-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDe novo non-alcoholic fatty liver disease after liver transplantation and liver fibrosis diagnosed by magnetic resonance over 2 years
De novo tuková choroba pečene po transplantácii pečene a fibróza pečene diagnostikovaná magnetickou rezonanciou počas dvoch rokov
Úvod: Nealkoholová tuková choroba pečene (NAFLD) je najrýchlejšie rastúcou príčinou ochorení pečene. Pri vzniku po transplantácii pečene (LT) pre iné indikácie nesie názov de novo NAFLD. Cieľ: Stanoviť incidenciu de novo NAFLD a jej asociáciu s BMI a fibrózou po LT v jednom transplantačnom centre. Metódy: Medzi januárom 2015 a decembrom 2020 sme realizovali propektívnu observačnú štúdiu u po sebe nasledujúcich pacientov po LT pre iné indikácie ako NAFLD. Sledovali sme demografické ukazovatele, etiológiu cirhózy pečene, MELD sklóre a Child Pugh skóre. Šesť, 12 a 24 mesiacov po LT sme hodnotili BMI, MR spektroskopiu (MRS, [≥ 5 % = NAFLD]) a MR elastografiu (MRE, [≥ 2,88 kPa = signifikantná fibróza, ≥ 3,54 kPa = pokročilá fibróza]). Výsledky: V sledovanom intervale sme do štúdie zaradili 164 pacientov po LT. Na základe vopred definovaných kritérií sme vylúčili 37 pacientov, do finálnej analýzy sme zaradili 104 pacientov s mediánom 53 rokov, 38 % žien, s mediánom MELD 15 bodov a BMI 25,4. Medián BMI – 6, 12 a 24 mes. po LT bol 25,5 vs. 27,3 (p = 0,032) a 26 vs. 27,8 (p = 0,062). MRS % – 6, 12 a 24 mes. po LT boli 4,5 oproti 5,1 (p = 0,2) a 4,4 oproti 7 (p = 0,012). Šesť, 12 a 24 mesiacov po LT sme signifikantnú fibrózu zistili u 27 %, 35 % a 46 % (p = 0,09) a pokročilú fibrózu u 4,7 %, 1,2 % a 15 % pacientov (p = 0,003). Závery: Počas dvoch rokov po LT pre iné indikácie ako NAFLD sme zaznamenali stúpajúci trend BMI, stúpajúci výskyt de novo NAFLD, signifikantnej a pokročilej fibrózy.
Klíčová slova:
transplantácia pečene – MR spektroskopia – MR elastografia – de novo NAFLD – fibróza pečene
Authors: S. Adamcová Selčanová 1
; L. Skladaný 1
; Škvarková B. 1; Bachova B. 1; L. Lafférs 2
; T. Koller 3
Authors place of work: HEGITO (Division of Hepatology, Gastroenterology and Liver Transplantation), FD Roosevelt Hospital, Banska Bystrica, Slovakia 1; Department of Mathematics, Faculty of Natural Sciences, Matej Bel University, Banska Bystrica, Slovakia 2; Department of Internal Medicine, Comenius University Faculty of Medicine, University Hospital Bratislava Ruzinov, Bratislava, Slovakia (Slovakia) 3
Published in the journal: Gastroent Hepatol 2022; 76(2): 112-120
Category: Původní práce
doi: https://doi.org/10.48095/ccgh2022112Summary
Background: Non-alcoholic fatty liver disease (NAFLD) is the fastest-growing cause of liver diseases; after liver transplantation (LT) for another indications bears the name de novo NAFLD. Aims: We set out to determine the incidence of de novo NAFLD and its associations with BMI and fibrosis in patients (pts) after LT at a single transplant centre. Methods: We organized an observational study of consecutive pts after LT for non-NAFLD causes between January 2015 and December 2020. At the baseline, we recorded the demographics, etiology of cirrhosis, MELD and Child-Pugh score; 6, 12 and 24 months after LT we recorded BMI, MR spectroscopy (MRS, [≥5% = NAFLD]) and MR Elastography (MRE, [≥2.88 kPa = significant fibrosis, ≥3.54 kPa = advanced fibrosis]). Results: We enrolled 164 pts after LT, excluded 37% for pre-defined criteria and analysed 104 pts with median age 53 years, 38% women, with median MELD 15 points and BMI 25.4. The median BMI – 6, 12 and 24 months after LT – were 25.5 vs 27.3 (P = 0.032) and 26 vs 27.8 (p = 0.062). MRS % – 6, 12, and 24 months after LT were 4.5 vs 5.1 (P = 0.2) and 4.4 vs 7 (P = 0.012). Significant fibrosis 6, 12 and 24 months after LT were found in 27%, 35% and 46%, respectively (P = 0.09), and advanced fibrosis in 4.7%, 1.2% and 15%, respectively (P = 0.003). Conclusions: Over 2 years after LT for various non-NAFLD indications, we identified rising BMI and rising incidence of de novo NAFLD and of significant and advanced fibrosis.
Keywords:
liver transplantation – liver fibrosis – MR spectroscopy – MR elastography – de novo NAFLD
Introduction
The prevalence of obesity and diabetes has reached an epidemic proportion in the past few years in the USA and throughout the Western world [1]. Non-alcoholic fatty liver disease (NAFLD) is considered a liver manifestation of the metabolic syndrome. Its more progressive form – NASH (non-alcoholic steatohepatitis) – via fibrosis, can lead to advanced chronic liver disease (ACLD) and hepatocellular carcinoma (HCC). Renamed to Metabolic dysfunction-associated fatty liver disease (MAFLD) with revised diagnostic criteria notwithstanding, NAFLD is likely to become the most frequent indication for liver transplantation (LT) in the Western and westernised world [2–4]. NAFLD-related liver transplants increased from less than 2% in 2002 to 19% in 2011, in Slovakia from 5% in 2014 to 14% in 2021 [5,6]. Additionally, NAFLD may arise after LT performed for another indication: then it is called de novo NAFLD.
