-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Biosimilar infliximab in anti-TNF-naïve IBD patients – 1-year clinical follow-up
Biosimilární infliximab v terapii anti-TNF naivních pacientů s IBD – jednoleté klinické sledování
Úvod:
Biosimilární infliximab (IFX) je pro léčbu idiopatických střevních zánětů (IBD) v Evropské unii schválen od září 2013. Schvalovací proces zahrnoval extrapolaci výsledků laboratorních a klinických zkoušek z jiných indikací, zejména revmatoidní artritidy a ankylozující spondylitidy. Ověření předpokládané účinnosti a bezpečnosti biosimilárního IFX u populace pacientů s IBD bylo očekáváno s výsledky z klinické praxe. Doposud jsou však pouze omezené zkušenosti s účinností a bezpečností přípravku z pohledu dlouhodobé udržovací terapie IBD.Metody:
Předmětem analýzy byla data získaná od naivních konsekutivních pacientů trpících Crohnovou chorobou (CD) či ulcerózní kolitidou (UC), kterým byla nově nasazena léčba biosimilárním IFX mezi lednem 2015 a květnem 2016. Na základě klinického stavu, laboratorních výsledků a endoskopického nálezu byli pacienti klasifikováni jako neodpovídající, částečně odpovídající či zcela odpovídající na léčbu. Kromě klinického a endoskopického hodnocení byla měřena hladina C reaktivního proteinu, fekálního kalprotektinu, hodnocen krevní obraz, sérové hladiny IFX a přítomnost protilátek proti IFX. Pravidelně byly zaznamenávány nežádoucí příhody. Analýza byla provedena k 54. týdnu léčby.Výsledky:
Do hodnocení bylo zařazeno 140 pacientů (CD 107; UC 33). Celkově na indukční terapii odpovědělo 94 % pacientů s CD a 82 % s UC. V týdnu 54 byla četnost odpovědi 87 % v případě CD a 60 % v případě UC; 47 %, resp. 36 % pacientů bylo ve stadiu remise. Ke slizničnímu hojení ve 14. týdnu došlo u 52 % pacientů s UC a 96 % pacientů s perianálním postižením zaznamenalo zlepšení nálezu, z toho u 59 % došlo k úplnému zastavení sekrece. Zaznamenán byl zřejmý steroidy šetřící efekt u obou skupin. Ke konci sledování terapie IFX pokračovala u 83 % pacientů (89 % CD, 64 % UC). Farmakokinetické vlastnosti, imunogenicita a četnost a charakter nežádoucích příhod byly plně srovnatelné s originálním přípravkem.Závěr:
Biosimilární IFX se dosud jeví v léčbě IBD jako účinná a bezpečná alternativa, která dosahuje srovnatelného terapeutického efektu bez dodatečných bezpečnostních rizik v porovnání s originálním přípravkem.Klíčová slova:
podobné biologické léčivé přípravky – infliximab – idiopatické střevní záněty
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Doručeno:
23. 10. 2016Přijato:
23. 11. 2016
Authors: M. Kolár 1; D. Ďuricová 1,2
; M. Bortlík 1,3; V. Hrubá 1
; N. Machková 1; K. Mitrová 1,4
; K. Malickova 5; M. Lukas Jr 1; M. Lukas 1,5
Authors place of work: IBD Clinical and Research Centre, ISCARE I. V. F. a. s., Prague, Czech Republic 1; Institute of Pharmacology, 1st Faculty of Medicine, Charles University in Prague and the General University Hospital in Prague, Czech Republic 2; Department of Internal Medicine, Charles University and Military Hospital in Prague, Czech Republic 3; Department of Paediatrics, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic 4; Institute of Medical Biochemistry and Laboratory Diagnostics, 1st Faculty of Medicine, Charles University in Prague and the General University Hospital in Prague, Czech Republic 5
Published in the journal: Gastroent Hepatol 2016; 70(6): 514-522
Category: IBD: původní práce
doi: https://doi.org/10.14735/amgh2016514Summary
Background:
Biosimilar infliximab (IFX) has been approved for the treatment of inflammatory bowel disease (IBD) in the European Union since September 2013. The approval process included extrapolation of clinical data from other indications, namely, rheumatoid arthritis and ankylosing spondylitis. Data from clinical practice are therefore desirable to confirm the efficacy and safety of biosimilar IFX in the IBD population. Evidence of the long-term efficiency and safety of maintenance treatment with biosimilar IFX in patients with IBD is sparse.Methods:
Data from consecutive patients with Crohn’s disease (CD) and ulcerative colitis (UC) who were started on biosimilar IFX between January 2015 and May 2016 at our center were analyzed. Patients were assessed as non-responders, partial responders, or complete responders based on clinical, endoscopic, and laboratory parameters. Besides clinical and endoscopic evaluation, C reactive protein levels, fecal calprotectin levels, blood counts, IFX trough levels, and anti-IFX antibody levels were measured. All adverse events were recorded. Final analysis was performed at week 54.Results:
