-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCommunity health promotion and medical provision for neonatal health—CHAMPION cluster randomised trial in Nagarkurnool district, Telangana (formerly Andhra Pradesh), India
In CHAMPION, a cluster-randomized trial carried out in rural India, Diana Elbourne and colleagues investigate the benefit of a package of interventions for neonatal care.
Published in the journal: . PLoS Med 14(7): e32767. doi:10.1371/journal.pmed.1002324
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002324Summary
In CHAMPION, a cluster-randomized trial carried out in rural India, Diana Elbourne and colleagues investigate the benefit of a package of interventions for neonatal care.
Introduction
Worldwide, nearly 40% of deaths in children under 5 years occur in the neonatal period (first 28 days of life) [1–3]: over 2.8 million neonates died in 2010 [4]. A review of global progress on the Millennium Development Goals found that for goal 4 (reducing child mortality), mortality in children under 5 years fell by 28% between 1990 and 2008, but reductions in neonatal mortality remained slow [5,6].
In India, 1.68 million children aged under 5 years died in 2010, with 52% of the deaths occurring in the first 28 days [1]—a neonatal mortality rate (NMR) of 39/1,000 live births [7]. Worldwide, India contributed nearly 25% of neonatal deaths [6], and NMRs in many regions of India remained near the highest rates worldwide [8]. Three-quarters of neonatal deaths in India were attributed to prematurity, low birthweight, neonatal infections, birth asphyxia, and birth trauma [9]. These deaths often occurred in rural environments, where children are commonly born at home and, while healthcare facilities were available, their quality could be inadequate [10]; in addition, poor health knowledge and practices including health service usage may be contributory factors [6].
Studies [8] have shown the potential for reducing neonatal deaths through providing home-based neonatal care, including management of sepsis [11]; behaviour change interventions with a focus on hypothermia [12]; community mobilisation through participatory discussion groups (PDGs) [13]; and implementation of the Indian Integrated Management of Neonatal and Childhood Illness programme for neonates born at home, which provides improved treatment of illness for children and home visits for newborn care [14]. An additional strategy in the developing world has been to use health providers not affiliated with the government to deliver services, particularly in areas where government service delivery may be underperforming [10]. Evidence about the effectiveness of such strategies is scarce [15–17].
The Lancet Neonatal Survival Series recommended future studies implement and evaluate packages of care that provide family, community, outreach, and facility-based care [18]. A subsequent systematic review [19] concluded that ‘the empirical evidence for the impact of neonatal health care packages is a weak base on which to build effective programmes…. An evidence-based approach to packaging interventions is clearly needed but requires substantial investment in high-quality research’.
The CHAMPION cluster randomised controlled trial (RCT) was initiated in 2007 to evaluate such a package of interventions, comprising community health promotion (including health education delivered via village health worker [VHW]–led PDGs) and provision of health services including outreach (mobile teams providing antenatal check-ups) and facility-based care (specifically subsidised access to pregnancy-related care provided by non-public health centres [NPHCs]). The primary hypothesis was that this package could reduce neonatal mortality by 25%. A cluster RCT design was needed as the interventions were delivered at the village level. An important element was an integral economic evaluation.
Around the time the trial was being designed and implemented, the Indian government introduced a number of maternal and child health programmes, including incentives to encourage institutional deliveries and care. One of the key elements of these programmes is the involvement of the Accredited Social Health Activists (ASHAs), who were trained to refer expectant women to medical institutions for delivery, and who also received incentives accordingly. These initiatives were not, however, fully established in most of the CHAMPION villages until well after the end of the trial (see Methods and Discussion).
The CHAMPION trial was implemented in Nagarkurnool district, Telangana. This was then Nagarkurnool division, Mahabubnagar district, in the state of Andhra Pradesh (AP). AP had one of the highest NMRs in India at the time, with 35% of neonatal deaths among children who were premature or of low birthweight [20]. An additional 14.1% of neonatal deaths involved neonatal infections, and 21.7% involved birth asphyxia and/or birth trauma [5,21,22]. The estimated NMR in rural regions of AP was between 49.2 [7] and 33 per 1,000 live births [23]. Mahabubnagar was among the 100 most disadvantaged districts in the country [24]; it was 90% rural [25] and had low levels of female literacy [26]. Nagarkurnool had a high tribal population [25,27] and a particularly high mortality rate [28]. Since June 2014, this division has been part of Telangana.
The aim of the CHAMPION trial was to investigate whether a package of interventions comprising community health promotion and provision of health services including outreach and facility-based care could lead to a reduction of the order of 25% in neonatal mortality.
Methods
Research ethics approvals
Approvals were obtained from the institutional review board of the L V Prasad Eye Institute, Hyderabad, India (number LEC07002; February 2007) and from the ethics committee of the London School of Hygiene & Tropical Medicine (LSHTM) (number 5166; June 2007).
The methods have been detailed [29], and are summarised below.
Eligibility
Clusters
The CHAMPION trial was conducted in 464 villages (clusters) in Nagarkurnool with a population below 2,500, as larger villages generally have better access to health services, lower neonatal mortality rates, and therefore less potential to benefit from the interventions.
Women
A list of eligible women in each village was compiled from a baseline survey (enumeration) conducted before randomisation. A woman was eligible at enumeration if she was married and aged less than 50 years, neither she nor her husband had had a family planning operation, she was resident in the village at the time of the baseline survey, and she fulfilled the criteria for informed consent described below.
For analysis, women were not added, moved from one village to another, or removed from the list as a result of either temporary or permanent migration from the village where they were initially registered. The only permitted additions to enrolment were women who newly married into a trial village after enumeration (as ascertained in the monthly visits by the enumerators) and who were enrolled at that point.
Children
The protocol specified that children born to eligible women were to be included in the primary trial analysis if their estimated delivery at enumeration was at least 6 months after randomisation, to allow time for the interventions to be implemented and to have an impact on neonatal mortality. In fact, the intervention took longer than anticipated to implement, and a decision was taken by the trial steering committee (TSC) in autumn 2008 (without access to outcome data) to delay the date from which data would contribute to the analysis to 1 December 2008 (9.5 months after randomisation, and 4 months after first initiation of the intervention).
Consent
State approval was obtained from the Andhra Pradesh Department of Health & Family Welfare. Village consent was obtained from each panchayat (smallest unit of democratically elected government in rural India). The trial protocol was explained to villagers in their local language. Oral consent was given by the panchayat and sarpanch (panchayat leader) during a meeting before randomisation, with written documentation of approval by the sarpanch. Women provided oral consent at enumeration. Women who married into a trial village post-randomisation were informed about the trial, and their consent obtained.
Randomisation
The villages were stratified by travel time to the nearest designated NPHC (less than or greater than 1 hour) and tribal status (whether the village was a thanda [tribal village 2–3 km from the main village, with around 15 families], a penta [tribal village 20–30 km from the main village, with around 4–5 families], or non-tribal). Within each stratum, half the villages were allocated to the intervention arm and half to the control arm by the LSHTM statistician using computer-generated random numbers.
Masking
After randomisation, participants, data collectors, and data analysts were not masked to allocation. Data collectors could not be masked as they were going into villages where the interventions were obvious and collecting data from participants who were not masked. Given this necessary lack of masking in the field, we felt that trying to ensure the masking of data analysts would add complexity for little benefit. The regular communication between the data analysts and the data collectors would have made such masking very difficult to implement. Further, the anticipated large difference in health knowledge between the arms would have made the randomisation clear.
Interventions
Intervention villages
A package consisting of community health promotion and provision of health services was delivered in the intervention villages. The intervention teams were selected and managed by the NICE (Neonatal Intensive Care and Emergencies) Foundation (http://nicefoundation.in/about.html). Members of the intervention team were responsible for all service delivery activities in the intervention villages. Team members included a programme coordinator who led the team and coordinated care with the NPHCs; 2 programme officers who managed the field supervisors, midwives, and VHWs; field supervisors who were responsible for community mobilisation, mainly via PDGs, and for monitoring the VHWs; and midwives and VHWs. Regular intervention team meetings were held to share lessons learned and for future planning.
Health promotion activities
Each intervention village received a health education campaign during the trial. Folk culture (Kalajatha) in the form of song and dance was used to promote key maternal and child health themes. The intervention team worked with a group of artists for a month to finalise the messages and ways to disseminate them. They produced the final format after 2 pilot sessions in villages. The songs carried messages about safe maternal and child health practices, focusing on clearing misconceptions about maternal and child health, danger signs when pregnancies go wrong, trained birth assistance, safe motherhood, newborn care, the advantages of institutional delivery, the importance of fixed day health services (FDHSs), and the PDGs.
