-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Experimental Evolution of Pathogenesis: “Patient” Research
article has not abstract
Published in the journal: . PLoS Pathog 9(5): e32767. doi:10.1371/journal.ppat.1003340
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003340Summary
article has not abstract
Overview
Twenty-five years and over 50,000 bacterial generations ago, one of the longest open-ended experiments in modern times began when the Lenski laboratory started passaging Escherichia coli under conditions of limited glucose as a carbon source [1]. Later that same year, a paper was published describing a new method for DNA sequencing, called pyrosequencing, that would ultimately light a path toward massively parallel (next-generation) sequencing [2]. As of 2013, a raw megabase of DNA costs less than $0.10 [3], or roughly equivalent to the cost of one petri dish. Combined with access to this affordable, high-throughput sequencing, experimental evolution [4] represents a sophisticated approach to dissecting host-pathogen interactions and testing models of host range and pathogen adaptation. Here, we review how experimental evolution has been used to directly test models of host-pathogen interactions (Figure 1). We discuss parallels between these laboratory studies and the real-world evolutionary trajectories that have been uncovered through the direct sequencing of clinical isolates.
Fig. 1. Some of the pathogenic characteristics explored by laboratory passage models. 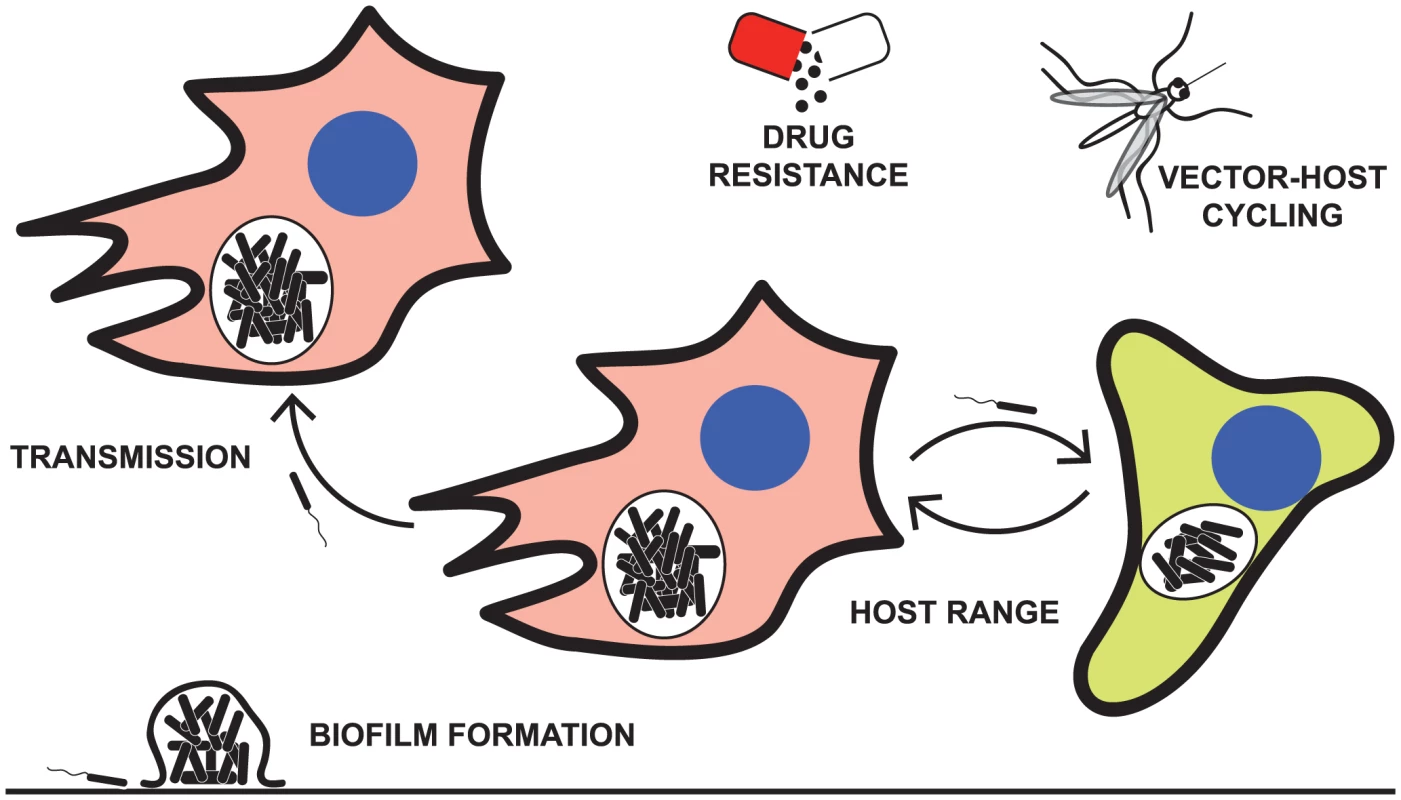
Examples of the application of experimental evolution to the study of pathogens include modeling the evolution of drug resistance [18]–[20], trade-offs between generalist versus specialist adaptations to the host [9], [12], pathogen transmission [13], [14], and biofilm formation [17]. Experimental Adaptation of Host Range
Laboratory passage has long been recognized as an effective means to modify the host range of pathogens, with successive passage of viruses in nonhuman hosts an early strategy for generating live attenuated vaccines [5]. The principle behind this attenuation is that confined passage in one host species can modify the host range of a pathogen such that it no longer efficiently causes disease in the original host [6]. Armed with modern molecular and genomic tools, several groups have revisited the basic outlines of this approach to directly test how natural host diversity and host cycling influence the evolutionary trajectory of pathogens.
Why do some pathogens specialize to one particular host while others remain generalists, able to infect a broad range of host species? One model is that exposure to diverse environmental hosts drives the expansion of host range and prevents specialization to any single host [7]. In this model, generalist pathogens fail to specialize because acquired host-specific adaptations likely result in antagonistic pleiotropy [8] in other hosts encountered during the pathogen life cycle, leading to their rapid exclusion from future generations. We recently used this approach to uncover fitness trade-offs in the broad host-range bacterial pathogen Legionella pneumophila during its adaptation to mouse macrophages [9]. L. pneumophila normally replicates in freshwater amoebae [10], and our model is that its broad host range is maintained due to environmental diversity of amoebae within its natural reservoirs. Consistent with this model, several identified mutations selected for in the macrophage-adapted strains came at the expense of reduced fitness in two natural amoebal hosts [9].
