-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Synthesis and biological activity of selected cinnamic acid derivatives
Authors: Martin Gazvoda; Slovenko Polanc
Authors place of work: Faculty of Chemistry and Chemical Technology, University of Ljubljana, Slovenia
Published in the journal: Čes. slov. Farm., 2015; 64, 294-295
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
The enzymes AKR1C1 AKR1C4, members of the aldo-keto reductase superfamily, catalyse the interconversions of 3-, 17 - and 20-ketosteroids with the corresponding 3α/β-, 17β-, 20α-hydroxysteroids to varying extents, using NADPH as a cofactor1). In this way they can control the ligand occupancy and trans-activation of androgen, estrogen and progesterone receptors by modulating the concentrations of the active steroids. The AKR1C enzymes are also involved in the prostaglandin and neurosteroid production and inactivation, and in the metabolism of xenobiotics. Among them, AKR1C3 preferentially acts as a 17β-ketosteroid reductase and converts a weak androgen 4-androstene-3,17-dione to a potent androgen testosterone, and estrone to a potent estrogen 17β-estradiol. AKR1C3 also catalyses the reduction of prostaglandin H2 (PGH2) into PGF2α, and PGD2 into 11β-PGF2, thereby diverting the biosynthesis of prostanoids away from the antiproliferative J-series. Catalysing these reactions, AKR1C3 represents an important target enzyme for the development of potential drugs for a treatment of the hormone dependent and hormone independent forms of cancer.
Experimental methods
General procedure for the synthesis of acids: Potassium acetate (982 mg, 10 mmol) or triethylamine (1.5 mL, 10.75 mmol) was added to a stirred mixture of the appropriate arylacetic acid (7.5 mmol), aromatic aldehyde (7.5 mmol) and acetic anhydride (3 mL, 32 mmol). The reaction mixture was stirred at 100–140 °C for 3.5‒–70 h. Then, it was cooled to rt, water was added (40 mL) and the mixture was treated with a solution of 10 M NaOH until pH 12 was reached, followed by conc. HCl (to pH 1). The resulting mixture was extracted with dichloromethane (3 . 80 mL). Organic phases were extracted with 2 M NaOH (3 . 80 mL) and water (80 mL). The combined aqueous solutions were washed with dichloromethane (100 mL) to remove the impurities and traces of starting materials, then acidified with conc. HCl (until pH was 1), and finally extracted with dichloromethane (4 . 50 mL). The organic phase was washed with brine (100 mL), dried over anhydrous sodium sulphate, evaporated to dryness and the residue was crystallized from a suitable solvent.
Cinnamic acids were assayed for their inhibition of the enzymes AKR1C1‒AKR1C3 at the Institute of Biochemistry, Faculty of Medicine, University of Ljubljana.
Results and discussion
Recently, α-methylcinnamic acid was reported as a promising inhibitor of AKR1C3 enzyme3). This prompted us to design the synthesis of a series of cinnamic acids with bulky aryl substituents on the α-position (α-arylcinnamic acids) (Fig. 1) and to evaluate their AKR1C1‒AKR1C3 inhibitory activities4).
Fig. 1. Design of α-arylcinnamic acids 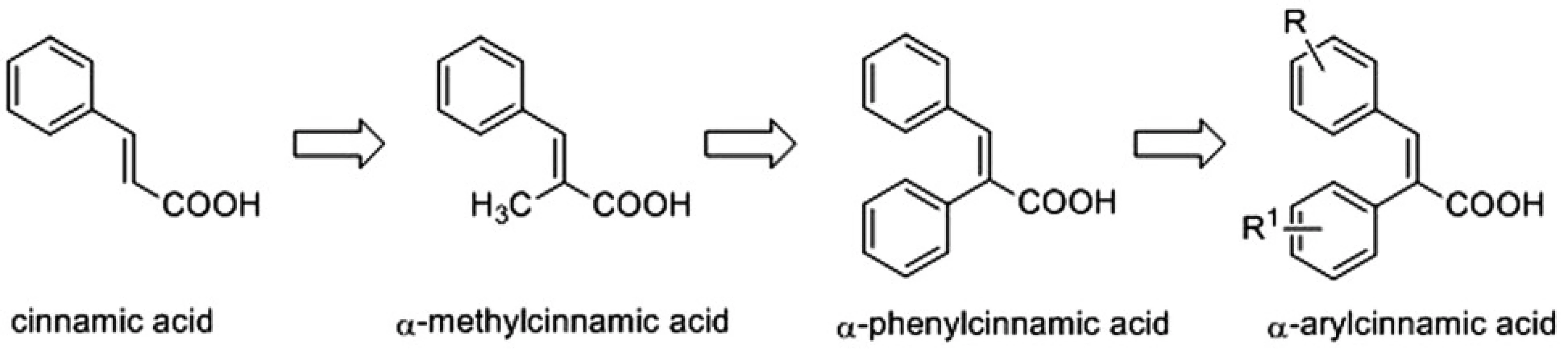
Acids were available with the Perkin reaction between substituted benzaldehydes and properly functionalized arylacetic acids in acetic anhydride using either potassium acetate or triethylamine as a base that led to the desired α-arylcinnamic acids (2,3-diarylpropenoic acids) as the final products (Fig. 2)4).
