from the acquisition of mutations within the pbp2b, pbp2x, and pbp1a genes, causing multiple amino acid changes in PBP2b, PBP2x, and PBP1a, which decrease the enzyme affinity for βLs [2]. Exogenous DNA containing pbp mutations can be provided by β-lactam resistant (βLR) strains of S. pneumoniae or by other Streptococcus species that cohabit the same niche, and is incorporated into the chromosome by homologous recombination [3].
The rise in βL resistance detected among circulating strains is due to their ability to acquire pbp genes, as mentioned above, in association with the wide therapeutic use of βL compounds, which could act as a selective pressure [4]. In the absence of antibiotic therapy, bacterial antibiotic resistance genes commonly result in reduced fitness. Nevertheless, by acquiring intra or intergenic compensatory mutations, certain clinical and laboratory strains are able to restore this fitness cost [5], [6]. Presumably, this compensation allows these strains to compete with susceptible strains in their natural niches. In S. pneumoniae, ciprofloxacin-resistant isolates have a low prevalence due to a fitness cost imposed by point mutations in the genes that encode DNA polymerase and DNA gyrase [7], [8]. A putative relationship has also been suggested between βL resistance and the loss of virulence in pneumococcal clinical strains [9], [10]. Likewise, Rieux and coworkers [11] described a loss of virulence in single and double isogenic βLR mutants, obtained by the transformation of pbp2x and pbp2b mutations amplified from βLR clinical isolates. Related to this, Trzcinski and coworkers, studying clinical isolates [12], reported an increase in fitness cost when βL resistance was conferred by the pbp2b pbp2x pbp1a mutations acquired by sequential transformation events.
In this work, we studied the clinical βLR strains belonging to a new serotype-14 variant of the Spain9V-3 international clone. This variant was isolated in Argentina and is indistinguishable by MLST and sequence analysis of the cps genes from those isolated in Baltimore, USA [13]. We demonstrated that the sequential acquisition of pbp mutations to develop βL resistance is closely associated to an intergenic compensatory process, which can restores the fitness cost imposed by pbp2b mutations and may favor the persistence and spread of βL resistance in S. pneumoniae.
Results
Fitness cost of β-lactam resistance in S. pneumoniae
The fitness cost imposed by pbp2b, pbp2x and pbp1a mutations that confer βL resistance in S. pneumoniae was evaluated. These pbp alleles were obtained from βLR clinical strains that belonged to a new serotype-14 variant of the Spain9V-3 international clone, identified and characterized in our laboratory [13]. To generate strains bearing alleles of pbp2b, pbp2x, and pbp1a from clinical strains that conferred resistance, we used PCR to amplify the DNA region that encodes the transpeptidase domain of each mutated pbp gene (in pbp1a, the glycosyltransferase domain was also included; Fig. S1 in Supporting Information S1), where the βL resistance mutations are commonly localized. These PCR products containing the pbp mutations were introduced by transformation into the βL-susceptible laboratory strain Cp1015, and βLR mutants were selected by growth on agar plates supplemented with piperacillin for pbp2b, or with cefotaxime for pbp2x and pbp1a (Table S1 in Supporting Information S2). The pbp mutations were integrated into the chromosome by homologous recombination.
Although no growth alterations for single pbp2x or pbp1a mutants were found, which presented similar growth curves to that of Cp1015, pbp2b mutants revealed an uncommonly prolonged lag-phase (Fig. S2 in Supporting Information S1). Then, due to the fact that clinical strains (from which pbp genes were amplified to generate single pbp mutants) grew as well as the wild-type strain (data not shown), we decided to investigate whether the fitness cost resulting from pbp2b mutations was compensated for by associating the pbp2x and pbp1a genes amplified from the same βLR strain donor of pbp2b. For this purpose, double and triple pbp mutants were obtained by sequential transformation of single and double pbp mutants (in that order), and the βLR mutant selection was made by using the MIC (Minimal Inhibitory Concentration) increment produced by the acquisition of different pbp mutations (Table 1). For example, a 14-fold increase occurred in the piperacillin MIC level for the pbp2b mutant; a 10-fold increase was found in the cefotaxime MIC level for the pbp2x or pbp1a mutants (single or double pbp mutants), and a 14-fold increase resulted in the cefotaxime MIC level for triple pbp mutants. The individual and double pbp mutants displayed a transformation rate higher than 10−2/µg of DNA, similar to the wild-type strain. Natural competence was also assayed, but no alterations in spontaneous transformability were detected (Fig. S3 in Supporting Information S1). We observed a partial restitution of the growth curve for the double pbp2b pbp2x and pbp2b pbp1a mutants, while a clear restoration was found for the triple pbp mutant (Fig. S2 in Supporting Information S1). Interestingly, all seven pbp2b mutants constructed by transformation with different mutated pbp2b genes obtained from other non-susceptible clinical isolates (Cba strains, Table S1 in Supporting Information S2) and the Spain9V-3 ATCC strain displayed similar growth alterations (data not shown). These pbp2b alleles were sequenced, and a comparison between them revealed that the DNA sequences obtained from Cba-19, Cba-28, Cba-52, Cba-62 and ATCC 700671 (clone Spain9V-3) were identical (Fig. S4 in Supporting Information S1). It was also found that 5 amino acids were conserved in the transpeptidase domain for all the pbp2b sequences (P417, E443, I460, G481 and A494) obtained from the different βLR clinical strains.
Tab. 1. Relative competitive fitness of Streptococcus pneumoniae pbp mutants. 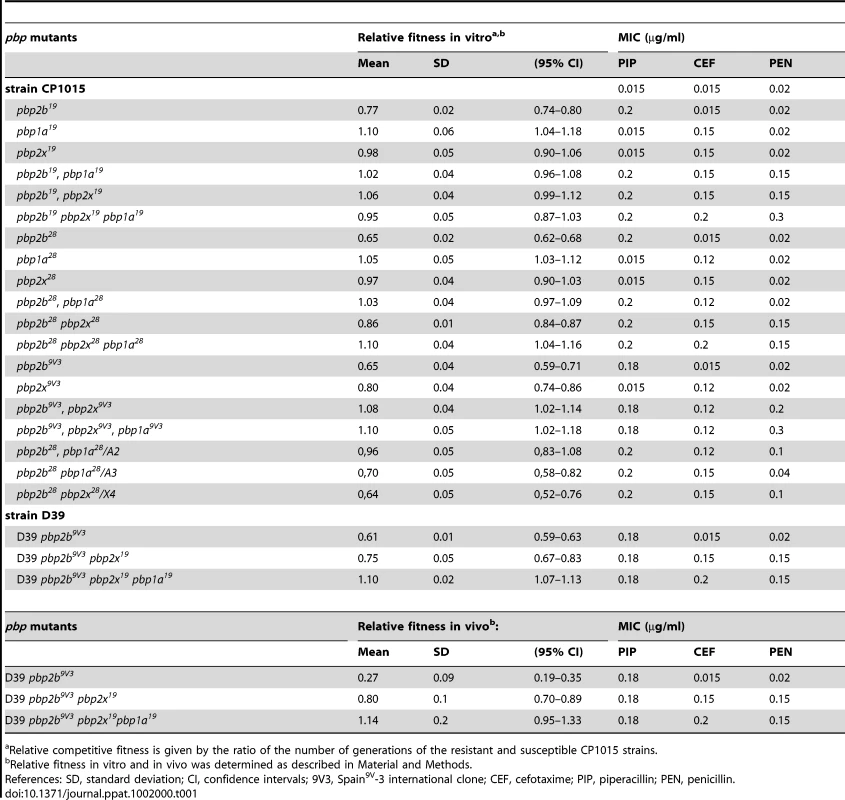
Relative competitive fitness is given by the ratio of the number of generations of the resistant and susceptible CP1015 strains. These results suggest that these substitutions could be responsible for the fitness cost detected in the pbp2b mutants. The pbp2b sequences of mutants constructed in the Cp1015 strain were identical to those obtained from the clinical strains, with each pbp2b sequence being coincident with the original pbp2b from the clinical strains used to transform the wild-type strain. Hereafter, the strain reference will be expressed as superscripts in the pbp mutations; for example, the pbp2b mutation from strain Cba-28 that was transformed into the Cp1015 strain will be indicated as pbp2b28.
Fitness cost compensation between pbp mutations associated to β-lactam resistance
All findings about compensation of growth alterations were similar to the results obtained by competitive fitness assays in vitro, when comparing the βL-susceptible Cp1015 strain with isogenic pbp mutants. The compensatory effect among pbp mutations was evident not only in triple, but also in pbp2b19 pbp1a19, pbp2b19 pbp2x19, pbp2b28 pbp1a28, and pbp2b9V3 pbp2x9V3 double mutants, recovering their values of relative fitness of 0.95–1.10 in the case of the triple pbp mutants (Table 1). We also observed that single pbp1a mutations obtained from Cba-19 and Cba-28 increased fitness in the wild-type strains (Table 1), and therefore should be considered as increasing-fitness mutations. Interestingly, we found a direct correlation between fitness compensation and an increase in the spectrum and level of βL resistance (Fig. 1; Table 1).
