-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification, Replication, and Functional Fine-Mapping of
Expression Quantitative Trait Loci in Primary Human Liver Tissue
The discovery of expression quantitative trait loci (“eQTLs”) can
help to unravel genetic contributions to complex traits. We identified genetic
determinants of human liver gene expression variation using two independent
collections of primary tissue profiled with Agilent
(n = 206) and Illumina (n = 60)
expression arrays and Illumina SNP genotyping (550K), and we also incorporated
data from a published study (n = 266). We found that
∼30% of SNP-expression correlations in one study failed to replicate
in either of the others, even at thresholds yielding high reproducibility in
simulations, and we quantified numerous factors affecting reproducibility. Our
data suggest that drug exposure, clinical descriptors, and unknown factors
associated with tissue ascertainment and analysis have substantial effects on
gene expression and that controlling for hidden confounding variables
significantly increases replication rate. Furthermore, we found that
reproducible eQTL SNPs were heavily enriched near gene starts and ends, and
subsequently resequenced the promoters and 3′UTRs for 14 genes and tested
the identified haplotypes using luciferase assays. For three genes, significant
haplotype-specific in vitro functional differences correlated
directly with expression levels, suggesting that many bona fide
eQTLs result from functional variants that can be mechanistically isolated in a
high-throughput fashion. Finally, given our study design, we were able to
discover and validate hundreds of liver eQTLs. Many of these relate directly to
complex traits for which liver-specific analyses are likely to be relevant, and
we identified dozens of potential connections with disease-associated loci.
These included previously characterized eQTL contributors to diabetes, drug
response, and lipid levels, and they suggest novel candidates such as a role for
NOD2 expression in leprosy risk and
C2orf43 in prostate cancer. In general, the work presented
here will be valuable for future efforts to precisely identify and functionally
characterize genetic contributions to a variety of complex traits.
Published in the journal: . PLoS Genet 7(5): e32767. doi:10.1371/journal.pgen.1002078
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002078Summary
The discovery of expression quantitative trait loci (“eQTLs”) can
help to unravel genetic contributions to complex traits. We identified genetic
determinants of human liver gene expression variation using two independent
collections of primary tissue profiled with Agilent
(n = 206) and Illumina (n = 60)
expression arrays and Illumina SNP genotyping (550K), and we also incorporated
data from a published study (n = 266). We found that
∼30% of SNP-expression correlations in one study failed to replicate
in either of the others, even at thresholds yielding high reproducibility in
simulations, and we quantified numerous factors affecting reproducibility. Our
data suggest that drug exposure, clinical descriptors, and unknown factors
associated with tissue ascertainment and analysis have substantial effects on
gene expression and that controlling for hidden confounding variables
significantly increases replication rate. Furthermore, we found that
reproducible eQTL SNPs were heavily enriched near gene starts and ends, and
subsequently resequenced the promoters and 3′UTRs for 14 genes and tested
the identified haplotypes using luciferase assays. For three genes, significant
haplotype-specific in vitro functional differences correlated
directly with expression levels, suggesting that many bona fide
eQTLs result from functional variants that can be mechanistically isolated in a
high-throughput fashion. Finally, given our study design, we were able to
discover and validate hundreds of liver eQTLs. Many of these relate directly to
complex traits for which liver-specific analyses are likely to be relevant, and
we identified dozens of potential connections with disease-associated loci.
These included previously characterized eQTL contributors to diabetes, drug
response, and lipid levels, and they suggest novel candidates such as a role for
NOD2 expression in leprosy risk and
C2orf43 in prostate cancer. In general, the work presented
here will be valuable for future efforts to precisely identify and functionally
characterize genetic contributions to a variety of complex traits.Introduction
Genome-wide association studies have uncovered numerous robust associations between common variants and complex traits, but only a minority of these can be traced to protein-altering polymorphisms [1]. It is likely that most of these associations result from non-coding variants. One hypothesis is that such variants modify cis-regulatory sequences and thereby change the expression levels of one or more target genes. Variance in gene expression plays essential roles in numerous important processes and is highly heritable in human populations [2].
Considering this, the discovery of genetic variants that have a functional impact on gene expression is a potentially powerful means to facilitate more accurate and robust identification of associations between variants and disease. Such discoveries may also provide mechanistic insight into otherwise anonymous genotype-phenotype correlations that often span many correlated variants across multiple genes. In large part due to this potential there has been recent substantial interest in the identification of expression quantitative trait loci (eQTLs) [3]–[10].
Regulation of gene expression in the liver is of particular interest given its vital roles in maintaining homeostasis and health, including synthesis of most essential serum proteins, the production of bile and its carriers, and the regulation of nutrients. The liver is also the predominant organ in xenobiotic metabolism, and it has been estimated that 75% of the 200 most widely prescribed drugs are eliminated from the body through liver metabolism [11]. Altered metabolism by genetic factors affects the systemic availability and residence time of xenobiotics and hence their toxic and pharmacologic effects [12].
While eQTL studies have made valuable contributions to genetic research (e.g., [13]), there exist several practical limitations to consider. First, most eQTL studies are conducted in immortalized, lymphoblastoid cell lines (LCLs), which clearly have utility for the interpretation of human disease associations, particularly with immunity-related phenotypes [14], [15]. However, the use of such cell lines potentially introduces artifacts associated with immortalization, subsequent passage, and growth conditions prior to harvest [16]. Second, eQTLs may exhibit spatiotemporal specificity [17], [18], presumably driven by polymorphisms located within tissue specific regulatory elements, and eQTL studies may be maximally informative for any given trait when conducted in a relevant, non-transformed cell type. Third, environmental factors and other, mostly hidden, confounding variables are known to significantly affect gene expression levels and measurements [19]–[23]. Fourth, most eQTL studies fail to provide replication on an independent set of samples with independent experimental assessment (see [24]–[27], [23] for exceptions).
We sought to address these limitations, and conducted two independent eQTL studies and compared these results to a third, published study. Genetic analyses were performed using Bayesian regression [28], [29] after controlling for age, sex, ancestry, and unmeasured confounding variables [20]. Using the UC liver panel as a ‘discovery’ cohort and the UW and Merck data as replication panels, we found that ∼30% of eQTLs identified at stringent thresholds failed to replicate in either of the two replication studies. We show that this is likely due to several factors, including SNPs in probes, but the effects of unmeasured confounding variables were particularly pronounced. We also found that reproducible eQTL associations were enriched near proximal promoters and 3′ UTRs. Through targeted resequencing and luciferase experiments, we identified 3 significant haplotype-specific in vitro functional effects that directly support a liver eQTL. These data functionally validate the enrichment for eQTLs near gene ends and suggest that many eQTLs can be rapidly fine mapped to a causative variant or haplotype. Finally, given our study design we identified hundreds of genes with reproducible SNP-associated expression levels, a subset of which provide strong mechanistic hypotheses for published associations between SNPs and disease.
Results
Three independent sample collections
We analyzed two independent sets of primary liver tissues at the University of Chicago (UC; n = 206) and University of Washington (UW; n = 60). We genotyped both sets of samples using Illumina SNP arrays (quad-610 and 550 k for UC and UW, respectively); to improve mapping power [30], [28] and replication ascertainment, additional genotypes were imputed using HAPMAP reference genotype panels (see Methods). Gene expression levels were analyzed using Agilent (UC) and Illumina (UW) expression arrays. We considered the UC liver collection as a ‘discovery’ set and used as replication panels the UW collection and a published set of liver eQTL data (Merck; n = 266) [31]. However, we note that the conclusions drawn below were robust to the choice of a ‘discovery’ set (Figure S1). All samples analyzed across all three studies were unique. Microarray expression probes from both platforms were remapped to RefSeq gene models to aid in cross platform comparisons. A total of 14,703 RefSeq genes were surveyed in the UC reference study while 11,245 RefSeq genes were present on all three platforms. We have made these data and results publicly available through the GEO and SCAN databases (http://www.scandb.org/) [32].
Demographic effects
After correcting for technical effects and unmeasured confounding variables, we found that thousands of gene expression traits were significantly associated with demographic variables. At a 5% false discovery rate (FDR), 769, 336, and 3,110 genes were significantly associated with ancestry, sex, and age, respectively within the UC livers. Genes significantly affected by sex or age (FDR<5%, Figure 1, Figure S2, examples displayed in Figure S3) have a marked enrichment for small p-values in both replication samples (Figure 1A, 1D). To lessen the influence of differential statistical power among the three studies (n = 206, 60, 266), we defined ‘replication’ as having a nominally significant p-value in the independent sample (p-value<0.05) and having a concordant effect direction (i.e., is YFG more highly expressed in males or females?). 29.9% and 32.1% of genes significantly affected by sex (UC sex t-test FDR<5%) replicated in the UW and Merck studies, respectively (Figure 1B). At more stringent thresholds, validation rates exceeded 80%, albeit with fewer included genes (Figure 1B). We also note that the sex-associated gene set was strongly enriched for genes on the X and Y chromosomes (Figure 1C; X chromosome, hypergeometric test, p-value = 1.72×10−14, Y chromosome, p-value<2×10−16), as would be expected for genes with sex-associated expression levels. Effects due to age were less reproducible: 13.2% and 21.5% of significantly age associated genes (UC age t-test FDR<5%) replicated in the UW and Merck studies, respectively (Figure 1E; an example of a replicated age-associated gene, TMEM22, is displayed in Figure 1F). Effect sizes for both sex and age were correlated across studies (Figure S4; Spearman's rho, UC-UW sex = 0.597, UC-Merck sex = 0.720, UC-UW age = 0.333, UC-Merck age = 0.159), underscoring the reproducibility of demographic effect estimates.
Fig. 1. Age and sex effect replication. 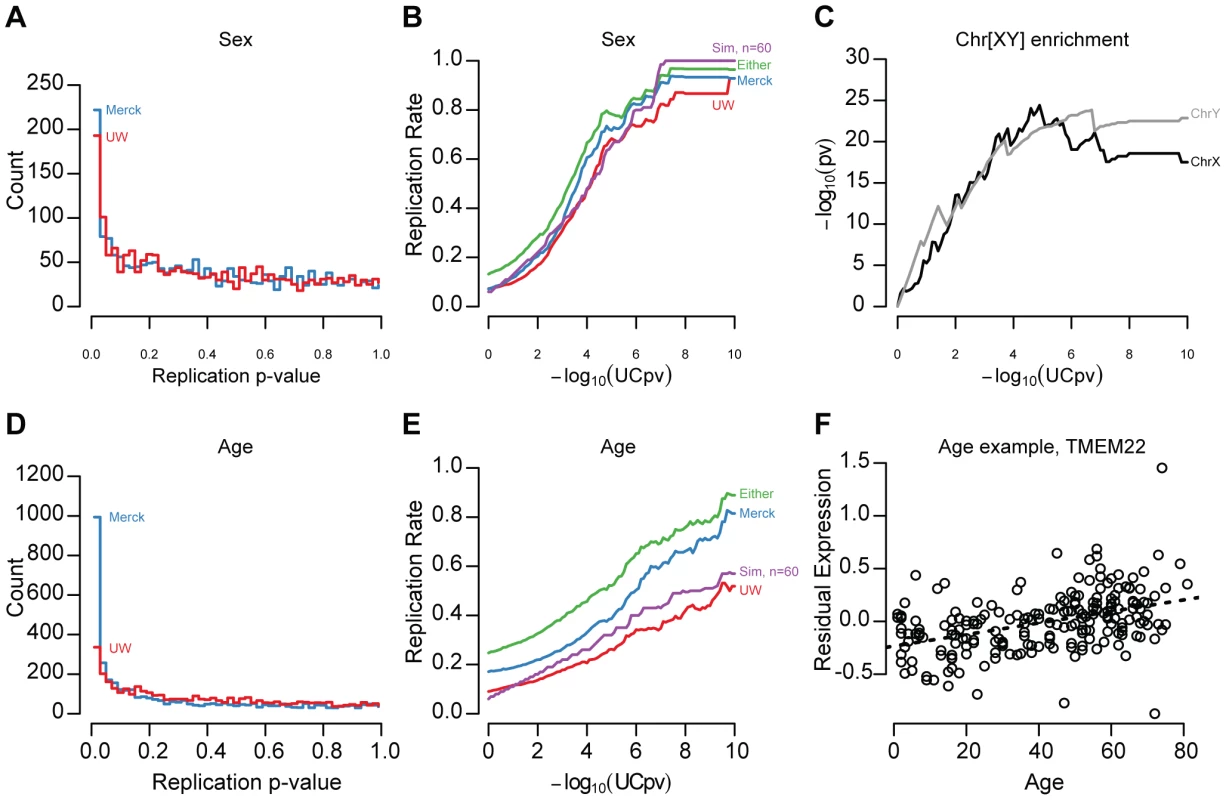
It is possible that both age and sex replication rates are downwardly biased due to differences in age and sex distributions (Table 1). To quantify the potential effects of heterogeneous sample sizes and unbalanced study designs, we performed resampling studies within the UC discovery cohort. Demographic effect replication rates were recalculated using 60 samples that were race, sex, and age (+/−3years) matched to the UW distribution (Figure 1B, 1E; see Methods). We found that 34% of sex effects and 15% of age effects replicated by simulation, supporting the conclusion that sample size and demographic heterogeneity do generate significant covariate associations that our replication studies are unable or underpowered to detect.
Tab. 1. Sample demographic summaries of all three studies. 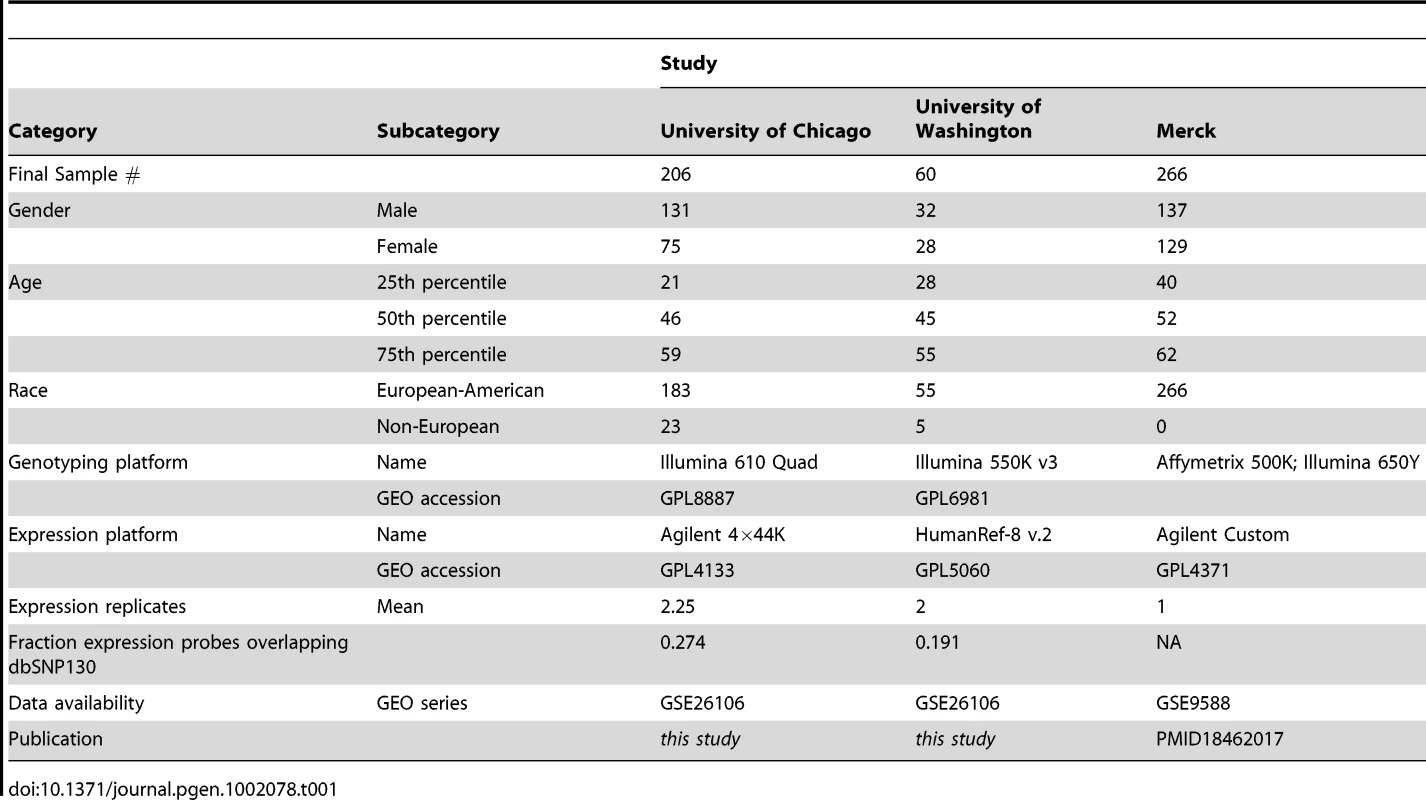
cis-eQTL mapping
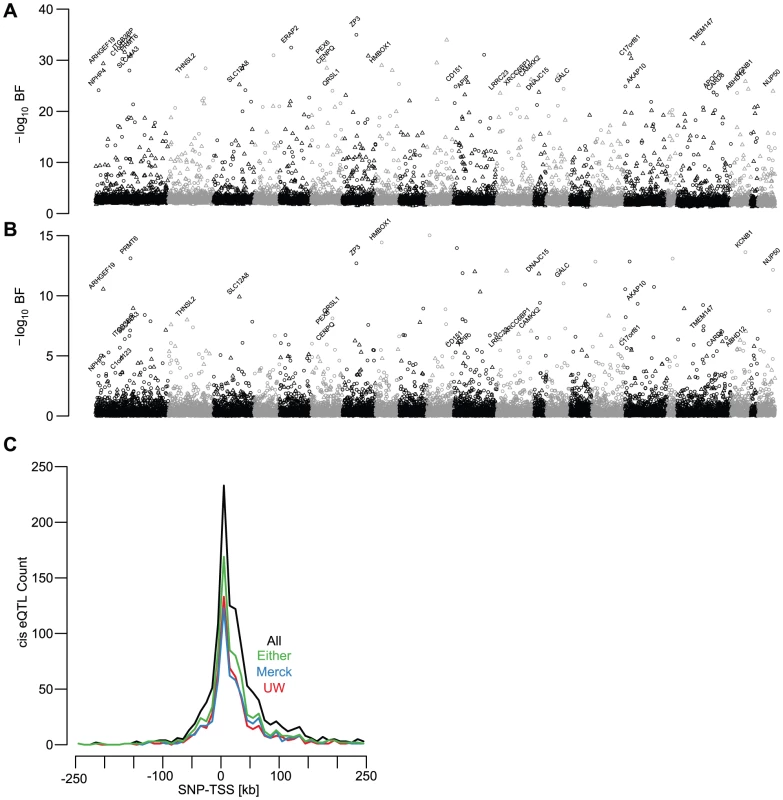
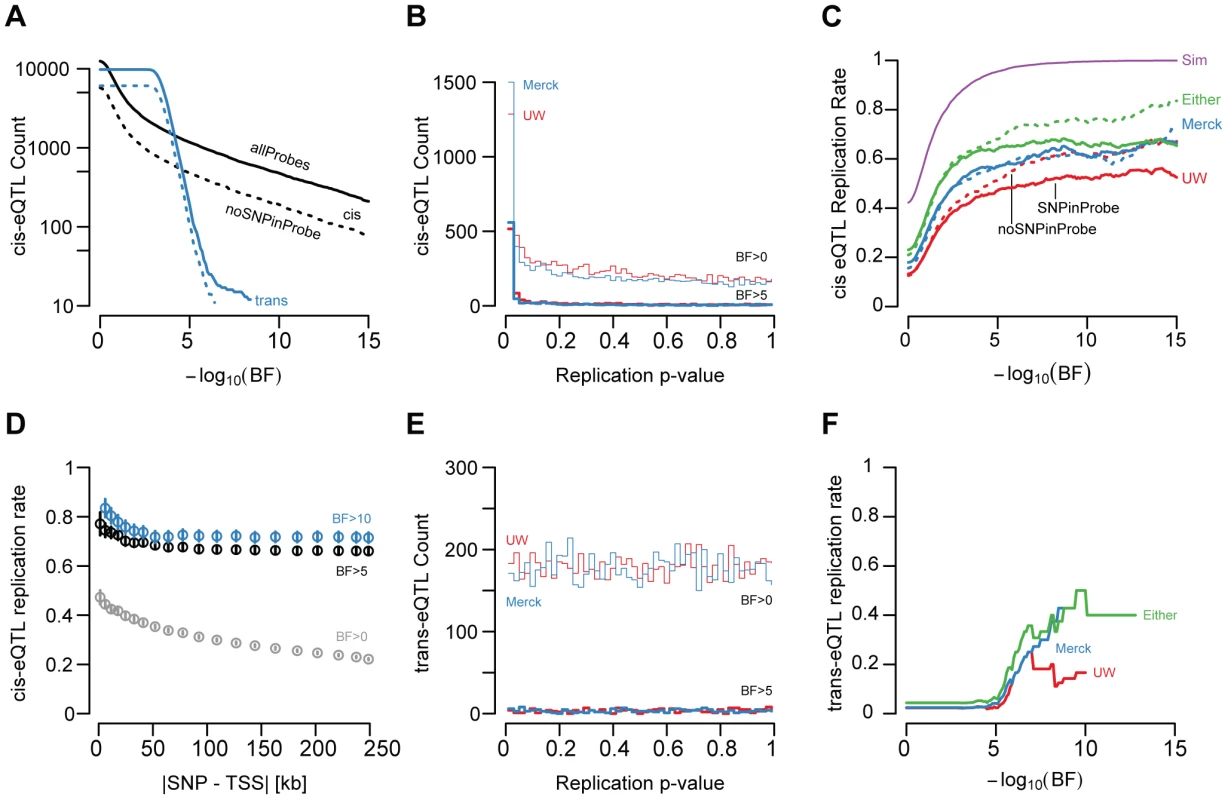
After adjusting for age, sex, ancestry, and unmeasured confounding variables (quantified by surrogate variable analyses, see Methods and [20]), we found 1,787 gene models with significant cis-linked genetic effects on expression levels (UC log10 Bayes Factor (BF)>5; SNP to TSS distance <250 kb; Figure 2A, Figure 3A, Table S1). The distribution of t-test p-values in the replication sets, adjusted for the same covariates, for the UC best associated gene-SNP pairs were significantly enriched for small values (Figure 3B), indicating that a large fraction of cis-eQTLs are reproducible in independent sample collections. As with demographic effects, we defined replication as a p-value<0.05 and a concordant allele effect direction (Figure 3C). While the significance of association in the discovery cohort has a large effect on replication probability, the relationship between significance and replication was effectively binary (Figure 3C). Cis-eQTLs with BFs>5 were much more likely to replicate than those with BFs<5 (chi square p-value<2×10−16). However, among genes with BFs>5, replication probability was only weakly dependent upon BF (Figure 3C; logistic regression chi-squared p-value = 0.00319).
We found that 49.1% and 57.6% of significant cis-eQTLs (UC BF>5) replicated in the UW and Merck studies, respectively (i.e., p-value<0.05 and concordant effect directions; Figure 2A–2B, Figure 3C). The lower observed replication rate for the UW study is partially attributable to the smaller sample size (60 vs 266), but may also reflect platform-dependencies. 66.7% of significant cis-eQTL associations replicated in at least one of the two replication cohorts, while 47.6% replicated in both cohorts. Cis-eQTLs that replicated in one replication study were significantly more likely to replicate in the second replication study than expected by chance (chi-squared p-value<2×10−16) and twice-replicated eQTLs had larger effect sizes than eQTLs that replicate in only one study (Wilcoxon rank-sum test p-value<2×10−16; Figure S5, examples of non-replicated cis-eQTLs displayed in Figure S7).
Sample size, statistical power, and winner's curse
Given differences in sample sizes among these studies, we sought to define a baseline replication rate against which to compare the observed levels of reproducibility. We therefore conducted a re-sampling experiment in which, for each gene expression trait, 100 sets of 60 sex and age (+/−3 years) matched samples were selected at random and used to define replication (i.e. concordant effect direction and p<0.05). We found that simulated replication rates increase dramatically near a BF of 5 (95.5% replication at BF>5; Figure 3C) and are effectively 100% at higher thresholds. These observations suggest that power differential among the studies cannot alone explain the observed rates of replication, as there are many genes with effect sizes in one study that should be readily detected in both (let alone either) replication panels. This is further supported by the observation that concordance alone (i.e. no p-value threshold) yielded similar levels of reproducibility, as did direct comparisons of allelic coefficients (Spearman's rho of 0.663 and 0.681 for UC–UW and UC-Merck comparisons, respectively; Figure S6).
We next sought to evaluate whether ‘winner's curse’ [33], [34] was deflating replication rates. Therefore, we extracted simulations in which the estimated coefficients randomly decreased and found that simulated replication remained >90% at BF>5 and near 100% at higher BF even when the effect size declined substantially (e.g. 30% drop in regression coefficient; Figure S9). Effect sizes would need to be over-estimated by 2-fold or greater across the entire set of eQTLs with UC BF>5 to result in the observed rates of replication. Furthermore, two lines of reasoning suggest winner's curse is not a major contributor to the observed rates of non-replication. First, we note that bias resulting from winner's curse should be progressively less pronounced as the true effect size increases, which in turn will correlate with significance estimates in the discovery panel [34]. However, replication rate was essentially flat even at extremely stringent thresholds (Figure 3C). Additionally, the resampling experiments demonstrated that, in direct contrast with a winner's curse prediction, effect sizes would need to be increasingly more severely over-estimated at higher thresholds (3-fold or more) to result in the observed rates of replication (Figure S8). Second, the definition of replication (concordance and p-value<0.05) is relatively loose when applied to eQTLs with a BF>5 (typical linear regression p-values<5×10−8) and should accommodate substantial drops in effect sizes for both replication panels but especially for the larger Merck dataset. This is further supported by the observation that concordance alone yielded similar rates of replication (Figure S6). We conclude that statistical power and winner's curse cannot explain the observed rates of non-replication for eQTLs with BF>5.
On reproducibility failures due to hybridization artifacts
One possible explanation for non-replication is that SNPs within sequences targeted by expression probes may change hybridization efficiency in an allele-specific manner; if that SNP is also correlated with a genotyped variant, false positive eQTLs may result [35]. While 45.3% and 37.2% of Agilent and Illumina probes overlap with a polymorphism found in dbSNP131 or the one thousand genomes project (2010.08.04 release), the frequency distribution of polymorphisms in and around probe sequences differs markedly between the Agilent (UC) and Illumina (UW) platforms (Figure S9); Illumina expression probes have clearly been designed to avoid common polymorphisms.
The presence of SNPs in expression probes had a larger effect on reproducibility at extremely high thresholds (Figure 3C). For example, the replication rate for cis-eQTLs with BF>5 is not significantly affected by the presence of SNPs in probes (p-value = 0.189); however, replication rate for cis-eQTLs thresholded at BF>10 is significantly affected by probe SNPs (p-value = 0.0354; 65.6% with, 74.9% without SNP) and replication rate is significantly associated with an interaction between probe SNPs and eQTL significance (logistic regression BF-SNP interaction p-value = 0.0224). These results suggest the proportion of non-reproducible cis-eQTLs increases with eQTL significance such that, for eQTLs with BF>10, ∼27% of the non-replication rate can be explained by the presence of hybridization artifacts caused by known polymorphisms. To investigate the potential confounding role of unannotated polymorphisms in eQTL ascertainment, we re-sequenced 15 expression probes for genes that had large discrepancies in correlation measurements between the UW and UC studies that did not overlap a known SNP (9 probes with strong UW correlation but low UC correlation, 6 of the converse; Table S2). We found that none of these 15 probes harbored SNPs in the 60 UW liver samples or a panel of 35 CEU HapMap samples. Collectively, our data suggest future array designs/eQTL studies would benefit from more aggressive avoidance of known SNPs, but current SNP annotations are sufficiently comprehensive that unknown variants are of little concern to eQTL analyses.
Surrogate variable analysis dramatically improves eQTL reliability
We next quantified the role of several additional factors that may generate spurious associations. Most strikingly, failure to control for unknown or unmeasured confounding variables by surrogate variable analysis (SVA) produced a large decrease in the number of significant (BF>5) cis-eQTL signals (1,787 vs. 873; Figure 4A; McNemar's chi-squared test p-value<2×10−16), similar to a recent study of gene expression within twins [23]. Not only did SVA produce a larger number of significant cis-eQTL associations, but these associations were also significantly more likely to replicate (McNemar's Chi-squared test p-value≪2×10−16; Figure 4B). While it has been shown that unknown or unquantified confounders can lead to unreliable genetic predictions [19], [36], [2], our data show that such factors, if unaccounted for, dramatically decrease the number of eQTL signals and their reproducibility across multiple independent collections of primary human tissues.
Fig. 4. SVA improves eQTL reproducibility. 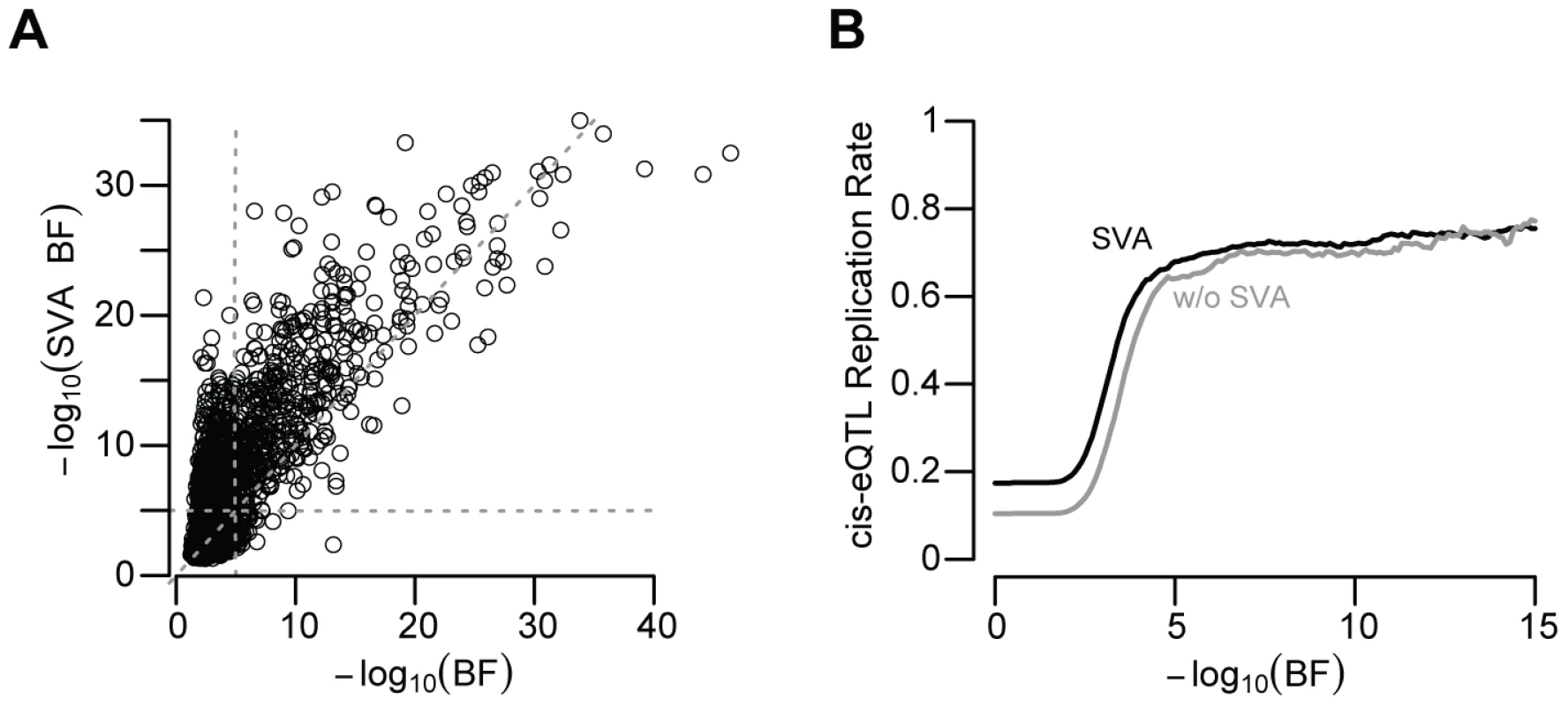
Other factors influencing reproducibility
Several additional aspects of the gene expression measurements correlated with cis-eQTL replication rate. Cis-eQTL replication rate was significantly associated with mean gene expression level and, independently, inter-individual expression coefficient of variation (Figure S10; multivariate logistic regression chi-squared p-value = 3.44×10−3 and 1.41×10−4, respectively); more highly expressed and highly variable genes were more likely to replicate. Further, we found that expression variance unexplained by age, sex, race, and surrogate variables was negatively correlated with expression level (Spearman's rho = −0.302, p-value<2×10−16). These data suggest greater measurement accuracy at higher expression levels that leads to more robust eQTL identification.
We also found that the best associated SNP for each gene expression trait was frequently immediately upstream or downstream from the transcription start site (TSS) (Figure 2C, [37]). Replication rate of significant cis-eQTLs was associated with absolute SNP to TSS distance (logistic regression chi-squared p-value = 5.35×10−3). 74.5% of cis-eQTLs within 5 kb of the TSS replicated, compared with only 60.6% located more than 100 kb from the TSS. Thus while distal regulatory elements are clearly important for human gene expression regulation, robustly quantifiable, segregating expression polymorphism was more likely to be found in SNPs very close to the TSS of genes.
Interestingly, significant cis-eQTLs were no more likely to replicate when analyses were restricted to probes targeting the same exon (chi-squared p-value = 0.759), demonstrating that most non-replicating eQTLs (in our study design) can not be accounted for by differential splicing or isoform usage. Similarly, replication was not improved when analyses were restricted to gene expression measurements derived from more than one expression probe (chi-squared p-value = 0.919). Additionally, the minor allele frequency of the associated SNP did not have a significant effect on replication rate (logistic regression chi-squared p-value = 0.600; Figure S10), and eQTLs at imputed SNPs replicated at similar rates to directly genotyped SNPs (logistic regression chi-squared p-value = 0.574; Figure S10). Uncertainty at imputed SNPs does not appear to have a significant effect on cis-eQTL replication rate, as the ratio of observed to expected genotype variance was not associated with replication rate in any of the three sample sets (logistic regression chi-squared p-values all >0.152; Figure S12).
Examination of the interplay of the factors influencing eQTL replication revealed several interesting trends. As mentioned above, replication probability was significantly associated with SNP to TSS distance, but this association decreases with increasing cis-eQTL significance (distance×BF interaction logistic regression p-value = 3.98×10−5). Thus, location information can help to differentiate real from false positive correlations of modest effect, but is less important for very strong correlations. We constructed stepwise multivariate logistic regression models, restricted to associations with BF>5, and confirmed that BF (logistic regression chi-squared p-value = 7.32×10−3), SNP to TSS distance (p-value = 2.33×10−3), gene expression (p-value = 0.0230), gene expression CV (p-value = 1.33×10−4), and probe SNP×BF interaction (p-value = 0.0207) all have significant effects on the cis-eQTL replication rate. In contrast, SNP minor allele frequency, SNP type (imputed or direct), and genotype variance do not substantially influence replication rate (p-values>0.5).
Trans-eQTLs
We also conducted genome-wide scans for associations between gene expression traits and unlinked SNPs. Such trans-eQTLs may represent regulatory interactions between transcription factors, signaling molecules, or chromatin regulators and their target genes. After adjusting for demographic variables as above, we found 353 gene expression traits with significant (BF>5) trans-linked genetic effects. The replication behavior of trans-eQTLs was markedly different from cis-eQTLs (compare Figure 3B, 3C with Figure 3E, 3F). First, the distribution of t-test p-values derived from the UW replication set, for each best associated gene-SNP pair identified in the UC set, was effectively uniform (Figure 3E). Second, in contrast to cis-effects, which rapidly approach an asymptotic replication rate at BF 5, trans-eQTLs almost completely failed to replicate (6.14%; Figure 3F) at a BF threshold of 5. At greater significance thresholds, trans effects did replicate more frequently (e.g., at BF> = 9.5, 50.0% replicate), however, these rates never approached those observed for cis-eQTLs. It is plausible that surrogate variable correction may mask true ‘master’ regulator effects, but as for cis-effects we identified more trans-eQTLs with surrogate variable correction than without and these associations were more likely to replicate (data not shown). While it is perhaps surprising that even extremely significant trans effects frequently fail to replicate, we note that this behavior is, to a certain extent, to be expected [27].
Fine-mapping and functional characterization
As the eQTLs we identified are associations between effectively anonymous SNPs and expression of a nearby gene, we were also interested in fine-mapping the associations, ideally to a causal variant (expression quantitative-trait-nucleotide or eQTN) or haplotype. We therefore re-sequenced the promoter and 3′UTR sequences for 18 genes with strong cis-eQTLs within the 60 UW livers (Table S3). Thirteen of these genes harbored a common SNP or indel within the proximal promoter or 3′UTR that correlated strongly (p-value<1×10−8) with the expression level of that gene, while 17 of 18 harbored a variant with at least a modest correlation (p-value<0.001). Of these 17 genes, the most strongly correlated SNP was within the 3′UTR for 11 genes and within the promoter region for 6 genes. Moreover, 10 of the 17 best SNPs were not within HapMap, indicating that a majority of the most strongly associated promoter/3′UTR variants were neither genotyped directly nor imputed and therefore not detectable in the original eQTL analysis.
We subsequently sought experimental support for the functional nature of the most strongly associated SNPs. Therefore, for 14 genes, we cloned (and sequence-verified) common haplotypes existing in the UW liver samples into a customized luciferase reporter vector, and tested the function of each haplotype using high-throughput, transient transfection reporter assays (Table S3; 9 of 14 underlying cis-eQTLs replicated in the UC or Merck samples). For each haplotype, multiple independent vector (mode of 3) preparations were made, and for each plasmid preparation 4 transfection replicates were performed (mode of 12 measurements per haplotype). We analyzed the resulting data using a random-effects model that accounted for both variation in transfection replicates and variation in vector preparations. Our results underscore the need to perform multiple independent DNA preparations to reliably infer sequence-specific functional effects with this system (Figure 5 and data not shown).
Fig. 5. Fine-mapping functional results. 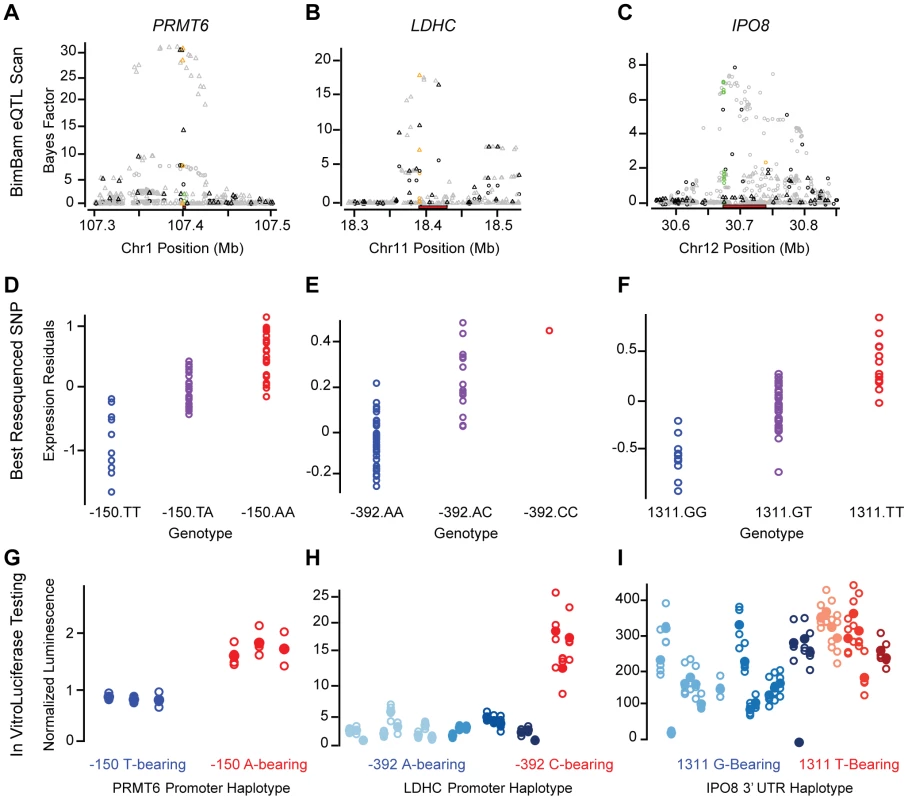
We identified three regions where the haplotype sequence had a significant (p-value<0.001) effect on reporter activity (luminescence) in the same allelic direction as the expression measurements, including two promoters and one 3′UTR region (Figure 5 and Figure S12). No significant but discordant effects were observed. Variants near PRMT6, which encodes a protein-arginine methyltransferase and has been associated with HIV infection progression [38], scored highly in both the UW and UC eQTL analysis (Figure 5A). Resequencing of the PRMT6 promoter yielded two common haplotypes defined by two perfectly correlated SNPs located 406 and 150 bp upstream from the TSS. The minor haplotype (40% frequency) correlated with a strong additive decrease in PRMT6 liver expression (t-test p-value = 6.4×10−14 for UW), and relative to the major haplotype, we found a concomitant decrease in luminescence for reporter constructs harboring the minor haplotype (p-value = 0.0002). A similar result was obtained for promoter haplotypes of the LDHC (lactate dehydrogenase C) gene in which six common variants defined 7 common haplotypes, five of which were successfully cloned and tested. The strongest expression correlation was observed for a SNP located 392 bp upstream of the TSS (15% MAF), and the luciferase data strongly support the functional effect of this variant (p-value = 8.7×10−9; Figure 5B).
Finally, we identified a significant haplotype-specific effect within the 3′UTR for IPO8 (importin 8), a protein that interacts with Argonaute proteins to direct miRNA mediated gene expression regulation [39]. There were nine common 3′UTR haplotypes defined by 13 variants for IPO8. The two haplotype groups defined by the most strongly expression-associated SNPs (two perfectly correlated variants at positions 1147 and 1195 relative to the 3′UTR start) have significantly different (p-value = 9.5×10−4) functional effects. However, unlike LDHC, there remained a substantial amount of variance within the haplotypes defined by alleles at these two SNPs, suggesting other variants may also have a functional role. Alternatively, the data gathered from 3′UTRs were generally noisier than that for promoters (Figure 5 and data not shown), and may not be as sensitive for identifying sequence-specific 3′UTR effects. Due to the increased noise, we repeated the analysis and performed new clone preparations and transfections for a subset of the IPO8 haplotypes. The replicate data also show a significant (p-value = 0.007) difference, in the same direction, between haplotypes defined by their 1147 (or 1195) allele (Figure S12).
Discussion
Genetic analyses of gene expression have great potential to facilitate insights into the genetic basis of complex traits. However, the utility of these data are limited by the extent to which the discovered associations correspond to legitimate, reproducible associations. Our estimates of 49% (UC vs. UW), 57% (UC vs. Merck), and 67% (UC vs. either) cis-eQTL reproducibility are substantially lower than two recent reports between two mouse crosses (76%, [27]), two independent sets of lymphoblastoid cell lines (83%, [25]), and two sets of primary human skin (>99%, [26]). Several non-exclusive possibilities likely contribute to these discrepancies. First, different discovery methodologies and replication criteria were employed in each study. Second, our studies were performed on different expression platforms (Agilent and Illumina), which reduces the influence of reproducible platform-specific errors but may result in missing splice-variant-specific eQTLs [40], [41], [10] as array manufacturers often target different exons in a given gene. However, this is likely to have a limited effect, as we found that the replication rate was not significantly different for genes assessed by probes within the same exon (Figure S10). Third, we compared three independent collections of primary human tissues (see Methods), not transformed cell lines or mouse tissues, and, despite the interpretive advantages associated with the former, our replication rate estimate is possibly downwardly biased by cell type heterogeneity. Finally, other systematic differences between studies, including protocols for sample collection and storage, clinical interventions taken by patients prior to death and autopsy, causes of death, life histories, etc., may contribute to non-reproducibility. This hypothesis is supported by the observation that drug exposures and other clinical covariates, for which data limitations prevent comprehensive analysis, have substantial effects on gene expression; for example, we found that drug metabolism genes were significantly up-regulated in barbiturate-exposed vs non-exposed livers (data not shown). The striking difference in reproducibility between the results reported here and a recent report quantifying the overlap of human skin eQTLs [26], suggests that the degree of functional tissue heterogeneity may vary substantially across tissues.
An important caveat is that these estimates of reproducibility are less meaningful for sequence-based studies of gene expression, which offer advantages in dynamic range and measurement accuracy [9], [10]; sequencing is also largely immune to the SNP-in-probe effect that significantly inflates false positives in our data (Figure 3C). However, the observation that age, sex, race, drug exposures, clinical covariates, and other global factors have such strong influences on expression (e.g., this study and [36], [2]) coupled with observations in other studies and different tissues that factors like cause of death are relevant [42], suggests that much of the non-reproducibility is in fact driven by systematic differences in tissue source. Such differences will likely be important to all studies of primary tissue samples, whether assayed by arrays or by sequencing. The reproducibility of future results would benefit from analysis of samples from multiple centers with as much clinical information as possible. Furthermore, our results confirm previous observations that the effects of unknown, unmeasured, or unquantified covariates can confound genetic effects with structured error sources [19], [36], [2], and that controlling for these hidden confounders substantially boosts the rate of eQTL discovery [23]. Importantly, we demonstrated that not only are more eQTLs detected but that their reproducibility in independent collections of primary human tissue was also significantly higher.
Finally, through resequencing and a widely used in vitro assay system [43], we found that of 14 tested genes, two genes harbored functional eQTNs in the proximal promoter and one gene harbored functional eQTNs in the 3′UTR. The success rate of 3 in 14 suggests that a substantial number of eQTLs, and by extension any complex traits that they may influence, can be functionally isolated using the scalable assay system that we employed or potentially higher-throughput assays [44]. We note that some truly functional variants will not be detectable in these assays, either from being tested out of their genomic context or having effect sizes below the limit of detection afforded by the number of replicates used (e.g. [45]), and that the actual fraction of eQTLs with promoter or 3′UTR functional variation may be substantially higher. Considering that replication was significantly greater for eQTLs near the ends of genes relative to those further away (Figure 3D), our functional analysis also strongly supports the use of SNP to gene distance as an important contributor to the prior probability that any given SNP is a cis-eQTN [37]. While some eQTNs clearly reside outside these regions (e.g., [46]), the heavy enrichment for reproducible and experimentally tractable eQTNs, coupled with historical evidence supporting disease relevance [47], [48], suggests that the relatively small ‘promoter’ - or ‘3′UTR’-ome target spaces may be valuable additions to exome-based disease resequencing efforts [49].
Given the ubiquitous importance of gene expression variance to phenotype, the known heritability of gene expression variance, and the great preponderance of non-coding functional elements in the genome [50], complex disease studies can benefit from eQTL analyses. Towards that end, we searched for correlations between replicated eQTL SNPs identified here and complex trait associated SNPs (R2>80%; Table 2, Table S4) in the NHGRI GWAS catalog (http://www.genome.gov/gwastudies/). These included several previously characterized mechanistic links to complex traits, such as VKORC1 expression and warfarin drug response [51] and SORT1 expression correlations with lipid levels and heart disease [13], both of which were originally identified using the UW liver panel described here. Additionally, these data support a relationship, which had previously been speculated but not shown to exist, between NOD2 expression levels and leprosy risk [48], and novel hypotheses such as a link between expression of the uncharacterized C2orf43 gene and prostate cancer risk [52].
Tab. 2. Selected overlap between liver eQTLs and GWAS SNPs. 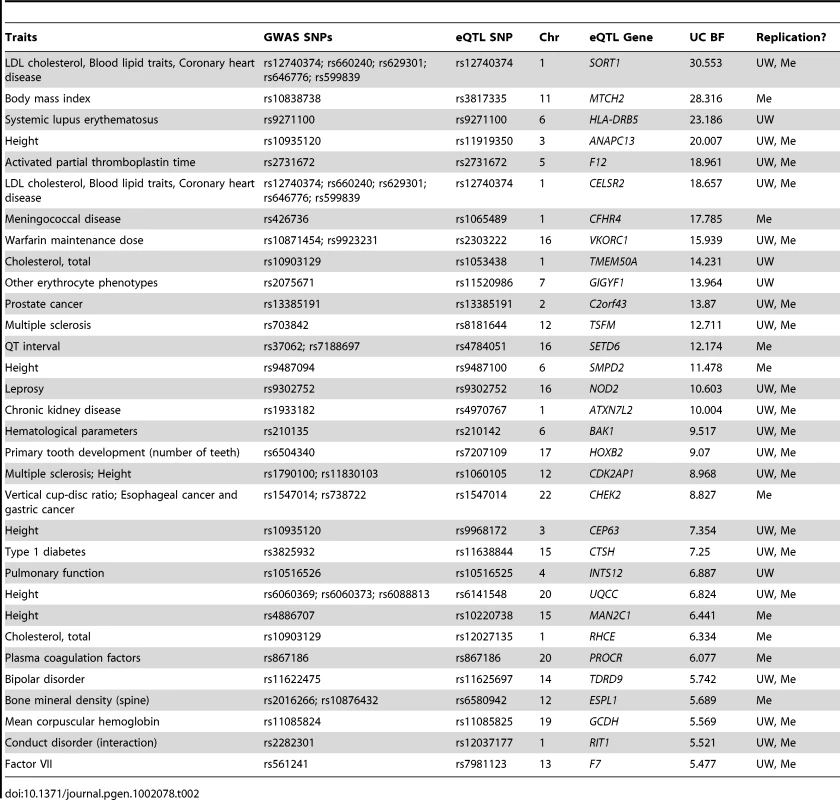
In summation, our data facilitate insights into the factors and experimental design criteria that affect eQTL reproducibility and may improve future eQTL studies, replicate many published but nonreplicated eQTLs (e.g. from [31]), support and extend eQTLs identified in other tissues like brain (e.g. FAM119B [53]), identify many novel reproducible liver eQTLs, show that promoters and 3′UTRs are enriched for experimentally accessible functional variation, and support or suggest numerous mechanistic links to biomedically important phenotypes. We believe that this study and others like it will be valuable to the robust discovery and fine-mapping of the genetic basis for complex human diseases.
Methods
Ethics statement
Research conducted in this study was performed on deceased, anonymous individuals and is therefore not considered to involve ‘human subjects.’ Samples were collected with approval of institutional review boards (IRBs) and the University of Chicago and University of Washington IRBs approved their use for the purpose of this study.
Tissue procurement—UC
Livers were processed through Dr. Mary Relling's laboratory at St. Jude Children's Research Hospital, part of the Pharmacogenetics of Anticancer Agents Research (PAAR) Group, and were provided by the Liver Tissue Cell Distribution System funded by NIH Contract #N01-DK-7-0004/HHSN267200700004C and by the Cooperative Human Tissue Network. Samples were collected with approval of institutional review boards (IRBs) and the University of Chicago IRB has approved their use for the purpose of this study.
Analysis began with 240 normal (non-diseased) livers that were collected from unrelated donors of self-reported European and African descent. Most of the liver tissue samples come from donor livers that were not used for whole organ transplants, the remainder being from liver tissue which remains following a partial graft into a smaller recipient, usually a pediatric patient. As such, each liver is procured with the intent to transplant under the best possible conditions to maintain cell viability. Standardized procedures have been in place for handling, freezing and storage of the livers and their subcellular fractionation and enzyme characterization. Demographic information is summarized in Table 1.
Tissue procurement—UW
The University of Washington IRB approved the collection of the liver tissues and their subsequent use for the purposes of this study. Samples of human liver were obtained from organ donors through the University of Washington Transplant Program and the Northwest Organ Procurement Agency. Consent for research was obtained in all cases. Standard procedures were employed for the handling, freezing and storage of the livers.
Gene expression analysis—UC
Gene expression microarray experiments were conducted with biological replication in all samples. Sample processing order was randomized. For each sample, total RNA was extracted at least twice independently, from tissue homogenized in TRIzol reagent, followed by Qiagen RNAeasy cleanup (Qiagen). RNA quality was assessed by Bioanalyzer (minimum RIN = 7). cRNA was produced using the Agilent Low-Input Linear amplification and labeling kit.
Array hybridizations (Agilent-014850 4×44 k arrays, GPL4133) were performed at The University of Chicago, Argonne National Labs high throughput genome analysis core facility, according the manufacturers instructions. The Agilent FE software was used to extract feature intensities and to flag saturated, non-uniform, and outlier features. Probe intensity was adjusted by subtracting background intensity using the minimum method [54], [55] and quantile normalized between arrays [56]. Dixon's outlier test was used to remove 13 arrays (out of a total of 517) based on total number of flagged probes, intra-array variance, inter-array variance, biological replicate variance, and spike-in linearity [57].
Probes were grouped into probe sets by aligning first to RefSeq gene annotations and then aligning unmapped probes to the human reference genome (build 36). All probes with non-unique best alignments were excluded from further analysis. Multiprobe probesets were hierarchically clustered using one minus the pearson correlation coefficients as a distance matrix. Clusters were divided into groups by cutting clusters at a dendrogram height of 0.5 (roughly producing clusters with internal correlation coefficients >0.5). All downstream analyses were performed independently on each resulting cluster and all single probe probesets.
Gene expression analysis—UW
Total RNA was extracted from 60 human liver tissue samples from the University of Washington School of Pharmacy Human Liver Bank as previously described [51], [13]. Genome wide expression analysis was performed using 750 ng of total RNA on the Illumina HumanRef-8 v.2 platform (GPL5060). All liver samples were analyzed with technical replicates that were randomized between processed batches of 24 arrays performed on different days. Raw signal intensity measurements from each sample were processed using the Illumina BeadStudio software v. 2.3.41 using the ‘average’ normalization function. Replicate data from each liver was averaged prior to statistical analysis. All samples and replicates passed quality-control measures.
Gene expression analysis—Merck
Processed gene expression data from the published Merck liver eQTL study [31] were downloaded from GEO (GSE9588, GPL4371). Based on available sample metadata, 266 samples had (a) unambiguous sample ID, age and sex assignments (b) expression data, (c) genotype data, and (d) did not overlap with the UC study. Probes were grouped into RefSeq gene annotation probe sets based on the array manifest. Probesets were further clustered and split following the methodology used for the UC array set.
Genotyping—UC
From the same liver samples received from the Liver Tissue Resource, DNA was obtained from 240 samples for genotyping. Genotyping was performed on the Illumina human 610 quad beadchip platform (GPL8887) at the Northwestern University Center for Genetic Medicine Genomics Core Facility according to the manufacturer's instructions. One sample was removed because it had a no call rate >10%. The initial marker set comprised 620,901 markers. 8,300 markers were removed because they showed significant deviation from Hardy-Weinberg equilibrium (HWE, Fischer's exact test, p<0.001). 29,705 SNPs were removed from the analysis because they had a no call rate in more than >10% of the samples. Hence, our final marker set is comprised of 583,073 SNPs. Identity by descent analysis, performed in Plink, revealed 14 pairs of duplicated samples. Erroneous, redundant sample collection was later confirmed by the tissue bank. Genotype and expression data for these samples were merged for all downstream analyses. The final sample set therefore consisted of 225 unique samples.
Genotyping—UW
Genotyping was performed on each liver sample using the Illumina HumanHap550 (GPL6981) Beadchip platform. Genotyping calls were made using GenomeStudio. After raw genotyping data were loaded into the software, pre-defined cluster definitions were applied and genotype calls were determined. Clusters were checked for separation, deviation from HWE, and lack of variation (i.e., monomorphic). Poorly assigned clusters were modified manually and sites were re-called with corrected cluster definitions. All samples had call rates greater than 98%.
Genotyping—Merck
Genotype data were generated as described [31].
Sex confirmation
The sex of each sample was imputed by K-means clustering of Y-linked gene expression levels and X - and Y-linked genotypes. 3 UC samples, 0 UW samples, and 0 Merck samples had mismatched imputed and annotated sexes, and were therefore excluded from all analyses.
Genotype imputation
For all three studies, care was taken to translate all genotypes to reference genome (b36) forward strand alleles, as subtle errors in genotype strand inference will downwardly bias replication rate estimates. Additional genotypes were imputed with Bimbam (v 0.99) [58], using HAPMAP release 27, build 36 unphased genotypes as reference panels. European American genotypes were imputed with a CEPH reference panel, while African American genotypes were imputed with a combined CEPH and YRI panel. Imputation was run with default Bimbam parameters, and mean imputed genotypes were recorded and used for all downstream analyses.
Quantification of ancestry—UC
We performed a principal component analysis (PCA) based quantification of race using the African and European populations from the Human Genome Diversity panel as reference populations. The SNP set was trimmed using linkage disequilibrium (LD)-based SNP pruning, removing all SNPs for with high pairwise LD (R2>0.8), as in [59]. PCA was performed using smartpca, as implemented in EIGENSOFT [60]. Four samples were flagged as outliers and removed from all further analyses. As expected, the first principal component separated African from non-African individuals. We therefore used this loading vector as an estimated quantification of African ancestry for further analyses.
Quantification of ancestry—UW
PCA was performed using the multi-dimensional scaling procedure implemented in PLINK v1.06 (http://pngu.mgh.harvard.edu/purcell/plink/) [61]. The vast majority of samples resided in a single cluster including all the individuals of self-reported European ancestry, with several moderately outlying samples corresponding to self-reported Hispanic and African ancestry. No samples were excluded from further analyses; the vectors determined for the first two principal components were used as ancestry control for all statistical analyses.
Quantification of ancestry—Merck
All 266 samples included from the published Merck study were self-reported Caucasians. The SNP set was trimmed using linkage disequilibrium (LD)-based SNP pruning, removing all SNPs for with high pairwise LD (R2>0.8), as in [59]. PCA was performed using the multi-dimensoinal scaling procedure implemented in PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) [61]. No outliers were detected; the vectors determined for the first four principal components were used as ancestry control for all statistical analyses.
Covariate modeling—UC
For each probeset, surrogate variable analysis (SVA) [20] was performed on the matrix of expression measurements, after controlling for the effects of hybridization protocol, age, sex, and a principal component analysis based quantification of genetic ancestry. For each probeset, we then constructed a linear mixed effects model y ∼ m + P + A + C + R + I + W + SVi..n + e, where y is the log2 transformed probe intensity, m is the expected probe intensity, P is a factor controlling for the effect of subtle variations in hybridization protocol (e.g., the identity of the technician who performed the experiment), A is the effect of individual age, and C is the effect of individual sex, and R is the effect of genetic ancestry. I is the random effect of each individual, W is the random effect of the oligonucleotide probe, SVi..n represents the effects of a matrix of 55 surrogate variables, and e is the residual error. The model was fitted to each gene by residual maximum likelihood using the lmer function in the R package lme4 (v 0.999375-32) [62], [63]. Fixed effect p-values were estimated using the pvals.fnc function in the languageR package (v 1.0) [64]. The significance of covariate effects was assessed by estimating false discovery rates, using Storey's q-value method [65]. To further control for the effects of outliers and population stratification, prior to eQTL mapping, the distribution of estimated individual effects, for each gene expression trait, was normal quantile transformed, within populations.
Covariate modeling—UW
SVA [20] was performed on the matrix of expression measurements, after controlling for the effects of age, sex, and a multidimensional scaling based quantification of genetic ancestry. For each probe, we constructed a linear model y ∼ m + A + C + R + SVi..n + e, where y is the log2 transformed probe intensity, m is the expected probe intensity, A is the effect of individual age, and C is the effect of individual sex, and R is the effect of genetic ancestry, SVi..n represents the effects of a matrix of surrogate variables, and e is the residual error. Models were implemented with the lm function in R. The residuals from this regression were used as the phenotype values for all subsequent analyses.
Covariate modeling—Merck
SVA [20] was performed on the matrix of expression measurements, after controlling for the effects of age, sex, and a principal component analysis based quantification of genetic ancestry; 54 significant surrogate variables were identified. For each probeset, we then constructed a linear model y ∼ m + A + C + R + W + SVi..n + e, where y is the log2 transformed probe intensity, m is the expected probe intensity, A is the effect of individual age, and C is the effect of individual sex, and R is the effect of genetic ancestry, W is the effect of the oligonucleotide probe, SVi..n represents the effects of a matrix of surrogate variables, and e is the residual expression. Models were implemented with the lm function in R. The residuals from this regression were used as the phenotype values for all subsequent analyses.
eQTL mapping
For each gene expression trait, residual expression variance was treated as a quantitative trait and tested for association with all markers genome-wide. Association testing was performed by Bayesian regression, as implemented in Bimbam (v 0.99), using mean imputed genotypes and default priors [28], [29]. Genotypes with minor allele frequencies less than 1% were excluded.
Probe resequencing
For 15 probes that showed discrepant eQTL scores between the UC and UW analyses (i.e. BF>4 in one study and BF<4 in the other), we designed primers to capture the relevant expression array probe and amplified and Sanger-sequenced the resulting PCR products in each of the 60 UW liver samples and 35 CEU HapMap samples. SNPs were identified as previously described (http://pga.gs.washington.edu/) including both automated prediction and manual curation.
Fine-mapping
We resequenced the promoter and 3′UTR regions within the 60 UW liver samples and 35 CEU HapMap samples for 18 genes that showed strong expression-SNP correlations within the UW data (selected before replication information was available). We used PCR amplification and Sanger-sequencing, identifying SNPs using both automated prediction and manual curation as previously described (http://pga.gs.washington.edu/). 3′UTRs were defined using the appropriate gene models, while promoters were defined as the 1 kb segment upstream of the annotated transcriptional start site. We subsequently defined haplotypes within each promoter and 3′UTR as previously described using Phase [58], and designated as common all haplotypes present in at least two samples.
Common haplotypes for each of 14 promoter and UTR regions were PCR-amplified and cloned into luciferase-reporter vectors. Promoter haplotypes were cloned immediately upstream of the luciferase reporter gene, while 3′UTRs were placed at the 3′ end of a luciferase gene whose expression is driven by the RPL10 promoter that has strong constitutive activity (vector maps available from SwitchGear Genomics, http://switchgeargenomics.com/resources/vector-maps/). We then transfected each of these constructs into HEPG2 cells, a liver-derived cell line, and measured luminescence. Each haplotype was tested using multiple (mode = 3) vector preparations and 4 technical transfection replicates measurements were obtained for each vector preparation (12 or more measurements for most haplotypes).
Transient transfection reporter assays were all performed in 96-well format. Transfection complexes were formed by incubating 100 ng of each individual promoter construct with 0.3 µL of Fugene 6 transfection reagent and Opti-MEM media in a total volume of 5 µL and incubated for 30 min. Transfection complexes (5 uL) were added to 10,000 HepG2 cells in 96-well format that had been seeded 24 h prior to transfection in a white tissue-culture treated plate.
After seeding and transfection, cells were incubated for 48 h before freezing at −80 degrees overnight. To read luminescent activity, plates were thawed for 45 min at room temperature. Then 100 µL of Steady-Glo reagent (Promega #E2520) was added and incubated for 30 min at room temperature. Then luminescence was read for 2 s per well on a 384-well compatible plate luminometer (Molecular Devices LMax384).
To identify significant in vitro effects of haplotype on luminescence, we employed a mixed-effects model using the lmer package [63] within R [62], grouping the replicate luminescence measurements by mini-prep identifier (treating the mini-prep as a random effect). The haplotype identifier has a significant effect on luminescence at p-value<0.001 for each of the three reported associations between haplotype sequence and luminescence measurement. No additional correlations were significant at this threshold.
Supporting Information
Zdroje
1. KuCSLoyEYPawitanYChiaKS
2010
The pursuit of genome-wide association studies: where are we
now?
J Hum Genet
55
195
206
2. StoreyJDMadeoyJStroutJLWurfelMRonaldJ
2007
Gene-expression variation within and among human
populations.
Am J Hum Genet
80
502
509
3. BremRBYvertGClintonRKruglyakL
2002
Genetic dissection of transcriptional regulation in budding
yeast.
Science
296
752
755
4. SchadtEEMonksSADrakeTALusisAJCheN
2003
Genetics of gene expression surveyed in maize, mouse and
man.
Nature
422
297
302
5. MonksSALeonardsonAZhuHCundiffPPietrusiakP
2004
Genetic inheritance of gene expression in human cell
lines.
Am J Hum Genet
75
1094
1105
6. MorleyMMolonyCMWeberTMDevlinJLEwensKG
2004
Genetic analysis of genome-wide variation in human gene
expression.
Nature
430
743
747
7. CheungVGSpielmanRSEwensKGWeberTMMorleyM
2005
Mapping determinants of human gene expression by regional and
genome-wide association.
Nature
437
1365
1369
8. StrangerBENicaACForrestMSDimasABirdCP
2007
Population genomics of human gene expression.
Nat Genet
39
1217
1224
9. MontgomerySBSammethMGutierrez-ArcelusMLachRPIngleC
2010
Transcriptome genetics using second generation sequencing in a
Caucasian population.
Nature
464
773
777
10. PickrellJKMarioniJCPaiAADegnerJFEngelhardtBE
2010
Understanding mechanisms underlying human gene expression
variation with RNA sequencing.
Nature
464
768
772
11. WienkersLCHeathTG
2005
Predicting in vivo drug interactions from in vitro drug discovery
data.
Nat Rev Drug Discov
4
825
833
12. LinJH
2007
Pharmacokinetic and pharmacodynamic variability: a daunting
challenge in drug therapy.
Curr Drug Metab
8
109
136
13. KathiresanSMelanderOGuiducciCSurtiABurttNP
2008
Six new loci associated with blood low-density lipoprotein
cholesterol, high-density lipoprotein cholesterol or triglycerides in
humans.
Nat Genet
40
189
197
14. NicaACMontgomerySBDimasASStrangerBEBeazleyC
2010
Candidate causal regulatory effects by integration of expression
QTLs with complex trait genetic associations.
PLoS Genet
6
e1000895
doi:10.1371/journal.pgen.1000895
15. NicolaeDLGamazonEZhangWDuanSDolanME
2010
Trait-associated SNPs are more likely to be eQTLs: annotation to
enhance discovery from GWAS.
PLoS Genet
6
e1000888
doi:10.1371/journal.pgen.1000888
16. ChoyEYelenskyRBonakdarSPlengeRMSaxenaR
2008
Genetic analysis of human traits in vitro: drug response and gene
expression in lymphoblastoid cell lines.
PLoS Genet
4
e1000287
doi:10.1371/journal.pgen.1000287
17. CheslerEJLuLShouSQuYGuJ
2005
Complex trait analysis of gene expression uncovers polygenic and
pleiotropic networks that modulate nervous system function.
Nat Genet
37
233
242
18. GerritsALiYTessonBMBystrykhLVWeersingE
2009
Expression quantitative trait loci are highly sensitive to
cellular differentiation state.
PLoS Genet
5
e1000692
doi:10.1371/journal.pgen.1000692
19. AkeyJMBiswasSLeekJTStoreyJD
2007
On the design and analysis of gene expression studies in human
populations.
Nat Genet
39
807
808; author reply 808–809
20. LeekJTStoreyJD
2007
Capturing heterogeneity in gene expression studies by surrogate
variable analysis.
PLoS Genet
3
e161
doi:10.1371/journal.pgen.0030161
21. IdaghdourYStoreyJDJadallahSJGibsonG
2008
A genome-wide gene expression signature of environmental
geography in leukocytes of Moroccan Amazighs.
PLoS Genet
4
e1000052
doi:10.1371/journal.pgen.1000052
22. StegleOPartsLDurbinRWinnJ
2010
A Bayesian framework to account for complex non-genetic factors
in gene expression levels greatly increases power in eQTL
studies.
PLoS Comput Biol
6
e1000770
doi:10.1371/journal.pcbi.1000770
23. NicaACPartsLGlassDNisbetJBarrettA
2011
The Architecture of Gene Regulatory Variation across Multiple
Human Tissues: The MuTHER Study.
PLoS Genet
7
e1002003
doi:10.1371/journal.pgen.1002003
24. PeirceJLLiHWangJManlyKFHitzemannRJ
2006
How replicable are mRNA expression QTL?
Mamm Genome
17
643
656
25. DimasASDeutschSStrangerBEMontgomerySBBorelC
2009
Common regulatory variation impacts gene expression in a cell
type-dependent manner.
Science
325
1246
1250
26. DingJGudjonssonJELiangLStuartPELiY
2010
Gene expression in skin and lymphoblastoid cells: Refined
statistical method reveals extensive overlap in cis-eQTL
signals.
Am J Hum Genet
87
779
789
27. van NasAIngram-DrakeLSinsheimerJSWangSSSchadtEE
2010
Expression Quantitative Trait Loci: Replication, Tissue - and
Sex-Specificity in Mice.
Genetics
28. ServinBStephensM
2007
Imputation-based analysis of association studies: candidate
regions and quantitative traits.
PLoS Genet
3
e114
doi:10.1371/journal.pgen.0030114
29. GuanYStephensM
2008
Practical issues in imputation-based association
mapping.
PLoS Genet
4
e1000279
doi:10.1371/journal.pgen.1000279
30. MarchiniJHowieBMyersSMcVeanGDonnellyP
2007
A new multipoint method for genome-wide association studies by
imputation of genotypes.
Nat Genet
39
906
913
31. SchadtEEMolonyCChudinEHaoKYangX
2008
Mapping the genetic architecture of gene expression in human
liver.
PLoS Biol
6
e107
doi:10.1371/journal.pbio.0060107
32. GamazonERZhangWKonkashbaevADuanSKistnerEO
2010
SCAN: SNP and copy number annotation.
Bioinformatics
26
259
262
33. GoringHHTerwilligerJDBlangeroJ
2001
Large upward bias in estimation of locus-specific effects from
genomewide scans.
Am J Hum Genet
69
1357
1369
34. ZollnerSPritchardJK
2007
Overcoming the winner's curse: estimating penetrance
parameters from case-control data.
Am J Hum Genet
80
605
615
35. AlbertsRTerpstraPLiYBreitlingRNapJP
2007
Sequence polymorphisms cause many false cis
eQTLs.
PLoS ONE
2
e622
doi:10.1371/journal.pone.0000622
36. SpielmanRSBastoneLABurdickJTMorleyMEwensWJ
2007
Common genetic variants account for differences in gene
expression among ethnic groups.
Nat Genet
39
226
231
37. VeyrierasJBKudaravalliSKimSYDermitzakisETGiladY
2008
High-resolution mapping of expression-QTLs yields insight into
human gene regulation.
PLoS Genet
4
e1000214
doi:10.1371/journal.pgen.1000214
38. Le ClercSLimouSCoulongesCCarpentierWDinaC
2009
Genomewide association study of a rapid progression cohort
identifies new susceptibility alleles for AIDS (ANRS Genomewide Association
Study 03).
J Infect Dis
200
1194
1201
39. WeinmannLHockJIvacevicTOhrtTMutzeJ
2009
Importin 8 is a gene silencing factor that targets argonaute
proteins to distinct mRNAs.
Cell
136
496
507
40. HullJCampinoSRowlandsKChanMSCopleyRR
2007
Identification of common genetic variation that modulates
alternative splicing.
PLoS Genet
3
e99
doi:10.1371/journal.pgen.0030099
41. KwanTBenovoyDDiasCGurdSProvencherC
2008
Genome-wide analysis of transcript isoform variation in
humans.
Nat Genet
40
225
231
42. LiJZMengFTsavalerLEvansSJChoudaryPV
2007
Sample matching by inferred agonal stress in gene expression
analyses of the brain.
BMC Genomics
8
336
43. TrinkleinNDAldredSJSaldanhaAJMyersRM
2003
Identification and functional analysis of human transcriptional
promoters.
Genome Res
13
308
312
44. PatwardhanRPLeeCLitvinOYoungDLPe'erD
2009
High-resolution analysis of DNA regulatory elements by synthetic
saturation mutagenesis.
Nat Biotechnol
27
1173
1175
45. CarlsonCSAldredSFLeePKTracyRPSchwartzSM
2005
Polymorphisms within the C-reactive protein (CRP) promoter region
are associated with plasma CRP levels.
Am J Hum Genet
77
64
77
46. MusunuruKStrongAFrank-KamenetskyMLeeNEAhfeldtT
2010
From noncoding variant to phenotype via SORT1 at the 1p13
cholesterol locus.
Nature
466
714
719
47. TreismanROrkinSHManiatisT
1983
Specific transcription and RNA splicing defects in five cloned
beta-thalassaemia genes.
Nature
302
591
596
48. ZhangFRHuangWChenSMSunLDLiuH
2009
Genomewide association study of leprosy.
N Engl J Med
361
2609
2618
49. NgSBTurnerEHRobertsonPDFlygareSDBighamAW
2009
Targeted capture and massively parallel sequencing of 12 human
exomes.
Nature
461
272
276
50. BirneyEStamatoyannopoulosJADuttaAGuigoRGingerasTR
2007
Identification and analysis of functional elements in 1%
of the human genome by the ENCODE pilot project.
Nature
447
799
816
51. RiederMJReinerAPGageBFNickersonDAEbyCS
2005
Effect of VKORC1 haplotypes on transcriptional regulation and
warfarin dose.
N Engl J Med
352
2285
2293
52. TakataRAkamatsuSKuboMTakahashiAHosonoN
2010
Genome-wide association study identifies five new susceptibility
loci for prostate cancer in the Japanese population.
Nat Genet
42
751
754
53. HandelAEHandunnetthiLBerlangaAJWatsonCTMorahanJM
2010
The effect of single nucleotide polymorphisms from genome wide
association studies in multiple sclerosis on gene
expression.
PLoS ONE
5
e10142
doi:10.1371/journal.pone.0010142
54. GentlemanR
2005
Bioinformatics and computational biology solutions using R and
Bioconductor
New York
Springer Science+Business Media
xix, 473
55. RitchieMESilverJOshlackAHolmesMDiyagamaD
2007
A comparison of background correction methods for two-colour
microarrays.
Bioinformatics
23
2700
2707
56. BolstadBMIrizarryRAAstrandMSpeedTP
2003
A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics
19
185
193
57. SokalRRRohlfFJ
1995
Biometry : the principles and practice of statistics in biological
research
New York
W.H. Freeman
xix, 887
58. ScheetPStephensM
2006
A fast and flexible statistical model for large-scale population
genotype data: applications to inferring missing genotypes and haplotypic
phase.
Am J Hum Genet
78
629
644
59. NovembreJJohnsonTBrycKKutalikZBoykoAR
2008
Genes mirror geography within Europe.
Nature
456
98
101
60. PriceALPattersonNJPlengeRMWeinblattMEShadickNA
2006
Principal components analysis corrects for stratification in
genome-wide association studies.
Nat Genet
38
904
909
61. PurcellSNealeBTodd-BrownKThomasLFerreiraMA
2007
PLINK: a tool set for whole-genome association and
population-based linkage analyses.
Am J Hum Genet
81
559
575
62. Team RDC
2008
R: Language and environment for statistical
computing
63. BatesDMaechlerM
2010
Linear mixed-effects models using S4 classes
64. BaayenRH
2009
Data sets and functions with “Analyzing Linguistic Data: A
practical introduction to statistics.”
65. StoreyJD
2002
A direct approach to false discovery rates.
Journal of the Royal Statistical Society Series B-Statistical
Methodology
64
479
498
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 5- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- Structural and Functional Differences in the Long Non-Coding RNA in Mouse and Human
- Identification, Replication, and Functional Fine-Mapping of Expression Quantitative Trait Loci in Primary Human Liver Tissue
- A −436C>A Polymorphism in the Human Gene Promoter Associated with Severe Childhood Malaria
- A Decline in p38 MAPK Signaling Underlies Immunosenescence in
- The Operon Balances the Requirements for Vegetative Stability and Conjugative Transfer of Plasmid R388
- Novel and Conserved Protein Macoilin Is Required for Diverse Neuronal Functions in
- Ixr1 Is Required for the Expression of the Ribonucleotide Reductase Rnr1 and Maintenance of dNTP Pools
- Genome of Strain SmR1, a Specialized Diazotrophic Endophyte of Tropical Grasses
- A Deficiency of Ceramide Biosynthesis Causes Cerebellar Purkinje Cell Neurodegeneration and Lipofuscin Accumulation
- A Latent Pro-Survival Function for the Mir-290-295 Cluster in Mouse Embryonic Stem Cells
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- DNA Methylation Dynamics in Human Induced Pluripotent Stem Cells over Time
- Prion Formation and Polyglutamine Aggregation Are Controlled by Two Classes of Genes
- Integrated Genome-Scale Prediction of Detrimental Mutations in Transcription Networks
- Post-Embryonic Nerve-Associated Precursors to Adult Pigment Cells: Genetic Requirements and Dynamics of Morphogenesis and Differentiation
- A Novel Mouse Synaptonemal Complex Protein Is Essential for Loading of Central Element Proteins, Recombination, and Fertility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
- A Genetic and Structural Study of Genome Rearrangements Mediated by High Copy Repeat Ty1 Elements
- A Missense Mutation in Causes a Major QTL Effect on Ear Size in Pigs
- A Flurry of Folding Problems: An Interview with Susan Lindquist
- Meiotic Recombination Intermediates Are Resolved with Minimal Crossover Formation during Return-to-Growth, an Analogue of the Mitotic Cell Cycle
- A Nervous Origin for Fish Stripes
- The ISWI Chromatin Remodeler Organizes the hsrω ncRNA–Containing Omega Speckle Nuclear Compartments
- The Telomerase Subunit Est3 Binds Telomeres in a Cell Cycle– and Est1–Dependent Manner and Interacts Directly with Est1
- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Characterizing Genetic Risk at Known Prostate Cancer Susceptibility Loci in African Americans
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Nodal-Dependent Mesendoderm Specification Requires the Combinatorial Activities of FoxH1 and Eomesodermin
- SHINE Transcription Factors Act Redundantly to Pattern the Archetypal Surface of Arabidopsis Flower Organs
- Association of Genetic Variants in Complement Factor H and Factor H-Related Genes with Systemic Lupus Erythematosus Susceptibility
- STAT Is an Essential Activator of the Zygotic Genome in the Early Embryo
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání