-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Multifaceted Intervention to Implement Guidelines and Improve Admission Paediatric Care in Kenyan District Hospitals: A Cluster Randomised Trial
Background:
In developing countries referral of severely ill children from primary care
to district hospitals is common, but hospital care is often of poor quality.
However, strategies to change multiple paediatric care practices in ruralhospitals have rarely been evaluated.
Methods and Findings:
This cluster randomized trial was conducted in eight rural Kenyan district
hospitals, four of which were randomly assigned to a full intervention aimed
at improving quality of clinical care (evidence-based guidelines, training,
job aides, local facilitation, supervision, and face-to-face feedback;
n = 4) and the remaining four to
control intervention (guidelines, didactic training, job aides, and written
feedback; n = 4). Prespecified
structure, process, and outcome indicators were measured at baseline and
during three and five 6-monthly surveys in control and intervention
hospitals, respectively. Primary outcomes were process of care measures,
assessed at 18 months postbaseline.
In both groups performance improved from baseline. Completion of admission
assessment tasks was higher in intervention sites at 18 months
(mean = 0.94 versus 0.65, adjusted difference 0.54
[95% confidence interval 0.05–0.29]). Uptake of
guideline recommended therapeutic practices was also higher withinintervention hospitals:
adoption of once daily gentamicin (89.2%
versus 74.4%; 17.1%
[8.04%–26.1%]); loading dose quinine
(91.9% versus 66.7%, 26.3% [−3.66% to
56.3%]); and adequate prescriptions of intravenous fluids for
severe dehydration (67.2% versus 40.6%; 29.9%
[10.9%–48.9%]). The proportion of children
receiving inappropriate doses of drugs in intervention hospitals was lower
(quinine dose >40 mg/kg/day; 1.0% versus 7.5%;
−6.5% [−12.9% to 0.20%]), and
inadequate gentamicin dose (2.2% versus 9.0%;
−6.8% [−11.9% to−1.6%]).
Conclusions:
Specific efforts are needed to improve hospital care in developing countries.
A full, multifaceted intervention was associated with greater changes in
practice spanning multiple, high mortality conditions in rural Kenyan
hospitals than a partial intervention, providing one model for bridging the
evidence to practice gap and improving admission care in similarsettings.
Trial registration:
Current Controlled Trials ISRCTN42996612
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(4): e32767. doi:10.1371/journal.pmed.1001018
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001018Summary
Background:
In developing countries referral of severely ill children from primary care
to district hospitals is common, but hospital care is often of poor quality.
However, strategies to change multiple paediatric care practices in ruralhospitals have rarely been evaluated.
Methods and Findings:
This cluster randomized trial was conducted in eight rural Kenyan district
hospitals, four of which were randomly assigned to a full intervention aimed
at improving quality of clinical care (evidence-based guidelines, training,
job aides, local facilitation, supervision, and face-to-face feedback;
n = 4) and the remaining four to
control intervention (guidelines, didactic training, job aides, and written
feedback; n = 4). Prespecified
structure, process, and outcome indicators were measured at baseline and
during three and five 6-monthly surveys in control and intervention
hospitals, respectively. Primary outcomes were process of care measures,
assessed at 18 months postbaseline.
In both groups performance improved from baseline. Completion of admission
assessment tasks was higher in intervention sites at 18 months
(mean = 0.94 versus 0.65, adjusted difference 0.54
[95% confidence interval 0.05–0.29]). Uptake of
guideline recommended therapeutic practices was also higher withinintervention hospitals:
adoption of once daily gentamicin (89.2%
versus 74.4%; 17.1%
[8.04%–26.1%]); loading dose quinine
(91.9% versus 66.7%, 26.3% [−3.66% to
56.3%]); and adequate prescriptions of intravenous fluids for
severe dehydration (67.2% versus 40.6%; 29.9%
[10.9%–48.9%]). The proportion of children
receiving inappropriate doses of drugs in intervention hospitals was lower
(quinine dose >40 mg/kg/day; 1.0% versus 7.5%;
−6.5% [−12.9% to 0.20%]), and
inadequate gentamicin dose (2.2% versus 9.0%;
−6.8% [−11.9% to−1.6%]).
Conclusions:
Specific efforts are needed to improve hospital care in developing countries.
A full, multifaceted intervention was associated with greater changes in
practice spanning multiple, high mortality conditions in rural Kenyan
hospitals than a partial intervention, providing one model for bridging the
evidence to practice gap and improving admission care in similarsettings.
Trial registration:
Current Controlled Trials ISRCTN42996612
: Please see later in the article for the Editors' SummaryIntroduction
Common illnesses including pneumonia, malaria, and diarrhea remain major contributors to child mortality in low-income countries [1]. Hospital care of severe illnesses may help improve survival, and disease-specific clinical guidelines have been provided by the World Health Organization (WHO) for more than 15 y [2], and as collated texts since 2000 [3],[4]. These guidelines form part of the Integrated Management of Childhood Illnesses (IMCI) approach adopted by over 100 countries. However, in contrast to its primary care aspects [5],[6], implementation of IMCI at district hospitals has not been evaluated. Paediatric hospital care is often inadequate in our setting and also in other low-income countries both in Africa and Asia [7]–[10], with most inpatient deaths occurring within 48 h of admission [11].
