-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study
In a case-crossover study, Mélanie Née and colleagues examines the association between use of presciption medicine by pedestrians and risk of injurious road traffic crashes.
Published in the journal: . PLoS Med 14(7): e32767. doi:10.1371/journal.pmed.1002347
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002347Summary
In a case-crossover study, Mélanie Née and colleagues examines the association between use of presciption medicine by pedestrians and risk of injurious road traffic crashes.
Introduction
Walking is rightly being promoted for its benefits for physical and mental health and for the environment. This comes, however, with a caveat: pedestrians are among the most vulnerable road users [1]. The World Health Organization’s 2013 global status report on road traffic safety revealed that pedestrians account for 22% of the world’s road traffic deaths [2].
A pedestrian crash is defined as any incident occurring on a public thoroughfare that involves at least 1 person on foot and 1 or more vehicles, at least 1 of which is moving. Cognitive, perceptive, and motor skills are involved in a pedestrian’s ability to move safely in a traffic setting. A decrease in motor skills may prevent pedestrians from crossing the road in time. For instance, a study in England compared the walking speed in the population with the speed required to utilize pedestrian crossings among 3,145 adults aged 65 and older and concluded that most of them were unable to cross the road in time [3]. Identified risk factors for the occurrence and severity of injury include environmental factors (e.g., lighting conditions, weather), roadway characteristics (e.g., speed limit, lack of pedestrian facilities), and pedestrian characteristics and behaviors (age, sex, maneuvers, alcohol intoxication) [4–8]. Because medicines have the potential to impair the skills needed to perform road users’ tasks safely, the association between the use of medicines and the risk of road traffic crash has been studied among vehicle drivers. The association between drivers’ use of benzodiazepines and the risk of road crash is now consistently documented [9–14]. Other medicines have also been studied: antidepressants [10,13–17], opioids used in pain treatment and in substitution treatment for opioid dependence [11,13,18,19], and cardiovascular drugs [20,21]. A positive association was found in these studies, except for 1 study [20] that found no association between calcium channel blockers and the risk of crash, and 2 studies [11,19] in which there was an increased risk with opioid use, but the increase was not statistically significant.
Very few studies have investigated the association between the use of medicinal drugs and the risk of road traffic crashes involving pedestrians, and available data remain descriptive, with no comparison group. One study analyzed postmortem blood and urine specimens from victims of road traffic fatalities between 2000 and 2006 in England and Wales. Benzodiazepines and antidepressants were detected in 30% and 20% of pedestrians’ blood and urine specimens, respectively [22]. In 2003, the Australian Transport Safety Bureau reported that 5.4% of male pedestrians aged 15–54 years killed in a road safety crash between 1997 and 1999 had taken benzodiazepine tranquilizers, and 1.3% had taken an antidepressant [23]. The non-comparative design of these studies excludes all conclusions about the actual role of medication use.
The aim of this study was to investigate the association between the use of medicines and the risk of road traffic crash in pedestrians.
Methods
We extracted and matched data from 3 French nationwide databases: the national healthcare insurance database, police reports, and the police national database of injurious crashes. Drivers were included by means of their national healthcare ID number, extracted from police reports by an automatic procedure. This national ID number was used to link pedestrians to medicine reimbursement data around the crash date. Police reports were matched to records in the injurious crashes database by a probabilistic linkage method [24].
Ethics statement
Confidentiality was ensured by using the personal information anonymization function of the healthcare insurance system [25]. The study was approved by the French Data Protection Authority.
Data sources
Police reports
French police officers are required to fill out a police report for each injurious crash occurring in the country (about 70,000 reports each year). An injurious crash is defined as a crash occurring in a road open to public circulation involving at least 1 vehicle and resulting in at least 1 victim needing medical attention or being killed. All reports are scanned and stored as image files. A manual validation study of 141 police reports involving pedestrians showed that national ID number was recorded for 39% of the pedestrians involved in these injurious road traffic crashes. These ID numbers were extracted from image files for later matching against dispensing records in the healthcare insurance database. All police reports available from 1 July 2005 to 31 December 2011 were compiled.
Police national database of injurious crashes
Information collected by the police for each injurious road traffic crash is stored in the police national database of injurious crashes, including information on the crash, vehicles, and persons involved. The variables used in this study were sex and age of the pedestrian, presumed responsibility for the crash as assessed by police officers, injury severity, weather, season, hour, day of week, lighting conditions, pedestrian action, and pedestrian location.
National healthcare insurance database
The healthcare insurance database covers the entire population of France. A record is added each time a reimbursed prescription medicine is dispensed to an outpatient at a pharmacy; the record includes national ID number, date of dispensing, and the 7-digit code that identifies medicines.
Participant inclusion
A pedestrian was excluded if the police report did not contain his or her national ID number or if the extraction procedure failed or a link could not be established with the corresponding record in the police national database of injurious crashes. If a pedestrian was involved in several crashes during the study period, only the first crash was considered, to ensure that the dispensing of a drug was not a consequence of a previous crash.
Participant and crash characteristics
Pedestrian characteristics (including age, sex, responsibility attributed by police officers, injury severity, and the pedestrian’s action and location) and crash characteristics (including weather, season, time and day of the crash, and lighting) were described. These characteristics were also used to investigate the factors associated with the probability of being part of the study.
Medicine and exposure periods
In France, a classification of medicines that could affect the ability to drive, use machines, and implement tasks requiring attention and precision has been developed by the French National Agency for Medicines and Health Products Safety (ANSM) and gradually applied since 2005 [26]. This classification, with its 4-level ranking from 0 (no or negligible risk) to 3 (major risk), has been validated in a study on drivers using the same databases as used in this study [24]. For the present study, only prescription medicines ranking from level 1 to 3 were included in the analysis. These medicines account for 37% of the medicines sold in France [27].
The exposure periods were determined for each pharmacotherapeutic class according to the Anatomical Therapeutic Chemical (ATC) classification (fourth level). Medication exposure was defined as starting 1 day after dispensing, and exposure duration was estimated from median values reported in a survey on medicine prescription in France [28]. To ensure that the medicines were not prescribed as a consequence of injuries sustained in the crash, medicines dispensed on the day of the crash were not considered in the analysis.
Case-crossover analysis
The case-crossover design allows study of the effect of transient exposure on the risk of acute events. The exposure frequency during a period just before the crash (case period) is compared with the exposure frequency during an earlier period (control period) in the same individual [29]. In the case-crossover analysis, only records with discordant exposures in case and control windows (either exposed on the case day and unexposed on the control day or unexposed on the case day and exposed on the control day) contribute to the analysis. The period between the case and control periods is called the washout. It corresponds to the period needed to ensure that exposure in the control period is not mixed with exposure in the case period.