Recurrence of NAFLD after LT
Following LT, the use of immunosuppressant drugs such as corticosteroids, calcineurin inhibitors and mammalian target of rapamycin inhibitors is known for its significant impact on the metabolic balance and is associated with the development of insulin resistance, diabetes, hypertension, obesity and hyperlipidaemia [7–9]. Many post-transplant patients (pts) fulfill the criteria for NAFLD recurrence after LT [10]. The recurrence rates for hepatic steatosis and steatohepatitis 5 years after LT are reported in the range from 10% to 100% and from 4 to 33%, respectively [11–15]. In the Czech Liver Transplant Centre at the Institute of Clinical and Experimental Medicine (IKEM), NAFLD was detected by liver biopsy in 30% and 48% of the patients 1 and 10 years after LT, respectively [16]. The driving force behind the NAFLD recurrence-associated poor clinical outcome is the development of liver fibrosis. Severe fibrosis was detected in 20% of patients six months post-LT and significant fibrosis of the F2 and more METAVIR in up to 33% of patients one year post-LT [13,17].
De novo NAFLD after LT
In contrast to the relatively abundant data on recurrent post-LT NAFLD, only a few studies have assessed the prevalence of de novo NAFLD after LT [18–21]. Post-LT diabetes, hypertension, hyperlipidaemia, obesity and immunosuppressant use seem to play a significant role in de novo NAFLD. Donor graft steatosis is also a predominant risk factor for de novo NAFLD [22–24]. The incidence of de novo NAFLD ranges from 9% to 48%, with progression to fibrosis and cirrhosis in 20–40% of the affected patients [22–24]. In some patients, coexisting NAFLD/NASH could remain undiagnosed before LT, thus de novo NAFLD could actually be a recurrence. Even if NAFLD after LT does not progress to ACLD, the patients are at an increased risk of cardiovascular and renal morbidity and mortality [17,25,26].
Diagnosis of NAFLD after LT
Liver biopsy is the gold standard test to determine fibrosis stage in NAFLD patients [27,28]. Non-invasive tools have been developed to diagnose NAFLD and fibrosis, including transient elastography and serum marker algorithms [29–33]. The most frequently used and most accurate methods for non-invasive diagnosis of NAFLD are fatty liver index (FLI) and magnetic resonance spectroscopy (MRS); the sensitivity and specificity of the FLI calculator ranges from 92% to 100% (Fatty Liver Index. [online]. Available from: https: //www.mdapp.co/fatty-liver-index-flicalculator-356/. [21,34–37]).
In addition to its diagnostic accuracy, the advantage of MRS is the ability to perform magnetic resonance elastography (MRE) with sensitivity and specificity of almost 100% [38–42].
Aims
In this study, we set out to determine the incidence of de novo NAFLD and liver fibrosis in patients up to 2 years after LT.
Methods
In this prospective study, we analysed the data of consecutive patients from the Hospital Information System “Care Center®” and the Liver Transplant Database of the F. D. Roosevelt Univ Hospital, Banska Bystrica, Slovakia. We enrolled patients transplanted for cirrhosis of non-NAFLD etiology during the interval from January 2015 to December 2020. We excluded patients who had LT performed for NAFLD, who had died or have had severe complications during the first 3 months after LT. We recorded the demographics (age, sex), anthropometrics (height, weight, waist circumference, BMI [Body Mass Index in kg/m2]), laboratory (gama-glutamyltransferase, triacylglycerides and calculated FLI, MELD [Model for End-stage Liver Disease]) and imaging variables (Magnetic Resonance Imaging [MRI]) – MRS and MRE – performed at the Jessenius Diagnostic Centre, Ltd., Nitra, Slovakia. The measurements were collected 3, 6, 12 and 24 months after LT. FLI was interpreted as suggestive of the diagnosis of NAFLD, if ≥ 60 [26]. The interpretation of the MRS was diagnostic of NAFLD if ≥5%, the MRE of significant fibrosis if ≥2.88 kPa, and advanced fibrosis ≥3.54 kPa.
For statistical analysis, we used legally obtained statistical software R [43].
All the participants signed an informed consent before the LT and agreed with the data publication. The study was carried out in accordance with the proceedings of the Declaration of Helsinki. All participants signed an informed consent prior to anonymised data recording and agreed with data publication.
Results
Baseline characteristic
During the study interval, LT were performed in 164 patients. We excluded 5 patients (3%) transplanted for NAFLD and 55 (34%) for other pre-defined criteria. We included 104 patients (63%) with a median age of 53 years (39–61), 38% women, the median MELD was 15 points (12–18), and BMI 25.4 (Scheme 1).
Scheme 1. Cohort analysis.
Schéma 1. Kohortová analýza.
The etiologies of ACLD before LT were alcohol-associated liver disease in 46 patients (44%), autoimmune etiologies (AIH – autoimmune hepatitis, PSC – primary sclerosing cholangitis, PBC – primary biliary cholangitis) in 32 (31%), miscellaneous in 13 (12%), HCC (hepatocellular carcinoma) in 9 (9%), viral hepatitis in 4 (4%), (Graph 1). Summary statistics and study group characteristics are displayed in Tab. 1.
Graph 1. Etiology of advanced chronic liver disease before liver transplantation.
Graf 1. Etiológia pokročilého chronického ochorenia pečene pred transplantáciou pečene.
Tab. 1. Baseline characteristics.
