-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Against Exogenous Gliotoxin
Gliotoxin, and other related molecules, are encoded by multi-gene clusters and biosynthesized by fungi using non-ribosomal biosynthetic mechanisms. Almost universally described in terms of its toxicity towards mammalian cells, gliotoxin has come to be considered as a component of the virulence arsenal of Aspergillus fumigatus. Here we show that deletion of a single gene, gliT, in the gliotoxin biosynthetic cluster of two A. fumigatus strains, rendered the organism highly sensitive to exogenous gliotoxin and completely disrupted gliotoxin secretion. Addition of glutathione to both A. fumigatus ΔgliT strains relieved gliotoxin inhibition. Moreover, expression of gliT appears to be independently regulated compared to all other cluster components and is up-regulated by exogenous gliotoxin presence, at both the transcript and protein level. Upon gliotoxin exposure, gliT is also expressed in A. fumigatus ΔgliZ, which cannot express any other genes in the gliotoxin biosynthetic cluster, indicating that gliT is primarily responsible for protecting this strain against exogenous gliotoxin. GliT exhibits a gliotoxin reductase activity up to 9 µM gliotoxin and appears to prevent irreversible depletion of intracellular glutathione stores by reduction of the oxidized form of gliotoxin. Cross-species resistance to exogenous gliotoxin is acquired by A. nidulans and Saccharomyces cerevisiae, respectively, when transformed with gliT. We hypothesise that the primary role of gliotoxin may be as an antioxidant and that in addition to GliT functionality, gliotoxin secretion may be a component of an auto-protective mechanism, deployed by A. fumigatus to protect itself against this potent biomolecule.
Published in the journal: . PLoS Pathog 6(6): e32767. doi:10.1371/journal.ppat.1000952
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000952Summary
Gliotoxin, and other related molecules, are encoded by multi-gene clusters and biosynthesized by fungi using non-ribosomal biosynthetic mechanisms. Almost universally described in terms of its toxicity towards mammalian cells, gliotoxin has come to be considered as a component of the virulence arsenal of Aspergillus fumigatus. Here we show that deletion of a single gene, gliT, in the gliotoxin biosynthetic cluster of two A. fumigatus strains, rendered the organism highly sensitive to exogenous gliotoxin and completely disrupted gliotoxin secretion. Addition of glutathione to both A. fumigatus ΔgliT strains relieved gliotoxin inhibition. Moreover, expression of gliT appears to be independently regulated compared to all other cluster components and is up-regulated by exogenous gliotoxin presence, at both the transcript and protein level. Upon gliotoxin exposure, gliT is also expressed in A. fumigatus ΔgliZ, which cannot express any other genes in the gliotoxin biosynthetic cluster, indicating that gliT is primarily responsible for protecting this strain against exogenous gliotoxin. GliT exhibits a gliotoxin reductase activity up to 9 µM gliotoxin and appears to prevent irreversible depletion of intracellular glutathione stores by reduction of the oxidized form of gliotoxin. Cross-species resistance to exogenous gliotoxin is acquired by A. nidulans and Saccharomyces cerevisiae, respectively, when transformed with gliT. We hypothesise that the primary role of gliotoxin may be as an antioxidant and that in addition to GliT functionality, gliotoxin secretion may be a component of an auto-protective mechanism, deployed by A. fumigatus to protect itself against this potent biomolecule.
Introduction
Gliotoxin, which has a molecular mass of 326 Da and is an epipolythiodioxopiperazine (ETP), contains a disulphide bridge of unknown origin and has been shown to play a significant role in enabling the virulence of Aspergillus fumigatus [1]–[3]. The cytotoxic activity of gliotoxin is generally mediated by direct inactivation of essential protein thiols [4] and by inhibition of the respiratory burst in neutrophils by disrupting NADPH oxidase assembly, thereby facilitating in vivo fungal dissemination [5], [6]. The enzymatic machinery responsible for gliotoxin biosynthesis, and metabolism, is encoded by a multi-gene cluster in A. fumigatus which is coordinately expressed during gliotoxin biosynthesis [7], [8]. This cluster encodes gliP, a bimodular nonribosomal peptide synthetase (NRPS) which has been conclusively shown to be responsible for the biosynthesis of a Phe-Ser dipeptide, a gliotoxin precursor, by gene disruption (ΔgliP mutant) [9]–[12]. In fact, disruption of gliP within the gliotoxin biosynthetic cluster has resulted in the effective inhibition of all cluster gene expression in a ΔgliP mutant [9]. A putative transporter, encoded by gliA, has been shown to facilitate gliotoxin efflux, and increased tolerance to exogenous gliotoxin, when expressed in Leptosphaeria maculans [13]. sirA is a gliA ortholog in this organism and L. maculans ΔsirA was more sensitive to exogenous gliotoxin and sirodesmin than wild-type, however restoration of sirA in the mutant led to greater tolerance towards these metabolites [13]. Bok et al. [14] have demonstrated that disruption of a fungal Zn(II)2-Cys(6) binuclear cluster domain transcription factor (gliZ) results in the complete inhibition of all gliotoxin cluster gene expression and effective diminution of gliotoxin production [14]. Although GliP has been shown to activate and condense L-Phe and L-Ser to form a precursor diketopiperazine moiety, no information relating to subsequent modification (e.g., thiolation) is available [9]–[12], [15] and it is also unclear if A. fumigatus might need to protect itself against potential gliotoxin cytotoxicity [13].
Interestingly, addition of gliotoxin (up to 5 µg/ml) to A. fumigatus ΔgliP resulted in the up-regulation of selected gene expression (gliI, J, T and N) within the gli cluster and Cramer et al. [9] noted complete activation of the gene cluster (except gliP) following gliotoxin exposure (20 µg/ml). However, exposure of wild-type A. fumigatus Af293 to gliotoxin (20 µg/ml), for 24 h, did not result in any significant alteration in gliotoxin cluster expression [9]. The biological significance of these observations is unclear, apart from implying a role for gliotoxin in the regulation of the gli cluster in the absence of gliotoxin production.
It has recently been demonstrated that gliotoxin and sporidesmin, also an ETP toxin containing a disulphide bridge, are both substrates and inactivators of glutaredoxin (Grx1) [16]. These authors also confirmed that the intact disulphide form of these ETP moieties was essential for Grx1 inactivation and that prior reduction of sporidesmin, using glutathione, prevented subsequent Grx1 inactivation. Oxygen presence was also required for Grx1 inactivation by sporidesmin and mass spectrometric analysis confirmed the formation of mixed disulphides between one molecule of Grx1 and either gliotoxin or sporidesmin, respectively. Combined, these data suggest interplay between oxygen availability and selective protein inactivation in the presence of oxidised ETP-type molecules. This indirectly suggests either a protective, or neutral, involvement of the oxidised forms of gliotoxin or sporidesmin in protecting against the deleterious effects of oxygen by selective protein inactivation.
In mammalian cells it has been demonstrated that the oxidized form of gliotoxin is actively concentrated in a glutathione-dependent manner and that it then exists within the cell almost exclusively in the reduced form [17]. As glutathione levels fall due to apoptosis, the oxidized form of gliotoxin effluxes from the cell where the cytocidal effects of gliotoxin are perpetuated in a pseudocatalytic manner. Conversely, it has been shown that gliotoxin may substitute for 2-cys peroxiredoxin activity in HeLa cells by accepting electrons from NADPH via the thioredoxin reductase–thioredoxin redox system to reduce H2O2 to H2O. In this way, nanomolar levels of gliotoxin may actually protect against intracellular oxidative stress [18].
Although the cytotoxic effects of gliotoxin on mammalian cells have been extensively investigated, and yeast have been deployed as a model system to study this interaction [19], no direct investigation of any self-protective mechanism used by A. fumigatus against this intriguing molecule has been undertaken. Here, we demonstrate that deletion of gliT results in transformants which cannot grow in the presence of even modest levels of exogenous gliotoxin and that exogenous gliotoxin up-regulates gene expression within the gliotoxin cluster, especially that of gliT. We propose that GliT is the key cellular defence against gliotoxin in A. fumigatus and that this finding yields a new selection marker system for detecting transformation.
Results
Deletion and complementation of gliT in A. fumigatus
ΔgliT mutants were generated by transformation of A. fumigatus strains ATCC46645 and ATCC26933, respectively, as described in Materials and Methods, using the bipartite marker technique and pyrithiamine selection, with modifications [20], [21] (Figure S1). Deposition number: IMI CC 396691 (CABI Bioscience Centre, Egham, Surrey, UK). These two strains were chosen because ATCC26933 is a gliotoxin producer, whereas ATCC46645 lacks significant gliotoxin production using the Minimal Media described in Materials and Methods (see below). Complementation of gliT mutant strains was carried out as described in Materials and Methods and Figure S1). Complemented strains (gliTC) (Deposition number: IMI CC 396692) exhibited wild-type like features in all subsequent experiments, demonstrating that the occurrence of a single ectopic integration of a gliT fragment is insignificant in the A. fumigatus ATCC26933 background.
Gliotoxin prevents growth of ΔgliT strains
ΔgliT protoplasts grew and regenerated mycelia perfectly in the absence of gliotoxin (Figure 1A). The ΔgliT strain grew at identical rates to wild-type (data not shown). However, ΔgliT protoplasts were unable to grow in the presence of gliotoxin (10 µg/ml) (Figure 1A) whereas exogenous gliotoxin had no effect on wild-type growth. Subsequent phenotypic analysis of A. fumigatus ATCC46645, ATCC26933, and respective ΔgliT conidia (ΔgliT46645 and ΔgliT26933) demonstrated that gliotoxin (5 µg/ml) significantly inhibited ΔgliT growth on minimal medium and completely inhibited ΔgliT growth on both AMM and Sabouraud medium (gliotoxin, 10 µg/ml) (Figure 1B & C; p<0.0001 and Figure S2). Moreover, germination rates of ΔgliT strains were comparable to those of wild-type A. fumigatus, even in the presence of gliotoxin up to 10 µg/ml. These results clearly indicated that ΔgliT was highly sensitive to exogenous gliotoxin. Consequently, ΔgliT46645 and ΔgliT26933 mutant complementation was carried out by introducing gliT only (no antibiotic resistance gene) to complement ΔgliT with selection in the presence of gliotoxin (10 µg/ml). Transformants, which had recovered resistance to exogenous gliotoxin, were confirmed by Southern analysis to have an intact and functional copy of gliT present (Figure S1). This result confirms that gliT confers resistance to gliotoxin in A. fumigatus and that ΔgliT mutants have significant potential for future functional genomic studies involving A. fumigatus since gene deletions in this strain are selectable by gliT reintroduction, with selection in the presence of gliotoxin.