We therefore set out to develop and test a strategy to improve paediatric care in district hospitals in partnership with the Kenyan government [12]–[14]. We considered a trial of alternative interventions necessary for ethical reasons and because systematic reviews indicated uncertainty in the value of multicomponent interventions [15]. Our evaluation is based on the classical Donabedian approach—assessing structure, process, and valued health system outcome measures [16]. We randomised hospitals, rather than individuals, to intervention groups because the intervention was designed to influence how the paediatric teams provided care. Secondly, the cluster randomised trial offered logistical convenience in implementing certain intervention components, which by their nature (training, feedback, supervision) are easier to administer to groups rather than on an individual basis. To provide data to inform debate on the plausibility of any cause–effect relationship arising from the trial data, we also planned that evaluation spanned a realistic timescale, evaluated possible postintervention deterioration, and assessed intervention context, adequacy, and barriers to implementation [12],[17]–[20].
Methods
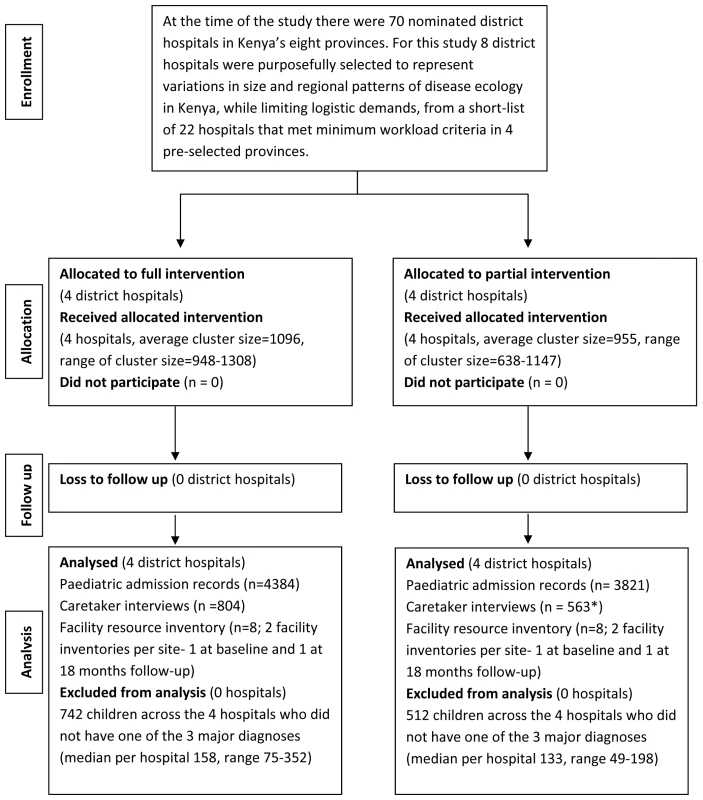
Study Sites and Participants
Eight rural hospitals (H1 to H8) were chosen purposefully from four of Kenya's eight provinces to provide some representation of the variety of rural hospital settings encountered in Kenya (Table 1) [12]. Hospitals admitting a minimum of 1,000 children and conducting at least 1,200 deliveries per year were eligible for inclusion. Prior to the study, medical records documenting admission information were written as nonstandard, free-text notes in all eight hospitals. The Ministry of Health usually aims to disseminate national guidelines aimed at hospital care to facilities through distribution of some print materials and ad hoc or opportunistic workshops or seminars. It had not previously been able to augment this approach with systematic efforts or provide specific supervision to support paediatric hospital care. Further, none of the eight hospitals themselves had explicit procedures for implementing new clinical guidelines.
Tab. 1. 
Number of staff trained exceeded target. We collected data from medical records of paediatric admissions aged 2–59 mo to describe paediatric care practices of clinicians and nursing staff targeted by the guidelines, training, and feedback. The Kenya Medical Research Institute National Ethics and Scientific review committees approved the study (Texts S1 and S2).
Randomization and Masking
Prior to inclusion in the study the eight shortlisted hospitals were visited and meetings were held with the hospital management team. At these meetings, the study design, randomization, potential inputs, approach to data collection, and longevity were explained. All hospital management teams subsequently assented to their hospital's participation and randomization after internal discussions. Assent from the hospital's catchment population was not sought. Staff in all hospitals were made aware of the study's overall aims to explore ways to improve care and need for data collection through specific presentations made after randomization at the start of introductory training and using written information sheets. After obtaining the hospitals' assent we allocated eight hospitals (clusters) to a full (intervention group, hospitals H1–H4) or partial (control group, hospitals H5–H8) package of interventions using restricted randomization. Of 70 possible allocations, seven defined two relatively balanced groups (Table 1). These allocations were written on identical pieces of paper, with hospitals represented by codes, and one allocation was randomly selected using a “blind draw” procedure. Participating hospitals and the research team could not be masked to group allocation. However, information on group allocation was not publicly disseminated and the geographic distance between hospitals was large. We therefore do not feel that users of the hospitals were aware of or influenced by the form of intervention allocated to the hospital.
