-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Synthesis and antimicrobial activity of novel sulfonamide derivatives
Authors: Martin Krátký 1; Jiřina Stolaříková 2; Jarmila Vinšová 1
Authors place of work: Department of Inorganic and Organic Chemistry, Faculty of Pharmacy, Charles University, Hradec Králové, Czech Republic 1; Laboratory for Mycobacterial Diagnostics and Tuberculosis, Regional Institute of Public Health in Ostrava, Ostrava, Czech Republic 2
Published in the journal: Čes. slov. Farm., 2015; 64, 289-290
Category: 44<sup>th</sup> Conference drug synthesis and analysis
Introduction
The alarming progression of drug resistance among human pathogens justifies the development of novel antimicrobial agents. Research studies should provide new antimicrobial molecules active against, e.g., Mycobacterium tuberculosis, nontuberculous mycobacteria, methicillin-resistant Staphylococcus aureus (MRSA) and polyresistant Gram-negative species1).
The modification of known drugs represents an effective approach in drug design. Sulfonamides have been widely used for the therapy of various bacterial infections. However, they share some disadvantages. More recently, sulfonamides were considered useless for the treatment of tuberculosis but concomitantly useful for the therapy of some nontuberculous mycobacterial infections. Based on the findings that M. tuberculosis strains are susceptible in vitro to clinically achievable concentrations of sulfamethoxazole 1, this molecule has been “resurrected” and novel sulfonamides have recently been reported as potential antimycobacterial agents1, 2). Additionally, a wide range of urea derivatives were found to display antimycobacterial activity3).
That’s why sulfamethoxazole-based ureas 2 and their cyclic analogues imidazolidine-2,4,5-triones 3 have been designed, synthesised, and evaluated as potential antimicrobial agents. In our previous paper1), we found that N-heptyl sulfamethoxazole-based urea displayed a significant activity against nontuberculous mycobacteria. Based on this finding, we involved also N-alkyl derivatives with a varying length of the alkyl chain.
Experimental methods
Sulfamethoxazole 1 was dissolved in dry acetonitrile and appropriate isocyanate (1.1 equivalent) was added. The solution was heated under reflux for 3.5 h and then stirred at ambient temperature for 8 h. In the case of decyl urea derivative, isocyanate was generated in situ from decylamine and triphosgene in dichloromethane in the presence of triethylamine under anhydrous conditions.
Oxalyl chloride (1.2 equivalent) was added to sulfonamide-based ureas 2 dissolved or suspended in dry tetrahydrofuran and the mixture was heated under reflux for 2 h. The crystallisation was initiated by an addition of hexane.
The synthetic pathway and the overview of designed derivatives is depicted in Figure 1. Some of the presented compounds were reported previously by our group1).
Fig. 1. Synthesis of sulfamethoxazole derivatives 2 and 3 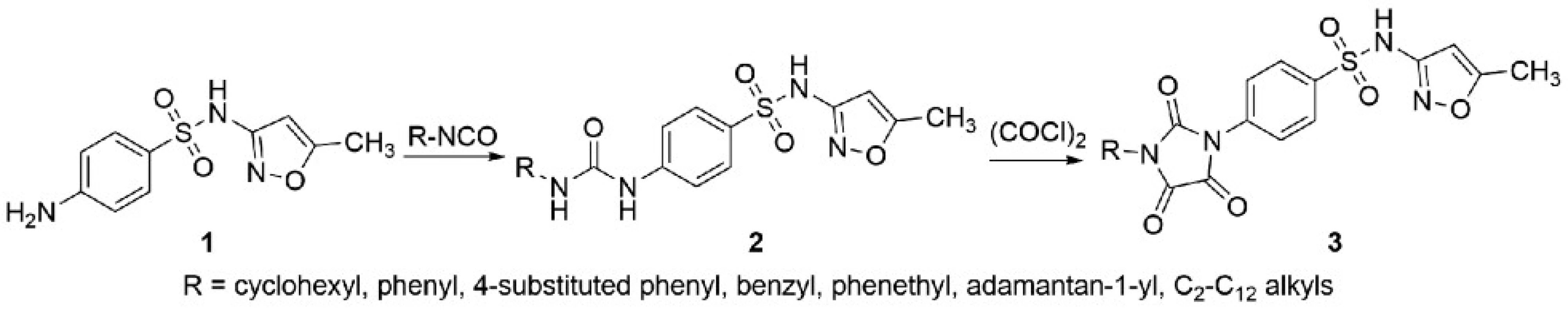
The in vitro antimycobacterial activity was evaluated against M. tuberculosis H37Rv, M. avium and two strains of M. kansasii. The antibacterial activity was assayed against eight Gram-positive and Gram-negative strains: Staphylococcus aureus, MRSA, Staphylococcus epidermidis, Enterococcus sp.; Escherichia coli, Pseudomonas aeruginosa and two strains of Klebsiella pneumoniae. The in vitro antifungal activity was evaluated against four Candida strains, Trichosporon asahii, Aspergillus fumigatus, Absidia corymbifera and Trichophyton mentagrophytes. Cytotoxicity was determined for the human hepatocellular liver carcinoma cell line (HepG2).
Results and discussion
Sulfamethoxazole-based ureas 2 were obtained in satisfactory yields (40–96%) similarly to corresponding imidazolidine-2,4,5-triones 3 (47–95%).
Derivatives 2 and 3 exhibited antimycobacterial activity with minimum inhibitory concentrations (MICs) of 4 μM or higher. M. tuberculosis showed the lowest susceptibility (MICs ≥ 62.5 μM), whereas M. kansasii was the most susceptible species. Nontuberculous mycobacteria were suppressed most effectively by 4-(3-heptylureido)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (2; R = n-heptyl) with MICs of 4–62.5 μM. Several n-alkyl ureas were inactive against one or more mycobacterial strains. None of the novel derivatives 2 and 3 exceeded significantly the activity of the parent sulfamethoxazole 1 and isoniazid.
The investigation of the influence of N3-substitution of ureas 2 on the activity for M. tuberculosis revealed that the highest activity is conferred by a shorter alkyl (R = ethyl, butyl), heptyl and 4-substituted phenyl. However, longer alkyls than heptyl did not produce an improvement of antitubercular activity.
The cyclisation of more efficient ureas 2 to imidazolidine-2,4,5-triones 3 resulted in derivatives with similar or lower activity whereas the modification of ureas with weaker antimycobacterial activity resulted in cyclized products 3 with beneficially decreased MIC values.
With respect to the bacterial strains, none of the derivatives significantly inhibited the growth of P. aeruginosa, K. pneumoniae and E. coli. Gram-positive cocci were susceptible with MICs from 125 μM. S. aureus exhibited the highest susceptibility with no marked difference between methicillin-susceptible and MRSA strains. Several derivatives avoided any antibacterial action. N-Heptyl urea 2 showed activity against S. aureus comparable or slightly superior to that of the parent sulfamethoxazole 1; nevertheless, other alkyl ureas 2 were completely inactive.
With respect to antifungal properties, sulfamethoxazole derivatives 2 and 3 showed almost no antifungal activity. IC50 values for HepG2 cells were within the range of ≥ 103.3 μM indicating a low cytotoxicity. N-Heptyl urea provided sufficient selectivity indexes ( > 10) for nontuberculous mycobacteria.
Conclusion
We designed and synthesized more than thirty sulfamethoxazole derivatives as potential antimicrobial agents. They were evaluated against four mycobacterial strains, a panel of Gram-positive, Gram-negative bacteria and fungi as well as for their cytotoxicity.
The highest in vitro activity was found against M. kansasii, whereas fungi and Gram-negative bacteria were practically resistant. We identified several derivatives with a promising in vitro activity profile.
The work was financially supported by the Research Project IGA NT 13346 (2012).
Conflicts of interest: none.
PharmDr. Mgr. Martin Krátký, Ph.D.
Department of Inorganic and Organic Chemistry
Faculty of Pharmacy, Charles University
Heyrovského 1203, 500 05 Hradec Králové, Czech Republic
e-mail: martin.kratky@faf.cuni.cz
Zdroje
1. Krátký M., Mandíková J., Trejtnar F., Buchta V., Stolaříková J., Vinšová J. Synthesis and antimicrobial activity of sulfamethoxazole-based ureas and imidazolidine-2,4,5-triones. Chem. Pap. 2015; 69, 1108–1117.
2. Krátký M., Vinšová J., Volková M., Buchta V., Trejtnar F., Stolaříková, J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur. J. Med. Chem. 2012; 50, 433–440.
3. Brown J. R., North E. J., Hurdle J. G., Morisseau C., Scarborough J. S., Sun D., Kordulakova J., Scherman M. S., Jones V., Grzegorzewicz A., Crew R. M., Jackson M., Mc-Neil M. R., Lee, R. E.. The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg. Med. Chem. 2011; 19, 5585–5595.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 6- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Antibakteriální účinky přírodních látek – silice
- Organická syntéza, Laboratórny manuál
- Cholinergický systém srdca
- Proběhl 42. mezinárodní kongres k dějinám farmacie
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Stable gold nanoparticles – synthesis, bioconjugation and application
- Determination of antigripal drugs (pheniramine, phenylephrine) in biological samples by on-line CITP-CZE coupled with tandem mass spectrometry
- Development of the hydrocortisone butyrate qualitative determination method
- Estimation of lipohydrophilic properties of molecules with potential β3-agonistic activity
- Determination of the colorants in vitamin E by HPLC with photodiode array detection
- Analysis of flavonoids in grape leaves by HPLC-DAD-MS/MS
- Antioxidative protection of inactivated rabies vaccine with squalene adjuvant by β-carotene
- From an old drug to a new one: Synthesis of valproate from 5,5-dipropylbarbituric acid
- Synthesis and antimicrobial activity of novel sulfonamide derivatives
- Synthesis and antioxidant activity of phenylcarbamic acid derivatives acting on the cardiovascular system
- Synthesis and biological activity of selected cinnamic acid derivatives
- Synthesis and biological properties of chosen symmetrical amides and thioamides of terephthalic acid
- Synthesis of quinoline derivatives using a nano-Pd/Cu catalyst in the search of new fluorophores
- Synthesis of triclosan derivatives and their antimycobacterial effect
- The development of a dental drug in the form of medicated chewing gum
- Sympozia Sekce dějin farmacie ČFS v roce 2015
- Autorský rejstřík
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Antibakteriální účinky přírodních látek – silice
- Povrch těla a tělesná hmotnost dospělé české onkologické populace
- Cholinergický systém srdca
- Organická syntéza, Laboratórny manuál
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