Fig. 1. Exogenous gliotoxin specifically inhibits growth of A. fumigatus ΔgliT. 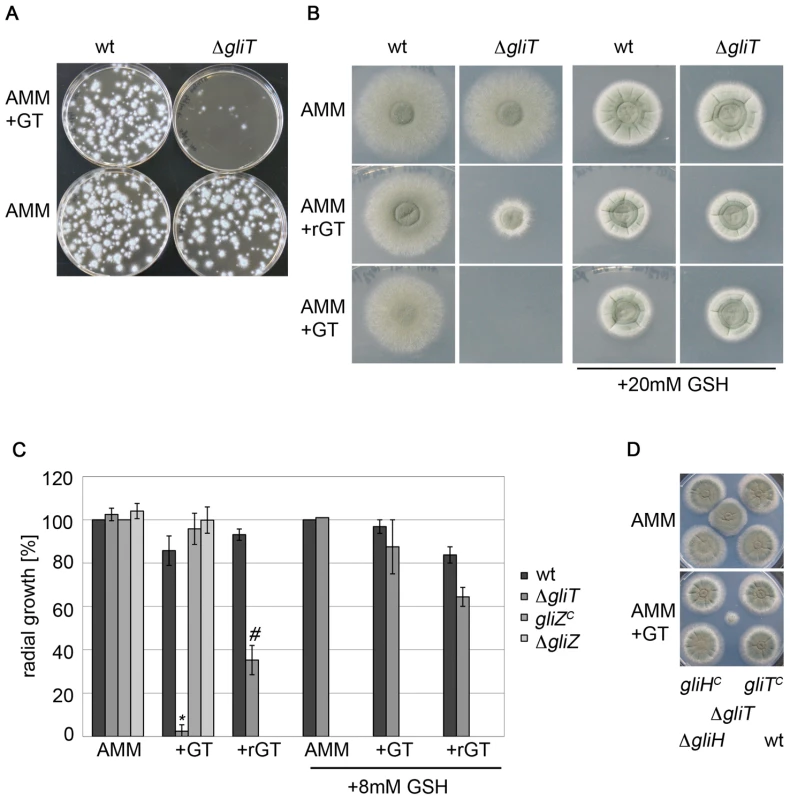
(A) Protoplasts of A. fumigatus wild-type (ATCC46645) and ΔgliT were poured onto AMM plates, in the presence or absence of gliotoxin (10 µg/ml). Plates were incubated for 48 h at 37°C. (B) Conidia (A. fumigatus ATCC26933 and ΔgliT; 104/spot) were dotted on AMM plates containing the indicated supplement and incubated for 48 h at 37°C. To obtain reduced gliotoxin (rGT) free of additional thiols, 10 µg/ml GT was reduced with 50 mM NaBH4 for 60 min at room temperature. Gliotoxin (GT) and rGT were added at a final concentration of 10 µg/ml. (C) Quantification of radial growth of A. fumigatus ATCC26933, ΔgliT, ΔgliZ and complemented ΔgliZ (gliZc) [14] in the presence of gliotoxin and rGT, with and without exogenous GSH. Strains (104 conidia) were dotted on AMM containing GT (gliotoxin; 10 µg/ml), rGT (10 µg/ml), or reduced glutathione (8 mM), respectively. Colony diameter was measured after 48 h of incubation at 37°C and experiments were repeated in triplicate. * indicates a significance level of p<0.0001 and # indicates p<0.05. Note: For clarity, A. fumigatus ΔgliZ and gliZc (gliZ complemented) data are only shown for AMM +/− GT only, as their growth was unaffected by all conditions tested. (D) A. fumigatus ΔgliH is unaffected by gliotoxin presence. 104 conidia were spotted on AMM with GT (10 µg/ml, bottom) or without GT (top). Plates were incubated for 72 h (hence the visible background growth of ΔgliT). A. fumigatus ΔgliH did not show any sign of an altered growth phenotype in the presence of gliotoxin. Remarkably, addition of reduced glutathione (GSH; 20 mM)) to test plates completely abolished the cytotoxic effects of exogenous gliotoxin which indicated that gliT loss resulted in depletion of intracellular GSH, when exposed to gliotoxin, or that only the oxidized form of gliotoxin is imported into A. fumigatus (Figure 1B & C). Prior reduction of gliotoxin, using 50 mM NaBH4, resulted in a statistically significant inhibitory effect of gliotoxin on growth of ΔgliT26933 (p<0.05) (Figure 1B & C). NaBH4 was selected as reductant as it avoided complications associated with the introduction of additional thiols, or GSH, and the formation of gliotoxin conjugates, which may have resulted from GSH, DTT or β-mercaptoethanol-mediated reduction. It was also observed that GSH presence (8 mM) partially alleviated the growth inhibitory effects of gliotoxin (with or without prior reduction; p<0.01 and p<0.005, respectively) (Figure 1C). However, wild-type levels of growth were only achieved in the presence of 20 mM GSH (Figure 1B). The enhanced GSH-mediated alleviation of gliotoxin-induced cytostatic effects observed in ΔgliT, strongly suggest that depletion of intracellular glutathione may be a consequence of gliT loss. GSH-mediated relief of A. fumigatus ΔgliT growth inhibition, by exogenous NaBH4-reduced gliotoxin, indicates that intracellular GSH depletion plays a role in the inhibitory effect of gliotoxin - and not that GSH is merely acting to reduce exogenously added gliotoxin and prevent uptake (Figure 1B & C). Exogenous gliotoxin or reduced gliotoxin had no effect on growth of ΔgliZ and gliZc (gliZ complemented strain) [14] (kind gifts from Professor Nancy Keller, University of Wisconsin-Madison) and an identical pattern was observed in the presence of GSH (data not shown). Moreover, A. fumigatus ΔgliT did not exhibit any phenotype when exposed to either H2O2 or phleomycin (data not shown). A. fumigatus gliTC strains were resistant to exogenous gliotoxin (Figure 1D).
Gliotoxin induces expression of the gliotoxin gene cluster
gliZ, A and G encode the gliotoxin cluster transcription factor, transporter and a putative glutathione s-transferase (generally a detoxification enzyme), respectively, and all are conceivably involved in protection against gliotoxin toxicity [3], [8], [22]. Northern analysis showed that expression of these 3 genes plus gliT, from the gliotoxin gene cluster, was induced in A. fumigatus ATCC46645 within 3 h following gliotoxin (5 µg/ml) addition at 21 h (Figure 2A). No gliT expression was detectable in ΔgliT whereas the expression of all other genes was identical to the wild-type, including the continued absence of expression at 24 h in the absence of added gliotoxin (Figure 2A). Expression of gliT was restored in pyrithiamine-resistant A. fumigatus gliTC derived from both ATCC46645 and ATCC26933 strain backgrounds, which unambiguously confirms restoration of gliT expression in complemented strains (Figure 2B). Moreover, gliT expression was inducible by addition of gliotoxin (5 µg/ml), as had been observed in both wild-type strains, thereby convincingly demonstrating that the wild-type phenotype had been entirely restored (Figure 2B). As noted above, no significant growth inhibition of A. fumigatus ΔgliZ in particular, or gliZc, was observed in the presence of gliotoxin or reduced gliotoxin (Figure 1C). These observations further confirm the minimal role played by any other component of the gli gene cluster in protection against gliotoxin presence since gliZ absence results in complete cluster attenuation [14]. Significantly, Northern analysis confirmed gliotoxin-induced gliT expression in ΔgliZ, which indicates the independent regulation of gliT with respect to other gli cluster components, such as gliG and gliA which are not expressed by A. fumigatus ΔgliZ following exposure to gliotoxin (Figure 2C). These observations are in complete accordance with proteomic data which demonstrated a threefold up-regulation of GliT expression (33% sequence coverage) in A. fumigatus ATCC26933, and the absence of detection of any other gli cluster component, following 3 h exposure to exogenous gliotoxin (14 µg/ml) (Figure 2D and Figure S3).
Fig. 2. gliT expression. 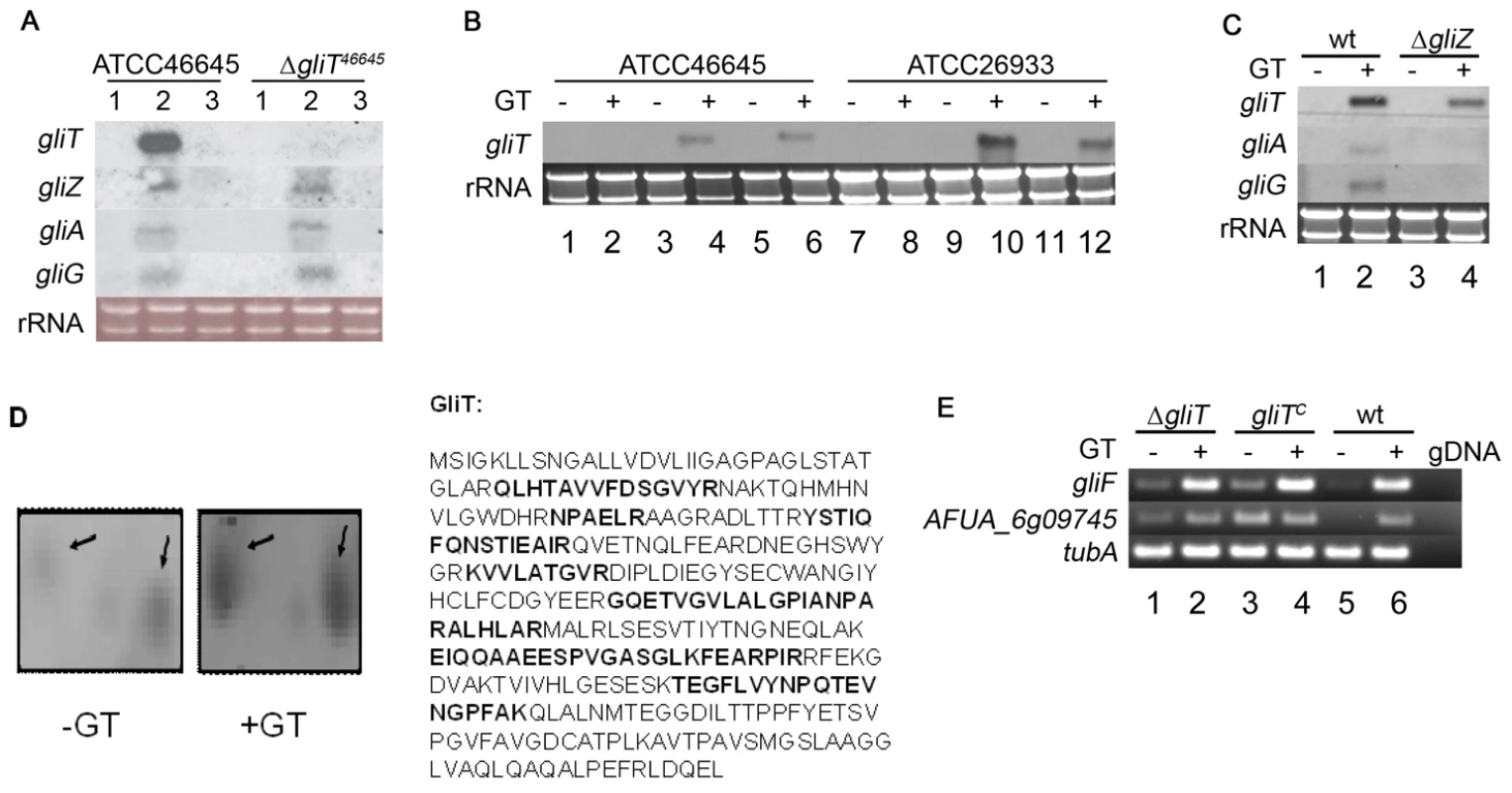
(A) Northern analysis of the induction of gliotoxin gene cluster expression in A. fumigatus ATCC46645 and ΔgliT. Lanes 1, 2 and 3 correspond to A. fumigatus RNA extracts from 21 h AMM, 21 h AMM shifted to gliotoxin (5 µg/ml) for 3 h and 24 h AMM, respectively. (B) Gliotoxin induction of gliT expression in A. fumigatus gliTC strains. Lanes 1–6 and 7–12 contain RNA from strains in the ATCC46645 and ATCC26933 backgrounds, respectively. Lanes 1 and 7: A. fumigatus ΔgliT 24 h AMM; Lanes 2 and 8: A. fumigatus ΔgliT 21 h AMM+3 h gliotoxin (5 µg/ml); Lane 3 and 9: A. fumigatus gliTC 24 h AMM; Lanes 4 and 10: A. fumigatus gliTC 21 h AMM+3 h gliotoxin (5 µg/ml); Lanes 5 and 11: A. fumigatus wt 24 h AMM; Lane 6 and 12: A. fumigatus wt 21 h AMM+3 h gliotoxin (5 µg/ml). (C) Expression of gliT in ΔgliZ following exposure to gliotoxin. Cultures of A. fumigatus ATCC46645 (lanes 1 and 2) and ΔgliZ (lanes 3 and 4) were grown for 24 h in AMM (Lane 1 and 3) or pulsed with gliotoxin (5 µg/ml) after 21 h and cultured for a further 3 h (Lane 2 and 4). Although gliotoxin induced expression of gliA and gliG in wild-type, neither gliA or gliG are expressed in ΔgliZ. All Northern analyses were performed with 10 µg of total RNA isolated from strains grown in AMM for 24 h with or without gliotoxin. (D) A. fumigatus GliT expression and identification. Quantitative 2D-PAGE analysis confirmed increased expression (threefold) of GliT following exogenous gliotoxin (GT) addition to A. fumigatus cultures (GliT appears to exist as two isoforms of different pI (5.5–5.6) and Mr). Peptides identified by MALDI-ToF mass spectrometry are highlighted in bold (33% sequence coverage) and mass spectrum is given in Figure S3. (E) Semi-quantitative RT-PCR of gliT adjacent genes in A. fumigatus wild-type (wt) (ATCC26933) and isogenic mutant strains. Expression of gliF and AFUA_6g09745 (gliH) was examined. As a control tubA expression was monitored. As a negative control genomic DNA (gDNA) was used as template. Lane 1: A. fumigatus ΔgliT26933 24 h AMM. Lane 2: A. fumigatus ΔgliT26933 21 h AMM+3 h gliotoxin (5 µg/ml). Lane 3: A. fumigatus gliTC 24 h AMM. Lane 4: A. fumigatus gliTC 21 h AMM+3 h gliotoxin (5 µg/ml). Lane 5: A. fumigatus wt 24 h AMM. Lane 6: A. fumigatus wt 21 h AMM+3 h gliotoxin (5 µg/ml). Genes immediately adjacent to gliT in the gliotoxin gene cluster do not mediate resistance to exogenous gliotoxin
Sequence analysis of the 5′ and 3′ regions adjacent to the original gliT locus in A. fumigatus ΔgliT26933 confirmed that gliF was intact but revealed two mutations (C23R and E160G) in the open reading frame of a gene (AFUA_6G09745; identified as a conserved hypothetical protein at http://www.cadre-genomes.org.uk (but here termed gliH), located 3′ with respect to the gliT locus. Although expression of gliF and gliH was confirmed by RT-PCR in A. fumigatus ΔgliT26933 (Figure 2E), there was concern that the altered sequence of gliH may have resulted in a mutant enzyme, which could possibly have also contributed to gliotoxin sensitivity in ΔgliT26933. However, A. fumigatus ΔgliH26933 grew in the presence of gliotoxin (10 µg/ml) (Figure 1D) which completely eliminated the possibility that this gene, located adjacent to gliT in the A. fumigatus genome, contributed to gliotoxin resistance and established, beyond question, the key role of gliT in mediating resistance to exogenous gliotoxin. A. fumigatus gliHC (Figure S1) was also resistant to exogenous gliotoxin, as expected (Figure 1D).
