-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Decline in Diarrhea Mortality and Admissions after Routine Childhood Rotavirus Immunization in Brazil: A Time-Series Analysis
Background:
In 2006, Brazil began routine immunization of infants <15 wk of age with a
single-strain rotavirus vaccine. We evaluated whether the rotavirus
vaccination program was associated with declines in childhood diarrhea
deaths and hospital admissions by monitoring disease trends before and after
vaccine introduction in all five regions of Brazil with varying disease
burden and distinct socioeconomic and health indicators.Methods and Findings:
National data were analyzed with an interrupted time-series analysis that
used diarrhea-related mortality or hospitalization rates as the main
outcomes. Monthly mortality and admission rates estimated for the years
after rotavirus vaccination (2007–2009) were compared with expected
rates calculated from pre-vaccine years (2002–2005), adjusting for
secular and seasonal trends. During the three years following rotavirus
vaccination in Brazil, rates for diarrhea-related mortality and admissions
among children <5 y of age were 22% (95% confidence
interval 6%–44%) and 17% (95% confidence
interval 5%–27%) lower than expected, respectively. A
cumulative total of ∼1,500 fewer diarrhea deaths and 130,000 fewer
admissions were observed among children <5 y during the three years after
rotavirus vaccination. The largest reductions in deaths
(22%–28%) and admissions (21%–25%)
were among children younger than 2 y, who had the highest rates of
vaccination. In contrast, lower reductions in deaths (4%) and
admissions (7%) were noted among children two years of age and older,
who were not age-eligible for vaccination during the study period.Conclusions:
After the introduction of rotavirus vaccination for infants, significant
declines for three full years were observed in under-5-y diarrhea-related
mortality and hospital admissions for diarrhea in Brazil. The largest
reductions in diarrhea-related mortality and hospital admissions for
diarrhea were among children younger than 2 y, who were eligible for
vaccination as infants, which suggests that the reduced diarrhea burden in
this age group was associated with introduction of the rotavirus vaccine.
These real-world data are consistent with evidence obtained from clinical
trials and strengthen the evidence base for the introduction of rotavirus
vaccination as an effective measure for controlling severe and fatalchildhood diarrhea.
:
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(4): e32767. doi:10.1371/journal.pmed.1001024
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001024Summary
Background:
In 2006, Brazil began routine immunization of infants <15 wk of age with a
single-strain rotavirus vaccine. We evaluated whether the rotavirus
vaccination program was associated with declines in childhood diarrhea
deaths and hospital admissions by monitoring disease trends before and after
vaccine introduction in all five regions of Brazil with varying disease
burden and distinct socioeconomic and health indicators.Methods and Findings:
National data were analyzed with an interrupted time-series analysis that
used diarrhea-related mortality or hospitalization rates as the main
outcomes. Monthly mortality and admission rates estimated for the years
after rotavirus vaccination (2007–2009) were compared with expected
rates calculated from pre-vaccine years (2002–2005), adjusting for
secular and seasonal trends. During the three years following rotavirus
vaccination in Brazil, rates for diarrhea-related mortality and admissions
among children <5 y of age were 22% (95% confidence
interval 6%–44%) and 17% (95% confidence
interval 5%–27%) lower than expected, respectively. A
cumulative total of ∼1,500 fewer diarrhea deaths and 130,000 fewer
admissions were observed among children <5 y during the three years after
rotavirus vaccination. The largest reductions in deaths
(22%–28%) and admissions (21%–25%)
were among children younger than 2 y, who had the highest rates of
vaccination. In contrast, lower reductions in deaths (4%) and
admissions (7%) were noted among children two years of age and older,
who were not age-eligible for vaccination during the study period.Conclusions:
After the introduction of rotavirus vaccination for infants, significant
declines for three full years were observed in under-5-y diarrhea-related
mortality and hospital admissions for diarrhea in Brazil. The largest
reductions in diarrhea-related mortality and hospital admissions for
diarrhea were among children younger than 2 y, who were eligible for
vaccination as infants, which suggests that the reduced diarrhea burden in
this age group was associated with introduction of the rotavirus vaccine.
These real-world data are consistent with evidence obtained from clinical
trials and strengthen the evidence base for the introduction of rotavirus
vaccination as an effective measure for controlling severe and fatalchildhood diarrhea.
:
Please see later in the article for the Editors' SummaryIntroduction
In July 2009, the World Health Organization recommended introduction of rotavirus vaccines into national immunization programs of all countries worldwide for controlling severe rotavirus disease, which accounts for approximately one-third of the 1.34 million diarrhea deaths and 9 million hospital admissions worldwide among children younger than 5 y of age [1]–[3]. Evaluating the health impact of large-scale programs, and ensuring their equity, is considered a top priority in global health for well-informed policy decisions [4]–[6]. This is particularly important for rotavirus vaccine programs because challenges remain concerning performance of the vaccine in regions with the highest morbidity and mortality [7],[8].
The protective effect of rotavirus vaccines has been assessed in various high-, middle-, and low-income settings. For unclear reasons, efficacy of live, oral rotavirus vaccines is location-dependent, with a gradient in immune response and protective efficacy that correlates with the socioeconomic status of the population [7],[8]. In a clinical trial from Latin America, the single-strain human rotavirus vaccine Rotarix (GlaxoSmithKline Biologicals) showed a protective efficacy of 85% against severe rotavirus disease (i.e., hospital admissions) [9],[10]. Efficacy was lower in the middle-income settings of South Africa (77%) and El Salvador (76%) and the low-income setting of Malawi (49%) [11],[12]. A similar gradient in efficacy has also been shown for the pentavalent rotavirus vaccine RotaTeq (Merck) in clinical trials [13]–[15] and post-licensure evaluations [16]–[18].