PDGs were conducted at least monthly and, in contrast to the health education campaign, primarily targeted women to improve mothers’ and caretakers’ health knowledge, increase each community’s awareness of maternal and child health, and encourage greater use of available health services, safe delivery techniques, and referral of high-risk women and babies to more specialist centres. The PDGs also gave mothers a forum to discuss solutions to important maternal and newborn health issues. Ten key discussion themes promoting good health practices including health service usage during and after pregnancy were repeated over the duration of the trial. These included recognising risk factors for baby and mother, drawing up birth plans, safe delivery techniques, family health, and immunisation. Other issues that were important in the villages were identified and delivered as a session. There was 1 PDG session (module) per theme. Posters, flip charts, and flash cards were used, supplemented by examples of actual foodstuffs at the nutrition session. Eligible women and other members of the community could attend the sessions, which happened on the day of the FDHS. Each session lasted approximately an hour and took place at a village venue suitable to most women. Sessions contained icebreakers, a review of the previous meeting, and new themes, with reinforcement through activities, demonstrations, and games.
Approximately 230 women were selected in consultation with the community and trained to serve as VHWs. Although an option in the protocol was for VHWs to be auxiliary nurse midwives (ANMs) or traditional birth attendants, in practice none were. They were resident women from or marrying into the village (‘daughter-in-laws of the village’), capable of reading and writing in Telugu. Most had a 10th grade education.
Health service activities
The aim of the CHAMPION trial interventions was not to run a parallel health service system but to strengthen existing government services, and so, where possible, the trial intervention team converged with the government flagship programmes, for instance, utilising ANM services to ensure tetanus vaccinations for pregnant women and administering child vaccinations through the government ANMs. However, many of the government initiatives were not in place in the trial villages during the period of the trial (see Discussion). In their absence, VHWs were the primary contact point for the intervention team in the village, responsible for delivering antenatal and health education in their allocated villages. Their major role was to identify and track eligible women in their village, visiting the houses of pregnant women and newborns, and documenting the events that occurred. They counselled pregnant women and their family members, mobilised pregnant women to attend the FDHS, supported midwives during the FDHS and provided follow-up care as advised by nurse midwives, provided case briefings to the midwife at each FDHS consultation, and supported the FDHS team in mobilising target audiences to PDG sessions. The VHWs were also involved in preparation for, and conducting or assisting at, normal deliveries in the village and providing follow-up care for postnatal women, including 3 visits during the first week, and 2 visits per week from the second to fourth week. An important role involved tracking high-risk mothers (identified based on vaginal bleeding; headache; persistent fever; abdominal cramps; dizziness; loss of consciousness or blurring of vision; convulsions; shakes; shivering; poor circulation and puffiness of the face, hands, or feet; lack of fetal movements over 4 hours; sudden weight gain or loss; previous history of fetal loss; obstructed labour; or signs of malaria; S1 Text), as well as detecting danger signs in newborns and facilitating timely referral and transport of pregnant woman or newborns, if needed. VHWs were provided with mucous suckers, mackintosh sheets, gauze pieces, rolls of cotton, brushes and antiseptic lotions (povidone-iodine for cord care), birthing kits, weighing scales, measuring tapes, and fetoscopes. VHWs received payment proportional to village size, to a maximum of 1,100 rupees for villages with close to 2,500 inhabitants.
VHWs underwent a 1-week residential training followed by quarterly 1-day refresher training. The training topics included anaemia and complaints in pregnancy such as fever, persistent headache, abdominal pain, and vaginal bleeding. VHWs were trained to monitor potential breast problems, oedema, fetal heartbeat, and fetal movements. They were taught to clinically assess fetal position, abnormal presentations, fundal height, and abnormal observations. The curriculum topics also included postnatal care, conducting safe home deliveries, home-based newborn care practices, recognising danger signs in pregnant women and newborns, and arranging referrals. VHWs were taught how to monitor and document implementation of the intervention, and how to conduct PDG sessions. Training kits included manuals, forms, flip charts, and delivery kits. Regular refresher sessions were held during the monthly meetings, which reviewed any issues raised in the previous PDG meetings.
Sixteen qualified midwives visited intervention villages regularly throughout the trial. Three were based at an NPHC. All midwives underwent a 10-day residential theoretical and practical training conducted by experts in maternal and neonatal health. Every fortnight, teams of 2 midwives (and a field supervisor) visited intervention villages and delivered the FDHS. One midwife delivered the antenatal and postnatal health checks, including pregnancy confirmation; assessment of fetal position; fundal height; gestational age; vaginal examination; abnormal observations from physical examination and investigations such as haemoglobin and urine analysis (sugar and albumin); and postnatal care and newborn care (head to foot examination). The other midwife monitored the delivery of health education by the VHW. Midwives also accompanied women with high-risk pregnancies to the NPHCs, conducted emergency deliveries at the village with the help of the VHWs, and, if needed in cases of complications, were responsible for referring the mother/neonate in distress to the next level of care accompanied by the VHW or the birth attendant.
Four NPHCs were identified to provide services that an expectant mother might require during pregnancy and for the first month of her baby’s life. The NPHCs had approximately 20 beds, with facilities and a qualified doctor for normal deliveries and cesarean sections and a paediatrician to see ‘problem’ newborns. NPHCs were selected based on criteria such as professional competence, existing infrastructure, and public perception of the facility. An initial contract of 3 months was agreed, after which favourable review led to a 6-month renewal. These NPHCs were expected to provide around-the-clock service, admission to the general ward, food for the patient and 1 attendant, and drugs and disposables to women from intervention villages. This contract was subject to a per-patient cost ceiling. A rigorous monitoring system implemented by the intervention team was part of the continuous quality assessment process that buttressed against overbilling and over-provision of NPHC services. Transport was not part of this contract, but 8 CHAMPION trial ambulances were provided by the NICE intervention team directly at the village level to pick up and drop off patients at the NPHCs. Women and their family members were also encouraged to use the emergency transport system provided by the state government [30]. However, this transport system was not operational at the trial start, and only inconsistently thereafter.
All eligible women in the intervention villages were issued a health card (S1 and S2 Figs) that tracked the various services they received under the programme, including immunisations, participation in health groups, regular check-ups, and hospitalisations.
Control villages
CHAMPION control villages were not offered the CHAMPION interventions. However, children between the ages of 6 and 9 years in 107 of the 232 CHAMPION control villages were provided with the Naandi Foundation’s Ensuring Children Learn (ECL) programme as part of a parallel trial (STRIPES [31]) utilising the same randomisation as in CHAMPION, but with CHAMPION controls receiving ECL. A village was eligible for inclusion in STRIPES if it was already participating in the CHAMPION trial, the village had at least 1 public primary school for both boys and girls, the school operated in the 2007–2008 academic year and was likely to continue operations during the following 2 years, and at least 15 children in total were present in classes 2, 3, and 4 in the school at the time of the baseline survey. Children were eligible for inclusion in the analysis of the trial if they were resident in an eligible village, they were recorded in the enumeration conducted in January 2008 as planning to be enrolled in class 2, 3, or 4 at the government school located in their village in the 2008–2009 academic year, and if, after hearing an explanation of the trial, their parent(s) or guardian(s) did not choose to opt out of the trial.
The remaining 125 CHAMPION control villages did not meet the eligibility requirements for STRIPES but were nevertheless offered ECL outside the trial. A key purpose of implementing ECL in the CHAMPION control villages was to give Naandi a presence in the control villages, hence helping to ensure that CHAMPION data collection was carried out to the same standard in intervention and control villages. We anticipated that the primary-school-level ECL educational intervention would not affect neonatal mortality in the control arm.
Outcomes
Primary outcome
The primary outcome for the trial was neonatal mortality defined as death in the first 28 days of life of a live-born baby from a pregnancy (regardless of whether singleton or multiple) with expected delivery date (EDD) on or after the assessment start date.
Secondary outcomes
Secondary outcomes included age at neonatal death, and cause as classified by verbal autopsy (VA) [32,33], and maternal mortality, defined as the death of a woman during pregnancy or within 42 days of the end of pregnancy from any cause related to or aggravated by the pregnancy or its management, among pregnancies with EDD on or after the assessment start date (the cause was classified by VA [32]). Other outcomes included health knowledge (including risk symptoms for neonates), health service usage (pregnancy care, care at delivery, place of delivery, care after delivery), and satisfaction with care. The costs of the interventions per life year saved (LYS) and per neonatal death averted are discussed in the economic evaluation below. Neonatal and maternal morbidity were listed in the protocol as potential outcomes, but, for logistical reasons, these data were not collected. Death in, or in transit to, an NPHC was considered a serious adverse event.
Sample size
Making the simplifying assumption that each village had an average population of 659 [28] with a birth rate of 23 per 1,000 population per year [25], 38 births per village over the trial period were expected. Assuming an intra-cluster correlation coefficient (ICC) of 0.00644 [34] for the primary outcome, 330 villages would give 80% power (5% 2-sided significance) to detect a 25% reduction in neonatal mortality from 4.38% [28] to 3.29%. To account for possible migration (e.g., to their mother’s village around 1 to 3 months before the EDD and/or during the summer to work in bigger towns) and for possible losses to follow-up, the target size was increased to 464 villages.
Trial management
The trial was managed by a TSC and a trial management group (TMG). The TSC met periodically to discuss overall progress. The TMG met monthly to ensure efficient day-to-day running of the trial. The Naandi Foundation was the local trial coordinating centre and was responsible for the research components and data management in the field. The trial database was designed and supported by Effective Intervention. LSHTM was responsible for all statistical aspects. The NICE Foundation was subcontracted to implement the interventions.
