-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The development of a dental drug in the form of medicated chewing gum
Authors: Oleksii Yakovenko; Julia Masliy
Authors place of work: National university of Pharmacy, Kharkiv, Ukraine
Published in the journal: Čes. slov. Farm., 2015; 64, 303-304
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Every year the number of people with diseases of the mouth cavity is growing rapidly. Therefore, the necessity for dosage forms which would effectively contribute to the treatment of these diseases is an urgent problem today. The medicated chewing gum (MCG), which carries out the role of oral drug delivery, is the optimal formulation for the prevention and treatment of dental diseases due to its advantages – fast local effect, no need to swallow, the possibility of applying in any convenient place for the patient, as it does not need water, reduced the first-pass metabolism, as well as a better perception of the patient and a more pleasant mode of administration. Besides that, MCGs increase of secretion of saliva, which helps digestion, cleaning and remineralization of teeth, effective in the prevention and treatment of xerostomia, the process of mastication has a massaging effect on the gingiva, which leads to greater blood flow and microcirculation of periodontal tissues. Moreover, properly selected active pharmaceutical ingredients (APIs) in the composition of the dosage form provide the appropriate comprehensive treatment and preventive effect on hard tissues of teeth, periodontal tissues and mucous. That is why the aim of our work was to develop a dental drug of comprehensive action in the form of the medicated chewing gum.
Experimental methods
The objects of research were a mixture of proteolytic enzymes, the composition of Health in gum (HIG)-01 and the auxiliary substances that are necessary for the development of the drug in the form of medicated chewing gums. The investigations of technological parameters developed composition and MCG; fluidity, angle of repose, abrasion and crushing resistance were carried out.
Results and discussion
Taking into account the etiology and pathogenesis of dental diseases, as active pharmaceutical ingredients we chose the proteolytic enzymes of animal and plant origin – lysozyme and papain that are used in dental practice widely and effectively because of antimicrobial, anti-inflammatory, immunostimulating, whitening, anti-viral and reparative properties. A wide range of pharmacological properties of enzymes explains their complex action on the dental hard and periodontal tissues.
For our research as the base for the chewing gum the composition HIG-01 (Spain, Cafosa) was taken, which allows to receive MCGs by the method of direct compression. Chemically, composition of Health in gum is a mixture of polyols with gumbase (elastomers), plasticizers and anticaking agents.
In order to select a method of administration of APIs in the base and a method for obtaining MCGs, we have studied pharmaco-technological parameters of lysozyme, papain and their mixture, the results of which are presented in Table 1.
Tab. 1. Pharmaco-technological properties of APIs and their mixtures 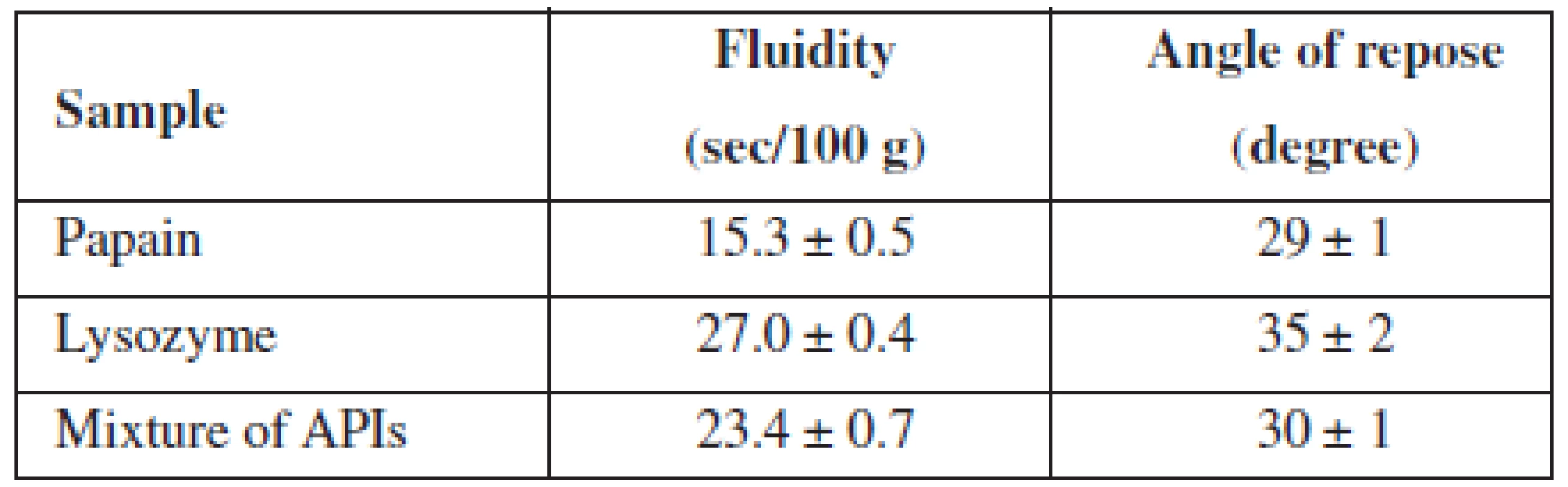
n = 5, P = 95% The data from Table 1 indicated that all test samples have satisfactory fluidity.
For an improvement of this indicator, we investigated the influence of the lubricant on the fluidity of APIs. With this aim, we introduced Aerosil, which is also linked to its ability to absorb moisture (because into the composition a flavouring «Melon» in the liquid form is introduced) and added it in various concentrations. The data are represented in Table 2.
Tab. 2. Influence of Aerosil amount on the fluidity of APIs 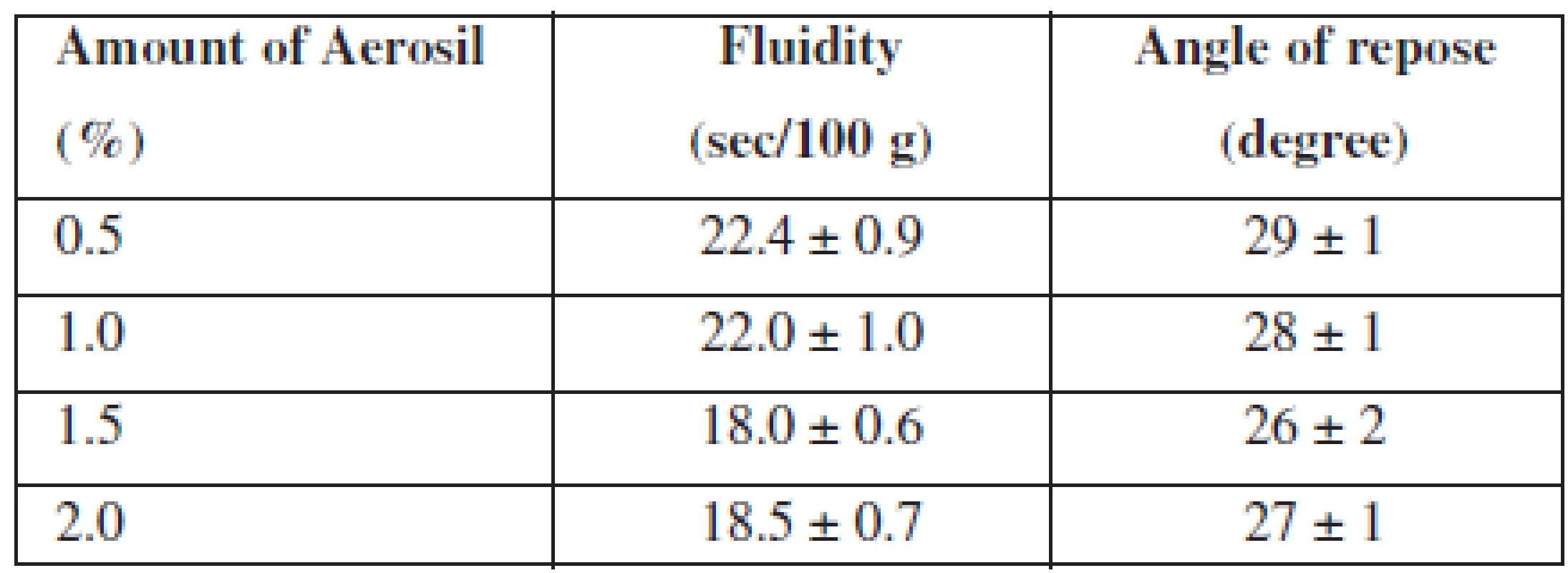
n = 5, P = 95% According to the results, we found that all samples have good fluidity and a satisfactory angle of repose. As we can see from the results, the optimal amount of Aerosil in composition of MCG is 1.5%.
As the glidant, preventing sticking mass to a press tool, we have taken calcium stearate in an amount of 1%. As an intense sweetener and at the same time a filler we have chosen sorbitol – sugar substitute, which has pleasant sweet taste, freshness and anticaries properties. As the flavouring agent, «Melon».
We also investigated the fluidity of the developed tabletting mass in comparison with the composition Health in gum. The results of these tests are given in Table 3.
Tab. 3. Pharmaco-technological properties of HIG and tabletting mass 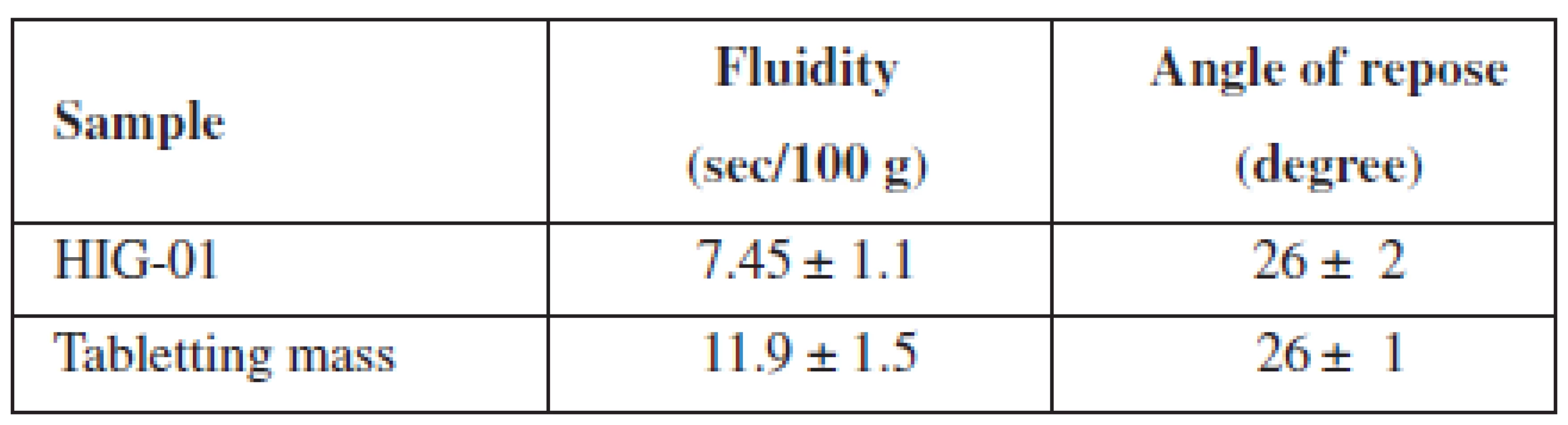
n = 5, P = 95% After obtaining chewing gum on a conventional tabletting machine, we investigated crushing and abrasion resistance of MCGs, the results of which are shown in Table 4.
Tab. 4. Crushing and abrasion resistance of MCGs 
As we can see from the results, the developed MCGs comply with the requirements of SPhU for these indicators.
Conclusions
All things considered, we have developed a composition and proved the technology of obtaining MCGs with proteolytic enzymes for use in dental practice. Using the composition Health in gum will provide easy and quick production of medicated chewing gums by the method of direct compression without the purchase and installation of a complex technological equipment, which, in turn, will make this dosage form more perspective for implementation into industrial production in Ukraine.
The future aim of our work will be continuing research of a drug in the form of MCG, which we developed.
Conflicts of interest: none.
dr. Oleksii Yakovenko
National University of Pharmacy
pr. Traktorostroitelei 85V, kv. 101, 66123, Kharkiv, Ukraine
e-mail: realmanutd.ua@gmail.com
Zdroje
1. General Monograph on Dosage Forms. Chewing gums, Medicated. Thr State Pharmacopoeia of Ukraine, 2nd ed.; Kh.: The Pharmacopoieal Center of Ukraine 2008.
2. Belmar J., Ribé M. Eye on excipients. Health in Gum by Cafosa. Barcelona, Spain 2013.
3. Basani G., Venkata R. D., Madhusudan R. Y. Medicated chewing gum – a novel approach to improve patient compliance. International Journal of Research in Pharmaceutical and Biomedical Sciences 2011; 2(1), 23–32.
4. General Monograph on Dosage Forms. Chewing gums, Medicated. In European Pharmacopoeia, 6th ed.; European Directorate for the Quality of Medicines, Council of Europe: Strasbourg, France 2008.
5. Kornev M. Medicated chewing gum: for and against. Chemistry and chemists 2010; 3, 65–71.
6. Naik Heema, Gupta Stuti. Medicated chewing gums – updated review. International Journal of Pharmaceutical Research and Development 2010; 2(11), 66–76.
7. Shivang A. Chaudhary, Aliasgar F. Shahiwala. Directly compressible medicated chewing gum formulation for quick relief from common cold http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3555007/
8. Health in Gum® .
http://www.cafosa.com/EN_Health_in_Gum
9. Roser Amposta. Medicated chewing gum.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Antibakteriální účinky přírodních látek – silice
- Organická syntéza, Laboratórny manuál
- Cholinergický systém srdca
- Proběhl 42. mezinárodní kongres k dějinám farmacie
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Sympozia Sekce dějin farmacie ČFS v roce 2015
- Autorský rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antibakteriální účinky přírodních látek – silice
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Cholinergický systém srdca
- Organická syntéza, Laboratórny manuál
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



