-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
Meiosis is a specialized cell division required for the formation of gametes (sperm and egg). Early in meiosis, the chromosome pairs that we inherit from our mother and father become linked and genetic material is exchanged. This is a remarkable process as every gamete that we make is unique, and the unison between a sperm and egg will create a new individual that harbors novel combinations of characteristics from each parents' family tree. Linkage and genetic exchange between chromosomes is facilitated by a linear protein scaffold structure. A group of protein complexes known as cohesins are a key component of the protein scaffold. To date, there are 4 meiosis-specific cohesin complexes identified. Only one cohesin component known as STAG3 is represented in all meiosis-specific cohesins. We mutated the gene that encodes for STAG3 in mouse and discovered that it results in meiotic failure and absence of gametes. From careful analysis we have determined that STAG3 is required for the stability of meiosis-specific cohesins, which ensure that chromosomes are paired and genetic material is exchanged. Our findings imply that abnormalities in human STAG3 will give rise to chromosome defects, infertility and gonad atrophy.
Published in the journal: . PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004413
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004413Summary
Meiosis is a specialized cell division required for the formation of gametes (sperm and egg). Early in meiosis, the chromosome pairs that we inherit from our mother and father become linked and genetic material is exchanged. This is a remarkable process as every gamete that we make is unique, and the unison between a sperm and egg will create a new individual that harbors novel combinations of characteristics from each parents' family tree. Linkage and genetic exchange between chromosomes is facilitated by a linear protein scaffold structure. A group of protein complexes known as cohesins are a key component of the protein scaffold. To date, there are 4 meiosis-specific cohesin complexes identified. Only one cohesin component known as STAG3 is represented in all meiosis-specific cohesins. We mutated the gene that encodes for STAG3 in mouse and discovered that it results in meiotic failure and absence of gametes. From careful analysis we have determined that STAG3 is required for the stability of meiosis-specific cohesins, which ensure that chromosomes are paired and genetic material is exchanged. Our findings imply that abnormalities in human STAG3 will give rise to chromosome defects, infertility and gonad atrophy.
Introduction
During mitosis, chromosomes are replicated and the resulting sister chromatids are segregated, generating two genetically identical daughter cells. Meiosis, on the other hand, is a specialized cell division that involves chromosome replication and two rounds of chromosome segregation (meiosis I and II), resulting in the formation of up to four haploid gametes. Meiosis I differs from mitosis because homologous chromosomes segregate, whereas sister chromatids remain associated until meiosis II. In order to ensure successful chromosome segregation during meiosis I, three coordinated events occur during prophase I, namely homologous chromosome pairing, synapsis, and recombination [1].
In mitotic cells, a structural maintenance of chromosomes (SMC) complex known as cohesin is required to hold sister chromatids together prior to the metaphase to anaphase I transition. The mammalian mitotic cohesin complex is composed of a heterodimer between SMC1α and SMC3 that form a V-shaped structure that is bridged by an α-kleisin known as RAD21 (Radiation Sensitive 21) and a stromal antigen protein (STAG1 or STAG2) [2]. Meiosis-specific cohesin subunits have been characterized for most model organisms, and are required for the unique events that occur during prophase I. In mammals there is a meiosis-specific SMC1 subunit (SMC1β), two additional α-kleisins (RAD21L and REC8) and another stromal antigen protein (STAG3) [3]–[6]. Based on interaction studies there are at least five species of cohesin complex associated with chromosomes during meiosis, including the mitotic cohesin (SMC1α-SMC3 bridged by STAG1 or 2 and RAD21), meiosis-specific SMC1β-containing cohesins (SMC1β-SMC3 bridged by STAG3 and either RAD21, REC8 or RAD21L), and meiosis-specific SMC1α-containing cohesins (SMC1α-SMC3 bridged by STAG3 and RAD21L or possibly REC8). From these interaction data, STAG3 is the only subunit that it is present in all meiosis-specific cohesin complexes [3], [7], [8].
Mitotic and meiosis-specific cohesin components first localize to chromatin during pre-meiotic S phase (also known at the pre-leptotene stage [2], [3], [8]–[12]. During pre-leptotene, telomeres become anchored to the nuclear envelope and rapid chromosome movements that facilitate initial pairing of homologous chromosomes are observed [13], [14]. Meiosis-specific cohesins localize to the telomeres at this stage and are required for stable telomere anchoring to the nuclear periphery [15]–[17]. All mouse chromosomes are telocentric and STAG3, REC8 and RAD21L cohesins also localize to the heterochromatin rich pericentromeric clusters (“chromocenters”) that form during pre-leptotene and are thought to be required for chromosome pairing [3], [15]. Another SMC protein complex known as SMC5/6 was recently shown to localize to the chromocenters at this stage of meiotic progression [18], [19]. In mitotic cells, it has been shown that chromocenters play a critical role in centromere function, inhibition of DNA recombination and chromosome segregation [20], [21]. When mitotic cells progress through prophase, pericentromeric regions of each chromosome dissociate from one another [20]. In contrast, chromocenters remain prevalent throughout prophase I of meiosis and these clusters may be required for chromosome pairing and inhibition of aberrant DNA recombination events at highly repetitive sequences [18], [19].
During the leptotene sub-stage of prophase I the meiosis-specific topoisomerase II-like enzyme, SPO11 introduces DSBs. These DSBs stimulate a DNA damage response (DDR) signaling cascade directed by ataxia telangiectasia mutated (ATM) and Rad3-related (ATR) kinases. ATM and ATR phosphorylate histone H2AFX (γH2AX) [22], [23] and recruit other DDR proteins including the ATR interacting protein (ATRIP) [24]. Concurrently, all cohesin complexes together with HORMA (Hop1-Rev7-Mad2) domain containing proteins HORMAD1 and 2 and the synaptonemal complex (SC) proteins SYCP2 and SYCP3 form axial elements between sister chromatids [25]–[27]. DSB repair is initiated at the zygotene stage where DNA pairing and strand-exchange proteins RAD51 and DMC1 (disrupted meiotic cDNA) initiate inter-homolog recombination. The inter-homolog interactions are coupled with the formation of the SC, whereby axial elements become lateral elements of the SC, and central region proteins including SYCP1 and testis expressed protein 12 (TEX12) link the homologues together [28]. During SC formation HORMAD1 and 2 disassemble from regions that have synapsed [27]. At the pachytene stage, homologous chromosomes are fully synapsed, and DSB repair is complete, resulting in the formation of non-crossover and crossover events. During spermatogenesis, the largely non-homologous X-Y chromosomes synapse at the pseudo-autosomal region (PAR) and are transcriptionally silenced to form the X-Y body [29], [30]. SC disassembly occurs during the diplotene stage, when central region proteins only remain at sites of crossovers and chromosome ends, whereas lateral element components including cohesin remain associated along the length of the chromosomes [3]–[6], [31]. At the final sub-stage of prophase I, diakineses, lateral element proteins such as SYCP3 and cohesin components SMC1β, RAD21, REC8, STAG3 and RAD21L become more punctate on chromosome arms and more prominent at the centromeres [3]–[6]. However, there is currently an inconsistency in localization data reported for cohesin component RAD21L as it has also been shown to be removed from the chromosome arms either at mid-to late pachytene stage [8], [32] or by diakinesis [33].
Homozygous mouse mutants for meiosis-specific cohesin subunits Smc1β, Rec8 and Rad21L have been characterized in both male and female mice. The aberrant meiotic phenotypes observed for each mutation were not identical. Mutation of Smc1β causes a mid-pachytene arrest in primary spermatocytes with shortened axial elements and failure to form crossovers [34] Female Smc1β mouse mutants on the other hand are fertile, but show correlation between increased incidence of non-disjunction and age, suggesting that there is a cohesin dependent mechanism for stabilizing sites of crossovers and centromeric cohesion [35]. Male mutants for Rad21l have a morphologically different zygotene-like arrest, exhibiting incomplete synapsis between homologues, a degree of synapsis between non-homologues and the absence of crossovers [16]. Rad21l female mutants are fertile, but they have premature ovarian failure which is linked to a defect in synapsis but not maintenance of chiasmata [16]. Male and female mouse mutants for Rec8 result in a meiotic arrest characterized by an aberrant zygotene-like stage with synapsed sister chromatids and the absence of crossovers [36], [37]. Rec8, Rad21l double mutants result in a leptotene-like arrest and immunofluorescence observations suggest that only the mitotic cohesin localizes to the axial elements [12]. Localization of STAG3 to chromosome axes is observed in Smc1β, Rec8 and Rad21L mutants, whereas a chromatin bound STAG3 signal was absent in the Rec8, Rad21l double mutants [12], [16], [34]–[37]. STAG3 is unique, as it is a component of all meiosis-specific cohesin complexes [3], [7], [8]. It is of great interest to assess how mutation of Stag3 effects meiotic progression, in comparison to the other cohesin mutants previously characterized.
We used two independently created null mutations for Stag3 and determined that STAG3 is required for clustering of pericentromeric heterochromatin, maintenance of centromere cohesion between sister chromatids, synapsis between homologues and repair of SPO11-induced DSBs. We show that STAG3 is required for normal axial localization and stability of meiosis-specific cohesin subunits SMC1β, REC8 and RAD21L. Mutation of Stag3 results in a zygotene-like stage arrest, which is less severe than that reported for the Rec8, Rad21l double mutants. We hypothesize that localization of REC8 and RAD21L cohesins to chromosome axes are stabilized by STAG3.
Results
Stag3 mutation results in sterility in male and female mice
We used two independently created Stag3 mutant mouse lines, one created by lentiposon induced mutagenesis (Stag3Ov allele) and the other by targeted mutation (Stag3JAX allele, see Materials and Methods and Fig. S1). Mice homozygous for either mutation and mice containing a combination of both mutant alleles resulted in matching phenotypes with respect to fertility and meiotic defects (Table S1 and Fig. S2). Mice that were heterozygous for the Stag3 mutations were phenotypically indistinguishable from their wild type littermates. Both female and male Stag3 homozygous mutant mice were sterile (Table S1). For 8 week old Stag3Ov mutant mice, the average testis weight was 24.8% of their control litter mates (Fig. 1A, N = 6, SD = 1.77%). Testis sections stained with haemoxylin and eosin (H&E) showed a complete absence of secondary spermatocytes, round spermatids or elongated spermatids (Fig. 1B). Assessment of adult and juvenile testis sections with TUNEL and H&E staining showed that tubule degeneration was first evident during the first wave of spermatogenesis when mid-prophase I is reached (Fig. 1C and D). Spermatid counts from 30 day old mutant and control mice showed that no spermatids were present in the Stag3 mutant tubules (106/1200 cells for heterozygote Vs 0/1200 for the Stag3 mutant). In addition, sperm isolation from the epididymis of 80 day old mice showed that sperm were completely absent in the Stag3 mutant. In 8 week old Stag3Ov mutant mice the average ovary weight was 10.9% of the size of their control litter mates (Fig. 1E, N = 6). H&E stained sections from adult and neonatal Stag3 mutant ovaries showed the complete absence of oocytes (Fig. 1 F and G).
