-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Response Regulator Heterodimer Formation Controls a Key Stage in S Development
Two-component signal transduction systems are a primary means of regulating gene expression in bacteria. Recognizing the diversity of mechanisms associated with these systems is therefore critical to understanding the full signaling potential of bacterial cells. We have analyzed the behavior of two orphan, atypical response regulators that play key roles in controlling morphological differentiation in the filamentous bacteria Streptomyces-BldM and WhiI. We demonstrate that BldM activates its Group I target promoters as a homodimer, but that it subsequently activates its Group II target promoters by forming a functional heterodimer with WhiI. BldM-WhiI heterodimer formation thus represents an unusual mechanism for the coactivation of target genes and the integration of regulatory signals at promoters, enhancing the known repertoire of signaling capabilities associated with two-component systems.
Published in the journal: . PLoS Genet 10(8): e32767. doi:10.1371/journal.pgen.1004554
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004554Summary
Two-component signal transduction systems are a primary means of regulating gene expression in bacteria. Recognizing the diversity of mechanisms associated with these systems is therefore critical to understanding the full signaling potential of bacterial cells. We have analyzed the behavior of two orphan, atypical response regulators that play key roles in controlling morphological differentiation in the filamentous bacteria Streptomyces-BldM and WhiI. We demonstrate that BldM activates its Group I target promoters as a homodimer, but that it subsequently activates its Group II target promoters by forming a functional heterodimer with WhiI. BldM-WhiI heterodimer formation thus represents an unusual mechanism for the coactivation of target genes and the integration of regulatory signals at promoters, enhancing the known repertoire of signaling capabilities associated with two-component systems.
Introduction
Two-component signal transduction systems are of central importance in regulating gene expression in bacteria. Canonically they consist of a response regulator, which functions as a homodimer, and a cognate sensor histidine kinase (which may also function as a cognate phosphatase). The activity of the kinase/phosphatase is modulated in response to a perceived stimulus. The sensor kinase autophosphorylates on a conserved histidine residue, and the phosphoryl group is then transferred to a conserved aspartate in the response regulator. The addition of the phosphoryl group stabilizes a conformation of the response regulator that drives an output response, most often the activation of gene expression. However, the intrinsic modularity of these systems has allowed bacteria to evolve variations on this basic theme, including more complex multicomponent phosphorelays, and changes in the nature of the response regulator effector domain such that the output can be, for example, an enzymatic activity rather than DNA binding [1]–[3]. Recognizing the diversity of mechanisms associated with these systems is therefore critical to understanding the full potential of the signaling capabilities of bacterial cells. This study concerns the behavior of two response regulators required for morphological development in the filamentous bacteria Streptomyces.
When streptomycete spores germinate, one or two germ tubes emerge and grow by tip extension and branching to form an extensive, multicellular vegetative mycelium [4]–[6]. Streptomycetes differentiate by forming specialized reproductive structures called aerial hyphae, which emerge from the colony surface into the air. The formation of aerial hyphae requires the activity of a class of developmental master regulators encoded by the bld (bald) genes [4]–[6]. Subsequently, in the most dramatic event of the lifecycle, each multigenomic aerial hypha arrests tip growth and undergoes a massive, synchronous septation event, giving rise to ∼50–100 unigenomic prespore compartments that ultimately develop into mature, pigmented exospores [4]–[6]. The differentiation of aerial hyphae into mature spores is coordinated by the activity of a second class of developmental master regulators encoded by the whi (white) genes. The focus of this work is the interaction between two of these global regulators, BldM and WhiI.
BldM and WhiI are both atypical response regulators (ARRs). In canonical response regulators, the aspartate residue that is subject to phosphorylation sits in a highly conserved pocket within the N-terminal receiver domain. ARRs usually lack essential residues within this phosphorylation pocket, suggesting that their activity is not controlled by phosphorylation [7]–[11]. WhiI has a degenerate phosphorylation pocket, lacking a universally conserved lysine and one of a pair of adjacent aspartate residues essential for binding Mg2+ [12], [13]. Although BldM does have a conserved phosphorylation pocket, a bldM allele carrying a D54A substitution at the putative site of phosporylation fully complements a bldM null mutant [14]. Moreover, BldM could not be phosphorylated in vitro [14]. Finally, BldM and WhiI are both ‘orphan’ response regulators – their genes are not adjacent to a sensor kinase gene, as is most often the case for canonical response regulators. Taken together, these observations strongly suggest that BldM and WhiI are not controlled by phosphorylation as part of conventional two-component systems. BldM and WhiI both belong to the NarL/FixJ subfamily of response regulators. bldM and whiI are found in all sequenced streptomycete genomes and their chromosomal context is conserved throughout. Strikingly, the amino acid sequence of BldM is 100% identical across all sequenced streptomycetes (the only response regulator that is 100% identical across all streptomycetes). WhiI is at least 93% identical, with amino acid variations found mainly in the linker region between the degenerate receiver domain and the DNA-binding domain. All sequenced WhiIs have a degenerate phosphorylation pocket.
During differentiation, the whiI and bldM genes are activated by two cognate, development-specific sigma factors, σWhiG and σBldN, respectively. whiI expression is activated from a single σWhiG target promoter, and thus whiI is not expressed in a whiG mutant [12]. σBldN directs transcription of the p1 promoter of bldM (the other promoter, bldMp2, is σBldN-independent, and so transcription of bldM is developmentally activated from only one of its two promoters in a bldN mutant [15], [16]). In addition to bldM, the other key targets of σBldN are the genes encoding the chaplins and rodlins, the major proteins of the hydrophobic sheath that coats the aerial hyphae and spores in Streptomyces [16]–[19].
Streptomyces venezuelae has recently emerged as an attractive new model system for the analysis of Streptomyces development because it sporulates in liquid culture [16], [20]. Here we take advantage of the S. venezuelae system to apply global microarray transcriptional profiling and ChIP-Seq to characterize the BldM and WhiI regulons. Through this route we go on to show that a key stage in Streptomyces development is controlled by response regulator heterodimer formation between BldM and WhiI, and to greatly expand our understanding of the regulatory network that controls morphological differentiation in these multicellular bacteria. The BldM-WhiI system thus exemplifies the potential of response regulator heterodimer formation as a mechanism to expand the signaling capabilities of bacterial cells.
Results
BldM directly activates the expression of key developmental genes
Having established conditions in which S. venezuelae sporulates abundantly in liquid culture [16], [20], immunoblotting of samples taken at 14, 15 and 16 h of growth in MYM liquid sporulation medium showed that BldM was abundant at each of these time points (Figure S1). To gain greater insight into BldM function, we defined the genome-wide BldM regulon using ChIP-Seq. As described in Materials and Methods, wild-type S. venezuelae was subjected to formaldehyde cross-linking, lysis and sonication after 16 h of growth. After immunoprecipitation using a BldM-specific polyclonal antibody, the resulting DNA was subjected to deep sequencing. As a negative control, a ChIP-Seq experiment was performed on the congenic bldM null mutant. Several well-characterized developmental loci, including ssgR, rshA, smeA-sffA, whiB and whiE, were among the direct BldM targets identified.
Next, in order to determine how BldM influences the expression of its target genes, wild-type S. venezuelae and the congenic ΔbldM mutant were subjected to time-resolved, genome-wide transcriptional profiling during vegetative growth and sporulation. Strains were grown under the same conditions used for the ChIP-Seq experiments. RNA samples were prepared at 2-hour intervals from 8 to 20 hours, by which time sporulation was nearing completion, and following cDNA synthesis and labeling, samples were hybridized to Affymetrix DNA microarrays. Three independent biological replicates were performed for each strain, and analysis of the resulting data showed that the expression of 131 direct BldM targets was significantly down-regulated in the ΔbldM mutant (p<0.01) in comparison to the wild type. In contrast, only six genes were up-regulated in the ΔbldM mutant (p<0.01) in comparison to the wild type. These results suggest that BldM functions mainly as a transcriptional activator.
Many BldM target genes cluster into two discrete groups based on whi gene dependency
We next determined the time-resolved transcriptional profiles of the BldM target genes in seven constructed white mutants: ΔwhiA, ΔwhiB, ΔwhiD, ΔwhiG, ΔwhiH and ΔwhiI. Strikingly, many of the BldM target genes clustered into two well-defined groups according to their dependencies on the whi genes. Group I genes consisted of developmentally induced genes that depend on bldM, but were activated normally in all the whi mutants (Figure 1A and Table S1). Group II BldM target genes were also developmentally induced, but depended not only on bldM, but also on whiG and whiI (Figure 1B and Table S2).