Gliotoxin is not produced by A. fumigatus ΔgliT
Gliotoxin (580 ng/ml) was detectable in organic extracts from A. fumigatus ATCC26933 but not ΔgliT26933 cultures, grown under identical conditions, by RP-HPLC and LC-MS analysis (Figure 3). Gliotoxin production was recovered in A. fumigatus ATCC26933 gliTC (Figure S4) Interestingly, ΔgliT26933 exhibited an identical phenotype to ΔgliT46645 which was generated from A. fumigatus ATCC46645, yet gliotoxin production was undetectable, under the culture conditions employed, in both A. fumigatus ATCC46645 and ΔgliT46645 indicating that sensitivity to exogenous gliotoxin is not associated with a de novo gliotoxin biosynthetic capacity. A metabolite with retention time (Rt) = 11.7 min (A220 nm) was apparent in ΔgliT26933 extracts which was absent in wild-type extracts (Figure 3). This material was purified to assess any growth inhibitory effect, however when added to AMM cultures of ΔgliT or wild-type no alteration of growth rates was observed (data not shown). High resolution LC-ToF MS analysis of the metabolite (from Figure 3B) confirmed the presence of a molecular ion with a mass of 279.0796 m/z ((M+H)+) (Figure S4). This accurate mass value (279.0796 m/z) corresponded to a predicted molecular formula of C13H15N2O3S for the ion whereby the calculated exact mass for C13H15N2O3S + H+ was 279.0798 Da using Agilent Technologies Masshunter workstation software. This result suggests that a monothiol form of gliotoxin could have been secreted from A. fumigatus ΔgliT26933. A molecular species of m/z 279, which yielded daughter ions of m/z 261.1, 231.0 and 203.1, upon MS2 analysis, was also detected by LC-MS analysis of the purified gliotoxin-related metabolite from A. fumigatus ΔgliT26933 (Figure S4). Gliotoxin was not produced by A. fumigatus ΔgliH26933 (Figure S4) which strongly supports a role for this gene in gliotoxin biosynthesis or secretion, but not protection against exogenous gliotoxin. This result was further consolidated whereby no gliotoxin production was detectable, by HPLC-DAD or LC-MS, in A. fumigatus ΔgliT26933gliH (data not shown), which was generated by restoration of the fully intact gliH in gliT-deficient A. fumigatus (Figure S1).
Fig. 3. Gliotoxin is not produced by A. fumigatus ΔgliT. 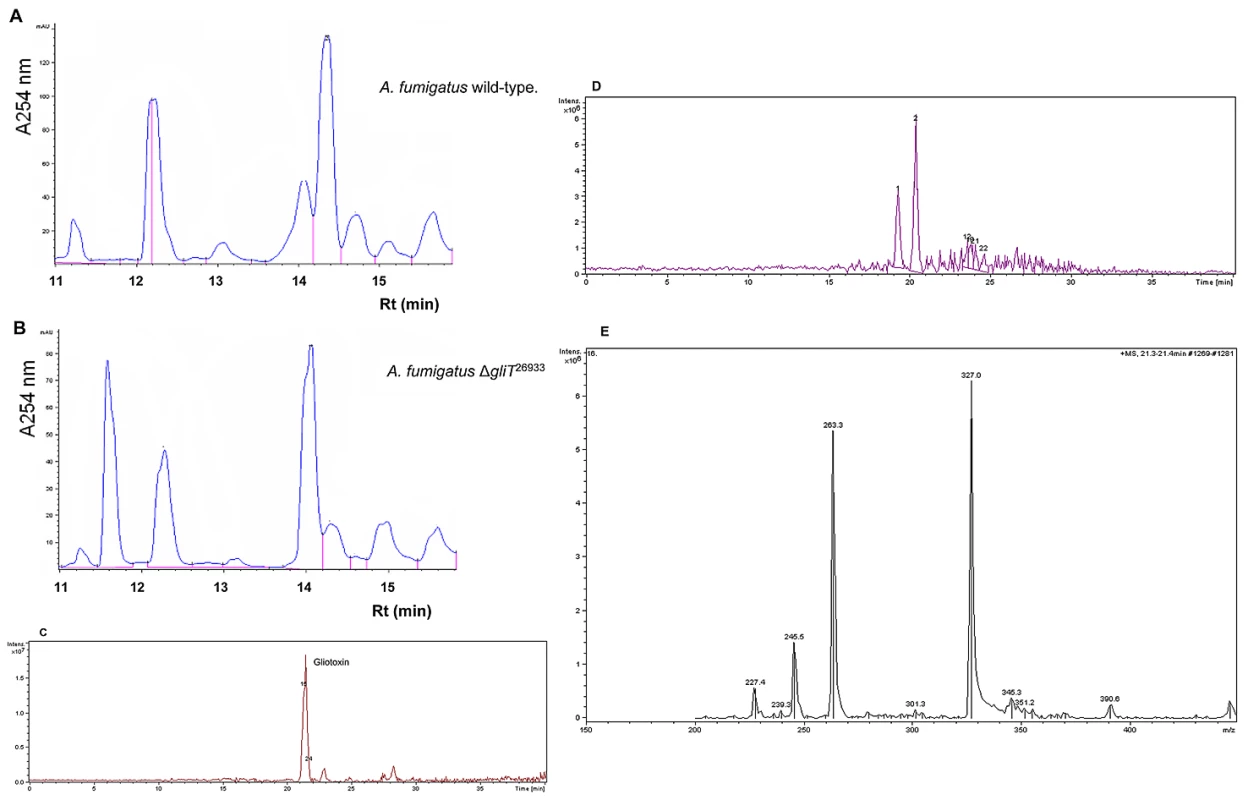
(A) HPLC chromatogram of an organic extract from A. fumigatus ATCC26933 indicating gliotoxin presence with Rt = 14.5 min. (B) HPLC chromatogram of organic extracts from A. fumigatus ΔgliT26933 indicating gliotoxin absence but a new metabolite with Rt = 11.7 min. (C) LC-MS analysis of an organic extract from A. fumigatus ATCC26933 indicating gliotoxin presence. (D) LC-MS analysis of A. fumigatus ΔgliT26933 confirms absence of secreted gliotoxin. (E) MS2 of gliotoxin present in wild-type A. fumigatus indicating expected sub-fragments as noted previously [10]. GliT exhibits a gliotoxin reductase activity
Recombinant GliT was expressed in, and purified by differential extraction from, E.coli with a yield of approximately 5.7 mg per gram of cells. However the protein was completely insoluble and was refractory to any attempts at refolding for activity analysis (data not shown). SDS-PAGE analysis confirmed a subunit molecular mass of 38 kDa for recombinant GliT (Figure S5), which appears to migrate as a dimer under non-reducing conditions (Figure S5), and protein identity was unambiguously confirmed by MALDI-ToF MS whereby peptides (following tryptic digestion) were identified yielding 21% sequence coverage (Figure S6). Immunoaffinity purification of GliT-specific human IgG was achieved by incubation of human sera with Sepharose-coupled recombinant GliT. The specificity of this GliT-specific human IgG was confirmed by the successful detection of native GliT in both A. fumigatus cell lysates, and partially-purified extracts of A. fumigatus (Protocol S1; Figure 4). Notably, GliT was not detectable in A. fumigatus ΔgliT (Figure 4).
Fig. 4. Immunoaffinity purified human IgG detects native GliT in A. fumigatus. 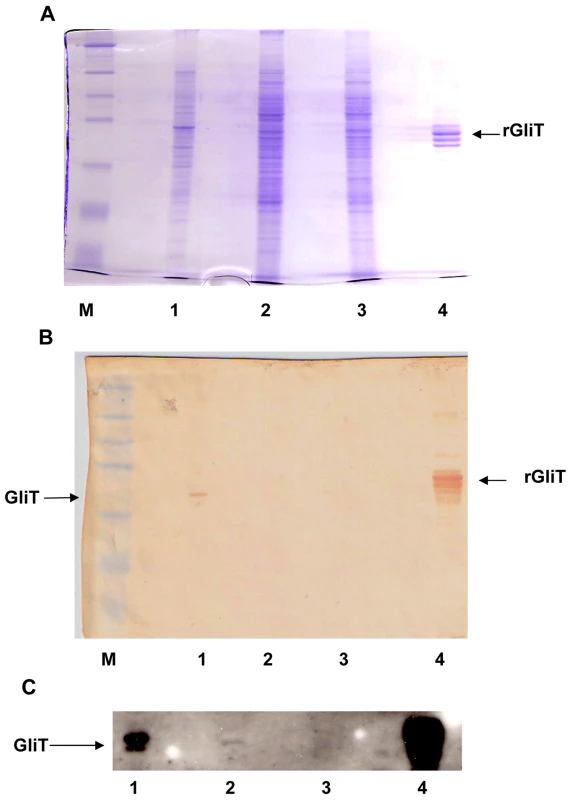
(A) SDS-PAGE and (B/C): Western blot analysis of A. fumigatus cell lysates. Immuno-affinity purified human IgG[anti-GliT] was used for Western analysis followed by anti-human IgG-HRP conjugate with visualization by either (B) diaminobenzidine or (C) ECL detection. Lane M: Mr marker: Lane 1: A. fumigatus ATCC26933 lysate (72 h culture); Lane 2: A. fumigatus ATCC46645 lysate (24 h culture); Lane 3: A. fumigatus ATCC46645 ΔgliT lysate (24 h culture) and Lane 4: Recombinant GliT (2 µg). Immunoaffinity purified human IgG to GliT identified GliT in all except A. fumigatus ΔgliT, however ECL substrate was required to detect low level GliT expression in A. fumigatus ATCC46645 (lane C.2). Previous hypotheses have suggested that GliT may only exhibit gliotoxin oxidase activity (responsible to disulphide bridge closure during biosynthesis) (3, 8, 22). However, following gliotoxin induction of A. fumigatus ATCC46645, enhanced GliT activity was evident in cell lysates and native GliT was partially purified by ammonium sulphate precipitation and ion-exchange chromatography (Figure S7). Data presented in Figure 5A confirm that partially-purified native GliT specifically catalyses the NADPH-mediated reduction of oxidized gliotoxin, whereby NADPH oxidation is only evident in the presence of both gliotoxin (9 µM) and GliT-containing lysates. Hence, GliT appears to exhibit gliotoxin reductase activity which can catalyse disulphide bridge cleavage, at concentrations up to 9 µM gliotoxin (Figure 5B). This activity is inhibited at higher gliotoxin concentrations (>12 µM). Not unexpectedly, A. fumigatus cell extracts appear to contain basal NADPH oxidase activity which yields background, non-specific NADPH oxidation (Figure 5A). Thus, A. fumigatus ATCC46645 and ΔgliT lysates, generated without prior gliotoxin induction of GliT expression, exhibit near-identical activity. However, significantly greater gliotoxin reductase activity (2 : 1) was apparent in A. fumigatus ATCC46645, than ΔgliT, cell lysates following gliotoxin exposure (Figure 5C). Immunoprecipitation of GliT from partially purified A. fumigatus cell lysates (Figure S7) using human IgG [anti-GliT] resulted in a 51% reduction of gliotoxin reductase (NADPH oxidase) activity (Figure 5D), in complete accordance with data in Figure 5C, further confirming enzyme specificity. Interestingly, GliT activity was not enhanced in the presence of thioredoxin from Spirulina sp., in activity assays, which indicates that GliT is specific for gliotoxin reduction and that it may operate independently of cellular thioredoxin reductase/thioredoxin systems.