Fig. 1. Stag3 mutation results in gonadal failure. 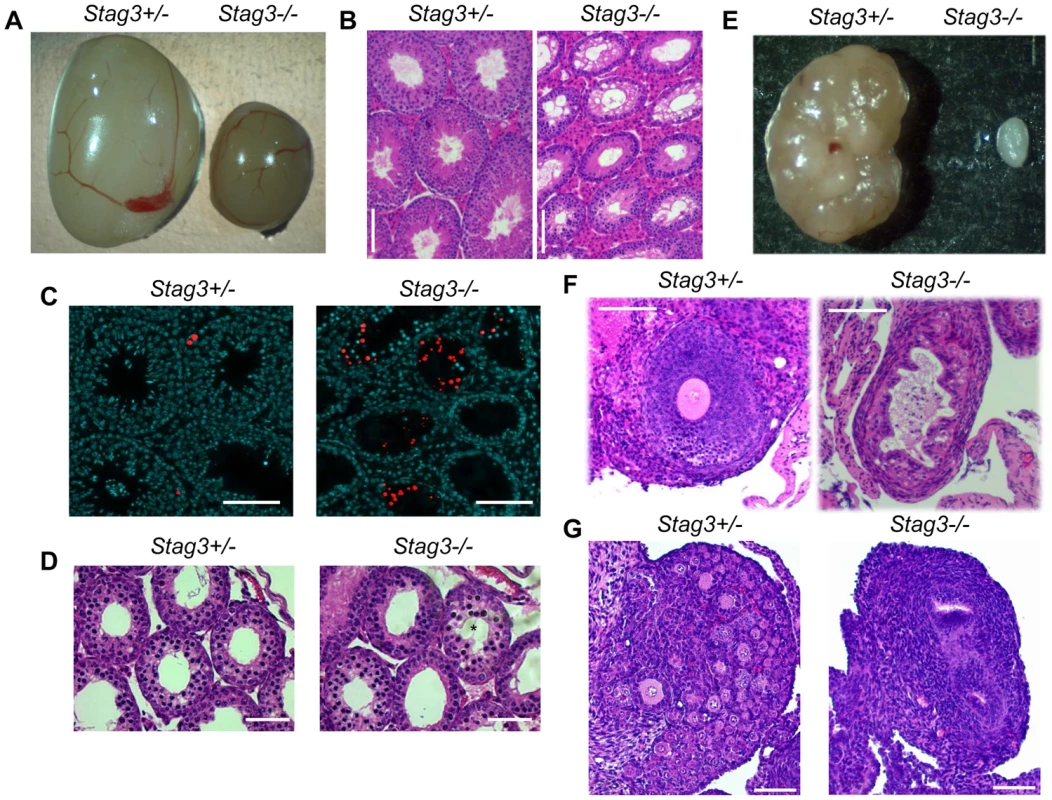
(A) Image of Stag3+/− and Stag3−/− testes at 8 weeks of age. The average testis to body weight ratio of six Stag3+/− and Stag3−/− 8 week old mice was 0.72% (+/− 0.05%) and 0.18% (+/− 0.02%) respectively. (B) Haemoxylin and eosin staining of 5 micron thick testis sections of 8 week old Stag3+/− and Stag3−/− mice, scale bar = 100 µm. (C) TUNEL staining of paraffin embedded 5 micron thick testis sections of 8 week old Stag3+/− and Stag3−/− mice; scale bar = 100 µm. (D) Haemoxylin and eosin staining of 5 micron thick testis sections of 18 days postpartum (dpp) Stag3+/− and Stag3−/− mice. The star represents a tubule that contains germ cells undergoing apoptosis, scale bar = 100 µm. (E) Image of Stag3+/− and Stag3−/− ovaries at 8 weeks of age. The average ovary to body weight ratio of six Stag3+/− and Stag3−/− 8 week old mice was 0.044% (+/−0.0064%) and 0.0048% (+/−0.001%) respectively. (F) Haemoxylin and eosin staining of 5 micron thick ovary sections of 8 week old Stag3+/− and Stag3−/− mice; scale bar = 50 µm. (G) Haemoxylin and eosin staining of 5 micron thick ovary sections of 6 dpp Stag3+/− and Stag3−/− mice, scale bar = 100 µm. All images in this figure are from mice with the Stag3OV mutant allele. Stag3 mutation results in a zygotene-like stage arrest in male and female germ cells
Mouse mutants for all other meiosis-specific cohesin components display defects during meiotic prophase I in spermatocytes [16], [34], [36], [37]. To assess the meiotic defect of the Stag3 mutants more closely, we assessed the formation of chromosome axes using immunofluorescence microscopy of spread chromatin. We staged the progression of prophase I using antibodies against axial/lateral element, SYCP3, and the central region protein SYCP1. Stag3 male and female mutant primary germ cells show aberrancies in leptotene and zygotene stages and fail to reach the pachytene stage (Fig. 2 and Fig. S2). The leptotene stage in control spermatocytes is characterized by many short stretches of SYCP3 (axial elements between sister chromatids) and the absence of SYCP1 (Figure 2A and C; average for Stag3+/Ov control = 154 SYCP3 stretches, N = 40 nuclei). However, the Stag3 mutants display a leptotene-like stage that has fewer SYCP3 stretches (Fig. 2A and C; average for Stag3Ov/Ov mutant = 41 SYCP3 stretches, N = 69 nuclei). At the early zygotene stage, control spermatocytes display fewer, longer stretches of SYCP3, some of which colocalize with SYCP1 indicating that homologous chromosomes are beginning to synapse (Fig. 2A, C and D; average for Stag3+/Ov control = 43 SYCP3 stretches, N = 50 nuclei). During later stages of zygotene, more extensive chromosome synapsis is evident and the number of SYCP3 stretches continues to decrease (Fig. 2A and C; average for Stag3+/Ov control = 25.5 SYCP3 stretches, N = 50 nuclei). Finally, at the pachytene stage, autosomes of the control spermatocytes are completely synapsed and the XY chromosomes are paired within the sex body (Fig. 2A and C; average for Stag3+/Ov control = 20 SYCP3 stretches, N = 40 nuclei). Chromatin spreads of the Stag3 mutant spermatocytes showed SYCP1 loading and we consider these as a zygotene-like stage (Fig. 2A). However, as the extent of SYCP1 loading increased, the number of SYCP3 stretches did not decrease (Fig. 2A and C, most right panel; average for Stag3Ov/Ov mutant = 42 SYCP3 stretches, N = 51 nuclei). Furthermore, the length of the SYCP3 stretches at the zygotene-like stage was approximately 66% shorter than the average length of SYCP3 stretches in wild type chromatin spreads (Fig. 2D). Similar differences in SYCP3 stretch length and number were measured between oocytes from control and Stag3 mutant mice (Fig. 2B and Fig. S3).