One hundred and forty IBD patients (CD, 107; UC, 33) were included in the analysis. In total, 94% of CD patients and 82% of UC patients responded to induction therapy with biosimilar IFX. At week 54, the response rates were 87% in CD patients and 60% in UC patients, and 47% and 36% of CD and UC patients, respectively, were in remission. Fifty-two percent of UC patients demonstrated mucosal healing at week 14. Perianal disease improved in 96% of CD patients at week 54, including 59% with complete cessation of drainage. A marked steroid-sparing effect was observed in all patients. Therapy was continued in 83% of patients at the end of the follow-up (CD, 89%; UC, 64%). Pharmacokinetic properties, immunogenicity, and the frequencies and characteristics of adverse events were similar to those previously observed during treatment with the original IFX.Conclusion:
Biosimilar IFX seems to be effective and safe in IBD patients; it achieves similar results as the original preparation with no additional safety issues.Key words:
biosimilar pharmaceuticals – infliximab – inflammatory bowel diseasesIntroduction
Biologic therapy is an effective, but also expensive treatment of different autoimmune diseases, including inflammatory bowel diseases (IBD). Biosimilar is a term referring to another version of an already licensed biologic drug with no meaningful differences from the reference product in terms of quality, physicochemical properties, biologic activity, safety or efficacy. Introduction of the first biosimilar infliximab (IFX) (CT-P13) was aimed at reducing the health care cost burden and to increase the number of treated individuals with similar treatment results. CT-P13 has been given authorisation in the European Union in September 2013 and the Food and Drug Administration authorised the product for use on the US market in April 2016. More recently, in May 2016, the European Medicines Agency (EMA) authorised for use the second biosimilar IFX named Flixabi®.
After thorough initial physicochemical, pharmacodynamic and pharmacokinetic comparative studies, two randomised controlled studies showed that CT-P13 has comparable safety and efficacy to the originator product in patients with ankylosing spondylitis and rheumatoid arthritis [1–3]. Subsequently, these findings led the EMA to extrapolation of the clinical data to other indications of the reference product, including IBD, even though previous studies in this specific patient population were not available. Since then, the use of biosimilar IFX has grown extensively, and several reports have confirmed comparable efficacy and safety with the originator product in anti-TNF-a naïve patients with IBD [4–8].
Even though the information in anti-TNF-a naïve patients with IBD are becoming quite solid, there is still a lack of sufficient data on long-term results in IBD patients on maintenance treatment with biosimilar IFX. Our aim was thus to evaluate the efficiency and safety of biosimilar IFX one year after routine usage in clinical practice in anti-TNF-a naïve IBD patients.
Patients and Methods
Consecutive patients with Crohn’s disease (CD) and ulcerative colitis (UC) naïve to anti-TNF-a in whom IFX therapy was indicated during a period from January 2015 to May 2016 were included.
Patients were followed in regular intervals coincident with infusion applications. At each visit a blood sample was taken for analysis of blood count, biochemistry and IFX pharmacokinetics (trough levels (TL) and anti-drug antibodies (ATI)) and a stool sample was collected for measurement of faecal calprotectin (FC). Intervals between infusions as well as dose were determined by a treating physician according to the patients’ needs. Response of both CD and UC patients naïve to biosimilar IFX was assessed retrospectively at weeks 14 (W14) and 54 (W54) based on clinical, endoscopic and biologic markers. In addition, endoscopic response in UC patients measured by change in Mayo score from baseline was assessed at W14. Adverse events were registered at each visit.