The optimal washout period for a given medicine depends on its indication and prescription patterns. Since all prescribed drugs with non-null risk level were considered here, we could not make any assumption concerning the choice of the washout period. This period was thus varied from 30 to 119 days before from the crash day, resulting in the implementation of 90 case-crossover designs with distinct washout periods (Fig 1). Data on reimbursed medicines dispensed within 6 months before the crash were available. To ensure that the pedestrian was not exposed to a medicine at the beginning of the observation period without us knowing (dispensing date before the beginning of the observation period), we chose a 4-month study period. This approach of using multiple washout periods provided an indication of the robustness of the observed associations.
Fig. 1. The case-crossover design of the study, with multiple drug exposures and a varying washout period. 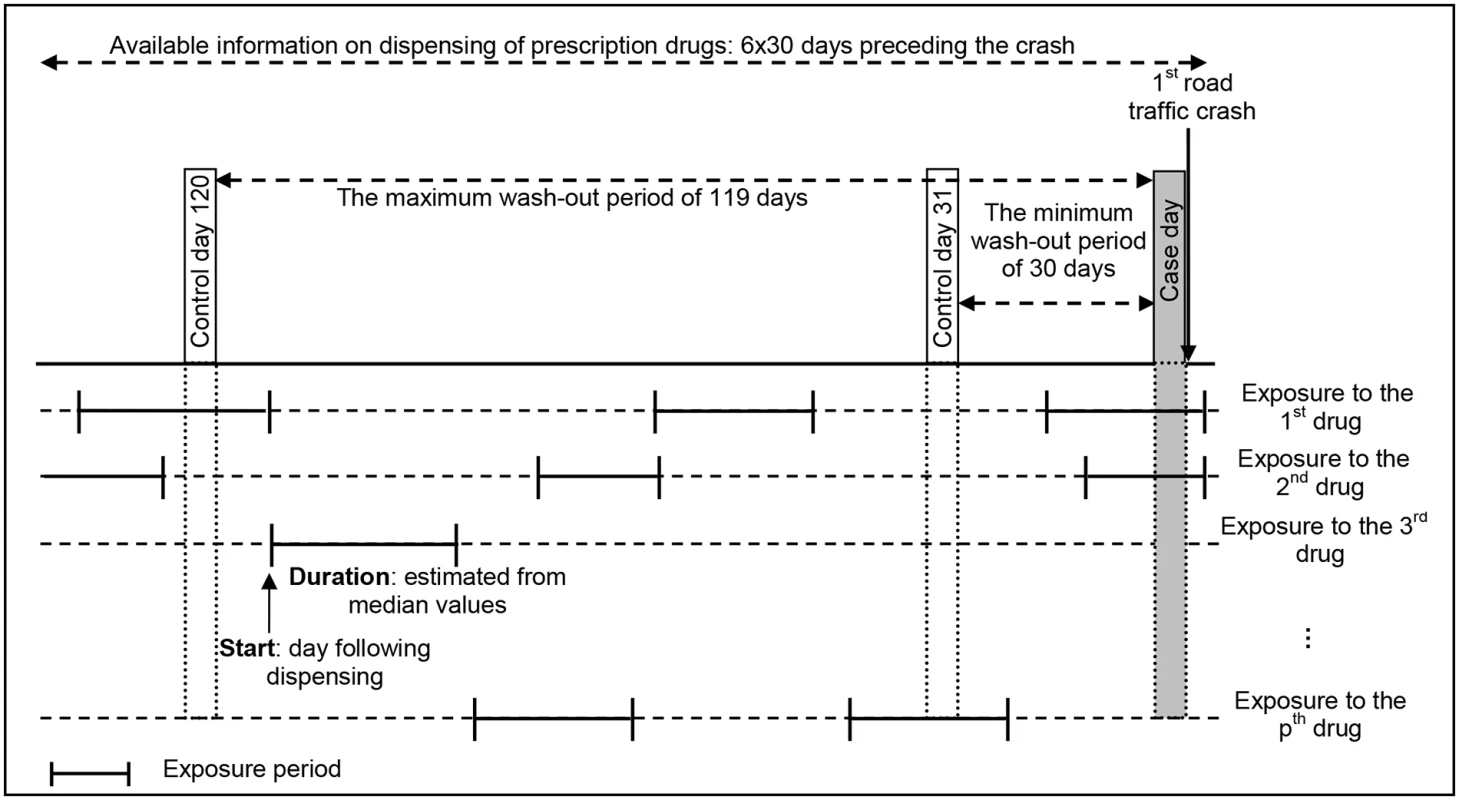
In the case-crossover design, only individuals with unequal exposures for the control period and the case period contribute to the analysis. For instance, with a control day defined at 120 days before the crash day, the individual shown in this figure has unequal exposures for the second drug (exposed during case day and unexposed during control day), but concordant exposures for the first drug (exposed both days) and the third drug (unexposed both days). In a case-crossover analysis, only the exposure to the second drug is used. The conditional logistic regression model was the standard tool used for the analysis.
Lasso analysis
We designed the analysis protocol assuming that few medicines increased the risk of road crash. A variable selection procedure thus had to be performed to identify these medicines from a large list of potential candidates. To address multiple testing problems, we implemented a Lasso (least absolute shrinkage and selection operator) regression analysis [30] for each washout period. In this multivariable modeling approach, a penalty term shrinks each coefficient estimate and sets some of them to zero. Estimation and selection of the subset of covariates showing the strongest association from a longer list of covariates occur simultaneously.
The Lasso approach tends to retain not only the relevant exposures but also a few additional irrelevant ones (though typically their estimates are small). To circumvent this problem, we used the Bolasso (bootstrap-enhanced least absolute shrinkage operator) method [31]. Only exposures frequently chosen by the Lasso analysis over the bootstrap samples were selected, which improved the stability of the results [32]. In the Bolasso procedure, 2 parameters have to be tuned. The optimal amount of shrinkage was estimated by cross-validation. The frequency threshold was tuned with the Akaike information criterion over 1,000 bootstrap samples. To correct bias in the estimated coefficients, we fitted the unpenalized logistic regression model with the exposures retained in the model (those having a nonzero point estimate of log-odds ratio) [33].
Results for each of the 90 control periods were combined in a figure to investigate whether patterns could be found according to the length of the washout period (e.g., medicines selected with narrow washout). A colored square was used to indicate that a medicine class was selected by the model. The color intensity and the size of this square were proportional to the bias-corrected odds ratio obtained at the end of the Bolasso procedure.
All statistical analyses were performed using the package Penalized in R statistical software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria). The bootstrap was implemented manually.
Results
National ID number, sex, and date of birth of individuals involved in a traffic crash were extracted for 215,032 of the 439,518 police reports of traffic crashes from 1 July 2005 to 31 December 2011. Among these, 186,636 (86.8%) were matched with a corresponding record in the police national database of injurious crashes. The linkage failed for national ID numbers corresponding to road users involved in a crash but not captured in the police national injurious crashes database and for individuals not involved in the crash (e.g., witnesses).
