-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
Authors: Lukáš Stanzel 1; Matej Maruniak 1; Ivan Malík 1; Ľubica Havranová Sichrovská 1; Iva Kapustíková 1; Eva Sedlárová 1; Jozef Csöllei 2
Authors place of work: Department of Pharmaceutical Chemistry, Faculty of Pharmacy Comenius University, Bratislava, Slovak Republic 1; Department of Chemical Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic 2
Published in the journal: Čes. slov. Farm., 2015; 64, 291-293
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Reactive oxygen species are formed during many metabolic reactions–metabolism of nutrients, biosynthesis of certain substances, electron transport in the mitochondria, for instance phagocytosis and inflammatory processes, trauma and physical exercise. To get rid of reactive oxygen species, the human body uses various antioxidant systems. Antioxidants used1) are hydrophilic, i. e., water soluble, which are greater in the extracellular fluid (vitamin C, uric acid, selenium, bioflavonoids, and i.) and the lipophilic ones, i. e., fat-soluble, which act mainly on the membranes within cells (vitamin E, beta-carotene, ubiquinone Q10, etc.)1). Antioxidant activity is defined as the ability of a particular compound or a mixture of compounds to inhibit oxidative degradation. One should distinguish two concepts, namely antioxidant capacity and activity. Antioxidant capacity provides information on the duration of the antioxidant effect, whereas the activity is characterized by an initial process dynamics during the antioxidant at a specific concentration of an antioxidant2).
The β-adrenergic receptors antagonists (β-ARAs) have been primarily used as drugs in the treatment of cardiovascular diseases such as ischaemic heart disease, angina pectoris, arrhythmia, hypertension, cardiomyopathy, or as prevention after myocardial infarction3). In addition, more recently they have been accepted in the heart failure therapy. Part of the beneficial cardiovascular effects shown by such a group of compounds has already been associated with the antioxidant properties that some of them have possessed – carvedilol, atenolol, carteolol ormetipranolol4).
Experimental methods
Compounds under the study:
Alkylesters of 4{2-hydroxy-3-[(4-pyridine-2-yl)piperazine-yl]-propoxy}phenylcarbamic acid monochloride.
ABTS method
Chemicals: Potassium dihydrogen phosphate (CentralChem, SR), potassium persulfate (Sigma-Aldrich, USA), 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), (ABTS, Sigma-Aldrich, USA), ethanol (CentralChem, SR), distilled water. Method used: Into a 10 ml volumetric flask an amount of 0.038 g of ABTS radical was added and was made up with distilled water. Into another 10 ml flask, 0.007 g of potassium dihydrogen phosphate and water were added as well. Prepared solutions were mixed in a ratio of 1 : 1 to give a blue-green colour, and left to stand for 24 h in the dark at room temperature. After 24 h, a volume of 1.1 ml of concentrated radical was transferred to a 50 ml volumetric flask and ethanol was added up to the mark. To the volume of 2 ml of ABTS solution, 100 μl of the sample (c = 10–4 mol/l) was added. After 5 minutes, the absorbance of each solution was measured at λ = 734 nm. As the reference solution, a reaction system without the sample was used. Ascorbic acid was applied as the control solution5).
Evaluation of results: Presence of active substances with antioxidant agent led to discolouring of the solution, which was measured spectrophotometrically.Antioxidant activity (%) = 100 × (Ap – A)/Ap, where Ap is the absorbance of the reference solution, A the absorbance of the samples DPPH method.
Chemicals: 1,1-Diphenyl-2 - (2,4,6-trinitrophenyl) hydrazyl (DPPH, Sigma-Aldrich, USA), methanol (CentralChem, SR).
Method used: DPPH (m = 1 mg) was dissolved in methanol and the solvent was made up to a 50 ml volumetric flask. To the 200 μl of evaluated compounds (c = 10–4 mol/l) was added 1.8 ml of DPPH radical. The activity of the samples was evaluated after 30 min by measuring the absorbance of each solution at λ = 517 nm. As referenced solution was considered reaction system without the sample. Ascorbic acid was applied as a control solution5).
Evaluation of results: After DPPH radical solution reduction, the colour changed from the original purple to yellow. The colour change was measured spectrophotometrically according to the equation given bellow.
Antioxidant activity (%) = 100 ⋅ (Ap – A)/Ap, where Ap is the absorbance of the reference solution, A the absorbance of the samples.
FRAP method
Chemicals: Sodium acetate (CentralChem, SR), 35% solution of hydrochloric acid (CentralChem, SR), ferric chloride (CentralChem, SR), 2,4,6-tris-(2-pyridyl)-s-triazine (TPTZ, Sigma-Aldrich, USA).
