-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Paediatric Intracranial Aneurysms
Dětská intrakraniální aneuryzmata
Cíle a předpoklady:
Cílem sdělení je rozbor anatomických a klinických charakteristik dětských aneuryzmat a prezentace našich zkušeností s jejich léčbou včetně dlouhodobých výsledků.Metody:
Do studie jsme zahrnuli 11 dětských pacientů s 13 intrakraniálními aneuryzmaty. Retrospektivně jsme analyzovali klinická data z chorobopisů a rentgenových snímků. Vyhodnocovali jsme výběr léčebné strategie. Výsledný outcome jsme hodnotili podle Rankinova skóre v období 0–144 měsíců.Výsledky:
U sedmi pacientů jsme aneuryzma vyhodnotili jako inoperabilní nebo vysoce rizikové pro chirurgický výkon. U těchto pacientů jsme preferovali endovaskulární léčbu. U tří pacientů jsme primárně indikovali chirurgickou léčbu. Jednoho pacienta v hlubokém komatu po SAK jsme léčili konzervativně, který zemřel do 24 hodin po krvácení. U pěti pacientů, kteří se klinicky prezentovali fokálním neurologickým deficitem bez SAK došlo po léčbě ke zlepšení a všichni při hodnocení dosáhli Rankin skóre 1. U čtyřech pacientů po SAK byl hodnocen klinický stav HH 1 až 3. Pouze u jednoho pacienta se zlepšil neurologický deficit a pacientův outcome dosáhl Rankin skóre 1. U zbývajících tří pacientů neurologický deficit, zvláště v oblasti psychiky, přetrvával i po léčbě a při hodnocení výsledného stavu dosáhli Rankin skóre 2 až 3.Závěr:
Rozhodnutí o volbě endovaskulární nebo chirurgické léčby je založeno na lokalizaci, velikosti aneuryzmatu, přítomnosti intracerebrálního hematomu a celkovém stavu pacienta. Při volbě typu léčby je doporučen multidisciplinární přístup.Klíčová slova:
dětská aneuryzmata – neurochirurgická a endovaskulární léčba – outcome
Authors: S. Rehak 1; A. Krajina 2; R. Taláb 3; M. Kanta 1; K. Zadrobilek 1; P. Ryska 2
Authors place of work: Charles University Teaching Hospital, Hradec Kralove, Czech Republic Department of Neurosurgery 1; Charles University Teaching Hospital, Hradec Kralove, Czech Republic Department of Radiology 2; Charles University Teaching Hospital, Hradec Kralove, Czech Republic Department of Neurology 3
Published in the journal: Cesk Slov Neurol N 2012; 75/108(1): 52-57
Category: Krátké sdělení
Summary
Aims and background:
The aim of this paper is to discuss the anatomical and clinical features of paediatric aneurysms and present our experience in their treatment, including that of long-term outcome.Methods:
Eleven paediatric patients with a total of 13 intracranial aneurysms were included in the study. The clinical data were retrospectively analysed from patient charts and imaging studies. The treatment strategy selected was evaluated. The resultant outcome was graded according to Rankin score during a follow-up ranging from 0 to 144 months.Results:
In seven patients the aneurysms were considered inoperable or the risk associated with surgery was too high, and therefore endovascular treatment was preferred. Three patients were treated primarily surgically. One patient in deep coma following SAH was treated conservatively and died 24 hours after the bleed. Clinical improvement followed treatment in five patients who presented with a focal neurological deficit and an absence of SAH, so they were all ranked as Rankin 1. Four patients presenting with SAH were graded HH 1 to 3. In only one case did the focal neurological deficit improve, enabling the patient to be ranked as Rankin 1. Neurological deficit persisted in the remaining three patients, particularly in psychological terms, leading to grades of Rankin 2 to 3.Conclusion:
The decision to perform endovascular or surgical treatment is based on location, aneurysm size, presence of intracerebral haematoma and overall patient condition. A multidisciplinary approach is recommended.Key words:
paediatric aneurysm – neurosurgical and endovascular treatment – outcomeIntroduction
With an incidence of less than 5% of all diagnosed intracranial aneurysms, paediatric intracranial aneurysms are rare [1–4]. In children it is almost certain that haemodynamic factors and vessel wall degeneration, i.e. hypertension, vascular disease, alcohol and tobacco use, do not play as significant a role in the pathogenesis of intracranial aneurysms as they do in adults. These factors are virtually absent in children, and it is therefore assumed that congenital factors play a dominant role, something that may also go some way towards explaining the differences in their localization, size, clinical presentation and tendency to rupture [1]. Paediatric aneurysms show a higher incidence of unusual positions, such as the posterior circulation, carotid bifurcation or periphery, as well as a greater incidence of giant aneurysms [1,4–7,10]. Additionally, intracranial aneurysms in children have a significantly higher incidence of cases presenting without subarachnoid haemorrhage (SAH) and thus have a more favourable outcome compared to those occurring in adults [1,10,11].