The procedure led to the inclusion of 16,458 pedestrians involved in a road traffic crash between 1 July 2005 and 31 December 2011 (19.1% of the pedestrians registered in the national database of injurious crashes; see Fig 2). Among these, 7,535 pedestrians (45.8%) were never exposed to any medicine under study during the time period considered (crash day and 120 days before), and 2,339 pedestrians (14.2%) were exposed to at least 1 medicine of interest, but without interruption. Consequently, these pedestrians were not included in the analyses. When varying the washout period, the sample size (pedestrians with unequal exposure in the case and control periods) varied from 5,009 (30.4% of pedestrians; washout = 37 days) to 5,315 (32.3% of pedestrians; washout = 107 days). The probability of being part of the study was associated with sex, age, injury severity, weather conditions, and day of the week (S1 Table).
Fig. 2. Flowchart of the inclusion procedure. 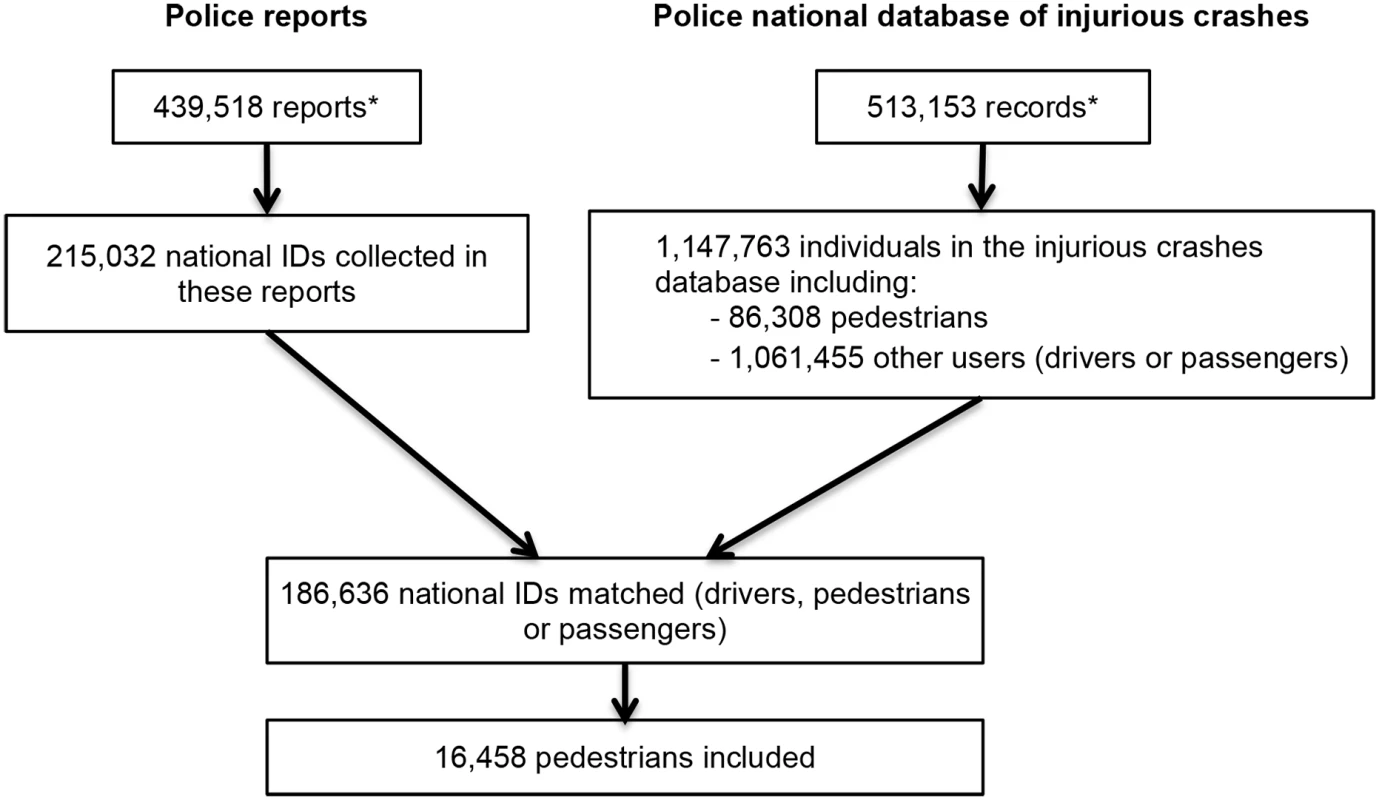
Note that the discrepancy between the number of police reports and the number of records in the police national database of injurious crashes is explained by the fact that a small proportion of unavailable reports were being used for ongoing legal investigations. *Modified from Orriols et al. [24]. Almost 60% of the participants were women, and almost one-third were 65 years old or older (Table 1). The majority of crashes occurred during favorable weather and lighting conditions. In all, 12,474 pedestrians (75.8%) were crossing the street at the time of the crash, and 7,823 (47.6%) were in a crosswalk. More precisely, most of the crashes occurred in a crosswalk with a traffic light (29.4%) or less than 50 m from a crosswalk (20.8%).
Tab. 1. Pedestrian and crash characteristics. 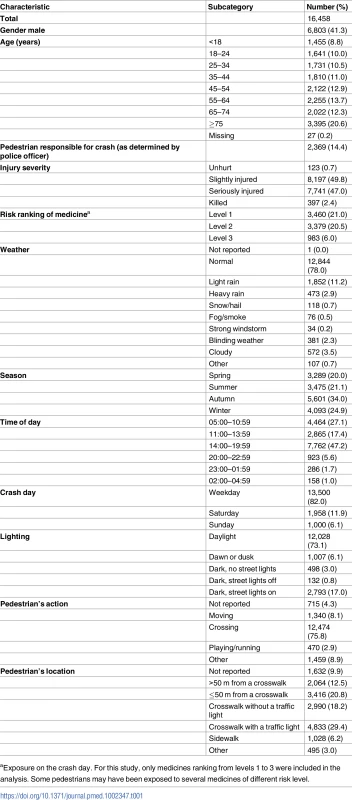
aExposure on the crash day. For this study, only medicines ranking from levels 1 to 3 were included in the analysis. Some pedestrians may have been exposed to several medicines of different risk level. When varying the washout period from 30 days (Fig 3, right) to 119 days (Fig 3, left), we found 48 medicine classes (ATC level 4) associated with an increased risk of being involved in a road crash as a pedestrian in at least 1 of the 90 models, and 4 association patterns (Fig 3). A pattern with a narrow washout period characterized 5 medicine classes (labeled with blue stars in Fig 3), including dopamine antagonists (N04BC) and piperazine derivatives (R06AE). A second pattern included 8 medicines that were consistently associated with increased risk in models with a wide washout period (yellow triangles). This pattern included 3 insulins and analogues (A10AC, A10AD, and A10BD). A third pattern included 3 medicine classes that were regularly associated with increased risk regardless of the washout period (green circles). Among these 3, alpha-adrenoreceptor antagonists (G04CA) showed the most regular association profile (showing association with increased risk in more than 90% of the designs). Finally, 32 medicines showed irregular associations with pedestrian crash risk (black squares) with respect to the length of the washout period.