Intuitively, such host dynamics should also shape the genomes of vector-borne diseases, where pathogens must balance fitness in both their vector and their other hosts. This hypothesis has been extensively tested in arthropod-borne viruses, where host cycling between insect vector and mammalian host should prevent the accumulation of mutations that result in fitness costs in either host, perhaps holding such pathogens in a sort of evolutionary stasis [11]. A recent in vivo experimental evolution study, however, showed that the evolution of West Nile virus, when passaged in either chicks or mosquitos, does not strictly fit the trade-off hypothesis [12]. Instead of each host selecting for unique host-specific adaptations, it was suggested that the mosquito host overall placed less stringent selection on the virus, allowing for expanded viral genetic diversity within the vector host. Once these viruses were transferred to the chick host, however, strong positive selection took over, selecting for virions carrying mutations that increased fitness within the vertebrate hosts. It will be particularly interesting to see whether similar complexities exist in the host-pathogen interactions of non–vector-borne diseases, such as L. pneumophila. Whether due to less stringent selection under some conditions, disruptions in environmental host diversity, increased mutation, or population bottlenecking, transient changes in pathogen diversity might subsequently influence the outcome of human exposure to these bacteria.
Experimental Modeling of Other Pathogenic Traits
Experimental evolution can also be used to modulate a pathogen's transmission between hosts. Usually, the conditions of serial passage (back dilution in growth media) in the laboratory do not recapitulate the nuances involved in natural transmission between hosts, selecting instead for mutations that increase within-host replication [6]. Nevertheless, recent work on H5N1 influenza, a deadly but poorly transmitted strain, indicates that this need not always be the case [13], [14]. Two independent groups used experimental evolution to select for transmissibility by serially passaging the virus between ferrets. One early model of host-pathogen interactions would have predicted a trade-off between within-host virulence and between-host transmissibility, but in recent years the universal applicability of this model has been questioned [15]. Indeed, these experimentally adapted viruses retained their ability to efficiently kill ferrets while showing dramatic improvements in their ability to spread through a population of hosts, which is inconsistent with the simple virulence/transmission trade-off model. Due to the recognized overlap between ferret-to-ferret and person-to-person spread, both the publication of these findings and the continuation of this research remain the subject of considerable debate.