Fig. 5. GliT exhibits a gliotoxin reductase activity. 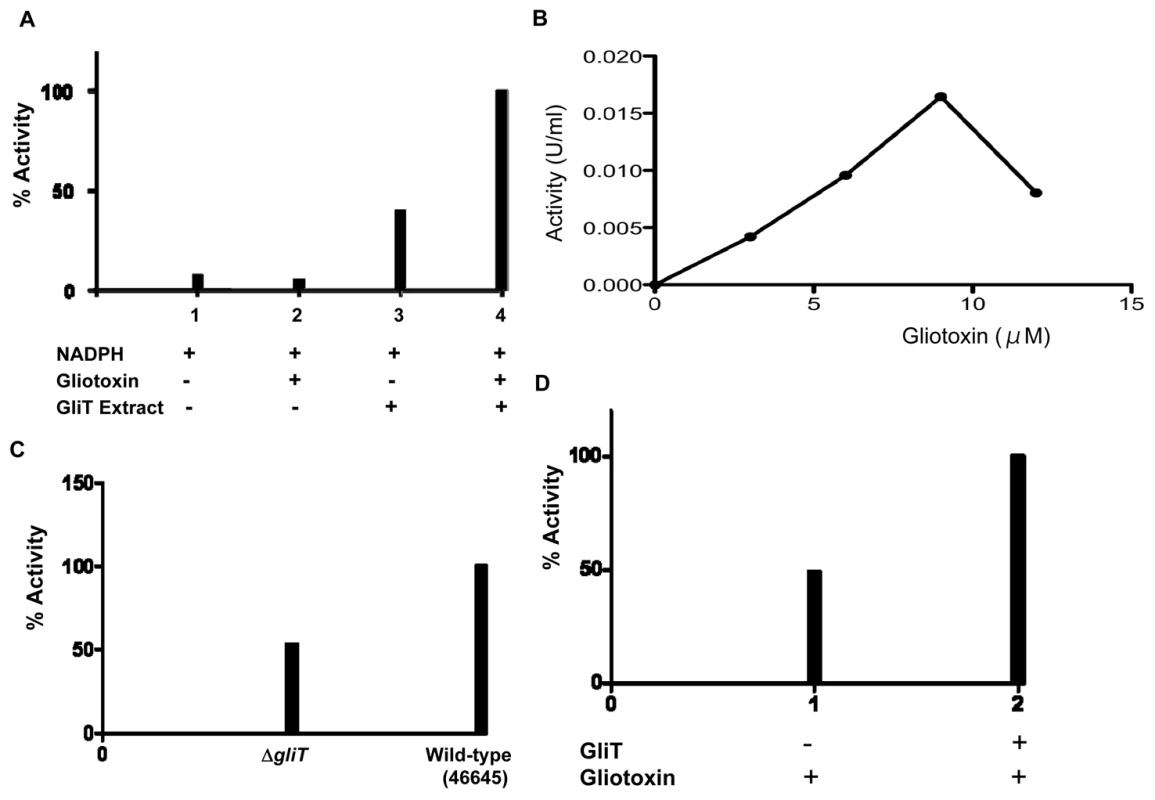
(A) No GliT activity (gliotoxin reductase) is detectable in the absence of gliotoxin or GliT (1 & 2). Background NADPH oxidase activity is detectable in semi-purified A. fumigatus cell extracts (3) (Figure S5), however, GliT-mediated gliotoxin reductase activity is detectable upon addition of gliotoxin (4). (B) In vitro, optimal GliT gliotoxin reductase activity is observed up to 9 µM gliotoxin. This activity is inhibited at higher gliotoxin concentrations (>12 µM). (C) Relative gliotoxin reductase activity in cell lysates from ΔgliT compared to A. fumigatus ATCC46645 with gliotoxin addition during culture. Wild-type lysates exhibit enhanced gliotoxin reductase activity (47%) consequent to elevated GliT expression. (D) Immunodepletion of GliT from semi-purified A. fumigatus cell extracts (Figure S5), using immunoaffinity purified human IgG [anti-GliT], results in a 51% decrease in gliotoxin reductase activity. Expression of GliT in A. fumigatus was further explored by fluorescence confocal microscopy. Data in Figure S8A-C confirm transformation of A. fumigatus ΔgliT46645 and that gliT-gfp expression is enhanced by gliotoxin addition. As shown in Figure S8A, it appears that low-level GliT expression is evident throughout mycelia without gliotoxin addition. However, following mycelial exposure to gliotoxin (5 µg/ml), an enhancement of GliT expression in the cytoplasm, and in nuclei, as shown by fluorescence intensities (Figure S8B & C), is observed - which is in complete agreement with proteomic, molecular and enzyme activity observations. Expression of GliT-GFP fusion protein completely restored gliotoxin resistance (10 µg/ml), although colonies appeared white (Figure S9).
The concordance of these data lead us to conclude that a GliT-mediated gliotoxin reductase activity is induced by exposure of A. fumigatus to gliotoxin.
GliT is not required for A. fumigatus virulence in Galleria mellonella
A prerequisite for testing A. fumigatus ΔgliT virulence was to evaluate the utility of our G. mellonella infection model. To this end, assessment of the relative virulence of A. fumigatus ΔgliZ and corresponding wild-type in G. mellonella, in either the presence or absence of added gliotoxin, was assessed (Figure S10). Here, all Galleria exposed to A. fumigatus ΔgliZ were alive at 24 h and the wild-type strain exhibited greater virulence than ΔgliZ (60% (12/20) versus 20% (4/20) mortality, respectively), at 48 h post-inoculation, thereby confirming the utility of the model system for detection of alteration in virulence associated with gliotoxin production. To assess now the relative contribution of gliT to virulence of A. fumigatus we compared the survival of larvae of the greater wax moth G. mellonella following infection with 106 conidia/larvae of A. fumigatus ATCC26933 and gliTC to that of larvae (n = 20) infected with the same dose of ΔgliT26933 (Figure S10). For all groups of infected larvae, 100% mortality was recorded after 72 h and the degree of melanisation was not distinguishable between these groups. Also, pretreatment of larvae with gliotoxin (0.5 µg/larva in 20 µl) did not lead to an attenuation of virulence of ΔgliT (Figure S10). Notably, similar results were obtained using ATCC46645 and ΔgliT46645 strains (data not shown). These results clearly show that, gliT has a minimal, if any, role to play in the virulence of A. fumigatus employing a Galleria model.
GliT confers protection against exogenous gliotoxin in Aspergillus nidulans and Saccharomyces cerevisiae
Reintroduction of gliT into A. fumigatus ΔgliT was selected for in the presence of gliotoxin and no additional selection marker was required (Figure S1 and Figure 1D). To further test the ability of gliT to confer resistance to gliotoxin, and its future potential as a selection marker gene, we introduced gliT into A. nidulans which does not produce gliotoxin and neither does it contain any genes involved in gliotoxin biosynthesis [22], [23]. The absence of gliT, and cognate gene expression, in A. nidulans was confirmed by Southern and Northern analysis (Figure 6A & B). Subsequent transformation of A. nidulans with A. fumigatus-derived gliT resulted in the generation of three transformants (AngliT 1, 2 and 3) (Deposition number: IMI CC 396693), which were shown by Northern analysis to express gliT to different extents (Figure 6B). This led to acquisition of resistance to high levels of exogenous gliotoxin (50 µg/ml) (Figure 6C) thereby confirming the key role of gliT in protection against gliotoxin toxicity in gliotoxin-naïve fungi. The gliT coding sequence was also transformed into the genetically distant yeast, S. cerevisiae BY4741, under control of the constitutive SSA2 promoter [24] in plasmid pC210. As can be seen in Figure 6D, yeast transformed with plasmid-encoded gliT were capable of growth in the presence of gliotoxin (16 and 64 µg/ml, respectively) depending on whether minimal or rich media was used to support growth, while those transformed with empty vector were unable to grow, irrespective of what media conditions were used. These observations further confirm the pivotal role of gliT in mediating resistance to gliotoxin, even in fungal species which do not normally contain the gene or biosynthesise gliotoxin.
Fig. 6. Transformation of A. nidulans and S. cerevisiae with gliT facilitates resistance to exogenous gliotoxin. 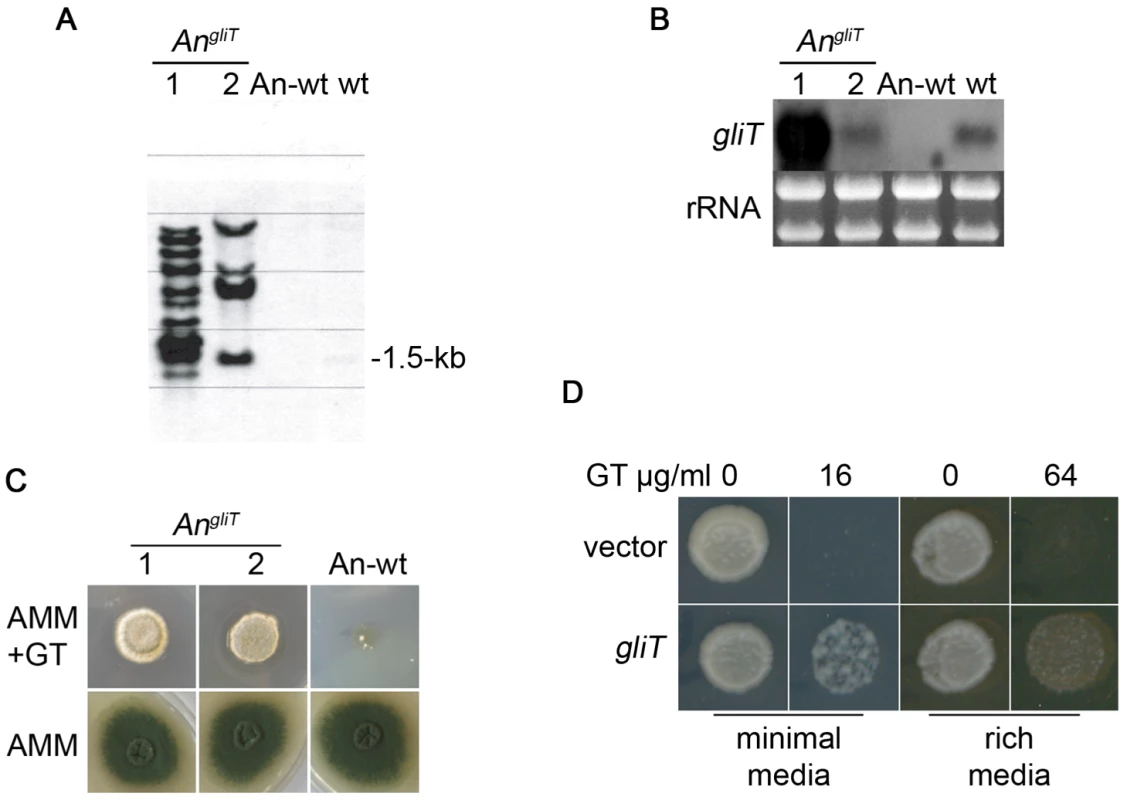
(A) Southern analysis confirms that two A. nidulans transformants contain gliT (AngliT 7 and 8) compared to A. nidulans wild-type (An-wt). wt: A. fumigatus (positive control for gliT). Genomic DNA was digested with PstI and probed for the presence of A. fumigatus gliT. (B) Northern analysis of gliT in An-wt, AngliT (7 and 8) and A. fumigatus wild-type (wt). RNA was isolated using TRI-reagent and 10 µg of total RNA were probed with a gliT-specific probe. (C) Plate assay of A. nidulans wild-type and A. nidulans expressing gliT (AngliT 7 and 8). A. nidulans wild-type and AngliT (104) conidia were dotted on AMM and on AMM containing gliotoxin (50 µg/ml). Growth was monitored over a period of 72 h at 37°C. Genotypes of strains are described in Table 1. (D) GliT confers gliotoxin resistance on S. cerevisiae. Spots represent equal numbers of yeast cells plated onto medium containing gliotoxin at the concentrations indicated. Plates were incubated at 30°C for two days followed by a further three days at room temperature. Discussion
Studies into the biosynthesis and pathogenicity of gliotoxin have attracted significant recent attention, stimulated in part by the plethora of fungal genome data now emerging [3], [22]. Here, we demonstrate for the first time that disruption of gliT, found within the gliotoxin biosynthetic cluster, but subject to differential regulation, completely sensitizes A. fumigatus to exogenous gliotoxin, and abolishes gliotoxin secretion. The possibility that genes adjacent to gliT in the gliotoxin gene cluster (gliF or gliH) play a role in auto-protection is excluded. Thus, we have elucidated a key cellular protective mechanism against the hitherto unknown, potent auto-toxicity of gliotoxin in A. fumigatus. Exposure of A. fumigatus ΔgliT to gliotoxin appears to result in depletion of intracellular GSH since the inhibitory phenotype can be completely relieved by GSH supplementation. Furthermore, we demonstrate the enzymatic functionality of GliT as a gliotoxin reductase and that GliT reactivity is evident in human sera. We demonstrate that gliT confers resistance to exogenous gliotoxin, independently of the extent of gliT expression, following transformation in naïve hosts, A. nidulans and S. cerevisiae. Finally, identification of gliT complementation in A. fumigatus ΔgliT46645 and 26933, respectively, was selected for in the presence of gliotoxin which supports a selection marker role for gliT in A. fumigatus transformation experimentation.
