-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPatient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
In a retrospective cohort study of 2,126 patients with multiple sclerosis, Joel Raffel of Imperial College London and colleagues showed that the scores from a patient-reported outcomes questionnaire were associated with the patients' 10-year risk of mortality.
Published in the journal: . PLoS Med 14(7): e32767. doi:10.1371/journal.pmed.1002346
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002346Summary
In a retrospective cohort study of 2,126 patients with multiple sclerosis, Joel Raffel of Imperial College London and colleagues showed that the scores from a patient-reported outcomes questionnaire were associated with the patients' 10-year risk of mortality.
Introduction
Patient-reported outcomes (PROs) are defined as ‘any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else’ [1]. They can offer significant advantages over assessment by a physician: they better capture the impact of disease on the person; they are often easier and cheaper to administer; and they can often be completed from the home environment, potentially allowing for long-term, geographically diverse, and large-scale observational and interventional studies [2]. They can also enhance routine clinical care in areas such as symptom screening, monitoring treatment response, care coordination, care systems assessment, and improving communication in the doctor–patient clinical encounter [3–6].
PROs are increasingly being used to complement traditional outcome measures in disciplines such as oncology, cardiology, and neurology. The increasing use of PROs in interventional trials is partly driven by the need for pharmaceutical companies to justify labelling and promotional claims in post-licensing marketing [1,7]. However, research and clinical practice, particularly in oncology, have led the way in proving that PROs can offer more than this: it is now well established in oncology that PROs are associated with hard clinical endpoints such as survival time (time from study entry until death) and can add prognostic value to the more traditional physician-reported outcome measures [8–10]. PROs are also well established as prognostic for survival time in heart failure [11–13]. However, such associations are more difficult to study in neurological research, in part because it is rarer for the clinical endpoint of trials in neurological disease to be survival.
The Multiple Sclerosis Impact Scale–29 (MSIS-29) is a PRO that attempts to assess both physical and psychological quality of life in multiple sclerosis (MS) [14]. It has the advantage of being self-reported and can be distributed by post. It could potentially be utilised in progressive MS trials, as it appears sensitive to clinically relevant change over short time frames [15]. This is in contrast to the traditional physician-assessed Expanded Disability Status Scale (EDSS), which is the primary outcome favoured in most MS trials despite several well-documented limitations, including poor interrater and intrarater reliability and a limited sensitivity to change over the time frame of 2–3 years, especially in progressive MS [16–20]. Though small studies have correlated MSIS-29 score with EDSS score, it is unknown how the MSIS-29 is linked to robust clinical endpoints such as survival time [21,22]. Indeed, to our knowledge, no PROs have been associated with survival in MS or any other neurological disease.
This study aimed to investigate whether MSIS-29 scores can be prognostic for survival time (time from MSIS-29 completion to death) in MS, using a large observational cohort of people with MS from the MS Society Tissue Bank (MSSTB). The primary study hypothesis was that MSIS-29 scores are associated with survival time in MS.
Methods
Study population
Since 1998, the MSSTB has operated a nationwide community-based scheme for people with MS and non-MS controls in the UK to donate their brain and spinal cord after death, by providing written consent while alive (ethics approval in 1998: London Multicentre Research Ethics Committee—MREC/02/2/39; then in 2008: Wales Research Ethics Committee 3–08/MRE09/31; then in 2013: Wales Research Ethics Committee 3–08/MRE09/31+5) [23]. This cohort is a unique population in that the participants are followed from registration to death, with eventual pathological confirmation [23]. On 15 July 2004, MSIS-29 questionnaires were sent out to all registered donors. For those who completed a MSIS-29 questionnaire at this time, a second MSIS-29 questionnaire was sent out 1 year later, to measure change in MSIS-29. In addition, since 15 July 2004, all new registered donors have been sent a MSIS-29 questionnaire at the time of registration, as well as a ‘patient-reported EDSS’ (prEDSS) [24,25]. This study included people with MS registered in the MSSTB up until 1 January 2014 who had completed at least 1 MSIS-29 questionnaire. Data were stored in the MSSTB facility at Imperial College London and were analysed in 2015–2016.
Outcome measures
The MSIS-29 consists of 29 questions answered on a 5-point Likert scale, giving 2 scores: the MSIS-29 physical (MSIS-29-PHYS) score (questions 1–20; therefore score range 20–100) and the MSIS-29 psychological (MSIS-29-PSYCH) score (questions 21–29; therefore score range 9–45) [14,26]. Imputation was used to address questionnaires returned with missing data using the following rule: if greater than 66% of questions had been answered within MSIS-29-PHYS or within MSIS-29-PSYCH, missing answers were imputed using the mean of the answered questions from the MSIS-29-PHYS or MSIS-29-PSYCH of that individual participant [27]. Otherwise, all data from that MSIS-29-PHYS or MSIS-29-PSYCH were excluded. The EDSS is a physician-reported gold standard for categorising disability in MS. The minimum score of 0 represents no impairment, 7 represents restriction to wheelchair, and the maximum score of 10 represents death. The prEDSS uses the same scale and can be completed without physician input, with good correlation [24]. A description of how to request access to the MSIS-29 questionnaire and the prEDSS questionnaire is available in S1 Appendix. Ten-year data on mortality were collected up until 1 June 2014. Survival time was defined using date of first MSIS-29 questionnaire as the entry point, date of death as the endpoint, and date of study completion (1 June 2014) as the censorship date for those still alive.
Baseline MSIS-29 scores were categorised into 5 equally spaced subgroups as follows: MSIS-29-PHYS scores—20–35, 36–51, 52–68, 69–84, 85–100; MSIS-29-PSYCH scores—9–16, 17–23, 24–30, 31–37, 38–45. In addition, for those with a repeat MSIS-29 questionnaire 1 year after baseline, the following subgroups were used: Subgroup 1—initial MSIS-29-PHYS score 20–84, no worsening after 1 year; Subgroup 2—initial MSIS-29-PHYS score 20–84, worsening after 1 year (≥1 point); Subgroup 3—initial MSIS-29-PHYS score 85–100, no worsening after 1 year; and Subgroup 4—initial MSIS-29-PHYS score 85–100, worsening after 1 year (≥1 point).