Fig. 3. Results of the 90 case-crossover designs obtained when varying the washout period from 30 days to 119 days. 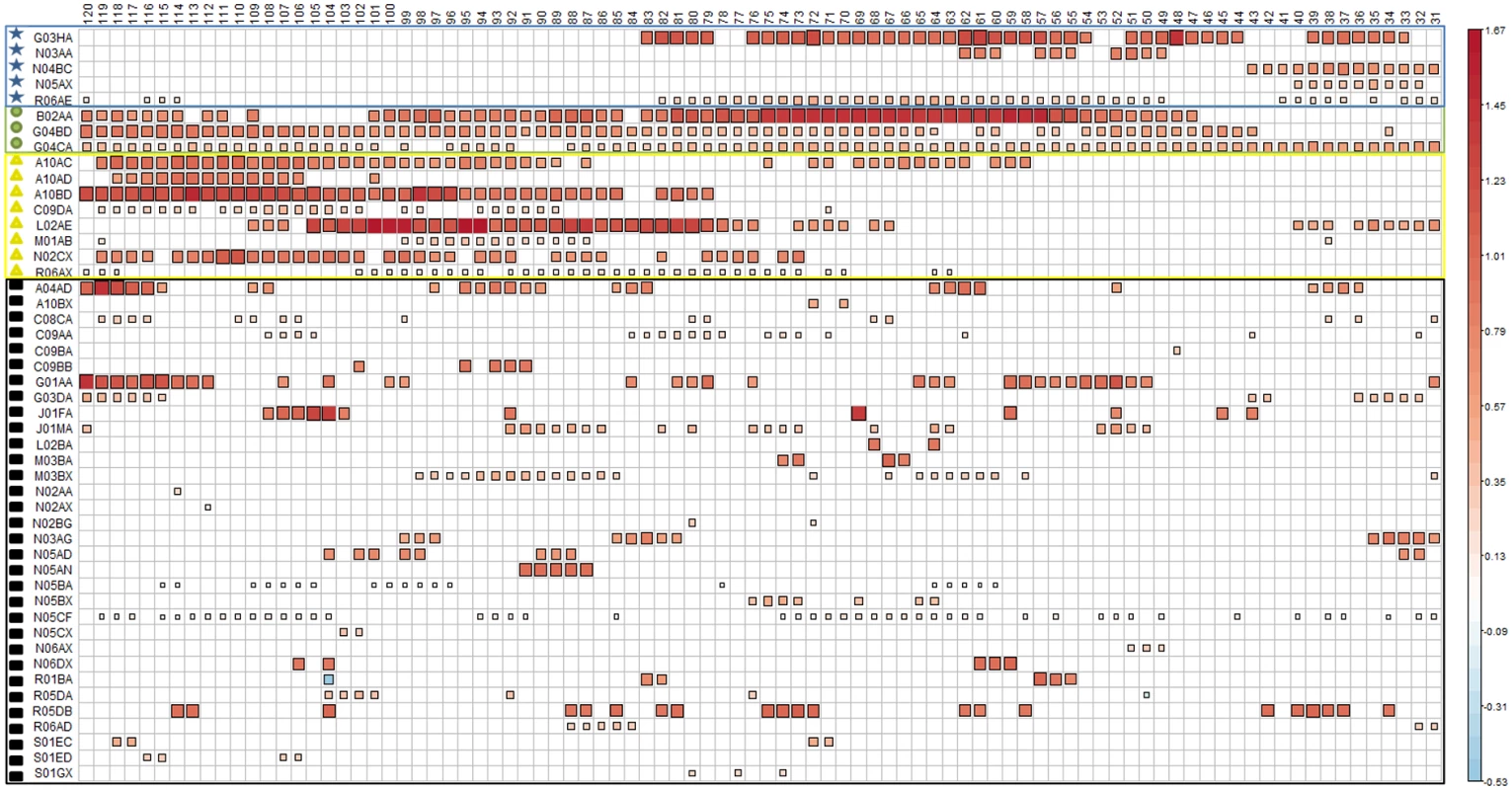
A blank cell means that the medicine class was not retained in the final model for this control period, and a colored square means that the medicine class was selected by the model. Both the size and color intensity of the squares depend on the absolute value of the bias-corrected estimated coefficients. When varying the washout period, the frequency thresholds estimated using the Akaike information criterion varied from a minimum of 50% (washout = 40) to a maximum of 74% (washout = 104). A frequency threshold of 74% means that medicines selected in at least 74% of the 1,000 bootstraps were considered as associated risk factors for pedestrian road crash. The different colored forms on the far left indicate groups of medicines according to the location of the control periods (with respect to the crash day) for which there was an association of the medicine with increased risk of being involved in a road crash as a pedestrian: blue stars indicate increased risk in control periods close to the crash; yellow squares indicate increased risk in control periods far from the crash; green circles indicate increased risk in control periods both close to and far from the crash; black squares indicate increased risk in discontinuous control periods. Table 2 presents the variation of the bias-corrected odds ratios and the total number of designs in which each exposure was selected (L value).
Tab. 2. Selection frequency of 48 medicine classes among the 90 case-crossover models, bias-corrected odds ratios, and unequal exposures. 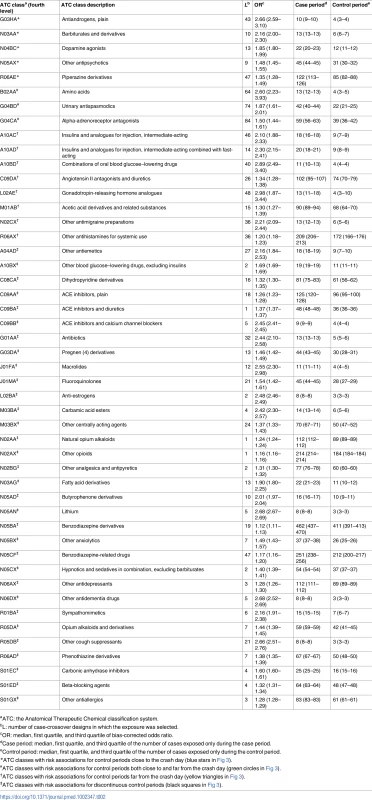
aATC: the Anatomical Therapeutic Chemical classification system. Table 3 shows that benzodiazepine derivatives and benzodiazepine-related drugs have the highest consumption rates in our cohort. Medicines with frequent use also include 4 classes of drugs used either as analgesics or as anti-inflammatory and antirheumatic drugs (R05DA, N02BG, N02AX, and M01AB), 2 classes of antihistamines (R06AX and R06AD), plain ACE inhibitors (C09AA), and 1 class of antidepressants (N06AX).
Tab. 3. The 10 most consumed medicines among those listed in Table 2 as associated with road traffic crash involvement. 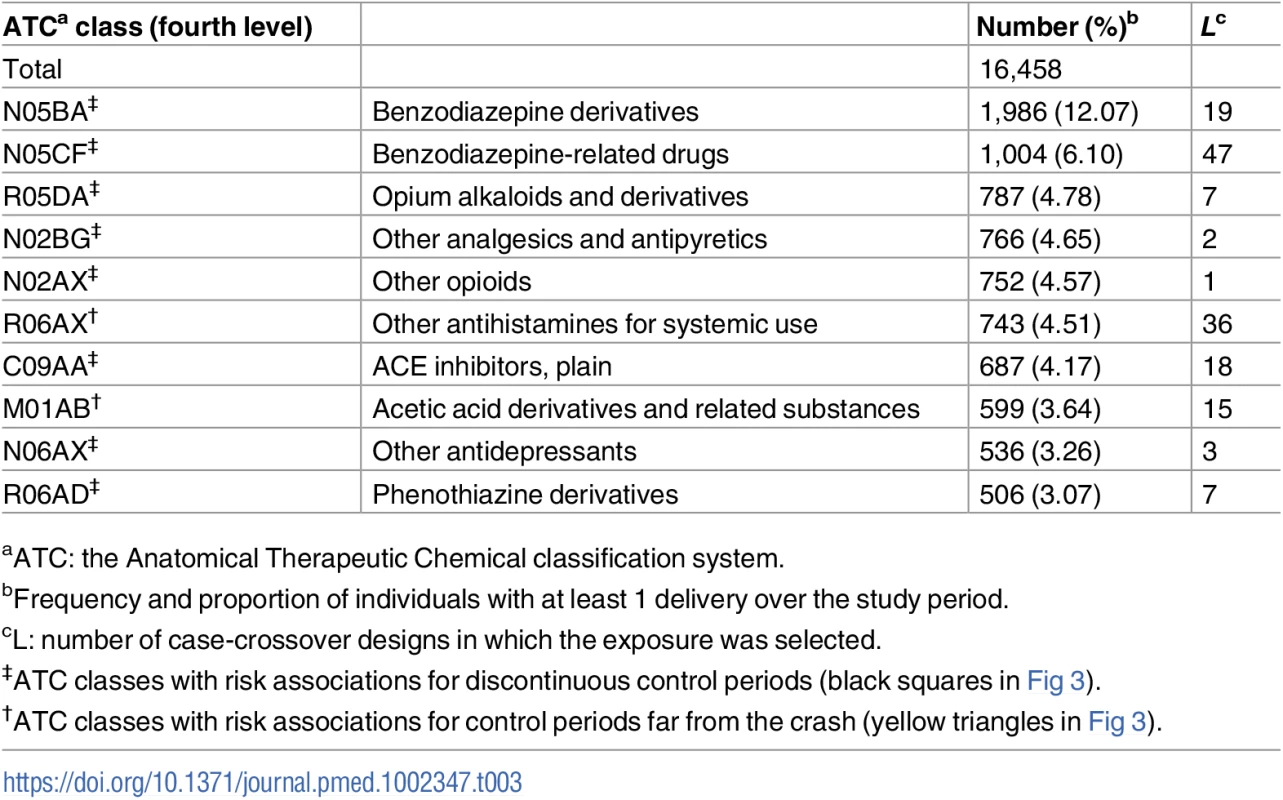
aATC: the Anatomical Therapeutic Chemical classification system. Discussion
The analysis of 16,458 pedestrians involved in a road crash between 1 July 2005 and 31 December 2011, among which 6,584 were included in the analyses, identified 48 medicine classes associated with an increased risk for pedestrians of being involved in an injurious road traffic crash. Among them, the 10 most consumed medicines included benzodiazepines and benzodiazepine-related drugs, antihistamines, and anti-inflammatory and antirheumatic drugs.
Injury severity and age were associated with the probability of being part of the study. This is due to 2 combined factors: (1) the healthcare number was more frequently noted for injured pedestrians admitted to hospital, leading to a slight overrepresentation of pedestrians injured in more severe crashes, and (2) older road users involved in a road crash are more likely to be more severely injured [34].
Computerized records of reimbursed prescriptions were used to determine exposure to medications. Compared with self-reporting methods, this method avoids memory bias. However, these databases have 2 main limitations. First, there is a lack of information about self-medication and the use of over-the-counter drugs because only dispensing of reimbursed drugs is recorded. Second, there is a lack of information about treatment compliance. Because of the use of the dispensing date to determine exposure periods, these limitations can result in exposure misclassification. A study using the same reimbursement database showed that healthcare insurance data are reliable indicators of actual exposure for medicines used over a long time, but less so for episodically used medicines [35].
In studies on medicines, both the treatment and the condition for which the treatment was prescribed can be associated with the outcome of interest. Because the case-crossover design compares each individual with himself/herself, it has the advantage of eliminating control selection bias and confounding by time-invariant factors such as age, sex, and chronic conditions [36]. However, confounding by indication remains for non-chronic conditions or if there is a change in the severity of the disease over time. Confounding may also remain for other time-varying factors such as increased enforcement of speeding laws. However, the effect on our estimates is likely insignificant because exposures of only the 4 months preceding the crash day were considered for each pedestrian. The probability of such an event occurring during these 4 months is therefore small. Another reason the effect of these potential confounding factors is likely to be insignificant is that these 4-month periods were different for every pedestrian and therefore were spread over a 6-year period. The choice of the control periods has to be independent of exposure, and only periods in which an event can occur must be considered [37].
One limitation of our study is that no information was available on how much the participants walked during the control versus case periods. Thus, we were not able to control for the amount of walking. If the probability of being a pedestrian is correlated with exposure, then the results may be biased. Indeed, consumption of some of the medicines under study may result in an increased probability of walking instead of driving because of the warning pictogram on medicine packaging. However, a recent study exploring trends in medicine consumption (restricted to hypnotics and anxiolytics) before and after the implementation of pictograms in France showed no impact of pictograms on exposure level to these medicines among drivers. Consequently, it is very unlikely that the pictogram system resulted in a substantial shift in transportation means for consumers of those medicines [38]. Medicines can also lead to an increase in walking due to an increased sense of well-being. However, there will be a bias only if the reason for taking this medicine already existed during the control period and led to a decrease in walking. This is unlikely, as control periods were at least 30 days before the crash, and there is no reason to think that people affected by a debilitating disease for at least 1 month would not take medicines. Consequently, taking medicines during the case period was assumed to enable the individual to get back to his or her previous walking habits.
Analyses were not adjusted for alcohol consumption because the information was not available during control periods. Alcohol has been found to be associated with a higher risk of crash for pedestrians [39] and could also be a confounding factor for medicines such as benzodiazepines. Information about blood alcohol level, as recorded in the police national database of injurious crashes, was unknown for 41.6% of the pedestrians. Among the 9,617 pedestrians with data for blood alcohol level, 95.7% had a level below the legal limit. A comparison between pedestrians with an alcohol level below the legal limit and pedestrians with missing alcohol status suggested that the latter were not tested for alcohol because the police officers did not consider it necessary. Pedestrians with a blood alcohol level above the legal limit were more likely to be male, aged 30 to 49 years, and presumed responsible for the crash, compared with pedestrians with an alcohol level below the legal limit or with missing alcohol status. They were also less likely to be exposed to level 1 medicines and more likely to be exposed to level 3 medicines. Removing the individuals with an alcohol level above the legal limit from the analysis would most likely be inconsequential because they represent only 412 individuals, thus resulting in a very small number of discordant exposures.