To date, the potential requirements for self-protection against gliotoxin, in A. fumigatus, have not been studied. The ETP toxin, sirodesmin, is produced by the fungus Leptosphaeria maculans with biosynthesis encoded by a multigene cluster similar to that responsible for gliotoxin production in A. fumigatus [13]. Deletion of the sirodesmin transporter gene, sirA, in L. maculans led to increased sensitivity to exogenous sirodesmin and gliotoxin, however the A. fumigatus gliotoxin transporter, GliA, was shown to confer resistance to exogenous gliotoxin (10 µM), but not sirodesmin, in L. maculans ΔsirA. Interestingly, production and secretion of sirodesmin was actually increased by 39% in L. maculans ΔsirA compared to wild-type and resulted in speculation as to the presence of alternative toxin efflux mechanisms [13]. Based on our observations, we hypothesise that in addition to the likely role of gliA in gliotoxin efflux in A. fumigatus, GliT may play an essential role in the auto-protective strategy against the deleterious effects of the ETP toxin. Moreover, we predict that gliT orthologs in other fungi [22] may play similar, if not identical roles.
Our results indicate that absence of GliT may lead to accumulation of intracellular gliotoxin which is reduced, non-enzymatically, by GSH, analogous to the situation in animal cells as demonstrated by Bernardo et al. [17]. The concomitant depletion of intracellular GSH levels, allied to the cytotoxicity of reduced gliotoxin, results in strong growth inhibition, possibly mediated by disruption of the cellular redox status and significant protein modification by gliotoxin. This conclusion is strongly supported by the observation that addition of GSH, during exposure of A. fumigatus ΔgliT to gliotoxin, effectively completely reverses the cytostatic effects of gliotoxin. While we cannot exclude the possibility that added GSH is merely reducing exogenously added gliotoxin and preventing import of the reduced form, it is clear from Figure 1 that addition of NaBH4-reduced gliotoxin results in significant growth inhibition of A. fumigatus ΔgliT (p<0.05). The observed alleviation of this inhibition (by NaBH4-reduced gliotoxin), in the presence of added GSH, supports the proposal that intracellular GSH depletion is a consequence of gliT disruption, when growth occurs in the presence of exogenous gliotoxin.
Addition of gliotoxin (up to 20 µg/ml) for 24 h resulted in the complete up-regulation of the gene cluster (except gliP) in A. fumigatus ΔgliP, but not in A. fumigatus wild-type [9]. We demonstrate that exposure to exogenous gliotoxin for 3 h does induce GliT expression in A. fumigatus wild-type at the transcript and protein level, in fact these data also represent the first confirmed identification of a protein encoded by the gliotoxin biosynthetic cluster. The discrepancy, possibly due to 3 versus 24 h experimental windows, nonetheless, indicates differential GliT expression relative to other gli genes. Disruption of gliZ, the transcriptional regulator of the gliotoxin biosynthetic cluster, has been shown to result in abolition of gliotoxin production and loss of gliotoxin cluster gene expression [14]. Our data demonstrate that although growth of A. fumigatus ΔgliZ and gliZc is unaffected by exogenous gliotoxin, gliZ expression is up-regulated in response to exogenous gliotoxin exposure in A. fumigatus ATCC46645, but to a lesser extent than that of gliT (Figure 2). In addition, we have shown that gliT expression is induced by gliotoxin addition to liquid cultures of A. fumigatus ΔgliZ thereby confirming the independent regulation of gliT expression to other cluster components (e.g., gliA and gliG). In combination, these observations further confirm the minimal role played by any other component of the gli gene cluster in protection against gliotoxin presence since gliZ absence results in complete cluster attenuation [14], except for gliT.
A thioredoxin system in A. nidulans has recently been described whereby a thioredoxin mutant exhibited decreased growth, impaired reproductive function and altered catalase activity [25]. These authors also identified a thioredoxin reductase (termed AnTrxR) which functions to regenerate reduced thioredoxin in A. nidulans. Our BLAST analysis indicates minimal identity between GliT and AnTrxR as well as between GliT and a second putative thioredoxin reductase in A. fumigatus (Genbank accession number: EAL85952; 30% identity). This strongly indicates distinct functionality of gliT and confirms that alternative thioredoxin reductase activities cannot compensate for loss of gliT in A. fumigatus. It further appears unlikely that thioredoxin is involved in mediating GliT activity since no thioredoxin reductase present in A. fumigatus cell lysates appears capable of compensating for GliT absence. Consequent to its bioinformatic classification as a thioredoxin reductase, GliT has been predicted by many authors to encode disulphide bond formation in gliotoxin and to play a role in gliotoxin biosynthesis [3], [8], [22]. While this ‘gliotoxin oxidase’ activity cannot be ruled out completely, our demonstration that GliT exhibits gliotoxin reductase activity (Figure 5) suggests that direct gliotoxin reduction is a pre-requisite for secretion from A. fumigatus via a GliT-mediated pathway or as a component of the auto-protective mechanism deployed against exogenous gliotoxin secreted by adjacent fungi in the environment (Figure 7). This hypothesis is firmly supported by the absence of gliotoxin secretion in A. fumigatus ΔgliT26933. Given the potential of reduced gliotoxin to thiolate cellular proteins, we speculate that reduced gliotoxin may be sequestered into intracellular vesicles where it is converted to the oxidized form, by an unidentified activity, prior to release from the cell by an exocytotic mechanism complementary to GliA-mediated efflux (Figure 7). It remains possible that GliT-mediated gliotoxin oxidase activity may be associated with disulfide bridge closure during gliotoxin biosynthesis when intracellular levels of gliotoxin can be regulated more precisely by the organism. Thus, GliT could be necessary to maintain a balance between reduced and oxidised gliotoxin in A. fumigatus. The detection of a molecular ion, with a molecular mass corresponding to a monothiol form of gliotoxin, in culture supernatants from A. fumigatus ΔgliT is interesting, and we hypothesize that this metabolite may represent a breakdown product of gliotoxin. Future work will involve purification and complete characterization of this molecule. The observation that GliT-specific IgG was present in human sera was unexpected and implies that GliT is either present in inhaled conidia or is expressed during abortive conidial germination in immunocompetent individuals. However, our observation suggests that the option of using normal human sera as a source of immunoaffinity antibodies, following Ig isolation and purification using a recombinant antigen (e.g., GliT), represents a novel approach for readily obtaining monospecific antisera against antigenic A. fumigatus proteins.
Fig. 7. A proposed model for GliT functionality in A. fumigatus based on experimental observations. 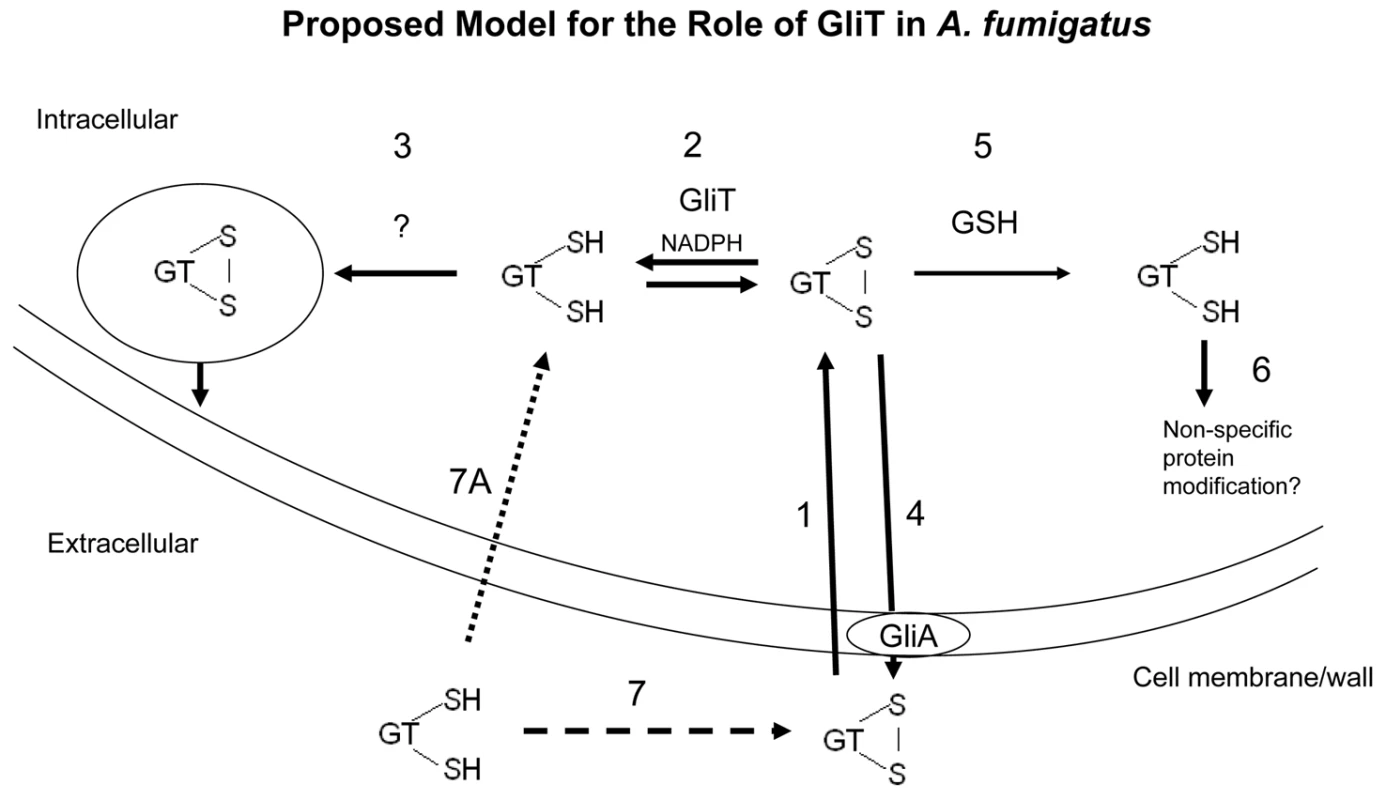
Exogenous gliotoxin enters A. fumigatus (1) and is converted to the reduced form intracellularly by GliT (gliotoxin reductase activity (2)). GliT may also be necessary to oxidize reduced gliotoxin during biosynthesis in A. fumigatus. Given the toxicity of the intracellular form of reduced gliotoxin, we predict that it may be imported into intracellular vesicles, possibly with concomitant oxidation for storage (3). GliA function to facilitate gliotoxin efflux (4) is extrapolated from the observation in L. maculans that this protein confers resistance to exogenous gliotoxin [13]. In the absence of GliT, gliotoxin may be alternately reduced by intracellular GSH (5) leading to a depletion in GSH and cell death/growth arrest and also modification of other cellular proteins leading to inactivation or activity modification (6). In this model, absence of GliT would lead to the build up of gliotoxin within the cell and also the inability to reduce exogenously added gliotoxin. Reduced gliotoxin may not enter but converts to the oxidized form in a time-dependent manner (7, 7A). The animal model system deployed herein appears to distinguish between virulence diminution associated with lack of gliotoxin production, since inoculation with A. fumigatus ΔgliZ resulted in reduced Gallerial mortality than exposure to wild-type A. fumigatus. This result extends previous observations with respect to the potential avirulence of A. fumigatus ΔgliZ [14]. However, the relatively equivalent virulence observed for A. fumigatus wild-type and ΔgliT, whereby the latter does not produce gliotoxin is somewhat at variance with the A. fumigatus ΔgliZ findings. We suggest that alterations in the levels of additional metabolites in A. fumigatus ΔgliZ, as noted in [14], or a possible cytotoxic role in G. mellonella for the putative monothiol form of gliotoxin secreted by A. fumigatus ΔgliT may account for this dichotomy. Our demonstration that gliT is expressed independently of other cluster components implies that previous virulence model experimentation, involving gliP - and gliZ –deficient mutants [9]–[12], may require interpretation in light of the possibility of independently regulated gliT expression, or GliT functionality. Indeed, if it is ever demonstrated that gliT expression occurs in the absence of gli cluster expression/gliotoxin biosynthesis (as has been demonstrated herein for A. fumigatus ΔgliZ), or is regulated by factors other than exposure to exogenous gliotoxin, then consideration may need to be given to this phenomenon in future studies. This consideration is based on the fact that independent regulation of gliT may have enabled acquisition of functionality beyond a role in gliotoxin biosynthesis or auto-protection.
