-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Bile Acid Recognition by the Germinant Receptor, CspC, Is Important for Establishing Infection
Clostridium difficile spores must germinate in vivo to become actively growing bacteria in order to produce the toxins that are necessary for disease. C. difficile spores germinate in vitro in response to certain bile acids and glycine. In other sporulating bacteria, proteins embedded within the inner membrane of the spore sense the presence of germinants and trigger the release of Ca++-dipicolinic acid (Ca++-DPA) from the spore core and subsequent hydrolysis of the spore cortex, a specialized peptidoglycan. Based upon homology searches of known germinant receptors from other spore-forming bacteria, C. difficile likely uses unique mechanisms to recognize germinants. Here, we identify the germination-specific protease, CspC, as the C. difficile bile acid germinant receptor and show that bile acid-mediated germination is important for establishing C. difficile disease in the hamster model of infection. These results highlight the importance of bile acids in triggering in vivo germination and provide the first description of a C. difficile spore germinant receptor. Blocking the interaction of bile acids with the C. difficile spore may represent an attractive target for novel therapeutics.
Published in the journal: . PLoS Pathog 9(5): e32767. doi:10.1371/journal.ppat.1003356
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003356Summary
Clostridium difficile spores must germinate in vivo to become actively growing bacteria in order to produce the toxins that are necessary for disease. C. difficile spores germinate in vitro in response to certain bile acids and glycine. In other sporulating bacteria, proteins embedded within the inner membrane of the spore sense the presence of germinants and trigger the release of Ca++-dipicolinic acid (Ca++-DPA) from the spore core and subsequent hydrolysis of the spore cortex, a specialized peptidoglycan. Based upon homology searches of known germinant receptors from other spore-forming bacteria, C. difficile likely uses unique mechanisms to recognize germinants. Here, we identify the germination-specific protease, CspC, as the C. difficile bile acid germinant receptor and show that bile acid-mediated germination is important for establishing C. difficile disease in the hamster model of infection. These results highlight the importance of bile acids in triggering in vivo germination and provide the first description of a C. difficile spore germinant receptor. Blocking the interaction of bile acids with the C. difficile spore may represent an attractive target for novel therapeutics.
Introduction
Clostridium difficile infections (CDI) are steadily increasing in the United States and other countries [1], [2]. The use of broad-spectrum antibiotics, often unrelated to CDI, leads to alteration of the colonic microbiota that normally provides resistance to C. difficile colonization [3]. In a host, C. difficile spores must germinate to form the actively growing, anaerobic bacteria that produce the two toxins that are necessary for disease (TcdA and TcdB) [4], [5], [6]. These two toxins are secreted by the bacterium where they then enter host epithelial cells by receptor-mediated endocytosis and, upon escape into the cytosol, glucosylate members of the Rho-family of GTPases [7]. The action of these toxins lead to symptoms normally associated with CDI (e.g. diarrhea) and release of C. difficile spores into the environment [8].
Metabolically dormant spores are formed by selected bacterial species in response to changes in environmental conditions, including nutrient availability [9]. During spore formation, the proteins required for germination are pre-packaged into the spore, priming the spore to germinate when conditions are appropriate [10]. In many spore-forming species, the interaction of the metabolically dormant spore with specific germination-inducing molecules (germinants) leads to the release of large amounts of Ca++-dipicolinic acid (DPA) from the dehydrated spore core in exchange for water. Subsequently, hydrolases embedded within the spore cortex, a specialized peptidoglycan, become activated and begin cortex hydrolysis. Once the core is rehydrated and the cortex is degraded, a vegetative cell begins to grow out from the germinated spore. This process is largely conserved among spore-forming bacteria, though the signals that initiate germination can vary. In Bacillus subtilis, L-alanine or a mixture of L-asparagine, glucose, fructose and potassium ions triggers germination, while spores of certain strains of Clostridium perfringens initiate germination in response to inorganic phosphate and sodium ions [11].
The proteins that respond to these signals, ger receptors, share homology among many spore-forming bacteria. However, based on homology searches, C. difficile is not among the spore-forming bacteria that have such canonical germinant receptors, suggesting that C. difficile responds to unique germinants or uses a novel mechanism for spore germination or both [12].
Approximately 30 years ago, Wilson and others showed that certain bile acids increased the frequency of C. difficile colony formation from environmental samples [13], [14], [15]. Bile acids are small amphipathic, cholesterol-based molecules that aid in the absorption of fats and cholesterol during digestion. Typically, the liver synthesizes two main bile acids, cholic acid (3α,7α,12α-trihydroxy-5β-cholanic acid) and chenodeoxycholic acid (3α,7α-dihydroxy-5β-cholanic acid), which are further modified with the addition of either a taurine or glycine amino acid [16]. Building on the work of Wilson and others, we demonstrated that all cholic acid derivatives can stimulate C. difficile colony formation from spores with approximately equal efficiency [17]. Further, we showed that exposure to the combination of taurocholic acid and glycine were required to initiate C. difficile spore germination [17]. Interestingly, chenodeoxycholic acid was unable to stimulate colony formation or the initiation of spore germination [17]. Subsequent studies identified chenodeoxycholic acid as a competitive inhibitor of cholic acid-mediated germination [18], [19]. While the chemical signals that promote the initiation of C. difficile spore germination are known, the proteins that respond to these germinants had not been identified.
Here, we applied a screen, previously used to identify loci involved in B. subtilis spore germination [20], to the identification of C. difficile germination-null phenotypes. Using a combination of traditional chemical mutagenesis and contemporary massively parallel DNA sequencing, we identified single nucleotide polymorphisms (SNPs) that give rise to ger phenotypes and characterized the resulting strains. We found that mutations in the C. difficile cspC gene can abrogate the initiation of C. difficile spore germination. Further, we identified a mutation in cspC that allows chenodeoxycholic acid to act as a spore germinant, instead of an inhibitor of germination. These results suggest that C. difficile CspC is the bile acid-sensing germinant receptor. The identification of the molecular target of bile acids on the C. difficile spore has allowed us to test, for the first time, the in vivo role of bile acid-mediated germination during C. difficile infection.
Results
Identifying germination-null phenotypes
Previously, we demonstrated that the cholic acid family of bile acids causes spores to initiate germination in rich medium [17]. To identify the C. difficile bile acid germinant receptor, we employed a strategy schematized in Figure 1A. We mutagenized C. difficile strain UK1 [19] using the DNA alkylating agent ethyl methanesulfonate (EMS). The EMS-mutagenized bacteria were allowed to recover during overnight incubation in fresh medium and spread on solid medium to allow efficient spore formation. Spores were purified and incubated overnight at 37°C in rich medium+10% w/v taurocholic acid [(TA); 185 mM] to germinate those spores that were still able to respond to TA as a germinant. The spore suspension (containing both germinated and non-germinated spores) was incubated at 65°C for 30 minutes to heat-kill the germinated spores; dormant, non-germinated spores are resistant to 65°C. The surviving spores were artificially germinated using thioglycollate and lysozyme [21] and then plated on rich medium to recover, as colonies, the artificially germinated spores. Mutants that failed to germinate under these conditions were enriched and 10 colonies, among thousands, were isolated and tested for the ability of their spores to germinate in response to TA.
Fig. 1. Isolation of C. difficile germination-null mutants. 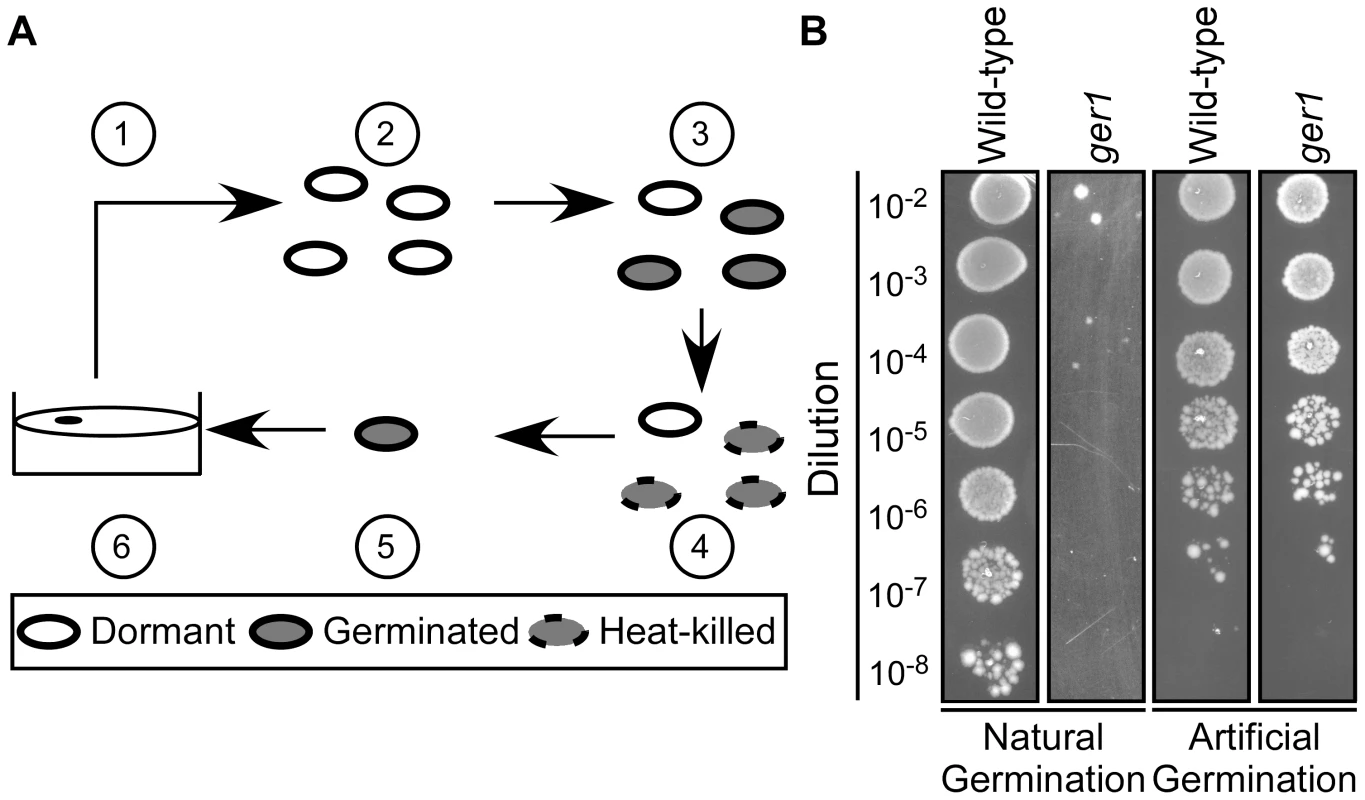
(A) Strategy to identify C. difficile ger phenotypes. Spores were generated (1) and purified (2). After purification, spores were germinated in BHIS medium supplemented with TA (3) and germinated spores heat-killed at 65°C (4). Spores that survived (4) were artificially germinated (5) before plating on BHIS medium (6). (B) C. difficile UK1 spores or C. difficile ger1 spores were serially diluted and spotted on BHIS medium supplemented with 0.1% TA or germinated by thioglycollate/lysozyme and were serially diluted and spotted on BHIS medium. Spores from all 10 isolates (ger1–ger10) were unable to form colonies on rich medium+TA, but did form colonies after artificial germination [21] (Figure 1B; only wild-type C. difficile UK1 and ger 1 are shown). This suggests that the ger mutants are either blocked at the outgrowth stage of germination (inability to grow as a vegetative cell from the germinated spore) or blocked in the initiation of germination (inability to respond to TA as a germinant)
Characterizing germination-null C. difficile strains
To determine at what stage the mutants are blocked, we analyzed the initiation of germination as measured by a decrease in A600 over time. Wild-type C. difficile UK1 initiated germination in the presence of 5 mM TA and 50 mM TA but not in the absence of TA (Figure 2A). However, spores derived from C. difficile ger1 (Figure 2B) did not initiate germination even at the highest TA concentration used (50 mM). Also, while wild-type C. difficile released DPA in response to TA and glycine, C. difficile ger1–ger10 spores were unable to release the majority of the stored DPA (Figure 2C); Ca++-DPA release from the spore core is one of the first measurable events in bacterial spore germination [10]. Together, these results suggest that the C. difficile ger isolates are defective in the earliest stages of spore germination and may be defective in recognizing TA as a germinant.