Among the 16,458 pedestrians included in the present study, older adults and women were overrepresented. These findings are in agreement with national statistics on injurious crashes [40]. This is probably the combined result of a greater fragility and exposure of the elderly, along with a higher proportion of women among the elderly. Two urological drugs (G04CA and G04BD) were selected for almost all case-crossover designs regardless of the washout period. This can be interpreted as the result of treatment initiation (they were exposed during the case period, but never before). These drugs can affect a pedestrian’s level of attention (e.g., reaction time) or mobility (e.g., walking speed). For instance, alpha-adrenoceptor antagonists induce vasodilatation and subsequently lower blood pressure, with potential symptoms including asthenia, dizziness, fatigue, and orthostatic hypotension, particularly upon treatment initiation [41–43]. Similarly, urinary antispasmodics have a high potential of producing anticholinergic effects, which can result in cognitive side effects including memory changes, blurred vision, somnolence, hallucinations, confusion, and delirium [44,45].
Eight medicines (ATC level 4) were selected in case-crossover designs with control periods away from the crash day. Risk associations with long washout periods may be the consequence of longer duration of medicine use, requiring a longer washout period in order to have discordant exposures between case and control periods. The exposures selected in this profile, including antidiabetic drugs (A10AC, A10AD, and A10BD) and antimigraine preparations (N02CX) prescribed in chronic treatment of migraine, tend to support this assumption. Hypoglycemia is a common adverse effect of antidiabetic treatment [46–48] and can result in decreased motor skills, visual acuity, or auditory processing and deterioration of a variety of cognitive processes [49,50]. However, in this pattern, the increased risk is most likely linked to disease-related complications. Indeed, in the case-crossover design, the longer the washout period, the higher the risk of potential confounding by unmeasured time-varying factors including disease severity. Medicines of the R06AX group were mostly second-generation antihistamines, which may have fewer sedative effects than those of the first generation. Rhinitis, for which they are prescribed, can affect the quantity and quality of sleep and so lead to fatigue and daytime somnolence [51,52]. Acetic acid derivatives and related substances (M01AB) are nonsteroidal anti-inflammatory drugs used in the treatment of various painful inflammatory conditions including rheumatoid arthritis and osteoarthritis. Rheumatoid arthritis, which may affect pedestrians’ walking ability, is characterized in most people by alternating periods of remission and relapse, which can explain the narrow washout despite it being a chronic condition [53]. Antihistamines (R06AX) and acetic acid derivatives (M01AB) were among the 10 most consumed medicines selected in our analyses.
Risk associations with exposure periods close to the crash suggest an effect of acute, short-term exposure to the medicine. Both Parkinson disease symptoms (e.g., muscle rigidity, slowing of physical movement) and side effects of dopamine agonists (N04BC)—including dizziness, somnolence, insomnia, and orthostatic hypotension—can affect a pedestrian’s walking ability. Because of the chronicity of Parkinson treatment, the increased risk of crash can be the result of a treatment change or a change in the disease condition. However, because of the proximity with the crash, the first possibility is more likely. This is also true for other antipsychotic drugs (N05AX) and barbiturates and derivatives (N03AA), 2 medicines used in chronic conditions. Moreover, lack of compliance with treatment is frequent in patients with schizophrenia, and barbiturates and derivatives (N03AA) have been found to be associated with drowsiness and cognitive effects, including diminished attention, executive function, and processing speed [54,55]. Piperazine derivatives (R06AE) were associated with an increased risk of crash, but with a low risk level (median bias-corrected odds ratio of 1.3). Like medicines grouped as “other antihistamines for systemic use drugs” (R06AX), they are prescribed for the treatment of allergic rhinitis and urticaria and are mostly second-generation antihistamines. However, cetirizine and levocetirizine (R06AE) were found to be associated with a higher risk of sedation than loratadine (R06AX) [56,57].
Among medicines showing risk associations with a less consistent pattern in terms of the length of the washout period, benzodiazepine derivatives (N05BA), benzodiazepine-related drugs (N05CF), and plain ACE inhibitors (C09AA) were 3 medicines inconsistently but frequently selected among the 90 models. Moreover, they were among the 10 most consumed medicines. The association between the use of benzodiazepines and the risk of road traffic crashes has been documented with consistent results among drivers. Benzodiazepines have also been shown to be associated with an increased risk of falling in the elderly, which may be due to attention, gait, and balance impairment [58], effects that might also increase the risk of crash for pedestrians. One study has reported a significantly higher risk of at-fault crash involvement with the use of ACE inhibitors in the elderly, medicines commonly used to treat hypertension [21].
Results for 3 medicines were unexpected: plain antiandrogens (G03HA), amino acids (B02AA), and gonadotropin-releasing hormone analogues (L02AE) showed a strong association with the risk of pedestrian crash. However, their levels of use in our sample of injured pedestrians were all under 0.3%, and their estimated odds ratios were based on a small number of discordant pairs. In addition, these medicines were all attributed a level 1 warning pictogram, which means that their risk is low, heterogeneous, and strongly dependent on individuals’ pharmacodynamic parameters.
In summary, our results suggest that several classes of medicine are associated with an increased risk of pedestrian crash. Improving awareness of this risk is, therefore, a necessity, as the risks of medicines in road safety have hitherto been thought to concern drivers only. Most of the crashes occurred when the pedestrian was crossing the street, which requires certain levels of physical ability (e.g., walking speed, head mobility), perception (e.g., vision, hearing), and cognitive processing (e.g., attention, processing speed) [59]. Despite an association between medicine use and pedestrian crashes, it is not recommended to drive instead.
Supporting Information
Zdroje
1. Constant A, Lagarde E. Protecting vulnerable road users from injury. PLOS Med. 2010;7:e1000228. doi: 10.1371/journal.pmed.1000228 20361017
2. World Health Organization. Global status report on road safety 2013. http://www.who.int/violence_injury_prevention/road_safety_status/2013/en/. Accessed 2015 Aug 26.