Study Intervention
The intervention delivered over 18 mo (from September 2006 to April 2008) aimed to improve paediatric admission care by promoting hospitals' implementation of best-practice guidelines and local efforts to tackle local organizational constraints. Before the trial commenced, a decision was made to adjust the timing of the primary endpoint for measuring intervention effectiveness, aligning it with the end of this 18-mo active intervention period. As part of this updated approach, monitoring of intervention sites was planned to continue for 12 mo after active intervention had ended. Funds were not available to support comparable extended monitoring in control sites. The intervention components are labeled 1–6 and a–c in Figure 1 [21] and included: (1) setting up a scheme for regular hospital assessment through surveys conducted six monthly, followed by (2) face-to-face feedback of findings in intervention sites, and (a) written feedback in both groups. The other components were: (3) 5.5-d training aimed at 32 health workers of all cadres approximately 6–10 wk after baseline surveys (July to August 2006) in intervention hospitals [13], (b) provision of clinical practice guidelines introduced with training, (c) job aides, (4) an external supervisory process, and (5) identification of a full-time local facilitator (a nurse or diploma-level clinician) responsible for promoting guideline use and on-site problem solving [19]. Supervision visits were approximately two to three monthly, but facilitation remained in place throughout the 18 mo. The package for control sites (H5–H8) included five components (1, 6, a, b, and c): (1) six-monthly surveys with written feedback only, provision of (b) clinical practice guidelines and (c) job aides, and (6) a 1.5-d initial guideline seminar for approximately 40 hospital staff. The design thus compares two alternative intensities of intervention, both providing considerably more than routinely delivered, although we refer to one arm as the “control.”
One of the job aides, introduced to all sites with all training and continuously supplied to improve documentation of illness, was a paediatric admission record (PAR) form. This was to replace traditional “blank paper” medical notes [22]. All hospitals were aware that their records and patient management were to be regularly evaluated. All job aides, training materials, and assessment tools are available online (http://www.idoc-africa.org/docs/list/cat/5/subcat/27).
Data Collection
Data were collected at baseline and then at six-monthly intervals during six and four surveys in intervention (surveys 1–6) and control hospitals (surveys 1–4), respectively (Figure 1). A single survey took approximately 2 wk with all sites surveyed within a maximum 6-wk consecutive period by employing up to four teams. The survey tools and team training have been described in detail elsewhere [14]. In brief, data were collected using three tools adapted from previous work [7],[8] then extensively pretested: a checklist of structure indicators, patient case-record data abstraction forms, and a structured parent/guardian interview tool. In the case of the parent/guardian interview formal, written consent was obtained prior to data collection with no parent/guardians refusing consent. Ethical approval was granted for confidential abstraction of data from archived case records without individuals' consent. Survey team leaders remained the same throughout the study and teams received 3 wk initial training that included a pilot survey. Data collectors could not be blinded to allocation, but all were guided by standard operating procedures and, for case records, a 10% sample were independently reevaluated by the survey supervisor during each survey. Agreement rates for data abstracted were consistently greater than 95%.
Case records from a random sample of calendar dates from the 6-mo intersurvey periods were selected with the proportion of dates sampled adjusted to yield approximately 400 records based on hospitals' admission rates. On the basis of prior experience we aimed to conduct interviews with 50 caretakers of admitted children during each 2 wk survey (surveys 1–4).
Performance Indicators
Primary effectiveness measures were 14 process indicators measured on paediatric admissions aged 2–59 mo at 18-mo post baseline (survey 4). Secondary measures were four valued system outcomes of admission and changes in structure measured at the hospital level. The trial was not designed to evaluate mortality effects.
Process indicators
Indicators reflected standards defined by the clinical guidelines focusing on: pneumonia, malaria and diarrhoea, and/or dehydration that account for more than 65% of paediatric admissions and deaths [13]. These span assessment, therapeutic, and supportive care. We defined dichotomous variables for process errors, e.g. wrong intravenous fluid prescription. However, to summarize assessment an aggregate assessment score for each child (range 0–1) was calculated by counting the number of features documented and dividing this by the total relevant for each child according to guidelines (pneumonia 8, malaria and diarrhoea/dehydration both 6). The denominator of the score was thus child specific, depended on the extent of comorbidity, and had a maximum value of 16 due to two shared features of severe illness.
Outcome indicators
These indicators reflected adherence to key policy recommendations and included vitamin A prescription, identifying missed opportunities for immunization, and universal provider initiated testing and counselling (PITC) for HIV. A fourth was based on a score (range 0–4) reflecting caretakers' correct knowledge, at discharge, of their child's diagnosis and number, duration, and frequency of discharge drugs.
Structure indicators
The availability of equipment, basic supplies, and service organization were evaluated using a checklist of 113 items needed to provide guideline directed care and representing seven logical groupings [23]. Data were collected by observation and interviewing senior hospital staff. A simple, unweighted proportion of the 113 items was derived, the change in proportion available from survey 1 to survey 4 was calculated for each hospital and the mean change in intervention and control groups compared.
Sample Size
There were 70 district hospitals in Kenya at the time of the study. Hospitals from four of Kenya's eight provinces without potentially confounding, nonstudy interventions and meeting the outlined eligibility criteria were shortlisted. Data on additional criteria felt to help define the range of contexts in Kenya were then evaluated, and eight hospitals from four provinces were purposefully selected to ensure that at least two out of these eight hospitals met each positive and negative criterion (Table 1), that two hospitals were from each of the four provinces and represented logistical implications of their location. The sample size of eight hospitals was estimated using two approaches to compare performance within each hospital (plausibility design) and across the two arms of the trial (cluster RCT analysis). Within hospitals, we estimated that 50% correct performance could be estimated with precision (95% confidence intervals [CIs]) of ±7% with 200 admission records (50% of 400 sampled admissions), or, ±10% with 100 admission records. The second calculation for group (C-RCT) comparisons accounted for the clustered nature of the data. The median intraclass correlation coefficient (ICC) for 46 quality of care variables estimated from a health facility cluster survey in Benin was ρ = 0.2 [24]. We estimated, employing this value for the ICC, that 100 observations per cluster would provide 80% power to detect a 50% or greater difference in proportions between intervention and control arms at 18 mo follow-up [25].