Tab. 1. Základné charakteristiky.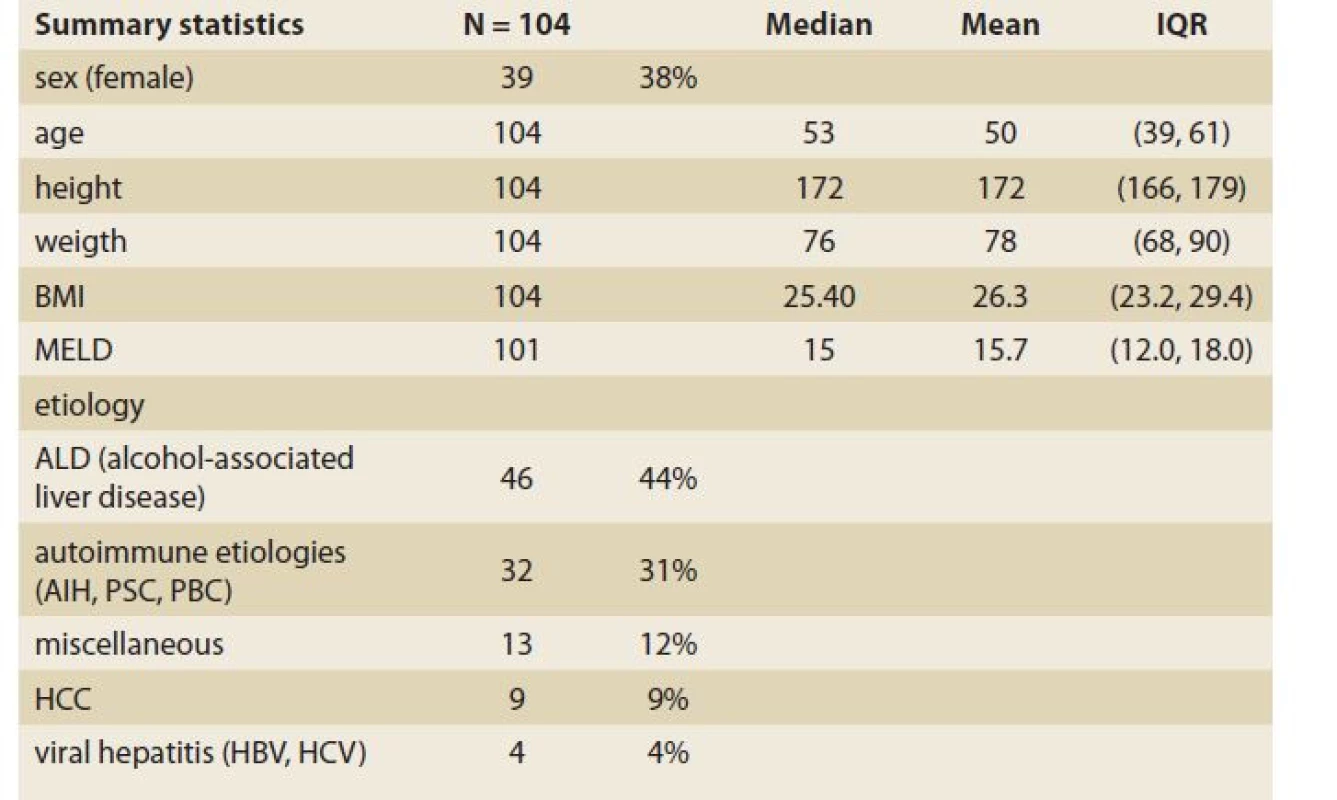
BMI – body mass index; MELD – model for end stage liver disease; ALD – alcohol-associated liver disease; AIH – autoimmune hepatitis, PSC – primary sclerosing cholangitis, PBC – primary biliary cholangitis, HCC – hepatocellular carcinoma, HBV – hepatitis B virus; HCV – hepatitis C virus The evolution of BMI performed in 104, 101, 95 and 47 patients at baseline, 3, 6, 12 and 24 months was as follows: 25.4 [23.2, 29.4] (kg/m2), 24.1 [21.7, 27.7], 24.9 [22.6, 28.0], 27.3 [24.0, 30.3] and 26.9 [24.5, 31.4] (Tab. 2, Fig. 1).
Tab. 2. Statistic analysis: body mass index – evolution in time.
Tab. 2. Štatistická analýza: index telesnej hmotnosti – vývoj v čase.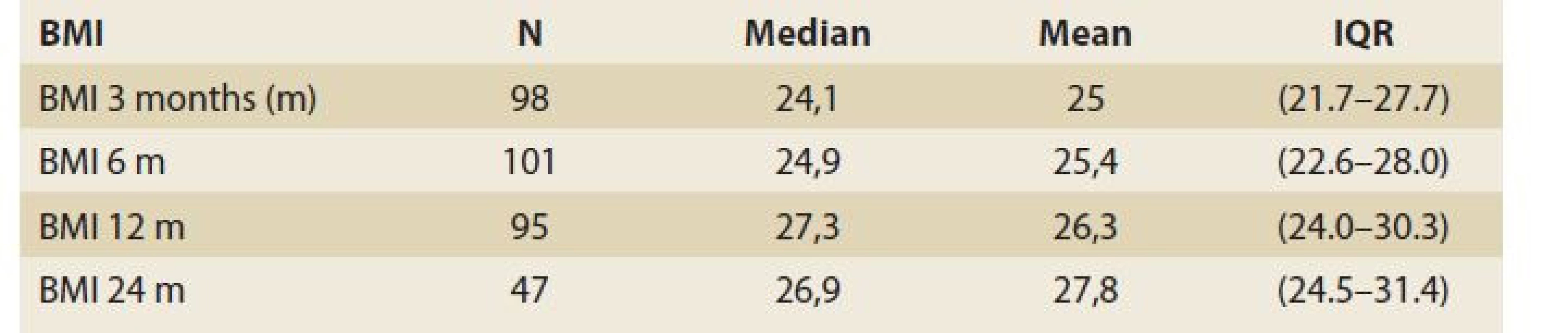
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 1. Body mass index evolution in time (baseline–6 months–12 months–24 months).
Obr. 1. Vývoj indexu telesnej hmotnosti v čase (základná hodnota–6 mesiacov–12 mesiacov–24 mesiacov).
The median body mass index at 6 vs.12 months and 6 vs. 24 after LT was 25.0 (22.5, 28.3) vs. 26.3 (24.0, 30.3), (P = 0.032), (Tab. 3, Fig. 2) and 25.0 (22.3, 29.9) vs. 26.9 (24.5, 31.4), P = 0.062 (Tab. 4, Fig. 3).
Tab. 3. body mass index in time – median and IQR, 6 vs. 12 months; P = 0,032.
Tab. 3. BMI – index telesnej hmotnosti v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,032.
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 2.BMI – body mass index in time – median and IQR, 6 vs. 12 months; P = 0,032.
Obr. 2. BMI – index telesnej hmotnosti v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,032.