3. Asher L, Aresu M, Falaschetti E, Mindell J. Most older pedestrians are unable to cross the road in time: a cross-sectional study. Age Ageing. 2012;41 : 690–4. doi: 10.1093/ageing/afs076 22695790
4. Sze NN, Wong SC. Diagnostic analysis of the logistic model for pedestrian injury severity in traffic crashes. Accid Anal Prev. 2007;39 : 1267–78. doi: 10.1016/j.aap.2007.03.017 17920851
5. Mueller BA, Rivara FP, Bergman AB. Factors associated with pedestrian-vehicle collision injuries and fatalities. West J Med. 1987;146 : 243–5. 3825131
6. Aziz HMA, Ukkusuri SV, Hasan S. Exploring the determinants of pedestrian–vehicle crash severity in New York City. Accid Anal Prev. 2013;50 : 1298–309. doi: 10.1016/j.aap.2012.09.034 23122781
7. Dai D. Identifying clusters and risk factors of injuries in pedestrian vehicle crashes in a GIS environment. J Transp Geogr. 2012;24 : 206–14. doi: 10.1016/j.jtrangeo.2012.02.005
8. Gårder PE. The impact of speed and other variables on pedestrian safety in Maine. Accid Anal Prev. 2004;36 : 533–42. doi: 10.1016/S0001-4575(03)00059-9 15094405
9. Kurzthaler I, Wambacher M, Golser K, Sperner G, Sperner-Unterweger B, Haidekker A, et al. Alcohol and/or benzodiazepine use: different accidents—different impacts? Hum Psychopharmacol. 2005;20 : 583–9. doi: 10.1002/hup.736 16317801
10. Ray WA, Fought RL, Decker MD. Psychoactive drugs and the risk of injurious motor vehicle crashes in elderly drivers. Am J Epidemiol. 1992;136 : 873–83. 1442753
11. Movig KLL, Mathijssen MPM, Nagel PHA, Van Egmond T, de Gier JJ, Leufkens HGM, et al. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36 : 631–6. doi: 10.1016/S0001-4575(03)00084-8 15094417
12. Orriols L, Philip P, Moore N, Castot A, Gadegbeku B, Delorme B, et al. Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clin Pharmacol Ther. 2011;89 : 595–601. doi: 10.1038/clpt.2011.3 21368756
13. Leveille SG, Buchner DM, Koepsell TD, McCloskey LW, Wolf ME, Wagner EH. Psychoactive medications and injurious motor vehicle collisions involving older drivers. Epidemiology. 1994;5 : 591–8. 7841240
14. Fournier JP, Wilchesky M, Patenaude V, Suissa S. Concurrent use of benzodiazepines and antidepressants and the risk of motor vehicle accident in older drivers: a nested case–control study. Neurol Ther. 2015;4 : 39–51. doi: 10.1007/s40120-015-0026-0 26847674
15. Orriols L, Wilchesky M, Lagarde E, Suissa S. Prescription of antidepressants and the risk of road traffic crash in the elderly: a case–crossover study. Br J Clin Pharmacol. 2013;76 : 810–5. doi: 10.1111/bcp.12090 24148104
16. Rapoport MJ, Zagorski B, Seitz D, Herrmann N, Molnar F, Redelmeier DA. At-fault motor vehicle crash risk in elderly patients treated with antidepressants. Am J Geriatr Psychiatry. 2011;19 : 998–1006. doi: 10.1097/JGP.0b013e31820d93f9 22123273
17. Orriols L, Queinec R, Philip P, Gadegbeku B, Delorme B, Moore N, et al. Risk of injurious road traffic crash after prescription of antidepressants. J Clin Psychiatry. 2012;73 : 1088–94. doi: 10.4088/JCP.11m07624 22967773
18. Drummer OH, Gerostamoulos J, Batziris H, Chu M, Caplehorn J, Robertson MD, et al. The involvement of drugs in drivers of motor vehicles killed in Australian road traffic crashes. Accid Anal Prev. 2004;36 : 239–48. 14642878
19. Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med. 2013;173 : 196–201. doi: 10.1001/2013.jamainternmed.733 23318919
20. Engeland A, Skurtveit S, Mørland J. Risk of road traffic accidents associated with the prescription of drugs: a registry-based cohort study. Ann Epidemiol. 2007;17 : 597–602. doi: 10.1016/j.annepidem.2007.03.009 17574863
21. McGwin G, Sims RV, Pulley L, Roseman JM. Relations among chronic medical conditions, medications, and automobile crashes in the elderly: a population-based case-control study. Am J Epidemiol. 2000;152 : 424–31. doi: 10.1093/aje/152.5.424 10981455
22. Elliott S, Woolacott H, Braithwaite R. The prevalence of drugs and alcohol found in road traffic fatalities: a comparative study of victims. Sci Justice. 2009;49 : 19–23. doi: 10.1016/j.scijus.2008.06.001 19418924
23. Australian Department of Infrastructure and Regional Development. Monograph 14—male pedestrian fatalities. http://www.infrastructure.gov.au/roads/safety/publications/2003/Ped_Male_1.aspx. Accessed 2015 Mar 3.
24. Orriols L, Delorme B, Gadegbeku B, Tricotel A, Contrand B, Laumon B, et al. Prescription medicines and the risk of road traffic crashes: a French registry-based study. PLOS Med. 2010;7:e1000366. doi: 10.1371/journal.pmed.1000366 21125020
25. Trouessin G, Allaert FA. FOIN: a nominative information occultation function. Stud Health Technol Inform. 1997;4(Pt A):196–200.
26. Riché C, Caulin C, Caron J, Chiffoleau A, Corbé C, Diquet B, et al. Medicinal products and driving. March 2009 update. Paris: Agence Nationale de Sécurité du Médicament et des Produits de Santé; 2009. http://ansm.sante.fr/var/ansm_site/storage/original/application/e5f2e48d5344bcfef6ca865ac63e7c3d.pdf. Accessed 2017 Jun 13.
27. Agence Française de Sécurité Sanitaire des Produits de Santé. Mise au point: Médicaments et conduite automobile. Paris: Agence Nationale de Sécurité du Médicament et des Produits de Santé; 2009. http://ansm.sante.fr/var/ansm_site/storage/original/application/faff1e402339cd443a9894792f20d31d.pdf. Accessed 2017 Jun 13.
28. IMS Health. Enquête Permanente sur la Prescription Médicale (EPPM). Danbury (Connecticut): IMS Health; 2005–2011.
29. Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21 : 193–221. doi: 10.1146/annurev.publhealth.21.1.193 10884952
30. Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B Methodol. 1996;58 : 267–88.
31. Bach F. Bolasso: model consistent Lasso estimation through the bootstrap. Ithaca (New York): arXiv.org; 2008. arXiv.0804.1302. http://arxiv.org/abs/0804.1302. Accessed 2017 Jun 13.
32. Avalos M, Orriols L, Pouyes H, Grandvalet Y, Thiessard F, Lagarde E, et al. Variable selection on large case-crossover data: application to a registry-based study of prescription drugs and road traffic crashes. Pharmacoepidemiol Drug Saf. 2014;23 : 140–51. doi: 10.1002/pds.3539 24136855
33. Belloni A, Chernozhukov V. Least squares after model selection in high-dimensional sparse models. Bernoulli. 2013;19 : 521–47. doi: 10.3150/11-BEJ410
34. Loo BPY, Tsui KL. Pedestrian injuries in an ageing society: insights from hospital trauma registry. J Trauma. 2009;66 : 1196–201. doi: 10.1097/TA.0b013e31817fdef6 19359937
35. Noize P, Bazin F, Dufouil C, Lechevallier-Michel N, Ancelin M-L, Dartigues J-F, et al. Comparison of health insurance claims and patient interviews in assessing drug use: data from the Three-City (3C) Study. Pharmacoepidemiol Drug Saf. 2009;18 : 310–9. doi: 10.1002/pds.1717 19241438
36. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133 : 144–53. 1985444
37. Mittleman MA, Mostofsky E. Exchangeability in the case-crossover design. Int J Epidemiol. 2014;43 : 1645–55. doi: 10.1093/ije/dyu081 24756878
38. Orriols L, Luxcey A, Contrand B, Gadegbeku B, Delorme B, Tricotel A, et al. Road traffic crash risk associated with benzodiazepine and z-hypnotic use after implementation of a colour-graded pictogram: a responsibility study. Br J Clin Pharmacol. 2016;82 : 1625–35. doi: 10.1111/bcp.13075 27544927
39. Öström M, Eriksson A. Pedestrian fatalities and alcohol. Accid Anal Prev. 2001;33 : 173–80. doi: 10.1016/S0001-4575(00)00028-2 11204887
40. L’Observatoire National Interministériel de la Sécurité Routière. Données brutes. http://www.securite-routiere.gouv.fr/la-securite-routiere/l-observatoire-national-interministeriel-de-la-securite-routiere/series-statistiques/donnees-brutes. Accessed 2017 Feb 7.
41. Bird ST, Delaney JAC, Brophy JM, Etminan M, Skeldon SC, Hartzema AG. Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40–85 years in the United States: risk window analyses using between and within patient methodology. BMJ. 2013;347:f6320. doi: 10.1136/bmj.f6320 24192967
42. Chrischilles E, Rubenstein L, Chao J, Kreder KJ, Gilden D, Shah H. Initiation of nonselective alpha1-antagonist therapy and occurrence of hypotension-related adverse events among men with benign prostatic hyperplasia: a retrospective cohort study. Clin Ther. 2001;23 : 727–43. doi: 10.1016/S0149-2918(01)80022-9 11394731
43. Oelke M, Gericke A, Michel MC. Cardiovascular and ocular safety of α1-adrenoceptor antagonists in the treatment of male lower urinary tract symptoms. Expert Opin Drug Saf. 2014;13 : 1187–97. doi: 10.1517/14740338.2014.936376 25073735
44. Geller EJ, Crane AK, Wells EC, Robinson BL, Jannelli ML, Khandelwal CM, et al. Effect of anticholinergic use for the treatment of overactive bladder on cognitive function in post-menopausal women. Clin Drug Investig. 2012;32 : 697–705. 22873491
45. Munjuluri N, Wong W, Yoong W. Anticholinergic drugs for overactive bladder: a review of the literature and practical guide. Obstet Gynaecol. 2007;9 : 9–14. doi: 10.1576/toag.9.1.009.27290
46. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157 : 1681–6. doi: 10.1001/archinte.1997.00440360095010 9250229
47. Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22 : 99–111. 10333910
48. Pramming S, Thorsteinsson B, Bendtson I, Binder C. Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet Med. 1991;8 : 217–22. 1828735
49. Sommerfield AJ, Deary IJ, McAulay V, Frier BM. Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care. 2003;26 : 390–6. 12547868
50. Brands AMA, Biessels GJ, de Haan EHF, Kappelle LJ, Kessels RPC. The effects of type 1 diabetes on cognitive performance a meta-analysis. Diabetes Care. 2005;28 : 726–35. 15735218
51. Colás C, Galera H, Añibarro B, Soler R, Navarro A, Jáuregui I, et al. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAAR study). Clin Exp Allergy. 2012;42 : 1080–7. doi: 10.1111/j.1365-2222.2011.03935.x 22251258
52. Muliol J, Maurer M, Bousquet J. Sleep and allergic rhinitis. J Investig Allergol Clin Immunol. 2008;18 : 415–9. 19123431
53. Van Laar M, Pergolizzi JV, Mellinghoff HU, Merchante IM, Nalamachu S, O’Brien J, et al. Pain treatment in arthritis-related pain: beyond NSAIDs. Open Rheumatol J. 2012;6 : 320–30. doi: 10.2174/1874312901206010320 23264838
54. Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10 : 885–91. doi: 10.1586/ern.10.71 20518605
55. Pellock JM. Treatment considerations: traditional antiepileptic drugs. Epilepsy Behav. 2002;3 : 18–23. doi: 10.1016/S1525-5050(02)00538-3 12609308
56. Salmun LM, Gates D, Scharf M, Greiding L, Ramon F, Heithoff K. Loratadine versus cetirizine: assessment of somnolence and motivation during the workday. Clin Ther. 2000;22 : 573–82. doi: 10.1016/S0149-2918(00)80045-4 10868555
57. Mann RD, Pearce GL, Dunn N, Shakir S. Sedation with “non-sedating” antihistamines: four prescription-event monitoring studies in general practice. BMJ. 2000;320 : 1184–6. 10784544
58. Rossat A, Fantino B, Bongue B, Colvez A, Nitenberg C, Annweiler C, et al. Association between benzodiazepines and recurrent falls: a cross-sectional elderly population-based study. J Nutr Health Aging. 2011;15 : 72–7. 21267523
59. Dommes A, Cavallo V. The role of perceptual, cognitive, and motor abilities in street-crossing decisions of young and older pedestrians. Ophthalmic Physiol Opt. 2011;31 : 292–301. doi: 10.1111/j.1475-1313.2011.00835.x 21470273
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Time for trauma immunology
- Translational approaches to coagulopathy after trauma: Towards targeted treatment
- The new survivors and a new era for trauma research
- Research questions in pre-hospital trauma care
- Major scientific lessons learned in the trauma field over the last two decades
- The science of rapid start—From the when to the how of antiretroviral initiation
- Reducing undiagnosed HIV infection among adolescents in sub-Saharan Africa: Provider-initiated and opt-out testing are not enough
- Community and health system intervention to reduce disrespect and abuse during childbirth in Tanga Region, Tanzania: A comparative before-and-after study
- Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study
- Trauma care: Finding a better way
- Risk of surgical site infection, acute kidney injury, and infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
- Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries
- Antimicrobial resistance in : Global surveillance and a call for international collaborative action
- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Cellular therapies in trauma and critical care medicine: Looking towards the future
- Leveraging peer-based support to facilitate HIV care in Kenya
- Trends in traumatic brain injury mortality in China, 2006–2013: A population-based longitudinal study
- Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study
- Community health promotion and medical provision for neonatal health—CHAMPION cluster randomised trial in Nagarkurnool district, Telangana (formerly Andhra Pradesh), India
- A comparison of Selective Aortic Arch Perfusion and Resuscitative Endovascular Balloon Occlusion of the Aorta for the management of hemorrhage-induced traumatic cardiac arrest: A translational model in large swine
- Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries
- Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study
- Cancer trials in sub-Saharan Africa: Aligning research and care
- Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study
- Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study
- Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial
- Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: A cross-sectional survey
- Timing of femoral shaft fracture fixation following major trauma: A retrospective cohort study of United States trauma centers
- IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model
- Long-term health status and trajectories of seriously injured patients: A population-based longitudinal study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