Fig. 2. Synthesis of α-arylcinnamic acids 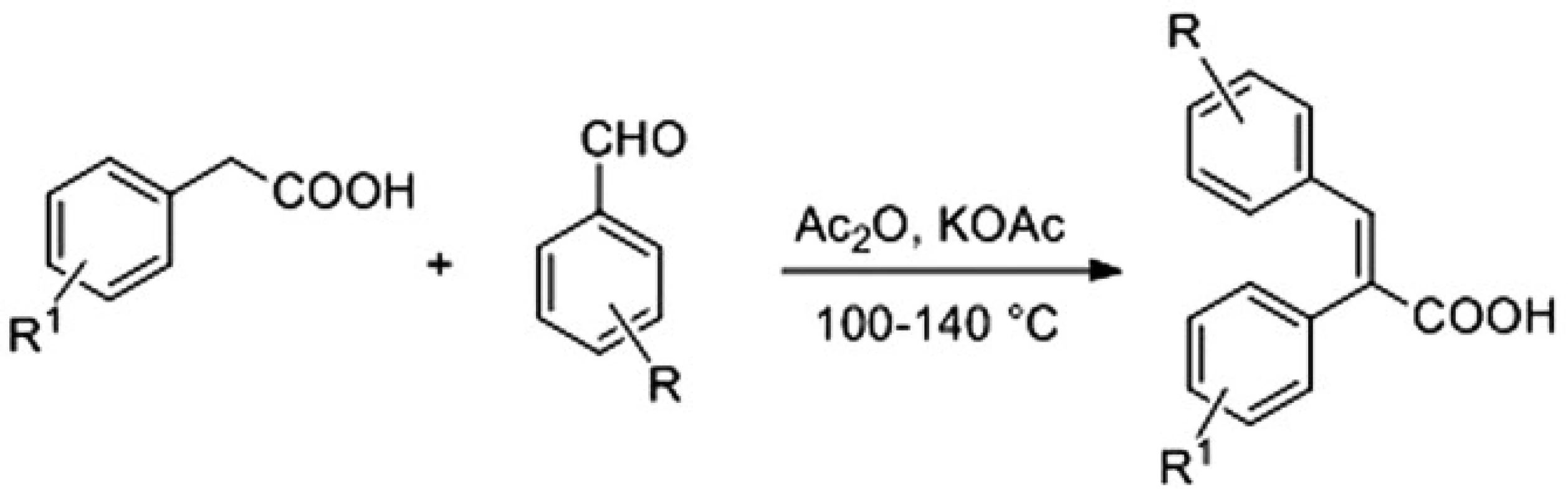
Following the above procedure, 42 acids were synthesized, and their activities against AKR1C enzymes were evaluated, for details see4). Some of the most active and selective AKR1C3 inhibitors are presented on Figure 3.
Fig. 3. Most active and selective AKR1C3 inhibitor 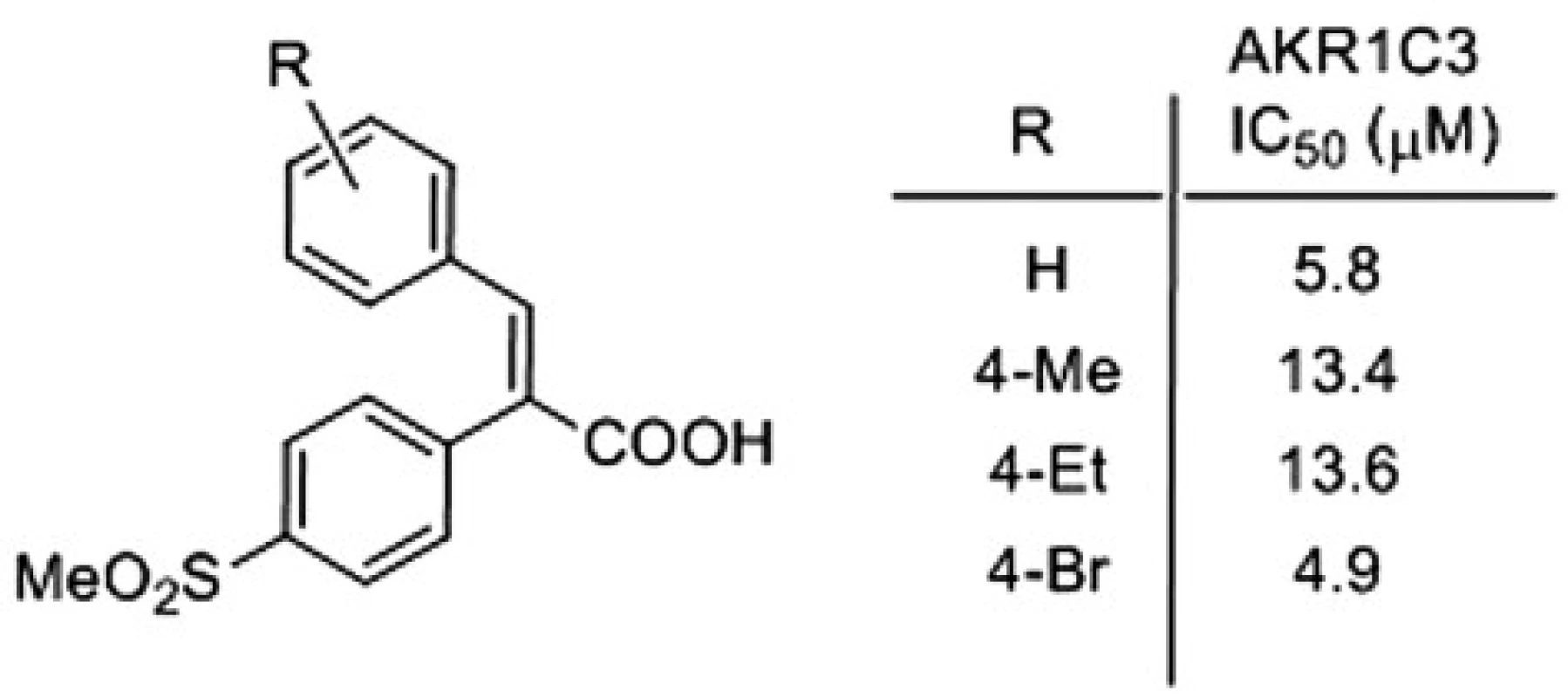
Conclusions
Several α-arylcinnamic acids were synthesized by the Perkin reaction between the substituted benzaldehydes and the properly functionalized arylacetic acids. They were evaluated as inhibitors of AKR1C1 ‒ AKR1C3. A modification of the structure revealed that the 4-methylsulfonylphenyl substituent at the position 2 of the 2,3-diarylpropenoic acid is an appropriate one to obtain a selective inhibition of AKR1C3. We found that the compound with 4-Br substituent (Fig. 2) is the best inhibitor of AKR1C3, and also shows very good selectivity. Its 4-methylphenyl and 4-ethylphenyl analogues, which exhibit a slightly lower activity against AKR1C3, are the most selective ones. Our data suggest that α-arylcinnamic acids (2,3-diarylpropenoic acids) represent a new class of selective inhibitors of AKR1C3 and may serve as a good starting point for the development of new antitumor agents for the treatment of the hormone-dependent and hormone-independent forms of prostate and breast cancers.
Conflicts of interest: none.
Dr. Martin Gazvoda
Faculty of Chemistry and Chemical Technology, University of Ljubljana
Večna pot 113, SI-1000 Ljubljana, Slovenia