Although rotavirus vaccines have only recently been introduced, the population-level effect of vaccination has been assessed in various settings. A large reduction in national diarrhea hospital admissions after the introduction of pentavalent rotavirus vaccine has been shown in high-income settings [19]–[22]. While these data are promising, evaluations from middle - and low-income settings, most of which are using the single-strain vaccine [23], are limited. A recent study from Mexico demonstrated significant reductions in infant diarrhea mortality early after rotavirus vaccine introduction [24]. Early evaluations of the impact of rotavirus vaccine in Brazil have shown a reduction in rotavirus and diarrhea hospitalizations during the first year after vaccine introduction [25]–[27]. However, Brazil is a large country with heterogeneous socioeconomic conditions and a high burden of diarrheal disease, where diarrhea deaths and admissions occur year-round and disease trends had been declining in recent years before vaccination [28]. In such settings, accounting for secular declines and monitoring diarrhea trends for a longer duration after vaccine introduction are necessary to isolate the effect of vaccine from other concurrently implemented interventions or changes in practice. Thus, we conducted a comprehensive national evaluation quantifying the sustained effect of a vaccination program in Brazil on both relevant outcomes, diarrhea deaths and diarrhea admissions from all causes, using an interrupted time-series analysis.
In 2006, the Brazilian Ministry of Health introduced the single-strain rotavirus vaccine, Rotarix, simultaneously in all 27 states through its national immunization program in order to accelerate reaching the fourth Millennium Development Goal of reduced child mortality. The primary objective of this study was to evaluate the effect of rotavirus vaccination by analyzing trends in mortality and hospital admissions for diarrhea due to all causes among young children in the five regions of Brazil.
Methods
Ethics Statement
This evaluation involved analysis of existing, publicly available datasets. Because these are publicly available non-identifiable data, the Brazilian Ministry of Health and the United States Centers for Disease Control and Prevention human subjects' offices deemed that an ethics statement was not required for this work.
Setting and Design
Brazil is a middle-income Latin American country with an annual birth cohort of ∼3 million infants and a gross domestic product per capita of US$8,300. Brazil's 27 states are divided into five geographic regions (North, Northeast, Southeast, South, and Central-West) with distinct socioeconomic and health indicators (Table 1). The United Nations Development Program classifies countries with a human development index (HDI) below 0.90 as “developing” [29]. In 2005, among 182 countries ranked in decreasing order of HDI, Brazil overall (HDI = 0.805) ranked 75th (for general comparison, Mexico [HDI = 0.844] ranked 53rd and the United Kingdom ranked 21st [HDI = 0.947]). The Northeast region of Brazil individually had an index (HDI = 0.720) below that of Bolivia (HDI = 0.722), a developing country that ranked 113th.
Tab. 1. 
Source: Ministry of Health, Brasilia, Brazil <em class="ref">[33]</em>. Rotavirus Vaccination
Brazil's national immunization program operates within the Sistema Unico de Saúde (SUS), Brazil's publicly funded health-care system, to provide universal access to recommended vaccines [30]. The federal government purchases vaccines, which are distributed to state and local immunization programs and provided at no cost at public health facilities throughout the country. In April 2006, a rotavirus vaccine (Rotarix) was introduced into Brazil's national immunization program. Vaccination is recommended at 2 and 4 mo of age. The first dose can be administered at 6–14 wk, and the second dose at 14–24 wk of age.
Rotavirus vaccination coverage estimates for 2007 through 2009 among children under 1 y of age were obtained for each region from the information department of the Ministry of Health [31]. Doses of rotavirus vaccine administered during the years 2007 through 2009, registered as a first or second dose, were recorded in a national electronic database by clinics providing immunization services. Coverage with two doses of oral rotavirus vaccine was estimated as the number of second doses administered divided by the population <1 y of age in the corresponding calendar year.
Diarrhea Admissions and Death Data
Data on diarrhea deaths were obtained from the Mortality Information System (Sistema de Informações sobre Mortalidade [SIM]) of the Brazilian Ministry of Health, the national database of information collected from death certificates [32]. This system is estimated to capture 90% of all deaths that occur in Brazil, with lower percentages (∼80%) in the North and Northeast regions. Capture of neonatal deaths is lower, but neonatal deaths account for a small proportion (∼3%–4%) of diarrhea deaths in Latin America [1]. Data on admissions were obtained from the electronic Hospital Information System (Sistema de Informações Hospitalares [SIH]) of SUS [32]. This system includes information on all hospital admissions authorized for payment by SUS, which covers approximately 70% of hospitalizations in Brazil. The hospitalization data are from public hospitals and some private hospitals that are paid by the government to care for patients. No changes occurred during the study period in terms of the number of hospitals that reported to this system. Both the SIM and SIH use the International Classification of Diseases (ICD-10) for causes of death (SIM) or admission diagnosis (SIH). In this analysis, we included deaths and admissions with the principal cause coded as diarrheal disease of any cause (ICD-10 codes: A00–A03, A04, A05, A06.0–A06.3, A06.9, A07.0–A07.2, A07.9, A08–A09) among children younger than 5 y from January 2002 through December 2009.