Fig. 1. Microarray transcription profiles of (A) Group-I and (B) Group-II genes in wild-type S. venezuelae during submerged sporulation and in congenic ΔbldM, ΔwhiA, ΔwhiB, ΔwhiD, ΔwhiG, ΔwhiH and ΔwhiI null mutants grown under identical conditions. 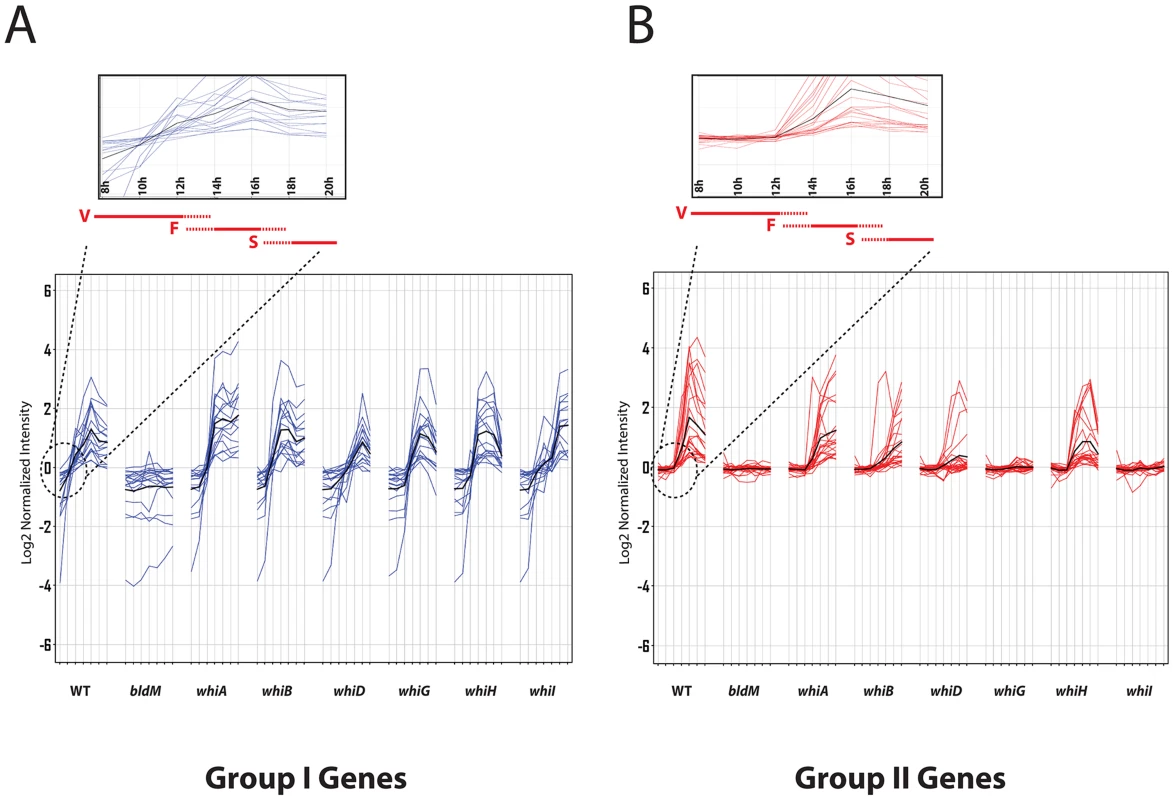
RNA samples were prepared at 2-hour intervals from 8 to 20 hours, by which time sporulation was nearing completion. For each panel, the x-axis indicates the age of the culture in hours, and the y-axis indicates the per gene normalized transcript abundance (log2). Group-I genes (blue) depend on bldM but are independent of all the whi genes. Group-II genes (red) depend on bldM but also depend whiG and whiI. The average expression profile is indicated by the black line. Group I genes are activated at least two hours earlier than Group-II genes (see insets). Red bars indicate vegetative growth (V), fragmentation of mycelium (F), and spore formation (S). Group I genes-identification of a consensus Group I BldM binding site
To gain further insight into Group I binding sites, we fed the sequences of Group I promoter regions into the MEME algorithm [21] to search for over-represented sequences, using as input the entire intergenic region in each case. This analysis revealed a well-conserved copy of a 16 bp palindromic sequence, 5′-TCACcCgnncGgGTGA-3′, for which the sequence logo is shown in Figure 2A. The palindromic nature of this sequence would be consistent with BldM binding as a homodimer to Group I promoters.
Fig. 2. BldM binds to the MEME-predicted Group-I consensus sequence. 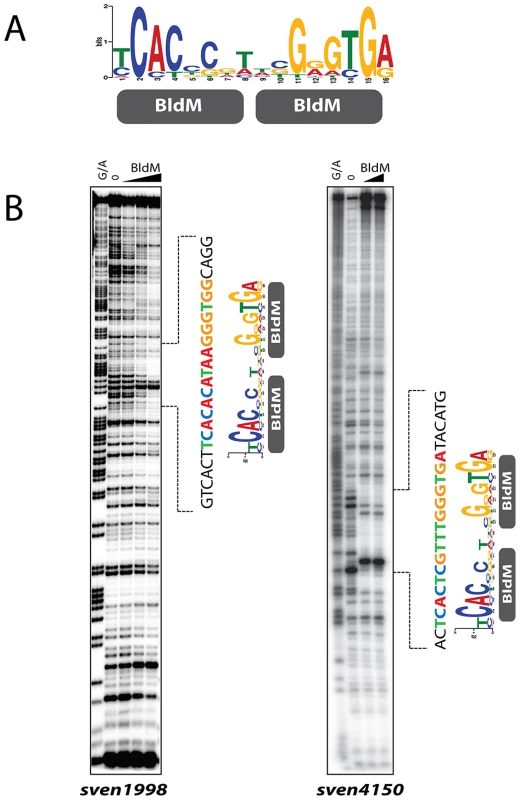
A. The MEME-predicted palindromic Group-I consensus sequence. The height of the letters in the sequence logo, in bits, is proportional to the frequency of the A, C, T, or G nucleotides at each position of the motif. B. DNase I footprinting analysis of BldM on two Group-I promoters: sven1998 and sven4150. The BldM protein concentrations used were 0, 100, 250 and 500 nM for sven1998 and 0, 250 and 500 nM for sven4150. G+A indicates the Maxam and Gilbert sequence ladder. The protected regions are indicated by dotted lines and the positions of the MEME-predicted Group I binding motif are shown. To test the validity of the MEME output, and to confirm and extend the ChIP-Seq analysis, we overexpressed and purified BldM from E. coli as a soluble, N-terminally His6-tagged protein. The resulting BldM protein was used in DNase I footprinting analysis on the intergenic regions upstream of two Group I BldM targets, sven1998 and sven4150. In both cases, BldM protected a region containing a well-conserved copy of the palindromic, MEME-predicted binding site (Figure 2B), consistent with this sequence serving as a high-affinity binding site for a BldM homodimer.
Group II genes
Group II BldM target genes were expressed later than Group I genes (see insets in Figure 1). Further, and in contrast to Group I, the expression of Group II genes depended not only on bldM but also on whiG and whiI (Figure 1 and Table S2). It is straightforward to account for the dependence of Group II gene expression on whiG. In S. coelicolor, whiI expression is activated from a single σWhiG target promoter, and thus whiI is not expressed in a whiG mutant [12]. This σWhiG target promoter appears well conserved at the sequence level in S. venezuelae (Figure S2A) and whiI is not expressed in an S. venezuelae whiG mutant (Figure S2B). Thus all genes that depend on whiI must necessarily also depend on whiG. Expression of whiI was not significantly affected in the ΔbldM mutant and vice versa (p<0.01) (Figure S2B), implying independent σWhiG-WhiI and σBldN-BldM regulatory pathways. The challenge then was to determine why Group II BldM target genes depend on whiI.
Group II genes are direct in vivo targets of WhiI
To determine if the dependence of Group II BldM target genes on WhiI was direct or indirect, we characterized the in vivo WhiI binding sites across the S. venezuelae genome using ChIP-Seq. Wild-type S. venezuelae was harvested at 16 h of growth and treated as described for the BldM ChIP-Seq, except that a WhiI-specific polyclonal antibody was used. As a control, a ChIP-Seq experiment was performed using the congenic whiI null mutant. The data showed that all of the BldM Group II targets were also direct targets of WhiI (Figure 3). As an independent confirmation, we repeated the ChIP-Seq experiment using a FLAG-tagged WhiI protein. An N-terminally 3xFLAG-tagged allele of whiI (TF-WhiI) was constructed such that it was expressed from its native promoter and cloned into the single-copy vector pMS82, which integrates site-specifically into the chromosome at the phage ΦBT1 attB site [22]. This construct fully complemented the phenotype of the whiI null mutant (Figure S3), and the complemented strain was used for the ChIP-Seq experiment, now using wild-type S. venezuelae as the negative control. The ChIP-Seq results seen using FLAG immunoprecipitation were almost identical to those obtained using WhiI polyclonal antibodies, confirming that Group II genes are directly regulated by both BldM and WhiI (Table S2).
Fig. 3. BldM and WhiI ChIP-Seq data for representative Group-I and Group-II promoters analysed in wild-type, ΔbldM and ΔwhiI backgrounds. 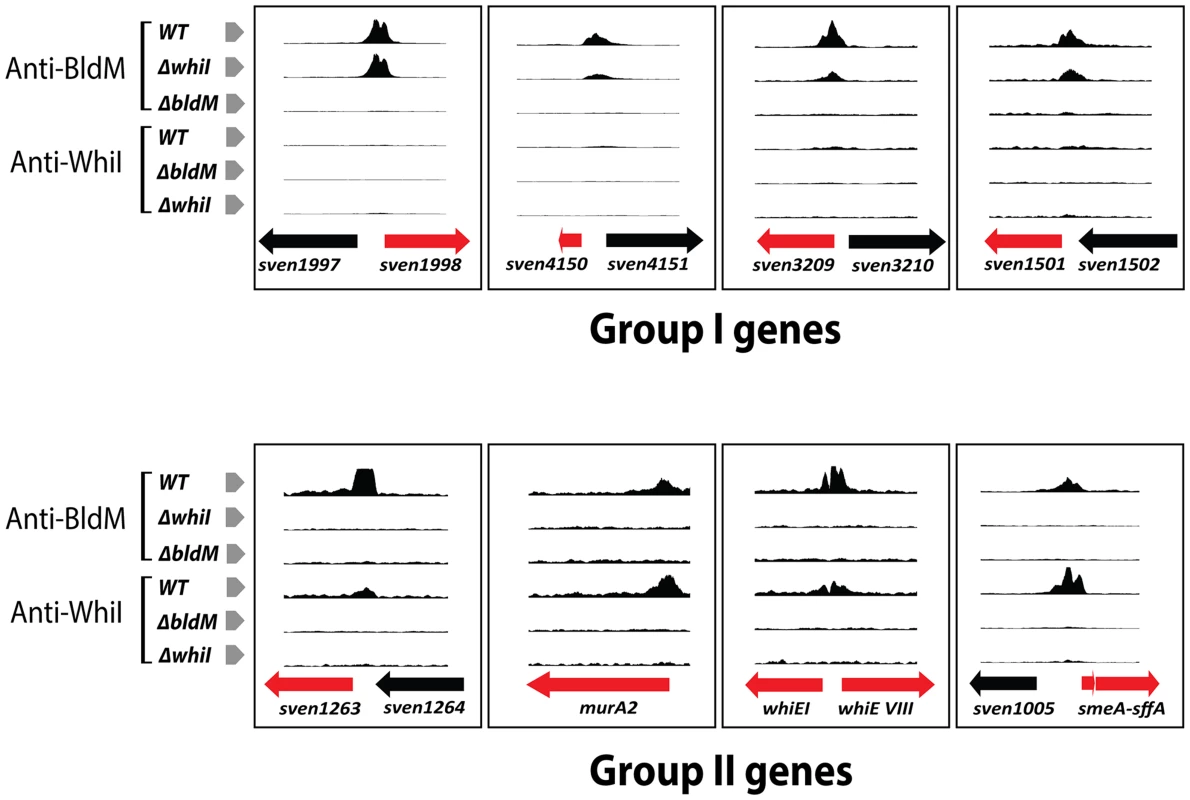
Only BldM binds at Group-I promoters and this occurs both in the wild type and in the ΔwhiI mutant. Both BldM and WhiI bind at Group-II promoters in the wild type, but BldM binding depends on WhiI and vice versa. The genes shown in red are the targets genes. At Group II promoters, in vivo WhiI binding depends on BldM and vice versa
Our data showed that expression of Group II genes depends on bldM and whiI and that both BldM and WhiI bind directly to the promoters of these genes. One possible model consistent with these observations would be that BldM and WhiI co-activate Group II promoters by binding as two separate homodimers. An alternative model would be that these two proteins activate Group II promoters by binding as a functional BldM-WhiI heterodimer. To begin to differentiate between these models, we performed BldM ChIP-Seq in a ΔwhiI mutant and WhiI ChIP-Seq in a ΔbldM mutant, using the same conditions described above. In a ΔwhiI mutant BldM binding was still observed at all Group I promoters (which depend solely on bldM), but no BldM binding was seen at Group II promoters (Figure 3). Equally, no WhiI binding to Group II promoters was observed in a ΔbldM mutant (Figure 3). Thus, in vivo, BldM and WhiI show mutual dependence for binding to Group II promoters.
BldM and WhiI interact in E. coli
To explore the possibility of BldM-WhiI heterodimer formation, we tested BldM and WhiI for direct interaction in E. coli using a bacterial two-hybrid (BACTH) system [23]. bldM was fused to the gene encoding the T18 fragment of adenylate cyclase in the vector pUT18 such that BldM was at the N-terminus of the fusion protein, and was also fused to the gene encoding the T25 fragment of adenylate cyclase in the vector pKT25 such that BldM was at the C-terminus of the fusion protein. Parallel pUT18 and pKT25 constructs were made carrying fusions to WhiI.
Interacting pairs of proteins were screened initially by transforming E. coli BTH101 with the appropriate plasmids and monitoring restoration of adenylate cyclase activity on X-gal indicator plates; clones of each pair were then assayed for β-galactosidase activity (Figure 4). Interaction of BldM with itself was readily observed (∼750 Miller units), but a stronger interaction (∼3000 Miller units), was observed between BldM and WhiI, regardless of which protein was fused to the T18 fragment of adenylate cyclase and which was fused to the T25 fragment (Figure 4). WhiI showed no detectable interaction with itself (Figure 4).
Fig. 4. Bacterial two-hybrid analysis of BldM-WhiI interaction. 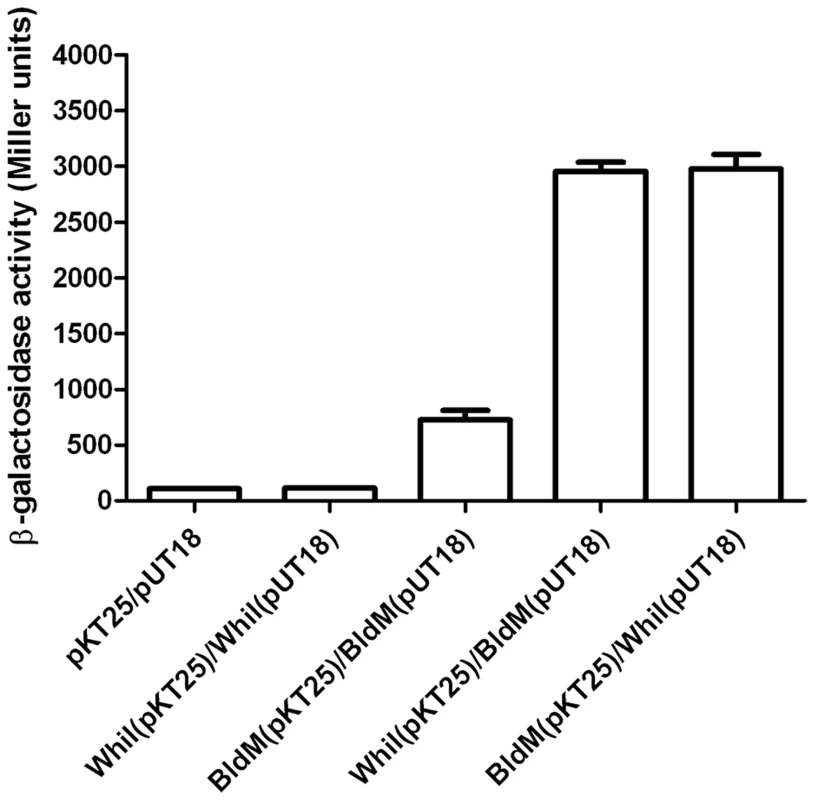
The listed pairs of constructs were transferred into the BACTH reporter strain E. coli BTH101 by transformation. The resulting transformants were selected on MacConkey-maltose agar containing 100 µg/ml carbenicillin and 50 µg/ml kanamycin and incubated at 30°C for two days before four independent clones were picked and subjected to β-galactosidase assays. Error bars show the standard error of the four replicates carried out for each pairwise combination. BldM and WhiI interact in Streptomyces
To confirm and extend the BACTH analysis, we tested the interaction of BldM and WhiI in Streptomyces by coimmunoprecipitation. A C-terminally 3xFLAG-tagged allele of bldM (BldM-TF) was constructed such that it was expressed from its native promoter and cloned into the integrative vector pMS82 [22]. This construct complemented the phenotype of the ΔbldM null mutant to wild-type levels of sporulation (Figure S3). This strain was used in conjunction with the ΔwhiI mutant complemented with the N-terminally 3xFLAG-tagged allele of whiI (TF-WhiI) described above. The BldM-TF and TF-WhiI proteins were immunoprecipitated directly from 16 h MYM liquid cultures using the M2 anti-FLAG monoclonal antibody. WhiI coimmunoprecipitated with BldM-TF and BldM coimmunoprecipitated with TF-WhiI, but neither was detected in the negative controls when the wild-type strain was used (Figure S4). Thus BldM and WhiI interact in Streptomyces in vivo.
Co-expression of BldM rescues WhiI from inclusion bodies
An early frustration in the in vitro analysis of WhiI function was that it overexpressed in an insoluble form in E. coli under all conditions tested. The realization that WhiI might act as part of a functional BldM-WhiI heterodimer led us to try an alternative approach. Where proteins form a complex, it is often observed that individual components are insoluble when expressed in isolation but become soluble when expressed with their cognate partner protein. Two examples are the α and β subunits of lambda integrase [24], and Streptomyces σBldN and its cognate anti-sigma factor, RsbN [16]. Accordingly, BldM and WhiI were co-expressed in E. coli using the pETDuet-1 system (Novagen). Initially, BldM was N-terminally His6-tagged and WhiI was left untagged. Co-expression of BldM was found to solubilize WhiI completely. Further, when His6-BldM was purified on a HisTrap Ni column, WhiI copurified with His6-BldM in approximately equal amounts, despite the fact that WhiI was untagged (Figure 5A) (the identity of the two proteins was confirmed by tryptic mass fingerprinting). Thus BldM rescues WhiI from inclusion bodies and the two proteins copurify in approximately stoichiometric amounts via a His6-tag present on BldM only.
Fig. 5. Affinity purification of the BldM-WhiI complex. 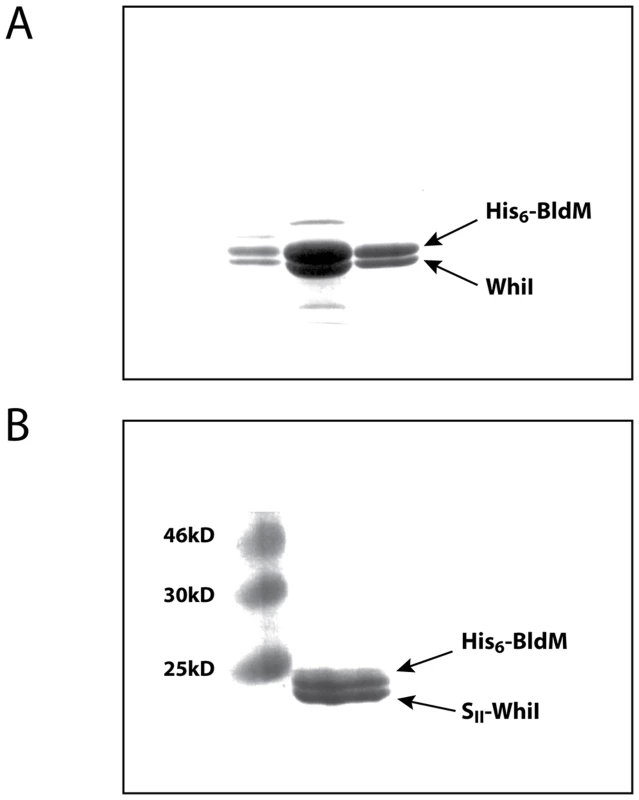
N-terminally His6-tagged BldM and either (A) untagged WhiI, or (B) N-terminally SII-tagged WhiI, were co-expressed from the pETDuet-1 vector. In both cases, co-expression of BldM solubilized WhiI, which formed inclusion bodies when expressed alone. The His6-BldMWhiI complex in (A) was purified over a HisTrap Ni affinity column, and the His6-BldM-SII-whiI complex in (B) was purified over consecutive HisTrap Ni and StrepTrap HP affinity columns. This approach was extended by coexpressing BldM and WhiI carrying two compatible affinity tags. N-terminally His6-tagged BldM and N-terminally StrepII-tagged WhiI were co-expressed from the pETDuet-1 vector. As before, both BldM and WhiI were found in the soluble fraction. The BldM-WhiI complex was then purified over consecutive HisTrap Ni and StrepTrap HP affinity columns and the BldM and WhiI proteins were found to be present in stoichiometric amounts in the resulting preparation (Figure 5B).
In silico analysis of Group II promoters
To further understand Group II binding sites, we searched for over-represented sequences in Group II promoter regions using MEME [21], again using as input the entire intergenic region in each case. This analysis revealed a well-conserved 16 bp non-palindromic sequence, 5′-TGnnCCGnnCGGGTGA-3′, for which the sequence logo is shown in Figure 6A. Strikingly, the 3′ half of the Group II logo was equivalent to a half-site of the Group I palindrome, but the other half was different in sequence, potentially consistent with a BldM-WhiI heterodimer binding to Group II targets.
Fig. 6. BldM-WhiI binds to the MEME-predicted Group-II consensus sequence. 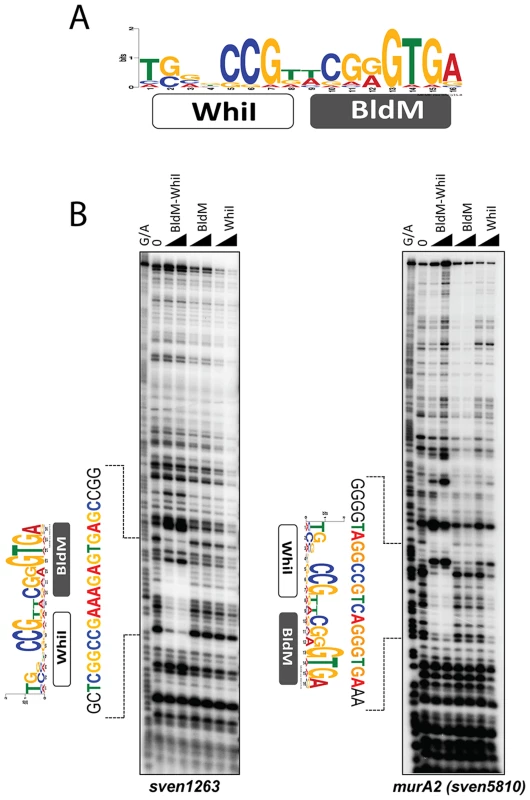
A. The MEME-predicted non-palindromic Group-II consensus sequence. The height of the letters in the sequence logo, in bits, is proportional to the frequency of the A, C, T, or G nucleotides at each position of the motif. B. DNase I footprinting analysis of BldM-WhiI, BldM and GST-WhiI binding to two Group-II promoters (sven1263 and murA2). The protein concentrations used were 100 and 200 nM. G+A indicates the Maxam and Gilbert sequence ladder. The protected regions are indicated by dotted lines and the positions of the MEME-predicted binding motifs are shown. BldM-WhiI binds to the MEME-predicted motifs in Group II promoters but BldM or GST-WhiI do not
To directly test the model that Group II promoters are controlled by a functional BldM-WhiI heterodimer, and to validate the MEME-predicted binding motif for these promoters, the doubly-tagged BldM-WhiI that had been purified over consecutive HisTrap Ni and StrepTrap HP affinity columns was used in DNase I footprinting analysis on the intergenic regions upstream of two Group II targets, sven1263 and murA2 (sven5810). BldM-WhiI footprinted on both promoters and in each case the protection region contained a copy of the non-palindromic, MEME-predicted binding site (Figure 6B), consistent with this sequence serving as a high-affinity binding site for a BldM-WhiI heterodimer. In contrast, neither BldM alone nor WhiI (produced as a soluble GST fusion), footprinted on either promoter (Figure 6B).
Discussion
The focus of this study is to elucidate the mechanism underlying the direct co-activation by BldM and WhiI of the Group II genes, required for the late stages of development. Our data show that BldM activates transcription of these Group II genes as a BldM-WhiI heterodimer, while activating transcription of the Group I genes required for the early stages of development as a BldM homodimer. This work also significantly expands our knowledge of the regulatory network that controls morphological differentiation in Streptomyces (Figure 7), an advance made possible by exploiting S. venezuelae as a new model species for the genus.
Fig. 7. Schematic representation summarizing the regulatory network involved in controlling BldM and WhiI expression and the activation of Group-I and Group-II genes. 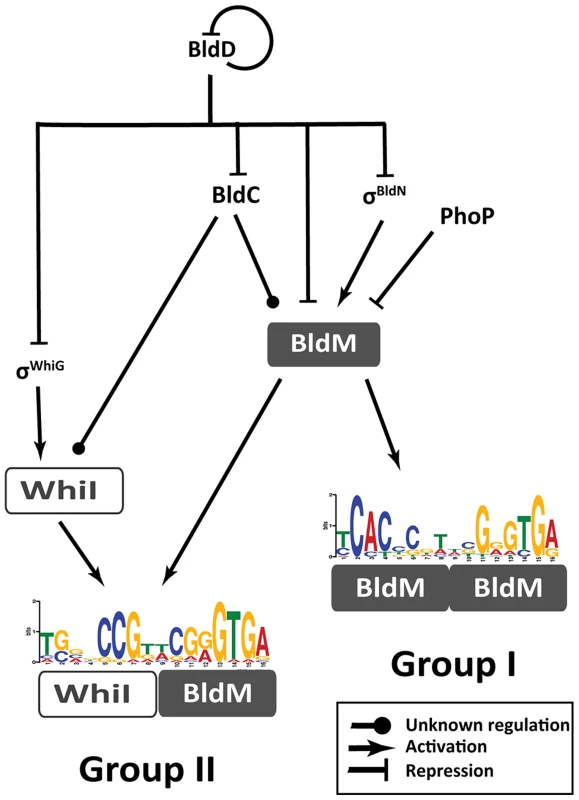
During vegetative growth, BldD represses bldC, bldN, whiG and bldM. At the onset of differentiation, this repression is relieved, σBldN activates bldM and σWhiG activates whiI. BldM homodimer activates early sporulation genes such as whiB and ssgR. Subsequently, BldM and WhiI form a heterodimer that activates late sporulation genes such as whiE and smeA-sffA. Thus heterodimer formation serves to integrate signals from two independent pathways (σWhiG-WhiI and σBldN-BldM). bldM expression is tightly controlled by at least four global regulators - BldD, BldC, σBldN and PhoP (a response regulator activated during phosphate depletion [59]). Group I genes
BldM homodimer activates several genes known to play key roles in the differentiation of aerial hyphae into spores, including whiB and ssgR. In addition to their positive regulation by BldM homodimer, these genes are also subject to repression during vegetative growth by the master regulator BldD, as is bldM itself (Figure 7) [25].
SsgA and SsgB are homologous proteins directly involved in the positive control of cell division in Streptomyces [26]. Sporogenic aerial hyphae undergo a synchronous round of cell division, initiated by the polymerization of a ladder of 50 or more FtsZ rings. SsgA and SsgB function in the recruitment and accurate positioning of these FtsZ rings, and ΔssgA and ΔssgB mutants of S. coelicolor lack sporulation septa [26]–[28]. ssgR encodes an IclR-family transcriptional regulator that directly activates the expression of ssgA in S. coelicolor [29]. Here we show that ssgR is directly activated by, and is completely dependent on BldM in S. venezuelae. ssgB is developmentally induced in S. venezuelae, but despite being a direct BldM target, its expression is only weakly affected in the ΔbldM mutant, suggesting complex regulation of this gene.
BldM homodimer also activates expression of whiB, which plays a vital role in developmentally controlled cell division. whiB null mutants fail to arrest aerial tip growth, the normal prelude to sporulation, and are completely blocked in the initiation of sporulation sepatation, producing abnormally long, undivided aerial hyphae [30]. WhiB is the founding member of a family of proteins confined to the actinomycetes, and several of these WhiB-like (Wbl) proteins have been shown to play key roles in the biology of streptomycetes and mycobacteria. Wbl proteins carry a [4Fe–4S] iron-sulfur cluster coordinated by four invariant cysteines in a C(X29)C(X2)C(X5)C motif [31]–[35], and although the biochemical role of these unusual proteins has been controversial [36], it seems increasingly certain that they function as transcription factors [33], [37]–[38].
Group II genes
Among the Group II targets controlled by the BldM-WhiI heterodimer are two loci with well characterized roles in sporulation: smeA-sffA and whiE. The smeA-sffA operon encodes a DNA translocase (SffA) involved in chromosome segregation into spores that is specifically targeted to sporulation septa by the small membrane protein SmeA [39]. Deletion of smeA-sffA in S. coelicolor results in a defect in spore chromosome segregation and has pleiotropic effects on spore maturation [39]. Like the Group I targets ssgR and whiB, expression of the smeA-sffA operon is also repressed by the master regulator BldD during vegetative growth [25]. whiE is a complex locus that specifies the spore pigment. The structure of the spore pigment has not been determined in any Streptomyces species but its polyketide nature was first predicted from the sequence of the whiE locus in S. coelicolor, because it encodes proteins that closely resemble the components of type II polyketide synthases involved in the synthesis of aromatic antibiotics [40]–[42]. Based on their coordinate regulation and proposed functions, we predict the whiE locus of S. venezuelae consists of an operon of seven genes (sven6798-6792) and the divergently transcribed gene sven6799. Two distinct ChIP-Seq peaks were seen in the intergenic region separating sven6799 and the sven6798-6792 operon, and all eight genes fail to be expressed in the bldM and whiI mutants, implying the BldM-WhiI heterodimer controls expression of the entire locus.
All WhiI-regulated genes belong to Group II
The work presented here suggests that there is no set of genes regulated by a WhiI homodimer and that WhiI functions as an auxiliary protein to modulate BldM binding specificity through heterodimerization. With no exceptions, all the genes down regulated in a ΔwhiI mutant were also down regulated in a ΔbldM mutant. Although some promoters were exclusively enriched as peaks in the WhiI ChIP-Seq experiment, without exception the transcriptional profile of such targets was unaffected in a ΔwhiI mutant, showing that WhiI has no regulatory influence on these genes. Further, in a bldM null mutant, some WhiI peaks are seen in ChIP-Seq, but these sites, often internal to ORFs, show no correlation with the wild-type WhiI regulon and the targets lacked a consensus binding motif. These results suggest that, in the absence of BldM, any DNA binding by WhiI is aberrant and unrelated to its behavior in the wild type. In contrast, in a ΔwhiI mutant, BldM fails to bind to Group II promoters but binds normally to its Group I promoters.
In a recent study, evidence was presented that the DNA-binding domain of S. coelicolor WhiI (in the absence of the receiver domain) can bind in vitro to the promoter of the sco3900-sco3899 operon encoding a transcriptional regulator (InoR) and an inositol 1-phosphate synthase (InoA), respectively [43]. Our ChIP-Seq data show that WhiI does not regulate these genes in S. venezuelae. Further, in wild-type S. venezuelae both genes are actively expressed during vegetative growth but are down-regulated during development, and this expression pattern is unaffected in a whiI mutant.
The potential of response regulator heterodimerization as a regulatory mechanism
Transcription factor heterodimerization can coordinate responses to different cues by integrating signals from distinct regulatory pathways. Although heterodimerization is prevalent as a regulatory mechanism in eukaryotes [44], it is rare in bacteria. Prior to the work described here, the only response regulator reported to heterodimerize with an auxiliary regulator was RcsB.
In Escherichia coli, the typical response regulator RcsB plays a central role in the regulation of capsule synthesis. Once phosphorylated by the histidine kinase RcsD, RcsB directly activates target genes including rprA, osmC, osmB and ftsZ, functioning as a homodimer [45], [46]. It also activates exopolysaccharide synthesis genes, required for capsule formation, as a heterodimer with RcsA, which is distantly related to response regulators (like RcsB, RcsA has a typical LuxR-type C-terminal DNA-binding domain, but its N-terminal domain is not related to typical response-regulator receiver domains). Like WhiI, RcsA appears to lack the capacity to activate genes by itself, and therefore functions solely as a modulator of RcsB binding specificity. RcsA is actively degraded by the Lon protease and in lon mutants capsule genes are highly upregulated causing a mucoid phenotype, due to enhanced activation by the stabilized RcsB-RcsA heterodimer [45], [46]. The capacity of RcsB to complex with other transcription factors is not limited to its interaction with RcsA, since RcsB also forms functional heterodimers with BglJ [47] and with GadE [48]. Using in vitro FRET analysis, weak hetero-pair interactions were detected between several members of the OmpR sub-family of response regulators from E. coli [49]. While these in vitro data may suggest potential crosstalk between distinct signaling pathways, their physiological significance has yet to be demonstrated.
The activities of numerous bacterial promoters respond to multiple cues, and there are many examples of promoters that depend on two activators for their activity. Several different regulatory mechanisms underpinning such codependence have been identified [50], [51]. The most widely documented is found at promoters where both activators bind independently, and both activators make independent contacts with RNA polymerase. However, there are rare examples of coactivators that exhibit cooperative binding, such as MelR and CRP at the E. coli melAB promoter [52]. There are also examples in which DNA binding by a secondary activator leads to the repositioning of the primary activator from a site where it cannot activate transcription to a site where it can, such as the repositioning of MalT by CRP at the malK promoter [53]. Response regulator heterodimer formation provides a new model for coactivation of target genes and the integration of regulatory signals at promoters. BldM-WhiI heterodimer formation serves to integrate signals from two independent pathways (σWhiG-WhiI and σBldN-BldM) and it may also function as a timing device, since Group II genes are activated later than Group I genes. Thus the BldM-WhiI system exemplifies the potential of response regulator heterodimer formation as a mechanism to expand the signaling capabilities of bacterial cells.
Materials and Methods
Bacterial strains, plasmids, oligonucleotides and growth conditions
Bacterial strains and plasmids are listed in Table S3 and the oligonucleotide primers with corresponding restriction sites used in cloning are listed in Table S4. For microarray and ChIP-Seq experiments, S. venezuelae strains were grown at 30°C in MYM liquid sporulation medium [16] made with 50% tap water and supplemented with 200 µl trace element solution [54] per 100 ml. The phenotypes of mutants and complemented strains were scored after 3–4 days growth on MYM-agar at 30°C. bldM and whiI deletion mutants were constructed by ‘Redirect’ PCR targeting [55] and their chromosomal structures were confirmed by PCR analysis and by Southern hybridization using the parental cosmids as probes.
ChIP (Chromatin immunoprecipitation)-Seq
For each strain, two flasks containing 35 ml of MYM were inoculated with spores (or mycelium in case of the ΔbldM mutant) to give an OD600 ∼0.35 after 8 h of growth. The crosslinking reagent formaldehyde was added to a final concentration of 1% (v/v) to the cultures at 16 h of growth and incubated at 30°C with shaking for 30 min before glycine was added to a final concentration of 125 mM to quench the crosslinking reaction. The samples were incubated at room temperature for 5 min and washed twice in PBS buffer pH 7.4 (Sigma). Mycelial pellets were resuspended in 0.5 ml lysis buffer (10 mM Tris-HCl pH 8, 50 mM NaCl, 15 mg/ml lysozyme, 1x protease inhibitor) and incubated at 37°C for 20 min. The lysate was resuspended in 0.5 ml IP buffer (100 mM Tris - HCl pH 8, 250 mM NaCl, 0.1% Triton-X-100, 1x protease inhibitor) and the lysate was kept on ice for 2 min before sonication. The samples were subjected to seven cycles of sonication, 15 s each, at 10 microns, to shear the chromosome into fragments ranging in size from 300–1000 bp. The sample was then centrifuged twice at top speed, 4°C for 15 minutes to clear cell extracts. To pre-clear non-specific binding, 90 µl protein A sepharose (Sigma) was added to cell lysate (about 900 µl) and incubated for 1 h at 4°C with mixing. The beads were cleared by centrifugation at top speed for 15 min. 100 µl BldM or WhiI antibodies were added to the corresponding cell lysates overnight at 4°C with mixing. 100 µl Protein A Sepharose 1∶1 suspension was added to immunoprecipitate antibody-BldM or WhiI chromatin complexes and incubated for 4 h at 4°C with mixing. The samples were centrifuged at 3500 rpm for 30 s and the beads were washed four times with IP buffer. The pellets were eluted in 150 µl IP elution buffer (50 mM Tris-HCl pH 7.6, 10 mM EDTA, 1% SDS) overnight at 65°C to reverse crosslink. The samples were centrifuged at top speed for 5 min to remove the beads and the pellets were re-extracted with 50 µl TE buffer (10 mM Tris-HCl pH 7.4, 1 mM EDTA). The supernatants were combined and incubated with 3 µl 10 mg/ml proteinase K (Roche) for 2 h at 55°C. The samples were extracted twice with phenol-chloroform to remove protein followed by chloroform extraction to remove traces of phenol and purified with Qiaquick columns (Qiagen). The IP DNA was eluted in 50 µl EB buffer (Qiagen). Sequencing libraries were generated and the IP DNA was sequenced as described previously [20]. The BayesPeak package was used to identify significantly enriched regions and the default parameters were applied [56].
Microarray transcriptional profiling
Microarray transcriptional profiling experiments were carried out as described previously [16], [20]. Multi-experiment viewer software (MeV 4.8) was used for viewing and statistical analysis [57]. The non-parametric tool ‘Rank Products’ [58] was used in MeV to assign ‘down regulated’, ‘up regulated’ and ‘not significant’ genes based on expression at 16, 18 and 20 h of growth. Group I genes were defined as direct BldM ChIP-Seq targets that were significantly down regulated in ΔbldM and not significantly changed in all Δwhi mutants (Table S1). Group II genes were defined as direct ChIP-Seq targets of both BldM and WhiI that were significantly down regulated in ΔbldM, ΔwhiG and ΔwhiI (Table S2).
Protein expression and purification
The open reading frame of interest was PCR-amplified using Expand High-Fidelity DNA polymerase (Roche). Plasmids containing the correct inserts were confirmed by sequencing and introduced into electrocompetent E. coli BL21(DE3)/pLysS. The transformed cells were spread on LB-carbenicillin/chloramphenicol and one colony was used for inoculation. Proteins were expressed in two 2.5 litre volumetric flasks each containing 400 ml LB culture and expression was induced with 0.25 mM IPTG. The optimised temperature for expression varied with the protein: His6-BldM was expressed at 25°C for 5 h; GST-WhiI was expressed at 15°C overnight; His6-BldM/WhiI or SII-WhiI were co-expressed at 30°C for 5 h. The pellets were lysed in a buffer containing 50 mM Tris-HCl pH 8, 250 mM NaCl, 10% glycerol, 0.1% Triton X100, protease inhibitor (complete mini, EDTA-free, Roche) and incubated at room temperature for 20 min. HisTrap HP Ni and StrepTrap HP affinity columns (GE Healthcare) were used to purify the His6 - and SII-tagged proteins in a tandem manner. The Gst-WhiI was purified with 1 ml GSTrap FF column (GE Healthcare).
DNase I footprinting
Singly 32P end-labelled probes (Table S4) were generated by PCR and purified using Qiaquick columns (Qiagen). Transcription factors were incubated with probe DNA (∼150,000 cpm) for 30 min at room temperature in 40 µl reaction buffer [50 mM Tris-HCl pH 7.5, 100 mM NaCl, 10% glycerol, 10 mM MgCl2, 2 mM dithiothreitol and 1 µg/reaction poly(dI-dC)], prior to treatment with 1 U DNase I (Promega) for 30–50 s in the case of group-II promoters and 3 U DNase I for 15–20 s in the case of group-I promoters. Reactions were terminated with 140 µl of stop buffer (192 mM sodium acetate, 32 mM EDTA, 0.14% SDS, 70 µg/ml yeast tRNA) and samples were extracted with phenol-chloroform prior to ethanol precipitation. Footprinting samples were loaded on 6% polyacrylamide sequencing gels, next to a G+A ladders prepared according to the Sure Track footprinting kit (Amersham Pharmacia Biotech).
Immunoprecipitation of BldM-FLAG and FLAG-WhiI proteins from S. venezuelae
C-terminally 3×FLAG-tagged bldM expressed from the native promoter was used to complement the ΔbldM mutant and N-terminal 3×FLAG-tagged whiI carrying native promoter was used to complement the ΔwhiI mutant. 50 µl dense spore suspension was used to inoculate 300 ml MYM in 2 litre flasks with spring baffles. After 17 h growth, cultures were harvested by centrifugation at 6000 rpm for 15 min at 4°C. Pellets were lysed in a buffer containing 50 mM Tris-HCl pH 8, 250 mM NaCl, 10% glycerol, 0.1% Triton X100, protease inhibitor (complete mini, EDTA-free, Roche) and sonicated for 5 cycles at 15-micron amplitude for 20 s. FLAG-tagged proteins were immunoprecipitiated using M2 beads [anti-FLAG antibodies covalently attached to agarose beads (Sigma)], the beads were washed using TBS buffer containing protease inhibitor (Roche) and 0.05% Triton X100, and the protein was eluted using FLAG peptides as recommended by the manufacturer.
Preparation of crude cell extracts and immunoblot analysis
S. venezuelae strains were grown in MYM medium. 10 ml samples were taken at 14, 15, and 16 hours of growth. Strains were harvested by centrifugation at 3000 rpm for 1 min, washed in 5 ml ice-cold washing buffer (20 mM Tris pH 8.0, 5 mM EDTA) and resuspended in 0.4 ml of ice-cold sonication buffer [20 mM Tris pH 8.0, 5 mM EDTA, 1x protease inhibitor (Roche)]. Samples were sonicated immediately for 5 cycles, 20 s at 10 microns with 1 min intervals of ice incubation, then centrifuged at 13000 rpm at 4°C for 15 min to remove cell debris. Protein concentrations of the supernatant crude cell extracts were measured by Bradford assay and samples (10 µg protein) were separated on a 12.5% SDS-PA gel and blotted onto nitrocellulose membrane. The membrane was incubated in blocking solution [10% dried milk powder in TBS (0.05 M Tris, 0.9% NaCl, pH 7.6, 0.1% Tween)] overnight and then incubated for 1 h at room temperature with the 1/2500 dilution of anti-BldM antiserum in blocking solution. The membrane was rinsed (twice for 10 min) in TBS and then incubated for 1 h with 1/5,000 dilutions of horseradish peroxidase-linked goat anti-rabbit immunoglobulin G antibody (GE Healthcare). Blots were developed using the ECL enhanced chemiluminescence system from GE Healthcare and were typically exposed to X - ray film for between 30 s and 5 min.
Supporting Information
Zdroje
1. CapraEJ, LaubMT (2012) Evolution of two-component signal transduction systems. Annu Rev Microbiol 66 : 325–347.
2. GaoR, StockAM (2009) Biological insights from structures of two-component proteins. Annu Rev Microbiol 63 : 133–154.
3. MitrophanovAY, GroismanEA (2008) Signal integration in bacterial two-component regulatory systems. Genes Dev 22 : 2601–2611.
4. Elliot MA, Buttner MJ, Nodwell JR (2008) Multicellular Development in Streptomyces. In: Whitworth DE, editor. Myxobacteria: Multicellularity and Differentiation. Washington D.C.: ASM Press. pp. 419–438.
5. FlärdhK, ButtnerMJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7 : 36–49.
6. McCormickJR, FlärdhK (2012) Signals and regulators that govern Streptomyces development. FEMS Microbiol Rev 36 : 206–231.
7. BourretRB (2010) Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol 13 : 142–149.
8. HongE, LeeHM, KoH, KimDU, JeonBY, et al. (2007) Structure of an atypical orphan response regulator protein supports a new phosphorylation-independent regulatory mechanism. J Biol Chem 282 : 20667–20675.
9. FraserJS, MerlieJPJr, EcholsN, WeisfieldSR, MignotT, et al. (2007) An atypical receiver domain controls the dynamic polar localization of the Myxococcus xanthus social motility protein FrzS. Mol Microbiol 65 : 319–332.
10. WangL, TianX, WangJ, YangH, FanK, et al. (2009) Autoregulation of antibiotic biosynthesis by binding of the end product to an atypical response regulator. Proc Natl Acad Sci U S A 106 : 8617–8622.
11. HickeyJM, LovellS, BattaileKP, HuL, MiddaughCR, et al. (2011) The atypical response regulator protein ChxR has structural characteristics and dimer interface interactions that are unique within the OmpR/PhoB subfamily. J Biol Chem 286 : 32606–32616.
12. AínsaJA, ParryHD, ChaterKF (1999) A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol Microbiol 34 : 607–619.
13. TianY, FowlerK, FindlayK, TanH, ChaterKF (2007) An unusual response regulator influences sporulation at early and late stages in Streptomyces coelicolor. J Bacteriol 189 : 2873–2885.
14. MolleV, ButtnerMJ (2000) Different alleles of the response regulator gene bldM arrest Streptomyces coelicolor development at distinct stages. Mol Microbiol 36 : 1265–1278.
15. BibbMJ, MolleV, ButtnerMJ (2000) σBldN, an extracytoplasmic function RNA polymerase sigma factor required for aerial mycelium formation in Streptomyces coelicolor A3(2). J Bacteriol 182 : 4606–4616.
16. BibbMJ, DomonkosÁ, ChandraG, ButtnerMJ (2012) Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by σBldN and a cognate anti-sigma factor, RsbN. Mol Microbiol 84 : 1033–1049.
17. ElliotMA, KaroonuthaisiriN, HuangJ, BibbMJ, CohenSN, et al. (2003) The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev 17 : 1727–1740.
18. ClaessenD, RinkR, de JongW, SiebringJ, de VreugdP, et al. (2003) A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev 17 : 1714–1726.
19. ClaessenD, StokroosI, DeelstraHJ, PenningaNA, BormannC, et al. (2004) The formation of the rodlet layer of streptomycetes is the result of the interplay between rodlins and chaplins. Mol Microbiol 53 : 433–443.
20. BushMJ, BibbMJ, ChandraG, FindlayKC, ButtnerMJ (2013) Genes required for aerial growth, cell division, and chromosome segregation are targets of WhiA before sporulation in Streptomyces venezuelae. MBio 4: e00684–13.
21. Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. Menlo Park: AAAI Press. pp 28–36.
22. GregoryMA, TillR, SmithMCM (2003) Integration site for Streptomyces phage ΦBT1 and development of site-specific integrating vectors. J Bacteriol 185 : 5320–5323.
23. KarimovaG, PidouxJ, UllmannA, LadantD (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA 95 : 5752–5756.
24. NashHA, RobertsonCA, FlammE, WeisbergRA, MillerHI (1987) Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J Bacteriol 169 : 4124–4127.
25. den HengstCD, TranNT, BibbMJ, ChandraG, LeskiwBK, et al. (2010) Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol Microbiol 78 : 361–379.
26. WillemseJ, BorstJW, de WaalE, BisselingT, van WezelGP (2011) Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev 25 : 89–99.
27. van WezelGP, van der MeulenJ, KawamotoS, LuitenRG, KoertenHK, et al. (2000) ssgA is essential for sporulation of Streptomyces coelicolor A3(2) and affects hyphal development by stimulating septum formation. J Bacteriol 182 : 5653–5662.
28. KeijserBJ, NoensEE, KraalB, KoertenHK, van WezelGP (2003) The Streptomyces coelicolor ssgB gene is required for early stages of sporulation. FEMS Microbiol Lett 225 : 59–67.
29. TraagBA, KelemenGH, van WezelGP (2004) Transcription of the sporulation gene ssgA is activated by the IclR-type regulator SsgR in a whi-independent manner in Streptomyces coelicolor A3(2). Mol Microbiol 53 : 985–1000.
30. FlärdhK, FindlayKC, ChaterKF (1999) Association of early sporulation genes with suggested developmental decision points in Streptomyces coelicolor A3(2). Microbiology 145 : 2229–2243.
31. JakimowiczP, CheesmanMR, BishaiWR, ChaterKF, ThomsonAJ, et al. (2005) Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. J Biol Chem 280 : 8309–8315.
32. SinghA, GuidryL, NarasimhuluKV, MaiD, TrombleyJ, et al. (2007) Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc Natl Acad Sci U S A 104 : 11562–11567.
33. SinghA, CrossmanDK, MaiD, GuidryL, VoskuilMI, et al. (2009) Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog 5: e1000545.
34. CrackJC, den HengstCD, JakimowiczP, SubramanianS, JohnsonMK, et al. (2009) Characterization of [4Fe-4S]-containing and cluster-free forms of Streptomyces WhiD. Biochemistry 48 : 12252–12264.
35. CrackJC, SmithLJ, StapletonMR, PeckJ, WatmoughNJ, et al. (2011) Mechanistic insight into the nitrosylation of the [4Fe-4S] cluster of WhiB-like proteins. J Am Chem Soc 133 : 1112–1121.
36. den HengstCD, ButtnerMJ (2008) Redox control in actinobacteria. Biochem Biophys Acta 1780 : 1201–1216.
37. SmithL, StapletonMR, FullstoneGJ, CrackJC, ThomsonAJ, et al. (2010) Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. Biochem J 432 : 417–427.
38. RybnikerJ, NowagA, van GumpelE, NissenN, RobinsonN, et al. (2010) Insights into the function of the WhiB-like protein of mycobacteriophage TM4 - a transcriptional inhibitor of WhiB2. Mol Microbiol 77 : 642–657.
39. AusmeesN, WahlstedtH, BagchiS, ElliotMA, ButtnerMJ, et al. (2007) SmeA, a small membrane protein with multiple functions in Streptomyces sporulation including targeting of a SpoIIIE/FtsK-like protein to cell division septa. Mol Microbiol 65 : 1458–1473.
40. DavisNK, ChaterKF (1990) Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol 4 : 1679–1691.
41. YuT-W, HopwoodDA (1995) Ectopic expression of the Streptomyces coelicolor whiE genes for polyketide spore pigment synthesis and their interaction with the act genes for actinorhodin biosynthesis. Microbiology 141 : 2779–2791.
42. KelemenGH, BrianP, FlärdhK, ChamberlinL, ChaterKF, et al. (1998) Developmental regulation of transcription of whiE, a locus specifying the polyketide spore pigment in Streptomyces coelicolor A3(2). J Bacteriol 180 : 2515–2521.
43. ZhangG, TianY, HuK, ZhuY, ChaterKF, et al. (2012) Importance and regulation of inositol biosynthesis during growth and differentiation of Streptomyces. Mol Microbiol 83 : 1178–1194.
44. ReményiA, SchölerHR, WilmannsM (2004) Combinatorial control of gene expression. Nat Struct Mol Biol 11 : 812–815.
45. MajdalaniN, GottesmanS (2005) The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol 59 : 379–405.
46. MajdalaniN, GottesmanS (2007) Genetic dissection of signaling through the Rcs phosphorelay. Methods Enzymol 423 : 349–362.
47. VenkateshGR, Kembou KoungniFC, PauknerA, StratmannT, BlissenbachB, et al. (2010) BglJ-RcsB heterodimers relieve repression of the Escherichia coli bgl operon by H-NS. J Bacteriol 192 : 6456–6464.
48. Castanié-CornetMP, CamK, BastiatB, CrosA, BordesP, et al. (2010) Acid stress response in Escherichia coli: mechanism of regulation of gadA transcription by RcsB and GadE. Nucleic Acids Res 38 : 3546–3554.
49. GaoR, TaoY, StockAM (2008) System-level mapping of Escherichia coli response regulator dimerization with FRET hybrids. Mol Microbiol 69 : 1358–1372.
50. LeeDJ, MinchinSD, BusbySJ (2012) Activating transcription in bacteria. Annu Rev Microbiol 66 : 125–152.
51. BrowningDF, BusbySJ (2004) The regulation of bacterial transcription initiation. Nat Rev Microbiol 2 : 57–65.
52. WadeJT, BelyaevaTA, HydeEI, BusbySJ (2001) A simple mechanism for co-dependence on two activators at an Escherichia coli promoter. EMBO J 20 : 7160–7167.
53. RichetE, Vidal-IngigliardiD, RaibaudO (1991) A new mechanism for coactivation of transcription initiation: repositioning of an activator triggered by the binding of a second activator. Cell 66 : 1185–1195.
54. Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces Genetics. The John Innes Foundation. Norwich, United Kingdom.
55. GustB, ChallisGL, FowlerK, KieserT, ChaterKF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100 : 1541–1546.
56. Cairns J, Spyrou C, Stark R, Smith ML, Lynch AG, et al. 2011. BayesPeak—an R package for analysing ChIP-seq data. Bioinformatics 27 : 713–714.
57. SaeedAI, BhagabatiNK, BraistedJC, LiangW, SharovV, et al. (2006) TM4 microarray software suite. Methods Enzymol 411 : 134–193.
58. BreitlingR, ArmengaudP, AmtmannA, HerzykP (2004) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573 : 83–92.
59. AllenbyNE, LaingE, BuccaG, KierzekAM, SmithCP (2012) Diverse control of metabolism and other cellular processes in Streptomyces coelicolor by the PhoP transcription factor: genome-wide identification of in vivo targets. Nucleic Acids Res 40 : 9543–9556.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 8- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Pánevní endometrióza spojená s volnou tekutinou v peritoneální dutině snižuje úspěšnost otěhotnění po intrauterinní inseminaci
-
Všechny články tohoto čísla
- DNA Double Strand Break Repair in Mitosis Is Suppressed by Phosphorylation of XRCC4
- Inference of Transposable Element Ancestry
- The Population Genetics of Evolutionary Rescue
- Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
- Pooled Segregant Sequencing Reveals Genetic Determinants of Yeast Pseudohyphal Growth
- Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
- SMA-Causing Missense Mutations in Display a Wide Range of Phenotypes When Modeled in
- Branch Migration Prevents DNA Loss during Double-Strand Break Repair
- Transcriptome Sequencing from Diverse Human Populations Reveals Differentiated Regulatory Architecture
- Genetic Deletion of SEPT7 Reveals a Cell Type-Specific Role of Septins in Microtubule Destabilization for the Completion of Cytokinesis
- Tethering Sister Centromeres to Each Other Suggests the Spindle Checkpoint Detects Stretch within the Kinetochore
- Global Genetic Variations Predict Brain Response to Faces
- Demography and the Age of Rare Variants
- The Response to High CO Levels Requires the Neuropeptide Secretion Component HID-1 to Promote Pumping Inhibition
- Sp6 and Sp8 Transcription Factors Control AER Formation and Dorsal-Ventral Patterning in Limb Development
- The Groucho Co-repressor Is Primarily Recruited to Local Target Sites in Active Chromatin to Attenuate Transcription
- A Transposable Element Insertion Confers Xenobiotic Resistance in Drosophila
- The Genomic Architecture of Population Divergence between Subspecies of the European Rabbit
- Human Social Genomics
- Gene Expansion Shapes Genome Architecture in the Human Pathogen : An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina)
- Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4
- Roles of Type 1A Topoisomerases in Genome Maintenance in
- The TRIM-NHL Protein LIN-41 Controls the Onset of Developmental Plasticity in
- Wnt-Mediated Repression via Bipartite DNA Recognition by TCF in the Hematopoietic System
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- Integration of UPR and Oxidative Stress Signaling in the Control of Intestinal Stem Cell Proliferation
- miR171-Targeted Scarecrow-Like Proteins Bind to GT -Elements and Mediate Gibberellin-Regulated Chlorophyll Biosynthesis under Light Conditions
- Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress
- LIN-3/EGF Promotes the Programmed Cell Death of Specific Cells in by Transcriptional Activation of the Pro-apoptotic Gene
- A System for Genome-Wide Histone Variant Dynamics In ES Cells Reveals Dynamic MacroH2A2 Replacement at Promoters
- Multiple Regulation of Rad51-Mediated Homologous Recombination by Fission Yeast Fbh1
- A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- Asymmetric Division and Differential Gene Expression during a Bacterial Developmental Program Requires DivIVA
- A Model-Based Approach for Identifying Signatures of Ancient Balancing Selection in Genetic Data
- Chromatin Insulator Factors Involved in Long-Range DNA Interactions and Their Role in the Folding of the Drosophila Genome
- Conditional Inactivation of Upstream Binding Factor Reveals Its Epigenetic Functions and the Existence of a Somatic Nucleolar Precursor Body
- Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in
- Patterns of Admixture and Population Structure in Native Populations of Northwest North America
- Response Regulator Heterodimer Formation Controls a Key Stage in S Development
- A Genetic Strategy to Measure Circulating Insulin Reveals Genes Regulating Insulin Production and Secretion
- EVA-1 Functions as an UNC-40 Co-receptor to Enhance Attraction to the MADD-4 Guidance Cue in
- Dysfunction of the CNS-Heart Axis in Mouse Models of Huntington's Disease
- An Otx/Nodal Regulatory Signature for Posterior Neural Development in Ascidians
- Phosphorylation of a Central Clock Transcription Factor Is Required for Thermal but Not Photic Entrainment
- Genome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
- Ku-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
- Functional Specialization Among Members Of Knickkopf Family Of Proteins In Insect Cuticle Organization
- Playing RNase P Evolution: Swapping the RNA Catalyst for a Protein Reveals Functional Uniformity of Highly Divergent Enzyme Forms
- The Translational Regulators GCN-1 and ABCF-3 Act Together to Promote Apoptosis in
- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- A -Regulatory Mutation of Causes Silky-Feather in Chickens
- VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by
- A Population Genetic Signal of Polygenic Adaptation
- A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Behavior
- The MAP Kinase p38 Is Part of Circadian Clock
- The Cohesin Subunit Rad21 Is Required for Synaptonemal Complex Maintenance, but Not Sister Chromatid Cohesion, during Drosophila Female Meiosis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