e-mail: martin.gazvoda@fkkt.uni-lj.si
Zdroje
1. Penning T. M., Byrns, M. C. Steroid hormone transforming aldo-keto reductases and cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 33‒42 and references therein.
2. Byrns M. C., Duan L., Lee S. H., Blair I. A., Penning T. M. Aldoeketo reductase 1C3 expression in MCF-7 cells reveals roles in steroid hormone and prostaglandin metabolism that may explain its over-expression in breast cancer. J. Steroid Biochem. Mol. Biol. 2010; 118, 177‒187.
3. Brožič P., Golob B., Gomboc N., Lanišnik-Rižner T., Gobec S. Cinnamic acids as new inhibitors of 17β-hydroxysteroid dehydrogenase type 5 (AKR1C3). Mol. Cell. Endocrinol. 2006; 248, 233‒235.
4. Gazvoda M., Beranič N, Turk S., Burja B., Kočevar M, Lanišnik-Rižner T., Gobec S., Polanc S. 2,3-Diarylpropenoic acids as selective non-steroidal inhibitors of type-5 17β-hydroxysteroid dehydrogenase (AKR1C3). Eur. J. Med. Chem. 2013, 62, 89–97.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Antibakteriální účinky přírodních látek – silice
- Organická syntéza, Laboratórny manuál
- Cholinergický systém srdca
- Proběhl 42. mezinárodní kongres k dějinám farmacie
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Sympozia Sekce dějin farmacie ČFS v roce 2015
- Autorský rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antibakteriální účinky přírodních látek – silice
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Cholinergický systém srdca
- Organická syntéza, Laboratórny manuál
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