Fig. 2. Stag3 mutation results in abnormal meiosis progression, atypical synapsis between sister chromatids, and absence of pachytene stage germ cells. 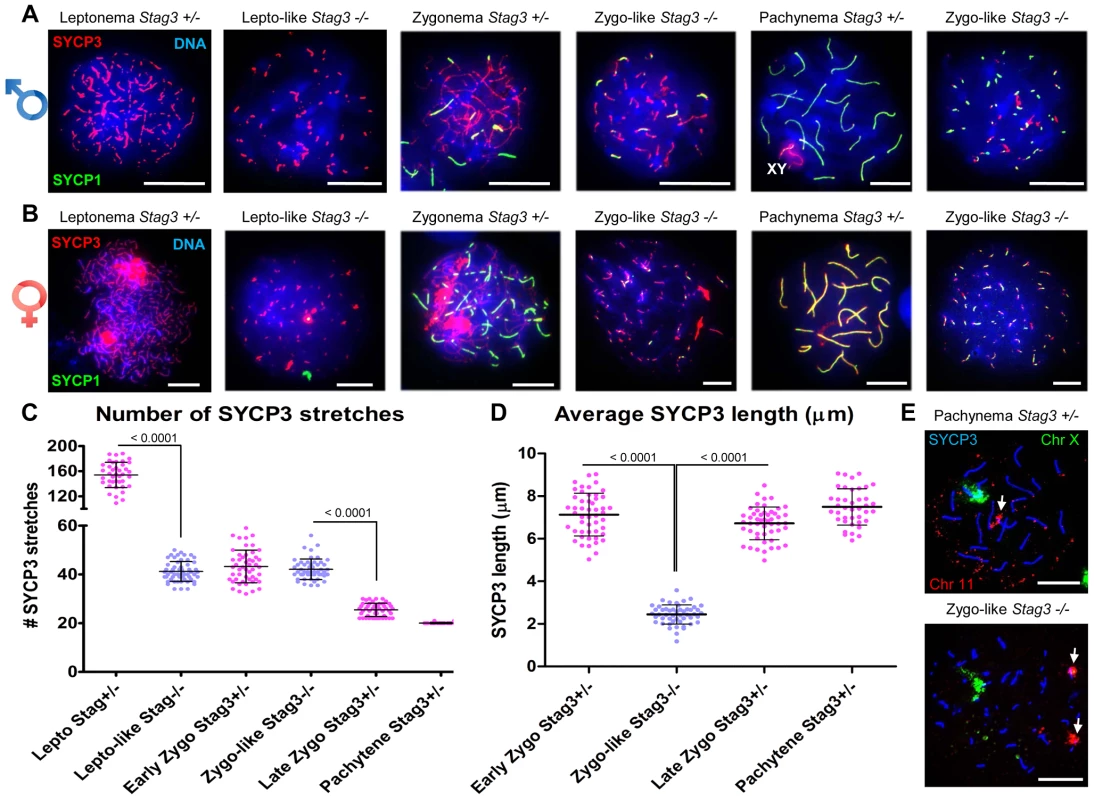
Chromatin spreads from (A) purified testicular germ cells of Stag3+/− and Stag3−/− mice aged 16 dpp and (B) embryonic ovarian germ cells of Stag3+/− and Stag3−/− mice aged 16.5 days post coitum were stained with DAPI (blue, DNA) and immunolabeled using antibodies against the SC lateral element protein SYCP3 (red) and the transverse filament of the central region of the SC SYCP1 (green). Meiotic prophase stages are indicated across the top; Stag3−/− spermatocytes and oocytes were deemed to be at a leptotene-like (lepto-like) stage when SYCP1 was not evident and at a zygotene-like stage (zygo-like) when SYCP1 colocalized with the SYCP3 signal. XY label represents the sex chromosome pair. Images in (A) and (B) are of spermatocytes carrying the Stag3OV mutant allele, but similar phenotypes were observed for spermatocytes with the Stag3JAX mutant allele and mice carrying the Stag3OV and Stag3JAX alleles combined (Fig. S2). (C) Scatter dot-plot graph of the number of SYCP3 linear stretches per spermatocyte chromatin spread during leptotene (lepto; average = 154, N = 40), early zygotene (early zygo; average = 43, N = 50), late zygotene (late zygo; average = 25, N = 50) and pachytene (average = 20, N = 40) stages for the Stag3+/− control and lepto-like (average = 41, N = 50) and zygo-like (average = 42, N = 51) stages for the Stag3−/− mice. Similar results were obtained when assessing oocyte chromatin spreads, summarized in Fig. S3. (D) Scatter dot-plot graph of the average SYCP3 length per spermatocyte chromatin spread during early zygo (7.1 µm), late zygo (6.7 µm) and pachytene (7.4 µm) stages for the Stag3+/− control and zygo-like (2.4 µm) stage for the Stag3−/− mice. Similar results were obtained when assessing oocyte chromatin spreads, summarized in Fig. S3. (E) Chromatin spreads from purified testicular germ cells of Stag3+/− and Stag3−/− mice aged 16 dpp were immunolabeled using an antibody against the SC lateral element protein SYCP3 (blue) and then hybridized to two pre-labelled FISH probes, one that detects the entire X chromosome (green) and the other detects 200 kilobases of mouse chromosome 11 (TK [11qE1]) distal to the centromere (red, white arrows). Mean and standard deviation of the columns of each graph are represented by the black bars and P values are given for indicated comparisons (Mann-Whitney, one-tailed). Experiments were performed using 4 separate littermate pairs of mutant and control mice. Scale bars = 10 µm Following pre-meiotic DNA replication, the number of sister chromatid pairs in mice is 40, which is similar to the number of SYCP3 stretches counted in prophase germ cells of the Stag3 mutant (Fig. 2C). Therefore the SYCP1 loading observed in the zygotene-like chromatin spreads may represent sister chromatid synapsis. To determine whether this was the case we employed fluorescence in situ hybridization (FISH) using two fluorescently labelled DNA probes, one specific to 200 kb of chromosome 11 and the other to detect the X chromosome (Fig. 2E). In spermatocyte chromatin spreads from control mice staged at pachytene, only one FISH signal for each probe was observed. In contrast chromatin spreads from the Stag3 mutant displayed two signals for chromosome 11. This suggests that the SYCP1 signals are indeed present on sister chromatids.
Mouse chromosomes are telocentric, and STAG3, REC8 and RAD21L cohesins localize to the telomeres at the pre-leptotene stage of meiosis [15], [38]. To characterize the Stag3 mutant chromosome axes further we assessed chromatin spreads immuno-stained for SYCP3, the centromere and telomeres (Fig. 3). In control chromatin spreads, a fully synapsed chromosome axis has a centromere and telomere signal at one end, and a telomere signal at the other (Fig. 3A). By analyzing chromatin spreads of the Stag3 mutant, we determined that SYCP3 stretches can indeed form along the entire length of the chromosomes (Fig. 3A middle and top right panel). We also observed circular SYCP3 stretches that were not observed in the control (Fig. 3A bottom right panel and 3B). Circular SYCP3 structures have also been observed in Smc1β mutants and they may be the result of telomere fusion [17].
Fig. 3. Stag3 mutation results in circular SYCP3 stretches, disrupted heterochromatin pericentromeric clustering (chromocenters), and premature loss of centromere cohesion between sister chromatids. 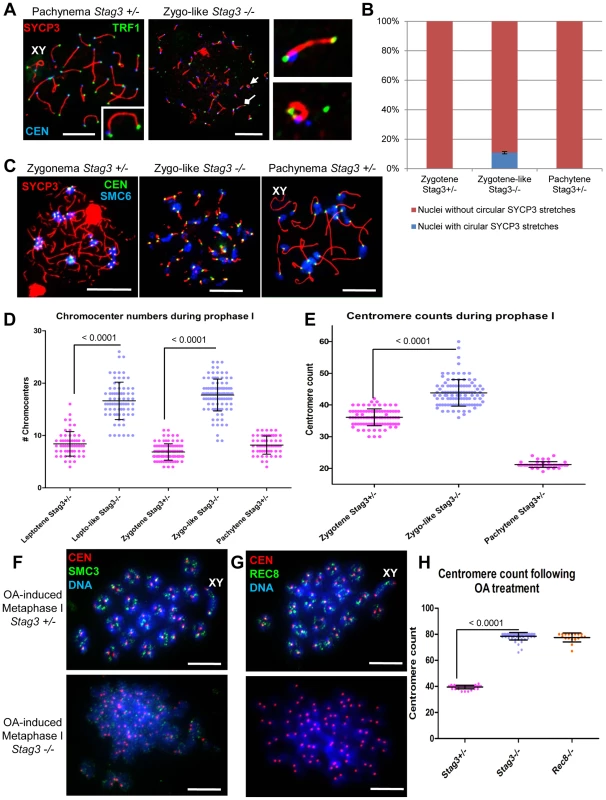
(A-E) Chromatin spreads were prepared from purified testicular germ cells of Stag3+/− and Stag3−/− mice aged 16 dpp. (A) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red), the centromere-kinetochore (blue, CEN) and the telomeric protein TRF1 (green). The left most panel is a Stag3+/− chromatin spread at pachytene stage. XY label represents the sex chromosome pair. Inset image on the bottom right corner is a 2× zoom of a synapsed autosome pair with a telomere signal at each end and a centromere signal at one end. The middle panel is a Stag3−/− chromatin spread at a zygo-like stage. The diamond and triangle arrow heads point to the SYCP3 stretches that are magnified in the right most panels. The top right most panel is a 5× zoom of a Stag3−/− SYCP3 stretch with a telomere signal at each end and a centromere signal at one end. The bottom right most panel is a 5× zoom of a Stag3−/− circular SYCP3 stretch. (B) Quantification of nuclei with circular SYCP3 stretches. No circular SYCP3 stretches were observed during zygotene or pachytene stages for the Stag3+/− control (N = 179 and 224 respectively), whereas 10.9% of zygo-like chromatin spreads from the Stag3−/− mice were recorded to have circular SYCP3 stretches (N = 212). This experiment was performed in triplicate and positive and negative error bars represent the highest and lowest percentage of circular SYCP3 stretches obtained. (C) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red), the centromere-kinetochore (green, CEN) and SMC6 protein which localizes to the pericentromeric heterochromatin clusters also known as “chromocenters” (blue). Meiotic prophase stages are indicated across the top. (D) Scatter dot-plot graph of the number of chromocenters per spermatocyte chromatin spread during leptotene (average = 8.4, N = 56), zygotene (average = 6.9, N = 89) and pachytene (average = 8.2, N = 55) stages for the Stag3+/− control and lepto-like (average = 16.6, N = 74) and zygo-like (17.7, N = 102) stages for the Stag3−/− mice. Similar results were obtained when assessing oocyte chromatin spreads, summarized in Fig. S4A and B. (E) Scatter dot-plot graph of the number of centromere-kinetochore signals per spermatocyte chromatin spread during zygotene (average = 36.1, N = 89) and pachytene (average = 21.2, N = 55) stages for the Stag3−/− mice and zygo-like stage (average = 43.8, N = 102) for the Stag3−/− mice. Experiments were performed using 3 separate littermate pairs of mutant and control mice. Images are from germ cells carrying the Stag3OV allele, comparable phenotypes were observed for germ cells carrying the Stag3JAX mutant allele (Fig. S2). Similar results for centromere counts were obtained when assessing oocyte chromatin spreads summarized in Fig. S4A and C. (F and G) Chromatin spreads from purified and short-term cultured testicular germ cells of Stag3+/− and Stag3−/− mice aged 20 dpp following treatment with 5 µM of okadaic acid. (F) Chromatin spreads stained with DAPI (blue, DNA) and immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and the pan-cohesin component SMC3 (green). (G) Chromatin spreads stained with DAPI (blue, DNA) and immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and the meiosis-specific α-kleisin cohesin component REC8 (green). (H) Scatter dot-plot graph of the number of centromere-kinetochore signals per spermatocyte chromatin spread following 5 hours of OA treatment for Stag3+/− (average = 39.5, N = 40), Stag3−/− (average = 78.5, N = 60) and Rec8−/− (average = 77.6, N = 18) mice. Mean and standard deviation of the columns of each graph are represented by the black bars and P values are given for indicated comparisons (Mann-Whitney, one-tailed). Scale bar = 10 µm Pericentromeric heterochromatin clustering is aberrant in a Stag3 mutant
STAG3, REC8 and RAD21L cohesins also localize to the heterochromatin rich pericentromeric clusters (“chromocenters”) at the pre-leptotene stage of meiosis [3], [15]. In nuclear spread preparations chromocenters can be easily distinguished from the rest of the chromatin by their more dense DAPI staining and can be further confirmed by the presence of the centromeres and SMC5/6 components (Fig. 3C) [18], [19]. From analysis of leptotene stage chromatin spreads, it is evident that there are chromocenter associations between non-homologous chromosomes as there are on average 8.4 chromocenter bodies per nucleus (Fig. 3C and D, N = 56 nuclei). At this stage dynamic chromosome movements are occurring and it has been proposed that these chromocenter associations are important for initial chromosome pairing, DNA repair, and synapsis between homologues [13], [14]. At zygotene stage, chromocenter associations are even more apparent with an average of 6.9 chromocenter bodies per nucleus (Fig. 3C and D; N = 89 nuclei). In contrast the Stag3 mutant shows reduced levels of chromosome associations within chromocenters at both leptotene-like and zygotene-like stages, showing on average 16.2 and 17.7 chromocenter bodies per nucleus respectively (Fig. 3C and D; N = 75 and 102 nuclei respectively). A similar trend for chromocenter counts was obtained from oocyte chromatin spreads of the Stag3 mutant and controls (Fig. S4A and B). This result suggests that STAG3 plays a role in mediating early prophase associations of pericentromeric chromosome ends into chromocenters.