Measurement of pharmacokinetic parameters
IFX TLs and ATIs of biosimilar IFX were measured by quantitative enzyme-linked immunosorbent assay (ELISA) kit SHIKARI Q-Inflixi and Q-ATI, respectively, manufactured by Matriks Biotek, Turkey. The lowest detectable limit of Q-Inflixi kit was 0.03 µg/ mL. The presence of ATIs was determined qualitatively by comparing the share of optical density of a sample at 450 nm and the mean optical density at 450 nm of negative controls with an index of 3. If the share was ≥ 3 the sample was positive for ATIs.
Statistical analysis
Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and calculation of mean and standard deviation and/ or range for continuous variables. The differences of continuous variables between baseline and W54 were analysed using the Mann-Whitney test. Using survival analysis, Kaplan-Meier curve was plotted to depict the proportion of patients remaining on the therapy. The IFX TL cut-off value predicting long-term response to therapy was calculated by receiver operating characteristic (ROC) in conjunction with Youden’s J statistic. Evaluation of categorical variables difference was performed by Fisher’s exact test. The statistical tests were performed using GraphPad Prism (version 6.05). A p-value < 0.05 was considered statistically significant.
Results
IFX treatment was newly initiated in 140 patients, 107 with CD and 33 with UC. Mean disease duration in these patients was 6.2 ± 6.5 years. Concomitant immunosuppression was present in 63 (58.9%) patients with CD and 9 (27.3%) with UC, 31 (29.0%) CD patients and 17 (51.5%) UC patients were on systemic corticotherapy and 18 (16.8%) and 2 (6.1%) patients had topical corticosteroids, respectively. Perianal disease was present at the time of treatment initiation or in disease history in 32 (29.9%) of CD patients and mean Mayo score in UC patients was 2.7. Baseline mean ± SD (range) C reactive protein (CRP) values in naïve patients were 13.3 ± 19.5 mg/ L (0.2–145.9) in CD patients and 9.6 ± 17.1 mg/ L (0.4–81.2) in UC patients. W0 FC levels were 558 ± 357 μg/ g (30–1,000) and 672 ± 425 mg/ L (30–1,000), respectively. The demographic and clinical characteristics of IFX naïve patients are outlined in Tab. 1.
Tab. 1. Demographic and clinical characteristics of patients at week 0. Tab. 1. Demografické a klinické charakteristiky pacientů v týdnu 0. 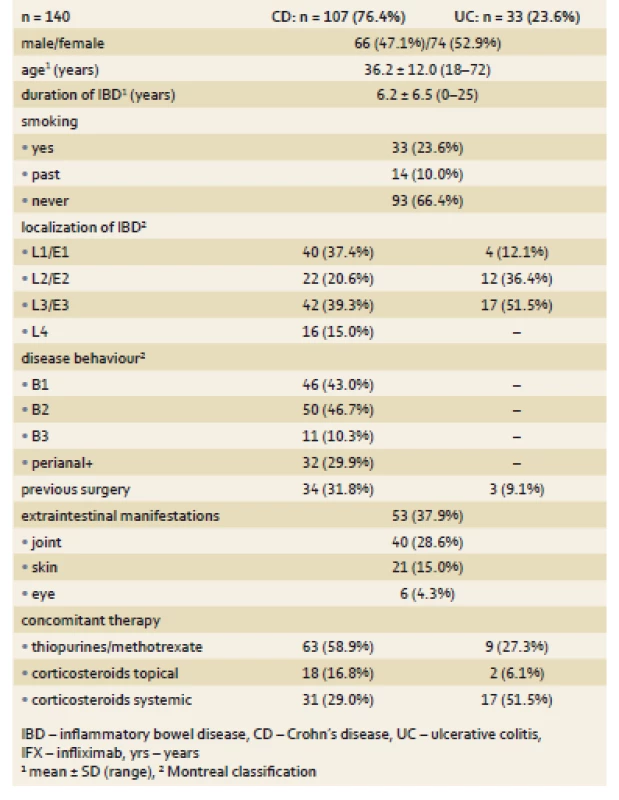
Efficiency
Response of all 140 naïve patients was evaluated at W14. Complete response to therapy was observed in 33 (30.8%) CD and 10 (30.3%) UC patients. Partially responded 67 (62.6%) CD and 17 (51.5%) UC patients, and no response occurred in 7 (6.5%) CD and 6 (18.2%) UC patients. Furthermore, response of 100 patients who reached W54 or who terminated the therapy early was evaluated. Of these patients, 35 (46.7%) CD and nine (36.0%) UC patients completely responded to the therapy. Partial response occurred in 30 (40.0%) CD and 6 (24.0%) UC patients, and no response was observed in 10 (13.3%) and 10 (40.0%) patients, respectively (Graph 1). Mean Mayo score in UC patients dropped from 2.7 at W0 to 1.6 at W16 (p = 0.0002). Mucosal healing (Mayo endoscopic subscore 0 or 1) was achieved in 13 (52.0%) of UC patients with Mayo endoscopic subscore of 3 or 2 at baseline (Graph 