Data collection
To reduce bias, the research/data collection team was selected and managed by the Naandi Foundation and was independent of the intervention teams. While the research team could not be masked to the intervention status of a village, they were trained to collect data in an identical manner in the intervention and control villages. No data from the research team were used by the intervention team.
The research team monitored and collected data about pregnant women and their babies in both intervention and control villages. The team included a research coordinator who led the team and was responsible for timely data entry; a research officer who validated the collected data and monitored field staff; 19 data supervisors who monitored enumerators, supervised the data collection process, obtained detailed follow-ups on pregnancy outcomes, and collected information for VAs when necessary; 432 enumerators, to cover the 464 villages, who identified eligible women and tracked pregnancies; and data entry operators who were responsible for data entry and generation of monitoring forms.
In each village, the enumerator conducted a monthly pregnancy check. The data were then transported to Hyderabad, where all data were double-entered into a database, from which forms were printed for the next monthly monitoring and taken to the field. If a woman was identified as pregnant, the database triggered a follow-up form after the EDD. Data supervisors conducted a follow-up interview 6 weeks after delivery. Information about women who could not be located or were known to have migrated beyond the trial area was sought through their relatives in the village. If a live-born baby was suspected to have died aged 28 days or less, or a woman known to be pregnant was suspected to have died during pregnancy or within 42 days of the end of pregnancy, a specially trained data supervisor interviewed the family and completed a VA form to enable independent assessors to assign the primary cause of death. Data about health knowledge, health practices including health service usage, and satisfaction with care were collected by the research team for women with an eligible pregnancy.
Data supervisors visited enumerators monthly and cross-checked a sample of their reports. The supervisors and enumerators met monthly at regional headquarters to deposit monthly survey results, check that forms were properly completed, and discuss any problems in the field. Flags were created in the database to check on inconsistencies or missing data in a timely manner.
Statistical analysis
Primary analysis of outcomes followed the intention to treat principle.
As this trial has a complex hierarchical structure, with multiple women per cluster, potentially multiple pregnancies per woman, and potentially multiple births per pregnancy, we used a generalised estimating equations (GEE) analysis approach [35]. This approach assumes non-independence of all observations from the same cluster, and so also accounts for non-independence of multiple outcomes from the same woman.
For the primary outcome, the relative risk [36] with a 95% confidence interval was obtained from a GEE model with a binary outcome, a log link, and a ‘working’ assumption of independence as recommended by Lee and Nelder [37], with robust standard errors to take account of clustering.
Although the protocol stated that we would not adjust for covariates in the primary analysis, in the final statistical analysis plan we amended this so that the model included the stratifying variables (travel time to nearest NPHC and tribal status of village). Secondary exploratory analyses extended the relative risk model by adding an interaction between treatment arm and timing of enrolment (pre - versus post-randomisation) to assess the possibility of bias in the primary outcome arising due to differential post-randomisation enrolment in the 2 arms. At the request of a reviewer, an additional stratified analysis by whether or not control villages were in the STRIPES trial was added.
The risk difference (and hence the estimated number of lives saved and the number needed to treat) was estimated using the same model (without an interaction), but with an identity rather than a log link. Although given as an option in the protocol, survival analysis was not used because the period when neonatal death was possible was very short and the precise date of death was not always known.
For secondary binary outcomes, relative risks were estimated using the same approach as for the primary outcome. Other outcomes are reported descriptively but without statistical testing due to the large number of comparisons.
Interim analyses were pre-specified and provided confidentially by the trial statisticians to the independent data monitoring committee (DMC), which was guided by the Haybittle–Peto rule [38].
Economic evaluation
A cost-effectiveness calculation in terms of cost per neonatal death averted and cost per LYS was conducted. (Cost per disability-adjusted life year saved could not be considered as no measure of future disability was available.) The sensitivity of these outcomes to the most important inputs—labour costs, fuel costs, and exchange rate movements—was examined.
The direct additional provider costs of the CHAMPION intervention activities compared to existing standard of care in the control arm was measured. Total spending was cross-checked with funding sources for accuracy. Spending was divided into running costs and capital costs. There were very limited start-up costs, which were assumed to be fully depreciated during the trial, because the NICE Foundation had previously implemented a pilot version of the programme elsewhere in India. Straight line depreciation of capital equipment (computers, ambulances, refurbishment costs for public health centres, and medical equipment for these clinics based on 3-, 4-, 6-, and 8-year lifespans, respectively) was allowed for, consistent with usual account practices. Capital spending outside these items was depreciated immediately. There were no contributions in kind. Office facility costs, which were shared with other projects managed by the Naandi Foundation, were allocated to the project according to estimated share of usage (30%).
Annual cost figures were adjusted by India’s GDP deflator in order to convert values to May 2011 rupees. Average exchange rates from May 2011 were used to convert rupee figures to US dollars.
Results
Enumeration was carried out between August and November 2007, with randomisation on 18 February 2008. After an inception period, the intervention was initiated from 1 August 2008. The assessment start date (see Methods) was 1 December 2008. The DMC met by teleconference to review interim analyses on 2 occasions but did not recommend early stopping. The intervention ended on 31 May 2011. Data collection was completed on 30 November 2011.
In the 464 villages, 20,282 enumerated women met the eligibility criteria (9,871 in control and 10,411 in intervention villages, a difference attributable to chance). Non-eligibility was mainly due to sterilisation. A further 9,387 newly married women were enrolled in the trial villages—more in intervention villages (4,266 control, 5,121 intervention). The total number of enrolled eligible women was thus 14,137 control and 15,532 intervention (Fig 1; S1–S4 Datas).
Fig. 1. Flowchart: Clusters, women, and children at trial entry and in final analysis. 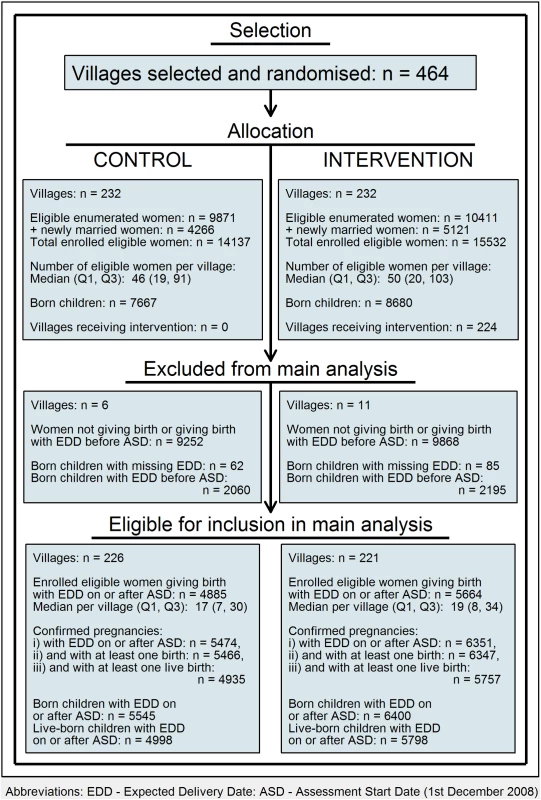
Based on all 464 villages, the number of births per village varied between 0 and 141 (median = 28, interquartile range [IQR] Q1 = 12, Q3 = 49); 17 villages (6 control, 11 intervention) had no live births during the assessment period (3 had stillbirths but no live births, and the other 14 had either only miscarriages or abortions or had no confirmed pregnancies [where a confirmed pregnancy was defined as either a live birth or a death where the pregnancy was confirmed by verbal autopsy]). Based on the 447 villages with at least 1 live birth, the number of live births per village varied between 1 and 92 (median = 21, IQR Q1 = 9, Q3 = 37) in the intervention villages and between 1 and 98 (median = 18, IQR Q1 = 7, Q3 = 31) in the control villages. In all, 4,885 (34.6%) of the control village women had 5,474 confirmed pregnancies with an EDD of 1 December 2008 or later, and gave birth to 4,998 eligible children. The corresponding numbers in the intervention villages were 5,664 (36.5%) women, 6,351 pregnancies, and 5,798 children.
Comparability of control and intervention villages is shown in Table 1 (see also S1 and S5–S7 Datas). None of the control villages and 224 of the 232 intervention villages received at least some of the intervention. There were no or very few women residing permanently in the 8 remaining villages due to seasonal migration specific to the tribal communities.