Tab. 4. BMI – body mass index in time – median and IQR, 12 vs. 24 months, P = 0,062.
Tab. 4. BMI – index telesnej hmotnosti v čase – medián a IQR, 12 vs. 24 mesiacov, P = 0,062.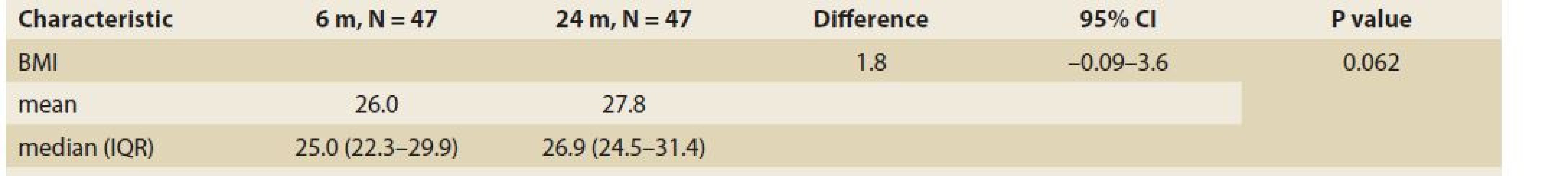
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 3. BMI – body mass index in time – median and IQR, 12 vs. 24 months, P = 0,062.
Obr. 3. BMI – index telesnej hmotnosti v čase – medián a IQR, 12 vs. 24 mesiacov, p = 0,062.
The diagnosis of de novo NAFLD
The median FLI results as calculated 6, 12 and 24 months after LT, perfomed in 100, 94 and 46 patients in these time periods increased, but did not differ significantly: 46 (22, 68), 48 (23, 74) and 63 (31, 81), (P = NS), (Tab. 5).
Tab. 5. Statistic analysis: fatty liver index in time.
Tab. 5. Štatistická analýza: index tuku v pečeni v čase.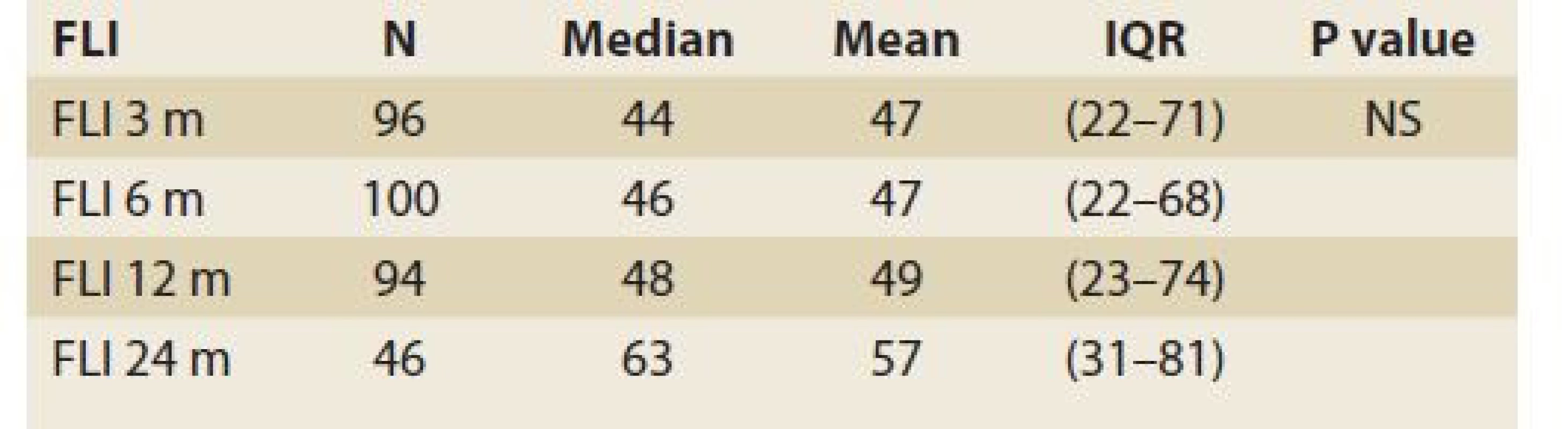
FLI – fatty liver index, N – number, IQR – interquartile range, m – month De novo NAFLD, as diagnosed by MRS (fat content ≥5%) at 6, 12 and 24 months was present in 24%, 26% and 38% (P = 0.17) of patients, respectively (Tab. 6).
Tab. 6. Statistic analysis: MR spectroscopy ≥5% in time.
Tab. 6. Štatistická analýza: MR spektroskopia ≥ 5 % v čase.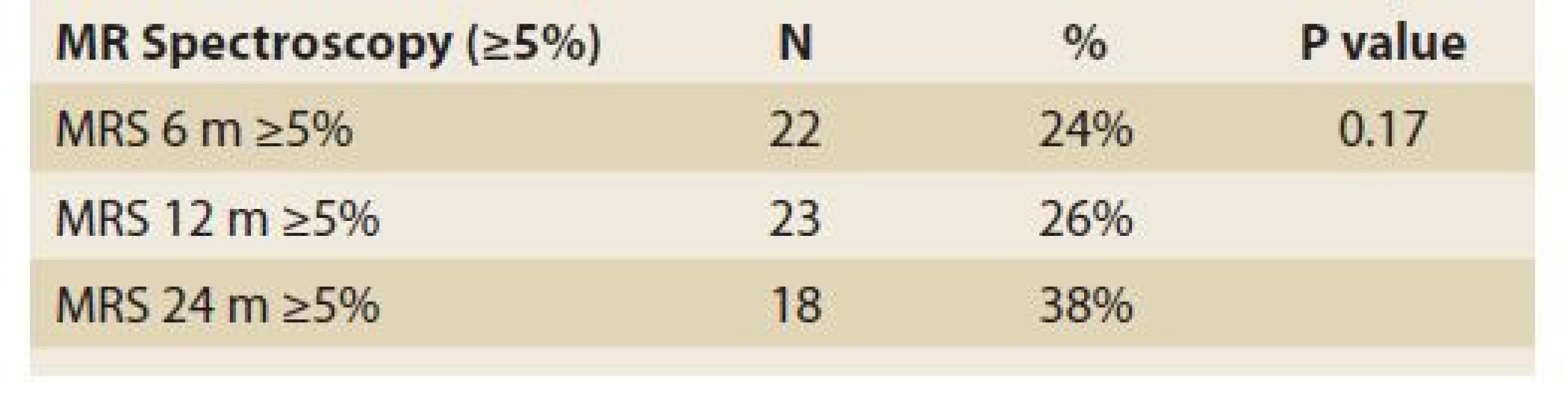
We observed a significant rise in the liver fat content by MRS (%) at 6 vs.12 months in 88 patients and 6 vs. 24 months in 47 patients. The median fat content was 3.8 (2.7, 5.0) vs. 4.0 (3.0, 5.2) (P = 0.2), (Tab. 7, Fig. 4) and 3.9 (2.9, 5.0) vs. 4.5 (3.5, 7.9) (P = 0.012), (Tab. 8, Fig. 5).
Tab. 7. MR spectroscopy (%) in time – median and IQR, 6 vs. 12 months, P = 0,2.
Tab. 7. MR spektroskopia (%) v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,2.
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 4. MRI spectroscopy (%) in time – median and IQR, 6 vs. 12 months, P = 0,2.
Obr. 4. MR spektroskopia (%) v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,2.
Tab. 8. MR spectroscopy (%) in time – median and IQR, 6 vs. 24 months, P = 0,012.
Tab. 8. MR spektroskopia (%) v čase – medián a IQR, 6 vs. 24 mesiacov, p = 0,012.
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 5. Significant rising of fat content diagnosed by MR spectroscopy two years after LT, P = 0,012.
Obr. 5. Signifikantný vzostup obsahu tuku diagnostikovaný MR spektroskopiou dva roky po LT, p = 0,012.
The median liver stiffness diagnosed by MRE at 6 vs.12 months and 6 vs. 24 months in 84 and 46 patients was 2.69 (2.34, 2.90) vs. 2.67 (2.39, 3.04) (P = 0.9), (Tab. 9, Fig. 6) and 2.70 (2.39, 2.83) vs. 2.87 (2.41, 3.20) (P = 0.11), (Tab. 10, Fig. 7).
Tab. 9. MR elastography (kPa) in time – median and IQR, 6 vs.12 months, P = 0,9.
Tab. 9. MR elastografi a (kPa) v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,9.
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 6. MR elastography (kPa) in time – median and IQR, 6 vs. 12 months, P = 0,9
Obr. 6. MR elastografia (kPa) v čase – medián a IQR, 6 vs. 12 mesiacov, p = 0,9.
Tab. 10. MR elastography (kPa) in time – median and IQR, 6 vs. 24 months, P = 0,11.
Tab. 10. MR elastografi a (kPa) v čase – medián a IQR, 6 vs. 24 mesiacov, p = 0,11.
BMI – body mass index, N – number, IQR – interquartile range, m – month Fig. 7. MR elastography (kPa) in time – median and IQR, 6 vs. 24 months, P = 0,11.
Obr. 7. MR elastografi a (kPa) v čase – medián a IQR, 6 vs. 24 mesiacov, p = 0,11.
Significant fibrosis at months 6, 12 and 24 was found in 27%, 35% and 46% of patients, respectively (P = 0.09), (Tab. 11, Fig. 8) and advanced fibrosis in 4.7%, 1.2% and 15% of patients, respectively (P = 0.003), (Tab. 12, Fig. 9).
Tab. 11. Statistic analysis: MR elastography ≥2,88 kPa in time.
Tab. 11. Štatistická analýza: MR elastografi a ≥ 2,88 kPa v čase.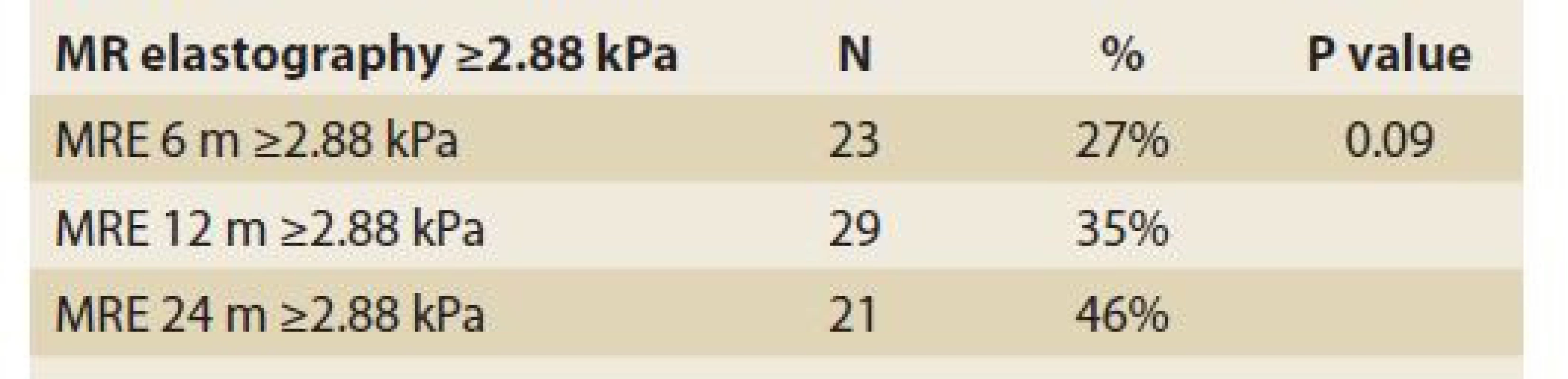
MRE – magnetic resonance elastography, N – number, m – month Fig. 8. Rising of significant fibrosis ≥2,88 kPa in pts with de novo NAFLD two years after LT.
Obr. 8. Vzostup signifikantnej fibrózy ≥ 2,88 kPa u pacientov s de novo NAFLD dva roky po LT.
Tab. 12. Statistic analysis: MR elastography ≥3,54 kPa in time.
Tab. 12. Štatistická analýza: MR elastografi a ≥ 3,54 kPa v čase.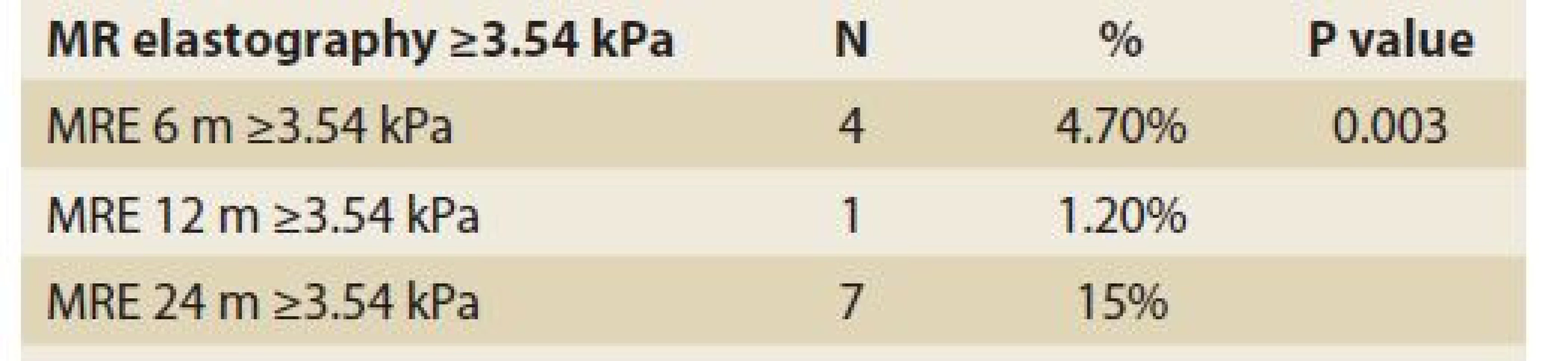
MRE – magnetic resonance elastography, N – number, m – month Fig. 9. Significant rising of advanced fibrosis ≥3,54 kPa in pts with de novo NAFLD two years after LT (P = 0,003).
Obr. 9. Signifikantný vzostup pokročilej fibrózy ≥ 3,54 kPa u pacientov s de novo NAFLD roky po LT (p = 0,003).
Discussion
The present study characterises the development of de novo NAFLD and assessment of significant and advanced fibrosis 2 years after LT with the non-invasive methods MRS and MRE.
The most important finding of this study was that the presence of de novo NAFLD was increasing: in 24% of pts 6 months and 38% of the pts 2 years after LT, respectively. Since our centre does not use protocol biopsies (performed each year, irrespective of clinical and laboratory findings), we diagnosed de novo NAFLD by MRS. In the current era of the search for non-invasive diagnostic tools for NAFLD, MRS has been described as the most promising instrument – with an accuracy similar, if not identical, to liver histology [36,37,42]. Following LT, Galvin et al. diagnosed de novo NAFLD in 48% after 3 years, Seo et al. reported 18% steatosis and 9% NASH in a similar cohort at a median of 28 months, Dumortier et al. studied the development of de novo NAFLD and reported 31.0% and steatohepatitis in 3.8% of 421 recipients at 3.3 years, and Kim et al. found de novo NAFLD incidence of 27% [22–24,41].
We also established that de novo NAFLD is associated with significant fibrosis (≥2.88 kPa on MRE) 6 months after LT in 27%, and 24 months after LT in 46% of pts, respectively (P = 0.09), and advanced fibrosis (≥3.54 kPa on MRE) in the same months after LT in 4.7% and 15% of pts after LT, respectively (P = 0.003).
The above-mentioned study by Galvin et al. showed significant fibrosis in 40.0% of the biopsies of patients with de novo NAFLD 3 years after LT, Tejedor-Tejada et al. diagnosed significant fibrosis (≥F2) in 58–86% of patients, employing a simple serum fibrosis score although the use of these fibrosis scores in a post-LT setting has been less promising than pre-LT NAFLD [24,28]. Morisaka et al. considered MRE to be a viable alternative to a liver biopsy for the staging of fibrosis [40]. Not using liver biopsies for comparison with MRI can be considered a limitation of our study. However, we believe that, in the near future, MRI at the head of non-invasive modalities will play an increasing role in diagnosing NAFLD as well as the staging of fibrosis [42].
There are other results in our study that add to the validity of the results in terms of increasing liver fat content over time after LT – BMI, and FLI. The BMI increased significantly during the 1st year (P <0.01). According to FLI measured at months 3, 6, 12 and 24, NAFLD was present in 39%, 39%, 42% and 53% of the patients, respectively. These results are higher than those achieved by the MRS but still comparable to the prevalence in literature.
Apart from the absence of systematic liver biopsies, there is another drawback to our study. We have not examined the main environmental, lifestyle, host and graft risk factors of de novo NAFLD. Sedentary lifestyle, obesity by BMI or waist circumference, high blood pressure, DM type 2, high triglycerides, low HDL-cholesterol, donor graft steatosis, hepatic denervation and immunosuppression after LT are the most important factors frequently cited by specialists in this field [24,28,44,45]. Immunosuppressive protocol in our TC includes a triple combination of intravenous methylprednisolone 500 mg in the anhepatic phase followed by a daily intravenous dose of 20 mg with a switch to oral dose at the resumption of oral intake, tapering over 3–6 months, tacrolimus 0.1 mg/kg/day, or ciclosporin 5–10 mg/kg/day and mycophenolate mofetil 2,000 mg/day.
It has been proved that tacrolimus increases hyperlipidaemia and incidence of obesity, DM (NODAT – new onset diabetes after transplantation) and arterial hypertension. Ciclosporin A has the same side effects, but has a more significant effect on hyperlipidaemia, while the incidence of NODAT is lower [24,28,45]. Glucocorticoids and weight gain also contribute to the development of de novo NAFLD and NODAT following LT [24,28,45]. Despite the fact that we have studied some of these associations in another work, we did not manage to link these risk factors to the occurrence of NAFLD in the current study [46]. Moreover, we have not examined the clinical impact of de novo NAFLD on the prognosis of our patients. Previous studies have indicated that patients with recurrent NAFLD are at increased risk of cardiovascular events and renal impairment [17,24,25]. Based on the shared metabolic risk factors, post‐LT patients with de novo NAFLD are likely to be at higher risk of cardiovascular death, in addition to progression of liver cirrhosis and subsequent complications of portal hypertension [24]. Early diagnosis and targeted therapy could potentially reduce the risk of the associated complications.
Conclusions
Over the 2 years after LT for various non--NAFLD indications, we identified rising BMI, rising incidence of de novo NAFLD and rising incidence of significant and advanced fibrosis.
ORCID authors
S. Adamcová Selčanová ORCID 0000-0001-8181-1937,
Ľ. Skladaný ORCID 0000-0001-5171-3623,
L. Lafférs ORCID 0000-0002-3141-3591,
T. Koller ORCID 0000-0001-7418-0073.
Doručené/Submitted: 21. 3. 2022
Prijaté/Accepted: 29. 3. 2022
Světlana Adamcová Selčanová, MD
HEGITO (Division of Hepatology, Gastroenterology and Liver Transplantation)
FD Roosevelt Hospital
L. Svobodu 1 Square
975 17 Banska Bystrica
Zdroje
1. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64 (6): 1388–1402. doi: 10.1016/j.jhep.2015.11.004.
2. Targher G. Concordance between MAFLD and NAFLD diagnostic criteria in „real-world“ data. Liver Int 2020; 40 (11): 2879–2880. doi: 10.1111/liv.14623.
3. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003; 37 (5): 1202–1219. doi: 10.1053/jhep.2003.50193.
4. Aller R, Fernández-Rodríguez C, Lo Iacono O et al. Consensus document. Management of non-alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol Hepatol 2018; 41 (5): 328–349. doi: 10.1016/j.gastrohep.2017.12.003.
5. Charlton MR, Burns JM, Pedersen RA et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141 (4): 1249–1253. doi: 10.1053/j.gastro.2011.06.061.
6. Agopian VG, Kaldas FM, Hong JC et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 2012; 256 (4): 624–633. doi: 10.1097/SLA.0b013e31826b4b7e.
7. Davidson JA, Wilkinson A. International Expert Panel on New‐Onset Diabetes after Transplantation. New‐Onset Diabetes After Transplantation 2003 International Consensus Guidelines: an endocrinologist’s view. Diabetes Care 2004; 27 (3): 805–812. doi: 10.2337/diacare.27.3.805.
8. Pham PT, Pham PM, Pham SV et al. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes 2011; 4 : 175–186. doi: 10.2147/DMSO.S19027.
9. Watt KD, Pedersen RA, Kremers WK et al. Evolution of causes and risk factors for mortality post‐liver transplant: results of the NIDDK long‐term follow‐up study. Am J Transplant 2010; 10 (6): 1420–1427. doi: 10.1111/j.1600-6143.2010. 03126.x.
10. Bhagat V, Mindikoglu AL, Nudo CG et al. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transplant 2009; 15 (12): 1814–1820. doi: 10.1002/lt.21927.
11. Contos MJ, Cales W, Sterling RK et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transplant 2001; 7 (4): 363–373. doi: 10.1053/jlts.2001.23011.
12. Ong J, Younossi ZM, Reddy V et al. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transplant 2001; 7 (9): 797–801. doi: 10.1053/jlts.2001.24644.
13. Charlton M, Kasparova P, Weston S et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transplant 2001; 7 (7): 608–614. doi: 10.1053/jlts.2001. 25453.
14. Kim WR, Poterucha JJ, Porayko MK et al. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation 1996; 62 (12): 1802–1805. doi: 10.1097/000078 90-199612270-00021.
15. Hanouneh IA, Macaron C, Lopez R et al. Recurrence of disease following liver transplantation: nonalcoholic steatohepatitis vs hepatitis C virus infection. Int J Organ Transplant Med 2011; 2 (2): 57–65.
16. Hejlova I, Honsova E, Sticova E et al. Prevalence and risk factors of steatosis after liver transplantation and patients outcomes. Liver Transpl 2016; 22 (5): 644–655. doi: 10.1002/lt.24393.
17. Dureja P, Mellinger J, Agni R et al. NAFLD recurrence in liver transplant recipients. Transplantation 2011; 91 (6): 684–689. doi: 10.1097/TP.0b013e31820b6b84.
18. Karam V, Sebagh M, Rifai K et al. Quality of life 10 years after liver transplantation: the impact of graft histology. World J Transplant 2016; 6 (4): 703–711. doi: 10.5500/wjt.v6.i4.703.
19. Andrade AR, Bittencourt PL, Codes L et al. New onset diabetes and nonalcoholic fatty liver disease after liver transplantation. Ann Hepatol 2017; 16 (6): 932–940. doi: 10.5604/01.3001. 0010.5285.
20. Gitto S, de Maria N, di Benedetto F et al. De novo nonalcoholic steatohepatitis is associated with long-term increased mortality in liver transplant recipients. Eur J Gastroenterol Hepatol 2018; 30 (7): 766–773. doi: 10.1097/MEG.000 0000000001105.
21. Skladany L, Adamcova Selcanova S, Ciefova J et al. De novo non-alcoholic fatty liver disease after liver transplantation – as diagnosed by magnetic resonance. Gastroent Hepatol 2020; 74 (2): 116–122. doi: 10.14735/amgh2020116
22. Seo S, Maganti K, Khehra M et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl 2007; (13) 6 : 844–847. doi: 10.1002/lt.20932.
23. Dumortier J, Giostra E, Belbouab S et al. Non-alcoholic fatty liver disease in liver transplant recipients: another story of „seed and soil”. Am J Gastroenterol 2010; 105 (3): 613–620. doi: 10.1038/ajg.2009.717.
24. Galvin Z, Rajakumar R, Chen E et al. Predictors of de novo nonalcoholic fatty liver disease after liver transplantation and associated fibrosis. Liver Transpl 2019; 25 (1): 56–67. doi: 10.1002/lt.25338.
25. Mikolasevic I, Orlic L, Hrstic I et al. Metabolic syndrome and non‐alcoholic fatty liver disease after liver or kidney transplantation. Hepatol Res 2016; 46 (9): 841–852. doi: 10.1111/hepr.12642.
26. Fatty Liver Index. [online]. Available from: https: //www.mdapp.co/fatty-liver-index-flical - culator-356/.
27. Seeff LB, Everson GT, Morgan TR et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol 2010; 8 (10): 877–883. doi: 10.1016/j.cgh.2010.03.025.
28. Tejedor-Tejada J, Valenzuela EF, Muňoz RN et al. De-novo nonalcoholic fatty liver disease at 5 years after liver transplantation: prevalence and predictive factors. Eur J Gastroenterol Hepatol 2021; 33 (3): 399–406. doi: 10.1097/MEG.0000000000001736.
29. Hernaez R, Lazo M, Bonekamp S et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011; 54 (3): 1082–1090. doi: 10.1002/hep.24452.
30. Petta S, Wong VW, Cammà C et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 2017; 65 (4): 1145–1155. doi: 10.1002/hep.28 843.
31. Myers RP, Pollett A, Kirsch R et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012; 32 (6): 902–910. doi: 10.1111/j.1478-32 31.2012.02781.x.
32. Karlas T, Kollmeier J, Böhm S et al. Noninvasive characterization of graft steatosis after liver transplantation. Scand J Gastroenterol 2015; 50 (2): 224–232. doi: 10.3109/00365521. 2014.983156.
33. Bhat M, Ghali P, Rollet-Kurhajec KC et al. Serum fibrosis biomarkers predict death and graft loss in liver transplantation recipients. Liver Transpl 2015; 21 (11): 1383–1394. doi: 10.1002/lt.24217.
34. Bedogni G, Bellentani S, Miglioli L et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006; 6 : 33. doi: 10.1186/1471-230X-6-33.
35. Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013; 58 (5): 1007–1019. doi: 10.1016/j.jhep.2012.11.021.
36. Idilman IS, Aniktar H, Idilman R et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013; 267 (3): 767–775. doi: 10.1148/radiol.13121360.
37. Gu J, Liu S, Du S et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol 2019; 29 (7): 3564–3573. doi: 10.1007/s00330-019-06072-4.
38. Yin M, Talwalkar JA, Glaser KJ et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 2007; 5 (10): 1207–1213.e2. doi: 10.1016/ j.cgh.2007.06.012.
39. Loomba R, Wolfson T, Ang B et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014; 60 (6): 1920–1928. doi: 10.1002/hep.27362.
40. Morisaka H, Motosugi U, Ichikawa S et al. Magnetic resonance elastography is as accurate as liver biopsy for liver fibrosis staging. J Magn Reson Imaging 2018; 47 (5): 1268–1275. doi: 10.1002/jmri.25868.
41. Kim H, Lee K, Lee KW et al. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin Transplant 2014; 28 (5): 521–529. doi: 10.1111/ctr.12343.
42. Newsome PN, Sasso M, Deeks JJ et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020; 5 (4): 362–373. doi: 10.1016/S2468-1253 (19) 30 383-8.
43. R Core Team. The R project for statistical computing. [online]. Available from: https: //www.R-project.org/.
44. Vallin M, Guillaud O, Boillot O et al. Reccurent or de novo non-alcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl 2014; 20 (9): 1064–1071. doi: 10.1002/lt.23936.
45. Shaker M, Tabbaa A, Albedawi M et al. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol 2014; 20 (18): 5320–5330. doi: 10.3748/wjg.v20.i18.5320.
46. Skladany L, Mesarosova Z, Bachova B et al. Alcohol-related liver disease as a new risk factor for post-transplant diabetes after liver transplantation. Transplant Proc 2019; 51 (10): 3369–3374. doi: 10.1016/j.transproceed.2019.07.021.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2022 Číslo 2- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Cinitaprid – v Česku nová účinná látka nejen pro léčbu dysmotilitní dyspepsie
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Cirrhosis-associated immune dysfunction (CAID) – causes, phenotypes and consequences
- De novo non-alcoholic fatty liver disease after liver transplantation and liver fibrosis diagnosed by magnetic resonance over 2 years
- Liver transplantation for hepatic PEComa mimicking hepatocellular carcinoma
- Hepato-renal manifestation of untreated severe hypothyroidism
- Ketoanalogue of essential amino acids in patients with inflammatory bowel disease (IBD) and chronic kidney disease (CKD) in preparation for kidney transplantation
- Recommended procedure of the Czech Society of Hepatology for the diagnosis and treatment of acute porphyrias – update 2022
- Primary biliary cholangitis (PBC) – Czech Society of Hepatology guidelines for dia gnosis and treatment – update (2022)
- Oral vitamin B12 therapy supplementation in patients with ileo-colonic resection for Crohn’s disease
- Low-volume polyethylene glycol ascorbate solution (PLENVUTM) – a new generation of intestinal cleansers with high efficiency and good tolerance
- Liver function test abnormality in paediatric patients with Covid-19 – a single-centre study in the south of Iran
- Tofacitinib in the treatment of ulcerative colitis – personal experience
- External meeting of the CGS ČLS JEP committee in České Budějovice
- The selection from international journals
- Prim. MUDr. Štefan Šafár (28. 7. 1938 – 19. 3. 2022)
- Life anniversary of the leading Slovak gastroenterologist Mária Zakuciová
- Správná odpověď na kvíz
- Budějovice gastroenterologické 2022 6.–7. dubna 2022
- Kreditovaný autodidaktický test: hepatologie
- Editorial
- Kvíz z klinické praxe
- Risk of liver fibrosis in patients undergoing surgical treatment of hip trauma
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Risk of liver fibrosis in patients undergoing surgical treatment of hip trauma
- Ketoanalogue of essential amino acids in patients with inflammatory bowel disease (IBD) and chronic kidney disease (CKD) in preparation for kidney transplantation
- Liver transplantation for hepatic PEComa mimicking hepatocellular carcinoma
- Oral vitamin B12 therapy supplementation in patients with ileo-colonic resection for Crohn’s disease
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