Genetic modification of filamentous fungi for the improved production of food additives, industrial enzymes or pharmaceuticals is an ongoing requirement of the biotechnological industry [26], [27]. Antibiotic-producing fungi are continually subjected to strain improvement, with a concomitant requirement for new selection markers, to increase product yield and decrease the level of unwanted side-products [28]. Our observation that gliT complementation in A. fumigatus can be selected for in the presence of gliotoxin, without the use of conventional selection markers, and that transformation of A. nidulans and S. cerevisiae with gliT confers enhanced resistance to gliotoxin offers the possibility of using the gliT/gliotoxin combination to select for fungal transformation. Moreover, acquired gliotoxin resistance in A. nidulans and S. cerevisiae resulting from gliT presence, underpins the important role played by this gene in mediating resistance to exogenous gliotoxin. Gliotoxin isolated from cultures of a marine fungus from the genus Pseudallescheria has been shown to possess both anti-bacterial and free-radical scavenging capability whereby an MIC50 of 1 µg/ml was observed against methicillin-resistant Staphylococcus aureus [29]. Gliotoxin may also provide a competitive advantage for A. fumigatus when grown in the presence of other fungi [30]. In this regard, gliotoxin production has been detected when A. fumigatus was co-cultured, at both 30 and 37°C, with a range of other Aspergillus spp., leading the authors to speculate that co-expression of resistance genes may allow toxin producers to resist the effects of their own biological arsenal in competitive co-culture situations [30]. The parallel between this supposition, and our observation of GliT-mediated resistance to exogenous gliotoxin, is vivid.
The vast majority of literature surrounding the role of gliotoxin in A. fumigatus focuses on its function as a cytotoxic molecule which has deleterious effects on cells within infected individuals and exhibits anti-microbial activity [5], [6], [9]–[12], [29], [30]. However, based on our observations and significant other literature [16], [18], [31], a credible alternative hypothesis is that gliotoxin may actually be part of the intracellular antioxidant defense system within A. fumigatus, and is a molecule, analogous to thioredoxin or 2-cys peroxiredoxin, which may undergo rapid changes in redox status to buffer against specific exogenous or endogenous oxidants. In other words, the cytotoxic effects of gliotoxin in infected host cells may actually be an indirect consequence of its role within A. fumigatus. This alternative hypothesis is not without support. Firstly, Watanabe et al. [31] have shown that the cytotoxicity of A. fumigatus culture filtrates was significantly attenuated, or absent, when cultures were grown under reduced aerobic or anaerobic conditions. Interestingly, gliotoxin production was detectable by GC-MS analysis from aerobic but not in reduced aerobic culture supernatants. Although Watanabe et al. concluded that their results indicated that gliotoxin production is increased to facilitate fungal pathogenicity (mimicking the aerobic lung environment), an alternative conclusion, which is in accordance with our thinking, is that gliotoxin production is actually elevated to cope with increased oxygen levels and that secretion of gliotoxin forms part of the gliotoxin homeostasis control mechanism within A. fumigatus to prevent the side-effect of intracellular oxidative stress. As noted earlier, in animal cells it has been shown that gliotoxin may substitute for 2-cys peroxiredoxin activity in HeLa cells by accepting electrons from NADPH via the thioredoxin reductase–thioredoxin redox system to reduce H2O2 to H2O. In this way, nanomolar levels of gliotoxin may actually protect against intracellular oxidative stress [18]. Additionally, as demonstrated by Srinivasan et al. [16], oxidized gliotoxin facilitates selective protein inactivation in the presence of molecular oxygen which, we hypothesise, could prevent global intracellular damage due to resultant reactive oxygen species. Moreover, a protective role for gliotoxin against environmental stress in A. fumigatus has been considered [2], [13]. Our observations and consequent hypothesis now provide a vehicle to explore this proposal.
In summary, we have demonstrated that GliT plays a major auto-protective role against gliotoxin toxicity in A. fumigatus which points to alternative gliotoxin functionality in A. fumigatus. From a utilitarian viewpoint, gliT/gliotoxin sensitivity represents a potential new selection marker strategy for fungal transformation. The trans-fungal implications of our observations remain to be explored.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. Ethical permission was obtained from The Ethics Committee of NUI Maynooth for the use of human serum specimens. Anonymous serum specimens were obtained with the signed agreement of the Irish Blood Transfusion Service.
Strains, growth conditions, and general DNA manipulation
In general, A. fumigatus strains (Table 1) were grown at 37°C in Aspergillus minimal media (AMM). AMM contained 1% (w/v) glucose as carbon-source, 5 mM ammonium tartarate as nitrogen-source, and trace elements according to Pontecorvo et al. [32]. Liquid cultures were performed with 200 ml AMM in 500 ml Erlenmeyer flasks inoculated with 108 conidia. For growth assays, 104 conidia of the respective strains were point inoculated on AMM plates, containing the relevant supplements and incubated for 48 h at 37°C.
Tab. 1. <i>A. fumigatus</i> and <i>A. nidulans</i> strains used in this study. 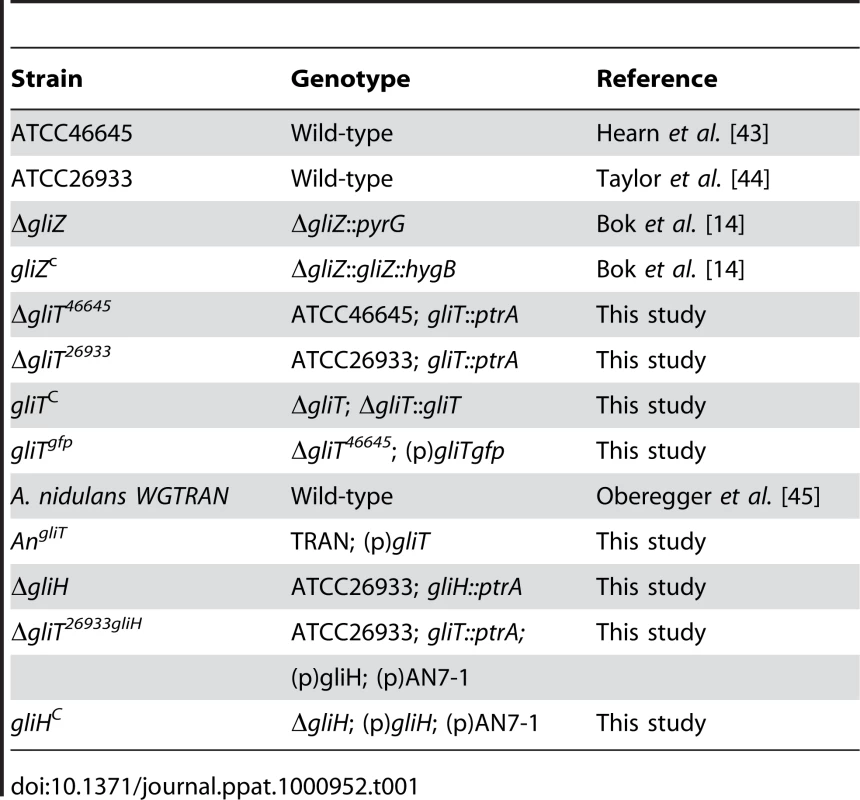
TOPO TA cloning system (Invitrogen) and TOP10 E. coli cells (F-mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 galU galK Δ (ara-leu)7697 rpsL (StrR) endA1 nupG) were used for general plasmid DNA propagation and A. fumigatus genomic DNA was purified using a ZR Fungal/Bacterial DNA Kit (Zymoresearch).
Generation of A. fumigatus mutant strains
For generating ΔgliT mutant strains, the bipartite marker technique was used [20]. Briefly, A. fumigatus strains ATCC46645 and ATCC26933 were co-transformed with two DNA constructs, each containing an incomplete fragment of a pyrithiamine resistance gene (ptrA) [21] fused to 1.2 kb, and 1.3 kb of gliT flanking sequences, respectively. These marker fragments shared a 557 bp overlap within the ptrA cassette, which served as a potential recombination site during transformation. During transformation, homologous integration of each fragment into the genome flanking gliT allows recombination of the ptrA fragments and generation of the intact resistance gene at the site of recombination. Two rounds of PCR generated each fragment. First, each flanking region was amplified from ATCC46645 genomic DNA using primer ogliT1 and ogliT4 for flanking region A (1.3 kb), and ogliT-2 and ogliT-3 for flanking region B (1.2 kb). Subsequent to gel-purification, the fragments were digested with SpeI and HindIII, respectively. The ptrA selection marker was released from plasmid pSK275 (a kind gift from Sven Krappmann, Goettingen, Germany) by digestion with SpeI and HindIII, and ligated with the two flanking regions A and B described above. For generation of ΔgliT, two overlapping fragments were amplified from the ligation products using primers ogliT-5 and optrA-2 for fragment C (2.6 kb) and primers ogliT-6 and optrA-1 for fragment D (2.2 kb). Subsequently ATCC46645 and ATCC26933 were transformed simultaneously with the overlapping fragments C and D. In the generated mutant allele of ΔgliT-ptrA the deleted region comprises amino acids 1–325 of gliT.
For reconstitution of the ΔgliT strain with a functional gliT copy, a 3.2 kb PCR fragment, amplified using primers ogliT-5 and ogliT-6, was subcloned into pCR2.1-TOPO (Invitrogen). The resulting 7.1 kb pgliT was linearised with AatII and used to transform A. fumigatus ΔgliT protoplasts. Taking advantage of the decreased resistance of the ΔgliT mutant to exogenous added gliotoxin ΔgliT protoplasts were transformed with pgliT and screened for wild-type resistance to gliotoxin for genetic complementation. Positive deletion - and reconstituted - strains were screened by Southern analysis (Figure S1) and DIG-hybridisation probes were generated using primers ogliT-5 and ogliT-4.
To obtain knock-out constructs for the deletion of gliH a 5′ flanking region with oligos ogliH1 and ogliH4 was amplified. For the 3′ flanking region a PCR with oligos ogliH2 and ogliH3 was performed. Amplicons were digested with SpeI and HindIII, respectively. Resulting fragments were ligated to a ptrA cassette, released from pSK275 via SpeI and HindIII digest. Final PCRs were obtained using oligos ogliH5/optrA2 and ogliH6/optrA1 and used for transformation.