Tab. 1. Comparability between trial arms. 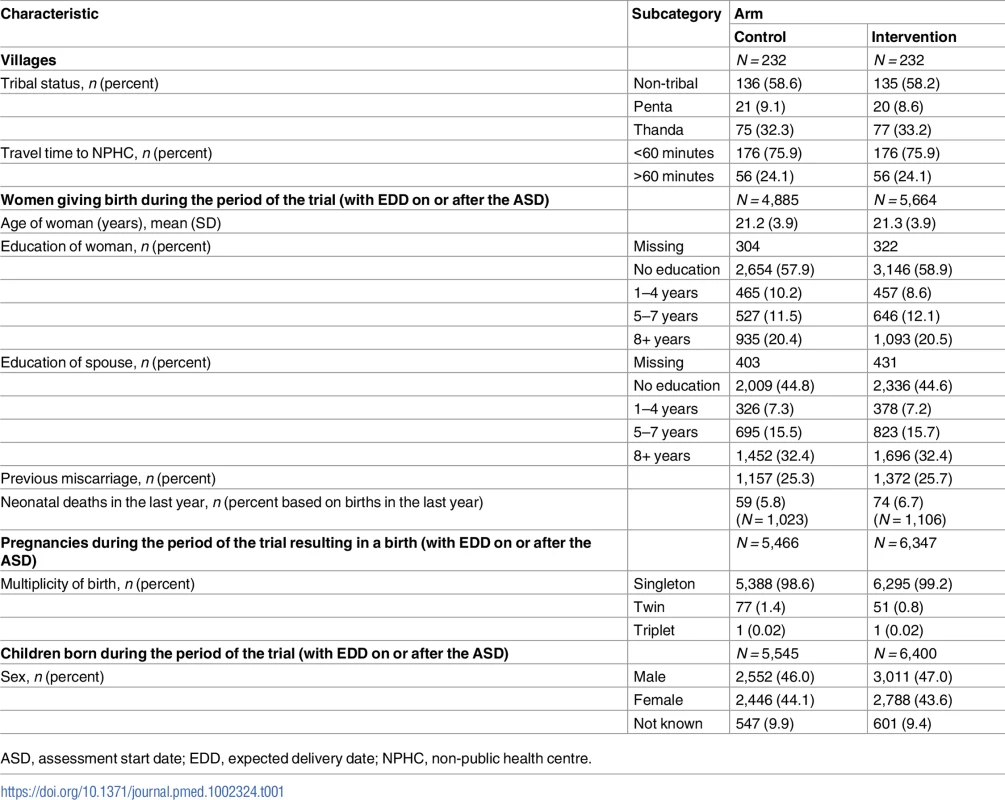
ASD, assessment start date; EDD, expected delivery date; NPHC, non-public health centre. Between 1 August 2008 and 31 May 2011, 9,939 PDGs were held in the intervention villages, at least monthly, and 12,195 FDHSs were conducted during the trial. The total number of pregnant women who attended the FDHSs was 4,492, against a potential number of 5,022.
Of the 4 NPHCs, 2 were managed by local private doctors, and 1 by midwives hired by the intervention team (with paediatric support available); the contract with the fourth was terminated due to quality concerns after supervisory visits, and the team referred cases to another NPHC. Over this period, there were 913 referrals (526 maternal and 387 neonatal—290 from a village to an NPHC or government facility, 429 from an NPHC to a tertiary care facility, and 194 from one NPHC to another or from a government facility to an NPHC or to a tertiary care centre); 24% of deliveries in the intervention arm occurred at one of the NPHCs, 21% at home, 29% at government-related facilities, and 28% at private facilities. In the control villages, 21% of deliveries were at home, 30% at government-related facilities, and 47% at private facilities.
Of the 11,945 children with an EDD of 1 December 2008 or later, 1,149 (602 in the intervention arm, 547 in the control arm) were stillborn or died earlier in the pregnancy. Of the live-born babies, 343 out of 4,998 (6.9%) in the control arm died in their first 28 days of life, and 303 out of 5,798 (5.2%) in the intervention arm (risk ratio 0.76, 95% CI 0.64 to 0.90, p = 0.0018; Table 2; S1, S9 and S10 Datas; risk difference −1.59%, 95% CI −2.63% to −0.54%), suggesting 92 fewer deaths (95% CI 31 to 152) as a result of the intervention. The ‘number needed to treat’ to prevent 1 neonatal death was 63 (95% CI 38 to 185). The babies in the control arm were more likely to die in the first week of life, but there were no clear differences between trial arms in terms of sex or cause of death.
Tab. 2. Neonatal mortality for births with expected delivery date after the assessment start date. 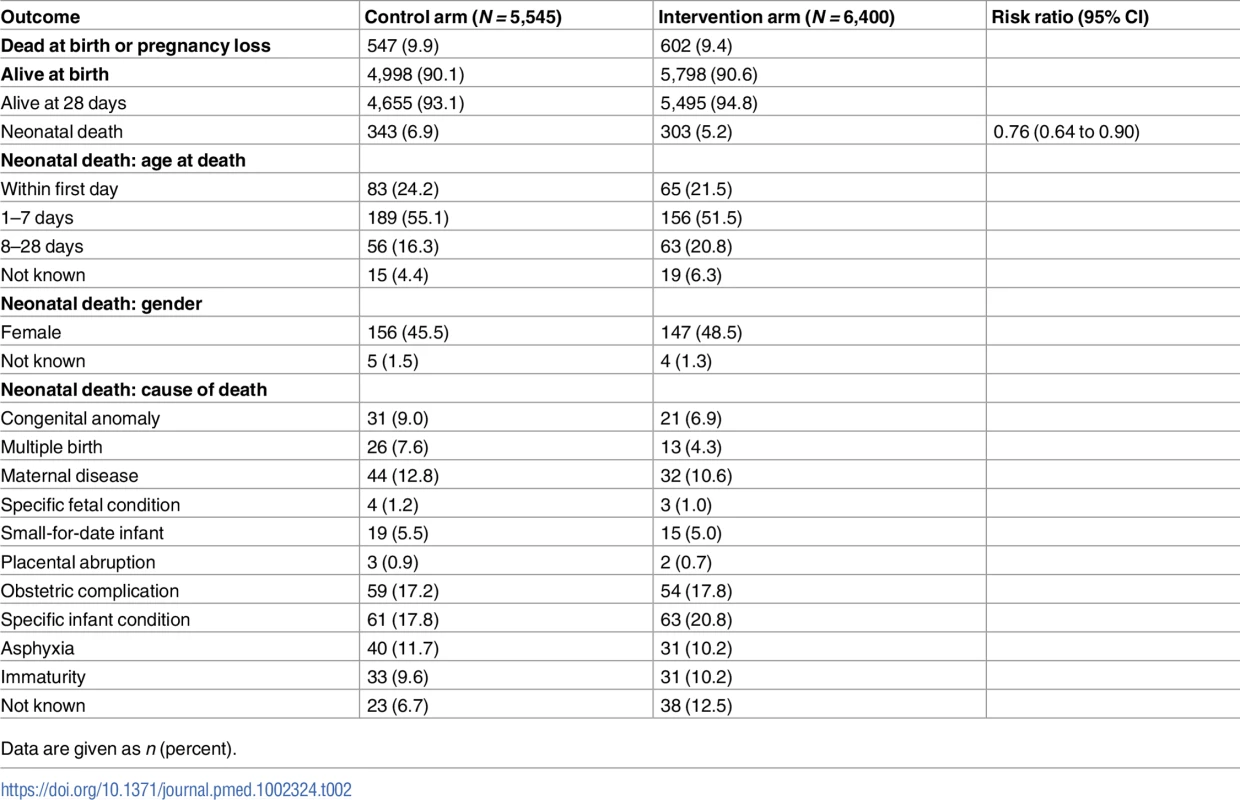
Data are given as n (percent). In exploratory analyses the effect of the intervention on neonatal mortality did not differ significantly by travel time to the nearest NPHC (interaction test p = 0.6516) or by tribal status of village (interaction test p = 0.3538). Nor was there a significant interaction by whether the village participated in the STRIPES education trial (interaction p = 0.2876) or according to timing of enrolment (investigated to assess the possibility of bias in the primary outcome due to differential post-randomisation enrolment between the 2 arms; pre-randomisation versus newly married post-randomisation, p = 0.1552). Table 3 shows the results in these strata (see also S1 and S11 Datas).