Mutation of Stag3 results in impaired centromere cohesion between sister chromatids
To count the number of centromere-kinetochore structures we used an anti-centromere autoantibody (CEN; also known as ACA and CREST). The average number of centromere-kinetochores counted in control zygotene and pachytene stage nuclei was 36.1 and 21.2 respectively (Figure 3C and E; N = 89 and 20 respectively), which corresponds well to the fact that the centromere-proximal ends are the last regions to synapse [39], [40]. In contrast the average number of centromeres counted in Stag3 mutant zygotene-like nuclei was 43.8 (Fig. 3C and E, N = 102). The centromere number corresponds well with the number of SYCP3 signals observed in the Stag3 mutant, also suggesting that synapsis is occurring between sister chromatids. In addition, 71% of zygotene-like Stag3 mutant nuclei had greater than 40 centromeres, suggesting that centromere cohesion between sister chromatids is compromised (Fig. 3C and E). To further assess this possibility we exposed spermatocytes to okadaic acid (OA), which stimulates an artificial chromatin transition from prophase to metaphase I [41]. When wild type spermatocytes are exposed to OA, they form 20 bivalents each consisting of a centromere-kinetochore pair (40 centromeres, Fig. 3F-H, N = 40). Conversely, 80 separated centromere-kinetochore signals were observed for the Stag3 mutant (N = 60), further demonstrating that STAG3 is required for centromere cohesion.
Absence of STAG3 destabilizes meiosis-specific cohesins
From physical interaction studies, it has been shown that there are up to 6 cohesin complexes present during meiosis, 5 of which are meiosis-specific [3], [7], [8], [34]. SMC3 is the only subunit that is present within all cohesin complexes. From our OA treatment studies we determined that SMC3 remains present on the Stag3 mutant chromatin (Fig. 3F), whereas REC8, a meiosis-specific kleisin subunit, was absent (Fig. 3G). This suggests centromere cohesion in this assay would also be lost in the absence of REC8, which was indeed the case (Fig. 3H).
STAG3 is the only meiosis-specific cohesin subunit that is present in all of the meiosis-specific cohesins [3], [7], [8]. Using antibodies raised against both mitotic and meiosis-specific cohesins, we assessed whether the localization and protein levels of cohesin components were affected in the absence of STAG3. We observed the mitotic cohesin components RAD21, SMC3 and SMC1α colocalize with SYCP3 during the zygotene and pachytene stages of meiosis in wild type spermatocyte and oocyte chromatin spreads (Fig. 4A-C, S5A and B, S6A). These mitotic cohesin components also localize with SYCP3 in the chromatin spreads of the Stag3 mutants. In addition, we immunoprecipitated SMC3 from germ cell extracts and assessed the co-immunoprecipitation of SMC1 and RAD21 (Fig. S7). From this we determined that the mitotic cohesin complex was not affected in the Stag3 mutant. The meiosis-specific cohesin subunits, SMC1β, REC8 and RAD21L also colocalize with SYCP3 during the zygotene and pachytene stages of meiosis (Fig. 4D-F, S5C and D, S6B and C). Strikingly the colocalization of SMC1β, REC8 and RAD21L with SYCP3 in both male and female meiotic chromatin spreads are greatly reduced in the absence of STAG3.
Fig. 4. Mutation of Stag3 does not affect the localization of components of the mitotic cohesin complex, but is required for the localization and stability of meiosis-specific cohesin subunits. 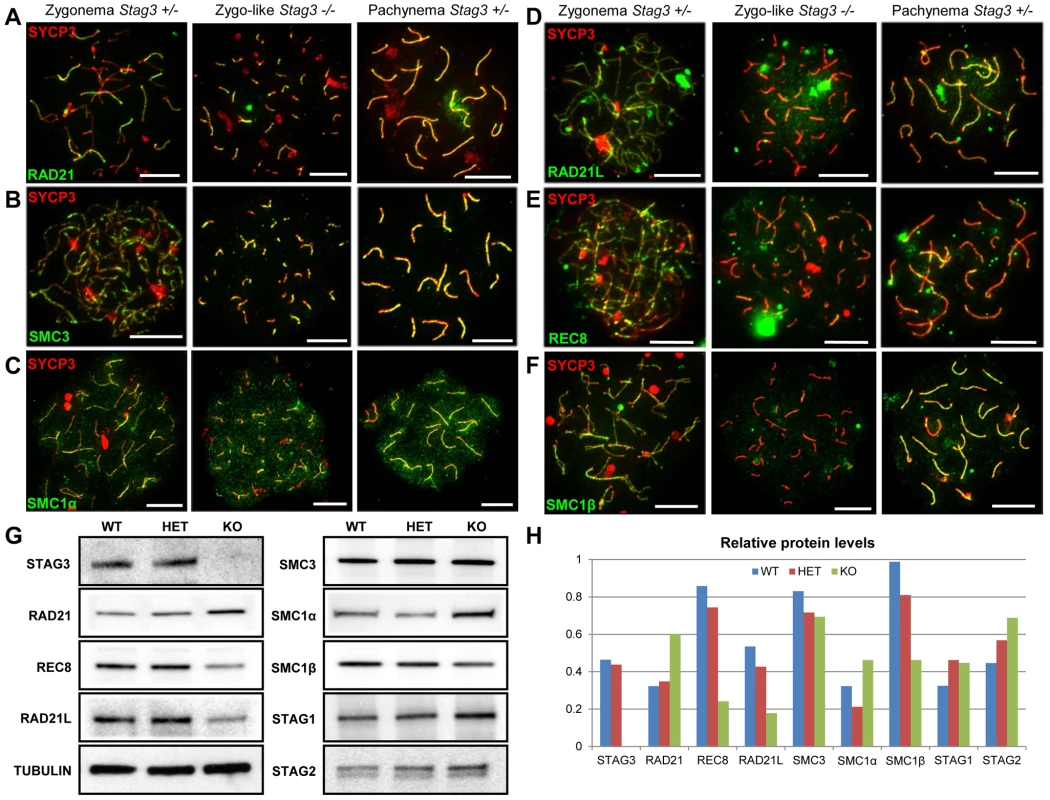
Chromatin spreads were prepared from purified testicular germ cells of Stag3+/− and Stag3−/− mice aged 16 dpp. Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and pan-cohesin component SMC3 (A), mitotic cohesin components SMC1α (B) and RAD21 (C) and meiosis-specific cohesin components RAD21L (D), REC8 (E) and SMC1β (F) in green. Meiotic prophase stages are indicated across the top. Experiments were performed using 3 separate littermate pairs of mutant and control mice. Images are from germ cells carrying the Stag3Ov allele. Similar results were obtained when assessing oocyte chromatin spreads, summarized in Fig. S5 and for the Stag3JAX allele mutants (Fig. S6). (G) Protein extracts from purified testicular germ cells of WT (Stag3+/+), HET (Stag3+/−) and KO (Stag3−/−) mice aged 16 dpp were prepared and western blot analyses performed for STAG3, RAD21, REC8, RAD21L, SMC3, SMC1α, SMC1β, STAG1 and STAG2. Tubulin was used as a loading control. (H) Quantification of protein levels of each cohesin component analyzed in (G). Tubulin was used to normalize the loading of each lane. Each western blot was repeated at least twice. Tubulin loading controls corresponding to each western blot analyzed is present in Fig. S7. Data shown for germ cell extracts from the Stag3OV homozygous mutants and littermate controls. Scale bar = 10 µm Taking advantage of the nearly synchronous first wave of spermatogenesis, we purified germ cells from mice that are enriched for early stages of prophase I (16 days postpartum). Using protein extracts from these cells we assessed protein levels of cohesin subunits. We did not detect STAG3 protein in the Stag3 mutant protein extracts. The Stag3 mutant mice exhibited protein levels for mitotic cohesin subunits SMC3, SMC1α, STAG1 and STAG2 that were equivalent to control littermates (Fig. 4G and H and S8). However, levels of the mitotic kleisin subunit RAD21 were higher in the Stag3 mutant. In contrast, levels of the meiosis-specific kleisin subunits RAD21L and REC8 were reduced in the Stag3 mutant extracts. Furthermore, the meiosis-specific SMC1 protein, SMC1β was also reduced in the Stag3 mutant extracts. From these observations it could be interpreted that STAG3 is required for the stability of the meiosis-specific cohesin components and is compensated for by an increase of mitotic cohesins in Stag3 mutants. Another possible contributing factor is that levels of meiosis-specific cohesin components are lower due to meiotic arrest, and therefore an increased mitotic to meiotic germ cell ratio. Nevertheless, taken together with the nuclear spread data, we propose that STAG3 is required for the stability of meiosis-specific cohesins that are loaded onto chromosome axes.
Mutation of Stag3 results in the inability to repair SPO11-induced DSBs
To assess the DNA repair pathway, we examined whether the ATM/ATR mediated phosphorylation of H2AFX histones (γH2AX) was present in the Stag3 mutants. In wild type spermatocyte chromatin spreads, γH2AX is widespread during the leptotene and zygotene stages (Fig. 5A). Following DNA repair the γH2AX signal is removed from the chromatin, remaining only on the X-Y chromatin by the pachytene stage (Fig. 5A). Chromatin spreads from male and female Stag3 mutants showed that γH2AX was widespread throughout the chromatin, which suggests SPO11-induced DSBs are being formed and that a DNA damage response was activated (Fig. 5A and S9). However, the γH2AX signal is not removed from the chromatin, which suggests that the DNA damage is not repaired in Stag3 mutants.