Statistical Analysis
Data were double entered and verified in Microsoft Access and analysed using Stata, version 10 (Stata Corp.) according to the prespecified analysis plan.
Descriptive analysis
We present characteristics of hospitals at baseline and of children contributing admission data during surveys 1–4. Process and outcome indicators are summarized as percentages and the absolute changes (95% CI) between survey 1 and 4 calculated for each hospital.
Comparison of intervention and control arms
Two approaches were used. The first approach was a cluster level analysis of mean change from baseline in intervention (n = 4) and control (n = 4) groups, a test of mean difference-in-difference, using an unpaired t-test (with individual sample variances if appropriate), which is reasonably robust to deviations from normality, even for a small number of clusters. The second approach compared the groups at survey 4 using a two-stage method [26]. In the initial stage, logistic or linear regression analyses were conducted for each outcome adjusting for hospital-level covariates (all-cause paediatric mortality, malaria transmission, and size) and gender, illness outcome (alive or died) at the patient-level but not study group. The observed events were then subtracted from predicted events in the regressions to obtain a residual for each cluster. The cluster residuals were then compared in the second stage using a t-test [26].
Performance post intervention period
Data from intervention hospitals (surveys 4–6) were analysed to determine the impact of intervention withdrawal by assessing trends graphically and using regression analysis. Linear and binomial regression analysis was used to assess whether the means or proportions changed over time; this was done by testing to see whether there was a linear trend associated with the postintervention period (surveys 4–6).
We acknowledge the use of multiple significance tests and report 95% CIs and exact p-values where appropriate noting that p-values lower than those traditionally considered “significant” might be given greater weight. We would, however, suggest consideration of the plausibility of the intervention's effectiveness should also take into account any consistency in effect across indicators.
Results
All hospitals participated in each survey as planned (Figure 2). The intervention's implementation is summarized in Table 1 and showed that intended training for at least 32 workers (the majority were nurses) was attained in three of the four intervention sites. No hospital received additional, nonstudy paediatric training during the study period. Staff turnover, which was of a like-for-like nature, was high in both intervention and control hospitals, especially in the larger hospitals (H3 and H7). At 18 mo only, 5% (2/35) to 13% (3/23) and 0 to 26% (6/23) of frontline clinical staff in the intervention and control hospitals, respectively, had received initial training (Table 1). As part of supervisory activities, the implementation team conducted an additional 10–12-h training session in two intervention hospitals and two to three small group sessions of 2–4 h in all four intervention hospitals over the 18 mo intervention period.
Intervention and control sites were similar at baseline (Table 1), although routinely reported prior paediatric mortality varied from 4.1% to 13.4%. Case records for primary process of care indicators were available for 1,130 and 1,005 records at baseline and 1,158 and 1,157 case records at 18 mo for intervention and control hospitals, respectively (Table 1). Additional data summarizing the patient populations at cluster level are provided in Tables S1 and S2.
Primary Effectiveness Measures
Results were similar from both approaches used to compare intervention arms, i.e., adjusted comparison at 18 mo and difference of differences. For brevity, we outline only the results of adjusted comparisons and present other data in Tables S3, S4, S5.
Process indicators
Of 14 process of care indicators, performance at hospital level for three indicators assessed for every admission were highly variable but often poor at baseline (Table 2): e.g., documentation of weight, 1%–95%, and mean assessment scores 0.26–0.44. In addition, disease-specific treatment practices at baseline were poor, rarely conforming to guideline recommendations (Table 2). For example, prescription of nationally recommended (since 1998) loading dose quinine for <7% appropriate cases in seven sites at baseline (Table 2).
Tab. 2. 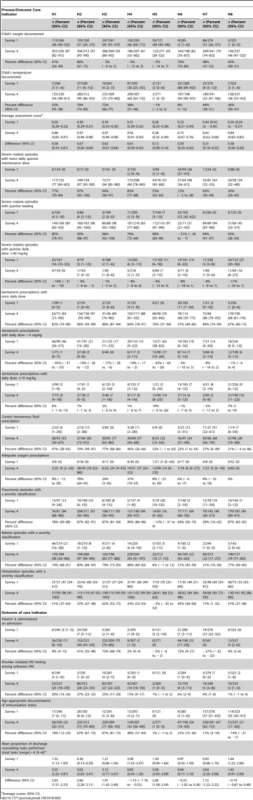
Average scores (95% CI). The proportion of admissions treated in line with clinical guidelines was substantially higher in intervention compared to control sites for prescription of twice rather than thrice daily quinine, once rather than thrice daily gentamicin, appropriate quinine and gentamicin dose/kg body weight, and the proportion of severely dehydrated children with correct intravenous fluid volumes (Table 3). There were no differences in proportions receiving possibly toxic gentamicin doses although this practice was relatively uncommon.
Secondary Effectiveness Measures
Outcome indicators
At baseline key child health policy interventions were rarely implemented. Vitamin A was prescribed only in H7 to 27% of admissions (Table 2). Health workers rarely documented missed opportunities for immunization (<9% across six sites) or offered PITC for HIV at baseline (all sites fewer than 4%).
At 18 mo the proportion of children offered PITC for HIV was significantly higher in intervention sites (adjusted difference, 19.4%; 95% CI 12.3%–26.4%), as was checking vaccination status (25.8%; 7.29%–44.4%]). Although, prescription of Vitamin A and counselling improved in some hospitals, differences between groups did not attain statistical significance (Table 3).
Structure indicators
Changes between baseline and 18 mo were positive in both groups for all domains. Improvements in intervention hospitals were, however, consistently greater than in control hospitals (Figure 3), with the mean difference of difference analysis showing a 21% greater overall improvement (p = 0.02, based on a simple t-test).
Performance within intervention sites during surveys 5 and 6
For most process indicators with improvement, and based on tests for trend between survey 4 and survey 5 or survey 6, no major decline in performance was noted even 12 mo after withdrawal of intervention and in the face of continuing staff turnover (Figures 4 and S1).
Fig. 4. Intervention effect on processes of care. 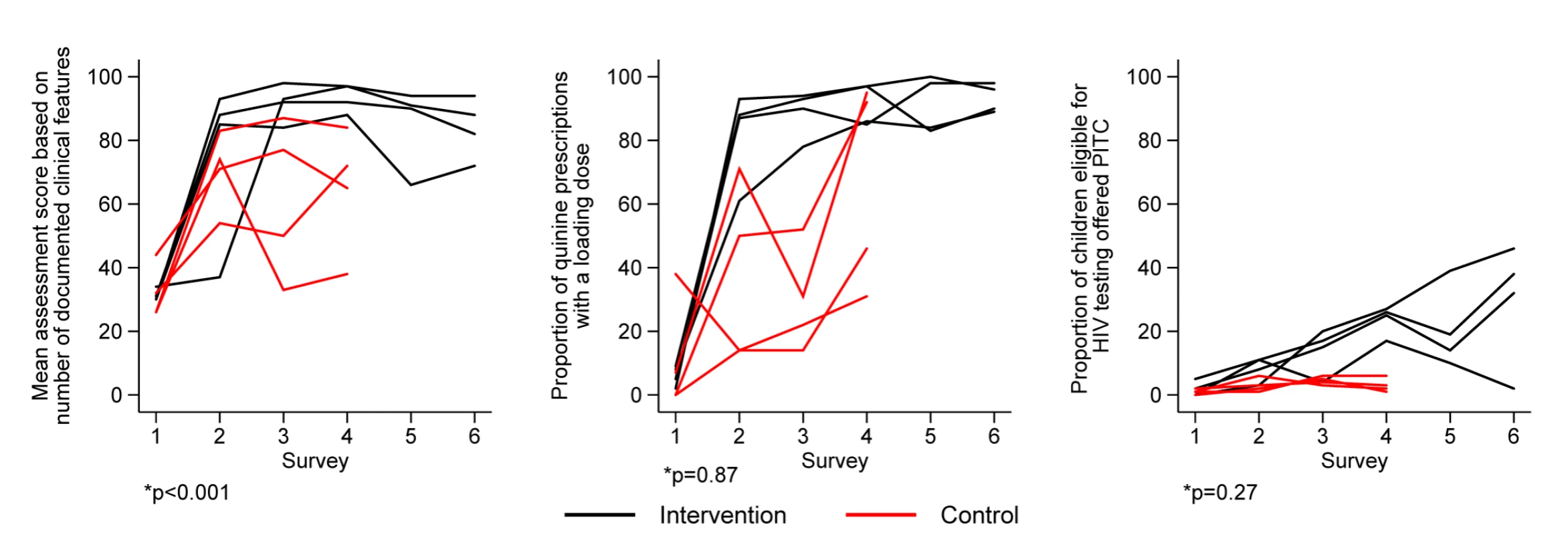
Discussion
We tested an approach to implementing clinical guidelines for management of illnesses that cause most deaths in children admitted to district hospitals in Kenya. Despite their modest success in developed countries [15], we used a multifaceted approach reasoning that deficiencies in knowledge, skills, motivation, resources, and organization of care would all need to be addressed. The intervention design was guided by experience in the setting [7],[8] and theories of change and culture of practice [13],[15],[27]–[29]. Our baseline data and other reports [7]–[10] suggest that the simple availability of authoritative WHO and national guidelines—for periods of more than 15 y—are currently having little impact on hospital care for children. So what did our interventions achieve?
The full intervention package resulted in significantly greater improvements in almost all primary and secondary effectiveness measures. Within specific hospitals performance of certain indicators, e.g., recording child's weight in H3, were already high at baseline. For these specific hospitals there was limited scope for improvement, but there remained significant potential for improvement at the group level since performance for most indicators was below the projected level of 50% at baseline. Substantial, clinically important changes occurred in processes of care despite very high staff turnover amongst the often junior clinicians responsible for much care in each site. Indeed, of 109 clinical staff involved in admitting patients sampled at survey 4 from intervention hospitals only nine (8.3%) had received any specific formal or even ad hoc training. At survey 6 this proportion had reduced to 4.4% (four out of 91) reflecting the typically high turnover of junior clinicians in such settings. As the training and guidelines were not being provided in preservice training institutions and as formal orientation periods are absent [14], we infer, but cannot confirm, that new staff learned correct practices more commonly from established clinicians or the facilitator in intervention hospitals. Improvement in structure indicators occurred without any direct financial inputs reflecting probably a small generalized improvement in resource availability and use of funding from user fees (total hospital incomes varied from US$57 to US$100 per bed per month [19]) that we feel was in part, in response to hospital feedback and the advocacy of the facilitator [14].
Improvements in quality of care thus occurred across a set of common, serious childhood conditions and over a prolonged period. These data are a major addition to reports from sub-Saharan Africa indicating that financial incentives can improve malaria-specific care and fatality [30] and that implementation of WHO guidelines can improve emergency triage assessment and treatment of children [31]–[33] and hospital care and outcomes for severe malnutrition [34]. They also complement evidence from middle-income settings where a multifaceted intervention resulted in substantial improvements in two key obstetric practices [35]. Our data however, to our knowledge, represent the first major report examining national adaptation and implementation of a broad set of rural hospital care recommendations. They are relevant to many of the 100 countries with IMCI programmes where rural hospitals have important roles supporting primary health care systems [36] and in helping to reduce child mortality [37],[38].
However, while change in simple process indicators was reasonably consistent in intervention sites, in control (partial intervention) sites, changes were more varied, even within hospitals (notably site H8). Certain indicators, e.g., PITC for HIV, also improved only in three of four intervention sites and steadily but slowly. Thus, while the full intervention may promote consistency, there was still substantial evidence of variation across indicators, across sites, and across time. Such variability is consistent with emerging debates drawing on theories of complexity, chaos, and change emphasizing the effect of interactions with contexts [39]–[41] and suggesting that understanding can be informed by parallel qualitative enquiry [42]. Data collected during this study on barriers to use of guidelines [18] and views on supervision, feedback, and facilitation [14] together with published literature [43] suggest to us that poor or slow uptake may be associated with a requirement for greater personal or organizational effort to change, the view that a task is not directly related to care of the immediate illness, or, in intervention sites, an area unlikely to be subject to local evaluation.
Limitations
Our study has limitations. Hospitals were not selected at random from a set of all eligible hospitals for logistic reasons and, because random selection of a small number of clusters may not have produced balance nor guaranteed representativeness at baseline. Hospitals assented to participation and randomization, but we were not able to engage communities in this process [44], and they and survey teams were aware of intervention allocation. The latter is a potential problem with results based largely on retrospective review of records. The discrepancy between documentation and performance presents a particular threat at baseline before efforts in all sites to improve clinical notes. Prescription data are less susceptible to this limitation however, and improved prescribing paralleled improvement in assessment indicators. Efforts to minimize possible observation bias at the point of data collection included the use of structured inventory forms, standard operating procedures, and extensive training in survey methods. With only four hospitals per group, attempts to adjust for baseline imbalance may also have only limited success. However, to facilitate scrutiny we report on the context of intervention [19],[20], its delivery and adequacy [12], the views of intervention recipients [18], and detailed site-specific data (see Tables S1, S2, S3, S4, S5) and suggest that all are considered for a complete interpretation of this study of a complex intervention.
Replication and Scaling Up
Demonstrations that a similar intervention package is effective in other settings would strengthen the evidence supporting widespread adoption. While there are few studies of this nature reported, we note the recently reported success of multifaceted interventions in middle - and high-income countries [35],[45]. However, standardizing complex interventions may be difficult, if not impossible, given the important role of context in shaping mechanisms and outcomes [46]. For this reason, future reports will attempt to provide detailed insight into how and why this intervention met with general but varying degrees of success. If our results are deemed credible, however, the data we present have a number of implications. Firstly, current efforts to implement and scale up improved referral care in low-income settings need to go beyond the existing tradition of producing and disseminating printed materials even when linked to training [15]. Instead broader health system efforts, guided by current understanding of local contexts and capabilities and theories of change, are required.
Within Kenya it would obviously be a mistake to consider that the intervention package tested can be scaled up simply by aiming for much broader coverage with the training course we designed. Effectiveness has been demonstrated only for the multifaceted intervention. Thus, scaling up should aim to provide all inputs not just guidelines, job aides, and introductory training. However, providing regular support supervision and performance feedback related to child and newborn care at first referral level are not routine. Resources and systems for supervision need strengthening and supervisors themselves will need training and organizing. Routine information systems are inadequate to generate the data required to evaluate care, and capacity for conducting and disseminating analyses as part of routine feedback is largely absent. The role of facilitators is also not one that currently exists. Although the roles required could perhaps be played by senior departmental staff, the lack of human resources means such tasks cannot simply be added to already busy jobs [19]. Furthermore the skills or desire to facilitate change are not necessarily present amongst such mid-level managers.
Countries other than Kenya considering adopting the approach may have similar limitations. In addition they may need to tailor some intervention components to their particular setting. For example, the detail of a clinical guideline or job aide or approach to training may need to reflect available resources or local evidence. However, such adaptation would need to be complemented by careful consideration of how systems can be made ready to support implementation of new practices and improved quality of care. We would suggest this includes due attention to influencing the institutional culture and context of rural hospitals although willingness to invest in more integrated approaches often seems lacking [47]. Finally, before making decisions on implementation, policy makers increasingly require carefully collected and reported cost-effectiveness data. Such a report is in preparation. Considering only the financial costs of specific inputs, for example the typical 5-d training course for 32 participants at approximately US$5,000 [13] or the annual cost of a facilitator at less than US$5,000 [18], while of some value, are insufficient for prioritizing resource use.
Conclusion
Our findings provide strong evidence that a multifaceted intervention can improve use of guidelines and, more generally, the quality of paediatric care. Cost data will help determine whether this implementation model warrants wider consideration as one approach to strengthening health systems in low-income settings.
Supporting Information
Zdroje
1. BryceJBoschi-PintoCShibuyaKBlackR
2005
WHO Child Health Epidemiology Reference Group. WHO estimates of
the causes of death in children.
Lancet
365
1147
1152
2. World Health Organisation
1990
Acute respiratory infections in children: case management in small
hospitals in developing countries: a manual for doctors and other senior
health workers
Geneva
WHO
3. World Health Organisation
2000
Management of the child with a serious infection or severe malnutrition.
Guidelines for care at first-referral level in developing countries
Geneva
WHO
4. World Health Organisation
2005
Pocket book of hospital care for children: guidelines for the management
of common illnesses with limited resources
Geneva
WHO
5. Armstrong SchellenbergJBryceJde SavignyDLambrechtsTMbuyaC
2004
The effect of Integrated Management of Childhood Illness on
observed quality of care of under-fives in rural Tanzania.
Health Policy Plan
19
1
10
6. PariyoGGouwsEBryceJBurnhamG
2005
Improving facility-based care for sick children in Uganda:
training is not enough.
Health Policy Plan
20
i58
i68
7. EnglishMEsamaiFWasunnaAWereFOgutuB
2004
Assessment of inpatient paediatric care in first referral level
hospitals in 13 districts in Kenya.
Lancet
363
1948
1953
8. EnglishMEsamaiFWasunnaAWereFOgutuB
2004
Delivery of paediatric care at the first-referral level in
Kenya.
Lancet
364
1622
1629
9. NolanTAngosPCunhaAMuheLQaziS
2001
Quality of hospital care for seriously ill children in
less-developed countries.
Lancet
357
106
110
10. ReyburnHMwakasungulaEChonyaSMteiFBygbjergI
2008
Clinical assessment and treatment in paediatric wards in the
north-east of the United Republic of Tanzania.
Bull World Health Organ
86
132
139
11. BerkleyJLBMwangiIWilliamsTBauniEMwarumbaS
2005
Community acquired bacteremia amongst children admitted to a
rural district hospital in Kenya.
N Engl J Med
352
39
47
12. EnglishMIrimuGWamaeAWereFWasunnaA
2008
Health systems research in a low-income country: easier said than
done.
Arch Dis Child
93
540
544
13. IrimuGWamaeAWasunnaAWereFNtoburiS
2008
Developing and introducing evidence based clinical practice
guidelines for serious illness in Kenya.
Arch Dis Child
93
799
804
14. NzingaJNtoburiSWagaiJMbindyoPMbaabuL
2009
Implementation experience during an eighteen month intervention
to improve paediatric and newborn care in Kenyan district
hospitals.
Implement Sci
4
45
15. GrimshawJThomasRMacLennanGFraserCRamsayC
2004
Effectiveness and efficiency of guideline dissemination and
implementation strategies.
Health Technol Assess
8
1
72
16. DonabedianA
1988
The quality of care: how can it be assessed?
JAMA
260
1743
1748
17. HabichtJVictoraCVaughanJ
1999
Evaluation designs for adequacy, plausibility and probability of
public health programme performance and impact.
Int J Epidemiol
28
10
18
18. NzingaJMbindyoPMbaabuLWariraAEnglishM
2009
Documenting the experiences of health workers expected to
implement guidelines during an intervention study in Kenyan
hospitals.
Implement Sci
4
44
19. EnglishMNtoburiSWagaiJMbindyoPOpiyoN
2009
An intervention to improve paediatric and newborn care in Kenyan
district hospitals: understanding the context.
Implement Sci
23
42
20. MbindyoPGilsonLBlaauwDEnglishM
2009
Contextual influences on health worker motivation in district
hospitals in Kenya.
Implement Sci
4
43
21. PereraRHeneghanCYudkinP
2007
Graphical method for depicting randomised trials of complex
interventions.
BMJ
334
127
129
22. MwakyusaSWamaeAWasunnaAWereFEsamaiF
2006
Implementation of a structured paediatric admission record for
district hospitals in Kenya–results of a pilot study.
BMC Int Health Hum Rights
6
doi:10.1186/1472-1698X-1186-1189
23. OpondoCNtoburiSWagaiJWafulaJWasunnaA
2009
Are hospitals prepared to support newborn survival? - An
evaluation of eight first-referral level hospitals in Kenya.
Trop Med Int Health
14
1165
1172
24. RoweALamaMOnikpoFDemingM
2002
Design effects and intraclass correlation coefficients from a
health facility cluster survey in Benin.
Int J Qual Health Care
14
521
523
25. HayesRBennettS
1999
Simple sample size calculation for cluster-randomized
trials.
Int J Epidemiol
28
319
326
26. HayesRMoultonL
2009
Cluster randomised trials
London
Chapman & Hall/CRC
27. EnglishM
2005
Child survival: district hospitals and
paediatricians.
Am J Public Health Arch Dis Child
9
28. FerlieEShortellS
2001
Improving the quality of health care in the United Kingdom and
the United States: a framework for change.
Milbank Q
79
281
315
29. MichieSJohnstonMAbrahamCLawtonRParkerD
2005
“Psychological Theory” Group. Making psychological
theory useful for implementing evidence based practice: a consensus
approach.
Qual Saf Health Care
14
26
33
30. BiaiSRodriguesAGomesMRibeiroISodemannM
2007
Reduced in-hospital mortality after improved management of
children under 5 years admitted to hospital with malaria: randomised
trial.
BMJ
335
Epub.862
31. GoveSTamburliniGMolyneuxEWhitesellPCampbellH
1999
Development and technical basis of simplified guidelines for
emergency triage assessment and treatment in developing countries. WHO
Integrated Management of Childhood Illness (IMCI) Referral Care
Project.
Arch Dis Child
81
473
477
32. MolyneuxE
2001
Paediatric emergency care in developing
countries.
Lancet
357
86
87
33. TamburliniGDi MarioSMaggiRVilarimJGoveS
1999
Evaluation of guidelines for emergency triage assessment and
treatment in developing countries.
Arch Dis Child
81
478
482
34. AshworthAChopraMMcCoyDSandersDJacksonD
2004
WHO guidelines for management of severe malnutrition in rural
South African hospitals: effect on case fatality and the influence of
operational factors.
Lancet
363
1110
1115
35. AlthabeFBuekensPBergelEBelizánJCampbellM
2008
A behavioral intervention to improve obstetrical
care.
N Engl J Med
68
48
54
36. World Health Organization
1992
The hospital in rural and urban districts: report of a WHO study group
on the functions of hospitals at the first referral level
Geneva
WHO
37. DarmstadtGBhuttaZCousensSAdamTWalkerN
2005
Evidence-based, cost-effective interventions: how many newborn
babies can we save?
Lancet
365
977
988
38. JonesGSteketeeRBlackRBhuttaZMorrisS
2003
How many child deaths can we prevent this year?
Lancet
362
65
71
39. LitakerDTomoloALiberatoreVStangeKAronD
2006
Using complexity theory to build interventions that improve
health care delivery in primary care.
J Gen Intern Med
Suppl 2
S30
S34
40. RhydderchMElwynGMarshallMGrolR
2004
Organisational change theory and the use of indicators in general
practice.
Qual Saf Health Care
13
213
217
41. RicklesDHawePShiellA
2007
A simple guide to chaos and complexity.
J Epidemiol Community Health
61
933
937
42. LewinSGlentonCOxmanA
2009
Use of qualitative methods alongside randomised controlled trials
of complex healthcare interventions: methodological study.
BMJ
339
b3496
43. ChandlerCJonesCBonifaceGJumaKReyburnH
2008
Guidelines and mindlines: why do clinical staff over-diagnose
malaria in Tanzania?
A qualitative study. Malar J
7
44. OsrinDAzadKFernandezAManandharDMwansamboC
2009
Ethical challenges in cluster randomized controlled trials:
experiences from public health interventions in Africa and
Asia.
Bull World Health Organ
87
772
779
45. ScalesDDaintyKHalesBPintoRFowlerR
2011
A multifaceted intervention for quality improvement in a network
of intensive care units: a cluster randomized trial.
JAMA
305
363
372
46. MackenzieMO'DonnellCHallidayESridharanSPlattS
2010
Do health improvement programmes fit with MRC guidance on
evaluating complex interventions?
BMJ
340
c185
47. EnglishMWamaeANyamaiRBevinsBIrimuG
2011
Implementing locally appropriate guidelines and training to
improve care of serious illness in Kenyan hospitals: a story of scaling-up
(and down and left and right).
Arch Dis Child
96
285
290
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Quality of Private and Public Ambulatory Health Care in Low and Middle Income Countries: Systematic Review of Comparative Studies
- A Multifaceted Intervention to Implement Guidelines and Improve Admission Paediatric Care in Kenyan District Hospitals: A Cluster Randomised Trial
- The Quality of Medical Care in Low-Income Countries: From Providers to Markets
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
- Improving Effective Surgical Delivery in Humanitarian Disasters: Lessons from Haiti
- Decline in Diarrhea Mortality and Admissions after Routine Childhood Rotavirus Immunization in Brazil: A Time-Series Analysis
- Effect of Pneumococcal Conjugate Vaccination on Serotype-Specific Carriage and Invasive Disease in England: A Cross-Sectional Study
- Effect of a Nutrition Supplement and Physical Activity Program on Pneumonia and Walking Capacity in Chilean Older People: A Factorial Cluster Randomized Trial
- Strategies and Practices in Off-Label Marketing of Pharmaceuticals: A Retrospective Analysis of Whistleblower Complaints
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Medical Complicity in Torture at Guantánamo Bay: Evidence Is the First Step Toward Justice
- A Public Health Emergency of International Concern? Response to a Proposal to Apply the International Health Regulations to Antimicrobial Resistance
- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- The African Women's Protocol: Bringing Attention to Reproductive Rights and the MDGs
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání