-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
Transposable elements represent a large proportion of the eukaryotic genomes. Long Terminal Repeat (LTR) retrotransposons are very abundant and constitute the predominant family of transposable elements in plants. Recent studies have identified chromoviruses to be a widely distributed lineage of Gypsy elements. These elements contain chromodomains in their integrases, which suggests a preference for insertion into heterochromatin. In turn, this preference might have contributed to the patterning of heterochromatin observed in host genomes. Despite their potential importance for our understanding of plant genome dynamics and evolution, the regulatory mechanisms governing the behavior of chromoviruses and their activities remain largely uncharacterized. Here, we report a detailed analysis of the spatio-temporal activity of a plant chromovirus in the endogenous host. We examined LORE1a, a member of the endogenous chromovirus LORE1 family from the model legume Lotus japonicus. We found that this chromovirus is stochastically de-repressed in plant populations regenerated from de-differentiated cells and that LORE1a transposes in the male germline. Bisulfite sequencing of the 5′ LTR and its surrounding region suggests that tissue culture induces a loss of epigenetic silencing of LORE1a. Since LTR promoter activity is pollen specific, as shown by the analysis of transgenic plants containing an LTR::GUS fusion, we conclude that male germline-specific LORE1a transposition in pollen grains is controlled transcriptionally by its own cis-elements. New insertion sites of LORE1a copies were frequently found in genic regions and show no strong insertional preferences. These distinctive novel features of LORE1 indicate that this chromovirus has considerable potential for generating genetic and epigenetic diversity in the host plant population. Our results also define conditions for the use of LORE1a as a genetic tool.
Published in the journal: . PLoS Genet 6(3): e32767. doi:10.1371/journal.pgen.1000868
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000868Summary
Transposable elements represent a large proportion of the eukaryotic genomes. Long Terminal Repeat (LTR) retrotransposons are very abundant and constitute the predominant family of transposable elements in plants. Recent studies have identified chromoviruses to be a widely distributed lineage of Gypsy elements. These elements contain chromodomains in their integrases, which suggests a preference for insertion into heterochromatin. In turn, this preference might have contributed to the patterning of heterochromatin observed in host genomes. Despite their potential importance for our understanding of plant genome dynamics and evolution, the regulatory mechanisms governing the behavior of chromoviruses and their activities remain largely uncharacterized. Here, we report a detailed analysis of the spatio-temporal activity of a plant chromovirus in the endogenous host. We examined LORE1a, a member of the endogenous chromovirus LORE1 family from the model legume Lotus japonicus. We found that this chromovirus is stochastically de-repressed in plant populations regenerated from de-differentiated cells and that LORE1a transposes in the male germline. Bisulfite sequencing of the 5′ LTR and its surrounding region suggests that tissue culture induces a loss of epigenetic silencing of LORE1a. Since LTR promoter activity is pollen specific, as shown by the analysis of transgenic plants containing an LTR::GUS fusion, we conclude that male germline-specific LORE1a transposition in pollen grains is controlled transcriptionally by its own cis-elements. New insertion sites of LORE1a copies were frequently found in genic regions and show no strong insertional preferences. These distinctive novel features of LORE1 indicate that this chromovirus has considerable potential for generating genetic and epigenetic diversity in the host plant population. Our results also define conditions for the use of LORE1a as a genetic tool.
Introduction
A large proportion of the eukaryotic genome is composed of transposable elements (TEs). In flowering plants, Long Terminal Repeat (LTR) retrotransposons have been regarded as the largest order of TEs [1],[2] and it has been suggested that the ratio between propagation and exclusion of LTR retrotransposons may have affected the size of host genomes [3],[4]. In line with this notion, large plant genomes usually contain substantially more LTR retrotransposons than small plant genomes [5],[6]. However, data from a wide range of flowering plants strongly suggest that LTR retrotransposons are not distributed evenly in genomes. Biased accumulation has led to the formation of LTR retrotransposon-rich, gene-poor heterochromatic blocks, which separate gene-rich euchromatic regions [7]. Thus, the activity of LTR retrotransposons has contributed remarkably towards generating the basic structure of current plant genomes.
In flowering plants, the LTR retrotransposons have been classified into two superfamilies, Gypsy and Copia, according to their structural features [8]. In many plants, Gypsy outnumbers Copia [9]–[13]. An exception is grapevine, in which the number of Copia elements exceeds that of Gypsy [14]. Chromovirus is a most widely-distributed lineage of Gypsy, characterized by a chromodomain at the carboxyl terminal of the ORF [15],[16]. It has been proposed that the insertion site preference of chromoviruses is controlled by the chromodomain [15],[16], and this suggestion has been supported by functional characterization of MAGGY, identified in the rice blast fungus Magnaporta grisea [17],[18]. The MAGGY chromodomain was shown to interact with histone H3 di - and tri-methyl K9, which are hallmarks of heterochromatin [18]. When it was fused to the integrase of Tf1 retrotransposon, the modified Tf1 preferentially transposed into heterochromatic regions in Schizosaccharomyces pombe genome [18]. In flowering plants, chromoviruses are phylogenetically distinct from the lineage containing MAGGY and they are classified into four clades, Reina, Tekay, Galadriel and CRM [16],[19]. Members of CRM were originally known as Gypsy elements which accumulate in centromeric and pericentromeric regions in plant genomes [20]–[23]. Since all four clades have been identified in both dicots and monocots, and Reina and CRM elements have been found in angiosperms and gymnosperms, these elements are likely to have an ancient origin within the seed plants [16],[19]. In order to complement these evolutionary studies, a precise characterization of retrotransposon transpositional activity is now being pursued by experimental analyses, and this activity represents one of the subjects that must be addressed if we are to develop a deeper understanding of plant genome dynamics and evolution.
Previously, most experimental studies of transpositional activity and the regulation of plant LTR retrotransposons were conducted using three Copia elements, Tnt1 and Tto1 in tobacco, and Tos17 in rice. Transpositions of these elements were observed only in cultured cells, where their transcriptional up-regulation occurs [24]–[26]. Since transpositional activity is immediately repressed in regenerated plants due to a decrease in transcription, transpositions in intact plants have not been well characterized. Thus far, transposition of Tos17 has been observed in intact transgenic plants in which the transcriptional level of a gene encoding histone H3K9 specific methylase was downregulated by RNA interference [27], but the spatio-temporal pattern of transposition remained unclear. Furthermore, little is known about the transpositional activity of plant Gypsy elements, including chromoviruses, despite their high abundance in plant genomes.
In more than a decade of studies, the model legume Lotus japonicus has facilitated dissection of the molecular mechanisms governing symbiotic nitrogen fixation with rhizobia. The L. japonicus genome has been sequenced and sequence data covering 67% of the genome (472 Mb), corresponding to 91.3% of the gene space, is now available [13]. From this model legume, we have identified two transpositionally active LTR retrotransposon families designated as LORE1 and LORE2 (Lotus Retrotransposon 1 and 2) [28],[29]. Both belong to the Gypsy superfamily and were first identified as insertions in symbiotic mutants isolated from a transgenic plant population established by tissue culture - mediated transformation [28]–[31]. However, the machinery underlying their activation remained to be characterized. Both LORE1 and LORE2 encode unique long open reading frames (ORFs) with a chromodomain at the carboxyl terminal ends, which suggests that they are chromoviruses (Figure 1A) [29]. Although this chromodomain was overlooked in the original characterization of LORE1 [28], Novikova et al. re-classified LORE1 as a member of the Reina clade of chromovirus [19].
Fig. 1. LORE1 transposition. 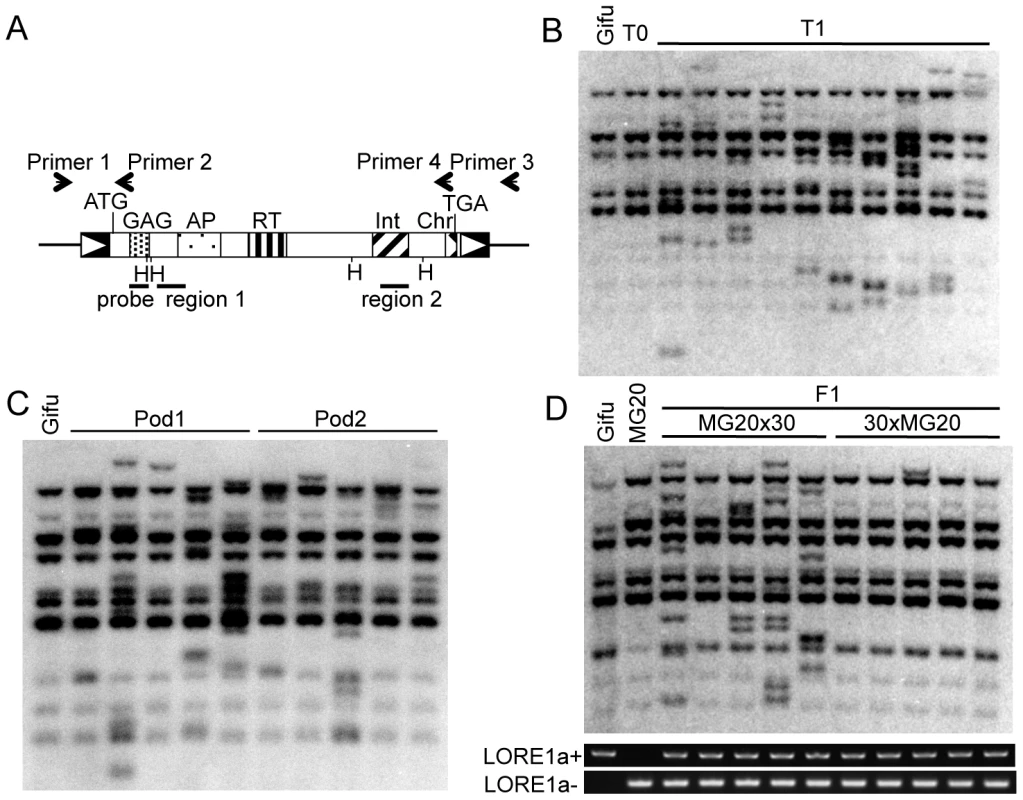
(A) Schematic representation of the LORE1a element. Boxes with triangles represent long terminal repeats (LTRs). Regions encoding functional domains predicted by Pfam are indicated as patterned boxes. GAG, Retrotransposon gag protein; AP, Aspartic protease; RT, reverse transcriptase; Int, integrase core domain; Chr, chromodomain. Positions of Primers 1, 2, 3, and 4 are indicated with arrows. Regions 1 and 2, containing LORE1a-specific SNPs, are indicated as bars. Positions of the DNA probes and Hind III sites used in the genomic Southern blot analyses shown in (B–D) are represented as bars and vertical lines with H, respectively. (B) Southern blot detection of transposed LORE1 elements in T1 siblings. LORE1 copies in control Gifu (Gifu), a T0 individual (T0), and 10 of its T1 siblings (T1) were analyzed. (C) LORE1 transposition in late development. Five siblings originating from each of two pods, pod1 and pod2, set at the top of the same inflorescence of a LORE1-activated T0 plant. (D) Southern blot analysis showing germline inheritance of LORE1 via male gametophytes. DNA from five F1 plants from each of the MG20 (female) × no. 30 (male) and no. 30 (female) × MG20 (male) crosses was analyzed. Gel images show PCR products obtained using Primers 1 and 2 detecting LORE1a (+LORE1a) from Gifu, and Primers 1 and 3 detecting absence of LORE1a (−LORE1a), the allele from MG20. Amplification of both bands confirmed the hybrid genotype of F1 plants. Previously, we estimated the number of “preexisting copies” (insertions that were already present in a plant accession) of LORE1 in the Gifu accession as ten, and obtained full or partial sequences for nine out of the ten preexisting LORE1 copies [28]. Nucleotide sequence polymorphisms among the nine copies enabled us to distinguish them from each other, and we designated them in alphabetical order as LORE1a, b, c, d, e, f, g, h, and i [28]. In this report, we show that in the Gifu accession, the preexisting LORE1a can be epigenetically de-repressed in standard tissue culture. However, transpositions per se occur primarily in pollen, i.e., male gametophytes, of regenerated intact plants, and so far new insertions generated in cultured cells have not been detected. We assume that the pollen-specific LTR promoter of LORE1a regulates the spatio-temporal pattern of transposition. Although LORE1 is a chromovirus, it does not appear to have a strong insertional preference for heterochromatin. These distinctive features of LORE1 underlie its ability to generate insertional polymorphisms, leading to a wide range of genetic and epigenetic diversity in a population. The results also define conditions for using LORE1 for insertion mutagenesis.
Results
Activation of LORE1 in regenerated plant populations
The transpositional activity of LORE1 was first demonstrated by the identification of four symbiotic mutant alleles, nin-7, symrk-1, nup133-3 and nap1-1, in which gene inactivation was caused by the insertion of LORE1 [28],[32]. As all four mutants were isolated from the same Ac/T-DNA tagging population established using the L. japonicus Gifu accession [30],[31], we screened other plants of the same population for LORE1 transpositions. Sequence-specific amplified polymorphism (SSAP) analysis of LORE1 insertion sites detected new transpositions in 32 plants out of a sub-population of 41 plants (Population 1 in Table 1), indicating that LORE1 was widely active in this population. Next, we investigated whether LORE1 transpositions were present in four transgenic or non-transgenic regenerated plant populations created using the Gifu accession. To detect new insertion sites of LORE1, we used SSAP to analyze the T1 and R1 progeny of primary transformants (T0) and of primary non-transgenic regenerated plants (R0). In addition to population 1 (Table 1), transpositions were detected in three of the other four populations. Importantly, transposition was detected in transgenic plants generated using six different constructs, as well as in non-transgenic regenerated plants. These results suggest that the simple process of in vitro tissue culture can activate LORE1 in a stochastic manner that is independent of the presence or absence of transgenes, antibiotic selection, and of the composition and contents of transgene constructs. The newly transposed LORE1 copies observed in R1/T1 plants might have resulted from transpositions in cultured cells and/or in the parental R0/T0 plants. However, LORE1 transposition was absent, infrequent, or below the detection levels of the SSAP method in a total of 27 plants from the initial R0/T0 plants from populations 2 and 3 (Table 1; data not shown). Previously, we observed the absence of obvious transcriptional or transpositional activation of LORE1 in cultured cells [28],[29]. These results suggest that even though LORE1 was apparently de-repressed in tissue culture, the transpositions per se appear to have occurred in regenerated intact plants, rather than in the cultured cells (see details in the next section).
Tab. 1. LORE1 transpositions in regenerated plant populations. 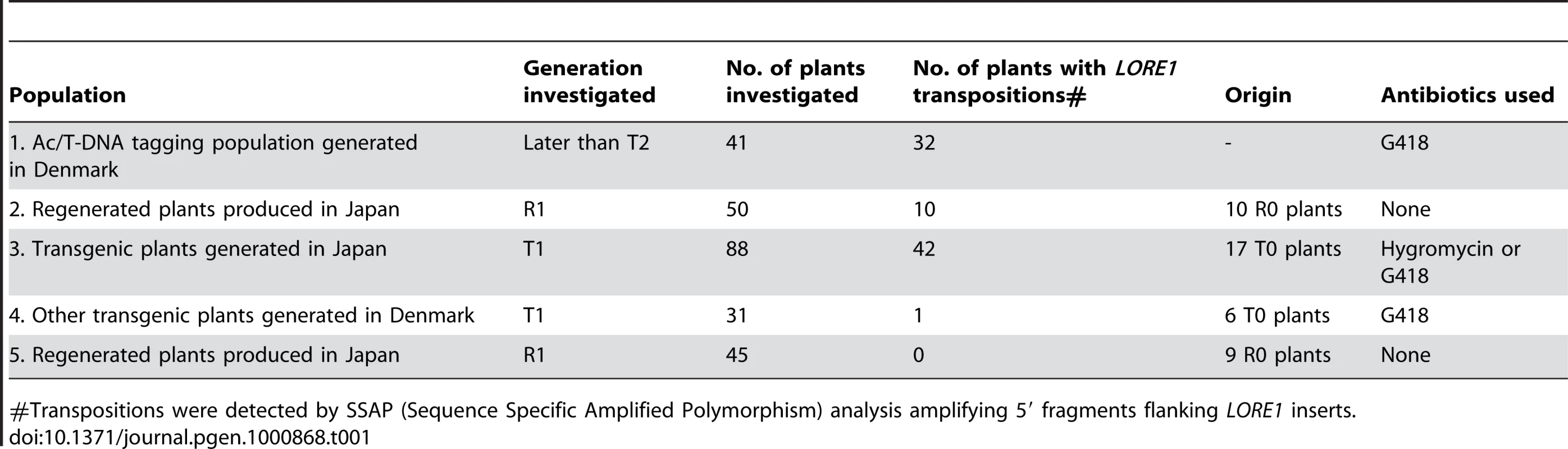
#Transpositions were detected by SSAP (Sequence Specific Amplified Polymorphism) analysis amplifying 5′ fragments flanking LORE1 inserts. Transposition of LORE1 in intact plants
To gain more precise information about transposition of LORE1 in intact plants, eight independent T0 plants were randomly selected from population 3 (Table 1) and investigated together with their T1 progeny. LORE1 transpositions were detected in the T1 progeny from 6 of the 8 T0 plants. A typical result of a genomic Southern blot analysis and SSAP analysis of a T0 and its 10 T1 progeny plants (in this instance plant line no. 30) are shown in Figure 1B and Figure S1, respectively. Notably, the banding pattern in the T0 plants was the same as in the control Gifu, again indicating absent or infrequent LORE1 transposition in the primary regenerated plants (Figure 1B). However, additional bands corresponding to newly transposed LORE1 copies were detected in the T1 progeny (Figure 1B and Figure S1). The highly polymorphic banding pattern indicates the occurrence of frequent independent transpositions of LORE1 in T1 plants (Figure 1B and Figure S1).
Next, we determined whether the new insertions of LORE1 found in the T1 plants were the result of transmission of previous transpositions in somatic cells from T0 forming sectors or of de novo transposition. We analyzed T1 plants originating from two seed pods at the top of the same shoot of the parental T0 plant (Figure 1C). We did not detect any new bands that were shared by the two neighboring pods, or T1 plants originating from the same pod. This result indicates that the majority of LORE1 transpositions occurred at late developmental stages in T0 plants. Reciprocal crosses between plant no. 30 (from the Gifu accession) and plants from the MG20 accession were used to determine if the new transposed copies detected in the T1 plant were transmitted via male or female gametes. Five F1 plants obtained from each reciprocal cross were analyzed for LORE1 copy number (Figure 1D). In total, 21 bands corresponding to new LORE1 transpositions were detected among the 5 F1 plants obtained from the MG20 (female) × no. 30 (male) cross. In contrast, only 1 newly transposed LORE1 copy was detected in 5 F1 plants from no. 30 (female) × MG20 (male) cross. We conclude that although LORE1 is active in both male and female gametophytes, its activity is much higher in male tissues. Next, we used parent-specific single nucleotide polymorphisms (SNPs) in the flanking regions to determine the parental origin of the seven new insertion sites in MG20x30 F1 plants. This analysis showed that all the new transpositions originated from Gifu, the pollen donor. Hence, the majority of LORE1 transpositions detected in the F1 plants seemed to occur before fertilization. Altogether, LORE1 was revealed to be robustly active especially in male gametophytes. Previous reports indicate that activated retrotransposons can be re-silenced again by activities such as copy number-dependent establishment of epigenetic silencing [33],[34]. However, LORE1 was still active in three T1 plants that already possessed an increased number of LORE1 copies (Figure S2A). This finding indicates that once activated, LORE1 was able to transpose over at least two successive generations. On the other hand, we also observed that LORE1 was inactivated in the nup133-3 mutant, in which a single new transposition was detected in the Nup133 gene (Figure S2B).
Activation of LORE1a in the initial generation of regenerated plants
Since the newly inserted LORE1 copies identified in the three symbiotic mutant alleles (nfr5-2, symrk-2, and nup133-3) were identical to one of the nine preexisting copies, LORE1a, we suspected that LORE1a was preferentially activated [28]. LORE1a-specific SNPs were identified in regions 1 and 2 (Figure 1A) and in all eight of the newly-transposed LORE1 fragments from population 3. This observation is consistent with our suggestion that LORE1a is responsible for the majority of LORE1 transpositions described here.
The transpositional activity of retrotransposons is often controlled at the transcriptional level [1], [2], [24]–[26]. We used RT-PCR to compare the levels of LORE1 transcription in mature flowers containing both male and female gametophytes (where LORE1 transposition presumably occurs). Among the eight T0 plants, including no. 30 from population 3 (Table 1), higher levels of LORE1 transcription were observed in the six T0 plants that possessed active LORE1 elements, compared to the control Gifu plant (Figure 2A). This finding indicated a correlation between the transcriptional and transpositional activities of LORE1. To determine which LORE1 family members were present in the transcript pool, RT-PCR products were TA cloned and sequenced. RT-PCR products spanning regions 1 and 2 were amplified separately from flowers of the control Gifu plant and two T0 plants (nos. 30 and 45) that exhibited LORE1 activity. LORE1a-specific SNPs were present in the region 1 of 7/16, 15/15 and 15/16 clones from the control Gifu, no. 30 and no. 45 plants, respectively. For region 2, LORE1a-specific SNPs were present in 3/12, 16/16 and 16/16 clones from the control Gifu, no. 30 and 45 plants, respectively. These data suggest that transcriptional activation is responsible for the preferential transposition of LORE1a among the family members. This expectation is supported by the following lines of evidence: i) a generally increased level of LORE1 transcripts in flowers of active lines; ii) a clear increase in LORE1a transcripts in two activated plants; and iii) all transposition events detected thus far are of LORE1a origin.
Fig. 2. Correlation between transcription level and transpositional activity of LORE1. 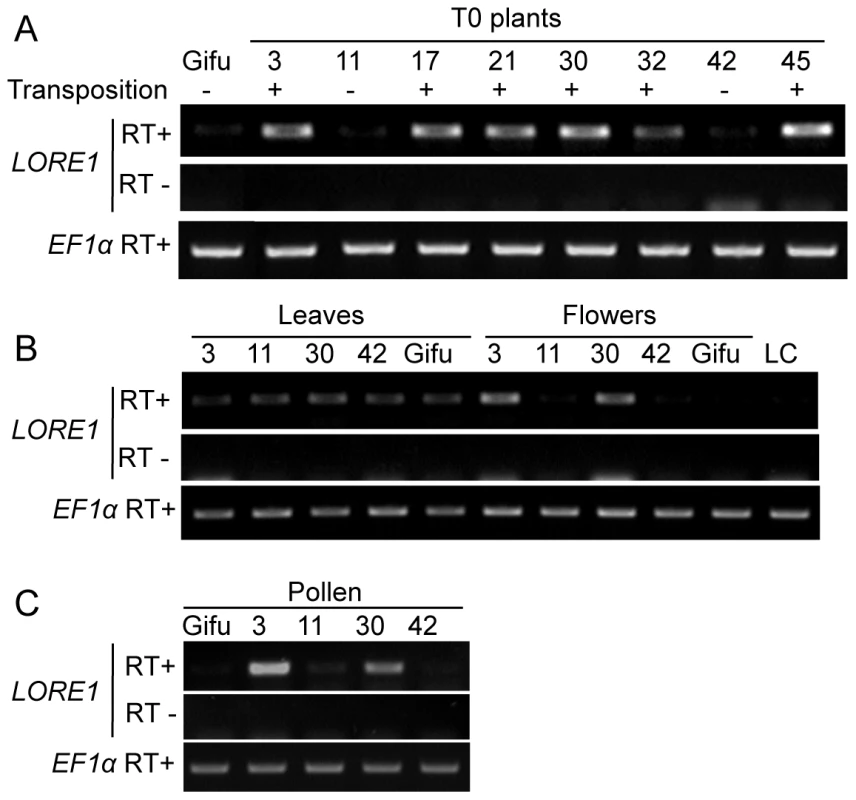
(A) LORE1 transcript levels in T0 plant nos. 3, 11, 17, 21, 30, 32, 42, and 45, as well as in a control Gifu plant. Plants marked with + show transpositional activity of LORE1, those marked with–do not. The upper panel (RT+) shows an image of an RT-PCR of LORE1 transcripts in flowers. The middle panel (RT−) shows negative control reactions without reverse transcriptase. The equal abundance of RNA among samples was confirmed by RT–PCR detection of elongation factor 1 alpha transcripts (EF1α). (B) LORE1 transcript levels among T0 plants vary in flowers, but not in leaves. The levels of LORE1 transcripts in flowers and leaves were determined by RT–PCR as in (A) using RNA samples extracted from four T0 plants, two with LORE1 activity (nos. 3 and 30) and two without LORE1 activity (nos. 11 and 42), together with a control Gifu plant and liquid-cultured cells (LC) from a Gifu plant. Images from a negative control experiment (RT−) and a control, to demonstrate equal abundance of RNA (EF1α), are also presented. (C) Transcriptional activation of LORE1 in pollen. Transcript levels in mature pollen grains isolated from LORE1-activated T0 plants (3 and 30), T0 plants without LORE1 activity (11 and 42), and control Gifu plants, were compared by RT–PCR. Images of a negative control experiment (RT−) and a control, to demonstrate equal abundance of RNA (EF1α), are also shown. Tissue specificity of LORE1 activation
The pattern of LORE1a activation via tissue culture is different from that of other well-characterized retrotransposons such as Tos17, Tto1, and Tnt1, which are activated and transpose during tissue culture, resulting in a copy number increase in the primary regenerated plants (R0) [24]–[26]. We hypothesized that tissue - or cell-specific transcription determines the unique spatio-temporal pattern of LORE1 transposition. To test this hypothesis, we compared LORE1 transcript levels in leaves and flowers among four T0 plants and a control Gifu plant, as well as its transcriptional level in cultured cells (Figure 2B). We found that there were no detectable differences in LORE1 transcript levels in the leaves of the four T0 plants or in the control Gifu plant. In contrast, high levels of LORE1 transcripts accumulated in the flowers of plant nos. 3 and 30 compared to nos. 11 and 42, or the control Gifu plants and cultured cells. Furthermore, high LORE1 transcript levels were detected in pollen from the two T0 plants exhibiting LORE1 activity, compared to the two T0 plants without LORE1 activity or the control Gifu plant (Figure 2C). These observations suggest that LORE1 has transpositional activity in pollen and that tissue specificity is controlled at the transcriptional level.
Since the 5′ LTR is known to function as a promoter for LTR retrotransposons [1], we determined promoter activity of the LORE1a LTR using a transgenic L. japonicus Gifu accession carrying LORE1a LTR fused to a GUS reporter gene. GUS activity was detected in mature pollen grains that were released from anthers and had accumulated at the tip on the inside of the keel (Figure 3A), as well as in isolated pollen grains (Figure 3B). We could not detect LTR-driven GUS activity in any other tissues (data not shown). A similar pattern of GUS activity was observed in three out of six independent transgenic plant lines. These results are in good agreement with the RT-PCR analyses, which indicate up-regulation of LORE1 transcription in pollen grains (Figure 2C). To investigate the LTR promoter activity in a heterologous system, we generated transgenic Arabidopsis plants carrying the same construct. Four out of the seven Arabidopsis transgenic lines showed GUS activity in hydrated pollen grains on stigmas and in pollen tubes (Figure 3F and 3G). Prolonged staining for GUS activity detected weaker expression in developing young anthers (Figure S3A). In the youngest anthers showing activity, GUS was detected primarily in cell layers around the developing pollen, rather than in the developing pollen grains (Figure S3C and S3E). No GUS activity was detected in other tissues. Taken together, these results indicate that the LORE1 LTR specifically promotes transcription in pollen and that the tissue specificity of the cis-elements may be operational in a wide range of flowering plants.
Fig. 3. Promoter activity of the LORE1a LTR. 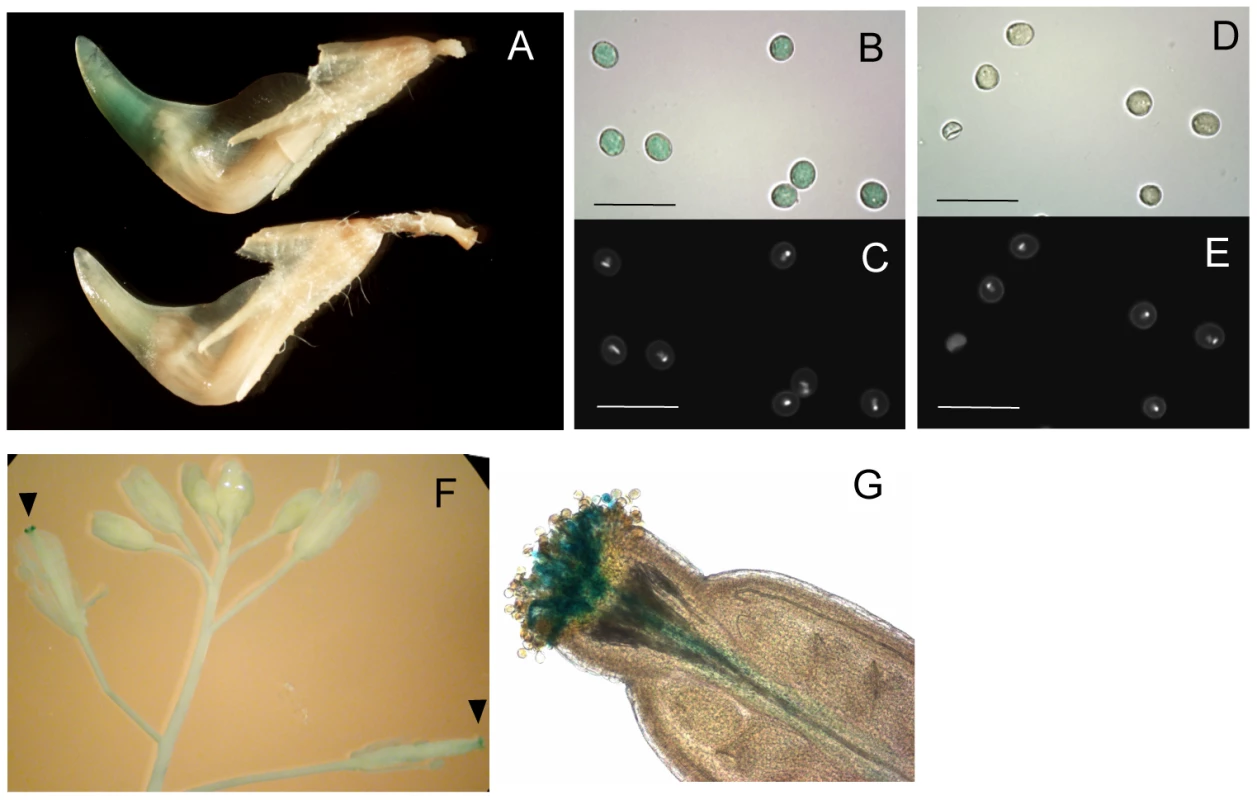
Histochemical GUS assay of transgenic plants containing a LORE1a LTR::GUS fusion. (A) GUS staining was observed at the tip of keel containing released pollen grains in a transgenic L. japonicus plant (upper), while no GUS activity was detected in the control Gifu (lower). (B,D) Close up of pollen grains after GUS staining of transgenic (B) and control Gifu (D) samples. Scale bars correspond to 50 µm. (C,E) Hoechst 33258 staining of the pollen grains shown in (B,D), respectively. (F) A transgenic Arabidopsis inflorescence incubated with GUS substrate for 12 h. Blue staining (GUS positive) was observed only at the pollinated pistils, indicated with arrowheads. GUS activity was not detected in other tissues. (G) Magnified image of a GUS-positive stigma. GUS activity was observed in pollen tubes penetrating the stigma and in hydrated pollen on the stigma. Distribution of newly-transposed LORE1 copies
The reported locations of several chromoviruses in the host plant genomes suggest that chromoviruses preferentially accumulate in heterochromatic regions [18], [20]–[23]. Of the nine preexisting LORE1 copies so far identified, the insertion sites of LORE1d, e, f, h, and i were found in genomic clones containing highly repetitive sequences, which were potential heterochromatic regions. However, the remaining four, LORE1a, b, c and g, were found in contigs that did not display any apparent heterochromatic characteristics (S. S. unpublished data). To investigate whether LORE1 exhibits a strong insertion site preference for heterochromatic regions, we used SSAP to obtain flanking sequences located immediately 5′ of new insertions in the T1 and R1 populations. A total of 97 SSAP fragments longer than 40 bp were analyzed by homology search using public databases including the L. japonicus genome sequence data obtained from the MG20 accession [13]. The absence of the 97 LORE1 insertions in the wild-type Gifu accession was confirmed by PCR (data not shown). In this analysis, only sequences showing homology higher than 77%, along stretches longer than 40 bp and with bit scores larger than 58, were considered homologous sequences. For the 75% of the LORE1 flanking sequences (73 out of the 97), homologous sequences including possible identical (allelic) sequences were identified from the published L. japonicus genome sequences (Table 2). The percentage (75%) is close to the coverage of the whole genome reported for the genome sequence project (67%) [13]. Among the 73 sequences, 37 were protein coding cellular genes or expressed sequence tags (ESTs), 11 were homologous to transposable elements (TEs), and the residual 25 did not show homology to genes or TEs and were categorized as unknown (Table 2). On the other hand, among the 24 fragments that did not show significant homology with L. japonicus sequences, 6 were classified as genes or ESTs, one was categorized as a TE, and the remaining 17 were classified as unknown (Table 2). Thus, a total of 43 sequences were assumed to be in genic regions. Among the 43, 31 were predicted to be exonic, since the insertion site was positioned in a region homologous to protein coding sequences and/or deposited ESTs. In contrast, 12% of the 97 LORE1 flanking sequences showed homology with TEs, which is lower than the predicted TE content of the L. japonicus genome (36%) derived from end-sequencing data of randomly selected BAC clones (S. S. unpublished data). Finally, we physically mapped 24 of the 73 SSAP sequences, and 4 of the 9 preexisting LORE1 members whose positions could be uniquely assigned, to the latest version of L. japonicus chromosome pseudo molecule [13] (Figure 4). This mapping indicated that the new insertion sites were distributed across the Lotus genome and no strong preference for LORE1 insertion sites was observed from those data.
Fig. 4. Linkage map positions of LORE1 insertion sites. 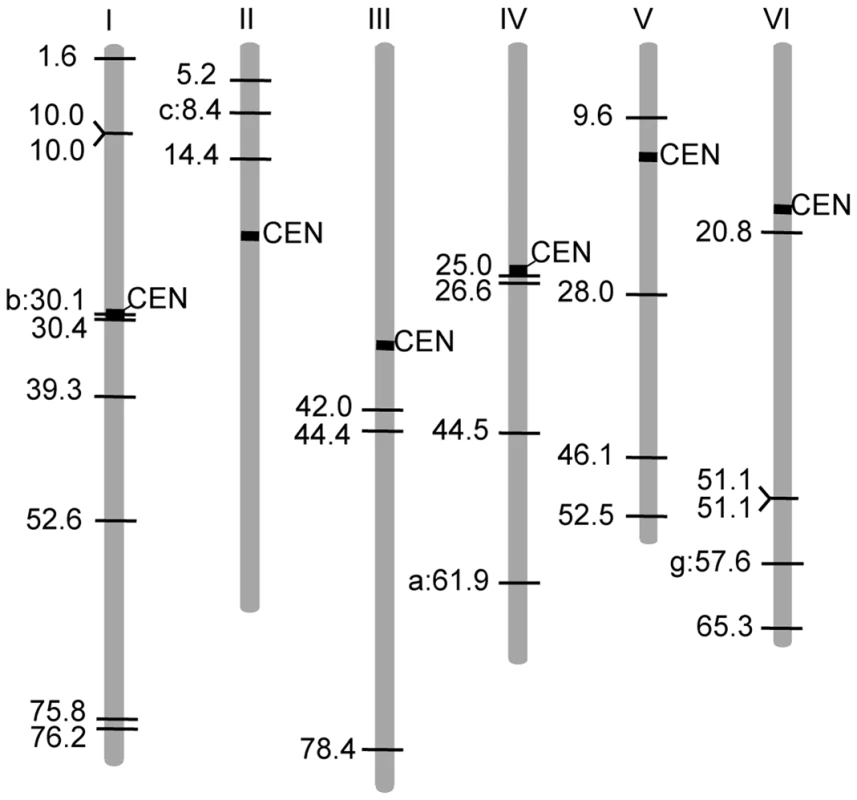
The Gifu map positions of 24 new insertion sites, together with the 4 preexisting LORE1 elements. CM positions are indicated. Vertical bars with numbers indicate chromosomes. Lengths of chromosomes are represented in proportion to their genetic distances (http://www.kazusa.or.jp/lotus/). Regions predicted as centromeric and pericentromeric are indicated as black boxes (S. S. unpublished data). New insertion sites are indicated with horizontal lines. Positions of the four preexisting LORE1 elements are indicated as horizontal lines with a letter identifying the individual copy. Tab. 2. Flanking sequences of new <i>LORE1</i> insertions. 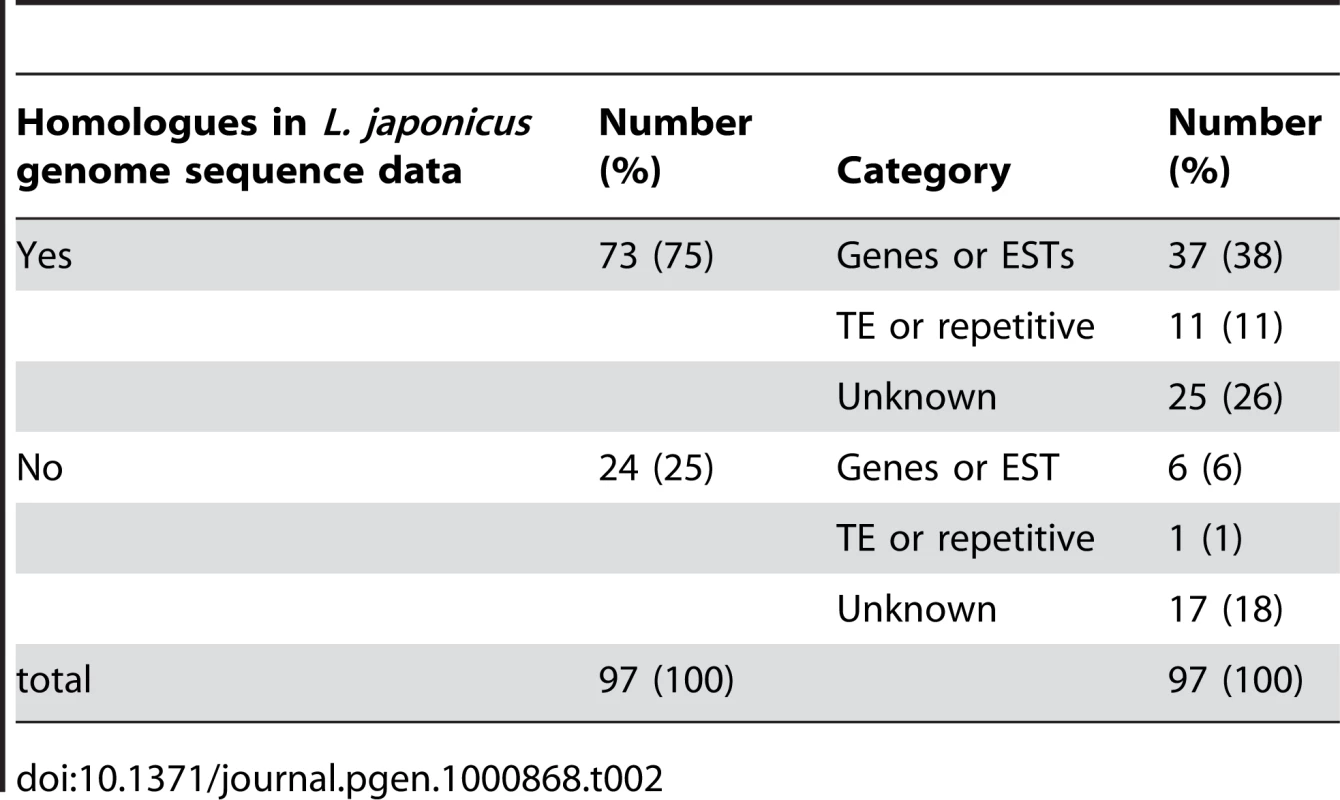
Variation in cytosine methylation patterns at LORE1a among regenerated plants
Because of the frequent but stochastic derepression of LORE1a in regenerated plant populations (Table 1), we predicted that LORE1a activation accompanying tissue culture was induced epigenetically rather than genetically. We examined the status of cytosine methylation around the 5′ end of LORE1a by Southern blot analysis using two restriction enzymes, Hind III and Alu I, which are sensitive to cytosine methylation at residues inside their recognition site [35]. We examined genomic DNA from five T0 plants (nos. 3, 11, 30, 42 and 45), together with the control Gifu (Figure 5A). When Hind III was used to digest genomic DNA from leaves, we observed distinct bands (approximately 1.5 kb) in all of the five plants, suggesting the absence of cytosine methylation at the two Hind III sites surrounding the region complementary to the DNA probe used in this analysis (Figure 5B). When genomic DNA samples were digested with Alu I, signals corresponding to approximately 300 and 650 bp DNA fragments were detected in each plant (Figure 5A). We assumed that the lower band signals represented a mixture of three Alu I fragments of 263, 284, and 306 bp, resulting from the digestion of the Alu I site 5′ adjacent to LORE1a and one of three Alu I sites in the 5′ LTR (Figure 5B). Thus, detection of the smaller hybridizing bands indicates the presence of hypomethylated Alu I sites in the 5′ LTR. On the other hand, the larger band was assumed to correspond to the 640 bp Alu I fragment, resulting from the absence of hypomethylated cleavable Alu I sites in the 5′ LTR (Figure 5B). Detection of signals from both high and lower sized DNA fragments indicates heterogeneity of the methylation status at the three Alu I sites in the 5′ LTR of each of the six investigated plants. However, the relative signal intensity of these DNA fragments showed variation among the five plants. The intensity of lower bands (corresponding to a hypomethylated status) was predominant in plant nos. 3 and 30, which have active LORE1a. However, the higher band (corresponding to hypermethylated alleles) was more intense in plant no. 11, which did not have active LORE1a. In plants nos. 42 and 45, both higher and lower bands were detected, with intensities similar to that of the control Gifu (Figure 5A). These trends in the relative signal intensity between the large and smaller sized bands were reproducible in independently extracted genomic DNA (Figure S4). The banding patterns observed in flowers, where transcriptional activation of LORE1a was observed, were similar to those observed in leaves (Figure S4). This finding suggests that no obvious changes in cytosine methylation pattern can be correlated to changes in LORE1 transcriptional level between the two tissues. Altogether, it would appear that T0 plants have a variable epigenetic status for LORE1a, and that it is different from Gifu control plants.
Fig. 5. Variation in cytosine methylation status observed at three Alu I sites in the 5′ LTR of LORE1a from five T0 plants. 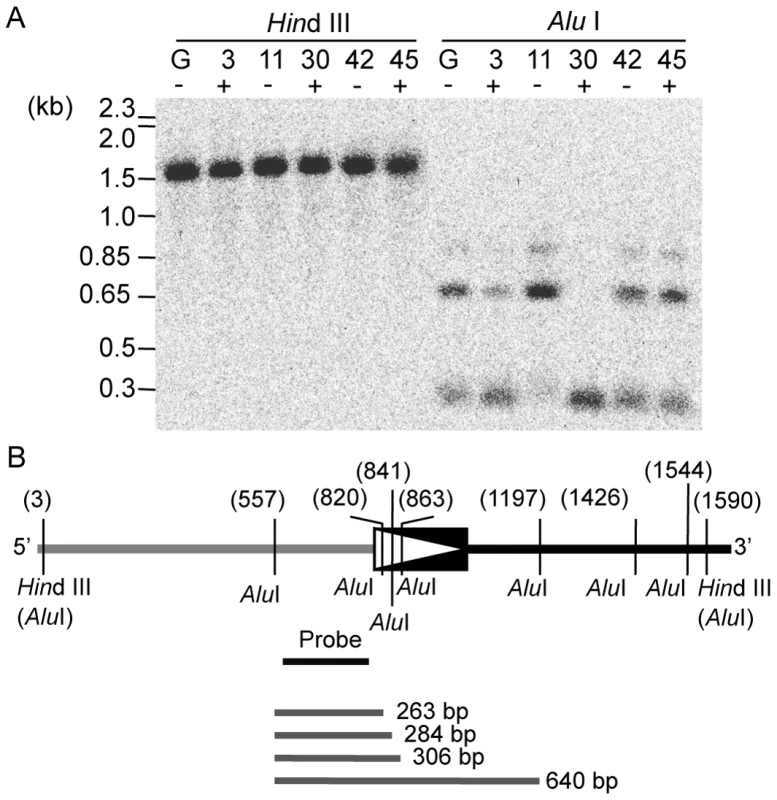
(A) Genomic Southern blot detecting fragments containing 5′ DNA flanking of LORE1a. Genomic DNA samples of the Gifu control (G) and five T0 plants (nos. 3, 11, 30, 42, and 45) were extracted from leaves. DNA was digested with Hind III (left) and Alu I (right). Plants marked with + show transpositional activity of LORE1, those marked with–do not. Molecular sizes of the DNA makers and the bands detected are indicated on the left. (B) A restriction map of the region around the 5′ LTR of LORE1a. 5′ LTR of LORE1a is indicated as a box with a triangle. The 5′ flanking region of LORE1a is indicated as a gray line on the left side of the LTR. The internal sequence of LORE1a is indicated as a black line on the right of the LTR. Two Hind III sites closest to the LTR also contain Alu I sites. The seven Alu I sites are shown as vertical lines. Numbers in parentheses indicate the relative positions of the restriction sites in base pairs. The probe is shown as a black bar below the schematic of the genome region. Four Alu I fragments expected to correspond to the bands detected in the Alu I-digested genome samples are indicated as grey lines below. An independent determination of the cytosine methylation status was obtained by bisulfite sequencing of the 5′ LTR of LORE1a in the five T0 plants and control Gifu. The same genomic leaf DNA samples used in the Southern blot in Figure 5 were analyzed, and twenty to twenty-four amplicons were sequenced from each plant line. This analysis revealed that cytosine residues in U3, the promoter region of LORE1a containing the three Alu I sites, are frequently methylated in control Gifu DNA, especially at CG and CHG sites (Blue bars in Figure 6A–6E). Graphical representation of the methylation status obtained from twenty amplicons showed some heterogeneity in the cytosine methylation patterns of the control Gifu (Figure S5A). This correlates with data obtained from the Hind III and Alu I digestion patterns (Figure 5 and Figure S4). LORE1a is activated in plant no. 30, and compared with control Gifu, this line showed a dramatic decrease in the cytosine methylation level throughout the investigated region (Figure 6C and Figure S5D). Plant no. 3 possesses activated LORE1a and it showed a general decrease in the methylation level in the U3 region; in three of twenty-three amplicons a complete loss of cytosine methylation in U3 was observed (Figure 6A and Figure S5B). LORE1a remains inactive in plant no. 11, and methylation at CG and CHG sites was maintained, as well as being very evident in the U3 (Figure 6B and Figure S5C). Plant nos. 42 and 45 showed similar methylation patterns when averaged among clones (Figure 6D and 6E). However, two amplicons corresponding to alleles that were completely demethylated in U3 were observed in plant no. 45, in which LORE1 is active, but not in no. 42, in which LORE1 remains inactive (Figure S5E and S5F). Among the T0 plants analyzed, these data support the idea that there may be a correlation between LORE1a activation and the presence of LORE1a alleles that have totally lost cytosine methylation in U3.
Fig. 6. Cytosine methylation status around the 5′ LTR of LORE1a, LORE1b, and LORE1f. 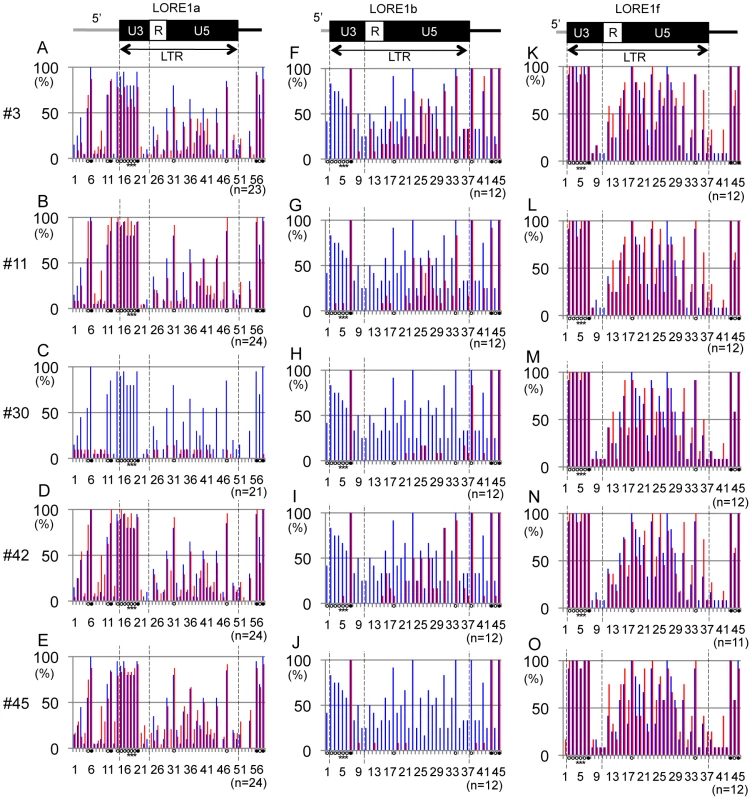
Bar graphs showing percentage of methylation at 58, 45, and 45 cytosines in the genomic regions around the 5′ LTR of LORE1a (A–E), LORE1b (F–J), and LORE1f (K–O), respectively. Methylation status in the Gifu control is represented by blue bars, while red bars indicate status in T0 plants. The positions of the 5′ flanking region, the 5′ LTR, and a part of the internal region of each LORE1 locus were analyzed and are shown at the top. Regions correspond to LTRs and U3 regions are indicated with dotted lines. Closed and open circles correspond to the cytosines in CG and CHG contexts, respectively. Cytosines without any marks are present as CHH. Asterisks indicate cytosines in Alu I sites in the LTRs. The numbers of the sequenced TA clones are indicated at the bottom right of each bar graph. To determine whether alteration in the methylation pattern occurs in the same region of other LORE1 loci, we used bisulfite sequencing to determine the methylation status of two LORE1 loci, LORE1b and LORE1f, which contain 5′ LTRs identical to that of LORE1a. This analysis revealed that the cytosine methylation profile of LORE1f is similar to that observed for LORE1a in control Gifu (blue bars in Figure 6K–6O). Specifically, it shows a higher level of methylation in the U3 region compared with the remaining regions in the investigated areas. However, in contrast to LORE1a, the methylation profile of LORE1f was largely unchanged among the five T0 plants investigated (red bars in Figure 6K–6O). LORE1b showed a moderate level of methylation throughout the investigated region in control Gifu, resulting in a flatter profile of methylation compared with LORE1a and LORE1f (blue bars in Figure 6F–6J). The significant decrease in methylation levels in LORE1b was observed in all the 5 T0 plants, even though the level of decrease differed (red bars in Figure 6F–6J). Taken together, the bisulfite sequencing unveiled variation of epigenetic status at LORE1 loci in control Gifu plants and indicated alteration of this status in the five T0 plants investigated. A characteristic observed with LORE1a was the variability of epigenetic changes among the T0 plants, whereas LORE1b and LORE1f exhibited stability or rather similar changes among the five T0 plants.
Discussion
In this study, we found that transposition of LORE1a, one of the LORE1 elements present in the Lotus japonicus accession Gifu, can be activated in plants regenerated from de-differentiated cells. In addition, we show that LORE1a transposes in the male germline, giving rise to independent insertions in the progeny. The frequency of activation differs between populations (Table 1), but was independent of construct or antibiotics used to select transgenes. Combining all the data, we infer that the phenomenon observed here is a result of a series of processes. The first is a tissue culture step that induces epigenetic changes in LORE1a. This alteration was documented by observing variation in the cytosine methylation patterns among the T0 plants investigated. In turn, this variation leads to the preferential transposition of de-repressed LORE1a in the pollen of intact regenerated plants, since the LORE1a LTR promoter is specifically active in pollen grains. Finally, newly transposed copies in the male germlines are inherited by the following generation. Our data suggests that mechanisms regulating the tissue-specific activity of TEs should be taken into account when considering the biology of TEs and their impact on genome dynamics and evolution. Activation of LORE1a appears to be an attractive system for investigating these mechanisms, as well as for the experimental analysis of plant chromovirus behavior.
Once de-repressed, the transpositional activity of LORE1a was maintained for at least two generations, indicating that the retrotransposon escaped the re-establishment of silencing during this period. One possible explanation for this escape from silencing is the low level of transcription of LORE1 in somatic cells, where de novo transcriptional silencing can be induced by an RNA-directed DNA methylation pathway. It has been shown that transcriptional gene silencing is inducible by artificial RNAi constructs utilizing the 35S promoter to drive the transgenes [36]. This promoter has been shown to be active in somatic tissues but not in pollen [37], and we do not know if de novo transcriptional silencing is inducible in pollen. Addition to that, it has been demonstrated that the RNA-directed transcriptional gene silencing and DNA methylation is less effective when the targets are located in the genic sequences, compared to those in the repetitive sequences [38]. Since LORE1a is located in an intron of a MAP kinase gene [28], efficiency of establishment of transcriptional gene silencing on once activated LORE1a may be low. An alternative possibility is that the increase in LORE1 copy-number was insufficient to induce copy number-dependent silencing [33],[34]. However, re-silencing of LORE1 was observed in the nup133-3 mutant, which also has a low LORE1 copy number. Interestingly, it was recently shown that pollen sperm cells accumulate transcripts of a set of genes involved in small RNA and DNA methylation pathways [39]. Furthermore, small RNAs of TEs originating from vegetative nuclei can transfer to sperm cells [40]. Investigation of the re-silencing of once-activated LORE1a in pollen, together with the steady state silencing of LORE1a, should provide new insights into the significance of epigenetic regulation in plant gametes.
Genetic changes, such as transposition of TEs and nucleotide substitutions and deletions generated during tissue culture, have been regarded as causes of the so-called somaclonal variations often observed as phenotypic changes in regenerated plant populations. Our investigation has unveiled another hidden layer of genetic changes creating phenotypic variation in regenerated plants. Epigenetic derepression of TEs induced via tissue culture can result in TE transpositions not in cultured cells but in regenerated plants. A similar behavior was observed for Karma, a rice LINE retrotransposon [41], and even though the underlying mechanism remains unclear, this observation indicates conservation of the feature. There are most likely other examples, but the temporal and spatial gaps between derepression and transposition of such TEs might have limited their detection. Our observation also indicates the potential use of tissue culture as a breeding method for generating epialleles of a gene of interest, even though these epialleles may not always be epigenetically stable, as demonstrated by recombinant inbred lines with epigenetically mosaic chromosomes consisting of wild-type and CG methylation-depleted segments [42]. Since epigenetic changes can be also generated in animal cells in culture [43], and considering the growing importance of the generative therapy using cultured stem cells, the risk of transposition of TEs after the regeneration of tissues should be given more attention and properly validated.
Even though we observed a good correlation between LORE1a activation and the presence of alleles with complete demethylation in the U3 of T0 plants, the presence of one amplicon of highly hypomethylated U3 in the control Gifu plant (completely hypomethylated except for one CG site, Figure S5A) suggests that demethylation alone might not be sufficient for LORE1a derepression. Therefore, there may be additional factors contributing to loss of LORE1a silencing in regenerated plants, but not in the Gifu plants. Alternatively, the changes in cytosine methylation pattern observed here may represent a by-product accompanying changes in chromatin states, such as histone modifications, which directly trigger LORE1a activation. Since the T0 plants analyzed in this study were selected with antibiotics during tissue culture, they are most likely of unicellular origin. Therefore, we suspect that the epigenetic variation at LORE1a that we observed among regenerated plants might already exist in cultured cells. Corroborating this suggestion is the finding that the epigenetic status of long-term cell cultures of Arabidopsis deviates from that of intact plants [44]. The range of epigenetic variation represented by the cytosine methylation pattern on LORE1a was more pronounced than in the other two LORE1 loci investigated in T0 plants. This suggests that although different members of a TE family may possess over 99% identity, their epigenetic regulation may differ and that tissue culture could influence the silencing variably. Position effects might represent a possible explanation for the different epigenetic changes among the three loci. Potential position effects have been observed in maize, which shows low heritability of silencing of a MuDR element induced by the Muk locus, a MuDR derivative producing a hairpin RNA molecule [45]. The transcriptional regulation of the neighboring MAP kinase gene might also affect expression of LORE1a. Although LORE1b was dramatically demethylated in regenerated plants, we have not yet observed transcriptional or transpositional activation of the copy. This finding indicates that silencing of LORE1b may be achieved by methods other than DNA methylation, such as histone modification, or even non-epigenetically via mechanisms influenced by the surrounding sequence, as demonstrated by Cheng et al. [46].
Chromodomains of chromoviruses are categorized into three groups, according to their structural features. Reina, Tekay, and Galadriel chromodomains are classified into group II, while the MAGGY chromodomain belongs to group I [18],[19]. Group I chromodomains contain three conserved aromatic residues that are necessary for interaction with methylated H3K9. Group II chromodomains only retain the second of these residues. Since CRM chromodomains differ more than those of groups I and II, they are referred to as CR motifs [18]. Even though neither group II chromodomains nor CR motifs interact with histone H3 methyl-K9, the interacting partner of group I chromodomains, they are able to target a YFP fusion to heterochromatic regions when expressed in plant cells, suggesting that they interact with an unknown partner present in plant heterochromatin [18]. Following the standard classification, the LORE1 and LORE2 chromodomains both belong to group II (Figure S6). Although the group II chromodomain in LORE1 appears canonical, we have not observed any strong global preference for insertion of LORE1 into heterochromatin. However, since the Lotus genome project was focused on euchromatic regions [13], we cannot exclude the possibility that LORE1 exhibits an insertional preference for gene-poor regions at a local level. In rice chromosome 1, the distribution pattern of chromoviruses possessing group II chromodomains suggest such a preference [18]. Future characterization of large numbers of new LORE1a insertion sites will, therefore, provide an opportunity to understand the biological function of the group II chromodomains. Gorinsek et al. pointed out that the genome of L. japonicus seems to contain a larger diversity of particular chromoviral clades than other plant species including Medicago truncatula, another model legume [16]. This may suggest that the L. japonicus genome was formed under the influence of the very active chromoviruses. Information on new insertion sites of LORE1a will also be useful for elucidating the survival strategy of these successfully propagated chromoviruses and the impact they have had on the current structure of the L. japonicus genome. From a different perspective, it might be interesting to see if the insertion site preference of LORE1 is affected by the chromatin structure in the pollen where it transposes, since the features of chromatin in plant sperm cells are distinct from somatic cells. Usually chromatin in pollen sperm cells is transcriptionally active at the same time as being highly condensed; it may use sperm-specific variants of histone H3.3, which is a hallmark of active chromatin [47],[48].
It is possible that in bisexual flowering plants, TEs like LORE1, which are active in germlines, could be strong generators of genetic variation over a short evolutionary period. Furthermore, the uniparental activity of these TEs, i.e., showing transposition mainly in male gametophytes, might provide an advantage as a survival strategy. Activity in pollen minimizes the risk of adversely affecting fertility because the number of pollen grains is usually large. Since particular TE families often show distinct biases for one of the two sex chromosomes, uniparentally-active TEs might also be involved in formation of sex chromosomes, which are evolutionarily recent events in flowering plants [49],[50]. On a shorter time-scale, as in the transpositional activity of LORE1, gametophytic transposition, as well as the lack of strong bias for insertion sites and frequent insertions into genes, indicates that this retrotransposon could be an ideal tool for establishing an insertional gene tagging system. We estimate that the population size necessary to obtain at least one insertion allele for all genes at a 95% probability is approximately 200,000 plant lines in L. japonicus. This calculation is based on the following assumptions: the value 2.7, the highest average number of new copies observed here in a T1 plant derived from a T0 plant; 2.9 kb as the average gene size; and 472 Mb as the genome size of L. japonicus [13]. As L. japonicus is a perennial plant and can be propagated by cuttings, harvesting 200,000 seeds from the identified plants possessing active LORE1 is feasible. We have started to establish a small-sized tagging population to test the system. Other transposable elements, activated in the same way as LORE1, might be identified in the course of establishment of this population; LORE2 [29] is one such candidate.
Note added during the production process
After the submission of this article, Tsukahara et al. reported the identification of a Gypsy element transposed in intact ddm1 mutant plants of Arabidopsis thaliana [51]. Precise characterization of the behavior of the Gypsy element, together with that of LORE1, will facilitate our understanding of the interaction between LTR retrotransposons and plant genomes.
Materials and Methods
Plant materials
The Gifu accession of Lotus japonicus was used to generate both the transgenic and regenerated populations. The MG20 accession was used in the reciprocal crosspollination experiment with the T0 plant exhibiting LORE1 activity. For promoter analysis of LORE1 LTR using Arabidopsis thaliana, the ecotype Columbia was used to generate transgenic plants.
Tissue culture methods
Transgenic and regenerated plant populations were produced from the Gifu accession using two different protocols. Populations 1 and 2 were generated according to the method described in [52]. Populations 3, 4, and 5 were generated following the method described in [53]. Antibiotic selection was not used when populations 2 and 5 were produced.
Transformation of Arabidopsis and L. japonicus with the LORE1 LTR::GUS fusion construct
The 225 bp LTR of LORE1a, corresponding to the region from 137 bp to 361 bp of the AJ966990 sequence, was cloned into a multi-cloning site upstream of an intron-containing GUS gene in the binary vector pZN-GUS [54]. The resulting plasmid was introduced into Agrobacterium tumefaciens strain EHA105. Arabidopsis thaliana ecotype Colombia was subsequently infected to generate transgenic plants following the method described in [55]. L. japonicus Gifu accession was infected with the same Agrobacterium strain and transgenic plants were generated following the method described in [53].
DNA methods
Genomic Southern blots were carried out following the method described in [29]. Hind III was used to digest genomic DNA in the Southern blot analyses shown in Figure 1 and Figure S2. Hind III and Alu I were used to digest genomic DNA in Southern blot analyses shown in Figure 5 and Figure S4. Washes were performed at high stringency (65°C, 0.1x SSC, 0.1% SDS). The DNA probe used in Figure 1 and Figure S2 was generated by PCR using the primer pair LORE1gagF (5′-GTTGCCAGTATCGCCATGGACG-3′) and LORE1gagR (5′-GGATTGAGGCCTCCAAGATAAC-3′), and BAC DNA containing LORE1a [28]. The DNA probe used in Figure 5 and Figure S4 was generated by PCR using the primer pair 5′FLKF (5′-TTGACCTGCTCTTCAGTGCATG-3′) and 5′FLKR (5′-GAATCCGGGTATAAGGGTTCC-3′). The Megaprime DNA Labeling System (GE Healthcare) was used for labeling the DNA probes with alpha-32P-dCTP.
SSAP analyses to detect new LORE1 insertions were conducted as described in [28]. In brief, genomic DNA was digested with Mse I (New England Biolabs), and ligated with Mse I adapters. The first PCR was conducted using a primer annealing to a internal region of LORE1 and oriented outward, and a primer specific to the Mse I adapters. A nested PCR was conducted using the first PCR reaction as template. The amplified SSAP fragments were electrophoresed on polyacrylamide sequencing gels, and detected by silver staining. Bands for putative new insertions, i.e., absent from control Gifu analyses, were excised using a scalpel, boiled in 1x PCR buffer, and then used as a template to reamplify the fragment using the same primer pairs as in the nested PCR of the SSAP reaction. The reamplified fragments were electrophoresed on 1% agarose gels, excised, and extracted from the gel using Wizard SV Gel and PCR Clean-up System (Promega). Cleaned fragments were sequenced using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). The reamplified fragments would be expected to contain the junction sequence between LORE1 and its flanking DNA, in which Mse I sites are absent. Sequences that contained Mse I sites were regarded as artifacts and not subjected to further analyses. To amplify junctions between the flanking DNA and LORE1, we designed primers specific to the flanking sequences obtained and oriented toward LORE1. When genome sequences corresponding to flanking DNA were available on the database, they were utilized to design primers. We confirmed that amplifications were successful for plants from which the SSAP fragments were recovered, but not from the parent plant or control Gifu accession.
DNA sequences corresponding to regions 1 and 2 in newly transposed LORE1 elements were obtained by direct sequencing of PCR products. These were amplified by primers specific to the 5′ flanking sequences of each LORE1 element and primer 4 (5′-CAACAGTAGTATCAAATGTAGG-3′), as indicated in Figure 1A, using a BigDye Terminator v3.1 Cycle Sequencing Kit. The primers used for sequencing region 1 were Reg1F (5′-AGTAGCACCTGTAACAGTGGAG-3′) and Reg1R (5′-CATTAAGAGAGACTTTAGGAAC-3′), and those for region 2 were Reg2F (5′-CCTCCAACATTGTCAGTGATAG-3′) and Reg2R (5′-TAGCTGTAAAGCTCCTGTCCAC-3′). In the reciprocal cross analysis shown in Figure 1D, PCR reactions were performed using Primer 1 (5′-GACTAAGTGCCTCTTCAACTGC-3′) and Primer 2 (5′-GACTAAGTGCCTCTTCAACTGC-3′) to amplify LORE1a from Gifu, and Primer 1 and Primer 3 (5′-CACCTGACGATGCTAGCCTTGG-3′) to amplify the region allelic to LORE1a (absence of LORE1) from MG20 (see Figure 1 legend).
Bisulfite sequencing
Genomic DNA samples were extracted from the leaves of T0 plants. Sodium bisulfite treatment of the DNA was conducted using a BisulFast Methylated DNA Detection Kit (TOYOBO), following the manufacturer's instructions. Briefly, 1 µg of column-purified genomic DNA was digested with Eco RI, treated with Proteinase K, and then subjected to bisulfite modification. Bisulfite-treated DNA (1 µl) was used as template for PCR reactions. Primary and nested PCR reactions were conducted for each LORE1 locus. The following primers were used for the primary PCR reactions: BSF R1 (5′-CTCTRAAACCTTRTTRCTTCARCCAT-3′) in combination with BSFa F (5′-TAAAAGAGAATYTGGGTATAAGGGAA-3′) for LORE1a; BSFb F (5′-TTYAAAGGTGYAGTYTYAATTGTATT-3′) for LORE1b; and BSFf F (5′-AGGGAGAYGAYAGTGATGGTGTTTT-3′) for LORE1f. For nested PCR reactions, 1 µl of the primary PCR reaction was used as template, with the following primers: for LORE1a, BSF R2 (5′ - CCATRATTCRCTCCTCCRCTTCAC-3′) and BSFa F; for LORE1b, BSF R2 and BSFb F; and for LORE1f, BSF R2 and BSFf F. PCR reactions (20 µl) were conducted as follows: incubation at 94°C for 2 min as an initial denaturation step, 30 cycles of 30 s at 94°C, 45 s at 55°C, and 45 s at 72°C for amplification, and incubation at 72°C for 5 min. Amplified fragments were TA cloned using the pGEM-T Easy Vector System (Promega). For LORE1a, 6 to 8 TA clones were obtained from each of three PCR reactions and, in total, between 20 and 24 clones were sequenced for each plant analyzed. For LORE1b, 12 clones obtained from a PCR reaction were analyzed for each plant examined. For LORE1f, 11 or 12 clones obtained from a PCR reaction were analyzed for each plant examined.
Total RNA extraction and RT–PCR
A method modified from [56] was used for RNA isolation from plant tissues. Ground tissues (∼0.1 g) were incubated with 700 µl of extraction buffer (2% ß-mercaptoethanol, 2% hexadecyltrimethylammonium bromide, 100 mM Tris-HCl [pH 8.0], and 25 mM EDTA) at room temperature for less than 5 min. The recovered RNA was treated with 5 U DNase I at 37°C for 30 min in a 100 µl reaction. DNase-treated RNA was purified and recovered using an RNeasy Mini Kit (QIAGEN), with additional DNase treatment performed on a column, following the manufacturer's instructions. For RT-PCR, cDNA was synthesized by ReverTra Ace α (TOYOBO) using 1 µg of purified total RNA and oligo (dT) 20 primer in a 20 µl reaction. A 5× dilution of the cDNA reaction (2 µl) was used as template for semi-quantitative RT-PCR in a 20 µl PCR reaction using Ex Taq (TaKaRa) and 5 pmoles of each primer. The primers LORE1gagF and LORE1gagR were used for detection of LORE1 transcripts and products were amplified with 28 PCR cycles. As a control, the primers EF1αF (5′-GTGAGGGACATGAGACAGACTG-3′) and EF1αR (5′-AAATAGCAGTGTAGGACAAGTC-3′) were used for detection of transcripts of elongation factor 1 alpha, and these reactions required 24 PCR amplification cycles. To identify the transcription of LORE1 members, RT-PCR amplifications of regions 1 and 2 were conducted using the primer pairs Reg1 F and Reg1 R, or Reg2 F and Reg2 R, respectively.
Sequence data analysis
BLAST searches were used to identify sequences homologous to SSAP fragments. These were conducted using Miyakogusa jp (http://www.kazusa.or.jp/lotus/), NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and Phytozome Glycine max (http://www.phytozome.net/soybean). Pfam was accessed at http://pfam.sanger.ac.uk/. Bisulfite sequencing data was analyzed using QUMA [57] and CyMATE [58].
Supporting Information
Zdroje
1. KumarA
BennetzenJL
1999 Plant retrotransposons. Annu Rev Genet 33 479 532
2. FeschotteC
JiangN
WesslerSR
2002 Plant transposable elements: where genetics meets genomics. Nat Rev Genet 3 329 341
3. VitteC
PanaudO
QuesnevilleH
2007 LTR retrotransposons in rice (Oryza sativa, L.): recent burst amplifications followed by rapid DNA loss. BMC Genomics 8 218
4. WangH
LiuJS
2008 LTR retrotransposon landscape in Medicago truncatula: more rapid removal than in rice. BMC Genomics 9 382
5. ZhangX
WesslerSR
2004 Genome-wide comparative analysis of the transposable elements in the related species Arabidopsis thaliana and Brassica oleracea. Proc Natl Acad Sci U S A 101 5589 5594
6. DuC
SwigonováZ
MessingJ
2006 Retrotranspositions in orthologous regions of closely related grass species. BMC Evol Biol 6 62
7. VitteC
PanaudO
2005 LTR retrotransposons and flowering plant genome size: emergence of the increase/decrease model. Cytogenet Genome Res 110 91 107
8. WickerT
SabotF
Hua-VanA
BennetzenJL
CapyP
2007 A unified classification system for eukaryotic transposable elements. Nat Rev Genet 8 973 982
9. Arabidopsis Genome Initiative 2000 Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 796 815
10. TuskanGA
DifazioS
JanssonS
BohlmannJ
GrigorievI
2006 The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313 1596 1604
11. Rice Annotation Project 2007 Curated genome annotation of Oryza sativa ssp. japonica and comparative genome analysis with Arabidopsis thaliana. Genome Res 17 175 183
12. MingR
HouS
FengY
YuQ
Dionne-LaporteA
2008 The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452 991 996
13. SatoS
NakamuraY
KanekoT
AsamizuE
KatoT
2008 Genome structure of the legume, Lotus japonicus. DNA Res 15 227 239
14. The French-Italian Public Consortium for Grapevine Genome Characterization 2007 The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449 463 467
15. MalikHS
EickbushTH
1999 Modular evolution of the integrase domain in the Ty3/Gypsy class of LTR retrotransposons. J Virol 73 5186 5190
16. GorinsekB
GubensekF
KordisD
2004 Evolutionary genomics of chromoviruses in eukaryotes. Mol Biol Evol 21 781 798
17. NakayashikiH
AwaT
TosaY
MayamaS
2005 The C-terminal chromodomain-like module in the integrase domain is crucial for high transposition efficiency of the retrotransposon MAGGY. FEBS Lett 579 488 492
18. GaoX
HouY
EbinaH
LevinHL
VoytasDF
2008 Chromodomains direct integration of retrotransposons to heterochromatin. Genome Res 18 359 369
19. NovikovaO
MayorovV
SmyshlyaevG
FursovM
AdkisonL
2008 Novel clades of chromodomain-containing Gypsy LTR retrotransposons from mosses (Bryophyta). Plant J 56 562 574
20. ChengZ
DongF
LangdonT
OuyangS
BuellCR
2002 Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 14 1691 1704
21. ZhongCX
MarshallJB
ToppC
MroczekR
KatoA
2002 Centromeric retroelements and satellites interact with maize kinetochore protein CENH3. Plant Cell 14 2825 2836
22. NagakiK
MurataM
2005 Characterization of CENH3 and centromere-associated DNA sequences in sugarcane. Chromosome Res 13 195 203
23. WeberB
SchmidtT
2009 Nested Ty3-gypsy retrotransposons of a single Beta procumbens centromere contain a putative chromodomain. Chromosome Res 17 379 396
24. CasacubertaJM
GrandbastienMA
1993 Characterisation of LTR sequences involved in the protoplast specific expression of the tobacco Tnt1 retrotransposon. Nucleic Acids Res 21 2087 2093
25. HirochikaH
1993 Activation of tobacco retrotransposons during tissue culture. EMBO J 12 2521 2528
26. HirochikaH
SugimotoK
OtsukiY
TsugawaH
KandaM
1996 Retrotransposons of rice involved in mutations induced by tissue culture. Proc Natl Acad Sci U S A 93 7783 7788
27. DingY
WangX
SuL
ZhaiJ
CaoS
2007 SDG714, a histone H3K9 methyltransferase, is involved in Tos17 DNA methylation and transposition in rice. Plant Cell 19 9 22
28. MadsenLH
FukaiE
RadutoiuS
YostCK
SandalN
2005 LORE1, an active low-copy-number Gypsy retrotransposon family in the model legume Lotus japonicus. Plant J 44 372 381
29. FukaiE
DobrowolskaAD
MadsenLH
MadsenEB
UmeharaY
2008 Transposition of a 600 thousand-year-old LTR retrotransposon in the model legume Lotus japonicus. Plant Mol Biol 68 653 663
30. ThykjærT
StillerJ
HandbergK
JonesJ
StougaardJ
1995 The maize transposable element Ac is mobile in the legume Lotus japonicus. Plant Mol Biol 27 981 993
31. SchauserL
HandbergK
SandalN
StillerJ
ThykjærT
1998 Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259 414 423
32. YokotaK
FukaiE
MadsenLH
JurkiewiczA
RuedaP
2009 Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21 267 284
33. HirochikaH
OkamotoH
KakutaniT
2000 Silencing of retrotransposons in Arabidopsis and reactivation by the ddm1 mutation. Plant Cell 12 357 369
34. Perez-HormaecheJ
PotetF
BeauclairL
Le MassonI
CourtialB
2008 Invasion of the Arabidopsis genome by the tobacco retrotransposon Tnt1 is controlled by reversible transcriptional gene silencing. Plant Physiol 147 1264 1278
35. McClellandM
NelsonM
RaschkeE
1994 Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 22 3640 3659
36. MetteMF
AufsatzW
van der WindenJ
MatzkeMA
MatzkeAJ
2000 Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J 19 5194 5201
37. WilkinsonJE
TwellD
LindseyK
1997 Activities of CaMV 35S and nos promoters in pollen: implications for field release of transgenic plants. J Exp Bot 48 265 275
38. FischerU
KuhlmannM
PecinkaA
SchmidtR
MetteMF
2007 Local DNA features affect RNA-directed transcriptional gene silencing and DNA methylation. Plant J 53 1 10
39. BorgesF
GomesG
GardnerR
MorenoN
McCormickS
2008 Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148 1168 1181
40. SlotkinRK
VaughnM
BorgesF
TanurdzicM
BeckerJD
2009 Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 461 472
41. KomatsuM
ShimamotoK
KyozukaJ
2003 Two-step regulation and continuous retrotransposition of the rice LINE-type retrotransposon Karma. Plant Cell 15 1934 1944
42. ReindersJ
WulffBB
MirouzeM
Mari-OrdonezA
DappM
2009 Compromised stability of DNA methylation and transposon immobilization in mosaic Arabidopsis epigenomes. Genes Dev 23 939 950
43. MeissnerA
MikkelsenTS
GuH
WernigM
HannaJ
2008 Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454 766 770
44. TanurdzicM
VaughnMW
JiangH
LeeTJ
SlotkinRK
2008 Epigenomic consequences of immortalized plant cell suspension culture. PLoS Biol 6 e302 doi:10.1371/journal.pbio.0060302
45. SinghJ
FreelingM
LischD
2008 A position effect on the heritability of epigenetic silencing. PLoS Genet 4 e1000216 doi:10.1371/journal.pgen.1000216
46. ChengC
DaigenM
HirochikaH
2006 Epigenetic regulation of the rice retrotransposon Tos17. Mol Genet Genomics 276 378 390
47. OkadaT
EndoM
SinghMB
BhallaPL
2005 Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J 44 557 568
48. IngouffM
HamamuraY
GourguesM
HigashiyamaT
BergerF
2007 Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol 17 1032 1037
49. SakamotoK
OhmidoN
FukuiK
KamadaH
SatohS
2000 Site-specific accumulation of a LINE-like retrotransposon in a sex chromosome of the dioecious plant Cannabis sativa. Plant Mol Biol 44 723 732
50. CermakT
KubatZ
HobzaR
KoblizkovaA
WidmerA
2008 Survey of repetitive sequences in Silene latifolia with respect to their distribution on sex chromosomes. Chromosome Res 16 961 976
51. TsukaharaS
KobayashiA
KawabeA
MathieuO
MiuraA
2009 Bursts of retrotransposition reproduced in Arabidopsis. Nature 461 423 426
52. HandbergK
StougaardJ
1992 Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J 2 487 496
53. ThykjærT
SchauserL
DanielsenD
FinnemanJ
StougaardJ
1998 Agrobacterium-mediated transformation of the diploid legume Lotus japonicus. Cell Biology: a Laboratory Handbook, Ed 23 518 525
54. AndersenSU
CvitanichC
GrønlundM
BuskH
JensenDB
2005 Vectors for reverse genetics and expression analysis. in: Lotus japonicus handbook Springer 289 292
55. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
56. ChangS
PuryearJ
CairneyJ
1993 A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11 113 116
57. KumakiY
OdaM
OkanoM
2008 QUMA: quantification tool for methylation analysis. Nucleic Acids Res 36 W170 W175
58. HetzlJ
FoersterAM
RaidlG
Mittelsten ScheidO
2007 CyMATE: a new tool for methylation analysis of plant genomic DNA after bisulphite sequencing. Plant J 51 526 536
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 3- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Parental Genome Dosage Imbalance Deregulates Imprinting in
- Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform
- HAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
- Initial Genomics of the Human Nucleolus
- Role of RecA and the SOS Response in Thymineless Death in
- PPS, a Large Multidomain Protein, Functions with Sex-Lethal to Regulate Alternative Splicing in
- Mislocalization of XPF-ERCC1 Nuclease Contributes to Reduced DNA Repair in XP-F Patients
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
- Local-Scale Patterns of Genetic Variability, Outcrossing, and Spatial Structure in Natural Stands of
- Arginylation-Dependent Neural Crest Cell Migration Is Essential for Mouse Development
- HP1 Recruitment in the Absence of Argonaute Proteins in
- MiR-218 Inhibits Invasion and Metastasis of Gastric Cancer by Targeting the Robo1 Receptor
- Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples
- Regulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
- KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading
- Identification of the Regulatory Logic Controlling Pathoadaptation by the SsrA-SsrB Two-Component System
- Drosophila Xpd Regulates Cdk7 Localization, Mitotic Kinase Activity, Spindle Dynamics, and Chromosome Segregation
- Multiple Signals Converge on a Differentiation MAPK Pathway
- In the Tradition of Science: An Interview with Victor Ambros
- Association of the Polymorphism His615Arg with Melanin Content in East Asian Populations: Further Evidence of Convergent Evolution of Skin Pigmentation
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