Fig. 2. C. difficile ger isolates fail to initiate germination. 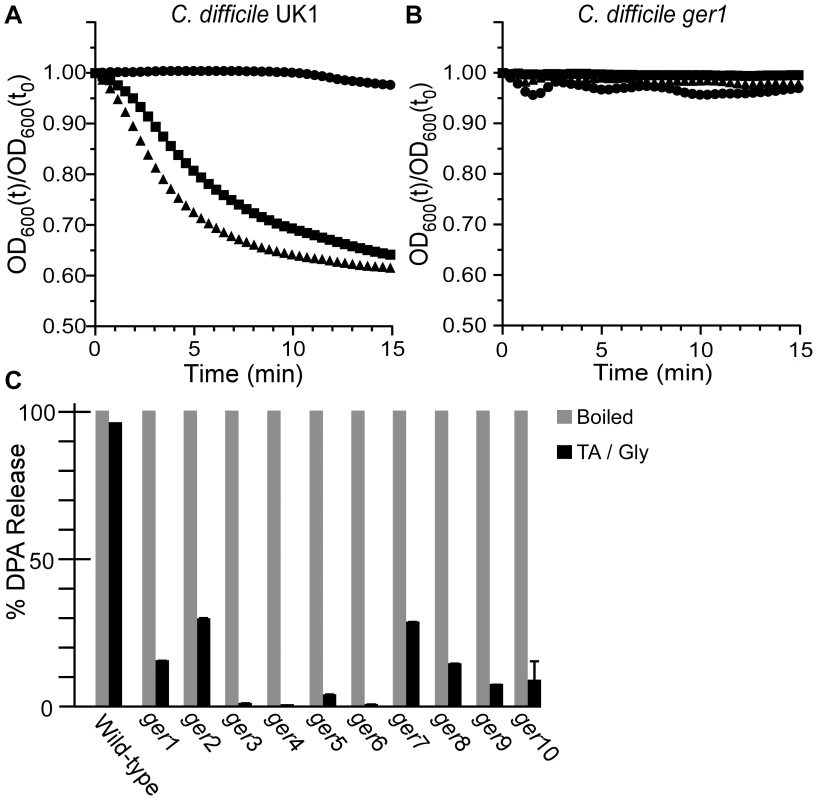
Purified C. difficile UK1 spores (A) or C. difficile ger1 spores (B) were suspended in BHIS medium (•) or BHIS medium supplemented with 5 mM TA (▪) or 50 mM TA (▴) and the initiation of germination was followed at A600. (C) DPA release from spores suspended in germination buffer supplemented with TA and glycine was analyzed at A270. Determining the locations of SNPs that give rise to ger phenotype
The locations of the SNP(s) that gave rise to the germination-null phenotypes were determined using Illumina sequencing technology (Table S1). The DNA sequence of the 10 ger isolates were compared to determine if all had mutations in the same locus or loci. All isolates had in common mutations in 7 loci, with 6 loci having conserved mutations among all isolates (Table 1). Interestingly, C. difficile ger1–ger10 had several different mutations in the cspBAC locus (Table 2). In Clostridium perfringens, CspA, CspB and CspC are germination-specific proteases that cleave the spore cortex lytic enzyme, SleC, to the active form [22], [23], [24]. This allows precise control of the timing of cortex hydrolysis during germination. C. perfringens CspA, CspB and CspC, all members of the subtilisin-family of proteases, have identifiable catalytic triads, while, in C. difficile, only CspB has obvious catalytic residues. In wild-type C. difficile, cspB and cspA coding sequences have been fused. Further, the CspA and CspC catalytic triads appear to have been lost (Figure S1). Eight of the 10 mutant strains had mutations in cspC (Table 2), suggesting that, despite the apparent absence of catalytic activity, wild-type CspC may still have a role in C. difficile spore germination.
Tab. 1. Locations of the SNPs common to all 10 C. difficile ger isolates. Gene numbering is based upon C. difficile R20291 gene numbering. 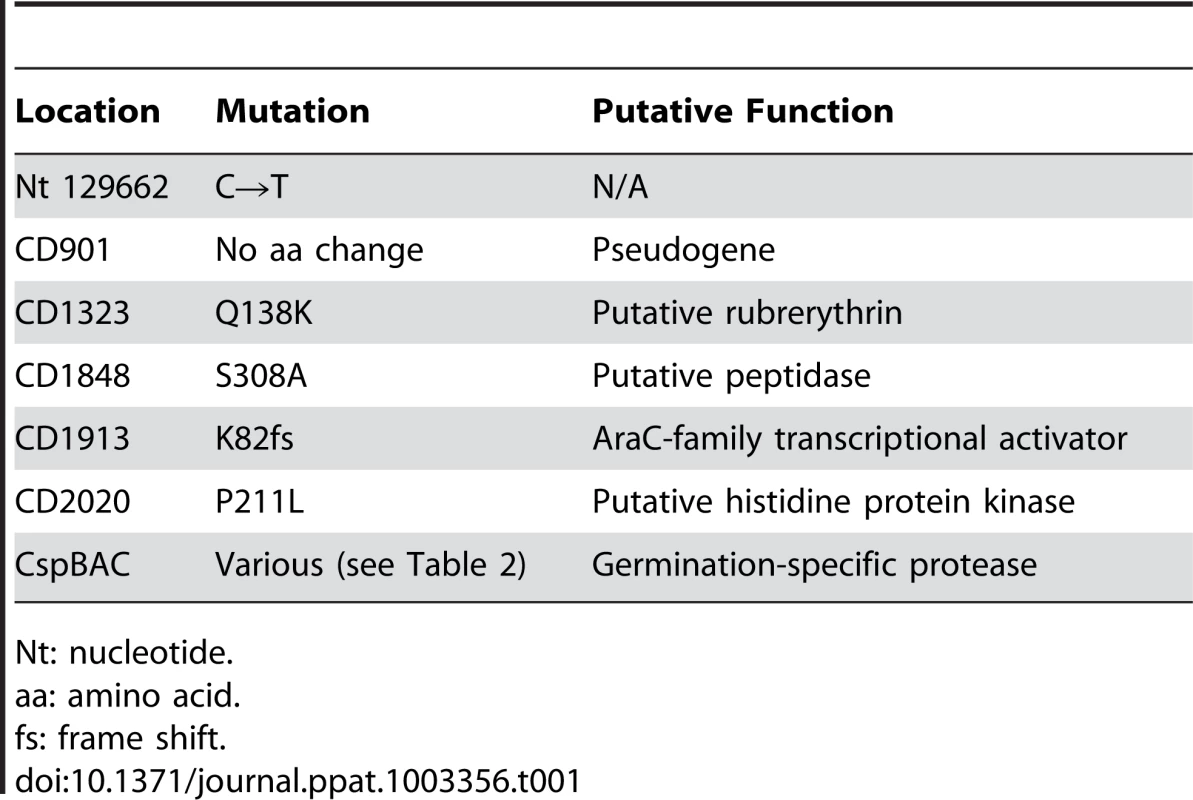
Nt: nucleotide. Tab. 2. Locations of C. difficile cspBAC mutations in the ger mutants. 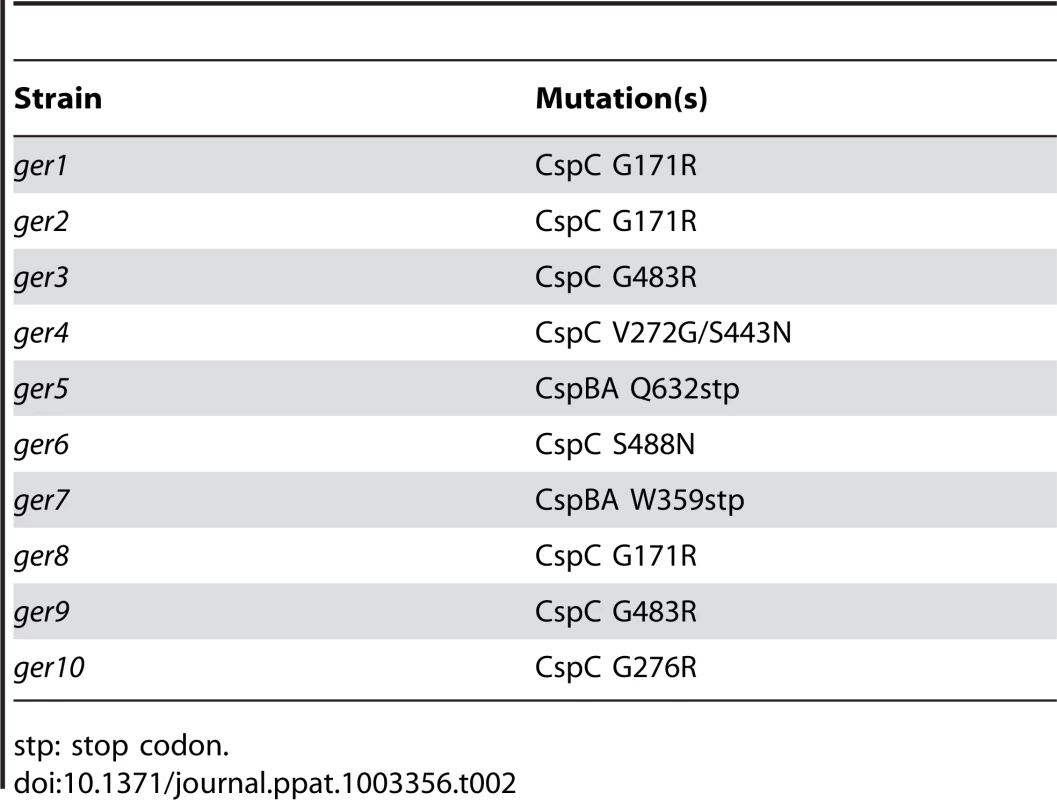
stp: stop codon. Defining the role of C. difficile cspC during spore germination
To investigate the role of CspC in C. difficile spore germination, we generated a site-directed mutation using TargeTron technology [4], [25], [26], [27], [28]. The resulting strain, C. difficile JSC10 (cspC::ermB), is unable to initiate germination in response to TA (Figure 3B) unless provided with the cspBAC locus expressed in trans from a plasmid (Figure 3C); wild-type C. difficile UK1 initiates germination in response to TA (Figure 3A). Interestingly, when C. difficile JSC10 was complemented with the cspBAC locus, spores generated from this strain appear to germinate more rapidly than do C. difficile UK1 spores. Further work will be needed to characterize the germination rates of these spores. When analyzed for DPA release, wild-type C. difficile UK1 and C. difficile JSC10 (pJS123) released DPA while C. difficile JSC10 was unable to release DPA (Figure 3D).