Statistical Analysis
Rates of all-cause diarrhea-related deaths and admissions among children younger than 5 y of age were examined before (2002 through 2005) and after (2007 through 2009) introduction of rotavirus vaccination using an interrupted time-series analysis. We excluded data from 2006, the year in which the vaccine was introduced. These rates were calculated from SIM and SIH figures for diarrhea-related events per month and population, using annual projections from the 2000 census [33].
For the regression analysis, a generalized linear model was fit to the time-series data, assuming that the diarrhea deaths and admissions were Poisson distributed. The standard error of the rate ratio was scaled to the Pearson chi-squared statistic divided by the residual degrees of freedom to account for over-dispersion of the monthly counts in the outcome data for all models [34]. We first computed monthly rates of diarrhea-related deaths and admissions “expected” to occur in the absence of a rotavirus vaccination program by fitting the model to pre-vaccine data (2002 through 2005). We adjusted for seasonality by including an indicator variable for each calendar month and for secular trends by including calendar year in the model. The sequential year term captured the linear slope of decreasing secular trend; we assumed that this linear trend continued into the vaccine era (2007 through 2009). This model based on pre-vaccine data (including a constant and terms for month and year) was used to estimate expected values in the vaccine era. We then compared the absolute number of diarrhea-related deaths and admissions observed in the vaccine era with those expected in the absence of vaccination, as computed by the model, to assess the potential impact of vaccination. Finally, we calculated the rate ratio of diarrhea deaths and admissions in the vaccine era with the inclusion of an indicator variable for the period after rotavirus vaccine introduction; again, we controlled for seasonal and secular trends.
We investigated changes in rates of diarrhea deaths and admissions by age groups (under 1 y, 1 to <2 y, 2 to 4 y) because vaccine coverage during the early years of an immunization program and disease rates vary substantially by age. Separate models were also fitted for each region of Brazil in order to investigate differential impact of the vaccine program and to allow for different seasonality of rotavirus and secular trends. Results are presented as percent decline (1 − rate ratio) and 95% confidence interval (CI). Analyses were done with Stata (version 11).
Results
Vaccination Coverage
In 2007, when an estimated 80% of children younger than 1 y of age received two doses of rotavirus vaccine, only 47% of those 1 to <2 y of age, and none of those 2 to 4 y of age had been completely immunized. By 2009, an estimated 84% of children younger than 1 y of age (Table 1) 81% of children 1 to <2 y of age, and 36% of children 2 to 4 y of age had received two doses of rotavirus vaccine.
Diarrhea-Related Deaths
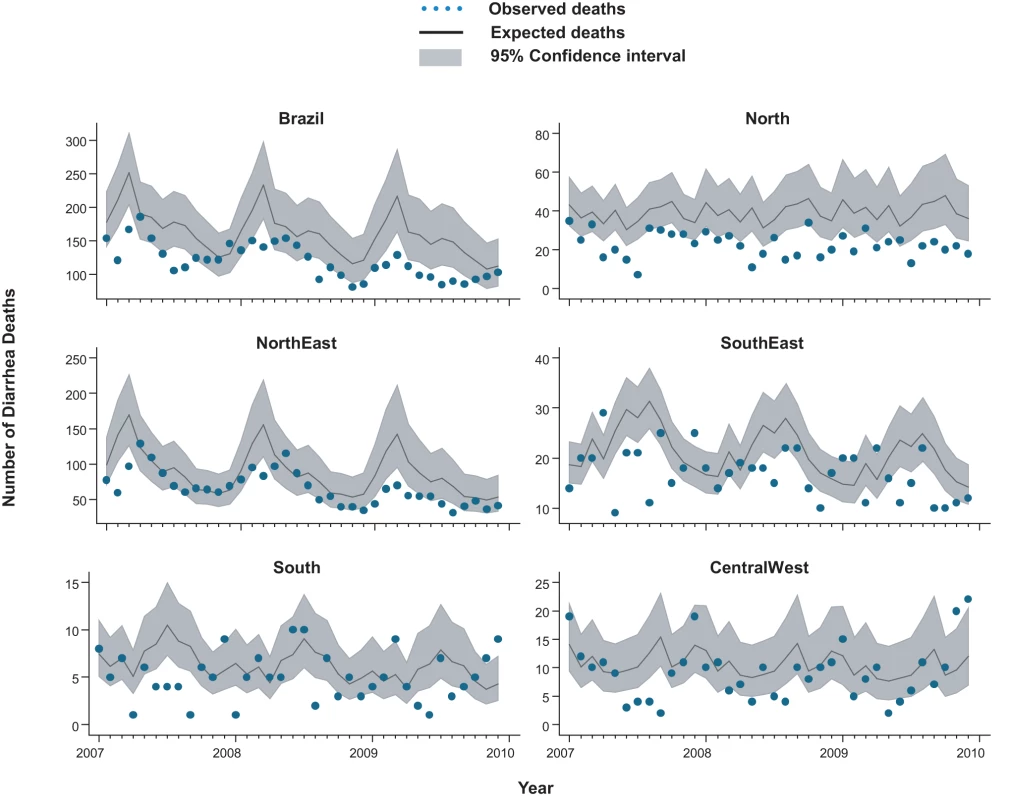
From 2002 through 2005, an annual median of 2,700 diarrhea-related deaths occurred among children younger than 5 y at an average annual rate of 16 deaths per 100,000, accounting for 14% of the 19,000 post-neonatal deaths (i.e., 30 d to 5 y of age) in Brazil each year. Prior to rotavirus vaccine introduction, there was a trend of declining diarrhea-related mortality among children younger than 1 y (relative reduction [RR] = 0.87/y; 95% CI 0.83–0.94; p<0.001), 1 to <2 y of age (RR = 0.96/y; 95% CI 0.91–1.02; p = 0.23) and 2 to 4 y of age (RR = 0.93/y; 95% CI 0.87–1.00; p = 0.06) (Figure 1). Diarrhea mortality rates and seasonality differed significantly by region (Figure 2). A majority (73%) of diarrhea-related deaths occurred in the North and Northeast, where mortality rates were four to five times higher than those in the Central-West, South, and Southeast.