Tab. 3. Neonatal mortality according to characteristics of villages/women for births with expected delivery date after the assessment start date. 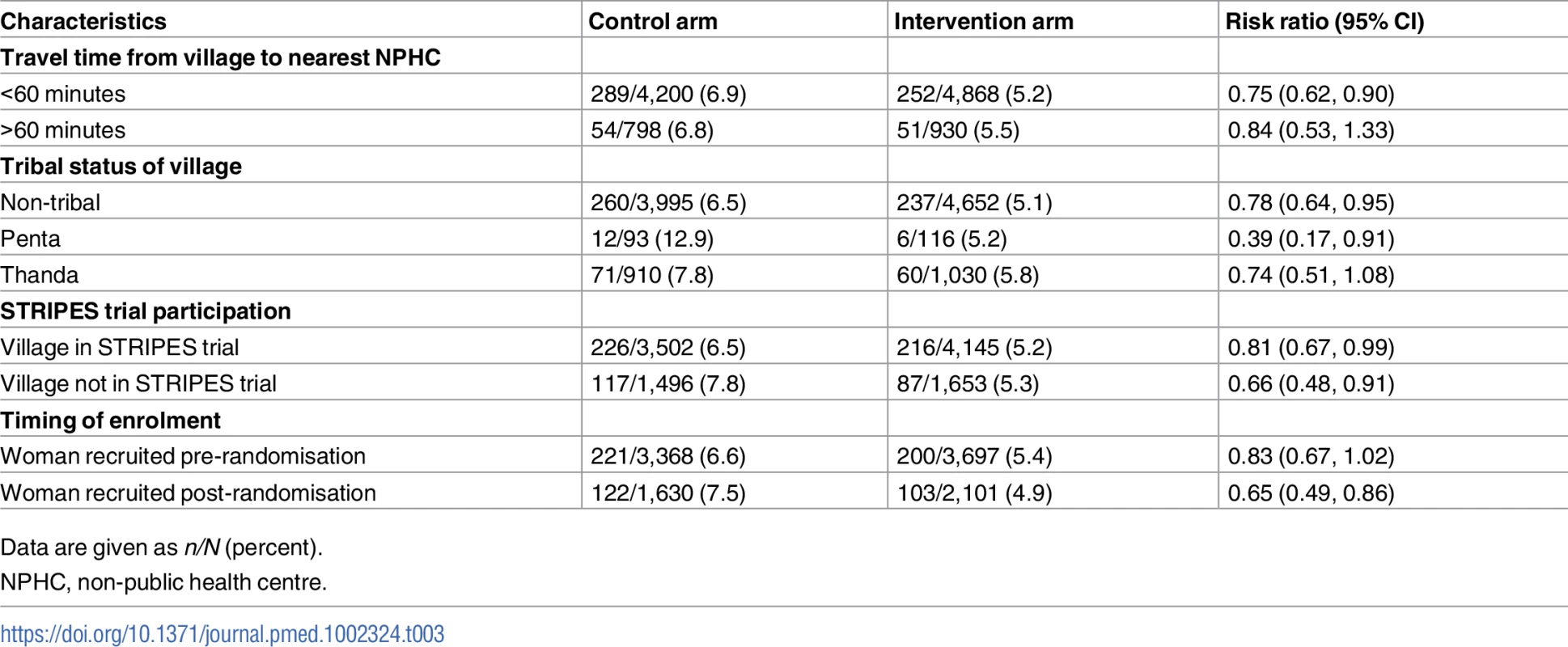
Data are given as n/N (percent). The estimated design effect for neonatal mortality was 1.306. There are complexities with defining an ICC for neonatal mortality in this study. The ICC has a simple definition only for a continuous outcome with a 2-level hierarchy. Here there is a 4-level hierarchy (potentially multiple children per pregnancy, multiple pregnancies per woman, and multiple women per village), and the outcome is binary (even for a 2-level hierarchy there are a number of different definitions of the ICC for a binary outcome [39], which can differ quite markedly). Ignoring these difficulties and estimating the ICC from the design effect and the mean number of live births per village (25.74) via the usual formula gives an estimated ICC of 0.012.
Table 4 shows maternal mortality based on pregnancies with an EDD of 1 December 2008 or later (see also S1 and S12 Datas). There were 9 (0.16%) deaths in the control arm compared to 13 (0.20%) in the intervention arm (risk ratio 1.24, 95% CI 0.53 to 2.90, p = 0.6176). In both arms, the main causes were haemorrhage and hypertensive disorders, although there were also cases of sepsis, obstructed labour, and other pregnancy-related conditions in the intervention arm.
Tab. 4. Maternal mortality for pregnancies with expected delivery date after the assessment start date. 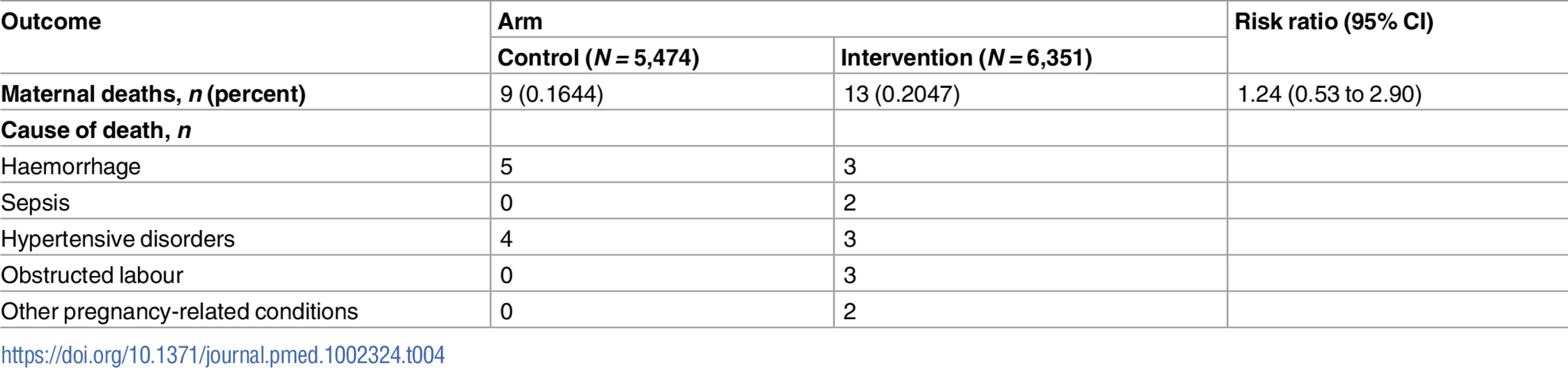
Harms potentially related to the CHAMPION intervention
One serious adverse event was reported in October 2008. Following a trial of labour at an NPHC, a woman died after transfer in a CHAMPION trial ambulance to a private hospital on her family’s wishes.
Health knowledge, health practices including health service usage, and satisfaction with care
Based on women with pregnancies that resulted in a live birth with EDD on or after the assessment start date, a higher proportion of women in the intervention arm than in the control arm gave correct answers on 20 out of 23 health knowledge items when asked about risk symptoms for neonatal problems (Table 5; S1 and S13 Datas).
Tab. 5. Mothers’ knowledge of risk symptoms for neonates (based on pregnancies that resulted in a live birth with estimated delivery date on or after the assessment start date). 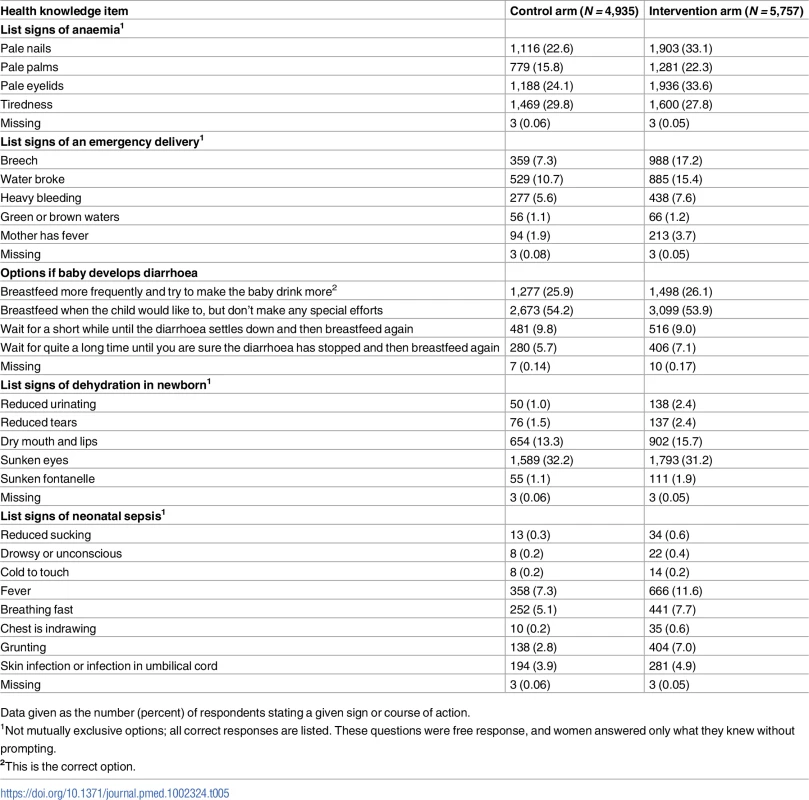
Data given as the number (percent) of respondents stating a given sign or course of action. Women with pregnancies with EDD on or after the assessment start date were asked about antenatal, delivery, and postnatal care experiences (although it was usually felt insensitive to ask these question of women whose deliveries did not lead to a live birth). Women in the intervention arm were more likely to have attended more antenatal care, with more testing and advice; have trained personnel at delivery; deliver in an institution; have antiseptic dressing for the umbilical cord; have their baby wiped and weighed; initiate breastfeeding soon after birth; and have trained personnel for postnatal checks (Table 6; S1 and S14 Datas). Women in the intervention arm were more likely to rate their delivery and postnatal care as good or very good (Table 7; S1 and S15 Datas).
Tab. 6. Antenatal, delivery, and postnatal care experience of women with pregnancies with estimated delivery date on or after the assessment start date. 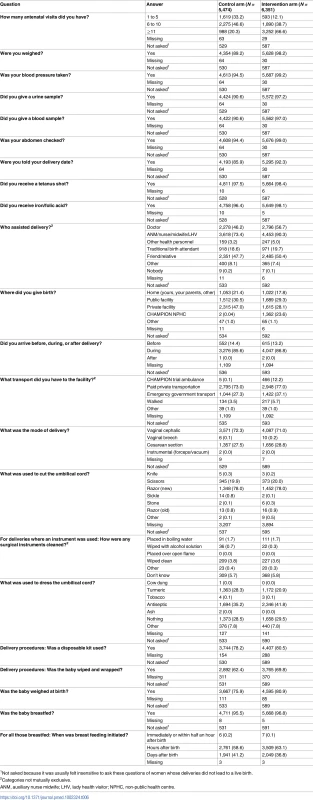
1Not asked because it was usually felt insensitive to ask these questions of women whose deliveries did not lead to a live birth. Tab. 7. Satisfaction with care (based on pregnancies with estimated delivery date on or after the assessment start date). 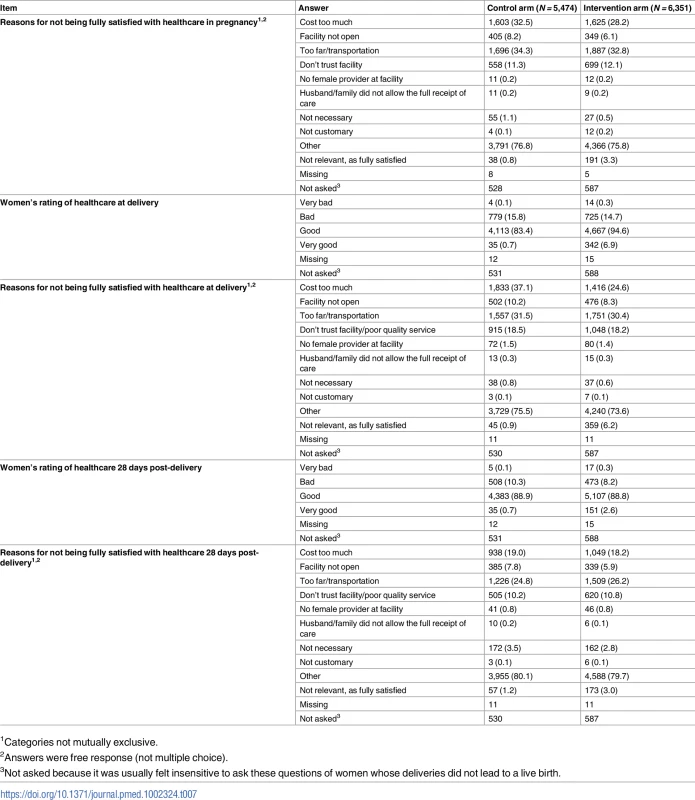
1Categories not mutually exclusive. Economic evaluation
The total cost of the CHAMPION interventions (US$1,084,955) was divided into spending on the clinical programme (63.4%—mainly financing mobile clinics, midwives, and emergency services, with around a quarter for the NPHCs), spending on the community mobilisation programme (mainly via PDGs; 20.2%), and administration costs (16.4%). Wages were 60% of total costs (Table 8). The total costs translate into a cost of US$11,769 per life saved (95% CI $7,115 to $34,653).
Tab. 8. Costs by category (calculated in May 2011 US dollars). 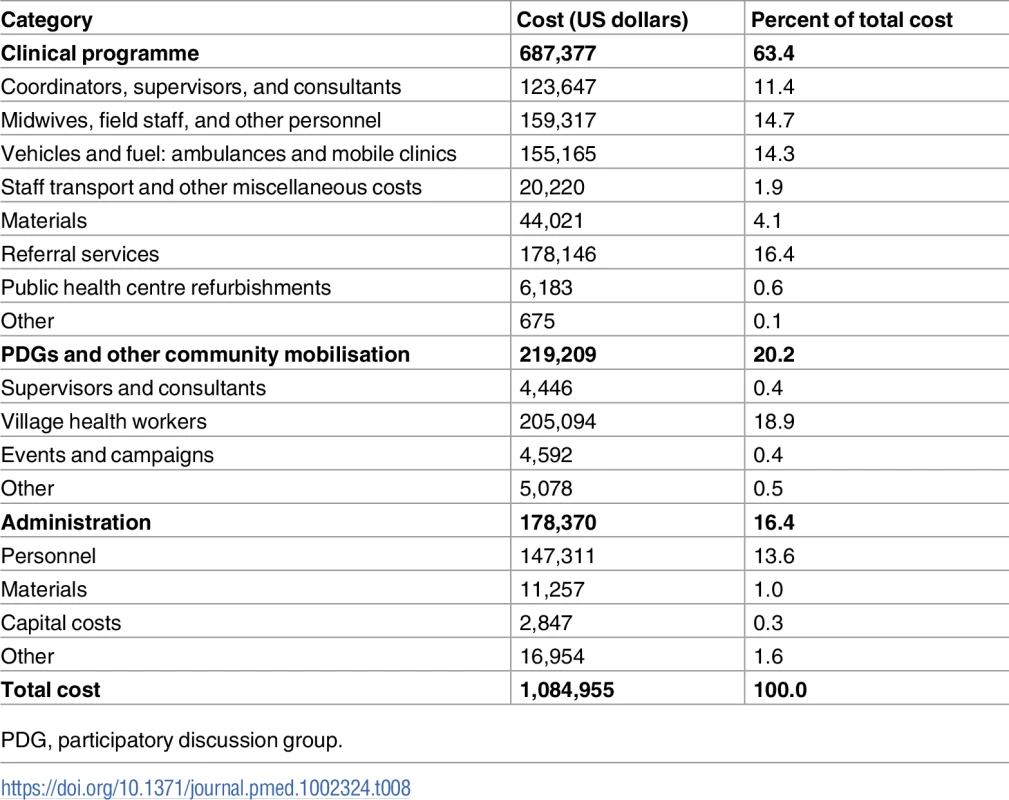
PDG, participatory discussion group. Though there were substantial distances between intervention villages due to the trial design, fuel costs represented only 9% of total costs. Since all costs were local, exchange rate movements have a one-to-one impact on cost-effectiveness measures when converted to dollars.
Life expectancy at birth for those living in rural AP was reported as 68.3 years [28] and, discounted at 3%, implies 28.8 LYS per child saved, giving a cost of US$409 (95% CI $247 to $1,203) per LYS (Table 9).
Tab. 9. Indicators of cost-effectiveness. 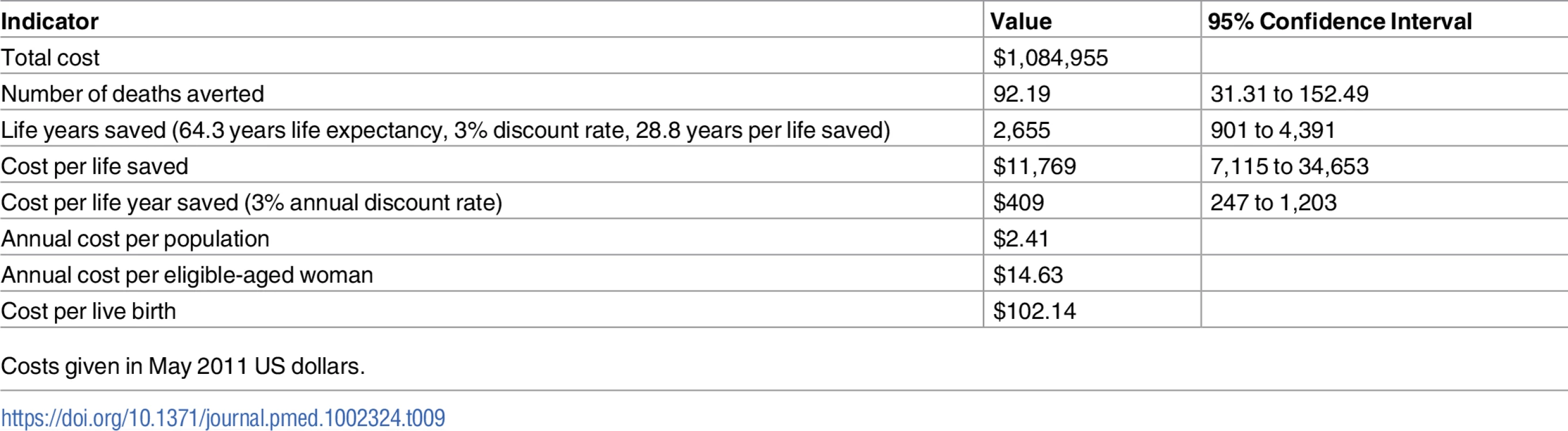
Costs given in May 2011 US dollars. Discussion
The CHAMPION trial showed that a package of interventions addressing health knowledge and health seeking behaviour, buttressing existing health services, and contracting out important areas of maternal and child healthcare led to almost the hypothesised 25% reduction in neonatal mortality in small villages in an Indian state with high mortality rates. This was in spite of large secular reductions (over 30%) in neonatal mortality in rural India over recent decades [40] (although the rate was still higher than assumed in the initial power calculations). Possible mechanisms for the excess reduction in the intervention arm may relate to the fact that women became more knowledgeable about risk symptoms for neonatal problems; had drawn up a specific birth plan; were proactively monitored, with appropriate referral and other actions; had the support of trained personnel available at delivery; had standardised care at the NPHCs; had antiseptic care for the umbilical cord; and initiated breastfeeding sooner than those in the control arm, However, the intervention was a ‘package’, and, while we could postulate particular causes for the large reduction, it is not possible to completely disentangle these in post hoc analyses. The benefits of the CHAMPION interventions were conservatively assumed to be limited to the 30 months for which statistical analysis of mortality was conducted. However, some of the intended benefits of this project are in terms of behaviour change and knowledge, which may have longer-term effects.
There were no clear effects of the intervention on maternal mortality, but the number of deaths meeting the WHO definition of deaths due to maternal causes over the period of the trial was low, and the confidence interval was wide. The rates were compatible with recent figures from AP [27].
The strengths of the study include its cluster RCT design, its size, and its focus on contracting out high-quality services. For instance, a team of doctors from the intervention team reviewed the quality of the care provided by NPHCs using stringent criteria before funds were released.
The potential for bias was reduced by separation of the research and intervention teams. They were housed in separate offices, did not train together or work together, were explicitly urged against communicating except when absolutely necessary, and had entirely separate reporting structures.
Villages in the control arm also received an active intervention (for primary-school-age children [31]) to reduce potential disappointment and increase the likelihood of receiving equally good quality data in both trial arms.
The 3-level consent process was agreed to by the relevant research ethics committees in 2007, and would also meet recent guidelines [41] for cluster RCTs.
A limitation of the study is that it could not be masked post-randomisation. It seems unlikely, however, that this led to ascertainment bias in the mortality outcomes given that these are ‘hard’ endpoints, and because of the training given to the data collectors and the standardised approaches used in the VAs. Also, if there was a bias in favour of the intervention villages, this might be expected to apply to both mortality outcomes, not only neonatal mortality.
A second limitation is that fetal losses were not divided into stillbirths and miscarriages based on gestational age, both because gestational age was not reliably reported and because of potential biases, as women in the intervention arm may have reported pregnancies earlier than control women. However, rigorous training was conducted to help data collectors to distinguish between neonatal and fetal losses.
Proportionately more women were recruited in the trial post-randomisation in the intervention arm, which might reflect women wishing to access the intervention package. While it seems implausible that women would marry into a village purely for that reason, the implications were nevertheless explored by an analysis of the interaction of arm with timing of enrolment, which was found to be significant.
It is possible that the effects of the intervention could have been diluted by other changes during the trial period, as the government introduced other maternal and child health programmes, including incentives to encourage institutional deliveries and care. Facility-based newborn care increased with the introduction of 2 government initiatives: the Janani Suraksha Yojna (JSY), a cash transfer incentive scheme for promoting institutional deliveries introduced in 2005, and the Janani Shishu Suraksha Karyakram, a free maternity and newborn programme in all government healthcare institutions introduced in 2011 under the National Rural Health Mission [42]. One of the key elements of the National Rural Health Mission is the involvement of the ASHAs. The CHAMPION trial cooperated with ASHAs in our villages when possible, but their presence and authority on maternal and child health was not fully established in most of our villages until well after the end of the trial. During the trial and afterwards, due partly to lack of manpower, blood storage facilities, and referral linkages, the public sector was often not in a position to provide emergency obstetric care services [43–47]; major problems were transportation distances and difficult terrains. The services offered under the CHAMPION trial improved access to maternal and newborn healthcare services that were not easily available to the marginalised populations living in difficult-to-reach villages.
As the aim of the CHAMPION trial interventions was not to run a parallel system, there was constant dialogue between personnel from the public health system and the trial intervention team to ensure that care of pregnant women and their babies did not fall between the gaps. Women delivering in private health facilities did not normally come under the ambit of the JSY scheme. However, those registered in the CHAMPION intervention villages could choose either the nearest government health facility or the NPHCs; many women with high-risk pregnancies opted for the NPHC, and an agreement was reached with government officials that women who gave birth at the NPHCs would be eligible to receive the JSY. Although not asked specifically about JSY, 85% of respondents in both the intervention and control arms said they expected to receive a subsidy.
The CHAMPION interventions may have reduced the demands on public health services, although the trial did not collect adequate usage and cost data to estimate the scale of this. Compared to similar interventions in India [48,49], the CHAMPION trial focused more on outreach services in the community, encouraging the majority of women to continue using existing care.
Recent reviews [50,51] seem to support the conclusions from the CHAMPION trial, in particular ‘the value of integrating maternal and newborn care in community settings through a range of interventions, which can be packaged effectively for delivery through a range of community health workers and health promotion groups’ [50].
In terms of the economic evaluation, investigators in a systematic review of cluster RCTs of women’s group interventions in India and Africa [52] found that the incremental cost per life saved ranged from $2,770 to $22,971 (2011 US dollars). In several cases, their costs were lower than the costs reported here; however, these other trials did not provide additional clinical services nor train VHWs. The CHAMPION trial was not able to determine the relative importance of the clinical services compared to the discussion groups offered. It is possible that there may be returns to scale that could further reduce the CHAMPION intervention costs, but total costs are most sensitive to wages (60% of total costs), and a scale-up of this project would not save on personnel and would not therefore lower costs significantly.
A cost-effectiveness ratio per LYS of double the local income per capita can be considered a threshold for public support [53]. This equates to US$3,180 in India (income per capita in India being US$1,590 in 2011). The cost per LYS of the CHAMPION intervention is approximately one-tenth of this threshold, suggesting that the intervention can be strongly justified on economic grounds. Given the successful results of the CHAMPION and STRIPES trials, we are continuing the interventions for a further 5 years, and scaling up the intervention to include all control villages.
In terms of generalisability, it is likely that the CHAMPION intervention can be strongly justified in much of rural India with disadvantaged populations (but with the work of the CHAMPION trial VHWs now being done by the ASHAs), and is of potential use in rural settings in other countries with similar socioeconomic and cultural patterns, similar neonatal mortality profiles, and the existence of some private health provision. Ongoing changes in maternal and child health programmes, both during the period of the trial and subsequently, make it imperative that a similar intervention that establishes ties between the community and health facilities is tested in different settings. We are in the process of designing a similar trial in a different region of India.
Supporting Information
Zdroje
1. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1 22579125
2. Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361 : 2226–34. doi: 10.1016/S0140-6736(03)13779-8 12842379
3. World Health Organization. Newborns: reducing mortality. Available from: http://www.who.int/mediacentre/factsheets/fs333/en/index.html. Accessed 2017 Oct 25.
4. Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–94. doi: 10.1016/S0140-6736(12)61719-X 23245603
5. Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, et al. Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. Lancet. 2010; 375(9730):2032–44. doi: 10.1016/S0140-6736(10)60678-2 20569843
6. Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 Suppl):519–617.
7. Ministry of Health and Family Welfare. Andhra Pradesh: National Family Health Survey (NFHS-3)—2005–06. Available from: http://rchiips.org/NFHS/NFHS-3%20Data/ap_state_report_printed_version_for_website.pdf. Accessed 2017 May 23.
8. Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5 15752534
9. Million Death Study Collaborators, Bassani DG, Kumar R, Awasthi S, Morris SK, Paul VK, et al. Causes of neonatal and child mortality in India: a nationally representative mortality survey. Lancet. 2010;376(9755):1853–60. doi: 10.1016/S0140-6736(10)61461-4 21075444
10. Chaudhury N, Hammer J, Kremer M, Muralidharan K, Rogers FH. Missing in action: teacher and health worker absence in developing countries. J Econ Perspect. 2006;20(1):91–116. 17162836
11. Bang AT, Bang RA, Baitule SB Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354(9194):1955–61. doi: 10.1016/S0140-6736(99)03046-9 10622298
12. Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet. 2008;372(9644):1151–62. doi: 10.1016/S0140-6736(08)61483-X 18926277
13. Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women’s groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet. 2010;375(9721):1182–92. doi: 10.1016/S0140-6736(09)62042-0 20207411
14. Bhandari N, Mazumder S, Taneja S, Sommerfelt H, Strand TA. Effect of implementation of Integrated Management of Neonatal and Childhood Illness (IMNCI) programme on neonatal and infant mortality: cluster randomised controlled trial. BMJ. 2012;344:e1634. doi: 10.1136/bmj.e1634 22438367
15. Loevinsohn B, Harding A. Buying results? Contracting for health service delivery in developing countries. Lancet. 2005;366(9486):676–81. doi: 10.1016/S0140-6736(05)67140-1 16112305
16. Lagarde M, Palmer N. The impact of contracting out on health outcomes and use of health services in low and middle income countries. Cochrane Database Syst Rev. 2009;7(4):CD008133.
17. Bellows NM, Bellows B, Warren C. Systematic review: the use of vouchers for reproductive health services in developing countries: systematic review. Trop Med Int Health. 2011;16(1):84–96. doi: 10.1111/j.1365-3156.2010.02667.x 21044235
18. Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365(9463):977–88. doi: 10.1016/S0140-6736(05)71088-6 15767001
19. Haws RA, Thomas AL, Bhutta ZA, Darmstadt GL. Impact of packaged interventions on neonatal health: a review of the evidence. Health Policy Plan. 2007;22(4):193–215. doi: 10.1093/heapol/czm009 17526641
20. Rani TU, Jampana VR, Machiraju VM (2014) Newborn survival in India—challenges and priorities for Andhra Pradesh. J Neonatal Biol 3 : 150.
21. International Institute for Population Sciences. District Level Household and Facility Survey: 2007–08: India. Andhra Pradesh: Mumbai: International Institute for Population Sciences; 2010. Available from: http://rchiips.org/pdf/INDIA_REPORT_DLHS-3.pdf. Accessed 2017 Jun 13.
22. National Rural Health Mission. Five years of NRHM. New Delhi: Ministry of Health and Family Welfare; 2009.
23. Indian Office of the Registrar General. Sample Registration System statistical report 2011. New Delhi: Indian Office of the Registrar General; 2013. Available from: http://www.censusindia.gov.in/vital_statistics/SRS_Report/1Contents%202011.pdf. Accessed 2017 Jun 13.
24. Ministry of Rural Development. National Rural Employment Guarantee Act 2005 (NREGA): report of the second year—April 2006–March 2007. New Delhi: Ministry of Rural Development; 2007. Available from: http://nrega.nic.in/NREGArpt_eng.pdf. Accessed 2017 Jun 9.
25. Mahabubnagar district. District statistics: Mahabubnagar district profile/statistics. Available from: http://mahabubnagar.nic.in/Resources/DistStat.doc. Accessed 2012 Oct 29.
26. Sahu D, Nair S, Singh L, Gulati BK, Pandey A. Levels, trends & predictors of infant & child mortality among Scheduled Tribes in rural India. Indian J Med Res. 2015;141 : 709–19. doi: 10.4103/0971-5916.159593 26139791
27. Census 2011. Nagarkurnool population census 2011. Available from: http://www.census2011.co.in/data/town/575744-nagarkurnool-andhra-pradesh.html/. Accessed 2017 Jun 13.
28. International Institute for Population Sciences, ORC Macro. National Family Health Survey (NFHS-2) 1998–99: India. Mumbai: International Institute for Population Sciences; 2000.
29. Boone P, Mann V, Eble A, Mendiratta T, Mukherjee R, Figueiredo R, et al. Community health and medical provision: impact on neonates (the CHAMPION trial). BMC Pediatr. 2007;7 : 26. doi: 10.1186/1471-2431-7-26 17625023
30. Prakassama M. Maternal mortality reduction programme in Andhra Pradesh. J Health Popul Nutr. 2009;27 : 220–34. 19489417
31. Lakshminarayana R, Eble A, Bhakta P, Frost C, Boon P, Elbourne D, et al. The Support to Rural India’s Public Education System (STRIPES) trial: a cluster randomised controlled trial of supplementary teaching, learning material and material support. PLoS ONE. 2013;8(7):e65775. doi: 10.1371/journal.pone.0065775 23874383
32. Baiden F, Bawah A, Biai S, Binka F, Boerma T, Byass P, et al. Setting international standards for verbal autopsy. Bull World Health Organ. 2007;85(8):570–1. doi: 10.2471/BLT.07.043745 17768508
33. Winbo IG, Serenius FH, Dahlquist GG, Kallenc BA. NICE, a new cause of death classification for stillbirths and neonatal and Intrauterine deaths. Int J Epidemiol. 1998;27(3):499–504. 9698143
34. Manandhar DS, Osrin D, Shrestha N, Mesko J, Morrison K, Tumbahangphe K, et al. Effect of a participatory intervention with women’s groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet. 2004;364(9438):970–9. doi: 10.1016/S0140-6736(04)17021-9 15364188
35. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73(1):13–22.
36. Sackett DL, Deeks JJ, Altman DG. Down with odds ratios! Evid Based Med. 1996;1(6):164–6.
37. Lee Y, Nelder JA. Conditional and marginal models: another view. Stat Sci. 2004;19(2):219–38.
38. Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34(6):585–612. 795448
39. Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33(5):869–80. doi: 10.1016/j.cct.2012.05.004 22627076
40. Oestergaard MZ, Inoue M, Yoshida S, Mahanani WR, Gore FM, Cousens S, et al. Neonatal mortality levels for 193 countries in 2009 with trends since 1990: a systematic analysis of progress, projections, and priorities. PLoS Med. 2011;8(8):e1001080. doi: 10.1371/journal.pmed.1001080 21918640
41. Weijer C, Grimshaw JM, Eccles MP, McRae AD, White A, Brehaut JC, et al. The Ottawa statement on the ethical design and conduct of cluster randomized trials. PLoS Med. 2012;9(11):e1001346. doi: 10.1371/journal.pmed.1001346 23185138
42. Ramji S, Modi M, Gupta N. 50 years of neonatology in India: progress and future. Indian Pediatr. 2013;50(1):104–6. 23396782
43. Lim SS, Dandona L, Hoisington JA, James SL, Hogan MC, Gakidou E. India’s Janani Suraksha Yojana, a conditional cash transfer programme to increase births in health facilities: an impact evaluation. Lancet. 2010;375(9730):2009–23. doi: 10.1016/S0140-6736(10)60744-1 20569841
44. Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet. 2011;377(9764):505–15. doi: 10.1016/S0140-6736(10)61894-6 21227492
45. Kumar S, Dansereau EA, Murray CJL. Does distance matter for institutional delivery in rural India? Appl Econ. 2014;46(33):4091–103.
46. Rammohan A, Iqbal K, Awofeso N. Reducing neonatal mortality in India: critical role of access to emergency obstetric care. PLoS ONE. 2013;8(3):e57244. doi: 10.1371/journal.pone.0057244 23544038
47. Randive B, San Sebastian M, De Costa A, Lindholm L. Inequalities in institutional delivery uptake and maternal mortality reduction in the context of cash incentive program, Janani Suraksha Yojana: results from nine states in India. Soc Sci Med. 2014;123 : 1–6. doi: 10.1016/j.socscimed.2014.10.042 25462599
48. Raman AV, Björkman JW. Public/private partnership in the provision of health care services to the poor in India. In: Dinello N, Shaoguang W, editors. China, India and beyond—development drivers and limitations. Cheltonham: Edward Elger Publishing; 2009. pp. 143–172.
49. Bhat R, Maheshwari S, Saha S. Contracting-out of reproductive and child health (RCH) services through mother NGO scheme in India: experiences and implications. Vastrapur: Indian Institute of Management Ahmedabad; 2007.
50. Lassi ZS, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database Syst Rev. 2015 : 3:CD007754.
51. Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam BA, Paul VK, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384(9940):347–70. doi: 10.1016/S0140-6736(14)60792-3 24853604
52. Prost A, Colbourn T, Seward N, Azad K, Coomarasamy A, Copas A, et al. Women’s groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet. 2013;381(9879):1736–46. doi: 10.1016/S0140-6736(13)60685-6 23683640
53. Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16 : 1–31. 10167341
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Time for trauma immunology
- Translational approaches to coagulopathy after trauma: Towards targeted treatment
- The new survivors and a new era for trauma research
- Research questions in pre-hospital trauma care
- Major scientific lessons learned in the trauma field over the last two decades
- The science of rapid start—From the when to the how of antiretroviral initiation
- Reducing undiagnosed HIV infection among adolescents in sub-Saharan Africa: Provider-initiated and opt-out testing are not enough
- Community and health system intervention to reduce disrespect and abuse during childbirth in Tanga Region, Tanzania: A comparative before-and-after study
- Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study
- Trauma care: Finding a better way
- Risk of surgical site infection, acute kidney injury, and infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
- Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries
- Antimicrobial resistance in : Global surveillance and a call for international collaborative action
- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Cellular therapies in trauma and critical care medicine: Looking towards the future
- Leveraging peer-based support to facilitate HIV care in Kenya
- Trends in traumatic brain injury mortality in China, 2006–2013: A population-based longitudinal study
- Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study
- Community health promotion and medical provision for neonatal health—CHAMPION cluster randomised trial in Nagarkurnool district, Telangana (formerly Andhra Pradesh), India
- A comparison of Selective Aortic Arch Perfusion and Resuscitative Endovascular Balloon Occlusion of the Aorta for the management of hemorrhage-induced traumatic cardiac arrest: A translational model in large swine
- Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries
- Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study
- Cancer trials in sub-Saharan Africa: Aligning research and care
- Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study
- Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study
- Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial
- Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: A cross-sectional survey
- Timing of femoral shaft fracture fixation following major trauma: A retrospective cohort study of United States trauma centers
- IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model
- Long-term health status and trajectories of seriously injured patients: A population-based longitudinal study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