To complement ΔgliH and ΔgliT26933 with a functional copy of gliH, oligos ogliT7 and M13 were used to amplify a PCR-fragment using pgliT as template. This fragment digested with EcoRI and SacII was cloned into pBS-KS (Stratagene), resulting in pgliH. Together with pAN7-1 [33], pgliH was used to complement A. fumigatus ΔgliH and ΔgliT26933.
GliT was C-terminally fused in frame to gfp (green fluorescent protein) to determine its subcellular localisation. To this end, a fragment containing gliT was amplified using oligos ogliT-5-SphI and ogliT-16. The resulting 2.2 kb fragment was sub-cloned into pCR2.1-TOPO (Invitrogen) and sequenced. Via SphI digest a fragment containing the gliT promoter region and the coding sequence was released and cloned into the corresponding SphI site of pgfp, resulting in pgliTgfp. To obtain pgfp, a gfp containing fragment was released from pUCG-H [34] via SmaI and SacI and subcloned into the corresponding EcoRV and SacI sites of pGEM5zf+ (Promega). The plasmid pgliTgfp was used to transform ΔgliT protoplasts via co-transformation using a phleomycin resistance gene. Phleomycin resistant transformants carrying an in-frame gliT-gfp fusion were used to localize GliT using fluorescence microscopy. Positive, GliT-GFP harbouring strains were screened by Southern analysis and hybridization probes were generated using oligos ogliT-7 and ogliT-8. A. fumigatus transformation was carried out according to Tilburn et al. [35]. In order to obtain homokaryotic transformants, colonies from single homokaryotic spores were picked and single genomic integration was confirmed by PCR (data not shown) and Southern blot analysis.
Northern analysis
RNA was isolated using TRI-Reagent (Sigma-Aldrich). Equal concentrations of total RNA (10 µg) were size-separated on 1.2% agarose-2.2 M formaldehyde gels and blotted onto Hybond N+ membranes (Amersham Biosciences). The hybridisation probes used in this study were generated by PCR using primers ogliA1 and ogliA2 for AFUA_6G09710, ogliG7 and ogliG8 for AFUA_6G09690, ogliT7 and ogliT8 for AFUA_6G09740, and ogliZ1 and ogliZ2 for AFUA_6G09630. All primers used in this study are listed in Table 2.
Tab. 2. Primers used in this study. 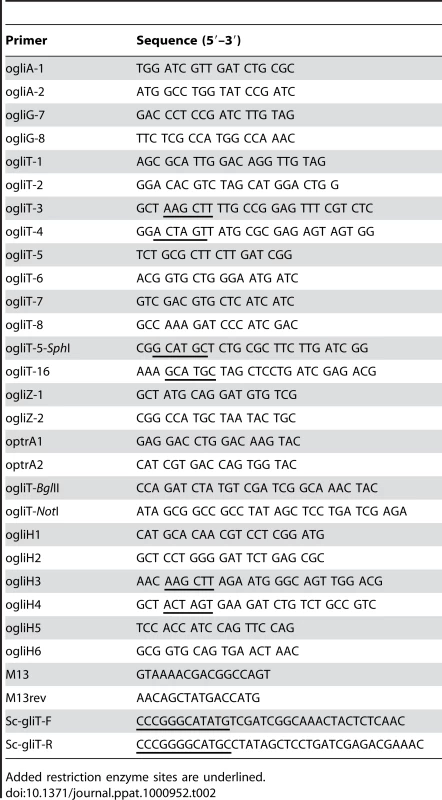
Added restriction enzyme sites are underlined. Proteomic analysis of GliT expression
A. fumigatus ATCC26933 was cultured (n = 3) for 21 h in Sabouraud media followed by gliotoxin addition for 3 h (final concentration: 14 µg/ml). Control cultures (n = 3), where gliotoxin was not added, were also performed. Mycelia were harvested, lysed and subject to MALDI-ToF mass spectrometric analysis as previously described [36] and Imagemaster analysis (GE Healthcare).
Analysis of gliotoxin production
To analyze gliotoxin, or related metabolite production, A. fumigatus wild-type and mutant strains were grown up at 37°C for 72 h in Czapeks-Dox. Supernatants were chloroform extracted overnight and fractions were lyophilized to complete dryness. Samples were resolubilised in MeOH and analysed using a reversed phase HPLC as described in [37] and LC-MS (Agilent 6340 ETD LC-MS system). Samples (1 µl) were loaded onto a Zorbax 300SB C-18 Nano-HPLC Chip (150 mm×75 µm, Agilent) with 0.1%(v/v) formic acid (0.6 µl/min), and compounds eluted by an increasing 0.1%(v/v) formic acid, acetonitrile gradient (90%(v/v) final). Eluted compounds were directly ionised and analysed by ion trap mass spectrometer (Agilent). For each round of MS the two most abundant compounds were automatically selected for MSn analysis. Gliotoxin was identified by its whole mass of 326.4 m/z and its characteristic MSn fragmentation pattern (263, 245 and 227 m/z). LC-ToF analysis was performed using an Agilent HPLC 1200 series using electrospray ionisation inputted into a ToF (Agilent). LC separation was via an XDB C18 column (4.6×150 mm) using a water/acetonitrile (both containing 0.1% (v/v) formic acid) gradient at a flow rate of 0.5 ml/min. The gradient was started at 50% (v/v) acetonitrile, which was increased to 100% acetonitrile in 10 min; 100% acetonitrile was maintained for 5 min before the gradient was returned to starting conditions. Spectra were collected at 0.99 spectra per second.
Cloning and expression of gliT
The gliT sequence was amplified from cDNA using primers incorporating terminal XhoI and HindIII sites to facilitate downstream cloning. PCR products were cloned into the pCR2.1 cloning vector (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. gliT was subsequently cloned into the pProEX-Htb expression vector (Invitrogen). Ligations were performed using Quickstick ligase (Bioline, London, UK) according to the manufacturer's instructions. pPXAgliT, the resultant expression vector was transformed into E. coli strain BL21 by standard protocols. Expression of GliT was induced by the addition of isopropyl β-D-thiogalactoside (IPTG; to 0.6 mM) and monitored by SDS-PAGE and Western blot analysis. Recombinant GliT purification was undertaken by differential extraction. Protein concentrations were determined using the Bradford method [38] with bovine serum albumin as a standard.
Purification of native GliT from A. fumigatus by ion-exchange chromatography
A. fumigatus ATCC46645 mycelia were ground in liquid nitrogen and lysed in ice-cold lysis buffer as described [36] following incubation with gliotoxin (10 µg/ml) for 3 h). Following centrifugation (12,000 g; 30 min), the lysate supernatant (176 ml) was ammonium sulphate precipitated (10, 20, 50 and 70% ammonium sulphate). The 50% pellet was resuspended in 20 mM Bis-Tris propane pH 7.6 and dialysed three times against 50 volumes of the same buffer at 4°C. The dialysate was centrifuged (12,000 g; 20 min) and filtered (0.45 µm) to remove particulates. The dialysate was loaded onto an equilibrated Q-Sepharose ion-exchange (IEX) column (4 ml) at a flow rate of 1 ml/min. The column was washed with 20 mM Bis-Tris propane pH 7.6 before bound protein was eluted using an NaCl gradient (0.5 M final). Absorbance detection was at 280 nm and 454 nm. Collected fractions were subjected to SDS-PAGE, Western blot and activity analysis for GliT.
Immunoaffinity purification of human IgG [anti-GliT]
Serum specimens (provided by the Irish Blood Transfusion Service, Dublin, Ireland according to institutional guidelines) containing high titer IgG [anti-GliT] were pooled, diluted 1 in 4 in PBS, and applied to a GliT-Sepharose affinity column (0.5 ml), prepared as per manufacturer's instructions. After removal of unbound proteins by PBS washing, immobilised IgG [anti-GliT] was eluted using 100 mM glycine pH 2.8, followed by immediate neutralization using 100 mM Trizma base pH 8.3. Resultant immunoaffinity purified (IAP) IgG [anti-GliT] was used to detect native GliT by Western analysis.
GliT activity assay and removal of native GliT from A. fumigatus by IAP pulldown
A. fumigatus ATCC46645 mycelia were ground in liquid nitrogen and lysed in ice-cold lysis buffer and bead-beating as described elsewhere [36]. Following centrifugation (12,000 g; 30 min), the lysate supernatants were used to determine gliotoxin reductase activity (ΔA340 nm) in the presence of gliotoxin (9 µM) and NADPH (200 µM) at pH 7.2 (a modified version of Hill et al. [39]). A. fumigatus cell lysates were also subjected to ion-exchange chromatography and a pooled IEX fractions (250 µl) incubated with IAP human IgG [anti-GliT] (100 µl) followed by Protein A-Sepharose addition and centrifugation (10,000 g; 10 min). Supernatant activity analysis as described above.
GliT-GFP confocal microscopy
A. fumigatus gliTgfp and ATCC46645 mycelia were grown in cell culture six well plates (Corning Inc.) for 21 h before induction with (or without) gliotoxin (5 µg/ml). Mycelia were removed from the wells and centrifuged (12,000 g; 5 min). Supernatants were stored while pellets were resuspended in DAPI staining solution and incubated (5 min) at room temperature. The stained mycelia were centrifuged and washed with deionised H2O before resuspension in the original supernatant. Aliquots of these preparations were analysed for GliT-GFP presence and DAPI fluorescence on an Olympus Fluoview 1000 confocal microscope.
Virulence model
G. mellonella larvae (n = 10) were inoculated into the hind pro-leg with 106 A. fumigatus conidia in 20 µl (per larva) [37]. In addition, one cohort of larvae was pre-treated with gliotoxin (0.5 µg/larva in 20 µl). Control treatments were included to ensure that neither the injection procedure, or the incubation period, were responsible for any mortality observed. These controls involved G. mellonella larvae injected with 20 µl of sterile PBS or gliotoxin alone. G. mellonella larvae were placed in Petri-dishes and incubated in the dark at 30°C. Mortality rates were recorded for 72 h post-injection. Mortality was assessed based on lack of movement in response to stimulation and discolouration (melanisation) of the cuticle.
Generation of gliT-encoding Aspergillus nidulans and Saccharomyces cerevisiae
To introduce gliT in A. nidulans TRAN, a plasmid containing gliT coding sequence under the control of a constitutive otef [40] promoter was used. Therefore, a 1.1 kb fragment containing gliT was amplified using ogliT-BglII and ogliT-NotI and subcloned into pCR2.1-TOPO (Invitrogen). A 0.9 kb fragment containing an otef promoter was released via BamHI/KpnI digest from plasmid pUCG-H [34] and cloned into the respective sites into pGliT-BglII-NotI. Transformation was performed as described for A. fumigatus.
The S. cerevisiae strain used in this study was BY4741 (MATa his3D1 leu2D0 met15D0 ura3D0) and was purchased from Euroscarf. Rich and minimal yeast medium was as described in [41], and gliotoxin was added to the desired concentration to cooled molten agar. To monitor the effects of GliT expression in S. cerevisiae gliT was amplified from A. fumigatus (ATCC46645) using PCR with primers Sc-gliT-F and Sc-gliT-R (Table 2), and cloned into the yeast shuttle vector pC210 [42]. Plasmids pC210 harbors the SSA1 coding sequence under control of the constitutive SSA2 promoter. Following digestion of pC210 with NdeI and SphI to remove the SSA1 coding sequence, similarly digested gliT PCR product was ligated into pC210 to create pC-GliT. Thus, pC-GliT harbors A. fumigatus gliT under control of the strong constitutive S. cerevisiae SSA2 promoter. The integrity of pC-GliT was confirmed by sequencing.
To test the sensitivity of yeast to gliotoxin, BY4741 harboring either vector alone (pRS315) or pC-GliT was grown to mid-exponential phase (3×106 cells/ml). Cells were harvested and resuspended in rich medium to a concentration of 5×106 cells/ml. Cells were serially diluted and were plated onto rich or minimal medium containing the desired concentration of gliotoxin, using a multi-pronged replicator. Plates were incubated at 30°C for 48 h with further monitoring of plates at room temperature for 72 h.
Accession numbers
The proteins named herein are available at Genbank under the following Accession numbers: GliA (AAW03302); GliF (AAW03300); GliG (AAW03304); GliH/AFUA_6G09745 (EAL88826); GliT (AAW03299) and GliZ (AAW03310).