Used method: Acetate buffer was prepared by adding 3.1 g of sodium acetate and 16 ml of concentrated acetic acid to a 1000 ml volumetric flask and made up with distilled water. TPTZ (m = 0.1562 g) and 0.177 ml of a 35% HCl solution were made up with distilled water into a 50 ml volumetric flask. FeCl3 (m = 0.2703 g) was dissolved in a 50 ml volumetric flask and made up to the mark with distilled water. FRAP solution was made by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ and 2.5 ml of FeCl3 solution. To 100 μl of a compound solution (c = 10–4 mol/l) 3 ml of FRAP solution were added. Absorbance was measured after 5 min at λ = 593 nm, using an ascorbic acid solution as the control solution5). Evaluation of results: The results were evaluated as the analogical amount of ascorbic acid (μg/ml) = A/0,134, where A is the absorbance of compounds.
Results and discusion
In the area of chemical analysis and biological evaluation of drugs, many methods have been developed to determine the total antioxidant activity of the sample (Total Antioxidant Activity – TAA). The purpose of these methods is to characterize compounds in such conditions close to the physiological environment of the antioxidant7).There is an effort to interpret antioxidant activity of the sample in a way that would be methodical, material and instrumental accessible and usable for numerous serial analyses. Their results should correspond to the biological value of the test substance. Chemical methods (TEAC, FRAP, DPPH, ABTS, ORAC or DCI) are based on the use of agents providing the oxygen free radical colour products which prevent the formation of antioxidants contained in the sample8).
The capability of inspected compounds to act as potential antoxidants was evaluated following the given methods (DPPH, ABTS and FRAP), respectively. The results obtained could be influenced by some physico-chemical aspects: lipohydrophilic properties of the molecules under the study, by isosteric replacement of ether bond for carbamoyloxy moiety, and by electrostatic and steric effects of pyridin-2-yl fragment9) (Table 1).
Tab. 1. The potential of the compounds 4a<sub>1</sub>–4d<sub>1</sub> to reduce the DPPH, ABTS and FRAP radicals 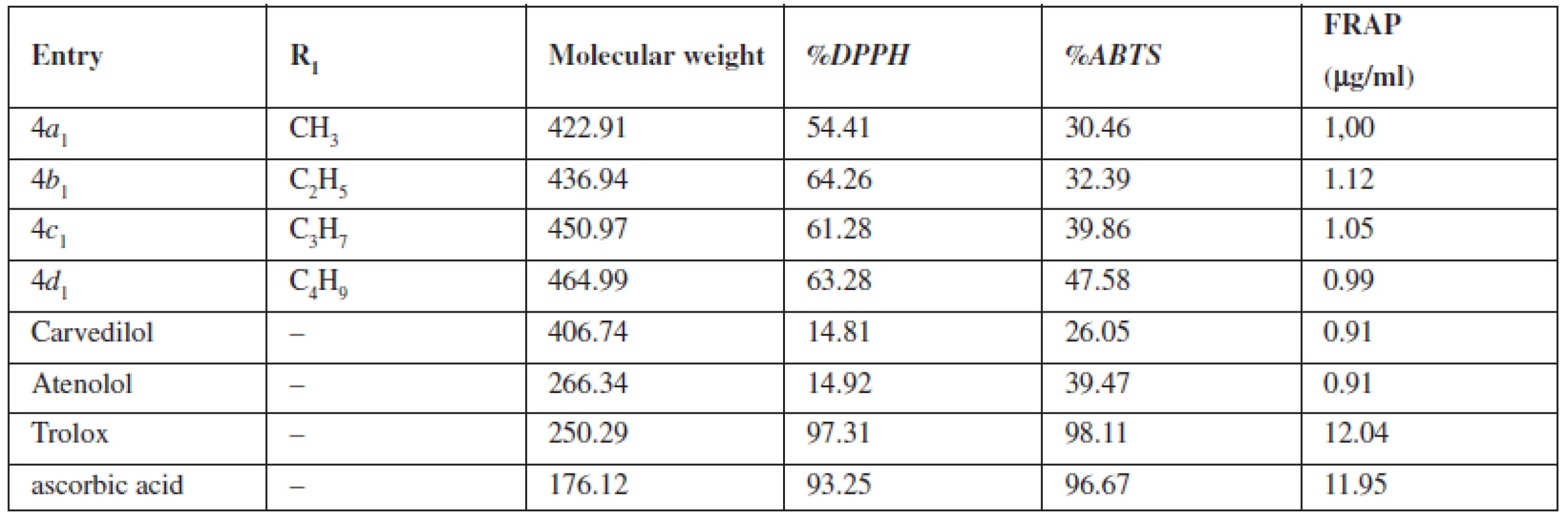
In terms of the chemical structures of tested compounds 4a1–4d1, the elongation of the alkyl side chain has not led to more promising antioxidative substances in all performed methods. Following the DPPH method in the compound 4b1 could be regarded as the most potent substance. On the other hand, results of ABTS testing have shown a connection between elongation of the alkyl side chain and activities of analyzed substances. The highest potential has been estimated for compound 4d1. Antioxidant properties of aryloxyaminopropanols 4a1, 4b1, 4c1, 4d1 determined by the FRAP method have not characterized their antioxidant activity because the method only describes the reduction potential of antioxidants and has not defined the ability to scavenge free radicals. Therefore the antioxidant activity of the inspected substances by the DPPH and ABTS method could not be compared to the antioxidant activity of the FRAP method. Nevertheless, any connection between the length of the alkyl side chain and the activity has not been revealed. The highest activity has been shown for the molecule 4c1, the lowest one for compound 4a1. For more convenient interpretation of the results, the outgoings of the analyzed substance have been compared to those of β-blockers in clinical use (carvedilol, atenolol) with antioxidative features and two strong antioxidants (Trolox, ascorbic acid).