2). All 22 patients with active perianal disease were adequately drained and observed by surgeons. Of these patients, the disease was completely resolved in 13 (59.1%) patients by W54, 8 (36.4%) patients experienced improvement of their condition, and 1 (4.5%) patient did not respond to biosimilar IFX treatment, however this patient had to be transferred to adalimumab early after the therapy initiation due to SLE-like symptoms. Comparing W0 and W54, a significant drop in CRP level was observed in CD patients (13.3 ± 19.5 mg/ L vs. 7.1 ± 12.2 mg/ L; p = 0.0010). In UC patients, only numerical decrease was present not reaching statistical significance (9.6 ± 17.1 mg/ L vs. 4.0 ± 4.7 mg/ L) (Graph 3). Similarly, the mean value of FC differed significantly between baseline and the time of evaluation in both CD and UC (558 ± 357 µg/ g vs. 290 ± 288 µg/ g; p = 0.0003 and 672 ± 425 µg/ g vs. 396 ± 392 µg/ g; p = 0.0476) (Graph 4).
Graph 1. Response to therapy with biosimilar IFX at weeks 14 and 54. Graf 1. Odpověď na léčbu biosimilárním IFX v týdnech 14 a 54. 
Graph 2. Change of Mayo endoscopic subscore in patients with UC between weeks 0 and 14. Graf 2. Změna Mayo endoskopického subskóreu pacientů s UC mezi týdny 0 a 14. 
Graph 3. Change of CRP during 54-week treatment with biosimilar IFX. Graf 3. Vývoj hladin CRP během 54týdenní léčby biosimilárním IFX. 
Graph 4. Change of FC during 54-week treatment with biosimilar IFX. Graf 4. Vývoj hladin FC během 54týdenní léčby biosimilárním IFX. 
The proportion of patients on systemic corticotherapy dropped from 29.0% at W0 to 1.6% at W54 in CD patients and from 51.5% to 6.3% in UC patients. Of 16.8% CD and 6.1% UC patients receiving topical corticosteroids at baseline, none had the topical steroids at W54. Concomitant immunosuppression remained stable during the time of observation, changing from 58.9% at W0 to 60.3% at W54 in patients with CD and from 27.3% to 31.3% of patients with UC.
In total, therapy with biosimilar IFX was stopped in 24 (17.1%) patients – 12 (11.2%) CD and 12 (36.4%) UC by the end of the follow-up. Estimated failure-free survival at 54 weeks of therapy is 89.2% for CD and 59.7% for UC (Graph 5). Two CD patients ended treatment due to infusion reaction and five for other adverse events. Three patients with CD stopped anti-TNF after undergoing IC resection and in two patients either follow-up was lost or they voluntarily quitted treatment. Of 12 UC patients, three were primary non-responders and seven patients failed during the maintenance phase. Two patients experienced adverse reactions that led to treatment stop.
Graph 5. Kaplan-Meier survival curves reflecting dropout rates during 54-week treatment. Graf 5. Kaplan-Meierovy křivky přežití znázorňující míry ukončení terapie během 54týdenního období léčby. 
Treatment optimisation
During the follow-up 36 (25.7%) patients in total underwent some treatment intensifying optimisation – dose increase, interval shortening or both. Six (18.2%) UC patients initiated the therapy with increased dose (10 mg/ kg) as a rescue therapy. Of these, two were primary non-responders and one lost response in a maintenance phase. Three patients continued treatment with regular dosing. At W54 17 (27.0%) CD and 9 (14.3%) UC patients had an intensified treatment regimen.
Safety
Three patients in whole cohort experienced infusion reaction. In total, 81 adverse events (AEs) were registered in 72 (51.4%) patients during the follow-up which represents 71.9 AEs per 100 patient-years. The frequency and type of adverse events were similar to those observed during the treatment with original IFX (Tab. 2) [9]. Seven patients in total discontinued biosimilar IFX due to adverse events other than infusion reaction – five patients discontinued therapy due to SLE-like reactions, severe arthralgia or skin rashes, one patient had severe infectious complications with formation of several abscesses, and the last patient suffered from palmoplantar pustulosis.
Tab. 2. Adverse events (AE) occurring during treatment with biosimilar IFX. Tab. 2. Nežádoucí příhody zaznamenané během terapie biosimilárním 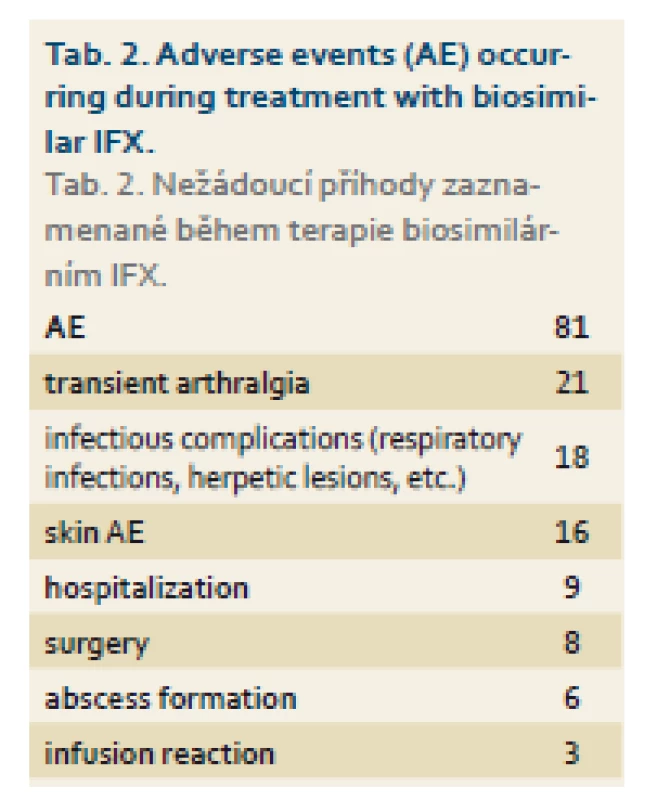
Pharmacokinetics and immunogenicity
Pharmacokinetic properties of biosimilar IFX were comparable to the original preparation. Mean IFX TLs were similar for both diagnoses and for whole cohort at W2, W6, and W14 were 21.9 ± 12.0 μg/ mL, 16.5 ± 12.0 μg/ mL, and 5.4 ± 5.7 μg/ mL, respectively. IFX TL at W54 was 4.4 ± 5.1 μg/ mL (Graph 6). ATI positivity was present in 2.4% of patients at W14 and in 12.1% at W54. During the follow-up, ATI positivity occurred in 26 (18.6%) patients, however in 13 (50%) of these the antibodies were only transient.
Graph 6. Observed mean trough serum concentrations of biosimilar IFX during 54-week treatment. Graf 6. Pozorované průměrné sérové koncentrace biosimilárního IFX během 54týdenního období léčby. 
Infliximab serum concentrations’ predictive role
IFX serum concentrations were available in every patient and the predictive role of early trough levels for long-term response could have thus beenestimated. IFX TLs of W2 were predictive for response in both W14 and W54 in CD. TLs at W6 were predictive for response at W54 for both CD and UC and were thus considered for cut-off value estimation where IFX TL of at least 8.9 μg/ mL at W6 was best predictive of response at W54 with a sensitivity of 70.0% and specificity of 74.1% (Graph 7–9).
Graph 7. Correlation of IFX serum levels and clinical response or mucosal healing at week 14. The box plots show a comparison of median IFX levels at weeks 2 and 6 between the responding and non-responding groups of CD and UC patients. Drug levels were compared using the Mann-Whitney test. Graf 7. Vztah sérových koncentrací IFX a klinické odpovědi nebo slizničního hojení v týdnu 14. Krabicové grafy znázorňují porovnání mediánových hladin IFX v týdnech 2 a 6 mezi skupinami pacientů s CD a UC s odpovědí a bez odpovědi. K porovnání hladin byl užit Mann-Whitneyův test. 
Graph 8. Correlation of IFX serum levels and clinical response at week 54. The box plots show a comparison of median IFX levels at weeks 2, 6 and 14 between the responding and non-responding groups of CD and UC patients. Drug levels were compared using the Mann-Whitney test. Graf 8. Vztah sérových koncentrací IFX a klinické odpovědi v týdnu 54. Krabicové grafy znázorňují porovnání mediánových hladin IFX v týdnech 2, 6 a 14 mezi skupinami pacientů s CD a UC s odpovědí a bez odpovědi. K porovnání hladin byl užit Mann-Whitneyův test. 
Graph 9. The ROC curve for IFX trough levels at week 6 predicting response to therapy at week 54 (AUC 0.75; p = 0.0092). Graf 9. ROC křivka pro sérové hladiny IFX v týdnu 6 ve vztahu k odpovědi na léčbu v týdnu 54 (AUC 0,75; p = 0,0092). 
Discussion
In our observation, the patient cohort included anti-TNF-a naïve patients only. In total, 94% of CD and 82% of UC patients responded to induction therapy (W14) with biosimilar IFX. Reported by Schnitzler et al., the initial response to IFX therapy in CD on the original IFX was 89.1% [10]. Response rates at W8 achieved in 5 mg/ kg groups of UC patients in ACT-1 and ACT-2 studies were 69% and 65%, respectively. [11]. At W54 the response rate in our cohort was 87% in CD and 60% in UC and 47% and 36% of patients were in remission, respectively. Previously in the analysis of Accent I trial response rate to IFX in CD at W54 was 63% and 41% of patients were in remission [12]. ACT-1 clinical response rate after 54 weeks was reported to be 46% and remission was reached by 35% of UC patients [11]. More than half of our UC patients experienced mucosal healing at W14 and improvement of perianal disease occurred in 96% of CD patients at W54, including 59.1% with complete cessation of drainage. In a study by Present et al. complete response of perianal fistulae to induction phase with original IFX occurred in 55% of patients [13]. Clear steroid-sparing effect was observed in all patients when steroids could have been almost completely tapered out during the follow-up period.
The therapy was continued in 83% of patients at the end of the follow-up (89% CD and 64% UC). Our group has previously published results from a cohort on original IFX where sustained response at one year was estimated to be 73%, however failed cases there also included patients with newly started corticosteroids or immunomodulators or their dose increase, as well as patients undergoing surgery [14]. On the other hand, a recently published study by Billiet et al. that includes patients on IFX from a 20-year timespan of clinical experience estimates 1 year failure-free survival of 94% [15].
Type and frequency of adverse events were comparable to original preparation as reported by cohort study with original IFX by Fidder et al. which focused on long-term safety [9].
Pharmacokinetic properties during maintenance phase of treatment remain stable and at W54 the mean IFX TL was 4.4 ± 5.1 μg/ mL, being similar to value observed in our previous cohort of patients on original IFX where median TL measured at W14–W22 was 4.04 μg/ mL. Similarly, cumulative ATI positivity occurred in 26 (18.6%) patients on biosimilar IFX during the follow-up compared to 17% recorded in Bortlik et al. [14].
It has already been reported that higher IFX TLs during induction are in association with long-term clinical response, mucosal healing and perianal fistulae closure [15–19]. We evaluated the predictive role of early TLs on long-term response in our cohort of IBD patients on biosimilar IFX. Trough level of at least 8.9 μg/ mL at W6 was best predictive of response at W54 which corresponds well with the results of previous studies on original IFX. Our findings thus suggest that the same association applies also to the biosimilar IFX.
Efficiency and safety of biosimilar IFX in IBD have already also been presented in previous studies. In a work by Jahnsen et al. from Norway [8], 79% of CD and 56% of UC patients achieved remission at W14. No unexpected adverse events were reported during the study. In a Hungarian multicentric nationwide study [5], efficiency in the induction of clinical remission and response in both CD and UC was described at W14 and W30. This study also included patients with previous IFX exposure in whom decreased response rates and higher probability of developing allergic reactions were observed. The biosimilar IFX has also been shown to be efficient in mucosal healing that was achieved in two-thirds of UC patients by the end of the induction period [6].
Despite similar clinical efficiency and safety of biosimilar and original IFX, even small differences in molecular structure might be responsible for different immunogenicity. Recent works have supported the immunogenic similarity between original and biosimilar IFX in IBD by proving that antibodies to original IFX similarly recognised and cross-reacted with the biosimilar IFX [20,21]. The usage of SHIKARI Q-Inflixi assay for the detection of biosimilar IFX concentration has been previously backed up by a study evaluating technical performance and confirming robustness and suitability of the assay for detection of biosimilar IFX serum levels [22].
According to our results and reports currently available, there is no negative or harmful signal about biosimilar IFX in anti-TNF-a naïve patients and its efficiency and safety seems to be comparable to the original preparation. The most important benefit from the introduction of biosimilars is a reduction of therapy costs. This is very important mainly in patients suffering from chronic diseases such as IBD who require long-standing treatment. An economic model for the introduction of biosimilar IFX in five West European countries showed potential considerable cost-saving effects even within one year of therapy [23]. Similarly, a 3-year budget impact analysis in countries from Central and Eastern Europe reported substantial cost savings by using biosimilar IFX [24]. This decrease in price might increase accessibility of the therapy for more patients.
Our observation has several limitations. Most importantly, we were not able to include a control group of naïve patients starting on the original product in order to perform a direct comparison between originator and biosimilar IFX in a randomised set of patients. Also, the number of patients enrolled into our cohorts is limited, especially those with UC.
In conclusion, biosimilar IFX in IBD patients seems to be effective and safe, gaining similar treatment results with no additional safety issues in comparison to the original preparation. However, there is a need for further mainly randomised controlled studies with larger patient populations and longer follow-up periods.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 23. 10. 2016
Accepted: 23. 11. 2016
Prof. Milan Lukas, MD, PhD
IBD Clinical and Research Centre, ISCARE I.V.F. a.s.
Jankovcova 1569/ 2c
170 04 Prague 7
Czech Republic
milan.lukas@email.cz
Zdroje
1. Park W, Yoo DH, Jaworski J et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther 2016; 18 : 25. doi: 10.1186/ s13075-016-0930-4.
2. Yoo DH, Racewicz A, Brzezicki J et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther 2016; 18 : 82. doi: 10.1186/ s13075-016-0981-6.
3. Choe JY, Prodanovic N, Niebrzydowski Jet al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2015. In press. doi: 10.1136/ annrheumdis-2015-207764.
4. Keil R, Wasserbauer M, Zádorová Z et al. Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn’s dis-ease and ulcerative colitis. Scand J Gastroenterol 2016; 51(9): 1062–1068. doi: 10.3109/ 00365521.2016.1149883.
5. Gecse KB, Lovász BD, Farkas K et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis 2016; 10(2): 133–140. doi: 10.1093/ ecco-jcc/ jjv220.
6. Farkas K, Rutka M, Golovics PA et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis 2016; 10(11): 1273–1278.
7. Jung YS, Park DI, Kim YH et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol 2015; 30(12): 1705–1712. doi: 10.1111/ jgh.12997.
8. Jahnsen J, Detlie TE, Vatn S et al. Biosimilar infliximab (CT-P13) in the treatment of inflammatory bowel disease: a Norwegian observational study. Expert Rev Gastroenterol Hepatol 2015; 9 (Suppl 1): 45–52. doi: 10.1586/ 17474124.2015.1091308.
9. Fidder H, Schnitzler F, Ferrante M et al.Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut 2009; 58(4): 501–508. doi: 10.1136/ gut. 2008.163642.
10. Schnitzler F, Fidder H, Ferrante M et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut 2009; 58(4): 492–500. doi: 10.1136/ gut.2008.155812. Epub 2008 Oct 2.
11. Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353(23): 2462–2476.
12. Rutgeerts P, Feagan BG, Lichtenstein GR et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004; 126(2): 402–413.
13. Present DH, Rutgeerts P, Targan S et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999; 340(18): 1398–1405.
14. Bortlik M, Duricova D, Malickova K et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis 2013; 7(9): 736–743. doi: 10.1016/ j.crohns.2012.10.019.
15. Billiet T, Cleynen I, Ballet V et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther 2016; 44(7): 673–683. doi: 10.1111/ apt.13754.
16. Papamichael K, Van Stappen T, Vande Casteele N et al. Infliximab Concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2016; 14(4): 543–549. doi: 10.1016/ j.cgh.2015.11.014.
17. Ungar B, Levy I, Yavne Y et al. Optimizing anti-TNF-a therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2016; 14(4): 550–557. doi: 10.1016/ j.cgh.2015.10.025.
18. Davidov Y, Ungar B, Bar-Yoseph H et al. Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis 2016. In press.
19. Yarur AJ, Jain A, Sussman DA et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2016; 65(2): 249–255. doi: 10.1136/ gutjnl-2014-308099.
20. Ben-Horin S, Yavzori M, Benhar I et al. Cross-immunogenicity: antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut 2016; 65 : 1132–1138. doi: 10.1136/ gutjnl-2015-309290.
21. Gils A, Van Stappen T, Dreesen E et al. Harmonization of infliximab and anti-infliximab assays facilitates the comparison between originators and biosimilars in clinical samples. Inflamm Bowel Dis 2016; 22 : 969–975. doi: 10.1097/ MIB.0000000000000709.
22. Malíčková K, Ďuricová D, Bortlík M et al. Serum trough infliximab levels: a comparison of three different immunoassays for the monitoring of CT-P13 (infliximab) treatment in patients with inflammatory bowel disease. Biologicals 2016; 44(1): 33–36. doi: 10.1016/ j.biologicals.2015.09.005.
23. Jha A, Upton A, Dunlop WC et al. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther 2015; 32(8): 742–756. doi: 10.1007/ s12325-015-0233-1.
24. Brodszky V, Rencz F, Péntek M et al. A budget impact model for biosimilar infliximab in Crohn’s disease in Bulgaria, the Czech Republic, Hungary, Poland, Romania, and Slovakia. Expert Rev Pharmacoecon Outcomes Res 2016; 16(1): 119–125. doi: 10.1586/ 14737167.2015.1067142.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2016 Číslo 6- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
- Pediatric gastroenterology and hepatology
- Bariatrics
- Jaká je Vaše diagnóza?
- Some genetic determinants of celiac disease, the role of HLA haplotyping in clinical settings and HLA-DQ haplotypes in a group of 306 pediatric patients
- Importance of faecal calprotectin in screening and clinical assessment of adult and pediatric patients with inflammatory bowel diseases
- Recent bariatric-metabolic surgery
- The duodenal-jejunal bypass liner (EndoBarrier®) for the treatment of type 2 diabetes mellitus in obese patients – efficacy and factors predicting optimal effects
- Results of interferon-free hepatitis C therapy in the Czech Republic in real-life
- Budesonide MMX (Cortiment® 9 mg) in the treatment of ulcerative colitis in real clinical practice
- Biosimilar infliximab in anti-TNF-naïve IBD patients – 1-year clinical follow-up
- Czech Society of Gastroenterology guidelines for diagnostic and therapeutic colonoscopy
- Echoes from Vienna
- 1st National Congress of Gastrointestinal Oncologywith international participation
- The selection from international journals
-
Správná odpověď na kvíz
Diferenciální diagnóza – Crohnova choroba nebo hypersekreční stav podmíněný hypergastrinemií? - Kreditovaný autodidaktický test: dětská gastroenterologie a hepatologie + bariatrie
- Combination of sofosbuvir and velpatasvir (Epclusa®)
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Importance of faecal calprotectin in screening and clinical assessment of adult and pediatric patients with inflammatory bowel diseases
- Budesonide MMX (Cortiment® 9 mg) in the treatment of ulcerative colitis in real clinical practice
- Czech Society of Gastroenterology guidelines for diagnostic and therapeutic colonoscopy
- The duodenal-jejunal bypass liner (EndoBarrier®) for the treatment of type 2 diabetes mellitus in obese patients – efficacy and factors predicting optimal effects
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