Fig. 3. C. difficile cspC is essential for bile acid-mediated spore germination. 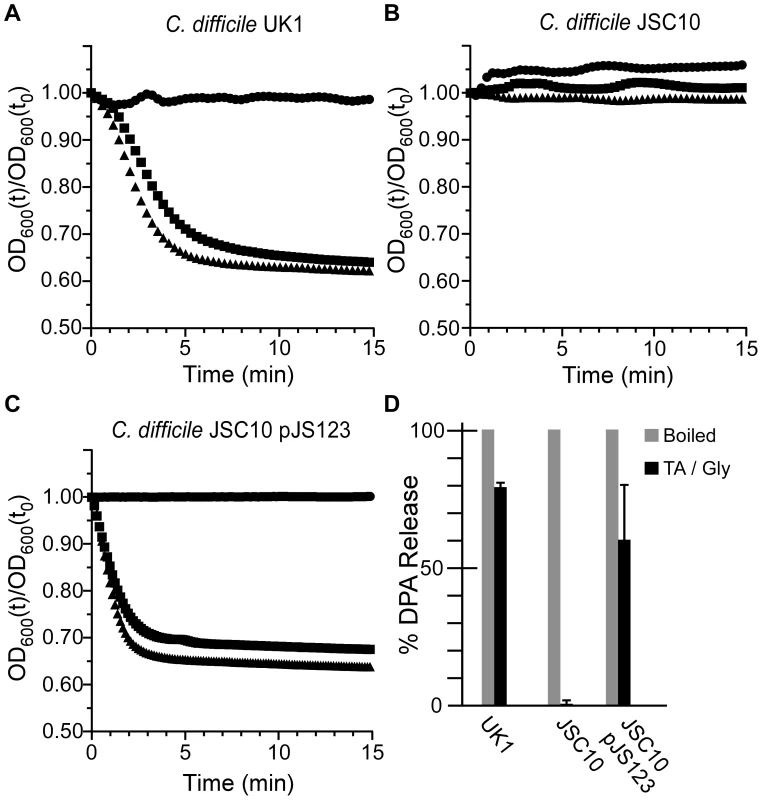
Purified C. difficile UK1 spores (A) or C. difficile JSC10 (cspC::ermB) spores (B) or C. difficile JSC10 (cspC::ermB) pJS123 (pcspBAC) (C) were suspended in BHIS medium (•) or BHIS medium supplemented with 5 mM TA (▪) or 50 mM TA (▴) and the initiation of germination was followed at A600. (D) DPA release from spores suspended in germination buffer supplemented with TA and glycine was analyzed at A270. Mutations in cspC can alter germinant specificity
It was previously reported that a mutation in sleC prevents C. difficile spore germination [27]. Thus, mutations that affect germination do not necessarily indicate that the gene in which the mutation lies normally codes for a germinant receptor. To test the hypothesis that C. difficile CspC is a bona fide germinant receptor, we again mutagenized C. difficile UK1 and allowed the mutagenized bacteria to form spores. The purified spores were plated on BHIS medium supplemented with 0.5 mM chenodeoxycholic acid. We looked for colony formation after 48 hours of incubation at 37°C. Chenodeoxycholic acid is a competitive inhibitor of cholic acid-mediated germination for C. difficile UK1 [18], [19] and other C. difficile strains [29]. Thus, in order to form colonies, these spores must have acquired an altered germinant specificity. Colonies were isolated and the phenotype confirmed as described above. We sequenced cspC from these newly generated strains and identified a single mutation, G457R. When the cspCG457R allele was used to complement C. difficile JSC10, we observed that this strain germinated in response to either TA or chenodeoxycholic acid (Figure 4). These results suggest that C. difficile CspC is a receptor for bile acid germinants.
Fig. 4. Mutations in C. difficile cspC can alter germination specificity. 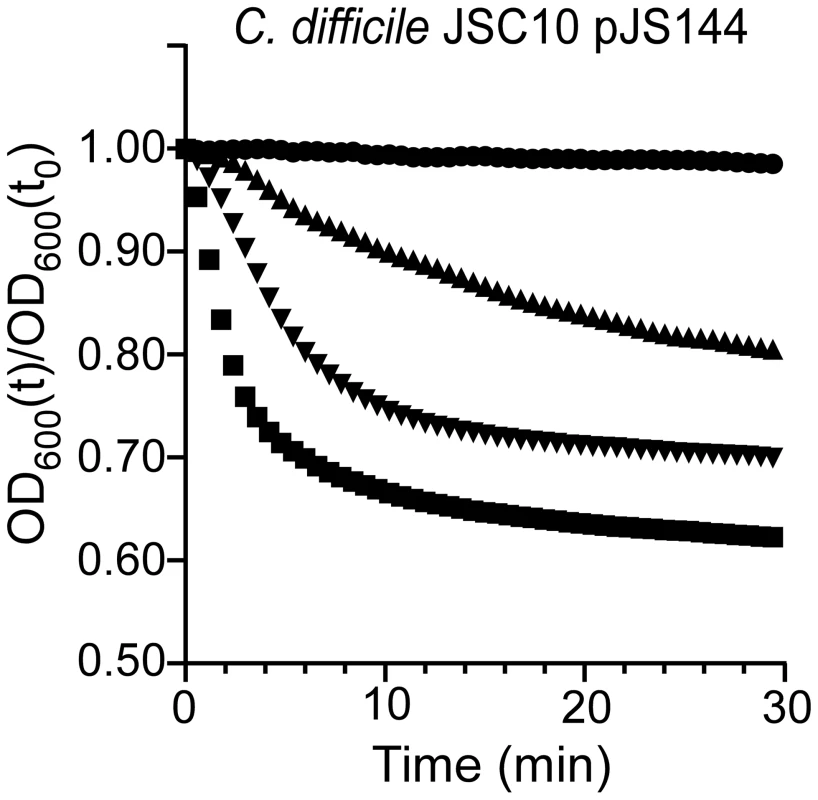
Purified C. difficile JSC10 (cspC::ermB) pJS144 (pcspBACG457R) spores were suspended in BHIS medium (•) or BHIS medium supplemented with 1 mM chenodeoxycholic acid (▴) or 5 mM chenodeoxycholic acid (▾) or 10 mM TA (▪) and the initiation of germination was followed at A600. Bile acid-mediated germination is important for C. difficile infection in hamsters
The in vivo signals that trigger C. difficile spore germination are unknown, though bile acids are obvious candidates [30]. To test whether bile acid-mediated germination is required for C. difficile infection, Syrian hamsters were treated with clindamycin to induce sensitivity to C. difficile colonization and infection; the Syrian hamster has been used for approximately 30 years to assess C. difficile virulence and recapitulates the most severe form of human C. difficile infection, pseudomembranous colitis [31]. Hamsters were gavaged with 1,000 C. difficile UK1 spores or C. difficile JSC10 spores or C. difficile JSC10 (pJS123) spores and monitored for signs of CDI. Animals infected with either C. difficile UK1 or C. difficile JSC10 (pJS123) rapidly succumbed to disease. However, C. difficile JSC10 was unable to cause fulminant CDI and exhibited reduced virulence (Chi-squared: p-value<0.02) (Figure 5). These results show that bile acid-mediated germination is important for C. difficile disease and suggest that inhibiting C. difficile spore germination may have therapeutic potential.
Fig. 5. Bile acid-mediated germination is required for virulence. 
Kaplan-Meier survival curve of clindamycin-treated Syrian hamsters inoculated with 1,000 spores of C. difficile UK1 or C. difficile JSC10 (cspC::ermB) or C. difficile JSC10 (cspC::ermB) pJS123 (pcspBAC). Animals showing signs of C. difficile infection (wet tail, poor fur coat, lethargy) were euthanized. Discussion
Classically, germinant receptors are embedded within the inner membrane of bacterial spores [10], [32]. Germinants must pass through layers of coat proteins, an outer membrane, the cortex and germ cell wall before interacting with their respective receptors. Upon interaction, Ca++-DPA is released from the spore core in exchange for water. This exchange is essential to rehydrate the core and allow metabolism to begin. In some bacteria, the release of Ca++-DPA triggers the activation of cortex hydrolases allowing a vegetative bacterium to grow from the germinating spore [33]. In C. perfringens the germination-specific proteases cleave the cortex hydrolase, SleC, to an active form [23], [24]. The signals that stimulate this proteolysis in C. perfringens are not known. In C. perfringens, CspA, CspB and CspC are all members of the subtilisin family of serine proteases and have complete catalytic triads, suggesting that any one of these proteins can activate SleC-mediated cortex hydrolysis. In C. difficile, the cspB and cspA coding sequences have been fused [34]. Only CspB contains a complete catalytic triad while CspA and CspC have lost their catalytic residues. Based on sequence analysis, one would predict that only CspB would have an active role in stimulating C. difficile cortex hydrolysis. Indeed, a recent study by Adams and colleagues has shown that CspBA undergoes autoprocessing to generate CspB, which can cleave the cortex hydrolase pro-SleC to an active form [34]. We have provided evidence that C. difficile CspC plays an active and essential role during germination by functioning as the bile acid germinant receptor.
In C. difficile CspC, two of the three catalytic residues have been lost, T170 (conserved H198 in C. perfringens CspC) and G485 (conserved S517 in C. perfringens CspC) (Figure S1 – red). Interestingly, several the SNPs identified in the germination-null screen lie near T170 or G485 (Figure S1 – green). When we screened for C. difficile mutants that germinated in response to an inhibitor of germination (chenodeoxycholic acid), we identified G457R (Figure S1 – yellow). This residue is approximately 30 amino acids removed from G485. G457R, being a fairly drastic substitution, may modify the bile acid binding pocket to allow for a less-stringent recognition of germination-inducing bile acids. The 12α-hydroxyl group that differentiates between cholic acid and chenodeoxycholic acid protrudes from the molecule. This hydroxyl, in wild-type CspC, may penetrate the hypothetical binding pocket, resulting in a conformational change that is transmitted to C. difficile CspB [34]. CspB would then cleave SleC, initiating cortex hydrolysis [34]. Two of the identified SNPs in the germination-null screen were nonsense mutations in cspBA (Q632stop and W359stop). In the CspBA hybrid protein, Q632 is located in CspA while W359 is in CspB. The generation of a premature stop codon in cspB would result in a truncated protein with an incomplete catalytic triad [34]. The precise role of CspA in C. difficile spore germination is unknown, though our data suggest that CspA may be important.
Our data indicate that host-derived bile acids mediate C. difficile spore germination and that recognition of bile acids is required for infection in the hamster model to be maximally effective. Still, 50% of the animals succumbed to disease when infected with the cspC mutant, suggesting (i) that enough spores spontaneously germinated in the GI tract of the animal to cause disease, or (ii) that other, as yet unidentified, host signals can stimulate spore germination. Lysozyme is able to induce germination of C. difficile spores in vitro [21]. However, recent evidence has suggested that lysozyme at physiological levels may not be able to stimulate C. difficile spore germination [35] and we observe most efficient lysozyme-mediated spore germination after spore coat removal. Further study will be needed to determine if other signals provided by the host can stimulate C. difficile spore germination.
Previously, we identified inhibitors of C. difficile spore germination that had increased potency when compared to chenodeoxycholic acid [19]. It is not yet known whether these germination inhibitors have therapeutic importance but a recent study by Howerton and coworkers has shown dosing antibiotic-treated mice with an inhibitor of germination can reduce disease severity [36]. The identification of the molecular target of bile acids in the C. difficile spore may allow even more potent inhibitors to be rationally designed. Further, these inhibitors may aid in the identification in the bile acid-binding pocket in CspC by providing high-affinity interaction, instead of the relatively low affinity (in the mM range) for taurocholic acid [19], [37].
The relative affinities of bile acids for the C. difficile spore were determined using kinetics of germination [18], [19], [37], [38], [39]. While these studies were important milestones in determining which bile acids affect C. difficile germination and what features are important for triggering or inhibiting germination, the precise interaction of the bile acid with the germinant receptor has not been analyzed. Also, it has been proposed that the bile acid germinant receptor binds taurocholic acid cooperatively [37]. The identification of the bile acid germinant receptor now permits testing these interactions.
Stimulation of cortex hydrolysis may not be sufficient to fully activate C. difficile spore germination. C. difficile spores suspended in buffered taurocholic acid alone do not initiate spore germination unless a co-germinant, glycine, is added [17]. This suggests that a second, glycine-sensing receptor is required to trigger germination and the return to vegetative growth. We hypothesize that this other receptor may be localized to the inner membrane to aid in the release of Ca++-DPA from the spore core during germination.
Materials and Methods
Ethics statement
All animal procedures were performed with prior approval from the Texas A&M Institutional Animal Care and Use Committee. Animals showing signs of disease were euthanized by CO2 asphyxia followed by thoracotomy as a secondary means of death, in accordance with Panel on Euthanasia of the American Veterinary Medical Association. Texas A&M University's approval of Animal Use Protocols is based upon the United States Government's Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training and complies with all applicable portions of the Animal Welfare Act, the Public Health Service Policy for the Humane Care and Use of Laboratory Animals, and all other federal, state, and local laws which impact the care and use of animals.
Bacterial strains and growth conditions
C. difficile UK1 [19] (Table 3) was grown in a Model B, Coy Laboratory Chamber at 37°C under anaerobic conditions (85% nitrogen, 10% hydrogen, 5% carbon dioxide) in BHIS medium (Brain Heart Infusion supplemented with 5 g/L yeast extract and 0.1% L-cysteine). Antibiotics were added as needed (20 µg/ml thiamphenicol, 10 µg/ml lincomycin, 5 µg/ml rifampin). E. coli DH5α [40] was routinely grown at 37°C in LB medium. Antibiotics were added as needed (50 µg/ml kanamycin or 20 µg/ml chloramphenicol). Bacillus subtilis was grown at 37°C in LB medium and antibiotics were added as needed (2.5 µg/ml chloramphenicol, 5 µg/ml tetracycline).
Tab. 3. Strains and plasmids used in this study. 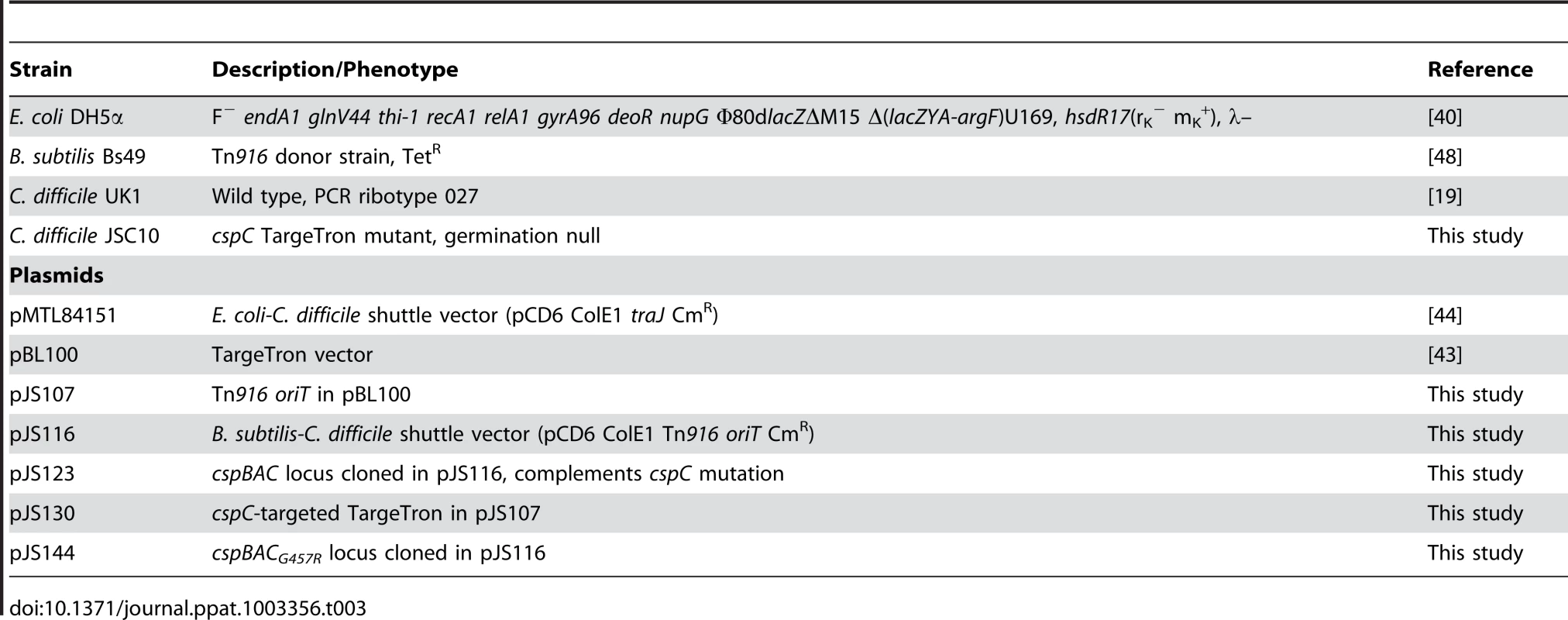
EMS mutagenesis
One overnight culture of C. difficile UK1 was diluted 1∶100 in 5 ml fresh medium and grown to OD600 = 0.5 before adding ethyl methanesulfonate (EMS) to 1% final concentration. The culture was incubated for 3 hours, washed in BHIS medium and recovered overnight in 40-ml BHIS. A sample was taken to score mutation frequency on rifampin-containing BHIS agar medium (Table S2) and 50-µL samples were spread on 20 BHIS plates to allow spore formation of the mutagenized bacteria. Plates were incubated for 4 days before spores were harvested and purified as described previously [19]. Purified spores were suspended in 40 ml BHIS+10% w/v taurocholic acid (TA) and incubated overnight at 37°C to germinate those spores that recognized TA as a germinant. Spores were collected and heated to 65°C for 1 hour to inactivate germinated spores (dormant spores are resistant to 65°C). To germinate the remaining dormant spores, spores were again collected and treated with 250 mM thioglycollate for 30 min at 50°C followed by incubation with 4 mg/ml lysozyme for 15 min at 37°C [21] and 25-µL aliquots were spread on BHIS agar plates to allow spore formation. To enrich for germination null phenotypes, spores were again collected and germinated as described above.
To select for mutations that change the affinity of the germinant receptor from TA to chenodeoxycholic acid, C. difficile UK1 was mutated as described above with the following modification. Purified spores generated from mutated bacteria were spread on BHIS medium supplemented with 0.5 mM chenodeoxycholic acid. Colonies from spores that germinated on this medium were purified and the germination phenotype of their spores was confirmed using standard germination techniques (below).
Illumina sequencing
High-quality, high-molecular weight genomic DNA was extracted, as described previously [41], [42], and submitted to Tufts University School of Medicine Genomics Core facility for Paired-End 50 Illumina re-sequencing. The samples were sonicated in a 4°C water bath with a Branson sonicator. Illumina libraries were then prepared using the Illumina TruSeq genomic DNA kit and tagged with individual Illumina barcodes. Final libraries were checked on said advanced analytical device, and then diluted to 10 nM prior to being loaded on a lane of an Illumina HiSeq2000. Illumina single-end sequencing was carried out for 50 cycles. The resulting sequence data in fastq format was aligned against the C. difficile R20291 genome using CLC Genomics Workbench, and SNPs were called at any position where more than 66% of the reads had an alternate base from the reference
Molecular biology
The Tn916 oriT from Bacillus subtilis Bs49 was amplified using oligonucleotides 5′Tn916SLIC and 3′Tn916SLIC (Table 4) and introduced into the BstAPI restriction site of pBL100 [43] using Sequence and Ligation Independent Cloning (SLIC), generating pJS107. The pJS107 plasmid was used as a TargeTron vector to introduce mutations in to C. difficile. The group II intron insertion sites for C. difficile cspC were identified using an algorithm that can be found at http://dna.med.monash.edu.au/~torsten/intron_site_finder/. The intron fragment was generated as described previously using oligonucleotides cspC (115) EBS2, cspC (115) IBS, cspC (115) EBS1 and EBSU, cloned into pCR2.1-TOPO and then sub-cloned at the HindIII and BsrGI sites of pJS107, yielding pJS130. The B. subtilis – C. difficile shuttle vector, pJS116, was generated through the introduction of the Tn916 oriT into the ApaI restriction site of the E. coli – C. difficile shuttle vector, pMTL84151 [44], using oligonucleotides 5′Tn916ApaI and 3′Tn916ApaI which amplify the Tn916 oriT. The C. difficile cspBAC loci were amplified with Phusion polymerase using 5′cspBA_CXbaI and 3′cspBA_CXhoI oligonucleotides and cloned into the B. subtilis – C. difficile shuttle vector, pJS116. The nucleotide sequences for all constructs were confirmed before use.
Tab. 4. Oligonucleotides used in this study. 
Conjugation and mutant selection
B. subtilis BS49 was used as a donor for conjugation with C. difficile. Plasmids were introduced into B. subtilis BS49 using standard techniques. Conjugation experiments were carried out as described previously [28]. C. difficile transconjugants were screened for the presence of Tn916 using tetracycline resistance. Thiamphenicol-resistant, tetracycline-sensitive (plasmid-containing, transposon negative) transconjugants were selected for further use. Potential TargeTron mutants were generated by screening lincomycin-resistant C. difficile for the insertion of the intron into C. difficile cspC using primers specific for full-length C. difficile cspC, the 5′ intron insertion site and the 3′ intron insertion site and a positive clone was identified, C. difficile JSC10.
Spore germination and DPA release
Spores were purified from BHIS agar medium as described previously [19] with the following modification. Spores from antibiotic-resistant strains (i.e. plasmid-containing or mutant strains) were generated on SMC medium [45] supplemented with appropriate antibiotics and purified as described previously. The initiation of spore germination was analyzed in a Lambda 25 Perkin Elmer spectrophotometer at A600 every 18 seconds, as described previously [17], [18], [19]. DPA release was measured by incubating purified spores at 37°C in germination salts (0.3 mM (NH4)2SO4, 6.6 mM KH2PO4, 15 mM NaCl, 59.5 mM NaHCO3 and 35.2 mM Na2HPO4) supplemented with 10% TA and 1 mM glycine for 1 hour. Equal aliquots were incubated at 100°C as a measure of 100% DPA release (positive control) or incubated at 37°C in germination salts without TA addition (negative control). Spores were sedimented and the supernatant was analyzed at A270 to measure the released DPA [46].
Virulence studies
Female Syrian golden hamsters, 80 g–120 g, were housed individually in cages and had ad libitum access to food and water for the duration of the experiment. To induce susceptibility to C. difficile infection, hamsters were gavaged with 30 mg/kg clindamycin [31], [47]. After 5 days, hamsters were gavaged with 1,000 spores of C. difficile UK1 or C. difficile JSC10 or C. difficile JSC10 pJS123, 10 animals per strain, and monitored for signs of disease (lethargy, poor fur coat and wet tail). Hamsters showing signs of disease were euthanized by CO2 asphyxia followed by thoracotomy as a secondary means of death in accordance with Panel on Euthanasia of the American Veterinary Medical Association. Fecal samples were collected daily and cecum samples were collected on those hamsters requiring euthanasia. All animal studies were performed with prior approval from the Texas A&M University Institutional Animal Care and Use Committee.
Statistical analyses
Experiments were performed in triplicate and, where indicated, error bars represent 1 standard deviation from the mean. A representative sample for the initiation of germination experiments at A600 is shown, error bars obscure the data. The data varied by <5%. Statistical significance of DPA release was performed using the Student's T-test. Differences in hamster survival between those infected with C. difficile JSC10 and either C. difficile UK1 or C. difficile JSC10 pJS123 were analyzed using the Log-rank test (GraphPad Prism).
Supporting Information
Zdroje
1. McDonaldLC, KillgoreGE, ThompsonA, OwensRCJr, KazakovaSV, et al. (2005) An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 353 : 2433–2441.
2. RedelingsMD, SorvilloF, MascolaL (2007) Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerging Infections Diseases 13 : 1417–1419.
3. WilsonKH, PeriniF (1988) Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun 56 : 2610–2614.
4. KuehneSA, CartmanST, HeapJT, KellyML, CockayneA, et al. (2010) The role of toxin A and toxin B in Clostridium difficile infection. Nature 467 : 711–713.
5. LyrasD, O'ConnorJR, HowarthPM, SambolSP, CarterGP, et al. (2009) Toxin B is essential for virulence of Clostridium difficile. Nature 458 : 1176–9 doi:10.1038/nature07822.
6. LawleyTD, CroucherNJ, YuL, ClareS, SebaihiaM, et al. (2009) Proteomic and genomic characterization of highly infectious Clostridium difficile 630 spores. J Bacteriol 191 : 5377–5386.
7. PruittRN, LacyDB (2012) Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol 2 : 28.
8. CarterGP, RoodJI, LyrasD (2012) The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol 20 : 21–29.
9. StragierP, LosickR (1996) Molecular Genetics of Sporulation in Bacillus subtilis. Annu Rev Genet 30 : 297–341.
10. SetlowP (2003) Spore germination. Curr Opin Microbiol 6 : 550–556.
11. Paredes-SabjaD, UdompijitkulP, SarkerMR (2009) Inorganic phosphate and sodium ions are cogerminants for spores of Clostridium perfringens type A food poisoning-related isolates. Appl Environ Microbiol 75 : 6299–6305.
12. SebaihiaM, WrenBW, MullanyP, FairweatherNF, MintonN, et al. (2006) The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat Genet 38 : 779–786.
13. WilsonKH (1983) Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol 18 : 1017–1019.
14. WilsonKH, KennedyMJ, FeketyFR (1982) Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol 15 : 443–446.
15. RailbaudP, DucluzeauR, MullerMC, SacquetE (1974) [Sodium taurocholate, a germination factor for anaerobic bacterial spores “in vitro” and “in vivo” (author's transl)]. Ann Microbiol (Paris) 125B: 381–391.
16. RidlonJM, KangD, HylemonPB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47 : 241–259.
17. SorgJA, SonensheinAL (2008) Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190 : 2505–2512.
18. SorgJA, SonensheinAL (2009) Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol 191 : 1115–1117.
19. SorgJA, SonensheinAL (2010) Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 192 : 4983–4990.
20. MoirA, LaffertyE, SmithDA (1979) Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J Gen Microbiol 124 : 165–180.
21. KamiyaS, YamakawaK, OguraH, NakamuraS (1989) Recovery of spores of Clostridium difficile altered by heat or alkali. J Med Microbiol 28 : 217–221.
22. MasayamaA, HamasakiK, UrakamiK, ShimamotoS, KatoS, et al. (2006) Expression of germination-related enzymes, CspA, CspB, CspC, SleC, and SleM, of Clostridium perfringens S40 in the mother cell compartment of sporulating cells. Genes Genet Syst 81 : 227–234.
23. Paredes-SabjaD, SetlowP, SarkerMR (2009) The protease CspB is essential for initiation of cortex hydrolysis and dipicolinic acid (DPA) release during germination of spores of Clostridium perfringens type A food poisoning isolates. Microbiology 155 : 3464–3472.
24. ShimamotoS, MoriyamaR, SugimotoK, MiyataS, MakinoS (2001) Partial characterization of an enzyme fraction with protease activity which converts the spore peptidoglycan hydrolase (SleC) precursor to an active enzyme during germination of Clostridium perfringens S40 spores and analysis of a gene cluster involved in the activity. J Bacteriol 183 : 3742–3751.
25. ZhongJ, KarbergM, LambowitzAM (2003) Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Research 31 : 1656–1664.
26. HeapJT, PenningtonOJ, CartmantST, CarterGP, MintonNP (2007) The ClosTron: A universal gene knock-out system for the genus Clostridium. J Microbiol Methods 79 : 452–464.
27. BurnsDA, HeapJT, MintonNP (2010) SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. J Bacteriol 192 : 657–664.
28. CarterGP, DouceGR, GovindR, HowarthPM, MackinKE, et al. (2011) The anti-sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS Pathog 7: e1002317.
29. HeegD, BurnsDA, CartmanST, MintonNP (2012) Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One 7: e32381.
30. GielJL, SorgJA, SonensheinAL, ZhuJ (2010) Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One 5: e8740.
31. ChangTW, BartlettJG, GorbachSL, OnderdonkAB (1978) Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun 20 : 526–529.
32. HudsonKD, CorfeBM, KempEH, FeaversIM, CootePJ, et al. (2001) Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. Journal of Bacteriology 183 : 4317–4322.
33. PaidhungatM, RagkousiK, SetlowP (2001) Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca(2+)-dipicolinate. J Bacteriol 183 : 4886–4893.
34. AdamsCM, EckenrothBE, PutnamEE, DoublieS, ShenA (2013) Structural and Functional Analysis of the CspB Protease Required for Clostridium Spore Germination. PLoS Pathog 9: e1003165.
35. Paredes-SabjaD, SarkerMR (2011) Germination response of spores of the pathogenic bacterium Clostridium perfringens and Clostridium difficile to cultured human epithelial cells. Anaerobe 17 : 78–84.
36. HowertonA, PatraM, Abel-SantosE (2013) A new strategy for the prevention of Clostridium difficile infections. J Infect Dis epub ahead of print.
37. RamirezN, LigginsM, Abel-SantosE (2010) Kinetic evidence for the presence of putative germination receptors in Clostridium difficile spores. J Bacteriol 192 : 4215–4222.
38. HowertonA, RamirezN, Abel-SantosE (2010) Mapping interactions between germinants and C. difficile spores. J Bacteriol 193 : 274–282.
39. AllenCA, BabakhaniF, SearsP, NguyenL, SorgJA (2013) Both Fidaxomicin and Vancomycin Inhibit Outgrowth of Clostridium difficile Spores. Antimicrob Agents Chemother 57 : 664–667.
40. HanahanD (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166 : 557–580.
41. BouillautL, McBrideSM, SorgJA (2011) Genetic manipulation of Clostridium difficile. Curr Protoc Microbiol Chapter 9: Unit 9A 2.
42. WrenBW, TabaqchaliS (1987) Restriction endonuclease DNA analysis of Clostridium difficile. J Clin Microbiol 25 : 2402–2404.
43. BouillautL, SelfWT, SonensheinAL (2012) Proline-Dependent Regulation of Clostridium difficile Stickland Metabolism. J Bacteriol epub ahead of print.
44. HeapJT, PenningtonOJ, CartmanST, MintonNP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78 : 79–85.
45. PermpoonpattanaP, TollsEH, NademR, TanS, BrissonA, et al. (2011) Surface layers of Clostridium difficile endospores. J Bacteriol 193 : 6461–6470.
46. Cabrera-MartinezR-M, Tovar-RojoF, VepacheduVR, SetlowP (2003) Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J Bacteriol 185 : 2457–2464.
47. SambolSP, TangJK, MerriganMM, JohnsonS, GerdingDN (2001) Infection of hamsters with epidemiologically important strains of Clostridium difficile. J Infect Dis 183 : 1760–1766.
48. HaraldsenJD, SonensheinAL (2003) Efficient sporulation in Clostridium difficile requires disruption of the sigmaK gene. Molecular Microbiology 48 : 811–821.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Plant Virus Ecology
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Volatile Metabolites of Pathogens: A Systematic Review
- Behind Closed Membranes: The Secret Lives of Picornaviruses?
- DNA from Skeletal Remains from the 6 Century AD Reveals Insights into Justinianic Plague
- Experimental Evolution of Pathogenesis: “Patient” Research
- Bile Acid Recognition by the Germinant Receptor, CspC, Is Important for Establishing Infection
- Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress
- The Secret Life of Viral Entry Glycoproteins: Moonlighting in Immune Evasion
- Uracil DNA Glycosylase Counteracts APOBEC3G-Induced Hypermutation of Hepatitis B Viral Genomes: Excision Repair of Covalently Closed Circular DNA
- Differences in Gastric Carcinoma Microenvironment Stratify According to EBV Infection Intensity: Implications for Possible Immune Adjuvant Therapy
- Polyphosphate and Its Diverse Functions in Host Cells and Pathogens
- Activation of the NLRP3 Inflammasome by IAV Virulence Protein PB1-F2 Contributes to Severe Pathophysiology and Disease
- Plant Virus Ecology
- Proving Lipid Rafts Exist: Membrane Domains in the Prokaryote Have the Same Properties as Eukaryotic Lipid Rafts
- Structural and Functional Basis for Inhibition of Erythrocyte Invasion by Antibodies that Target EBA-175
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Malaria Parasite cGMP-dependent Protein Kinase Regulates Blood Stage Merozoite Secretory Organelle Discharge and Egress
- The Secret Life of Viral Entry Glycoproteins: Moonlighting in Immune Evasion
- Structural and Functional Basis for Inhibition of Erythrocyte Invasion by Antibodies that Target EBA-175
- DNA from Skeletal Remains from the 6 Century AD Reveals Insights into Justinianic Plague
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