Fig. 1. Correlation plot between the fitness value of pbp mutants, obtained by using pbp genes from Cba-19 and Cba-28 clinical strains, and the penicillin MIC increase found (µg/ml). 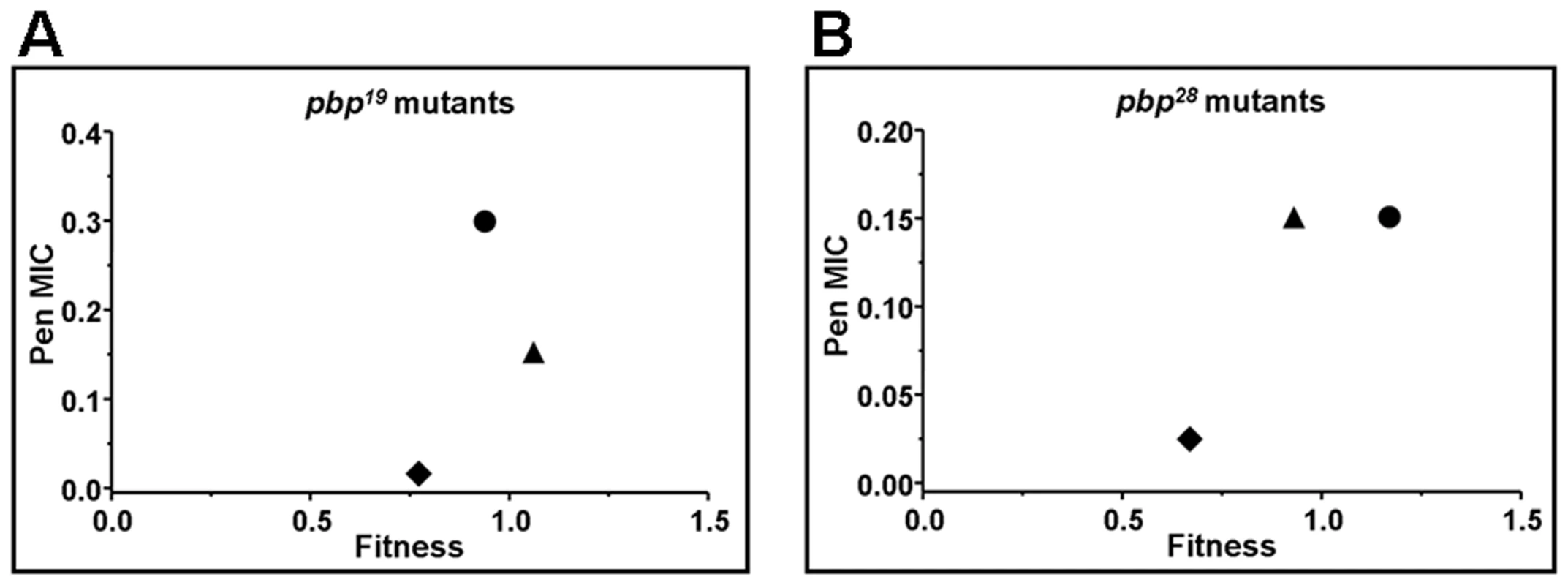
A) Correlation for Cp1015 pbp19 mutants, Cp1015 pbp2b19 (diamonds), Cp1015 pbp2b19 pbp2x19 (triangles), and Cp1015 pbp2b19 pbp1a19 pbp2x19 (circles). B) Correlation for Cp1015 pbp28 mutants, Cp1015 pbp2b28 (diamonds), Cp1015 pbp2b28pbp2x28 (triangles), and Cp1015 pbp2b28 pbp2x28 pbp1a28 (circles). The acquisition of pbp2x and pbp1a mutations from clinical βLR strains other than Cba-28 was also able to compensate the fitness cost found in pbp2b28 mutants. We observed that fitness cost was ameliorated in 7 of the 8 double pbp2b28 pbp2x mutants analyzed, which were constructed by transforming pbp2x genes from donor βLR strains different from Cba-28 into the pbp2b28 mutant (Table S2 in Supporting Information S2). When the transpeptidase regions of pbp2x sequences from the different mutants constructed in Cp1015 were compared, we observed that the pbp2x sequences in 4 mutants (pbp2b28 pbp2x12, pbp2b28 pbp2x52, pbp2b28 pbp2x54 and pbp2b28 pbp2x62) presented 10 conserved substitutions compared with Cp1015, with the exception of pbp2x52, which showed D278 in place of N278 (Fig. S5 in Supporting Information S1). Coincidently, pbp2x52 was the only one of these 4 pbp2x alleles that could not ameliorate the fitness cost of pbp2b28 (Table S2 in Supporting Information S2). This result suggests that the N278 residue might be involved in the compensatory mechanism of fitness, at least for the set of mutations present in pbp2x12, pbp2x54 and pbp2x62. Each pbp2x sequence used to transform Cp1015 was identical to the original pbp2x obtained from clinical strains (data not shown).
To corroborate the fitness compensation of pbp mutations observed in vitro, we also evaluated the relative fitness in vivo of single, double and triple pbp mutants using a model of intraperitoneal infection in C57BL/6 mice. Due to the fact that Cp1015 is an avirulent strain, we transferred the pbp mutations into the D39 strain, a virulent capsulated strain, which is able to infect C57BL/6 mice [14]. Single pbp mutants were obtained in D39 as described for Cp1015. Curiously, however, only crossed double and triple pbp mutants were recovered by transformation of the pbp2x and pbp1a genes from the Cba-19 strain into the D39 pbp2b9V3 mutant (Table 1), indicating that the βL resistance conferred by these pbp mutations was dependent on the genetic background of the recipient strain. In agreement with the data obtained by assays in vitro, we observed a similar compensatory phenomenon for the D39 pbp mutants in a mice model, with values of relative fitness of 0.27 for pbp2b9V3, 0.80 for pbp2b9V3 pbp2x19, and 1.14 for the triple pbp2b9V3 pbp2x19 pbp1a19 mutant being obtained (Table 1).
βL resistance is complex and it has been reported that is conferred by mosaic DNA fragments containing several mutations in the transpeptidase domain, which decrease the binding of βLs. With the purpose of identifying the amino acid substitutions that could be involved in the fitness compensation mechanism described, sub-parts of the pbp2x and pbp1a genes from the βLR Cba-28 strain were amplified by PCR (see schemes shown in Figs. S6 and S7 in Supporting Information S1), and PCR products were independently transformed into the pbp2b28 mutant, with the transformants being selected by cefotaxime resistance. We showed that fragments A2, A3 (both amplified from pbp1a28) and X4 (amplified from pbp2x28) conferred resistance, but only A2 could also compensate the fitness alterations (Table S3 in Supporting Information S2), reaching the same level as that obtained when pbp2b28 was transformed with the pbp1a28 fragment containing the glycosyltransferase and transpeptidase domains (Table 1). We analyzed the amino acid sequences deduced from the DNA sequences of A2 and A3, and found four substitutions shared between both regions (Fig. S8 in Supporting Information S1), which are probably involved in βL resistance. Interestingly, the E285Q mutation, localized in the transpeptidase domain [15], was only present in A2 (from pbp1a28). We propose that the E285Q mutation may have contributed to the fitness compensation mechanism. The presence of all these mutations was found in the double mutants pbp2b28 pbp1a28 and pbp2b28 pbp2x28 constructed in the Cp1015 genetic background as well as in the clinical Cba-28 strain.
In contrast with our results, Trzcinski and coworkers [12] reported that the fitness cost of pneumococcal penicillin-resistant strains increased with the number of resistant pbp alleles acquired. On comparing the predicted amino acid sequences of the pbp alleles studied here with those obtained by Trzcinski and coworkers, we found clear differences between them, detecting 9, 11 and 12 substitutions in PBP2x, PBP1a and PBP2b, respectively (Figs. S9, S10 and S11 in Supporting Information S1). This suggests that the discrepancy found between these works may be due to specific pbp mutations.
Cell division alterations associated to pbp mutations
It is known that PBPs are responsible for peptidoglycan synthesis. These proteins form the main component of the cell wall, and are involved in longitudinal wall growth and the cell division process. Morlot and coworkers [16] have proposed certain functions for PBP2b, PBP2a and PBP1a in S. pneumoniae, but the specific roles of PBPs in the growth and division phases are not yet well understood in bacteria. PBPs are also recognized as the targets for βLs, the most frequently used antimicrobials for treating bacterial infections. Therefore, considering that the interaction between drug resistance and the cell division processes remains unclear [4], we decided to investigate this connection. First, we examined the effects of pbp mutations on S. pneumoniae cell morphology, and then the putative cell division alterations. Microscopic examinations of single pbp1a28 and pbp2x28 mutants in Cp1015 strain did not display any morphological alterations, whereas the pbp2b28 mutant (Fig. 2A) and all 10 strains bearing pbp2b mutations from clinical strains showed a rod shape (data not shown). This atypical phenotype was also found for pbp2b mutants with a different genetic background to that of Cp1015, such as the R6 and D39 laboratory strains (data not shown).
Fig. 2. Effect of pbp mutations on cell morphology. 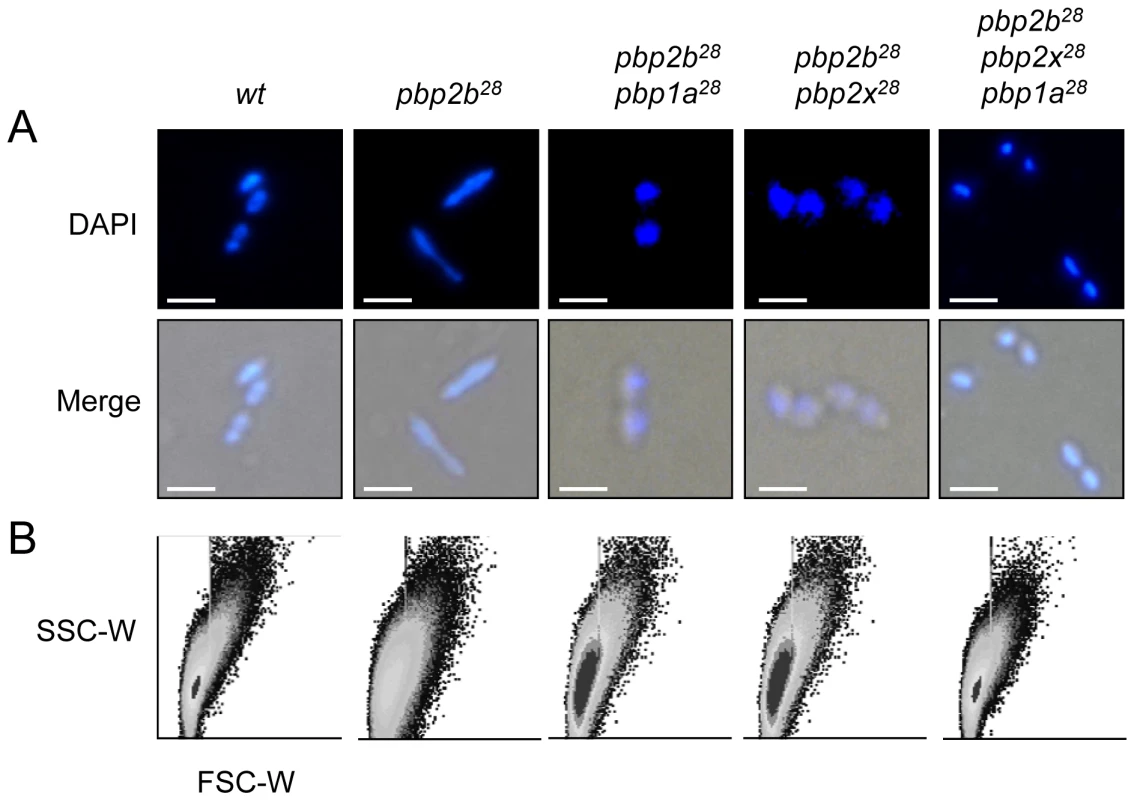
Cells were prepared for microscopy and stained with DAPI as described in Materials and Methods (bar scale, 5 µm). (A) Merged phase-contrast and DAPI fluorescence image of the pbp mutants showing a bacillar morphology, contrasting with the typical coccoid shape of the CP1015 (wt) strain. (B) Cell shape modifications of the cell populations detected by flow cytometry analysis. Exponentially growing bacterial cells were injected into a flow cytometer and the results were analyzed with the WinMDI software. To compare populations, the data for each strain were plotted on a two-dimensional graph (x-axis, forward scatter; y-axis, side scatter). Curiously, 46% of pbp2b28 cells had a rod-like shape (Fig. 2A), with the rest showing an apparent wild-type morphology. Electron microscopy revealed that the coccoid-shaped cells exhibited a variety of cell wall defects, including an abnormal septum position, atypical intracellular structures and frequent asymmetrical divisions (Figs. 3 and S13 in Supporting Information S1) compared with the wild-type strain (Figs. 3 and S12 in Supporting Information S1), with the rod-shaped cells exhibiting multiple septa (Figs. 3 and S13 in Supporting Information S1). Moreover, the double pbp2b28 pbp2x28 mutant displayed a coccoid morphology but with atypical septum localizations and peptidoglycan accumulation (Figs. 3 and S14 in Supporting Information S1). However, the triple pbp mutants had wild-type cell morphology and showed no ultrastructural alterations (Figs. 3 and S15 in Supporting Information S1).
Fig. 3. Electron microscopy analysis of cell morphology of pbp mutants. 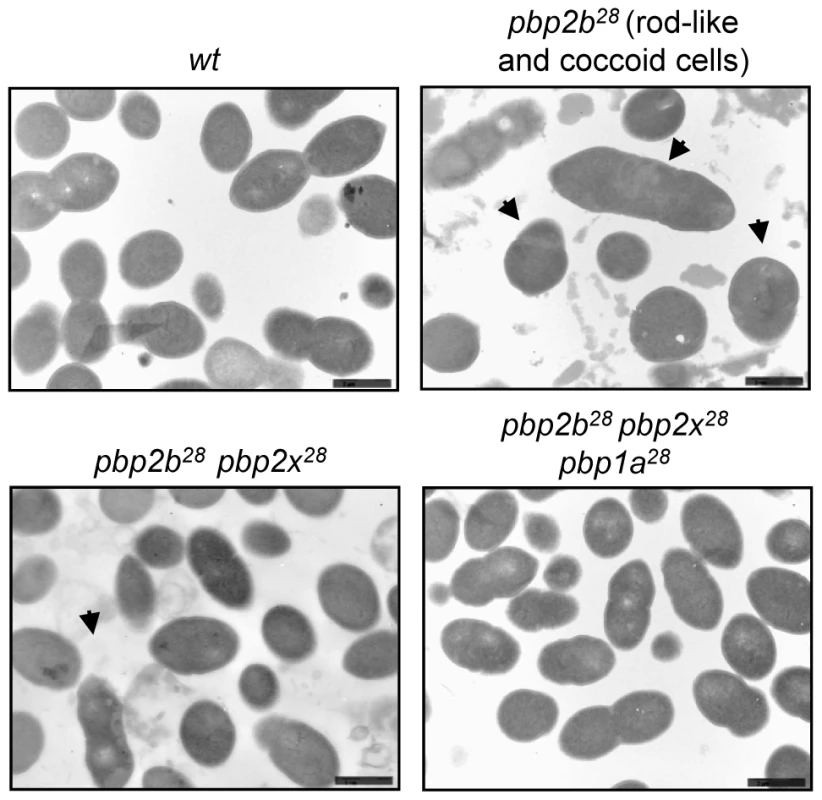
Microphotographs of the wild-type strain (Cp1015) revealed the typical morphology of S. pneumoniae, with a correct septum placement, division and symmetry of daughter cells. In the pbp2b28 mutant, two subpopulations were identified, namely rod-like and coccoid cells. The rod-like shaped cells showed multiple septa with incorrect formation and placement. The coccoid-shape cells exhibited multiple septa and intracellular accumulation of the cell wall, with the atypical septum localization resulting in irregular cell divisions and asymmetrical daughter cells (see arrows). The pbp2b28 pbp2x28 mutant partially restored the coccoid morphology, but conserved some cell alterations similar to those of the single pbp2b28 mutant. The triple pbp mutant showed a similar morphology to wt cells. Additional microphotographs are shown in Figs. S12, S13, S14 and S15 in Supporting Information S1 (bar scale, 1.6 µm). When cells were stained with fluorescein-labeled vancomycin (Van-FL), which localizes to sites of nascent peptidoglycan synthesis and clearly marks the septum location in the wild-type strain [17] (Fig. S16 in Supporting Information S1), an abnormal septum pattern was revealed in rod-shaped cells of the pbp2b28 mutants, suggesting a clear alteration in cell division (Fig. 4). However, this phenomenon was compensated in the double (pbp2b28 pbp2x28 or pbp2b28 pbp1a28) and triple pbp mutants (Fig. 4). The morphological variation was confirmed by flow cytometry analysis, which allowed determining the population distribution of pneumococci by cell size. These assays showed a displacement favoring a larger cell size in the pbp2b28 mutant, and the restoration of normal size in the triple pbp mutant (Fig. 2B). These results suggest that the septal alterations and the cellular enlargement found in the pbp2b28 mutant could have been responsible for its growth retardation.
Fig. 4. Septal localization in pbp mutants. 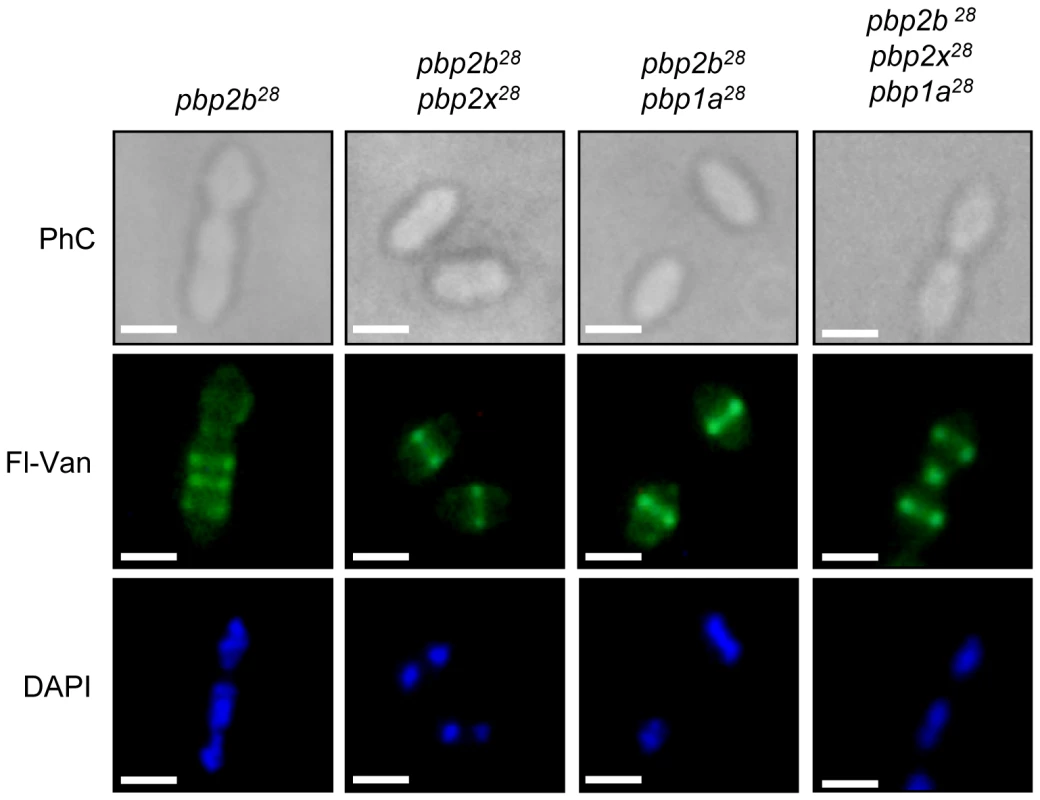
Exponentially grown cells were stained with DAPI and fluorescent vancomycin (Fl-Van, which labels nascent peptidoglycan synthesis and strongly marks septal localization) and analysed by using an epifluorescence microscope (see Material and Methods). The pbp2b showed a septal accumulation in rod-like shaped cells, whereas the double (pbp2b pbp2x or pbp2b pbp1a) and the triple pbp mutants restored their septal localization. Representative images are shown from experiments that were repeated independently three times (bar scale, 1 µm). To investigate the putative cause of these effects on cell morphology and fitness, we analyzed the stability of the proteins encoded by these pbp mutated genes using immunoblotting and inhibiting the protein synthesis by the addition of kanamycin. For these assays, we constructed C-terminal HA tagged PBPs to permit the detection of the proteins using an anti-HA monoclonal antibody. The gene constructs were inserted into the chromosome by insertion-duplication as described previously [18], and these genes were expressed as single copies under the control of their native promoters. These pbp-HA mutants showed the same phenotypes as the original pbp mutants, as well as the compensatory effects demonstrated in the double and triple pbp mutants (data not shown). We observed an increase in the half-life (>120 min) for PBP2b28 compared with the wild-type protein (21 min), not only in pbp2b28 (Fig. 5A) but also in the triple pbp mutant (Fig. 5C). Interestingly, we also detected an increased half-life for PBP1a28 (>120 min) and PBP2x28 (77 min) in the triple pbp mutant compared with half-life of PBP1a (31 min) and PBP2x (29 min) displayed in the wild-type strain (Figs. 5B and 5C). Our hypothesis is that these stability changes in the PBP mutants could have been responsible for the fitness/morphological alterations in the pbp2b mutant and the compensatory effects in the triple pbp mutant, and this will be discussed later.
Fig. 5. Stability in vivo of wild-type and mutant PBPs. 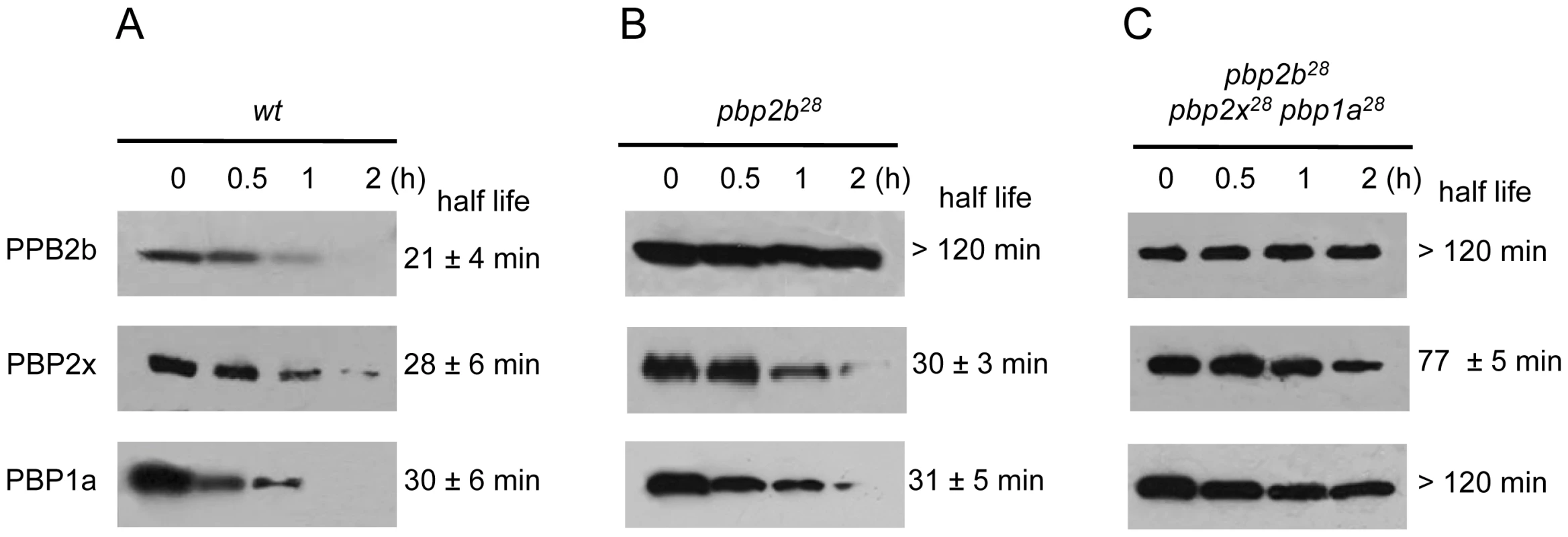
Strains Cp1015 (Panel A), Cp1015 pbp2b28 (Panel B) and Cp1015 pbp2b28pbp2x28 pbp1a28 (Panel C) expressing HA-tagged PBPs were grown in Todd Hewitt broth with 0.5% Yeast Extract to an OD600 nm of 0.2 prior to the addition of kanamycin (500 µg/ml) to inhibit protein synthesis. At the time intervals indicated, aliquots were withdrawn and analyzed by SDS-PAGE followed by immunoblotting with an anti-HA monoclonal antibody (see Material and Methods). We also investigated the putative cause of a morphological change in the pbp2b28 mutant, and particularly in the cell division process. It was previously proposed that the life cycle of bacterial cells consists of repeated controlled enlargement, septum formation, and cell division [19]. In this scenario, FtsZ is an essential protein, which was postulated as the force generator that drives the cell division process, since the correct localization of all proteins involved in this mechanism is dependent on FtsZ [19], [20]. To evaluate the impact of pbp mutations on cell division, we determined the FtsZ localization by immunofluorescence microscopy, using a polyclonal antibody against FtsZ. Before septum formation, the FtsZ division ring has been described to be localized at the mid cell, as we also observed for the wild-type strain (Fig. 6A). However, an atypical FtsZ placement was found in pbp2b cells, with an apparent helical structure rather than the mid-cell localization found in wild-type cells. In the pbp2b28 pbp2x28 mutants, although we observed a coccoid morphology, the FtsZ localization was still altered. In contrast, a total FtsZ placement restoration was found in the triple pbp mutants (Fig. 6A). The higher stability of PBP2b28 led us to speculate that this increased protein level could also cause a delocalization of this protein mutant. Therefore, we constructed the PBP2b-GFP and PBP2b28-GFP fusions, expressed ectopically from a multicopy plasmid, in order to study their localization in S. pneumoniae cells. We observed that PBP2b-GFP was localized equatorially in the wild-type strain as described previously [16]. However, PBP2b28-GFP revealed an atypical helical distribution, similar to FtsZ in the pbp2b28 mutant (Fig. 6B), suggesting that PBP2b could interact with FtsZ. To investigate this, we analyzed this putative interaction by using a bacterial two-hybrid system (Bacteriomatch II, Stratagene), confirming this hypothesis by the detection of a positive interaction between PBP2b28 (or PBP2b) and FtsZ, but not between PBP2b28 (or PBP2b) and PBP2x. This served as a control of specificity in addition to the positive and negative controls included in this system (Fig. 7).
Fig. 6. Protein localization in wild-type and pbp mutants by fluorescence microscopy. 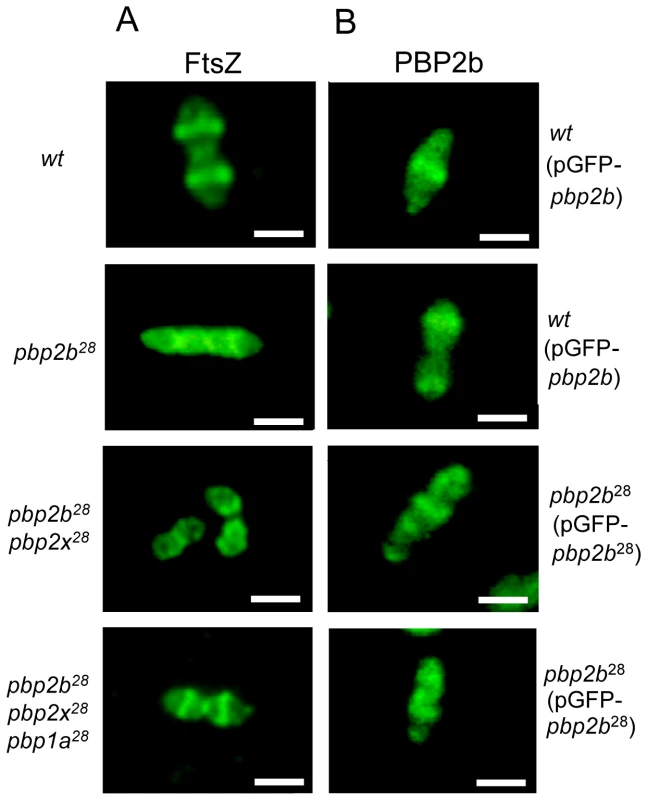
A) Localization of FtsZ. Microphotographs indicate the most common patterns of FtsZ localization through the cell cycle for wt and pbp mutants. Polyclonal antibody against FtsZ was used for its detection by immunofluorescence microscopy. In the Cp1015 (wt) strain, FtsZ is localized at the equatorial ring and septum, In Cp 1015 pbp2b28, the patterns suggest FtsZ delocalization and cell cycle disruption. The triple pbp mutant showed a wild-type FtsZ placement. B) Localization of PBP2b28-GFP. Microphotographs indicate the most common patterns found for PBP2b localization through the cell cycle in the wt and pbp2b28 mutant. Representative images are shown from experiments that were repeated independently three times (bar scale, 1 µm). Fig. 7. Bacterial two hybrid assay. 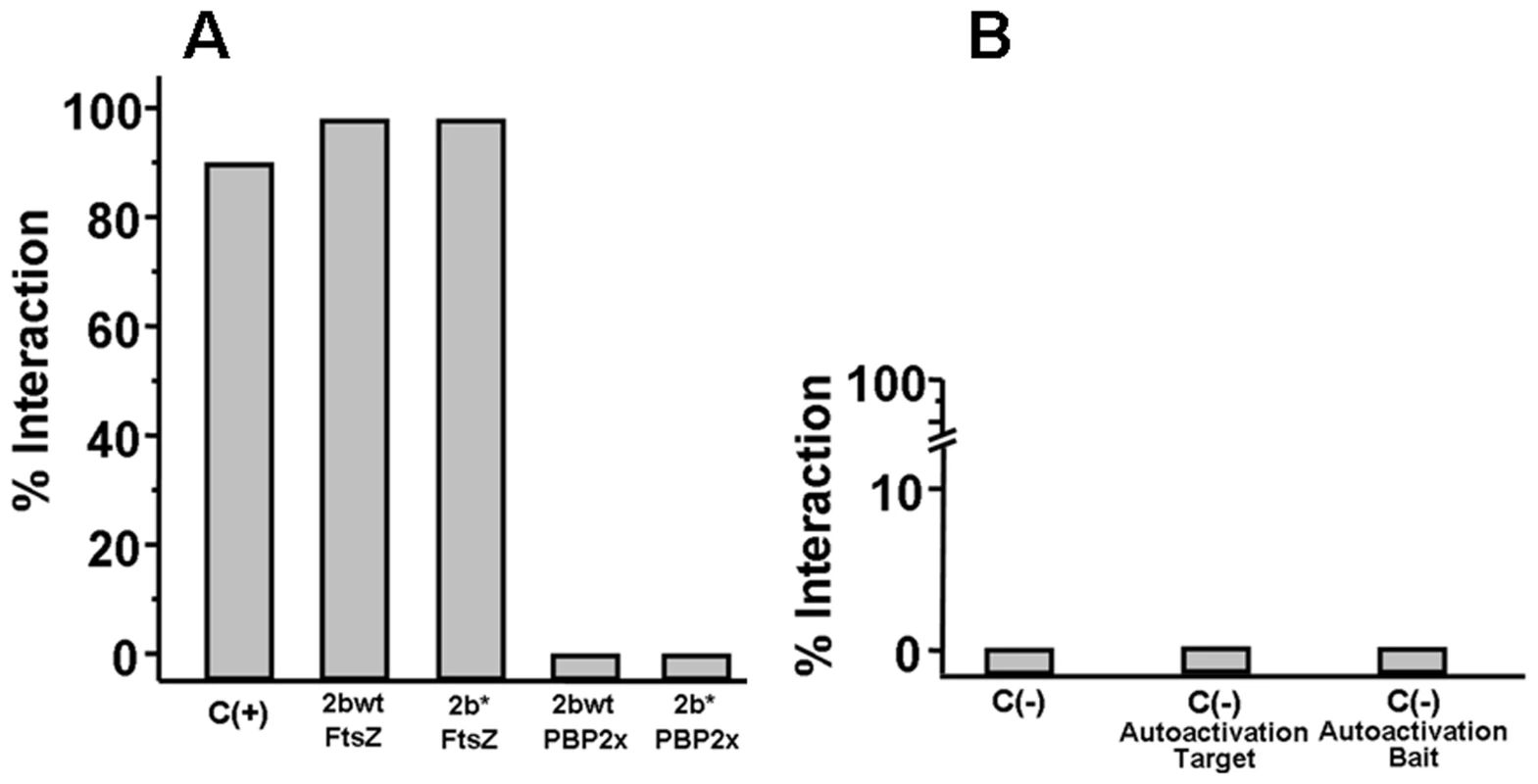
The percentage of interaction is defined as the ratio between the number of cells co-transformed able to grow in 3-AT selective medium and the total number of cells obtained in medium without 3-AT. The plasmid pair pBT-LGF2/pTRG-Gal11 (BacterioMatch II, Stratagene) was used as a positive control. A) Interaction of PBP2bwt-FtsZ and PBP2b28-FtsZ B) As negative controls, the following plasmid pairs were used: pBT/pTRG carrying codifying sequences of non-interacting proteins, C (-); pBT-empty/pTRG-recombinant, to evaluate the two-hybrid system auto-activation for the target proteins (-)/Autoactivation Target; pBT-PBP2b/pTRG-empty, as non-specific transcription activation control mediated by PBP2bwt or PBP2b28, C (-)/Autoactivation Bait. Discussion
Mutations associated with antibiotic resistance are known to impose a fitness cost in several bacterial species. Nevertheless, this physiological cost can be ameliorated by compensatory mutations in the same altered gene or in others involved in the developed resistance mechanism, thereby maintaining the same resistance level [21]. This compensatory phenomenon can occur with or without selective pressure. However, under antibiotic exposure, only those mutations able to maintain the resistance level will be selected, thus permitting the survival and persistence of resistant strains [22].
In S. pneumoniae, individual pbp2b or pbp2x mutations are considered primary βL resistance determinants that confer low-level resistance, whereas pbp1a mutations are acquired later and are responsible for increasing this level. In this work, only pbp2b mutations were related to a significant fitness cost, indicating that these mutations were a disadvantage when competing with wild-type strains. This finding indicates that the pbp2b mutants were only able to survive under antibiotic pressure due to their resistance. However, in the absence of antibiotics, the only way for these mutants to compete with wild-type strains is by improving their fitness by acquisition of compensatory mutations.
In this work, one of the most important findings is that these compensatory mutations are also responsible for an increase in βL resistance. It is well known that the combination of pbp mutations that confers βL resistance is very complex, and that pbp alleles have DNA mosaics with mutations in the region that encodes the transpeptidase domain. These DNA mosaics may differ by over 20% from DNA sequences from sensitive pbp genes and from each other, resulting in changes in amino acid of more than 10% [23]. In this scenario with PBPs showing multiple mutations, it is very difficult to distinguish those involved in resistance development from the alterations caused by the natural evolution of the genes. Therefore, the contribution of each mutation is unclear, with the exception of a few conserved mutations in the transpeptidase domains of PBPs. In an attempt to identify the amino acids involved in the compensatory mechanism described here, different fragments of pbp1a28 and pbp2x28 were transformed into the pbp2b28 mutant, and the resistance and fitness cost were evaluated. Although two fragments from the pbp1a28 gene were able to confer resistance, only A2 compensated the fitness cost caused by the pbp2b28 mutation. The amino acid sequence deduced from the DNA sequence of A2 revealed that the E285Q substitution might be involved in the compensatory mechanism.
On the other hand, when pbp2x alleles from different clinical strains were used to transform pbp2b28, only one of them (pbp2x52) was unable to ameliorate the fitness cost of this mutant. Four pbp2x alleles shared 9 substitutions compared with Cp1015, with the exception of pbp2x52, which differed in only 1 residue (D278 in the place of N278). This result suggests that N278, present in the pbp2x mutations that compensated the fitness cost of pbp2b28, could be also a compensatory mutation, at least in the set of mutations described for pbp2x12, pbp2x52, and pbp2x62.
Rieux and coworkers [11] studied the relationship between the acquisition of penicillin resistance and virulence, and demonstrated that the single and double pbp2b pbp2x mutants lost virulence in an intraperitoneal infection model in Swiss mice. After several passages in mice, the pbp2x mutations showed stability. However, for the pbp2b mutants, virulent revertants were recovered without intragenic modifications, suggesting that extragenic compensatory mutations were involved in this phenomenon. On the other hand, Trzcinski and coworkers [12] reported a rise in the βL resistance levels of the susceptible D39 strain, by the sequential transformation of the pbp genes obtained from clinical isolates. This event was accompanied by an increase in the fitness cost of a triple pbp mutant as determined by a rat nasal colonization model, but without growth alterations occurring in vitro. However, in the Trzcinski's work, no fitness compensation was detected among the pbp mutations obtained from isolates belonging to the Spain6B-2, Hungary19A-6, or serotype-9V Spain9V-3 international clones.
In contrast with these reports, we showed that pbp2x and pbp1a genes carrying specific mutations that conferred βL resistance, not only compensated the pbp2b-associated fitness cost in Cp1015 and D39 strains, but also increased the βL resistance levels. We also demonstrated that this that this compensatory mechanism was present in the clinical strains that belonged to the new serotype-14 variant of the Spain9V-3 pneumococcal clone identified in Argentina [13]. Related to this, several other studies have reported that the fitness cost associated to antibiotic resistant mutants depends on the specific mutations related to the resistant mechanism and the genetic background [5], [24], [25], [26], [27], [28]. Here, we also used strain D39, the same strain used by Trzcinski and coworkers [12], as an acceptor for pbp transformations. However, contrasting results were obtained, which might be explained by considering the different nature of the pbp mutations and the particular evolution of the clinical isolates carrying these mutations. These assumptions were supported by an amino acid sequence analysis of the PBPs encoded by the mutated pbp2b, pbp1a and pbp2x genes from the clinical strains used in this study, with and a clear difference being found with those described by Trzcinski and coworkers [12].
When the βL resistance of the double and triple pbp mutants was analyzed, we found an increase not only in its level, but also in its spectrum. We postulate a direct correlation between an increase in βL resistance and fitness cost compensation. A similar mechanism was previously shown for fluoroquinolone resistance, by analyzing the parC/E and gyrA mutations obtained from clinical pneumococcal strains using competitive fitness assays in vitro [8]. Recently, this phenomenon was also described for fluoroquinolone resistance in E. coli by fitness assays in vitro and in vivo using a laboratory strain [28].
Although the main focus of the present work was the compensatory evolution associated to the pbp mutations that conferred βL resistance, it is important to highlight another topic linked to PBPs, the cell division process. In particular, we studied the putative mechanism that caused morphological changes in pbp2b mutants. It is already known that PBPs play an important role in cell wall synthesis [29], but morphological alterations have not been previously described for pbp mutants in the absence of β-lactams. In this work, we described an atypical transition from a coccoid to a rod-like shape and also other alterations caused by pbp2b mutated genes obtained from βLR clinical isolates, with this morphological phenotype being reproduced in different laboratory strains. In a previous work, it was proposed that PBP2b, an essential class-B PBP, is involved in peripheral peptidoglycan synthesis in S. pneumoniae [16]. Related to this, bacterial cell morphogenesis is regulated by a controlled peptidoglycan synthesis, where PBP activity is essential for the normal progression of this process [30], and also by an orchestrated interaction of cytoskeleton proteins triggered by FtsZ polymerization [31]. The septal accumulation observed by Van-FL staining in the pbp2b mutants indicated an abnormal cell division. To investigate the impact of pbp2b mutations on this process, we analyzed the FtsZ localization by immunofluorescence in the pbp2b mutant and found an unusual localization pattern, revealing structures that resembled the helical Van-FL-stained sidewall shown by Bacillus subtilis [17]. To search for the reason why the pbp2b mutants showed these morphological alterations, we also measured the stability of PBP2b28 and found that PBP2b28 showed an increased half-life, in contrast with that observed in the wild-type strain. The fact that PBP2b28 displayed a higher protein level led us to investigate a putative delocalization of this protein in a pbp2b28 genetic background. Immunofluorescence assays showed that PBP2b-GFP fusion localized equatorially as FtsZ did in the wild-type strain described by Morlot and coworkers [16]. In contrast, PBP2b28-GFP fusion revealed an atypical helical distribution similar to FtsZ in the pbp2b28 mutant. It is possible that protein-protein interactions may have altered the localization of certain proteins, and we suspected that PBP2b28 could have interacted with FtsZ, thus modifying its normal emplacement. Using a bacterial two-hybrid system, we demonstrated that FtsZ was able to interact with PBP2b28 as well as with the wild-type protein, but not with PBP2x. Considering that PBP2b28 was delocalized, we propose that this interaction of PBP2b28 with FtsZ led to a misplacement of this protein, and consequently, to an aberrant cellular morphology that caused a decreased fitness. Moreover, these results support the idea that this interaction is essential for the control of cell morphogenesis in S. pneumoniae, as suggested for E. coli [32], [33]. In addition to this phenomenon, we suspect that PBP2b28 presented a decreased transpeptidase activity, as all the PBP mutants confer βLR by a decreased affinity to these compounds [34], [35]. Because PBP2b has been involved in the elongation stage during cell division [16], we propose that as PBP2b28 could not elongate efficiently then these cells accumulated septa, as demonstrated by Van-FL staining assays, and this could be another cause of the morphological alterations found in the pbp2b28 mutant. In our laboratory, work is now in progress in an attempt to gain a better understanding of this complex process and this not well understood molecular mechanism.
To try to explain the putative cause of compensation of the PBP2b28 alterations by PBP1a28 and PBP2x28, we also analyzed the stability of both mutant proteins. We observed that PBP1a28 and PBP2x28 displayed an increased half-life in the triple pbp mutant as we had also for PBP2b28. Considering that all these PBPs have transpeptidase activity, these results suggest that PBP1a28 and PBP2x28 have a complementary function on PBP2b28, based mainly on their increased protein levels. We propose that this event could have caused the compensatory effect on fitness and the morphological alterations in the pbp2b28 mutant.
In the current work, we reported compensatory extragenic mutations that restored the fitness alterations imposed by mutations related to βL resistance. Given that the fitness compensation determined by assays in vivo and in vitro were similar, our data suggest that the selection of fitter strains may have taken place in the natural habitat of S. pneumoniae. This phenomenon has an important clinical relevance, since these compensated pbp mutants cannot only maintain but also increase the βL resistance levels, in contrast with previous reports [22]. Our results also indicate that clinical strains acquired those pbp mutations which improved their resistance-associated fitness cost, producing a clear competitive and selective advantage, and thus raising the potential spreading of βL resistance. We think it is probable that S. pneumoniae exploited its high transformability to acquire the compensatory pbp mutations that are essential for its persistence and dissemination. This idea is supported by the experimental data, which indicated that the pbp mutants showed no transformability alterations, and that the pbp mutants could be selected at a transformation rate higher than 10-2/µg of DNA, thus demonstrating that compensatory mutations can be acquired in a one-step transformation. If this compensatory evolution was due to the sequential incorporation of adaptive mutations, then the occurrence of this phenomenon may increase rapidly under antibiotic pressure. Therefore, it is possible that certain mutations were selected for their compensatory effect on fitness in addition to their contribution in developing higher βL resistance levels.
It is known that the emergence and stability of antibiotic resistance is a complex biological process, being driven by different factors such as the volume of antibiotic used, the rate of resistant mutant formation, the fitness cost imposed and the compensatory mechanisms to improve that cost [22]. Many studies on the effect of a reduction in β-lactam consumption have reported a sustained resistance level to S. pneumoniae [22], suggesting that other factors in addition to antibiotic exposition are contributing to the persistence and the evolution of βL resistance. In the present work, the compensatory mechanism seems to be an important factor, favoring environmental long-term persistence and the spreading of βLR strains. Moreover, it is known that fitness compensation improves the dissemination ability of resistant strains, an essential trait that characterizes pneumococcal clones. However, we could not show that this phenomenon was particularly associated to the pbp genes for other successful international clones. As mentioned above, fitness restoration has not been reported for pbp mutations obtained from clinical strains that belong to the Spain6B-2, Hungary19A-6, and serotype-9V Spain9V-3 international clones [12]. Nevertheless, we demonstrated that a compensatory mechanism was present, at least in the new serotype-14 variant of the Spain9V-3 clone recently characterized in our laboratory [13], and also in a serotype-9V reference strain of this clone (ATCC 700671) isolated in France [36]. Therefore, this model contributes to the understanding of βL resistance evolution in S. pneumoniae.
Materials and Methods
Bacterial strains, growth conditions and susceptibility testing procedures
The bacterial strains used in this work are listed in Table S1 in Supporting Information S2. βLR pneumococcal strains were obtained from invasive infections of pediatric patients and belonged to the Spain9V-3 international clone [13]. Cells were routinely grown at 37°C in Todd-Hewitt broth supplemented with 1% bovine serum albumin. For antimicrobial susceptibility testing, strains were grown at 37°C in a 5% CO2 atmosphere on Mueller-Hinton agar with 5% defibrinated sheep blood. Penicillin, cefotaxime, and piperacillin MICs were determined by agar dilution following a CLSI protocol [37].
PCR conditions and transformation assays
To amplify the transpeptidase region of the pbp1a, pbp2b, and pbp2x genes by PCR, we used the F1a/R1a, F2b/R2b, and F2x/R2x [13] primer pairs, respectively (Fig. S1 in Supporting Information S1). The internal fragments of the pbp1a gene were amplified and sequenced with the following primer pairs: Fa1/Ra1 (from position 94 to 234); Fa2/Ra2 (from position 599 to 1002), Fa3/Ra3 (from position 881 to 1294), Fa4/Ra4 (from position 385 to 521) and Fa5-Ra5 (from position 476 to 629) (Fig. S6 in Supporting Information S1). The internal fragments of the pbp2x gene were amplified and sequenced with the following pair primers: Fx1/Rx1 (from position 125 to 271), Fx2/Rx2 (from position 238 to 370); Fx3/Rx3 (from position 320 to 469) and Fx4-Rx4 (from position 445 to 738) (Fig. S7 in Supporting Information S1). The internal fragments of the pbp2b gene were amplified and sequenced with the following primer pairs: Fb1/Rb1 (from upstream of pbp2b to 114); Fb2/Rb2 (from position 69 to 256); Fb3/Rb3 (from position 217 to 439); Fb4/Rb4 (from position 374 to 557) and Fb5/Rb5 (from position 502 to downstream pbp2b) (Fig. S17 in Supporting Information S1). The primers sequences are detailed in Table S4 in Supporting Information S2. PCR products were amplified using the following parameters: initial denaturation at 94°C for 4 min, 30 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 30 s, elongation at 72°C for 1 min, and a final extension at 72°C for 10 min. Chromosomal DNA was isolated using the Wizard Genomic DNA purification kit (Promega), following the manufacturer's instructions. The PCR products were amplified from chromosomal DNA and used to genetically transform the Cp1015 and D39 strains as described previously [38]. Transformants were selected on Mueller-Hinton agar plates supplemented with 5% defibrinated sheep blood containing 0.05 µg/ml piperacillin or 0.1–0.3 µg/ml cefotaxime. Natural competence was performed as described previously (3).
Flow cytometry analysis
Strain Cp1015 and its respective pbp mutants were individually grown to the mid-logarithmic phase in Todd-Hewitt broth at 37°C. Cells were then washed three times and resuspended in PBS. In order to analyze cell shape modifications by flow cytometry analysis, bacteria were injected into a FACS Aria. Between 50,000 and 100,000 events were counted and analyzed by using WinMDI software. To compare populations, the data for each strain were plotted on a two-dimensional graph (x-axis, forward scatter; y-axis, side scatter).
Determination of relative fitness in vitro
Relative fitness was quantified as the average number of surviving progeny of a particular genotype, and this was compared with average number of surviving progeny of competing genotypes after a single generation. The wild-type strain genotype was normalized at wt = 1 and the fitnesses of other genotypes were measured with respect to that genotype [39]. The cost of a resistance mutation was determined by direct competition against the susceptible Cp1015 strain as described previously [7]. Individual strains were exponentially grown to an OD600 nm of 0.2 and cultures were diluted 2,000-fold. Mixed cultures containing equivalent amounts of the Cp1015 and pbp mutant cells (about 5×104 CFU/ml) were incubated in antibiotic-free medium for 6 h. These mixed cultures were then diluted 1,000-fold to avoid the typical lysis of S. pneumoniae cultures at the stationary phase, and cells were cultured for an additional 6 h. The number of viable cells was determined at 0, 6 and 12 h by plating serial dilutions of the culture on BHI agar with 5% defibrinated sheep blood, containing 0.05 µg/ml piperacillin (for pbp2b simple mutants), 0.1 µg/ml cefotaxime (for pbp double and triple mutants), or with no antibiotic. The number of susceptible cells was calculated by subtracting the number of resistant cells from the total cell number revealed by the CFU counts of the plates without drug. To determine the CFU numbers, the mean of four counts was calculated. The number of generations of the resistant and Cp1015 strains in the mixed culture was calculated by using the following formula: (log B - log A)/(log 2), where A is the number of CFU/ml at time zero and B is the number of CFU/ml at the end of each cycle (6 h and 12 h). The relative fitness of each strain was determined from the ratio of the number of generations of the resistant strain and Cp1015. The mean of four to nine replicate competition assays were determined. Statistical tests were performed using Instat software.
Determination of relative fitness in vivo
These assays using a mice model were performed as described previously [40], but with specific modifications for S. pneumoniae. Seven male C57BL/6, 4 to 5 weeks old (obtained from Comision Nacional de Energia Atomica, Ezeiza, Argentina), were inoculated intraperitoneally (under isoflurane anesthesia) with a mix of equal parts of D39 strain and an isogenic pbp mutant (1×105 CFU) in 0.1 ml 50 mM glucose (in PBS). These mice were sacrificed by CO2 asphyxiation after 2 days, and strains were recovered from each homogenized liver by plating onto BHI agar containing piperacillin, cefotaxime, or no antibiotic, as described above. Relative fitness was determined from the ratio of the number of generations of the resistant strains and strain D39. ANOVA statistical tests were performed with Instat software.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care. The experimental protocols performed were reviewed and approved by the Ethic Committee of the Facultad de Ciencias Químicas, Universidad Nacional de Cordoba (Permit number 15-07-68964). The Ethic Committee is constituted by the following professors of the Facultad de Ciencias Químicas-UNC: Laura Chiapello, Claudia Sotomayor, Margarita Briñon, Teresa Scimonelli, Santiago Quiroga, Mariana Contin and Graciela Granero. The protocols and the Animal Laboratory of our Institute (CIBICI-CONICET, Argentina) obtained an Animal Welfare Assurance from NIH, USA (Assurance number A5802-01, see http://grants.nih.gov/grants/olaw/assurance/500index.htm?Country=AR#GridTop).
All the clinical strains used in this work were provided by the strain collection of the Centro de Estudios Avanzados en Pediatría (Córdoba, Argentina). The Ethics Committee declared in writing that no formal ethical approval was needed to use these clinically obtained materials, because the specimens were remnant from patient samples of blood and nasopharyngeal swabs collected from routine analysis in bacteriology laboratories of public hospitals, and the data were analyzed anonymously.
Van-Fl staining
Pneumococcal strains were routinely grown to an OD600 nm of 0.2 in Todd-Hewitt broth. Samples were collected and incubated with 2 µg/ml Van-FL (Molecular Probes) for 20 minutes at 37°C. Cells were centrifuged, washed three times with PBS and fixed with 3% paraformaldehyde. After several washes with PBS, cells were spotted on glass slides, air dried, dipped in methanol at −20°C for 10 min, and allowed to dry at room temperature. The cells were then stained with DAPI (Sigma) at a final concentration of 0.2 µg/ml for 10 min before being observed, and images were acquired using a Nikon Eclipse TE-2000 epifluorescence microscope fitted with a Nikon Digital Sight DS-U1 camera. This was performed using ACT-U software, and images were processed with Adobe Photoshop CS version.
Electron microscopy
Cp1015 and the isogenic mutants pbp2b28, pbp2b28pbp2x28, and pbp2b28pbp2x28pbp1a were exponentially grown at 37°C in Todd-Hewitt medium, and samples for electron microscopy were then collected, centrifuged, and fixed with 4% formaldehyde-2% formalin in 0.1 M cacodylate buffer for 1 hour at room temperature. An additional fixation with 1% osmium tetroxide in cacodylate buffer was carried out for 1 hour at room temperature. These fixed cells were dehydrated using an increasing concentration of acetone, and embedded in polymerized Araldite at 60°C for 48 hours. Thin sections were obtained using a JEOL JUM-7 microtome equipped with a glass or gem grade diamond knife, and microphotography was performed with a Zeiss LEO 906E microscope.
Immunolocalization of FtsZ protein
Exponentially growing cells (OD600 nm of 0.25) were fixed with 3% paraformaldehyde for 15 min at room temperature and incubated for 45 min on ice. The fixed bacteria were washed three times in PBS (pH 7.4) and resuspended in GTE (50 mM glucose, 20 mM Tris-HCl, 10 mM EDTA), with a freshly prepared lysozyme solution in GTE being added to a final concentration of 2 µg/ml. 10 µl samples were immediately distributed onto poly-L-lysine microscope slides and air dried. The slides were dipped in −20°C methanol for 5 min, in −20°C acetone for 30 s, and then allowed to dry completely. After rehydration with PBS, the slides were blocked for 1 hour at 37°C with 2% bovine serum albumin (Sigma) in PBS (BSA-PBS). Cells were then incubated with a 1∶100 dilution in BSA-PBS of rabbit polyclonal anti-FtsZ antibody [41] for 1 hour at 37°C. After washing 10 times with PBS, samples were incubated with a fluorescein-conjugated secondary antibody in PBS (Alexa Fluor 488, Molecular Probes) for 30 min at 37°C in the dark. After removing the secondary antibody by washing samples several times with PBS, DAPI was added at a final concentration of 0.2 µg/ml and the samples were incubated for 10 min at room temperature. Slides were washed again, allowed to dry, and then mounted using Dako fluorescent mounting medium (Invitrogen). The final slides were stored at −20°C for several days. Images were acquired using a Nikon Eclipse TE 2000 epifluorescence microscope, and processed with Adobe Photoshop CS version.
Construction of strains expressing GFP-PBP2b
The pbp2b gene was amplified from Cp1015 wt and Cba-28 clinical strains with primers F2bf and R2bf (Table S4 in Supporting Information S2). The gfpmut3 gene was amplified from the pCM18 [42] plasmid using the primer pair Fgfp3/Rgfp3 (Table S4 in Supporting Information S2) in order to obtain a gfp copy under the control of the pneumococcal constitutive promoter, Pc [43]. The pbp genes were cloned into pGEMT-easy (Promega) and the gfpmut3 gene into pCRTOPO 2.1 (Invitrogen), thereby generating the pGEM-pbp and pGFPTOPO plasmids, respectively (Table S4 in Supporting Information S2). The pbp genes were exscinded by XhoI digestion of pGEM-pbp and inserted into the SalI-restricted pGFP-TOPO plasmid. The SalI site was located in pGFP-TOPO upstream the transcription origin of gfpmut3 and downstream the Pc. The resultant plasmid pGFP-TOPO-pbp contained a pbp2b N-terminal fusion to the gfpmut3 gene under the control of Pc. This construction was then removed from pGFP-TOPO-pbp by EcoRI digestion and inserted into the same restriction site in the E. coli-S. pneumoniae shuttle vector plasmid pAT18 [44]. The resultant vector was named pAT18pbp-gfp, and was used to transform the wild-type strain (Cp1015) and the Cp1015 pbp2b mutants. The transformant selection was made in BHI agar plates supplemented with 5% blood sheep and 2.5 µg/ml erythromycin. All constructions were confirmed by PCR and sequencing with the same primers used for the amplification of each gene.
Construction of strains expressing HA-tagged PBPs
Insertion duplication mutagenesis was used to construct strains expressing HA-tagged PBPs. Primer pairs F2btag/R2bHA, F2xtag/R2xHA and F1atag/R1aHA (Table S4 in Supporting Information S2) were designed to amplify approximately the last 300–400 bp of the pbp2b, pbp2x and pbp1a genes fused to the sequence of the HA tag. The PCR products were cloned into the XhoI and XbaI sites of plasmid pEVP3 [43], and the final constructions (named pEVP32bHA, pEVP32xHA, pEVP31aHA) were used to transform the strains Cp1015, Cp1015 pbp2b28 and Cp1015 pbp2b28 pbp2x28 pbp1a28. Selection of the transformants was made in BHI 5% sheep blood agar supplemented with chloramphenicol (2 µg/ml). All constructions were confirmed by PCR and sequencing with the external primers for amplified regions, as mentioned above.
Protein stability analysis of PBPs
Protein expression was inhibited in exponentially growing cultures in Todd Hewitt broth with 0.5% Yeast Extract (total volume 400 ml) at an OD600nm of 0.2 by the addition of kanamycin (500 µg/ml). One-hundred ml aliquots were withdrawn at 0, 30, 60 and 120 min after the addition of the antibiotic. Cells were immediately harvested, washed once with 1 ml of ice cold PBS and resuspended in 400 µl of ice cold MQ water plus Complete protease inhibitor cocktail (Roche), before being subjected to five freeze-thaw cycles followed by sonication using a Sonics VibraCell VCX130 sonicator (20 cycles, 30 s ON 30 s OFF, 40% amplitude). To solubilize the membrane proteins, 100 µl of 5X RIPA buffer (250 mM Tris pH 7.4, 750 mM NaCl, 5%NP-40, 2.5% Sodium deoxycholate, 0.5% SDS) were added and the samples were heated for 10 min at 95°C, and cell debris was removed by centrifugation for 15 min at 20,000 g. The total protein content was determined by using bicinchoninic acid assay [45]. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to nitrocellulose membranes of 25 µg of the protein extract, the membranes were probed with a mouse anti-HA primary antibody (1∶2,000; Abacam) and with a goat anti-mouse immunoglobulin G secondary antibody conjugated to horseradish peroxidase (1∶2,500; Invitrogen). Detection was performed with an enhanced chemiluminescence substrate (SuperSignal West Pico Chemiluminescent Substrate; Pierce) and Hyperfilm CL film (GE) using exposures of between 1 and 10 min. Western Blot bands were quantified using the Gel-Pro Analyzer v3.1 software and the half-life was calculated with Graph Pad Prism v5.3 software.
Protein-protein interaction assays
The bacterial two-hybrid system BacterioMatch II (Stratagene) was used to screen for interactions of PBP2b with FtsZ protein and PBP2x. The pbp2b coding sequence was amplified by PCR with the primer pair F2bdh/R2bdh (Table S4 in Supporting Information S2) from the Cp1015 and Cba-28 strains, digested with XhoI/BamHI and cloned into the XhoI/BamHI restricted bacterial two-hybrid vector pBT. In addition, the coding sequences of ftsZ and pbp2x were amplified by PCR with F2xdh/R2xdh, FftsZdh/RftsZdh (Table S4 in Supporting Information S2), respectively, digested with XhoI/BamHI or XhoI/BglII, in that order, and cloned into the pTRG vector cleaved by XhoI/BamHI. The E. coli XL1 Blue MR, the BacterioMatch II Validation Reporter strain (Stratagene), was co-transformed with the two plasmids. Growth cultures of the clones on M9 minimal media agar plates containing 2.5 mM 3-amino-1,2,4-triazol (3-AT) and 10 µg/ml of streptomycin were assessed according to the manufacturer's instructions.
Supporting Information
Zdroje
1. JacobsMR 2008 Antimicrobial-resistant Streptococcus pneumoniae: trends and management. Expert Rev Anti Infect Ther 6 619 635
2. ZapunAContreras-MartelCVernetT 2008 Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev 32 361 385
3. ClaverysJPPrudhommeMMartinB 2006 Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol 60 451 475
4. MacheboeufPContreras-MartelCJobVDidebergODessenA 2006 Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev 30 673 691
5. Maisnier-PatinSAnderssonDI 2004 Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res Microbiol 155 360 369
6. AnderssonDI 2006 The biological cost of mutational antibiotic resistance: any practical conclusions? Curr Opin Microbiol 9 461 465
7. BalsalobreLde la CampaAG 2008 Fitness of Streptococcus pneumoniae fluoroquinolone-resistant strains with topoisomerase IV recombinant genes. Antimicrob Agents Chemother 52 822 830
8. RozenDEMcGeeLLevinBRKlugmanKP 2007 Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother 51 412 416
9. BrilesDECrainMJGrayBMFormanCYotherJ 1992 Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun 60 111 116
10. Azoulay-DupuisERieuxVMuffat-JolyMBedosJPValleeE 2000 Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob Agents Chemother 44 1575 1577
11. RieuxVCarbonCAzoulay-DupuisE 2001 Complex relationship between acquisition of beta-lactam resistance and loss of virulence in Streptococcus pneumoniae. J Infect Dis 184 66 72
12. TrzcinskiKThompsonCMGilbeyAMDowsonCGLipsitchM 2006 Incremental increase in fitness cost with increased beta -lactam resistance in pneumococci evaluated by competition in an infant rat nasal colonization model. J Infect Dis 193 1296 1303
13. Albarracin OrioAGCortesPRTregnaghiMPinasGEEcheniqueJR 2008 A new serotype 14 variant of the pneumococcal Spain9V-3 international clone detected in the central region of Argentina. J Med Microbiol 57 992 999
14. CortesPRAlbarracín OrioAGRegueiraMPiñasGEEcheniqueJ 2008 Characterization of in vitro-generated and clinical optochin-resistant strains of Streptococcus pneumoniae isolated from Argentina. J Clin Microbiol 46 1930 1934
15. Di GuilmiAMMouzNAndrieuJPHoskinsJJaskunasSR 1998 Identification, purification, and characterization of transpeptidase and glycosyltransferase domains of Streptococcus pneumoniae penicillin-binding protein 1a. J Bacteriol 180 5652 5659
16. MorlotCZapunADidebergOVernetT 2003 Growth and division of Streptococcus pneumoniae: localization of the high molecular weight penicillin-binding proteins during the cell cycle. Mol Microbiol 50 845 855
17. DanielRAErringtonJ 2003 Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113 767 776
18. DesaiBVMorrisonDA 2006 An unstable competence-induced protein, CoiA, promotes processing of donor DNA after uptake during genetic transformation in Streptococcus pneumoniae. J Bacteriol 188 5177 5186
19. LanGWolgemuthCWSunSX 2007 Z-ring force and cell shape during division in rod-like bacteria. Proc Natl Acad Sci U S A 104 16110 16115
20. LutkenhausJAddinallSG 1997 Bacterial cell division and the Z ring. Annu Rev Biochem 66 93 116
21. AnderssonDILevinBR 1999 The biological cost of antibiotic resistance. Curr Opin Microbiol 2 489 493
22. BjorkmanJAnderssonDI 2000 The cost of antibiotic resistance from a bacterial perspective. Drug Resist Updat 3 237 245
23. DowsonCGHutchisonABranniganJAGeorgeRCHansmanD 1989 Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci U S A 86 8842 8846
24. ReynoldsMG 2000 Compensatory Evolution in Rifampin-Resistant Escherichia coli. Genetics 156 1471 1481
25. GagneuxSLongCDSmallPMVanTSchoolnikGK 2006 The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science 312 1944 1946
26. BjorkholmBSjolundMFalkPGBergOGEngstrandL 2001 Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci U S A 98 14607 14612
27. BottgerECSpringerBPletschetteMSanderP 1998 Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat Med 4 1343 1344
28. MarcussonLLFrimodt-MollerNHughesD 2009 Interplay in the selection of fluoroquinolone resistance and bacterial fitness. PLoS Pathog 5 e1000541
29. ZapunAVernetTPinhoMG 2008 The different shapes of cocci. FEMS Microbiol Rev 32 345 360
30. MorlotCNoirclerc-SavoyeMZapunADidebergOVernetT 2004 The D,D-carboxypeptidase PBP3 organizes the division process of Streptococcus pneumoniae. Mol Microbiol 51 1641 1648
31. PophamDLYoungKD 2003 Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr Opin Microbiol 6 594 599
32. VarmaAYoungKD 2004 FtsZ collaborates with penicillin binding proteins to generate bacterial cell shape in Escherichia coli. J Bacteriol 186 6768 6774
33. VinellaDJoseleau-PetitDThevenetDBoulocPD'AriR 1993 Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J Bacteriol 175 6704 6710
34. PernotLChesnelLLe GouellecACroizeJVernetT 2004 A PBP2x from a clinical isolate of Streptococcus pneumoniae exhibits an alternative mechanism for reduction of susceptibility to beta-lactam antibiotics. J Biol Chem 279 16463 16470
35. LuWPKincaidESunYBauerMD 2001 Kinetics of beta-lactam interactions with penicillin-susceptible and -resistant penicillin-binding protein 2x proteins from Streptococcus pneumoniae. Involvement of acylation and deacylation in beta-lactam resistance. J Biol Chem 276 31494 31501
36. LefevreJCBertrandMAFauconG 1995 Molecular analysis by pulsed-field gel electrophoresis of penicillin-resistant Streptococcus pneumoniae from Toulouse, France. Eur J Clin Microbiol Infect Dis 14 491 497
37. CLSI CaLSI 2007 Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth edition Information Supplement. CLSI document M100-S17 [ISBN1-56238-625-5]. Wayne, Pennsylvania Clinical and Laboratory Standards Institute
38. PinasGECortesPROrioAGEcheniqueJ 2008 Acidic stress induces autolysis by a CSP-independent ComE pathway in Streptococcus pneumoniae. Microbiology 154 1300 1308
39. LenskiRE 1991 Quantifying fitness and gene stability in microorganisms. Biotechnology 15 173 192
40. BjorkmanJNagaevIBergOGHughesDAnderssonDI 2000 Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287 1479 1482
41. LaraBRicoAIPetruzzelliSSantonaADumasJ 2005 Cell division in cocci: localization and properties of the Streptococcus pneumoniae FtsA protein. Mol Microbiol 55 699 711
42. HansenMCPalmerRJJrUdsenCWhiteDCMolinS 2001 Assessment of GFP fluorescence in cells of Streptococcus gordonii under conditions of low pH and low oxygen concentration. Microbiology 147 1383 1391
43. ClaverysJPDintilhacAPestovaEVMartinBMorrisonDA 1995 Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164 123 128
44. Trieu-CuotPCarlierCPoyart-SalmeronCCourvalinP 1991 Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene 102 99 104
45. NobleJEBaileyMJ 2009 Quantitation of protein. Methods Enzymol 463 73 95
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding ModuleČlánek A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- A Fresh Look at the Origin of , the Most Malignant Malaria Agent
- In Situ Photodegradation of Incorporated Polyanion Does Not Alter Prion Infectivity
- Highly Efficient Protein Misfolding Cyclic Amplification
- Positive Signature-Tagged Mutagenesis in : Tracking Patho-Adaptive Mutations Promoting Airways Chronic Infection
- Charge-Surrounded Pockets and Electrostatic Interactions with Small Ions Modulate the Activity of Retroviral Fusion Proteins
- Whole-Body Analysis of a Viral Infection: Vascular Endothelium is a Primary Target of Infectious Hematopoietic Necrosis Virus in Zebrafish Larvae
- Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation
- STAT2 Mediates Innate Immunity to Dengue Virus in the Absence of STAT1 via the Type I Interferon Receptor
- Uropathogenic P and Type 1 Fimbriae Act in Synergy in a Living Host to Facilitate Renal Colonization Leading to Nephron Obstruction
- Elite Suppressors Harbor Low Levels of Integrated HIV DNA and High Levels of 2-LTR Circular HIV DNA Compared to HIV+ Patients On and Off HAART
- DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells
- Short-Lived IFN-γ Effector Responses, but Long-Lived IL-10 Memory Responses, to Malaria in an Area of Low Malaria Endemicity
- Induces T-Cell Lymphoma and Systemic Inflammation
- The C-Terminus of RON2 Provides the Crucial Link between AMA1 and the Host-Associated Invasion Complex
- Critical Role of the Virus-Encoded MicroRNA-155 Ortholog in the Induction of Marek's Disease Lymphomas
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Atypical/Nor98 Scrapie Infectivity in Sheep Peripheral Tissues
- Innate Sensing of HIV-Infected Cells
- BosR (BB0647) Controls the RpoN-RpoS Regulatory Pathway and Virulence Expression in by a Novel DNA-Binding Mechanism
- Compensatory Evolution of Mutations Restores the Fitness Cost Imposed by β-Lactam Resistance in
- Expression of Genes Involves Exchange of the Histone Variant H2A.Z at the Promoter
- The RON2-AMA1 Interaction is a Critical Step in Moving Junction-Dependent Invasion by Apicomplexan Parasites
- Epstein-Barr Virus Nuclear Antigen 3C Facilitates G1-S Transition by Stabilizing and Enhancing the Function of Cyclin D1
- Transcription and Translation Products of the Cytolysin Gene on the Mobile Genetic Element SCC Regulate Virulence
- Phosphatidylinositol 3-Monophosphate Is Involved in Apicoplast Biogenesis
- The Rubella Virus Capsid Is an Anti-Apoptotic Protein that Attenuates the Pore-Forming Ability of Bax
- Episomal Viral cDNAs Identify a Reservoir That Fuels Viral Rebound after Treatment Interruption and That Contributes to Treatment Failure
- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection
- The Genotype of Early-Transmitting HIV gp120s Promotes αβ –Reactivity, Revealing αβ/CD4 T cells As Key Targets in Mucosal Transmission
- Small Molecule Inhibitors of RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis
- The bZIP Transcription Factor MoAP1 Mediates the Oxidative Stress Response and Is Critical for Pathogenicity of the Rice Blast Fungus
- Entrapment of Viral Capsids in Nuclear PML Cages Is an Intrinsic Antiviral Host Defense against Varicella-Zoster Virus
- NS2 Protein of Hepatitis C Virus Interacts with Structural and Non-Structural Proteins towards Virus Assembly
- Measles Outbreak in Africa—Is There a Link to the HIV-1 Epidemic?
- New Models of Microsporidiosis: Infections in Zebrafish, , and Honey Bee
- The C-Terminal Domain of the Arabinosyltransferase EmbC Is a Lectin-Like Carbohydrate Binding Module
- A Viral microRNA Cluster Strongly Potentiates the Transforming Properties of a Human Herpesvirus
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- Secreted Bacterial Effectors That Inhibit Host Protein Synthesis Are Critical for Induction of the Innate Immune Response to Virulent
- Genital Tract Sequestration of SIV following Acute Infection
- Functional Coupling between HIV-1 Integrase and the SWI/SNF Chromatin Remodeling Complex for Efficient Integration into Stable Nucleosomes
- DNA Damage and Reactive Nitrogen Species are Barriers to Colonization of the Infant Mouse Intestine
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
- Targeted Disruption of : Invasion of Erythrocytes by Using an Alternative Py235 Erythrocyte Binding Protein
- Trivalent Adenovirus Type 5 HIV Recombinant Vaccine Primes for Modest Cytotoxic Capacity That Is Greatest in Humans with Protective HLA Class I Alleles
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genetic Mapping Identifies Novel Highly Protective Antigens for an Apicomplexan Parasite
- Type I Interferon Signaling Regulates Ly6C Monocytes and Neutrophils during Acute Viral Pneumonia in Mice
- Infections in Cells: Transcriptomic Characterization of a Novel Host-Symbiont Interaction
- The ESCRT-0 Component HRS is Required for HIV-1 Vpu-Mediated BST-2/Tetherin Down-Regulation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