In addition to transmission and host-range trade-offs, experimental evolution has also provided unique insight into other aspects of pathogenesis, including the fitness trade-offs between colonization and systemic infection [16], the community architecture during biofilm formation [17], and the development of drug resistance in both prokaryotic and eukaryotic pathogens [18]–[20]. Particular focus has been placed on the predominant bacterial pathogens affecting cystic fibrosis patients, as evolutionary trajectories within each patient are thought to have profound impacts on the severity of disease [21], [22]. Experimental passage of Pseudomonas aeruginosa in media designed to mimic the conditions encountered by bacteria in the lungs of cystic fibrosis patients selected for several mutations, including antibiotic resistance, that overlapped with known mutations previously identified in patient isolates [20]. Another study used experimental evolution to model the complex dynamics of biofilm production in a population of Burkholderia cenocepacia, another frequent pathogen of cystic fibrosis patients [17]. Rather than selecting for clones that followed one evolutionary trajectory to maximum fitness, careful examination of the population during this passage indicated that multiple types of clones evolved to occupy different environmental niches within the experiment. Several parallels were observed between the adaptations selected for in this in vitro model of biofilm formation and what has been learned from studying clinical isolates from chronic infections of the cystic fibrosis lung, including an increased ability to utilize limited iron and alterations in lipopolysaccharide production.
Patience versus Patients—Genomic Epidemiology
Many of the concepts of experimental evolution are also relevant to the expanding field of genomic epidemiology, where using whole-genome sequencing to track the spread of disease through a population can also provide real-world information about how pathogens adapt to their human host. In particular, concepts such as parallel genome evolution (repeated selection for mutations in the same genes or pathways in independent clones) and fitness trade-offs between human infection and environmental persistence are as important for understanding outbreaks of disease as they are to laboratory adaptation.
The life-long colonization of cystic fibrosis patients with Burkholderia dolosa [21] and Pseudomonas aeruginosa [22] has provided a unique window into the evolutionary trajectory of these pathogens during adaptation to the human host. In longitudinal studies of both pathogens, independent lineages acquired mutations in an overlapping set of genes, including gyrA (drug resistance) and global response regulators. In P. aeruginosa replicating for 200,000 generations, the observation of purifying negative selection suggested that these populations reached fitness peaks not previously observed during in vitro adaptation of the pathogen [22]. In another study that blurred the lines between experimental evolution and genomic epidemiology in patients, the adaptation of an asymptomatic bacteriuria strain of E. coli to the human host was monitored by sequencing a series of isolates taken from the bladders of six patients that were deliberately colonized with this strain [23].
Sequence First and Ask Questions Later
Like many fields, experimental evolution has not escaped the transformative effects of next-generation sequencing. By sequencing the genomes of laboratory-adapted strains, it is now possible to define the molecular basis of microbial adaptation to new environments or conditions. Given that, using current technologies—even large bacterial genomes currently cost less than $100 to sequence at >100× coverage—we favor what could best be described as a sequence first and ask questions later approach to understanding the laboratory evolution of pathogens. Indeed, complex population dynamics, including frequent clonal interference between competing clones within a population, are likely to be quite common during these experiments [9], [17]. To capture these dynamics, either several isolates from intermediate time-points should be sequenced, high-resolution genotyping should be performed [9], [17], or metagenomic sequencing of the population should be used to estimate allelic frequencies across the experimental time frame [17], [24].
What's Next?
With the increased ability to link laboratory adaptations to specific genetic lesions via affordable, widely accessible sequencing, the application of experimental evolution to study pathogen adaptation has never held more potential (Table 1). Future challenges in experimental design include the development of sequencing and analysis tools that are better designed to identify complex genomic rearrangements and gene duplication, as one recent paper elegantly demonstrated that copy number expansion can be a powerful driving force in the rapid adaptation of viruses to a suboptimal host [25]. Furthermore, as real-world pathogen adaptation is never as simple as one host, one pathogen, a logical next step will be to include coinfections with multiple pathogens [26] in these laboratory models to allow for interspecies competition as well as horizontal gene transfer between adapting pathogens. Lastly, as the price of sequencing continues to fall, studying the host genome's response to prolonged pathogen exposure will be a natural extension for the field.
Tab. 1. Considerations in designing a laboratory passage experiment. 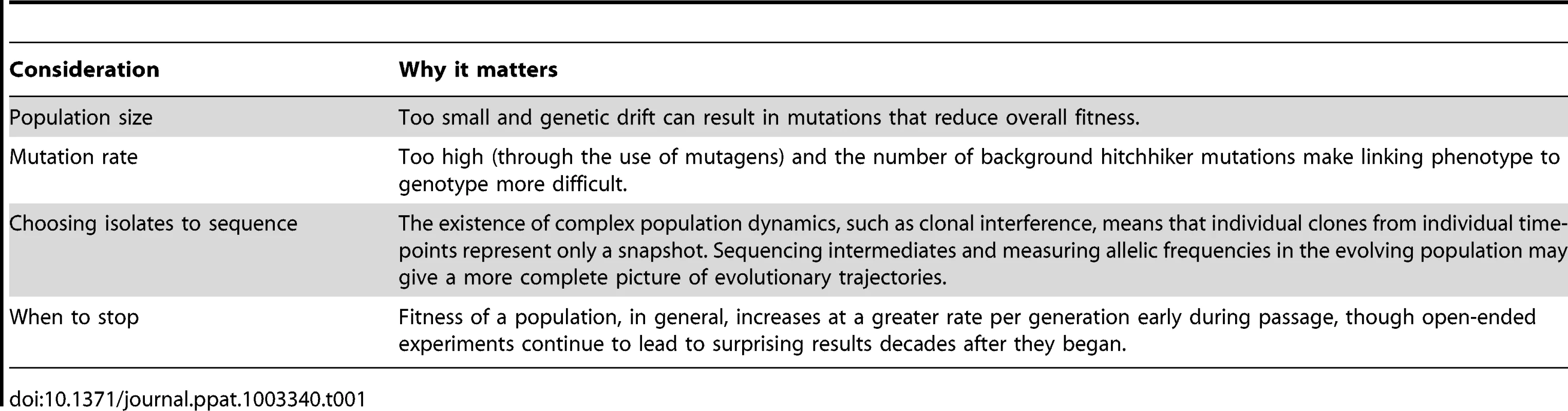
Zdroje
1. LenskiRE, TravisanoM (1994) Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc Natl Acad Sci U S A 91 : 6808–6814.
2. HymanED (1988) A new method of sequencing DNA. Anal Biochem 174 : 423–436.
3. Wetterstrand K. DNA sequencing costs: data from the NHGRI Genome Sequencing Program (GSP). Available: http://www.genome.gov/sequencingcosts/. Accessed 29 April 2013.
4. KaweckiTJ, LenskiRE, EbertD, HollisB, OlivieriI, et al. (2012) Experimental evolution. Trends Ecol Evol 27 : 547–560.
5. SabinAB, SchlesingerRW (1945) Production of immunity to dengue with virus modified by propagation in mice. Science 101 : 640–642.
6. EbertD (1998) Experimental evolution of parasites. Science 282 : 1432–1435.
7. KassenR (2002) The experimental evolution of specialists, generalists, and the maintenance of diversity. J Evol Biol 15 : 173–190.
8. CooperVS, LenskiRE (2000) The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407 : 736–739.
9. EnsmingerAW, YassinY, MironA, IsbergRR (2012) Experimental evolution of Legionella pneumophila in mouse macrophages leads to strains with altered determinants of environmental survival. PLoS Pathog 8: e1002731 doi:10.1371/journal.ppat.1002731
10. FieldsBS (1996) The molecular ecology of legionellae. Trends Microbiol 4 : 286–290.
11. CiotaAT, KramerLD (2010) Insights into arbovirus evolution and adaptation from experimental studies. Viruses 2 : 2594–2617.
12. DeardorffER, FitzpatrickKA, JerzakGVS, ShiP-Y, KramerLD, et al. (2011) West Nile virus experimental evolution in vivo and the trade-off hypothesis. PLoS Pathog 7: e1002335 doi:10.1371/journal.ppat.1002335
13. HerfstS, SchrauwenEJ, LinsterM, ChutinimitkulS, de WitE, et al. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336 : 1534–1541.
14. ImaiM, WatanabeT, HattaM, DasSC, OzawaM, et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486 : 420–428.
15. EbertD, BullJJ (2003) Challenging the trade-off model for the evolution of virulence: is virulence management feasible? Trends Microbiol 11 : 15–20.
16. PandyaU, SinhaM, LuxonBA, WatsonDA, NieselDW (2009) Global transcription profiling and virulence potential of Streptococcus pneumoniae after serial passage. Gene 443 : 22–31.
17. TraverseCC, Mayo-SmithLM, PoltakSR, CooperVS (2013) Tangled bank of experimentally evolved Burkholderia biofilms reflects selection during chronic infections. Proc Natl Acad Sci U S A 110: E250–259.
18. AlmahmoudI, KayE, SchneiderD, MaurinM (2009) Mutational paths towards increased fluoroquinolone resistance in Legionella pneumophila. J Antimicrob Chemother 64 : 284–293.
19. AndersonJB, SirjusinghC, ParsonsAB, BooneC, WickensC, et al. (2003) Mode of selection and experimental evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 163 : 1287–1298.
20. WongA, RodrigueN, KassenR (2012) Genomics of adaptation during experimental evolution of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Genet 8: e1002928 doi:10.1371/journal.pgen.1002928
21. LiebermanTD, MichelJB, AingaranM, Potter-BynoeG, RouxD, et al. (2011) Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet 43 : 1275–1280.
22. YangL, JelsbakL, MarvigRL, DamkiaerS, WorkmanCT, et al. (2011) Evolutionary dynamics of bacteria in a human host environment. Proc Natl Acad Sci U S A 108 : 7481–7486.
23. ZdziarskiJ, BrzuszkiewiczE, WulltB, LiesegangH, BiranD, et al. (2010) Host imprints on bacterial genomes—rapid, divergent evolution in individual patients. PLoS Pathog 6: e1001078 doi:10.1371/journal.ppat.1001078
24. HerronMD, DoebeliM (2013) Parallel evolutionary dynamics of adaptive diversification in Escherichia coli. PLoS Biol 11: e1001490 doi:10.1371/journal.pbio.1001490
25. EldeNC, ChildSJ, EickbushMT, KitzmanJO, RogersKS, et al. (2012) Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150 : 831–841.
26. LeggettHC, BenmayorR, HodgsonDJ, BucklingA (2013) Experimental evolution of adaptive phenotypic plasticity in a parasite. Curr Biol 23 : 139–142.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Volatile Metabolites of Pathogens: A Systematic Review
- Behind Closed Membranes: The Secret Lives of Picornaviruses?
- DNA from Skeletal Remains from the 6 Century AD Reveals Insights into Justinianic Plague
- Experimental Evolution of Pathogenesis: “Patient” Research
- Bile Acid Recognition by the Germinant Receptor, CspC, Is Important for Establishing Infection
- Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress
- The Secret Life of Viral Entry Glycoproteins: Moonlighting in Immune Evasion
- Uracil DNA Glycosylase Counteracts APOBEC3G-Induced Hypermutation of Hepatitis B Viral Genomes: Excision Repair of Covalently Closed Circular DNA
- Differences in Gastric Carcinoma Microenvironment Stratify According to EBV Infection Intensity: Implications for Possible Immune Adjuvant Therapy
- Polyphosphate and Its Diverse Functions in Host Cells and Pathogens
- Activation of the NLRP3 Inflammasome by IAV Virulence Protein PB1-F2 Contributes to Severe Pathophysiology and Disease
- Plant Virus Ecology
- Proving Lipid Rafts Exist: Membrane Domains in the Prokaryote Have the Same Properties as Eukaryotic Lipid Rafts
- Structural and Functional Basis for Inhibition of Erythrocyte Invasion by Antibodies that Target EBA-175
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress
- The Secret Life of Viral Entry Glycoproteins: Moonlighting in Immune Evasion
- Structural and Functional Basis for Inhibition of Erythrocyte Invasion by Antibodies that Target EBA-175
- DNA from Skeletal Remains from the 6 Century AD Reveals Insights into Justinianic Plague
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