Fig. 1. Trends in childhood diarrhea deaths and admissions in Brazil. 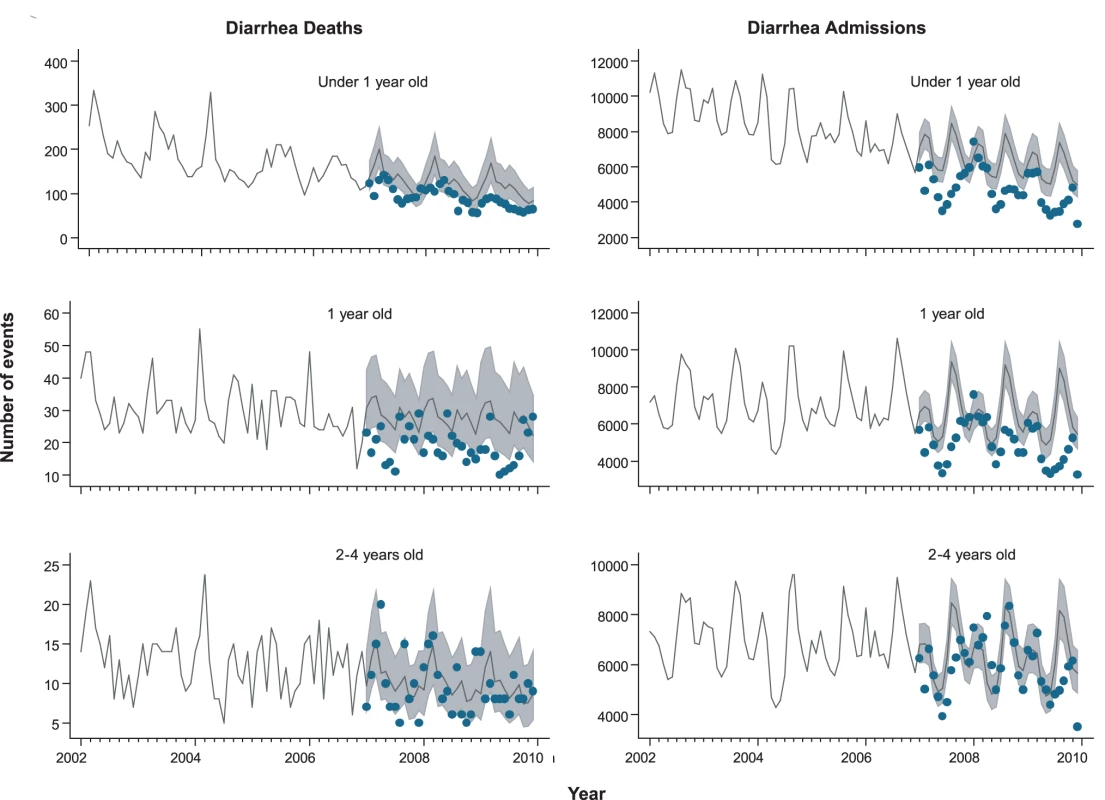
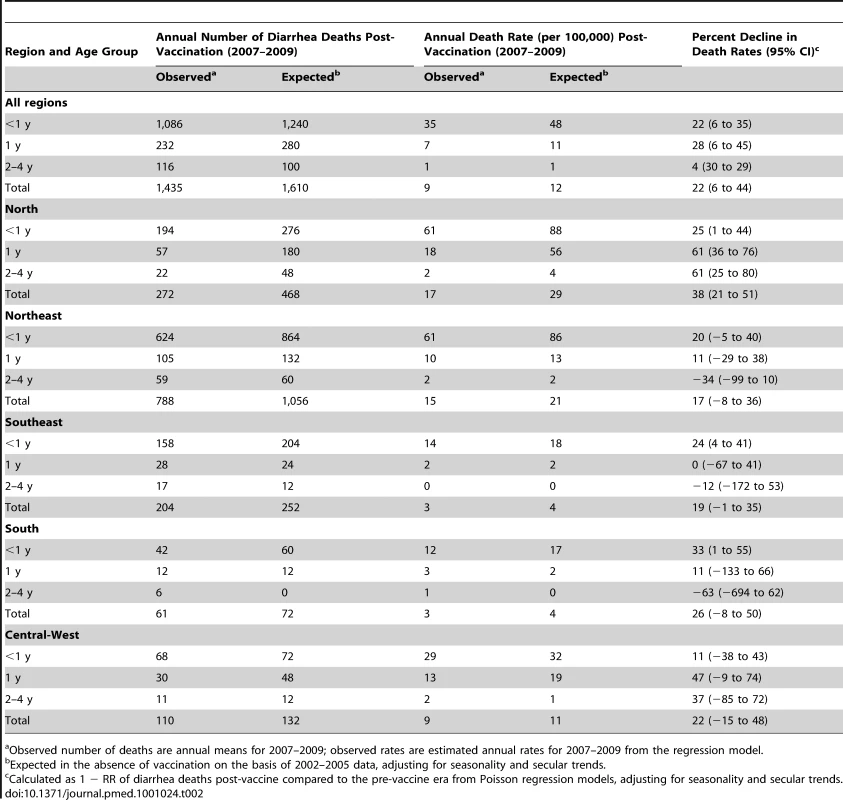
Compared to expected rates based on pre-vaccine trends, diarrhea-associated mortality among children younger than 5 y of age was 22% (95% CI 6%–44%) lower during the three years after rotavirus vaccination (2007 through 2009; Table 2). Additionally, in the month-to-month comparison of observed versus expected diarrhea mortality, a decline occurred during most months of the post-vaccination period (Figure 2), with most prominent reductions during January through March. Similar declines in diarrhea deaths among children younger than 5 y of age were observed during each post-vaccination year, with a 21% reduction (95% CI 6%–34%) in 2007, a 24% reduction (95% CI 7%–38%) in 2008, and a 33% reduction (95% CI 14%–47%) in 2009.