The final treatment strategy depends on the individual characteristics of both the child patient and the aneurysm itself. Experience with either surgical or endovascular treatment certainly lends some bias to such a decision. The aim of this paper is to discuss the anatomical and clinical features of paediatric aneurysms and present our experience in their treatment, including long-term outcome.
Methods
A total of 11 paediatric patients (less than 18 years of age), with a total of 13 intracranial aneurysms, hospitalized at our neurosurgical unit between 1994 and 2008 were included in the study. Patients with mycotic aneurysms and AVMs were excluded. The average age at time of presentation was 14.3 years, ranging from 9 to17 years (Tables 1, 2). The clinical data was retrospectively analysed from patient charts and imaging studies. Basic factors such as sex, age and year of treatment were analysed. From a clinical perspective, we then judged aetiology and neurological presentation as WFNS and Hunt-Hess (HH) scores. The imaging studies were analysed to quantify the extent of SAH as Fisher scores, while any presence of intracerebral haematoma or thrombus formation in the aneurysm was also noted. Each individual aneurysm was evaluated in terms of locality, size and shape. The chosen treatment strategy was re-evaluated. The resulting outcome was graded according to Rankin score during a follow-up ranging from 0 to144 months [12]. Foreign nationality limited the length of follow-up in five patients.
Results
Aetiology, position and size
Eleven patients with a total of 13 aneurysms were included in the study. One patient (No. 10) had three aneurysms (Table 1). Two patients (No. 1, 4) had incidental giant ICA aneurysms proven to be traumatic in origin; aetiology in the remaining patients the remains unknown. Six patients (No. 3, 7, 8, 9, 10, 11) had aneurysms located in the bifurcation of the MCA.
In four patients (No. 1, 2, 4, 10) the aneurysm was located on the ICA. Significantly fewer patients (No. 5, 6) had posterior circulation aneurysms and only one patient (No. 1) had an AcomA aneurysm (Fig. 1). In seven patients, i.e. 54%, the aneurysm was greater than 1 cm in size and in two cases (No.1, 3) the aneurysms were classified as giant.
Fig. 1. Stratification of paediatric aneurysms by location. 
Clinical presentation and imaging characteristics
Five patients (No. 1, 4, 5, 7, 8) presented without subarachnoid haemorrhage (HH = 0). Four of these cases were diagnosed in the light of neurological deficit caused by the mass effect of the aneurysm; in one patient (No. 7), the only manifestation was headache. The remaining six patients (No. 2, 3, 6, 9, 10, 11) did present with SAH, ranging in severity from transient headache to coma with decerebration rigidity. WFNS better characterizes patients in terms of consciousness and motor function, but in most cases it was in agreement with HH scores. One patient (No. 8) without SAH developed transient hemiparesis and speech difficulty secondary to severe migraine (WFNS = 3). The Fisher scale, which characterizes the amount of SAH clotting on the basis of CT, correlated well to WFNS, especially in the patients in serious clinical condition (No. 9, 11), in whom an intracerebral haematoma was present. Thrombi were found to be present in the aneurysms of four patients (No. 3, 5, 6, 7) and in most cases did not affect the clinical course, except in one patient (No. 3), in whom it led to ischemia in the basal ganglia.
Treatment strategy
The optimum type of treatment of child aneurysm patient depends on many factors – as it does in any case of intracranial aneurysm. These patients were hospitalized at our neurosurgical unit and the decision as to which type of treatment was most suitable was made by the neurosurgeon together with the interventional radiologist. In seven patients the aneurysms were considered inoperable or the risk associated with surgery too high, and therefore endovascular treatment was preferred (Tables 1, 2).
Tab. 1. Clinical and anatomical features of paediatric aneurysms. 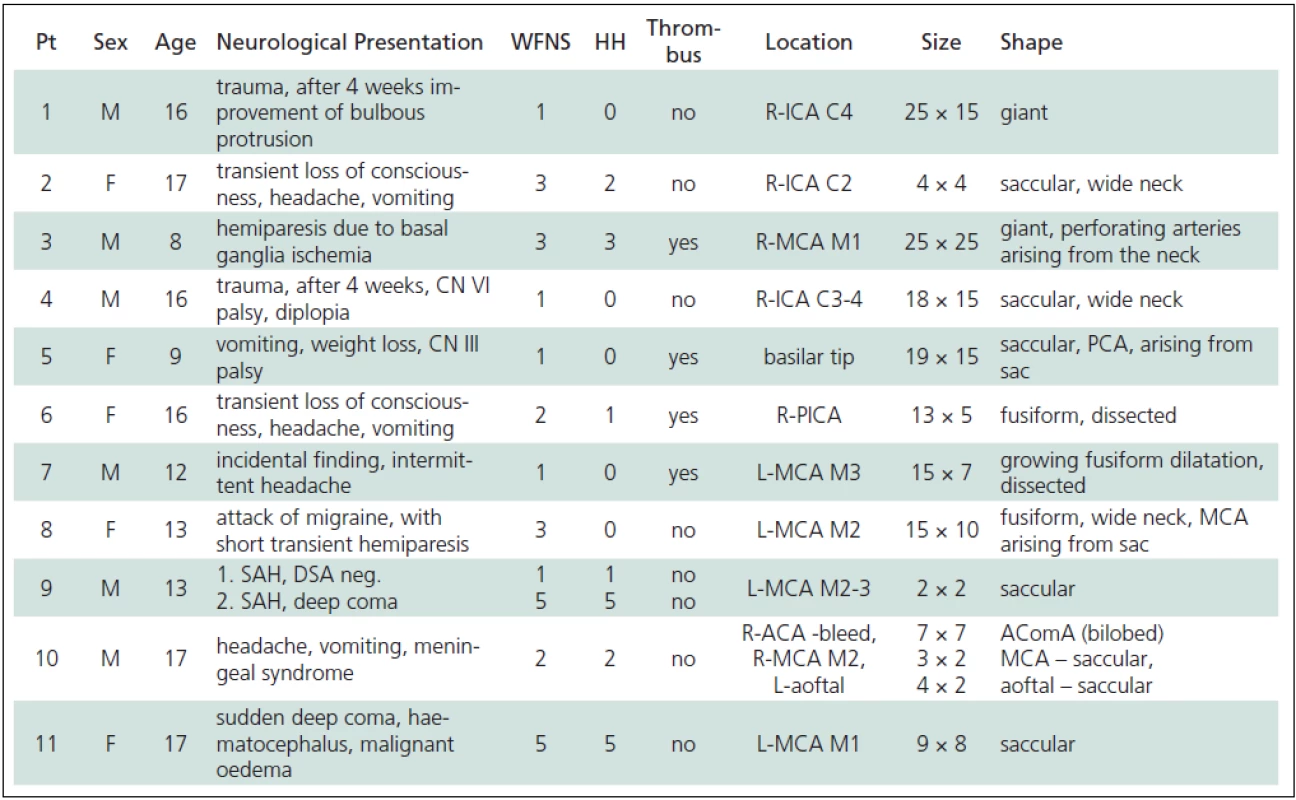
Tab. 2. Treatment and clinical outcome of paediatric aneurysms. 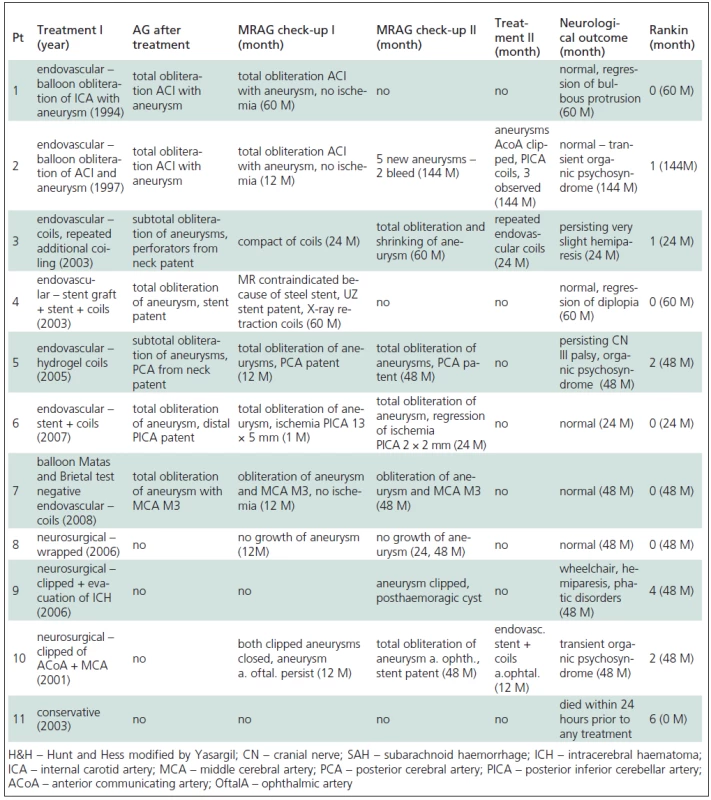
In two patients (No. 1, 2) endovascular treatment was chosen because the anatomical localization of the aneurysm was unfavourable, in the cavernous sinus in one patient (No. 1) and the lacerous segment of the ACI in the other (No. 2). Balloon occlusion was performed in both patients, which permitted sufficient collateral circulation without neurological deterioration. In both cases, a balloon was inserted into the sac of the aneurysm with another in the area of the neck of aneurysm, with sacrifice of the parent artery. Follow-up angiography revealed total occlusion of the ACI including the aneurysm, without neurological deterioration in both patients (No. 1, 2). Follow-up MRAG at 60 months in Patient 1 showed total obliteration of the aneurysm with flow stopped in the ACI in the area of the neck of the aneurysm. Clinically, there was an alleviation of focal neurological deficit. One month follow-up MRAG in Patient 2 showed total obliteration of the aneurysm, together with the parent artery, and no additional aneurysms. However, 144 months after treatment the same patient presented with SAH and angiography showed five new aneurysms. One aneurysm on AcomA was surgically clipped, one on PICA treated endovascularly and the other three smaller aneurysms are still being followed up.
In the other five patients treated endovascularly (No. 3, 4, 5, 6, 7), a reconstructive procedure was performed. In four cases (No. 3, 4, 6, 7) the aneurysm was simply coiled and in one case (No. 4), the coiling of the aneurysm was combined with a stent.
Endovascular treatment was preferred to surgical treatment in these five patients for the following reasons: Patient 3 had a partially thrombotized giant MCA M1 aneurysm with perforators leaving the neck (coiled with conserved patency of the perforators), Patient 4 had a large, traumatic, broad-necked ACI aneurysm (coiled in combination with a stent due to instability of coils), Patient 5 was a case of large, partially thrombotized basilar tip aneurysm with the PCA leaving the neck (obliterated with coils and conserved patency of the PCA). Patient 6 had a ruptured fusiform aneurysm of the medullary segment of the PICA (obliterated with coils and with conserved patency of PICA distally to the aneurysm). Patient 7 was referred for endovascular treatment due to a growing fusiform dissecting aneurysm on the MCA M3 (obliterated together with the parent artery following a negative balloon occlusion and Brietal test with no neurological sequelae).
A total of three patients (No. 8, 9, 10) were treated primarily surgically. The first patient (No. 8) had an unruptured fusiform MCA M2 aneurysm with a broad neck and a branch of the MCA leaving the sac of the aneurysm. An unfavourable configuration of the aneurysm prevented its being treated endovascularly, and neither could it be safely clipped, so it was wrapped.
Yearly MRAG follow-ups have not revealed any progression in the size of the aneurysm. The second surgically-treated patient (No. 9) presented with SAH, in good clinical condition, in which angiography did not reveal the source of bleeding. Three weeks later there was a recurrence of SAH with intracerebral haematoma and this time the patient was in poor clinical condition. Angiography revealed a small saccular aneurysm at MCA M2, which had not been visualized the first time due to vasospasm. The aneurysm was clipped emergently, but the patient remained in a vegetative state. The last surgically-treated patient (No. 10) had three saccular aneurysms. A ruptured AcomA aneurysm was clipped, as well as an ipsilateral coincidental MCA M2 aneurysm. A contralateral coincidental ophthalmic aneurysm with a broad neck was treated endovascularly 12 months later at the parent´s request. This was done in combination with a stent, since the neck of the aneurysm was broad. Follow-up MRAG after 48 months showed obliteration of all three aneurysms and patency of the stent. One patient (No. 11), in deep coma following SAH, was treated conservatively and died 24 hours after the bleed.
Follow-up and outcome
The aim of both surgical and endovascular treatment of intracranial aneuryms is obliteration of the lumen and thereby prevention of destructive haemorrhage. The final clinical outcome largely depends on the damage caused by the SAH. In none of the above-mentioned patients was there clinical deterioration as a consequence of active treatment.
Clinical improvement was evident following treatment in five patients (No. 1, 4, 5, 7 ,8) who had presented with a focal neurological deficit in the absence of SAH, and they were all thus ranked as Rankin 1. In only one case (No. 5) a mild focal deficit with psychological changes persisted and was thus ranked as Rankin 2 (Tables 1, 2).
Four patients (No. 2, 3, 6, 10) presenting with SAH were graded as HH 1 to 3. In only one case (No. 6) did the focal neurological deficit improve enabling the patient to be ranked Rankin 1. In the remaining three patients (No. 2, 3, 10) a neurological deficit persisted particularly in terms of psychological aspect, leading to rankings of Rankin 2 to 3.
Two patients (No. 9, 11) remained in deep coma following SAH (HH = 5). One patient died within 24 hours of SAH onset (No. 11) and one patient (No. 9) remained in a prolonged poor neurological condition after clipping; although there has been an improvement in the longer term, he remains carer-dependent i.e. Rankin 4.
Discussion
Childhood aneurysms differ from those in adults age in several ways. Unlike those in adults, paediatric intracranial aneurysms are not associated with haemodynamic factors or vessel wall damage i.e. hypertension, alcohol, tobacco use and high fat intake. According to the literature, paediatric aneurysms are predominantly associated with congenital systemic connective tissue disorders affecting the vessel wall [1,5,8]. However, this aetiology was not observed in our series. In paediatric aneurysms, traumatic aetiology is described at a range of 5%–35% and up to 35% develop on magistral arteries in the vicinity of the skull base [5,8,15–17]. In our series, two traumatic aneurysms were confirmed four weeks after mild head injury. Patient 6 presented with a focal neurological deficit due to the mass effect of a large aneurysm in the petrous segment of the ICA. Patient 1 presented in the same way due to a giant aneurysm in the cavernous segment of the ICA. Neither had CT signs of SAH.
A major difference in comparison with adult aneurysms is the juvenile predilection to occur in localities such as the ICA, MCA, the posterior circulation and the periphery [1,5,7,10]. Paediatric aneurysms have been described as occurring in the terminal segments of the ICA and the MCA at an incidence of 21–39% [1,7,10]. In our series, the most common localities were the MCA (46%) and the ICA (31%), while aneurysms on the AcomA were rare (one patient).
The incidence of giant aneurysms in children varies from 20% to 54% [1,6,7,10,18]. Typically, such aneurysms occur supratentorially or peripherally, are irregular in shape and lack a neck [5]. In our series, giant aneurysms were present in two patients. One proved to be traumatic in origin and both presented with focal neurological deficit without SAH. Another characteristic of paediatric intracranial aneurysms is their tendency to manifest with focal neurological signs in the absence of SAH: 19%–65% according to the literature [7,9,10]. This was the case in five of the 11 patients in our series, i.e. 45%.
The treatment strategy is multifactorial and the decision as to whether to opt for a surgical or endovascular approach depends on good communication between the neurosurgeon and the interventional radiologist. According to the literature, the percentage of endovascularly treated paediatric aneurysms ranges between 0% and 62.8% [1,6,7,10,13,14,18,19]. Surgical treatment is always suitable if technically feasible and the patient is in good clinical condition. In cases of MCA aneurysms that are surgically treatable, this method is to be preferred, However, in our series only two of seven such cases were treated surgically. In our series seven of 11 aneurysms were primarily treated endovascularly because the aneurysms were considered inoperable in the light of their locality, configuration, or size, or the complexity of the surgical approach.
Giant aneurysms make up a specific subgroup that is difficult to treat either surgically or endovascularly. In our series, both giant aneurysms were considered inoperable.
In the first patient (No. 3) the body of the aneurysm MCA was oriented towards the parenchyma with several perforators leaving the sac. In the second patient (No. 1), the aneurysm was located in the cavernous sinus and was therefore surgically inaccessible. The advantage of a clipped aneurysm is that it provides a definitive solution, whereas coiled aneurysms require follow-up and potentially re-embolization in the event of coil impaction. In the long term, only one of our seven endovascularly treated aneurysms (No. 3) required additional coiling due to impaction.
Our neurosurgical unit has six years of experience in stent usage in the cerebral vasculature. Data on stent usage in child patients is scarce in the literature. In our series, stents were used in two cases of broad-necked aneuryms in which there was a risk of coil instability within the sac of the aneurysm (No. 5, 11). In the long-term follow-up, both stents remain patent.
In a cooperative study of surgically treated paediatric intracranial aneurysms 63.4% of patients had no neurological sequelae [9]. In our series, nine patients were within Rankin 0–2 in the long term, i.e. 82%, and no patient deteriorated as a result of treatment. However, the subsequent clinical result is largely due to the initial damage arising out of SAH, but the plasticity and higher tolerance of the child brain to vasospasm partially explains the promising results seen in paediatric patients [1,5].
Assoc. Prof. Svatopluk Rehak, MD, PhD.
Charles University Medical School and Teaching Hospital
Sokolska 581
500 05 Hradec Kralove
e-mail: rehak@lfhk.cuni.cz
Accepted for review: 10. 3. 2010
Accepted for print: 17. 5. 2011
Zdroje
1. Lasjaunias P. Intracranial Aneurysms in Children. In: Lasjaunias P (ed). Vascular Diseases in Neonates, Infants and Children. Berlin, Heidelberg, New York: Springer 1997 : 374–387.
2. Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms, and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg 1966; 25(2): 219–239.
3. Patel AN, Richardson AE. Ruptured intracranial aneurysms in the first two decades of life. A study of 58 patients. J Neurosurg 1971; 35(5): 571–576.
4. Skotaková J, Prochazka J, Mach V. Intrakraniální aneuryzma u kojenců – 2 kazuistiky. Cesk Slov Neurol N 2005; 68/101(6): 399–401.
5. Allison JW, Davis PC, Sato Y, James CA, Haque SS, Angtuaco EJ et al. Intracranial aneurysms in infants and children. Pediatr Radiol 1998; 28(4): 223–229.
6. Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A. Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst 2005; 21(6): 437–450.
7. Laughlin S, Terbrugge KG, Willinsky RA, Armstrong DC, Montanera WJ, Humphereys RP. Endovascular Management of Paediatric Intracranial Aneurysms. Interventional Neuroradiology 1997; 3(3): 205–214.
8. Norris JS, Wallace MC. Pediatric Intracranial Aneurysms. Neurosurgery Clinics of North America 1998; 9(3): 557–563.
9. Roche JL, Choux M, Czorny P, Dhellemmes P, Fast M, Frerebeau P et al. L´anévrysme artériel intra-cranien che l´enfant. Etude Coopérative. A propos de 43 observations. Neurochirurgie 1988; 34(4): 243–251.
10. Meyer FB, Sundt TM, Fode NC, Morgan MK, Forbes GS, Mellinger JF. Cerebral aneurysms in childhood and adolescence. J Neurosurg 1989; 70(3): 420–425.
11. Kanaan I, Lasjaunias P, Coates R. The spectrum of intracranial aneurysms in pediatrics. Minim Invas Neurosurg 1995; 38(1): 1–9.
12. Rankin J. Cerebral vascular accidents in patients over the age of 60. Prognosis. Scott Med J 1957; 2(5): 200–215.
13. Hunt WE, Hess RH. Surgical risk as related to time of intervention in the repair of intracranial aneurysm. J Neurosurg 1968; 28(1): 14–20.
14. Yasargil MG, Smith RD, Young PH, Teddy PJ. Clinical Considerations. In: Yasargil MG (ed). Microneurosurgery. New York: Thieme 1984 : 1–12.
15. Morón F, Benndorf G, Akpek S, Dempsy R, Strother CM. Spontaneous Thrombosis of a Traumatic Posterior Cerebral Artery Aneurysm in a Child. Am J Neuroradiol 2005; 26(1): 58–60.
16. Suh CD, Alvarez H, Sainte Rose C, Lasjaunias P. Supraclinoid Internal Carotid Arterial Aneurysm Presenting as a Suprasellar Mass-like Lesion in a Child. Interv Neuroradiol 2001; 7(4): 357–361.
17. Ventureyra EC, Higgins MJ. Traumatic intracranial aneurysms in childhood and adolescence: case reports and review of the literature. Childs Nerv Syst 1994; 10(6): 361–379.
18. Ferrante L, Fortuna A, Celli P, Santoro A, Fraioli B. Intracranial Arterial Aneurysms in Early Childhood. Surg Neurol 1988; 29(1): 39–56.
19. Thrush AL, Marano GD. Infantile intracranial aneurysm: report of a case and review of the literature. AJNR Am J Neuroradiol 1988; 9(5): 903–906.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2012 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Webové okénko
-
Analýza dat v neurologii
XXXI. Bayesovská vs klasická statistika v medicínských aplikacích - Kdy vlastně začíná Alzheimerova nemoc – nová kritéria mírné kognitivní poruchy a Alzheimerovy nemoci
- Diabetes and Schizophrenia
- Prof. MUDr. Stanislav Němeček, DrSc., osmdesátníkem
- IX. medzinárodné afaziologické sympózium, Bratislava
- Mezinárodní sympozium Dystonie a dystonické syndromy
- Multimodal Imaging with Simultaneous EEG-fMRI
- Sonothrombolysis – Mechanisms of Action and its Use in the Treatment of Stroke
- Úvodník
- Fiber Dissection Technique of the Tracts of the Lateral Aspects of the Hemisphere
- XIV. European Congress of Neurosurgery – Řím
- Variability of Angiotensinogen and Susceptibility to Multiple Sclerosis
- Carpal Tunel Syndrome and Neurosurgeon – Experience after 2,200 Surgeries
- Paediatric Intracranial Aneurysms
- Visual Functions in Premature Children with Perinatal Brain Injury
- Accuracy of Prehospital Diagnosis of Stroke
- Neurological Occupational Diseases in the Czech Republic in 1994–2009
- Recanalization of Acute Occlusions of Cerebral Arteries Using a Retrievable Stent
- Vertebroplasty – Treatment Option for Structurally Insufficient Vertebras
- Paraneoplastic Polymyositis – a Case Report
- Guillain-Barré Syndrome in a Patient with a Renal Carcinoma – a Case Report
- Diagnostically Specific Findings in Posturography – Two Case Reports
- Guidelines for Pharmacotherapy of Neuropathic Pain
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Vertebroplasty – Treatment Option for Structurally Insufficient Vertebras
- Carpal Tunel Syndrome and Neurosurgeon – Experience after 2,200 Surgeries
- Guillain-Barré Syndrome in a Patient with a Renal Carcinoma – a Case Report
- Guidelines for Pharmacotherapy of Neuropathic Pain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