Statistical analysis
We did not preregister or publish a detailed analysis plan for this study. The statistical analysis is described below. Details on the history of this study and changes to the analysis plan are provided in S2 Appendix, while an early project outline is provided in S3 Appendix.
Population demographics are presented as mean (standard deviation [SD]) and frequency (percentage) for continuous and categorical variables, respectively. Differences between continuous variables were tested with the unpaired Student's t-test, or one-way analysis of variance (ANOVA) when more than 2 groups, while differences between categorical data were tested with the chi-square test. To assess whether MSIS-29-PHYS score, MSIS-29-PSYCH score, prEDSS score, and change in MSIS-29-PHYS score are associated with mortality, survival times were modelled using Cox proportional hazard models where the hazard ratios (HRs) for the respective instruments were adjusted for age and sex. The HRs are presented with 95% confidence intervals (95% CIs) and p-values testing the null hypothesis of the HRs being equal to 1. Survival curves within the subgroups were estimated using Kaplan–Meier estimators. Correlations between the 3 PRO scales (MSIS-29-PHYS, MSIS-29-PSYCH, and prEDSS) were estimated using rank-based Spearman correlations, which are reported with p-values testing the null hypothesis of no correlation. To investigate whether MSIS-29-PHYS and MSIS-29-PSYCH scores are associated with survival independent of prEDSS score, survival times were modelled using a Cox regression with prEDSS score, MSIS-29-PHYS score, MSIS-29-PSYCH score, age, and sex as independent variables. The SAS 9.4 platform was used for all statistical analysis.
Results
Population demographics
In all, 2,914 people with MS were enrolled in the MSSTB over the study period. 2,126 participants completed the MSIS-29 questionnaire for inclusion in this study (participation rate 73.0%). Of these, 2,119 participants completed both the MSIS-29-PHYS and MSIS-29-PSYCH questionnaire, and 7 participants completed only the MSIS-29-PHYS questionnaire. Data were imputed for 273 participants. A prEDSS assessment was available at the same time as the MSIS-29 assessment in 630 participants, and a repeat MSIS-29 questionnaire was completed 1 year after baseline by 872 participants. Differences in baseline characteristics between those included and those not included in the study, those with and without imputed data, those with and without prEDSS data, and those with and without longitudinal MSIS-29 data are presented in Table 1.
Tab. 1. Differences in baseline characteristics between those included and those not included in the study, those with and without imputed data, those with and without prEDSS data, and those with and without longitudinal MSIS-29 data. 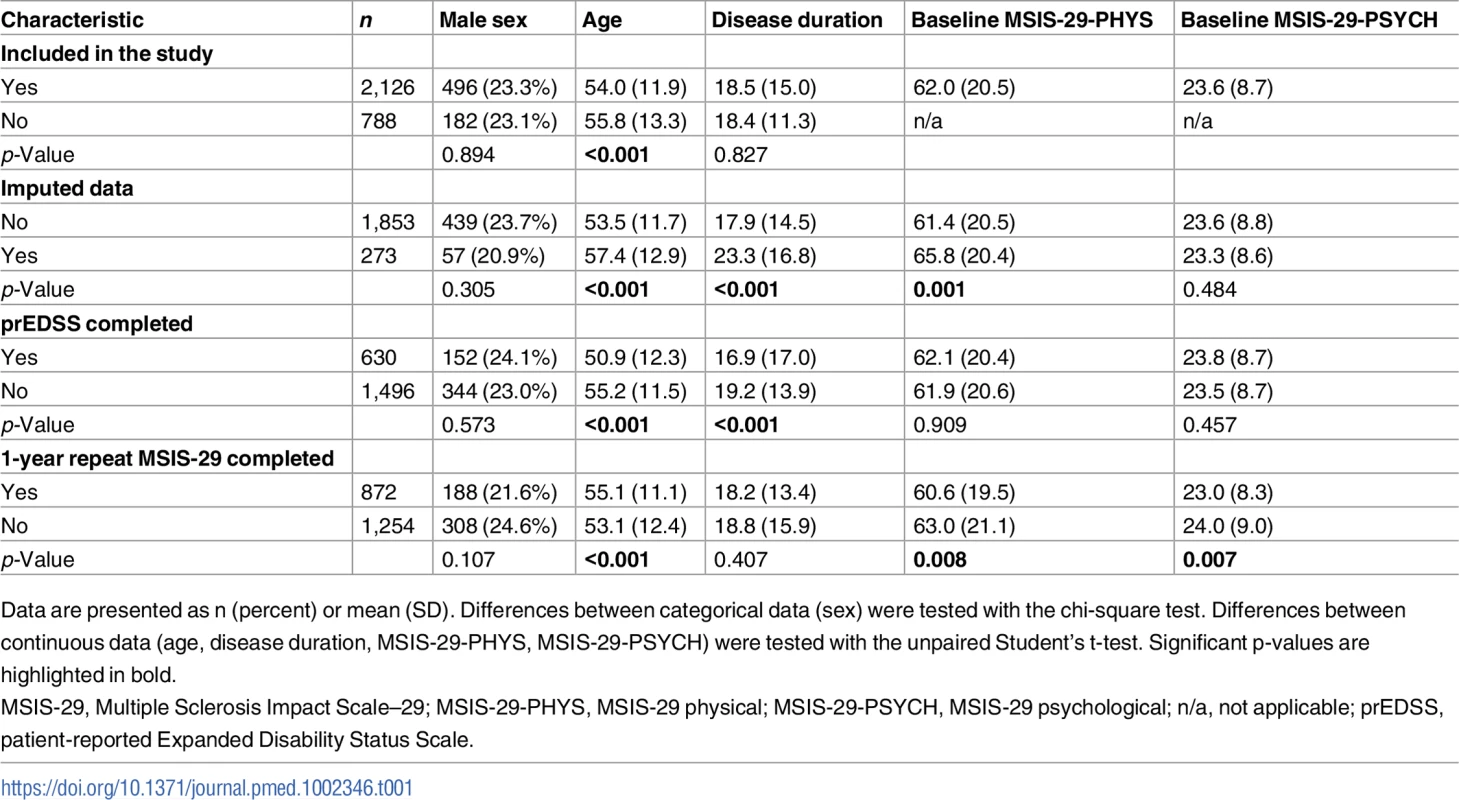
Data are presented as n (percent) or mean (SD). Differences between categorical data (sex) were tested with the chi-square test. Differences between continuous data (age, disease duration, MSIS-29-PHYS, MSIS-29-PSYCH) were tested with the unpaired Student's t-test. Significant p-values are highlighted in bold. Median follow-up time was 9 years, while 865 participants had the maximum 10 years of follow-up. Up until 1 June 2014, 264 (12.4%) of the total group had died. The mean population age at MSIS-29 assessment was 54 (SD 11.9) years, with a disease length of 18.5 (SD 15.0) years, and 1,630 (76.7%) were female. At baseline, mean MSIS-29-PHYS score was 62 (SD 20.5), mean MSIS-29-PSYCH score was 23.6 (SD 8.7), and mean prEDSS score was 5.7 (SD 2.2).
A higher MSIS-29-PHYS score is associated with reduced survival time
Cox regression models demonstrated that higher MSIS-29-PHYS score was associated with reduced survival time independently of age and sex (Wald chi-square, [degrees of freedom (df) = 4, n = 2,126] = 98.5, p < 0.001; Fig 1A). Older age at first MSIS-29 completion (Wald chi-square [df = 1, n = 2,126] = 88.6, p < 0.001) and male sex (Wald chi-square [df = 1, n = 2,126] = 24.8, p < 0.001) were also associated with reduced survival time in the model. HRs for death were greater, and reduced survival times were observed, with higher MSIS-29-PHYS score, using the ranges 20–35, 36–51, 52–68, 69–84, and 85–100 (Fig 1B). The HR for death was 5.7 in the subgroup with MSIS-29-PHYS score 85–100 compared with the subgroup with MSIS-29-PHYS score 20–35 (HR 5.7, 95% CI 3.1–10.5, p < 0.001). Those with higher MSIS-29-PHYS scores were more likely to be male (chi-square [df = 4, n = 2,126] = 25.2, p < 0.001) and had older age (F[df = 4, n = 2,121] = 16.1, p < 0.001) and longer disease duration (F[df = 4, n = 2,121] = 4.1, p < 0.01; Table 2).
Fig. 1. Higher MSIS-29-PHYS scores are associated with reduced survival time. 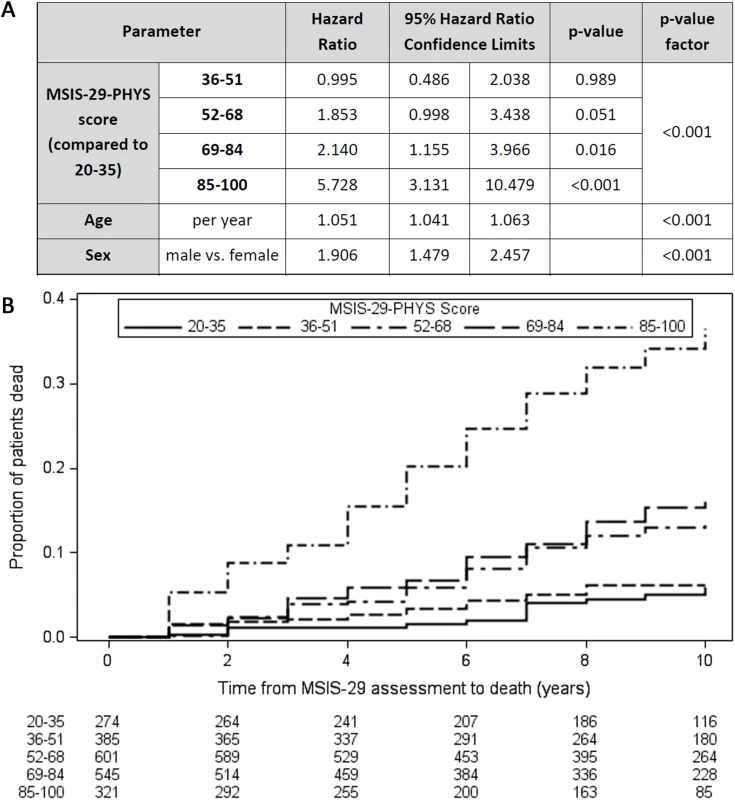
(A) Table: Higher MSIS-29-PHYS score was associated with reduced survival time (greater hazard ratio for death), as were older age at first MSIS-29 completion and male sex. (B) Kaplan–Meier failure curves (n = 2,126). Note that Kaplan–Meier curves do not account for the effect of age and sex on survival time. MSIS-29, Multiple Sclerosis Impact Scale–29; MSIS-29-PHYS, MSIS-29 physical. Tab. 2. Variation in characteristics between those with different baseline MSIS-29 scores. 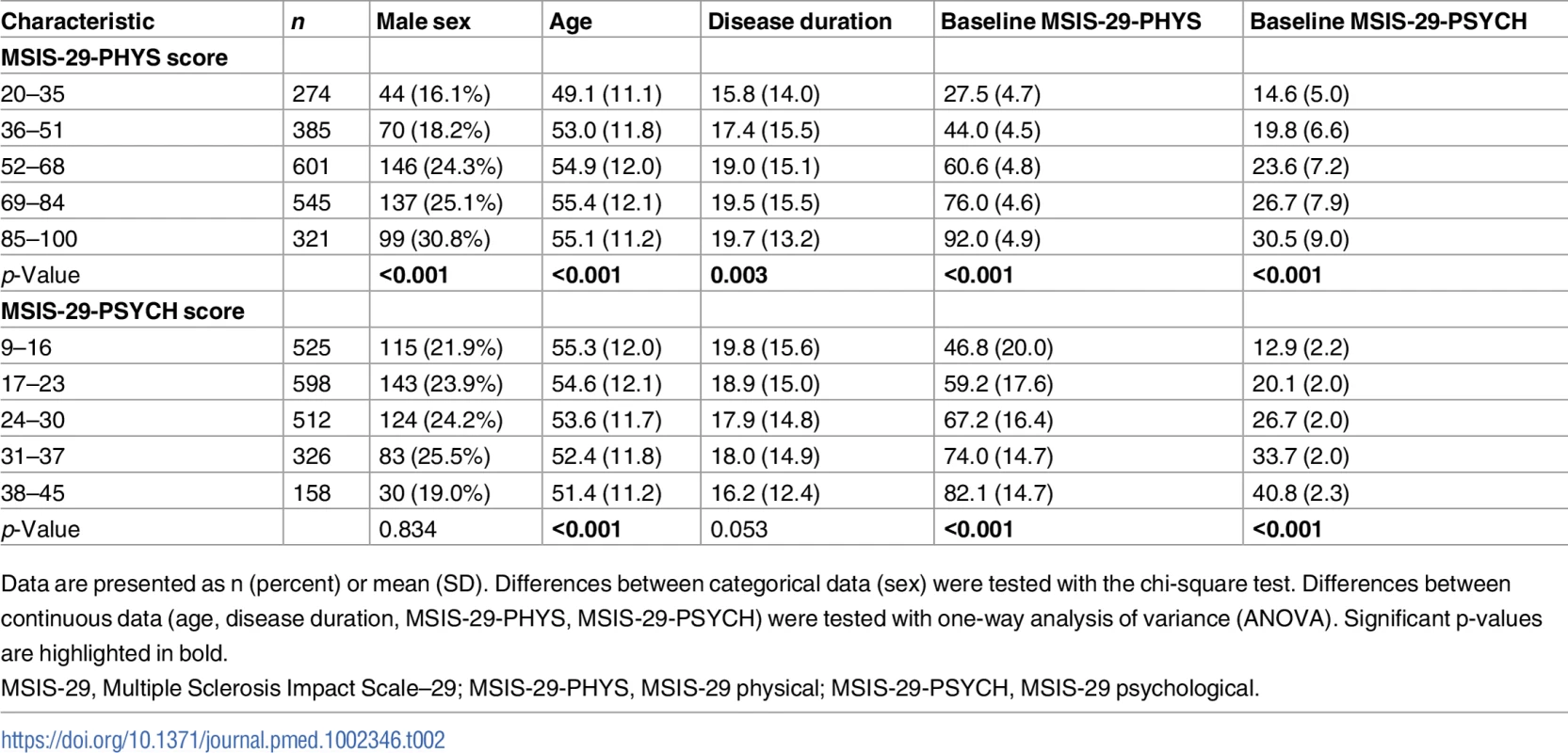
Data are presented as n (percent) or mean (SD). Differences between categorical data (sex) were tested with the chi-square test. Differences between continuous data (age, disease duration, MSIS-29-PHYS, MSIS-29-PSYCH) were tested with one-way analysis of variance (ANOVA). Significant p-values are highlighted in bold. A higher MSIS-29-PSYCH score is associated with reduced survival time
Similarly, Cox regression models demonstrated that higher MSIS-29-PSYCH score was associated with reduced survival time independently of age and sex (Wald chi-square [df = 4, n = 2,119] = 19.2, p < 0.001; Fig 2A), although the effect was less pronounced than with MSIS-29-PHYS. HRs for death were greater, and reduced survival times were observed, with higher MSIS-29-PSYCH score, using the ranges 9–16, 17–23, 24–30, 31–37, and 38–45 (Fig 2B). The HR for death was 2.8 in the subgroup with MSIS-29-PSYCH score 38–45 compared with the subgroup with MSIS-29-PHYS score 9–16 (HR 2.8, 95% CI 1.8–4.4, p < 0.001). In contrast to MSIS-29-PHYS score, higher MSIS-29-PSYCH score was not associated with male sex or longer disease duration and was associated with younger age (F[df = 4, n = 2,114] = 5.5, p < 0.001; Table 2).
Fig. 2. Higher MSIS-29-PSYCH scores are associated with reduced survival time. 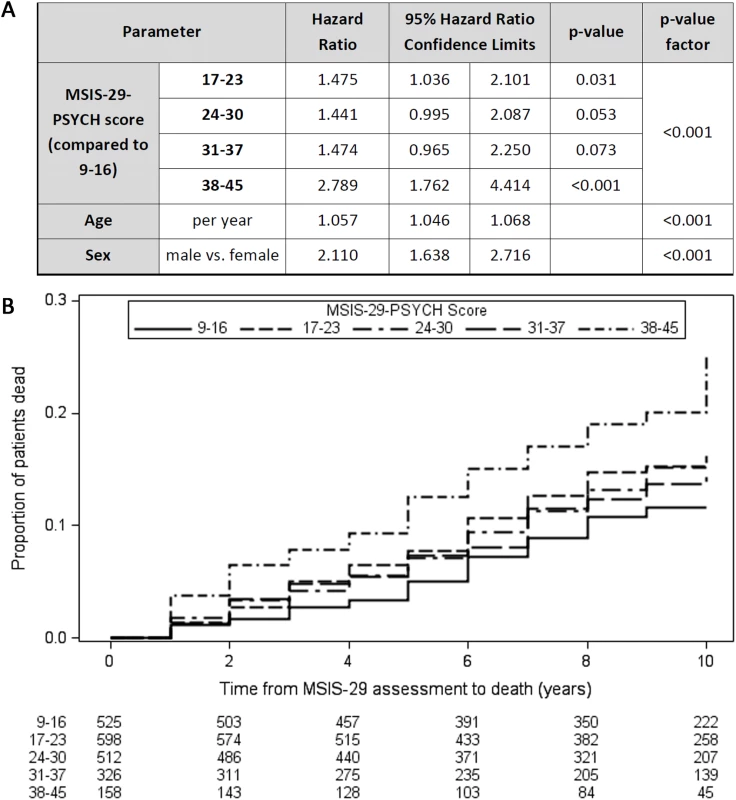
(A) Table: Higher MSIS-29-PSYCH score was associated with reduced survival time (greater hazard ratio for death), as were older age at first MSIS-29 completion and male sex. (B) Kaplan–Meier failure curves (n = 2,119). Note that Kaplan–Meier curves do not account for the effect of age and sex on survival time. MSIS-29, Multiple Sclerosis Impact Scale–29; MSIS-29-PSYCH, MSIS-29 psychological. MSIS-29-PHYS score is correlated with prEDSS score but is independently associated with survival time
There was a strong correlation between the MSIS-29-PHYS and MSIS-29-PSYCH scores (r[df = 2,117] = 0.54, p < 0.001). There was a strong correlation between the MSIS-29-PHYS score and the prEDSS score (r[df = 628] = 0.52, p < 0.001) and a weak correlation between the MSIS-29-PSYCH score and the prEDSS score (r[df = 623] = 0.19, p < 0.001). To determine whether the MSIS-29-PHYS and MSIS-29-PSYCH scores were associated with survival time independently of prEDSS score, all measures were included in a Cox regression model, along with age and sex, in the limited number of participants who completed all 3 measures (n = 625; Table 3). Reduced survival time was associated with older age at baseline (per year: HR 1.07, 95% CI 1.04–1.10, p < 0.001), a higher prEDSS score (per 1 SD [2.2]: HR 2.0, 95% CI 1.0–3.7, p < 0.05), and a higher MSIS-29-PHYS score (per 1 SD [20.3]: HR 1.8, 95% CI 1.1–2.9, p < 0.05).
Tab. 3. Reduced survival time (greater hazard ratio for death) was associated with older age, higher prEDSS score, and higher MSIS-29-PHYS score in the limited cohort with prEDSS score available (n = 625). 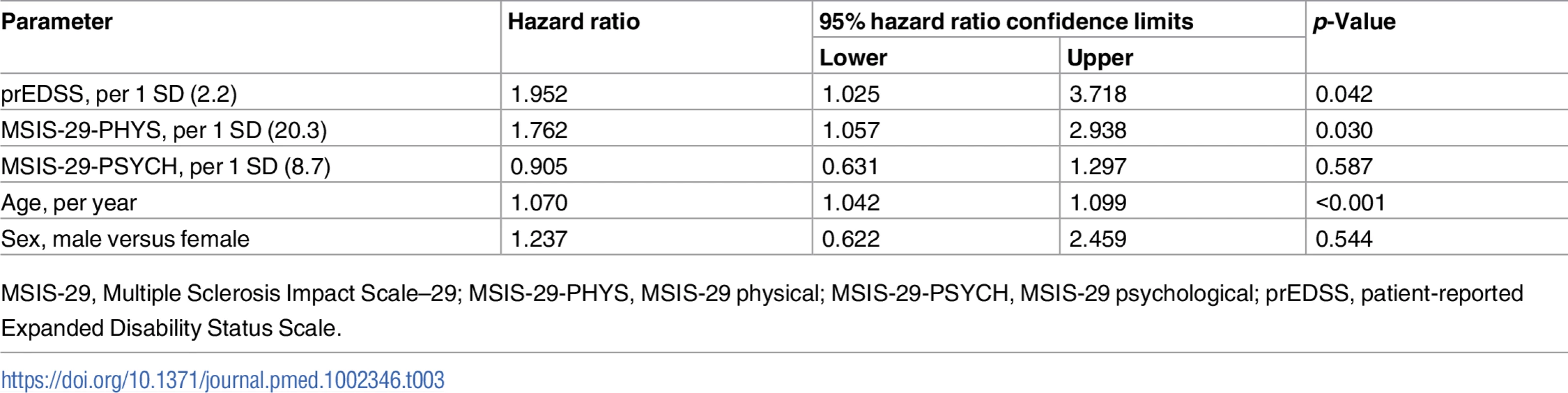
MSIS-29, Multiple Sclerosis Impact Scale–29; MSIS-29-PHYS, MSIS-29 physical; MSIS-29-PSYCH, MSIS-29 psychological; prEDSS, patient-reported Expanded Disability Status Scale. Worsening in the MSIS-29-PHYS score over 1 year is associated with reduced survival time
The MSIS-29 questionnaire was repeated after 1 year in a subgroup (n = 872) of those who had originally completed the MSIS-29 in 2004. Comparing MSIS-29-PHYS stable/improving score participants with MSIS-29-PHYS worsening score participants (change in MSIS-29-PHYS ≤ 0 versus > 0), there was no statistically significant difference in mortality (Cox regression adjusted for age and sex: n = 872, HR = 1.2, 95% CI 0.9–1.2, p = 0.28). However, in the subgroup of participants whose initial MSIS-29-PHYS score was 85–100, a longitudinal worsening of MSIS-29-PHYS score was associated with reduced survival time (HR = 2.3, 95% CI 1.2–4.4, p = 0.016; Fig 3). This was not apparent in the subgroup with initial MSIS-29-PHYS score 20–84 (HR = 1.3, 95% CI 0.8–2.0, p = 0.24; Fig 3). Fig 3B shows the survival curves for the 4 subgroups.
Fig. 3. Longitudinally worsening MSIS-29-PHYS scores are associated with reduced survival time. 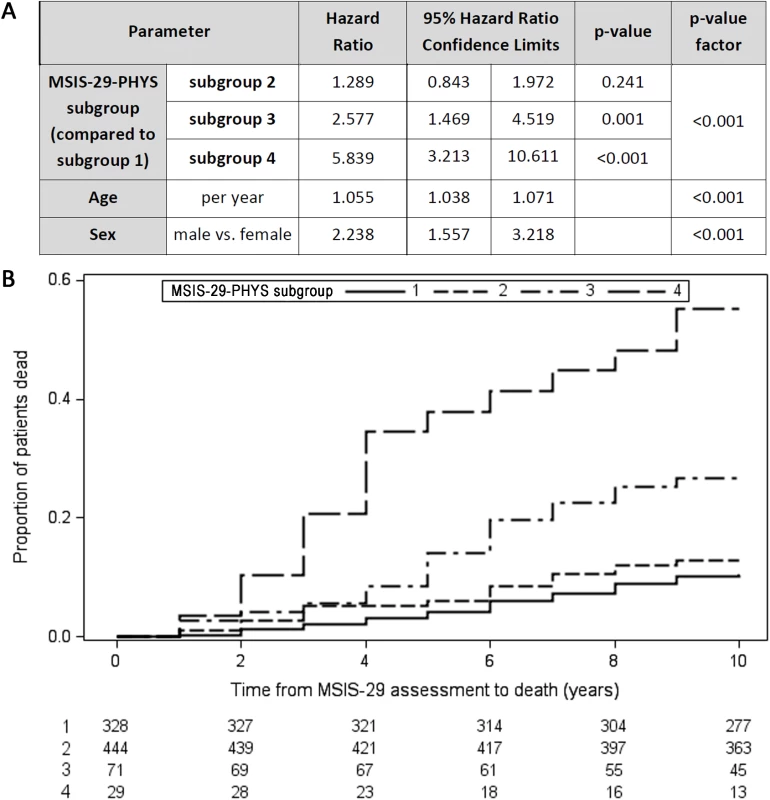
Four subgroups are presented in this figure: Subgroup 1—initial MSIS-29-PHYS score 20–84, no worsening after 1 year (solid line); Subgroup 2—initial MSIS-29-PHYS score 20–84, worsening after 1 year (short-dashed line); Subgroup 3—initial MSIS-29-PHYS score 85–100, no worsening after 1 year (dot-dashed line); Subgroup 4—initial MSIS-29-PHYS score 85–100, worsening after 1 year (long-dashed line). (A) Table: Subgroups 1 and 2 had no statistically significant difference in survival time (HR = 1.289, 95% CI 0.843–1.972, p = 0.241). Subgroup 4 had reduced survival time compared with subgroup 3 (HR = 2.266, 95% CI 1.163–4.413, p = 0.016). p < 0.001 for differences between the 4 subgroups. (B) Kaplan–Meier failure curves (n = 872). Note that Kaplan–Meier curves do not account for the effect of age and sex on survival time. HR, hazard ratio; MSIS-29, Multiple Sclerosis Impact Scale–29; MSIS-29-PHYS, MSIS-29 physical. Discussion
This study reports that higher MSIS-29 score is associated with reduced survival time in a large observational cohort of people with MS. MSIS-29-PHYS and MSIS-29-PSYCH scores were both associated with survival time, although MSIS-29-PHYS score has stronger prognostic value, and associates with survival time independently of age, sex, and prEDSS score in a Cox regression model. In addition, in the subgroup with initial MSIS-29-PHYS score 85–100, a 1-year longitudinal worsening of MSIS-29-PHYS score is associated with an even worse prognosis. This finding shows that how a person with MS answers these questions is important and relates to how well they may do in terms of health in the future. To our knowledge, this is the first study to associate PROs with survival outcomes in any neurological disease.
This study benefitted from the MSSTB cohort in several ways. We believe this is the largest cohort with reported MSIS-29 results to date, other than 1 web-based cohort [28,29], and the MSSTB has by far the longest follow-up after MSIS-29 completion (up to 10 years, median 9 years). This cohort had 264 deaths within the study period. This number of deaths allowed the prognostic value of MSIS-29 scores for survival time to be studied.
One should consider the external validity of this cohort’s results to the general MS population. The MSSTB recruitment strategy is based entirely in the community, relying upon community-based presentations and a quarterly magazine, MS Matters, distributed to approximately 30,000 members of the Multiple Sclerosis Society of Great Britain and Northern Ireland [30]. Moreover, the MSSTB population has previously been shown to be representative of the UK MS population in terms of disease characteristics and clinical milestones over the course of the disease [23]. The spread of MSIS-29 scores in this study was comparable with that reported in other large cohort studies [28,29]. In addition, factors that are known to associate with reduced survival, such as male sex, older age at baseline, and higher prEDSS score, were also found to associate with reduced survival time in this cohort, along with MSIS-29 score [31–33]. However, at the time of enrolment into the MSSTB, participants are often late in their disease course, as evidenced by this study’s mean disease duration of 18.5 years and mean prEDSS score of 5.7 at baseline MSIS-29 questionnaire. Therefore, this study likely underrepresents those with earlier disease and less disability, and it is uncertain how MSIS-29 scores and their prognostic value for mortality will vary in this group. One might hypothesize that those with earlier disease would have lower MSIS-29 scores and increased survival times, on average [29].
There are several other limitations to this study. Only a limited cohort completed the prEDSS questionnaire (n = 630), and only a limited cohort completed a longitudinal MSIS-29 (n = 872), mostly because of changes to the study protocol over time. Also, this study used a prEDSS rather than the traditional physician-reported EDSS, although these have previously been shown to correlate well [24]. Data on disease subtype, relapses, and disease-modifying therapy were not available for this study, and could influence the relationship between MSIS-29 score and survival time. Like most clinical outcome measures, PROs are susceptible to random measurement error, and hence regression dilution bias likely causes a decrease in the prognostic value of MSIS-29 for mortality [34]. However, previous studies have reported the test–retest reliability of MSIS-29-PHYS and MSIS-29-PSYCH to be high (intraclass correlation coefficients of 0.94 and 0.87, respectively), and so this effect is likely minimal [14].
In MS research, PROs such as MSIS-29 offer several advantages over traditional physician-assessed outcome measures such as the EDSS [2]. Interrater EDSS variability is high [17,35]. The EDSS also has a limited sensitivity to change over the 2 - to 3-year time scale of clinical trials [36,37]. This is especially true in progressive MS, where the EDSS often does not capture changes in arm function or subtle changes in mobility [38]. MSIS-29 can be more responsive to clinically relevant change over short time frames [15,39]. PROs including MSIS-29 can also be sent to large cohorts of people with MS, and completed by post or online [29]. PROs could therefore enable large cohort studies, which would otherwise be financially unfeasible, such as comparative clinical effectiveness research. PROs validated against hard clinical endpoints could also be incorporated into patient registries to help address key questions in personalised medicine relating to prognosis, predicting response to treatment, and assessing response to treatment. These are increasingly important issues in MS and other neurological diseases like epilepsy, stroke, Parkinson disease, and spasticity, where a range of therapies are now available but questions remain regarding how to treat the individual.
Outside of the research setting, PROs can benefit individual patients directly if they are utilized in routine clinical practice. Oncology has again led the way, where PROs are routinely used to enhance patient care [5]. PROs can help screen for changes in physical or psychological symptoms, and identify unmet health, care, and support needs. They can be used as a decision aid when devising or evaluating treatment plans [40]. Patients and doctors can have differing views on which outcomes matter most, and the effective use of PROs can help refocus care goals to the views of the individual patient [41,42]. This might also empower patients towards improved self-management of their condition [43]. When assessed in a randomised controlled trial, PROs enhanced doctor–patient communication and improved patient health-related quality of life and emotional well-being [4].
On a healthcare provision level, PROs can also be used to audit quality of care within a service or to compare quality of care between services [44]. PROs are often now incorporated into automated electronic systems for data collection, with high user compliance [45,46]. As well as providing direct benefit to patients, these systems can also feed into patient registries to help address research questions.
Further research questions emerge from this study. With this study having shown that MSIS-29 score is associated with death, similar methods could be used to investigate whether MSIS-29 score is associated with disability outcomes, such as time until wheelchair use. The effect of disease-modifying treatment on MSIS-29 and long-term clinical endpoints needs further attention, to assess whether MSIS-29 scores could be used as a surrogate for response to treatment. Multiple variables, including other PROs collected at multiple time points, could be incorporated into more complex models to better predict outcomes in large cohorts.
PROs will continue to be used in interventional studies, in part to satisfy labelling and promotional claims in post-licensing marketing [1,7]. This study argues that the MSIS-29 questionnaire can offer more than this, since its association with hard clinical endpoints supports its use as a meaningful clinical outcome to inform care decision-making. In oncology, PROs are now established as influential and clinically relevant measures, and it is accepted that the classic clinical endpoints do not fully capture the benefits, risks, and costs of treatment [8–10]. MS and neurology research will continue to rely upon clinical trials, as well as ‘big data’ gathered from clinical registries. The careful incorporation of PROs can enrich such datasets and allow the investigation of research questions beyond what traditional physician-based assessments can offer.
Supporting Information
Zdroje
1. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring (Maryland): Food and Drug Administration; 2009. Available from: https://www.fda.gov/downloads/Drugs/…/Guidances/UCM193282.pdf. Accessed 2017 Jun 14.
2. Fayers P, Machin D. Quality of life: the assessment, analysis and interpretation of patient-reported outcomes. Hoboken (New Jersey): John Wiley & Sons; 2013.
3. Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK. Use of health-related quality-of-life assessments in daily clinical oncology nursing practice—a community hospital-based intervention study. Cancer. 2008;113(3):628–37. doi: 10.1002/cncr.23623 18543317
4. Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22(4):714–24. doi: 10.1200/JCO.2004.06.078 14966096
5. Howell D, Molloy S, Wilkinson K, Green E, Orchard K, Wang K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26(9):1846–58. doi: 10.1093/annonc/mdv181 25888610
6. Snyder CF, Aaronson NK, Choucair AK, Elliott TE, Greenhalgh J, Halyard MY, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res. 2012;21(8):1305–14. doi: 10.1007/s11136-011-0054-x 22048932
7. McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–9. doi: 10.1586/erp.11.12 21476818
8. Secord AA, Coleman RL, Havrilesky LJ, Abernethy AP, Samsa GP, Cella D. Patient-reported outcomes as end points and outcome indicators in solid tumours. Nat Rev Clin Oncol. 2015;12(6):358–70. doi: 10.1038/nrclinonc.2015.29 25754949
9. Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin. 2007;57(5):278–300. doi: 10.3322/CA.57.5.278 17855485
10. Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–63. doi: 10.1200/JCO.2007.13.3439 18227528
11. Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115(15):1975–81. doi: 10.1161/CIRCULATIONAHA.106.670901 17420346
12. Tate CW, Robertson AD, Zolty R, Shakar SF, Lindenfeld J, Wolfel EE, et al. Quality of life and prognosis in heart failure: results of the Beta-Blocker Evaluation of Survival Trial (BEST). J Card Fail. 2007;13(9):732–7. doi: 10.1016/j.cardfail.2007.07.001 17996821
13. Heidenreich PA, Spertus JA, Jones PG, Weintraub WS, Rumsfeld JS, Rathore SS, et al. Health status identifies heart failure outpatients at risk for hospitalization or death. J Am Coll Cardiol. 2006;47(4):752–6. doi: 10.1016/j.jacc.2005.11.021 16487840
14. Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29)—a new patient-based outcome measure. Brain. 2001;124 : 962–73. 11335698
15. Bosma LVAE, Sonder JM, Kragt JJ, Polman CH, Uitdehaag BMJ. Detecting clinically-relevant changes in progressive multiple sclerosis. Mult Scler. 2015;21(2):171–9. doi: 10.1177/1352458514540969 25013153
16. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52. 6685237
17. Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the Expanded Disability Status Scale (EDSS) and Functional Systems (FS) in a multiple sclerosis clinical trial. Neurology. 1990;40(6):971–5. 2189084
18. Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain. 2000;123 : 1027–40. 10775547
19. Cohen JA, Reingold SC, Polman CH, Wolinsky JS, International Advisory Committee on Clinical Trials in Multiple Sclerosis. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol. 2012;11(5):467–76. doi: 10.1016/S1474-4422(12)70059-5 22516081
20. European Medicines Agency. Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis. London: European Medicines Agency; 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdf. Accessed 2017 Jun 14.
21. Hoogervorst ELJ, Zwemmer JNP, Jelles B, Polman CH, Uitdehaag BMJ. Multiple Sclerosis Impact Scale (MSIS-29): relation to established measures of impairment and disability. Mult Scler. 2004;10(5):569–74. doi: 10.1191/1352458504ms1078oa 15471375
22. McGuigan C, Hutchinson M. The multiple sclerosis impact scale (MSIS-29) is a reliable and sensitive measure. J Neurol Neurosurg Psychiatry. 2004;75(2):266–9. 14742602
23. Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O. The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol. 2011;122(2):155–70. doi: 10.1007/s00401-011-0840-0 21626034
24. Collins CD, Ivry B, Bowen JD, Cheng EM, Dobson R, Goodin DS, et al. A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult Scler. 2016;22(10):1349–58. doi: 10.1177/1352458515616205 26564998
25. Lechner-Scott J, Kappos L, Hofman M, Polman CH, Ronner H, Montalban X, et al. Can the expanded disability status scale be assessed by telephone? Mult Scler. 2003;9(2):154–9. doi: 10.1191/1352458503ms884oa 12708811
26. Ramp M, Khan F, Misajon RA, Pallant JF. Rasch analysis of the Multiple Sclerosis Impact Scale (MSIS-29). Health Qual Life Outcomes. 2009;7 : 58. doi: 10.1186/1477-7525-7-58 19545445
27. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;339:b2393. doi: 10.1136/bmj.b2393
28. Jones KH, Jones PA, Middleton RM, Ford DV, Lockhart-Jones H, Tuite-Dalton K, et al. Physical disability, anxiety and depression in people with MS: an internet-based survey via the UK MS Register. PLoS ONE. 2014;9(8):e104604. doi: 10.1371/journal.pone.0104604
29. Jones KH, Ford DV, Jones PA, John A, Middleton RM, Lockhart-Jones H, et al. The physical and psychological impact of multiple sclerosis using the MSIS-29 via the web portal of the UK MS Register. PLoS ONE. 2013;8(1):e55422. doi: 10.1371/journal.pone.0055422 23383186
30. MS Society. MS Matters—your MS magazine. Available from: https://www.mssociety.org.uk/get-involved/membership/ms-matters-magazine. Accessed 2017 Apr 30.
31. Weinshenker BG. Natural history of multiple sclerosis. Ann Neurol. 1994;36(Suppl):S6–11. doi: 10.1002/ana.410360704
32. Kingwell E, van der Kop M, Zhao Y, Shirani A, Zhu F, Oger J, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012;83(1):61–6. doi: 10.1136/jnnp-2011-300616 21865212
33. Levic ZM, Dujmovic I, Pekmezovic T, Jarebinski M, Marinkovic J, Stojsavljevic N, et al. Prognostic factors for survival in multiple sclerosis. Mult Scler. 1999;5(3):171–8. doi: 10.1177/135245859900500306 10408717
34. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289 20573762
35. Verdiertaillefer MH, Zuber M, Lyoncaen O, Clanet M, Gout O, Louis C, et al. Observer disagreement in rating neurologic impairment in multiple-sclerosis—facts and consequences. Eur Neurol. 1991;31(2):117–9. doi: 10.1159/000116658 2044616
36. van Winsen LML, Kragt JJ, Hoogervorst ELJ, Polman CH, Uitdehaag BMJ. Outcome measurement in multiple sclerosis: detection of clinically relevant improvement. Mult Scler. 2010;16(5):604–10. doi: 10.1177/1352458509359922 20086019
37. Kragt JJ, Thompson AJ, Montalban X, Tintore M, Rio J, Polman CH, et al. Responsiveness and predictive value of EDSS and MSFC in primary progressive MS. Neurology. 2008;70(13):1084–91.
38. Ontaneda D, Fox RJ, Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 2015;14(2):208–23. doi: 10.1016/S1474-4422(14)70264-9 25772899
39. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry. 2005;76(11):1539–43. doi: 10.1136/jnnp.2005.064584 16227547
40. Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res. 2009;18(1):115–23. doi: 10.1007/s11136-008-9430-6 19105048
41. Marshall S, Haywood K, Fitzpatrick R. Impact of patient-reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12(5):559–68. doi: 10.1111/j.1365-2753.2006.00650.x 16987118
42. Rothwell PM, McDowell Z, Wong CK, Dorman PJ. Doctors and patients don’t agree: Cross sectional study of patient’s and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ. 1997;314(7094):1580–3. 9169401
43. Stimson GV. Obeying doctor’s orders: a view from the other side. Soc Sci Med. 1974;8(2):97–104. 4821252
44. Devlin NJ, Appleby J. Getting the most out of PROMS: putting health outcomes at the heart of NHS decision-making. London: The King’s Fund; 2010. Available from: https://www.kingsfund.org.uk/publications/getting-most-out-proms. Accessed 2017 Apr 6.
45. Wright EP, Selby PJ, Crawford M, Gillibrand A, Johnston C, Perren TJ, et al. Feasibility and compliance of automated measurement of quality of life in oncology practice. J Clin Oncol. 2003;21(2):374–82. doi: 10.1200/JCO.2003.11.044 12525532
46. Jensen RE, Snyder CF, Abernathy AP, Basch E, Potosky AL, Roberts AC, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract. 2014;10(4):e215–22. doi: 10.1200/JOP.2013.001067 24301843
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Time for trauma immunology
- Translational approaches to coagulopathy after trauma: Towards targeted treatment
- The new survivors and a new era for trauma research
- Research questions in pre-hospital trauma care
- Major scientific lessons learned in the trauma field over the last two decades
- The science of rapid start—From the when to the how of antiretroviral initiation
- Reducing undiagnosed HIV infection among adolescents in sub-Saharan Africa: Provider-initiated and opt-out testing are not enough
- Community and health system intervention to reduce disrespect and abuse during childbirth in Tanga Region, Tanzania: A comparative before-and-after study
- Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study
- Trauma care: Finding a better way
- Risk of surgical site infection, acute kidney injury, and infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
- Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries
- Antimicrobial resistance in : Global surveillance and a call for international collaborative action
- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Cellular therapies in trauma and critical care medicine: Looking towards the future
- Leveraging peer-based support to facilitate HIV care in Kenya
- Trends in traumatic brain injury mortality in China, 2006–2013: A population-based longitudinal study
- Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study
- Community health promotion and medical provision for neonatal health—CHAMPION cluster randomised trial in Nagarkurnool district, Telangana (formerly Andhra Pradesh), India
- A comparison of Selective Aortic Arch Perfusion and Resuscitative Endovascular Balloon Occlusion of the Aorta for the management of hemorrhage-induced traumatic cardiac arrest: A translational model in large swine
- Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries
- Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study
- Cancer trials in sub-Saharan Africa: Aligning research and care
- Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study
- Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study
- Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial
- Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: A cross-sectional survey
- Timing of femoral shaft fracture fixation following major trauma: A retrospective cohort study of United States trauma centers
- IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model
- Long-term health status and trajectories of seriously injured patients: A population-based longitudinal study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