Among all children younger than 5 y of age, observed mortality rates post-vaccination declined significantly in all regions, with largest absolute reductions occurring in high mortality regions of North and Northeast Brazil (Table 2). Among children younger than 1 y of age, observed rates were significantly lower than expected in all regions. Among 1 - to <2-y-old children, the absolute declines in diarrhea-associated mortality in the North accounted for most of the overall declines in this age group. During each post-vaccination year, diarrhea-related mortality among children younger than 5 y of age was lower than expected in all but one region of Brazil (Figure 3). While a decline in mortality was not observed in the Central-West region (−2%; 95% CI −74% to 41%) in 2009 (Figure 3), overall diarrhea mortality during 2007–2009 for this region was non-significantly lower (22% decline; 95% CI −15% to 48%) than expected.
In total, during 2007–2009, approximately 1,500 fewer diarrhea-related deaths were observed in Brazil compared to that expected without a vaccine program among children under 5 y of age.
Diarrhea-Related Admissions
During the pre-vaccine period, there were a total of ∼1,091,000 hospital admissions for diarrhea from all causes reported through the SUS among children younger than 5 y, giving an average annual rate of 1,593 per 100,000. Similar to diarrhea-related deaths, significant declining trends prior to vaccine introduction were also observed in diarrhea-related admissions among children younger than 1 y of age (RR = 0.93/y; 95% CI 0.90–0.97; p<0.001), but not in children 1 to <2 y of age (RR = 0.99/y; 95% CI 0.94–1.04; p = 0.66) or 2 to 4 y of age (RR = 0.98/y; 95% CI 0.94–1.03; p = 0.59) (Figure 1). Unlike diarrhea-related deaths, which were mostly concentrated in the poorest North and Northeast regions, diarrhea-related hospital admissions occurred throughout Brazil. However, the highest expected diarrhea admission rates (per 100,000) were in the less developed North (2,915) and Northeast (2,070) and the lowest rates in the more developed Southeast (666) and South (892) regions of Brazil (Table 3).
During the three years following vaccine introduction, the observed annual hospital admission rates for diarrhea among children younger than 1 y and children 1 to <2 y of age were 637 and 601 per 100,000 lower than the expected rates without vaccination (Table 3), reductions of 25% (95% CI 14%–34%) and 21% (95% CI 7%–33%; p<0.001), respectively. Reduction in diarrhea-related hospital admissions was most prominent during May to October (Figure 4). Countrywide observed diarrhea-related hospital admissions among children younger than 5 y of age were lower than expected during each post-vaccination year, with a 21% reduction (95% CI 9%–31%) in 2007, an 11% reduction (95% CI −5% to 24%) in 2008, and a 24% reduction (95% CI 8%–37%) in 2009. Compared to expected rates, significantly lower diarrhea-related hospital admission rates were observed in four out of five regions (Table 3). In the South, diarrhea-related hospital admissions were 12% (95% CI −8% to 29%) lower than expected in 2007 and 11% (95% CI −19% to 33%) lower than expected in 2009, but above the expected by 27% (95% CI 0%–62%) during 2008 (Figure 3).
Overall, during 2007–2009, a 17% (95% CI 5%–27%) reduction, or approximately 130,000 fewer diarrhea-related hospital admissions (∼42,480 per year), was observed compared to the number of admissions expected in the absence of vaccination among children under 5 y of age in Brazil.
Discussion
The introduction of a single-strain rotavirus vaccine in the childhood immunization program in Brazil was associated with a significant nationwide decline in diarrhea-related deaths and hospital admissions among children younger than 5 y of age. Reductions in mortality are consistent with results from Mexico, where the single-strain rotavirus vaccine was introduced in the same year (2006) [24]. However, Brazil is one of several countries in which substantial reductions in hospital admissions due to diarrhea have been shown following introduction of rotavirus vaccination into the national immunization program [19],[20]. Prevention of rotavirus-related admissions is particularly important for middle - and high-income countries in which nonfatal rotavirus diarrhea is a common cause of childhood morbidity. The results from Brazil suggest protection of rotavirus vaccination against both diarrhea deaths and diarrhea-related hospital admissions, adding to the strength of evidence supporting investment in rotavirus vaccination to curtail the 1.3 million deaths and 9 million hospital admissions related to diarrhea that occur annually worldwide.