Supporting Information
Zdroje
1. GardinerDM
WaringP
HowlettBJ
2005 The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology 151 1021 1032
2. Kwon-ChungKJ
SuguiJA
2008 What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med Mycol 2 1 7
3. FoxEM
HowlettBJ
2008 Biosynthetic gene clusters for epipolythiodioxopiperazines in filamentous fungi. Mycol Res 112 162 169
4. HurneAM
ChaiCL
WaringP
2000 Inactivation of rabbit muscle creatine kinase by reversible formation of an internal disulfide bond induced by the fungal toxin gliotoxin. J Biol Chem 275 25202 25206
5. TsunawakiS
YoshidaLS
NishidaS
KobayashiT
ShimoyamaT
2004 Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect Immun 72 3373 3382
6. NishidaS
YoshidaLS
ShimoyamaT
NunoiH
KobayashiT
2005 Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase. Infect Immun 73 235 244
7. GardinerDM
CozijnsenAJ
WilsonLM
PedrasMS
HowlettBJ
2004 The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans. Mol Microbiol 53 1307 1318
8. GardinerDM
HowlettBJ
2005 Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol Lett 248 241 248
9. CramerRAJr
GamcsikMP
BrookingRM
NajvarLK
KirkpatrickWR
2006 Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell 5 972 980
10. KupfahlC
HeinekampT
GeginatG
RuppertT
HärtlA
2006 Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol 62 292 302
11. SuguiJA
PardoJ
ChangYC
ZaremberKA
NardoneG
2007 Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6 1562 1569
12. SpikesS
XuR
NguyenCK
ChamilosG
KontoyiannisDP
2008 Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 197 479 486
13. GardinerDM
JarvisRS
HowlettBJ
2005 The ABC transporter gene in the sirodesmin biosynthetic gene cluster of Leptosphaeria maculans is not essential for sirodesmin production but facilitates self-protection. Fungal Genet Biol 42 257 263
14. BokJW
ChungD
BalajeeSA
MarrKA
AndesD
2006 GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 74 6761 6768
15. BalibarCJ
WalshCT
2006 GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry 45 15029 15038
16. SrinivasanU
BalaA
JaoSC
StarkeDW
JordanTW
2006 Selective inactivation of glutaredoxin by sporidesmin and other epidithiopiperazinediones. Biochemistry 45 8978 8987
17. BernardoPH
BraschN
ChaiCL
WaringP
2003 A novel redox mechanism for the glutathione-dependent reversible uptake of a fungal toxin in cells. J Biol Chem 278 46549 46555
18. ChoiHS
ShimJS
KimJA
KangSW
KwonHJ
2007 Discovery of gliotoxin as a new small molecule targeting thioredoxin redox system. Biochem Biophys Res Commun 359 523 528
19. ChamilosG
LewisRE
LamarisGA
AlbertND
KontoyiannisDP
2008 Genomewide screening for genes associated with gliotoxin resistance and sensitivity in Saccharomyces cerevisiae. Antimicrob Agents Chemother 52 1325 1329
20. NielsenML
AlbertsenL
LettierG
NielsenJB
MortensenUH
2006 Efficient PCR-based gene targeting with a recyclable marker for Aspergillus nidulans Fungal Genet Biol 43 54 64
21. KuboderaT
YamashitaN
NishimuraA
2000 Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci Biotechnol Biochem 64 1416 1421
22. PatronNJ
WallerRF
CozijnsenAJ
StraneyDC
GardinerDM
2007 Origin and distribution of epipolythiodioxopiperazine (ETP) gene clusters in filamentous ascomycetes. BMC Evol Biol 7 174
23. GalaganJE
CalvoSE
CuomoC
MaLJ
WortmanJR
2005 Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438 1105 1115
24. Hjorth-SørensenB
HoffmannER
LissinNM
SewellAK
JakobsenBK
2001 Activation of heat shock transcription factor in yeast is not influenced by the levels of expression of heat shock proteins. Mol Microbiol 39 914 923
25. ThönM
Al-AbdallahQ
HortschanskyP
BrakhageAA
2007 The thioredoxin system of the filamentous fungus Aspergillus nidulans: impact on development and oxidative stress response. J Biol Chem 282 27259 27269
26. LeClerqueA
WanH
2007 Novel dominant selection marker for the transformation of fungi. US Patent 2007178594
27. ArcherDB
DyerPS
2004 From genomics to post-genomics in Aspergillus. Curr Opin Microbiol 7 499 504
28. Rodríguez-SáizM
LemboM
BertettiL
MuracaR
VelascoJ
2004 Strain improvement for cephalosporin production by Acremonium chrysogenum using geneticin as a suitable transformation marker. FEMS Microbiol Lett 235 43 49
29. LiX
KimSK
NamKW
KangJS
ChoiHD
2006 A new antibacterial dioxopiperazine alkaloid related to gliotoxin from a marine isolate of the fungus Pseudallescheria. J Antibiot (Tokyo) 59 248 250
30. LosadaL
AjayiO
FrisvadJC
YuJ
NiermanWC
2009 Effect of competition on the production and activity of secondary metabolites in Aspergillus species. Med Mycol 47 Suppl 1 S88 96
31. WatanabeA
KameiK
SekineT
WakuM
NishimuraK
2004 Effect of aeration on gliotoxin production by Aspergillus fumigatus in its culture filtrate. Mycopathologia 157 19 27
32. PontecorvoG
RroperJA
HemmonsLM
MacDonaldKD
BuftonAW
1953 The genetics of Aspergillus nidulans. Adv Genet 5 141 238
33. PuntPJ
OliverRP
DingemanseMA
PouwelsPH
van den HondelCA
1987 Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 56 117 124
34. LangfelderK
PhilippeB
JahnB
LatgéJP
BrakhageAA
2001 Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect Immun 69 6411 6418
35. TilburnJ
Sánchez-FerreroJC
ReoyoE
ArstHNJr
PeñalvaMA
2005 Mutational analysis of the pH signal transduction component PalC of Aspergillus nidulans supports distant similarity to BRO1 domain family members. Genetics 171 393 401
36. CarberryS
NevilleCM
KavanaghKA
DoyleS
2006 Analysis of major intracellular proteins of Aspergillus fumigatus by MALDI mass spectrometry: identification and characterisation of an elongation factor 1B protein with glutathione transferase activity. Biochem Biophys Res Commun 341 1096 1104
37. ReevesEP
MessinaCG
DoyleS
KavanaghK
2004 Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158 73 79
38. BradfordMM
1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248 254
39. HillKE
McCollumGW
BurkRF
1997 Determination of thioredoxin reductase activity in rat liver supernatant. Anal Biochem 253 123 125
40. SpelligT
BottinA
KahmannR
1996 Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Mol Gen Genet 252 503 509
41. LooversHM
GuinanE
JonesGW
2007 Importance of Hsp70 ATPase domain in prion propagation. Genetics 175 621 630
42. SchwimmerC
Masison
DC
2002 Antagonistic interactions between yeast [PSI+] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol 22 3590 3598
43. HearnVM
MackenzieDW
1980 Mycelial antigens from two strains of Aspergillus fumigatus: an analysis by two-dimensional immunoelectrophoresis. Mykosen 23 549 562
44. TaylorJJ
BurroughsEJ
1973 Experimental avian aspergillosis. Mycopathol Mycol Appl 51 131 141
45. ObereggerH
EisendleM
SchrettlM
GraessleS
HaasH
2003 4′-phosphopantetheinyl transferase-encoding npgA is essential for siderophore biosynthesis in Aspergillus nidulans. Curr Genet 44 211 215
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin NeutralizationČlánek Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane KinasesČlánek Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Cognitive Dysfunction Is Sustained after Rescue Therapy in Experimental Cerebral Malaria, and Is Reduced by Additive Antioxidant Therapy
- DNA Watermarking of Infectious Agents: Progress and Prospects
- Self-Protection against Gliotoxin—A Component of the Gliotoxin Biosynthetic Cluster, GliT, Completely Protects Against Exogenous Gliotoxin
- Modifies the Tsetse Salivary Composition, Altering the Fly Feeding Behavior That Favors Parasite Transmission
- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- The Enteropathogenic Effector EspF Targets and Disrupts the Nucleolus by a Process Regulated by Mitochondrial Dysfunction
- Epithelial p38α Controls Immune Cell Recruitment in the Colonic Mucosa
- Coexpression of PD-1, 2B4, CD160 and KLRG1 on Exhausted HCV-Specific CD8+ T Cells Is Linked to Antigen Recognition and T Cell Differentiation
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- EspA Acts as a Critical Mediator of ESX1-Dependent Virulence in by Affecting Bacterial Cell Wall Integrity
- An RNA Element at the 5′-End of the Poliovirus Genome Functions as a General Promoter for RNA Synthesis
- Tetherin Restricts Productive HIV-1 Cell-to-Cell Transmission
- Epstein-Barr Virus-Encoded LMP2A Induces an Epithelial–Mesenchymal Transition and Increases the Number of Side Population Stem-like Cancer Cells in Nasopharyngeal Carcinoma
- Protein Kinase A Dependent Phosphorylation of Apical Membrane Antigen 1 Plays an Important Role in Erythrocyte Invasion by the Malaria Parasite
- Requirement for Ergosterol in V-ATPase Function Underlies Antifungal Activity of Azole Drugs
- The SOCS-Box of HIV-1 Vif Interacts with ElonginBC by Induced-Folding to Recruit Its Cul5-Containing Ubiquitin Ligase Complex
- A Crucial Role for Infected-Cell/Antibody Immune Complexes in the Enhancement of Endogenous Antiviral Immunity by Short Passive Immunotherapy
- Modulation of the Arginase Pathway in the Context of Microbial Pathogenesis: A Metabolic Enzyme Moonlighting as an Immune Modulator
- Role of Abl Kinase and the Wave2 Signaling Complex in HIV-1 Entry at a Post-Hemifusion Step
- Serum-Dependent Selective Expression of EhTMKB1-9, a Member of B1 Family of Transmembrane Kinases
- Epigenetic Repression of by Latent Epstein-Barr Virus Requires the Interaction of EBNA3A and EBNA3C with CtBP
- Host Cell Invasion and Virulence in Sepsis Is Facilitated by the Multiple Repeats within FnBPA
- Role of PKR and Type I IFNs in Viral Control during Primary and Secondary Infection
- A Kinome RNAi Screen Identified AMPK as Promoting Poxvirus Entry through the Control of Actin Dynamics
- Cryptococcal Cell Morphology Affects Host Cell Interactions and Pathogenicity
- NleG Type 3 Effectors from Enterohaemorrhagic Are U-Box E3 Ubiquitin Ligases
- Human Cytomegalovirus UL29/28 Protein Interacts with Components of the NuRD Complex Which Promote Accumulation of Immediate-Early RNA
- Complement Receptor 1 Is a Sialic Acid-Independent Erythrocyte Receptor of
- A Viral microRNA Down-Regulates Multiple Cell Cycle Genes through mRNA 5′UTRs
- Immunotoxin Complementation of HAART to Deplete Persisting HIV-Infected Cell Reservoirs
- Protein Expression Redirects Vesicular Stomatitis Virus RNA Synthesis to Cytoplasmic Inclusions
- Entry and Fusion of Emerging Paramyxoviruses
- Paramyxovirus Entry and Targeted Vectors for Cancer Therapy
- Suppressing Glucose Transporter Gene Expression in Schistosomes Impairs Parasite Feeding and Decreases Survival in the Mammalian Host
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Fungal Cell Gigantism during Mammalian Infection
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
- Rotavirus Structural Proteins and dsRNA Are Required for the Human Primary Plasmacytoid Dendritic Cell IFNα Response
- The Epigenetic Landscape of Latent Kaposi Sarcoma-Associated Herpesvirus Genomes
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requirement of NOX2 and Reactive Oxygen Species for Efficient RIG-I-Mediated Antiviral Response through Regulation of MAVS Expression
- Formation of Complexes at Plasmodesmata for Potyvirus Intercellular Movement Is Mediated by the Viral Protein P3N-PIPO
- Insight into the Mechanisms of Adenovirus Capsid Disassembly from Studies of Defensin Neutralization
- Two Novel Point Mutations in Clinical Reduce Linezolid Susceptibility and Switch on the Stringent Response to Promote Persistent Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