Fig. 5. Stag3 mutants fail to repair meiotic DSBs and have an abnormal DNA damage response. 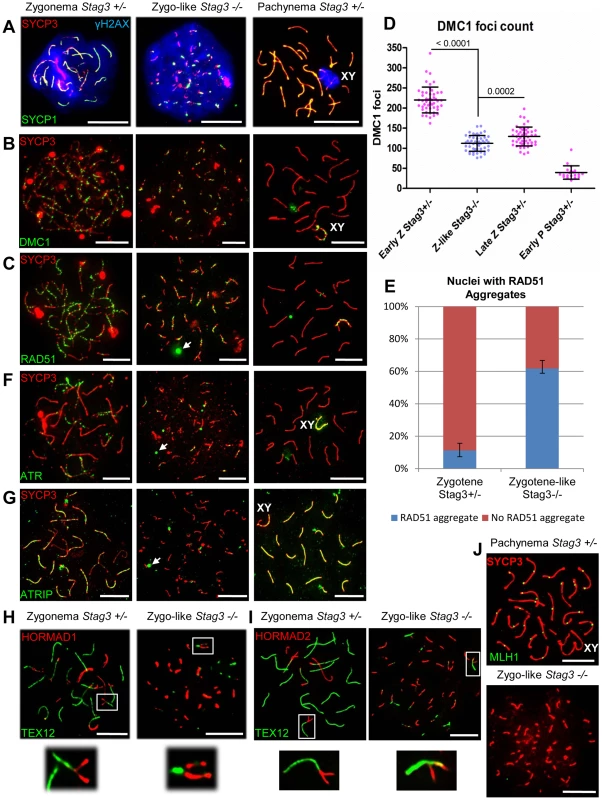
Chromatin spreads from purified testicular germ cells of Stag3+/− and Stag3−/− mice aged 16 dpp were prepared and immunolabeled. (A) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red), phosphorylated histone H2AFX (blue, γH2AX) and the transverse filament of the central region of the SC SYCP1 (green). (B) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and meiosis-specific single-end invasion protein DMC1 (green). (C) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and single-end invasion protein RAD51 (green). Arrows represent RAD51 aggregates not associated with SYCP3 stretches. (D) Scatter dot-plot graph of the number of DMC1 foci per spermatocyte chromatin spread during early zygotene (Early Z, average = 220, N = 50), late zygotene (Late Z, average = 129, N = 50) and early pachytene (Early P, average = 39.5, N = 20) stages for the Stag3+/− control and zygo-like stage (Z-like average = 112, N = 50) for the Stag3−/− mice. Mean and standard deviation of each column of the graph are represented by the black bars and P values are given for indicated comparisons (Mann-Whitney, one-tailed). (E) Bar graph of the percentage of chromatin spreads that contain RAD51 aggregates at the zygotene stage (average = 11.2%, N = 179) for the Stag3+/− control and zygotene-like stage (average = 61.8%, N = 212) for the Stag3−/− mice. The error bars represent the variation between three independent experiments. (F) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and DNA damage response protein ATR (green). Arrows represent ATR aggregates not associated with SYCP3 stretches. (G) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and DNA damage response protein ATRIP (green). Arrows represent ATRIP aggregates. (H and I) Chromatin spreads were immunolabeled using antibodies against the HORMA domain containing protein HORMAD1 (H, red) or HORMAD2 (I, red) and the SC central element protein TEX12 (green). The boxed regions are magnified 3× below the whole chromatin spread images. Images are from the Stag3Ov mutant allele, comparable phenotype was observed for the Stag3JAX mutant allele (Fig. S2). (J) Chromatin spreads were immunolabeled with antibodies against the SC lateral element protein SYCP3 (red) and crossover protein MLH1 (green). Each experiment was performed at least twice. Images are from cells with the Stag3Ov mutant allele. XY label represents the sex chromosome pair. Scale bars = 10 µm Stag3 mutants have a defective DNA damage response
To determine whether there are any defects in DNA repair by homologous recombination in meiosis, we assessed localization of the single-end invasion proteins, RAD51 and DMC1, in primary spermatocytes [42]. RAD51 and DMC1 load at DSB sites and promote DNA repair during zygonema (Fig. 5B and C). The number of DMC1 foci is highest during early zygotene stage for the control spermatocytes averaging 220 foci per nucleus (Fig. 5D, N = 50). DSBs begin to be repaired at the late zygotene stage and the average number of DMC1 foci reduces to 129 foci per nucleus (Fig. 5D, N = 50). DMC1 and RAD51 foci are mainly absent from the autosomes by early pachytene stage, but remain on the X-Y axes (Fig. 5B-D). DMC1 and RAD51 foci localized to the SYCP3 stretches in the Stag3 mutant, however the numbers of DMC1 foci were lower in comparison to the early zygotene stage of the control (Fig 5B-D, 112 foci per nucleus, N = 50). Furthermore, DMC1 and RAD51 foci remained present on the SYCP3 stretches in the Stag3 mutant, indicating that DSBs are not repaired. In addition, RAD51 aggregates were evident in more than 60% of the Stag3 mutant chromatin spreads suggesting that DNA repair processes are aberrant (Fig. 5E). Together with the persistence of γH2AX, these observations show that SPO11-induced DSBs are not repaired in primary germ cells of the Stag3 mutant.
It is known that ATR is responsible for a DNA damage checkpoint cascade which includes its interaction partner ATRIP [42]. During the zygotene stage, ATR-ATRIP signals the existence of recombination intermediates and activates the DNA damage response [24]. ATR localizes to unsynapsed regions of chromosome axes during zygonema, and then dissociate from the autosomes following synapsis (Fig. 5F) [43]. Unlike ATR and other ATR-mediated checkpoint proteins, ATRIP remains localized to the autosomes following synapsis (Fig5G) [24]. Localization of ATR and ATRIP to SYCP3 stretches in the Stag3 mutant was aberrant, and often formed large aggregates that were not associated with SYCP3 (Fig. 5F and G).
HORMAD1 and 2 are asynapsis surveillance proteins preferentially localize to unsynapsed chromosome axes (Fig. 5H and I) [27]. Both proteins are required to stimulate regular levels of SPO11 induced DSBs and to trigger the ATR-mediated asynapsis response [23], [44]–[46]. Our data suggests that sister chromatids are synapsed in the Stag3 mutant (Fig. 2). Therefore we wished to determine whether HORMAD1 and 2 proteins dissociate during this abnormal form of synapsis. We observed that the HORMAD proteins do dissociate from the synapsed regions of the chromosome axes (Fig. 5H and I), suggesting that the asynapsis surveillance mechanism does not distinguish between synapsis between homologues or sister chromatids.
In summary, meiotic DSBs formed in the Stag3 mutant, and the DNA damage response mechanisms such as H2AFX phosphorylation, RAD51 and DMC1 loading were apparent. However, meiotic DSBs were not repaired in Stag3 mutants and the ATR-mediated DNA damage response was abnormal.
Discussion
STAG3 - a conserved and essential meiosis-specific component
Stromal antigen (STAG) domain-containing cohesin subunits are common in eukaryotic model organisms including Saccharomyces cerevisiae, Schizosaccharomyces pombe, Caenorhabditis elegans, Drosophila melanogaster and mammals. Interestingly, there are meiosis-specific STAG domain proteins in a subset of these organisms. The fission yeast meiosis-specific STAG domain protein, Rec11 was shown to be a component of chromosome arm-specific cohesin with Rec8, whereas the mitotic STAG protein (Psc3) is a centromere cohesin component with Rec8 [47]. Rec11 cohesin is removed from the chromosome arms during the first meiotic division, whereas Psc3 cohesin remains until meiosis II. The localization pattern of STAG3 in primary spermatocytes is very similar to fission yeast Rec11, as STAG3 has been shown to localize to the axial/lateral elements during prophase and remains bound between sister chromatid arms at metaphase I [5]. The STAG3 arm cohesin is removed progressively from the arms during the metaphase to anaphase I transition, but a proportion of STAG3 remains in close proximity with the centromere until the onset of anaphase I during spermatogenesis [5]. However, the localization of STAG3 is sexually dimorphic, as it localizes between sister kinetochores from anaphase I to metaphase II in human oocytes [9]. Another meiosis-specific STAG protein is the Stromalin in Meiosis (SNM) protein of Drosophila. Surprisingly, SNM does not colocalize with SMC1, suggesting that its role is independent of cohesin [48]. In addition, SNM is specific to the male where meiosis is not coupled with homologue exchange, SC formation and chiasma formation [1]. SNM is required for linking achaismate homologous chromosomes during meiosis via “pairing sites” and ensures accurate chromosome segregation [48].
Here we have shown that mammalian Stag3 is required for normal SC formation between homologous chromosomes and sister chromatid cohesion. Mutation of fission yeast Rec11 resulted in impaired linear element formation and increased sister chromatid separation [49]. Furthermore, mutation of Rec11 causes reduced levels of recombination [50]. Our study has shown that Stag3 mutants are unable to form crossovers due to an inability to repair SPO11-induced meiotic DSBs. In summary, STAG3 and Rec11 have a number of similarities with respect to function during meiosis, whereas SNM is a divergent protein with unique functions specific to the Drosophila male. Nevertheless, each meiosis-specific STAG domain protein is essential for meiotic progression, and each has a conserved role in mediating pairing of homologous chromosomes.
Common and unique characteristics of the meiosis-specific cohesin mutants
Four cohesin subunits are meiosis-specific in mammals, namely SMC1β, RAD21L, REC8 and STAG3 (Fig. 6A). There are up to six cohesin complexes associated with chromosomes during meiosis, including the mitotic cohesin (SMC1α-SMC3 bridged by STAG1 or 2 and RAD21), meiosis-specific SMC1β-containing cohesins (SMC1β-SMC3 bridged by STAG3 and either RAD21, REC8 or RAD21L) and meiosis-specific SMC1α-containing cohesins (SMC1α-SMC3 bridged by STAG3 and RAD21L or possibly REC8) [3], [7], [8], [34]. Therefore, STAG3 is the only component that is present in all meiosis-specific cohesins. By analyzing the Stag3 mutant mouse, we have shown that STAG3 is required for stable localization of SMC1β, RAD21L and REC8 to chromosome axes, thus confirming their interaction in vivo.
Fig. 6. STAG3 is required for stability and loading of meiosis-specific cohesins to chromosome axes during meiosis. 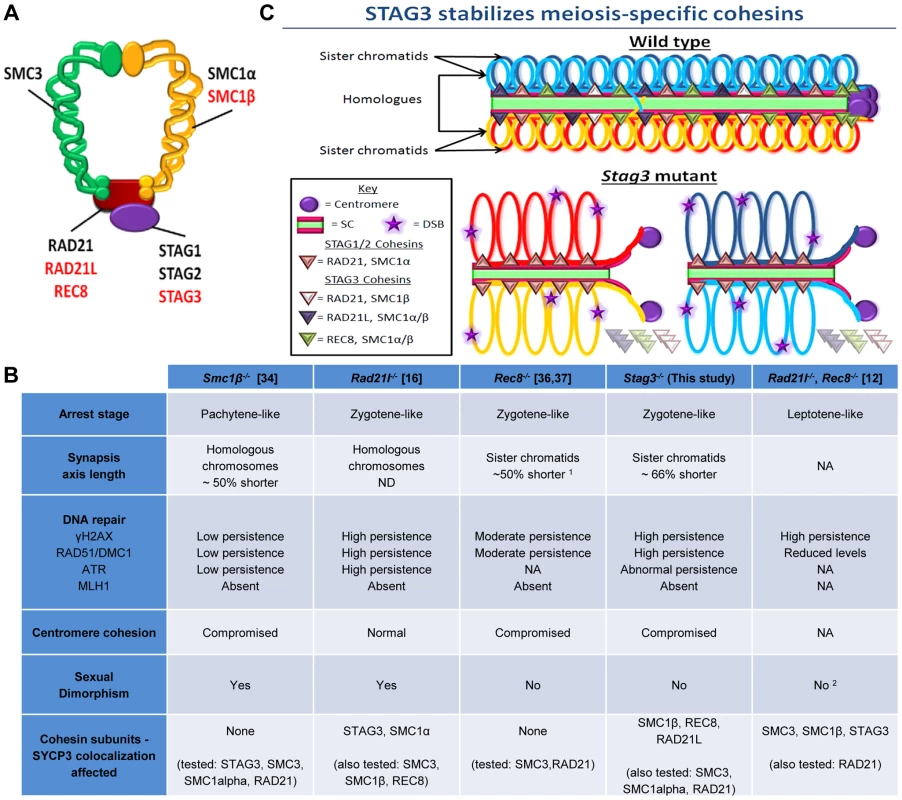
(A) Diagram depicting the ring-like structure of the cohesin complex. The mitotic cohesin and meiosis-specific components are written in black and red text respectively. (B) Summary table of the phenotypes recorded for mutants of the four meiosis-specific cohesin components. 1 The axis length was not defined in these studies, but from our analysis it is ∼50% shorter. 2 Although the female phenotype was not reported in this study, it can be implied due to the phenotype of the Rec8−/− mutant. (C) Cartoon of mid-prophase of a wild type (pachytene stage) and Stag3 mutant (zygotene-like stage). All features are described within the key. At pachytene stage, homologous chromosomes are fully synapsed and an obligate crossover has formed. In zygotene-like staged Stag3 mutant germ cells, localization and stability of meiosis-specific cohesin complexes is aberrant and leads to synapsis between sister chromatids, DNA double strand breaks (DSBs) are not repaired and centromere cohesion between sister chromatids is perturbed. Chromatin loops are depicted to be larger in the Stag3 mutant as their chromosome axes are shorter compared to wild type, and is supported by analysis of the Smc1β mutant mouse [70]. Mutants of all four mouse meiosis-specific cohesin subunits have now been characterized using similar phenotype analyses such as meiotic progression, chromosome synapsis, DNA repair, centromere cohesion and localization of other cohesin components (Fig. 6B). In the male, mutation of each meiosis-specific cohesin component results in a prophase I arrest prior to crossover formation, nevertheless there are distinct features for each mutant. For instance, Smc1β mutation results in a pachytene-like stage arrest with a majority of chromosome synapsis and DNA repair occurring between homologues [34]. However, the synapsed chromosomes in a Smc1β mutant are shorter than in wild type and it was demonstrated that chromosome loops are larger. Mutation of the α-kleisin, Rad21l, gives rise to an arrest at a zygotene-like stage where homologous chromosomes are partially synapsed, but there is also a degree of non-homologous synapsis and SPO11-induced DSBs are not efficiently repaired [16]. In contrast, mutation of the other meiosis-specific α-kleisin, Rec8, results in synapsis between sister chromatids and although still aberrant, DNA repair is more apparent [36, 37, unpublished data]. Rad21l, Rec8 double mutant spermatocytes arrest at a leptotene-like stage where the SYCP3 protein forms aggregates, showing that these α-kleisin subunits are both important for axial element formation [12]. The Stag3 mutation results in a zygotene-like stage arrest similar to the Rec8 mutant where sister chromatids are synapsed. However, the phenotype is more pronounced in the Stag3 mutant, with chromosome axis length being shorter and the level of residual DNA damage being greater (Fig. 6B and C). In addition, the Stag3 mutation caused the formation of circular SYCP3 axes, which are conceivably the result of telomere fusion, a phenomenon also observed in the Smc1β mutant [17]. Our data also suggests that STAG3 is required for maintenance of centromere cohesion between sister chromatids, which is a function shared by SMC1β and REC8, but not RAD21L [16], [34], [51]. If STAG3 is a component of all meiosis-specific cohesins, why is the Stag3 mutant phenotype less pronounced than the Rad21l, Rec8 double mutant? Based on the structure of cohesin complexes, the V-shaped heterodimer formed by SMC1 and SMC3 is bridged by one of the α-kleisins, and the STAG proteins interact with the α-kleisin (Fig. 6A) [52], [53]. Therefore, STAG proteins may not be required for cohesin ring formation per se, but required for cohesin ring stability (Fig. 6C). Our data supports this hypothesis as we observe a degree of SMC1β, REC8 and RAD21L loading onto the chromosome axes in the Stag3 mutant, whereas there is complete absence of STAG3 loading in germ cells of the Rad21l, Rec8 double mutant [12]. Also, the DNA damage response defect observed for the Rad21l, Rec8 double mutant is more severe than the Stag3 mutant (Fig 6B). Knockdown of single SMC complex components in tissue culture experiments has been shown to result in the decrease in protein stability of other components of the same cohesin complex. For example, RNAi mediated knockdown of SMC3 results in reduced SMC1 and RAD21 protein levels [54]. SMC1β, REC8 and RAD21L protein levels are decreased in a Stag3 mutant, which further supports the hypothesis that STAG3 is required for the stability of meiosis-specific cohesins. We also observed higher levels of mitotic cohesin components in germ cell protein extracts of the Stag3 mutant, particularly RAD21. It is possible that mitotic cohesins compensate for the loss of REC8 and RAD21L cohesin complexes in the Stag3 mutant, which may not be the case in the Rad21l, Rec8 double mutant. Mutational analyses combining the Stag3 mutant with mutants of the other meiosis-specific cohesin subunits and conditional mutants for mitotic cohesin components will help test our hypothesis further.
Mutants of all four mouse meiosis-specific cohesin subunits also display differing phenotypes in the female germline (Fig. 6B). The Rad21l mutant phenotype is the least severe, with females exhibiting subfertility after 6 months of age and become prematurely infertile [16]. While oocytes of the Smc1β mutants progress to metaphase II, they are grossly aneuploid [34]. On the other hand, both female Stag3 and Rec8 mutants display a phenotype analogous to that observed in males [36], [37]. This suggests that STAG3-REC8 cohesin complexes are the predominant cohesin required for meiotic progression in females. Heterozygous mutations for Smc1β and Rec8 have been reported to give rise to increased levels of premature sister chromatid separation in oocytes from adult mice [55]. It would certainly be interesting to determine whether this is the case for Stag3 heterozygote mutants.
Homologue recognition and cohesins
Homologue recognition/association initiates upon entry into meiotic prophase, prior to DSB formation and axis assembly [13], [14], [32]. As repetitive elements constitute 30–50% of the mammalian genome, it has been proposed that large chromosome elements such as sub-telomeric regions and peri-centromeric heterochromatin are crucial for initial homologue recognition [14]. Mouse chromosomes are telocentric, and it has been demonstrated that the peri-centromeric heterochromatin accumulates at the nuclear envelope during pre-leptotene and form clusters known as “chromocenters” [19]. During meiosis, STAG3, REC8 and RAD21L localize to the telomeres and chromocenters at pre-leptotene [3], [8], [15]. To facilitate initial pairing during pre-leptotene, telomere ends attach to the nuclear envelope. The notion that cohesins are required to stabilize these telomere attachments is supported by the fact that this event is partially defective in Smc1β and Rad21l mutants [16], [17]. Furthermore, it was demonstrated that STAG3 cohesins stabilize telomere attachment to the nuclear envelope via interaction with the telomere TRF1-TERB1 protein complex [15]. The TRF1-TERB1 protein complex also interacts with the nuclear membrane protein complex, SUN-KASH, which is required for stimulating chromosome movements that promotes chromosome pairing/synapsis [56]. In future work, it will be very interesting to assess the effect of the Stag3 mutation on telomere binding to the nuclear envelope.
Although telomere movement is important for facilitating efficient chromosome pairing/synapsis, a recent report showed that it is not essential for the initial stages of homologue recognition [32]. The researchers of this study found that RAD21L is required for DSB-independent homologue recognition, and proposed that initial homologue pairing is based on homology of chromosome architecture. As STAG3 is a component of the RAD21L cohesins, it is very likely that STAG3 is required for the initial homologue recognition. We have shown that mutation of Stag3 results in the dramatic decrease in pericentromeric heterochromatin clustering during meiotic prophase. The pericentromeric heterochromatin clustering phenomenon occurs at the same time as the initial homologue recognition [14], [32]. As STAG3 is required to ensure normal chromocenter formation, and STAG3-RAD21L localize to chromocenters, we propose that pericentromeric heterochromatin is a component of the chromosome architecture required for the initial homologue recognition. In addition, REC8 localizes to the pericentromeric chromatin. We have shown that STAG3-REC8 cohesins are required for maintaining sister chromatid cohesion. Robust sister chromatid cohesion at metaphase I may also require loading of cohesins to the chromocenters at meiotic entry. This is supported by the fact that cohesin loading at pericentromeric heterochromatin is important for maintenance of sister chromatid cohesion during mitosis [57], [58].
Cohesinopathies – meiotic-specific components
Cohesinopathies is a term coined to encompass all human disorders caused by mutations in genes encoding for cohesin components or cofactors [59]. Mutations in RAD21, SMC1α and SMC3 have been shown to result in Cornelia de Lange syndrome, which causes intellectual disability and growth retardation and as well as facial and limb anomalies [60]–[62]. Based on mouse studies, it has also been suggested that Stag1 mutation can cause Cornelia de Lange syndrome [63]. These disorders are attributed to the role of cohesin in regulating gene expression via interaction with CCCTC-binding factor sites or with mediator proteins, which repress or enhance gene expression respectively [59]. It is conceivable that expression of meiosis-specific cohesin subunits in mitotic cells could also give rise to cohesinopathies and cancer. For example, it has been shown that p53 mutated lymphoma cells express Rec8 and Stag3 [64] and an allele of Stag3 is linked with the development of epithelial ovarian cancer [65]. Furthermore, it was recently shown that an inherited mutation in human Stag3 that gives rise to infertility and gonadal failure [66].
Conclusion
Using two independently derived mutations of mouse Stag3, we have determined that STAG3 is essential for fertility. Mutation of Stag3 causes a zygotene-like meiotic prophase I arrest in both males and females. We show that STAG3 is required for the localization of the meiosis-specific subunits of cohesin, SMC1β, RAD21L and REC8, to chromosomal axes during meiotic prophase. STAG3 cohesins are required for DNA repair of SPO11-induced DSBs, synapsis between homologues, centromeric cohesion between sister chromatids, and heterochromatin-rich pericentromeric clustering between non-homologous chromosomes to form chromocenters.
Materials and Methods
Ethics statement
All mice were bred by the investigators at The Jackson Laboratory (JAX, Bar Harbor, ME) and Johns Hopkins University (JHU, Baltimore, MD) under standard conditions in accordance with the National Institutes of Health and U.S. Department of Agriculture criteria and protocols for their care and use were approved by the Institutional Animal Care and Use Committee (IACUC) of The Jackson Laboratory and Johns Hopkins University.
Mice
Two mutations for Stag3 were used in this study. 1) 1–8 cell stage FVB/N embryos were mutated by random insertion of the SB-cHS4core-SB-Tyro-WPRE-FUGW lentiposon transgene (LV2229). Using inverse PCR analysis, the lentiviral integration site was identified in intron 8 of the stromal antigen 3 gene (Stag3) on chromosome 5. The 3'-LTR is linked to the (+) strand of DNA at position 138,735,815 bp [NCB137/mm9; 3'-138,735,815(+)]. The lentivirus is inserted in the sense orientation relative to the disrupted mouse gene (Fig. S1A, http://www.mmrrc.org/catalog/sds.php?mmrrc_id=36275). The resulting heterozygote mice (FVB/N-Stag3TgTn(sb-cHS4,Tyr)2312COve/Mmjax) were bred together to create homozygote offspring which were compared to heterozygote and wild type littermate controls. 2) C57BL/6N-derived JM8.N4 embryonic stem (ES) cells that were targeted with a β-galactosidase containing cassette that generated a knockout first reporter allele for Stag3 that harbored a floxed exon 5 were sourced from the International Knockout Mouse Consortium [67], http://www.knockoutmouse.org/martsearch/project/22907). As part of the KOMP2 program (http://commonfund.nih.gov/KOMP2/) these ES cells were injected into B6(Cg) - Tyrc-2J/J blastocysts. The resulting chimeric males were bred to C57BL/6NJ females and then to B6N.Cg-Tg(Sox2-cre)1Amc/J mice to remove the floxed neomycin and exon 5 (Fig. S1B). Offspring were bred to C57BL/6NJ mice or to wildtype siblings to remove the cre-expressing transgene resulting in the heterozygote B6N(Cg)-Stag3tm1b(KOMP)Wtsi/2J strain used in this study. Offspring homozygous for the Stag3tm1b(KOMP)Wtsi/2J allele were compared to heterozygote and wild type littermate controls. To confirm the phenotypes, heterozygote B6N(Cg)-Stag3tm1b(KOMP)Wtsi/2J animals were bred to FVB/N-Stag3TgTn(sb-cHS4,Tyr)2312COve/Mmjax to create experimental offspring that harbored both alleles, which were compared to heterozygote offspring for either allele. The Rec8 mutant mice used in our study has previously been described [36].
Histological analysis and TdT-mediated dUTP nick end labelling (TUNEL) assay
Testis and ovary tissues were fixed in bouins fixative. Tissues were embedded in paraffin and serial sections 5 microns thick were placed onto slides and stained with hematoxylin and eosin. For the TUNEL assay, sections were deparafinnized and apoptotic cells were detected using the in situ BrdU-Red DNA fragmentation (TUNEL) assay kit (Abcam) and counterstained with DAPI.
Mouse germ-cell isolation and culture
Isolation of mixed germ cells from testes was performed using techniques previously described [68], [69]. Germ cells isolated from 16 day old male mice enriched for mid-prophase spermatocytes (2.5×106 cells/ml) were cultured for 10 hr at 32°C in 5% CO2 in HEPES (25 mM)-buffered MEMα culture medium (Sigma) supplemented with 25 mM NaHCO3, 5% fetal bovine serum (Atlanta Biologicals), 10 mM sodium lactate, 59 µg/ml penicillin, and 100 µg/ml streptomycin. To initiate the G2/MI transition, cultured pachytene spermatocytes were treated with 5 µM okadaic acid (OA) (CalBiochem).
Protein analyses
For protein level analyses, proteins were extracted from germ cells using RIPA buffer (Santa Cruz) containing 1× protease inhibitor cocktail (Roche). Protein concentration was calculated using a BCA protein assay kit (Pierce). Lanes of 4–15% gradient SDS polyacrylamide gels (Bio-Rad) were loaded with 20 µl of 1 mg/ml protein extract. Following protein separation via standard SDS PAGE, proteins were transferred to PVDF membranes using the Trans-Blot® Turbo™ western transfer system (Bio-Rad). Primary antibodies and dilution used are presented in Supplemental Table S2. At a 1∶20,000 dilution, Invitrogen horseradish peroxidase-conjugated antibodies rabbit anti-mouse (R21455), goat anti-rabbit (A10533), rabbit anti-goat (R21459) were used as secondary antibodies. The presence of antibodies on the PVDF membranes was detected via treatment with Pierce ECL western blotting substrate (Thermo Scientific) and captured using the Syngene XR5 gel documentation system. Protein levels were assessed using Image J (NIH). The SMC3 Co-IP experiment was performed using the Dynabead® Co-IP kit (Life Technologies). Each milligram of beads was covalently linked to 4 µg of SMC3 antibody (Abcam, ab9263) or corresponding IgG control antibody (Life Technologies, A10533).
Spread chromatin analyses
Germ cell chromatin spreads were prepared as previously described [19], [31]. Primary antibodies and dilution used are presented in Supplemental Table S2. Secondary antibodies against human, rabbit, rat, mouse and guinea pig IgG and conjugated to Alexa 350, 488, 568 or 633 (Life Technologies) were used at 1∶500 dilution. Chromatin spreads were mounted in Vectashield + DAPI medium (Vector Laboratories). For fluorescence in situ hybridization (FISH), we used a pre-labelled FISH probes, one probe was used to detect 200 kilobases of mouse chromosome 11 (TK [11qE1]) distal to the centromere, and the other to recognize the X chromosome (Creative Bioarray). Prior to performing FISH, nuclear spreads were immuno-stained with rabbit anti-SYCP3 followed by the corresponding secondary conjugated to Alexa 633. We performed FISH following the manufacturer's protocol for cell preparations. Briefly, slides were incubated in 10 mM sodium citrate (pH 6.0) at 96°C in for 15 min, dehydrated and air dryed. The FISH probes and chromatin spreads were co-denatured at 80°C for 10 min under a 22×22 mm coverslip sealed with Fixogum (Marabu GmbH & Co.). Following hybridization at 37°C overnight slides were washed in 0.4×SSC+0.1% Igepal for 2 min then 2×SSC+0.3% Igepal for 1 min. Slides were dehydrated and mounted in Vectashield. Nuclear spread images were captured using a Zeiss CellObserver Z1 linked to an ORCA-Flash 4.0 CMOS camera (Hamamatsu) and analyzed with the Zeiss ZEN 2012 blue edition image software including foci and length measurement capabilities and Photoshop (Adobe) was used to prepare figure images.
Supporting Information
Zdroje
1. JordanP (2006) Initiation of homologous chromosome pairing during meiosis. Biochem Soc Trans 34 : 545–549.
2. NasmythK (2011) Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13 : 1170–1177.
3. IshiguroK-i, KimJ, Fujiyama-NakamuraS, KatoS, WatanabeY (2011) A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 12 : 267–275.
4. GómezR, ValdeolmillosA, ParraM, VieraA, CarreiroC, et al. (2007) Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep 8 : 173–180.
5. PrietoI, SujaJA, PezziN, KremerL, Martinez-AC, et al. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3 : 761–766.
6. RevenkovaE, EijpeM, HeytingC, GrossB, JessbergerR (2001) Novel Meiosis-Specific Isoform of Mammalian SMC1. Mol Cell Biol 21 : 6984–6998.
7. Gutiérrez-CaballeroC, HerránY, Sánchez-MartínM, SujaJÁ, BarberoJL, et al. (2011) Identification and molecular characterization of the mammalian α-kleisin RAD21L. Cell Cycle 10 : 1477–1487.
8. LeeJ, HiranoT (2011) RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol 192 : 263–276.
9. Garcia-CruzR, BrienoMA, RoigI, GrossmannM, VelillaE, et al. (2010) Dynamics of cohesin proteins REC8, STAG3, SMC1β and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod 25 : 2316–2327.
10. ParraMT, VieraA, GomezR, PageJ, BenaventeR, et al. (2004) Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci 117 : 1221–1234.
11. XuH, B.M., VerschoorS, InselmanA, HandelMA, McKayMJ (2004) A new role for the mitotic RAD21/SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Rep 5 : 378–384.
12. LlanoE, HerránY, García-TuñónI, Gutiérrez-CaballeroC, de ÁlavaE, et al. (2012) Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol 197 : 877–885.
13. Boateng KingsleyA, Bellani MarinaA, Gregoretti IvanV, PrattoF, Camerini-OteroRD (2013) Homologous Pairing Preceding SPO11-Mediated Double-Strand Breaks in Mice. Dev Cell 24 : 196–205.
14. ScherthanH, WeichS, SchweglerH, HeytingC, HärleM, et al. (1996) Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 134 : 1109–1125.
15. ShibuyaH, IshiguroK-i, WatanabeY (2014) The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat Cell Biol 16 : 145–156.
16. HerranY, Gutierrez-CaballeroC, Sanchez-MartinM, HernandezT, VieraA, et al. (2011) The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 30 : 3091–3105.
17. AdelfalkC, JanschekJ, RevenkovaE, BleiC, LiebeB, et al. (2009) Cohesin SMC1β protects telomeres in meiocytes. JCB 187 : 185–199.
18. VerverDE, van PeltAM, ReppingS, HamerG (2013) Role for rodent Smc6 in pericentromeric heterochromatin domains during spermatogonial differentiation and meiosis. Cell Death Dis 4: e749.
19. GómezR, JordanPW, VieraA, AlsheimerM, FukudaT, et al. (2013) Dynamic localization of SMC5/6 complex proteins during mammalian meiosis and mitosis implies functions in distinct chromosome processes. J Cell Sci 125 : 5061–5072.
20. GuenatriM, BaillyD, MaisonC, AlmouzniG (2004) Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol 166 : 493–505.
21. LangeUC, SiebertS, WossidloM, WeissT, Ziegler-BirlingC, et al. (2013) Dissecting the role of H3K64me3 in mouse pericentromeric heterochromatin. Nat Commun 4 : 2233.
22. BellaniMA, RomanienkoPJ, CairattiDA, Camerini-OteroRD (2005) SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm-/ - spermatocytes. J Cell Sci 118 : 3233–3245.
23. RoyoH, ProsserH, RuzankinaY, MahadevaiahSK, CloutierJM, et al. (2013) ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev 27 : 1484–1494.
24. RefolioE, CaveroS, MarconE, FreireR, San-SegundoPA (2011) The Ddc2/ATRIP checkpoint protein monitors meiotic recombination intermediates. J Cell Sci 124 : 2488–2500.
25. MoensPB, HeytingC, DietrichAJ, van RaamsdonkW, ChenQ (1987) Synaptonemal complex antigen location and conservation. J Cell Biol 105 : 93–103.
26. OffenbergHH, SchalkJAC, MeuwissenRLJ, van AalderenM, KesterHA, et al. (1998) SCP2: A major protein component of the axial elements of synaptonemal complexes of the rat. Nucleic Acids Res 26 : 2572–2579.
27. WojtaszL, DanielK, RoigI, Bolcun-FilasE, XuH, et al. (2009) Mouse HORMAD1 and HORMAD2, Two Conserved Meiotic Chromosomal Proteins, Are Depleted from Synapsed Chromosome Axes with the Help of TRIP13 AAA-ATPase. PLoS Genet 5: e1000702.
28. HamerG, GellK, KouznetsovaA, NovakI, BenaventeR, et al. (2006) Characterization of a novel meiosis-specific protein within the central element of the synaptonemal complex. J Cell Sci 119 : 4025–4032.
29. TurnerJMA (2007) Meiotic sex chromosome inactivation. Development 134 : 1823–1831.
30. HandelMA (2004) The XY body: a specialized meiotic chromatin domain. Expt Cell Res 296 : 57–63.
31. JordanPW, KarppinenJ, HandelMA (2012) Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes. J Cell Sci 125 : 5061–5072.
32. IshiguroK-i, KimJ, ShibuyaH, Hernández-HernándezA, SuzukiA, et al. (2014) Meiosis-specific cohesin mediates homolog recognition in mouse spermatocytes. Genes Dev 28 : 594–607.
33. GaoY-F, LiT, ChangY, WangY-B, ZhangW-N, et al. (2011) Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat Cell Biol 13 : 924–933.
34. RevenkovaE, EijpeM, HeytingC, HodgesCA, HuntPA, et al. (2004) Cohesin SMC1β is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6 : 555–562.
35. HodgesCA, RevenkovaE, JessbergerR, HassoldTJ, HuntPA (2005) SMC1β-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 37 : 1351–1355.
36. BannisterLA, ReinholdtLG, MunroeRJ, SchimentiJC (2004) Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. Genesis 40 : 184–194.
37. XuH, BeasleyMD, WarrenWD, van der HorstGT, McKayMJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8 : 949–961.
38. LiebeB, AlsheimerM, HöögC, BenaventeR, ScherthanH (2004) Telomere Attachment, Meiotic Chromosome Condensation, Pairing, and Bouquet Stage Duration Are Modified in Spermatocytes Lacking Axial Elements. Mol Biol Cell 15 : 827–837.
39. QiaoH, ChenJK, ReynoldsA, HöögC, PaddyM, et al. (2012) Interplay between Synaptonemal Complex, Homologous Recombination, and Centromeres during Mammalian Meiosis. PLoS Genet 8: e1002790.
40. BisigCG, GuiraldelliMF, KouznetsovaA, ScherthanH, HöögC, et al. (2012) Synaptonemal Complex Components Persist at Centromeres and Are Required for Homologous Centromere Pairing in Mouse Spermatocytes. PLoS Genet 8: e1002701.
41. WiltshireT, ParkC, CaldwellKA, HandelMA (1995) Induced Premature G2/M-Phase Transition in Pachytene Spermatocytes Includes Events Unique to Meiosis. Dev Biol 169 : 557–567.
42. ZouL, ElledgeSJ (2003) Sensing DNA Damage Through ATRIP Recognition of RPA-ssDNA Complexes. Science 300 : 1542–1548.
43. PlugAW, PetersAH, KeeganKS, HoekstraMF, de BoerP, et al. (1998) Changes in protein composition of meiotic nodules during mammalian meiosis. J Cell Sci 111 : 413–423.
44. KogoH, TsutsumiM, InagakiH, OhyeT, KiyonariH, et al. (2012) HORMAD2 is essential for synapsis surveillance during meiotic prophase via the recruitment of ATR activity. Genes to Cells 17 : 897–912.
45. WojtaszL, CloutierJM, BaumannM, DanielK, VargaJ, et al. (2012) Meiotic DNA double-strand breaks and chromosome asynapsis in mice are monitored by distinct HORMAD2-independent and -dependent mechanisms. Genes Dev 26 : 958–973.
46. DanielK, LangeJ, HachedK, FuJ, AnastassiadisK, et al. (2011) Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 13 : 599–610.
47. KitajimaTS, YokobayashiS, YamamotoM, WatanabeY (2003) Distinct cohesin complexes organize meiotic chromosome domains. Science 300 : 1152–1155.
48. ThomasSE, Soltani-BejnoodM, RothP, DornR, LogsdonJJM, et al. (2005) Identification of Two Proteins Required for Conjunction and Regular Segregation of Achiasmate Homologs in Drosophila Male Meiosis. Cell 123 : 555.
49. MolnarM, DollE, YamamotoA, HiraokaY, KohliJ (2003) Linear element formation and their role in meiotic sister chromatid cohesion and chromosome pairing. J Cell Sci 116 : 1719–1731.
50. PonticelliAS, SmithGR (1989) Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics 123 : 45–54.
51. Biswas U, Wetzker C, Lange J, Christodoulou E, Seifert M, et al.. (2013) Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions. PLoS Genet 9 : p. e1003985.
52. SumaraI, VorlauferE, GieffersC, PetersBH, PetersJ-M (2000) Characterization of Vertebrate Cohesin Complexes and Their Regulation in Prophase. JCB 151 : 749–762.
53. PetersJ-M, TedeschiA, SchmitzJ (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22 : 3089–3114.
54. LaugschM, SeebachJ, SchnittlerH, JessbergerR (2013) Imbalance of SMC1 and SMC3 Cohesins Causes Specific and Distinct Effects. PLoS ONE 8: e65149.
55. MurdochB, OwenN, StevenseM, SmithH, NagaokaS, et al. (2013) Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior. PLoS Genet 9: e1003241.
56. HiraokaY, DernburgAF (2009) The SUN Rises on Meiotic Chromosome Dynamics. Dev Cell 17 : 598–605.
57. HahnM, DambacherS, DulevS, KuznetsovaAY, EckS, et al. (2013) Suv4-20h2 mediates chromatin compaction and is important for cohesin recruitment to heterochromatin. Genes Dev 27 : 859–872.
58. WhelanG, KreidlE, WutzG, EgnerA, PetersJ-M, et al. (2012) Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J 31 : 71–82.
59. BarberoJL (2013) Genetic basis of cohesinopathies. App Clin Genet 6 : 15–23.
60. Deardorff MatthewA, Wilde JonathanJ, AlbrechtM, DickinsonE, TennstedtS, et al. (2012) RAD21 Mutations Cause a Human Cohesinopathy. Am J of Human Genet 90 : 1014–1027.
61. DeardorffMA, KaurM, YaegerD, RampuriaA, KorolevS, et al. (2007) Mutations in Cohesin Complex Members SMC3 and SMC1A Cause a Mild Variant of Cornelia de Lange Syndrome with Predominant Mental Retardation. Am J of Human Genet 80 : 485–494.
62. MusioA, SelicorniA, FocarelliML, GervasiniC, MilaniD, et al. (2006) X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet 38 : 528–530.
63. RemeseiroS, CuadradoA, Gomez-LopezG, PisanoDG, LosadaA (2012) A unique role of cohesin-SA1 in gene regulation and development. EMBO J 31 : 2090–2102.
64. KalejsM, IvanovA, PlakhinsG, CraggM, EmzinshD, et al. (2006) Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC Cancer 6 : 6.
65. NotaridouM, QuayeL, DafouD, JonesC, SongH, et al. (2011) Common alleles in candidate susceptibility genes associated with risk and development of epithelial ovarian cancer. Int J Cancer 128 : 2063–2074.
66. Caburet S. ArboledaV, LlanoE, OverbeekP, BarberoJL, et al. (2014) Mutant Cohesin in Premature Ovarian Failure. NEJM 370 : 943–949.
67. SkarnesWC, RosenB, WestAP, KoutsourakisM, BushellW, et al. (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474 : 337–342.
68. Bellve AR (1993) Purification, culture, and fractionation of spermatogenic cells. Methods in Enzymology: Academic Press. 84–113 p.
69. La Salle, S., F Sun and M.A Handel. 2009. Isolation and Short-Term Culture of Mouse Spermatocytes for Analysis of Meiosis. Meiosis. Vol. 558 . J.M Walker, editor. Humana Press. 279–297 p.
70. NovakI, WangH, RevenkovaE, JessbergerR, ScherthanH, HöögC (2008) Cohesin Smc1β determines meiotic chromatin axis loop organization. JCB 180 : 83–90.
Štítky
Genetika Reprodukční medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 7- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