In Brazil, we observed a reduction of approximately 40,000 admissions per year (i.e., nearly one in five diarrhea-related hospital admissions averted) among children under 5 y of age during 2007–2009. Declines in diarrhea-related hospital admissions were substantial both in the more developed South and Southeast regions, as well as in the poorest regions of Brazil (North and Northeast), where socioeconomic and health indicators approximate those of less developed countries. Because the baseline burden of diarrhea is three to five times greater in the North and Northeast than in other regions of Brazil, the absolute reduction in diarrhea-related deaths and diarrhea-related hospital admissions was much greater in this population. Lower rotavirus vaccine efficacy and immunogenicity have been observed in impoverished populations of Latin America, Asia, and Africa that have the highest risk of severe disease [11],[13],[18]. Significant reductions in diarrhea-associated mortality in the North of Brazil suggest that children living in areas with limited access to health care who are at highest risk of dying from diarrheal illnesses benefitted from vaccination.
Several pertinent findings support rotavirus vaccination as the most likely explanation for the reduction in diarrhea-related deaths and admissions. First, our estimates of decline were adjusted for seasonal fluctuations and secular declines in trends of diarrhea deaths and admissions. Second, the decline among vaccinated age groups was sustained on a national level for three full years after vaccine introduction. Third, the reduction in diarrhea-related hospital admissions was larger in children aged 1 y or less, who had high rates of vaccination, than in those 2 to 4 y of age, who were not eligible for rotavirus immunization during the study period.
Lastly, seasonal blunting in mortality and admissions was noted in several regions. Seasonality in rotavirus disease in Brazil varies by region and study [35]–[37]. In the South, Central-West, and Southeast regions of Brazil, previous surveillance indicated that seasonal peaks in rotavirus admissions occur during May to October, although year-round detection of the virus has also been reported [35],[36]. Our observation of the largest declines in diarrhea admissions during these months of peak rotavirus activity supports the contention that vaccination might have had a causal role. Data are sparse on the seasonality of rotavirus in the North and Northeast regions of Brazil, although one study suggests year-round detection of rotavirus [37], which is consistent with our finding of year-round decreases in diarrhea mortality in these regions.
We were intrigued by the increase in diarrhea-related hospital admissions during 2008 in the South despite high vaccination coverage, which contributed to an overall lower decline in diarrhea-related hospital admissions nationally during the same year. In 2007 and 2008, G2P[4] was the predominant rotavirus strain circulating in Brazil, a strain fully heterotypic to the G1P[8] vaccine used in Brazil, prompting a debate about whether vaccine pressure contributed to its emergence [38]–[40]. While emergence of this strain might explain the higher diarrhea-related hospital admission rates in the South during 2008, we think this is unlikely because the single-strain rotavirus vaccine provided good heterotypic cross-protection against G2P[4] strains in Brazil [26], and a similar G2P[4] strain predominated in the South as well as in other regions that had sustained declines in diarrhea-related disease. The possibility also exists that an accumulation of older susceptible children in 2008 who were unexposed to rotavirus during 2007 because of the large declines in rotavirus disease during that year might have contributed to an increase in the intensity of transmission during 2008 [41]. However, we suspect this is also unlikely to fully explain the increase in the South because similar increases should have been observed across Brazil. One possible explanation for greater than expected diarrhea-related hospital admissions in 2008 in the South of Brazil is circulation of other enteric pathogens causing a regional epidemic of diarrhea.
Several factors could affect the interpretation of our findings, primarily relating to the ecological nature of our study. Ecological studies can be informative but have several shortcomings. Because these studies do not link an exposure to an outcome at an individual level, they may be prone to ecological fallacy (i.e., those unexposed get the disease). Confounding biases such as seasonal periodicity of disease, secular declines in trends, contribution of simultaneously implemented interventions, and changes in coding and health-care treatment patterns cannot be directly assessed with these studies because of the absence of a control group [42]. Although we adjusted for seasonality and secular declines in diarrhea mortality and admissions, we cannot be fully confident that changes in coding or treatment practices did not occur during the study period. While we do not have evidence suggesting that reporting of diarrhea deaths changed over the study period, a decrease in reporting of deaths after rotavirus vaccine introduction could be misinterpreted as vaccine effectiveness. However, any changes in reporting would likely have affected all age groups, and led to decline of mortality or admissions in both older and younger children. The persistent reductions in diarrhea deaths and diarrhea-related hospital admissions across all regions, and the gradient in observed declines, with the lowest reduction in the oldest children, who were not immunized with the rotavirus vaccine, are consistent with vaccine-associated effects. Programmatic realities are such that diagnostic testing is typically not done for etiologic confirmation of diarrhea. Thus, we used all-cause diarrhea codes to assess vaccine impact. Although this may provide less precision with regard to vaccine effect on rotavirus disease, we suspect that measuring impact on all-cause diarrhea is more valuable to decision makers and the public health community because it provides an estimate of the preventable fraction of diarrhea deaths and admissions attributable to rotavirus.
In summary, this time-series analysis provides evidence of substantial reductions following the introduction of rotavirus vaccination of both diarrhea-related deaths and diarrhea-related hospital admissions from a large middle-income country in the Americas with both developing and developed regions. These findings have important global health policy implications. In low-income countries, the main impetus for introduction of rotavirus vaccines has been the potential to prevent rotavirus deaths: the consistency in findings of a sustained reduction in diarrhea mortality after rotavirus vaccination in Mexico [24] and Brazil suggests that rotavirus vaccination is an important tool for reducing the global burden of diarrhea deaths. In middle-income countries that are not eligible for financial support from donors, the potential reductions in diarrhea-related hospital admissions and other health-care costs will be important for cost-effectiveness considerations to justify the purchase of these relatively expensive vaccines. The reductions in diarrhea-related hospital admissions observed in Brazil, especially in low-mortality and higher income regions, are also relevant for decisions in higher income countries that have not yet introduced rotavirus vaccines into their routine immunization programs.
Zdroje
1. BlackRECousensSJohnsonHLLawnJERudanI
2010
Global, regional, and national causes of child mortality in 2008:
a systematic analysis.
Lancet
375
1969
1987
2. ParasharUDBurtonALanataCBoschi-PintoCShibuyaK
2009
Global mortality associated with rotavirus disease among children
in 2004.
J Infect Dis
200
Suppl 1
S9
S15
3. World Health Organization
2009
Meeting of the Immunization Strategic Advisory Group of Experts,
April 2009—conclusions and recommendations.
Wkly Epidemiol Rec
84
220
236
4. [Anonymous]
2010
Evaluation: the top priority for global health.
Lancet
375
526
5. OxmanADBjorndalABecerra-PosadaFGibsonMBlockMA
2010
A framework for mandatory impact evaluation to ensure well
informed public policy decisions.
Lancet
375
427
431
6. ReidpathDDMorelCMMecaskeyJWAlloteyP
2009
The Millennium Development Goals fail poor children: the case for
equity-adjusted measures.
PLoS Med
6
e1000062
doi:10.1371/journal.pmed.1000062
7. PatelMShaneALParasharUDJiangBGentschJR
2009
Oral rotavirus vaccines: how well will they work where they are
needed most?
J Infect Dis
200
Suppl 1
S39
S48
8. PatelMMParasharUD
2009
Assessing the effectiveness and public health impact of rotavirus
vaccines after introduction in immunization programs.
J Infect Dis
200
Suppl 1
S291
S299
9. LinharesACVelazquezFRPerez-SchaelISaez-LlorensXAbateH
2008
Efficacy and safety of an oral live attenuated human rotavirus
vaccine against rotavirus gastroenteritis during the first 2 years of life
in Latin American infants: a randomised, double-blind, placebo-controlled
phase III study.
Lancet
371
1181
1189
10. Ruiz-PalaciosGMPerez-SchaelIVelazquezFRAbateHBreuerT
2006
Safety and efficacy of an attenuated vaccine against severe
rotavirus gastroenteritis.
N Engl J Med
354
11
22
11. MadhiSACunliffeNASteeleDWitteDKirstenM
2010
Effect of human rotavirus vaccine on severe diarrhea in African
infants.
N Engl J Med
362
289
298
12. de PalmaOCruzLRamosHde BairesAVillatoroN
2010
Effectiveness of rotavirus vaccination against childhood
diarrhoea in El Salvador: case-control study.
BMJ
340
c2825
13. ZamanKDangDAVictorJCShinSYunusM
2010
Efficacy of pentavalent rotavirus vaccine against severe
rotavirus gastroenteritis in infants in developing countries in Asia: a
randomised, double-blind, placebo-controlled trial.
Lancet
376
615
623
14. VesikariTMatsonDODennehyPVan DammePSantoshamM
2006
Safety and efficacy of a pentavalent human-bovine (WC3)
reassortant rotavirus vaccine.
N Engl J Med
354
23
33
15. ArmahGESowSOBreimanRFDallasMJTapiaMD
2010
Efficacy of pentavalent rotavirus vaccine against severe
rotavirus gastroenteritis in infants in developing countries in sub-Saharan
Africa: a randomised, double-blind, placebo-controlled
trial.
Lancet
376
606
614
16. BoomJATateJESahniLCRenchMAHullJJ
2010
Effectiveness of pentavalent rotavirus vaccine in a large urban
population in the United States.
Pediatrics
125
e199
e207
17. BoomJATateJESahniLCRenchMAQuayeO
2010
Sustained protection from pentavalent rotavirus vaccination
during the second year of life at a large, urban United States pediatric
hospital.
Pediatr Infect Dis J
29
1133
1135
18. PatelMPedreiraCDe OliveiraLHTateJOrozcoM
2009
Association between pentavalent rotavirus vaccine and severe
rotavirus diarrhea among children in Nicaragua.
JAMA
301
2243
2251
19. CurnsATSteinerCABarrettMHunterKWilsonE
2010
Reduction in acute gastroenteritis hospitalizations among US
children after introduction of rotavirus vaccine: analysis of hospital
discharge data from 18 US states.
J Infect Dis
201
1617
1624
20. LambertSBFauxCEHallLBirrellFAPetersonKV
2009
Early evidence for direct and indirect effects of the infant
rotavirus vaccine program in Queensland.
Med J Aust
191
157
160
21. TateJEPanozzoCAPayneDCPatelMMCorteseMM
2009
Decline and change in seasonality of US rotavirus activity after
the introduction of rotavirus vaccine.
Pediatrics
124
465
471
22. ZellerMRahmanMHeylenEDe CosterSDe VosS
2010
Rotavirus incidence and genotype distribution before and after
national rotavirus vaccine introduction in Belgium.
Vaccine
28
7507
7513
23. de OliveiraLHDanovaro-HollidayMCMatusCRAndrusJK
2008
Rotavirus vaccine introduction in the Americas: progress and
lessons learned.
Expert Rev Vaccines
7
345
353
24. RichardsonVHernandez-PichardoJQuintanar-SolaresMEsparza-AguilarMJohnsonB
2010
Effect of rotavirus vaccination on death from childhood diarrhea
in Mexico.
N Engl J Med
362
299
305
25. GurgelRGBohlandAKVieiraSCOliveiraDMFontesPB
2009
Incidence of rotavirus and all-cause diarrhea in northeast Brazil
following the introduction of a national vaccination
program.