It has been revealed that the antioxidant activity of atenolol and carvedilol determined by the DPPH method was notably lower than that of any other substance under study. Results from the ABTS study have pointed a similar antioxidant ability of carvedilol as compared to tested compounds. Atenol has reached the capability similar to the lowest active substance 4a1 only. Results from the FRAP determination have shown a lower redox potential of both analyzed β-blockers. On the other hand, trolox and ascorbic acid have shown a strong antioxidative potential in all performed methods.
Conclusion
Considering the important role of antioxidants in biological systems, series of original β-adrenergic receptor antagonists have been evaluated in vitro for their ability to reduce the DPPH, ABTS and FRAP radicals as well. The results might contribute to their cardiovascular therapeutic benefits.
Focusing on performed in vitro experiments, it has been suggested that a presence of the carbamoyloxy moiety could be regarded as favourable structural modification. However, relatively high lipophilicity of all the compounds under study might not be considered an explicit guarantee of their antioxidant potential. No significant relationships between elongation of the alkyl side chain and antioxidant activity have been revealed.
Potential therapeutical benefits based on current in vitro assays results should be the motivation for a closer study of other in vitro or in vivo systems and processes, in search of new cardiovascular therapeutics.
This work was supported by the Grant Projects: FaF UK/29/2015, UK/346/2015, FaF UK/28/2015, FaF UK/44/2015, FaF UK/63/2015; Comenius University in Bratislava Science Park supported by the Research and Development Operational Programme funded by the ERDF – Grant number: ITMS 26240220086.
Conflicts of interest: none.
PharmDr. Lukáš Stanzel
Department of Pharmaceutical Chemistry
Faculty of Pharmacy Comenius University
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: stanzel1@uniba.sk
Zdroje
1. Čepička J., Karabin M. Polyfenolové látky piva - přirozené antioxidanty (In Czech). Chem. Listy 2002; 96, 90–95.
2. Karabin M., Dostálek P., Hofta P. Přehled metod pro stanovení antioxidačnej aktivity v pivovarství (In Czech). Chem. Listy 2006; 100, 184–189.
3. Prichard B. N. C., Graham B., Cruickshank J. M. Beta-blockers in the third millennium – when are they really indicated. J. Clin. Basic Cardiol. 2001; 4, 3–9.
4. Gomes A., Costa D., Lima J. L. F. C., Fernandes E. Antioxidant activity of β-blockers: An effect mediated by scavenging reactive oxygen and nitrogen species. Bioorg. Med. Chem. 2006; 14, 4568–4577.
5. Kurin E., Mučaji P., Nagy P. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An Interaction Study. Molecules. 2012; 17, 14336–14348.
6. Šulc M., Lachmann J., Hamouz K., Orsák M., Dvořák P., Horáčková V. Selection and evaluation of methods for determination of antioxidant activity of purple and red-fleshed potato varieties. Chem. Listy 2007; 101, 584–591.
7. Vančo J., Švajlenová O., Račanská E., Muselík J.,Valentová J. Antiradical activity of different copper (II) Schiff base complexes and their effect on alloxaninduced diabetes. J. Trace Elem. Med. Biol. 2004; 18, 155–161.
8. Aruoma O. I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998; 75, 199–212.
9. Malík I., Muselík E., Sedlárová E., Csöllei J., Stanzel L. In vitro antioxidant properties of basic ortho-/meta-alkoxyphenylcarbamic acid esters bearing 4-(4-fluoro-/3-trifluoromethylphenyl)piperazin-1-yl fragment. Fresen. Environ. Bull. 2013; 22, 2689–2694.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Antibakteriální účinky přírodních látek – silice
- Organická syntéza, Laboratórny manuál
- Cholinergický systém srdca
- Proběhl 42. mezinárodní kongres k dějinám farmacie
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Sympozia Sekce dějin farmacie ČFS v roce 2015
- Autorský rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antibakteriální účinky přírodních látek – silice
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Cholinergický systém srdca
- Organická syntéza, Laboratórny manuál
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