Gastroenterology
137
1970
1975
26. CorreiaJBPatelMMNakagomiOMontenegroFMGermanoEM
2010
Effectiveness of monovalent rotavirus vaccine (Rotarix) against
severe diarrhea caused by serotypically unrelated G2P[4] strains
in Brazil.
J Infect Dis
201
363
369
27. LanzieriTMCostaIShafiFACunhaMHOrtega-BarriaE
2010
Trends in hospitalizations from all-cause gastroenteritis in
children younger than 5 years of age in Brazil before and after human
rotavirus vaccine introduction, 1998-2007.
Pediatr Infect Dis J
29
673
675
28. BarrosFCMatijasevichARequejoJHGiuglianiEMaranhaoAG
2010
Recent trends in maternal, newborn, and child health in Brazil:
progress toward Millennium Development Goals 4 and 5.
Am J Public Health
100
1877
1889
29. United Nations Development Programme
2010
Human development report 2010. Available: http://hdr.undp.org/en/reports/global/hdr2010/. Accessed 19
March 2010
30. TemporaoJG
2003
[The private vaccines market in Brazil: privatization of
public health.] Cad Saude Publica
19
1323
1339
31. Brazilian Ministry of Health
2011
Sistema de Informação do Programa Nacional de
Imunizações. Brasilia: Brazilian Ministry of Health. Available
at: http://pni.datasus.gov.br/inf_estatistica_cobertura.asp.
Accessed 19 March 2011
32. REDE Interagencial de Informação para a
Saúde
2008
Basic health indicators in Brazil: concepts and application,
2nd edition. Brasilia, Brazil: Pan American Health Organization. Available:
http://www.ripsa.org.br/php/index.php Accessed 19 March
2011
33. Brazilian Institute of Geography and Statistics
2010
Population estimates. Available at: http://www.ibge.com.br/english/estatistica/populacao/estimativa2009/default.shtm
[Last accessed March 19, 2011]
34. McCullaghPNelderJ
1989
Generalized linear models.
London
Chapman and Hall
35. MunfordVGilioAEde SouzaECCardosoDMCardosoDD
2009
Rotavirus gastroenteritis in children in 4 regions in Brazil: a
hospital-based surveillance study.
J Infect Dis
200
Suppl 1
S106
S113
36. LuzCRMascarenhasJDGabbayYBMottaARLimaTV
2005
Rotavirus serotypes and electropherotypes identified among
hospitalised children in Sao Luis, Maranhao, Brazil.
Rev Inst Med Trop Sao Paulo
47
287
293
37. LinharesACGabbayYBFreitasRBda RosaESMascarenhasJD
1989
Longitudinal study of rotavirus infections among children from
Belem, Brazil.
Epidemiol Infect
102
129
145
38. GurgelRQCuevasLEVieiraSCBarrosVCFontesPB
2007
Predominance of rotavirus P[4]G2 in a vaccinated
population, Brazil.
Emerg Infect Dis
13
1571
1573
39. NakagomiTCuevasLEGurgelRGElrokhsiSHBelkhirYA
2008
Apparent extinction of non-G2 rotavirus strains from circulation
in Recife, Brazil, after the introduction of rotavirus
vaccine.
Arch Virol
153
591
593
40. PatelMMde OliveiraLHBispoAMGentschJParasharUD
2008
Rotavirus P[4]G2 in a vaccinated population,
Brazil.
Emerg Infect Dis
14
863
865
41. United States Centers for Disease Control and Prevention
2009
Reduction in rotavirus after vaccine introduction—United
States, 2000-2009.
MMWR Morb Mortal Wkly Rep
58
1146
1149
42. World Health Organization
2008
Generic protocol for monitoring impact of rotavirus vaccination on
rotavirus disease burden and viral strains. Document WHO/IVB/08.16
Available: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.16_eng.pdf.
Accessed 16 March 2011. Geneva: World Health Organization
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Quality of Private and Public Ambulatory Health Care in Low and Middle Income Countries: Systematic Review of Comparative Studies
- A Multifaceted Intervention to Implement Guidelines and Improve Admission Paediatric Care in Kenyan District Hospitals: A Cluster Randomised Trial
- The Quality of Medical Care in Low-Income Countries: From Providers to Markets
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
- Improving Effective Surgical Delivery in Humanitarian Disasters: Lessons from Haiti
- Decline in Diarrhea Mortality and Admissions after Routine Childhood Rotavirus Immunization in Brazil: A Time-Series Analysis
- Effect of Pneumococcal Conjugate Vaccination on Serotype-Specific Carriage and Invasive Disease in England: A Cross-Sectional Study
- Effect of a Nutrition Supplement and Physical Activity Program on Pneumonia and Walking Capacity in Chilean Older People: A Factorial Cluster Randomized Trial
- Strategies and Practices in Off-Label Marketing of Pharmaceuticals: A Retrospective Analysis of Whistleblower Complaints
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Medical Complicity in Torture at Guantánamo Bay: Evidence Is the First Step Toward Justice
- A Public Health Emergency of International Concern? Response to a Proposal to Apply the International Health Regulations to Antimicrobial Resistance
- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- The African Women's Protocol: Bringing Attention to Reproductive Rights and the MDGs
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Global Health Philanthropy and Institutional Relationships: How Should Conflicts of Interest Be Addressed?
- A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance
- Claims about the Misuse of Insecticide-Treated Mosquito Nets: Are These Evidence-Based?
- Neglect of Medical Evidence of Torture in Guantánamo Bay: A Case Series
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání