-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Modelling the Evolutionary Dynamics of Viruses within Their Hosts: A Case Study Using High-Throughput Sequencing
Uncovering how natural selection and genetic drift shape the evolutionary dynamics of virus populations within their hosts can pave the way to a better understanding of virus emergence. Mathematical models already play a leading role in these studies and are intended to predict future emergences. Here, using high-throughput sequencing, we analyzed the within-host population dynamics of four Potato virus Y (PVY) variants differing at most by two substitutions involved in pathogenicity properties. Model selection procedures were used to compare experimental results to six hypotheses regarding competitiveness and intensity of genetic drift experienced by viruses during host plant colonization. Results indicated that the frequencies of variants were well described using Lotka-Volterra models where the competition coefficients βij exerted by variant j on variant i are equal to their fitness ratio, rj/ri. Statistical inference allowed the estimation of the effect of each mutation on fitness, revealing slight (s = −0.45%) and high (s = −13.2%) fitness costs and a negative epistasis between them. Results also indicated that only 1 to 4 infectious units initiated the population of one apical leaf. The between-host variances of the variant frequencies were described using Dirichlet-multinomial distributions whose scale parameters, closely related to the fixation index FST, were shown to vary with time. The genetic differentiation of virus populations among plants increased from 0 to 10 days post-inoculation and then decreased until 35 days. Overall, this study showed that mathematical models can accurately describe both selection and genetic drift processes shaping the evolutionary dynamics of viruses within their hosts.
Published in the journal: . PLoS Pathog 8(4): e32767. doi:10.1371/journal.ppat.1002654
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002654Summary
Uncovering how natural selection and genetic drift shape the evolutionary dynamics of virus populations within their hosts can pave the way to a better understanding of virus emergence. Mathematical models already play a leading role in these studies and are intended to predict future emergences. Here, using high-throughput sequencing, we analyzed the within-host population dynamics of four Potato virus Y (PVY) variants differing at most by two substitutions involved in pathogenicity properties. Model selection procedures were used to compare experimental results to six hypotheses regarding competitiveness and intensity of genetic drift experienced by viruses during host plant colonization. Results indicated that the frequencies of variants were well described using Lotka-Volterra models where the competition coefficients βij exerted by variant j on variant i are equal to their fitness ratio, rj/ri. Statistical inference allowed the estimation of the effect of each mutation on fitness, revealing slight (s = −0.45%) and high (s = −13.2%) fitness costs and a negative epistasis between them. Results also indicated that only 1 to 4 infectious units initiated the population of one apical leaf. The between-host variances of the variant frequencies were described using Dirichlet-multinomial distributions whose scale parameters, closely related to the fixation index FST, were shown to vary with time. The genetic differentiation of virus populations among plants increased from 0 to 10 days post-inoculation and then decreased until 35 days. Overall, this study showed that mathematical models can accurately describe both selection and genetic drift processes shaping the evolutionary dynamics of viruses within their hosts.
Introduction
Plant virus emergences represent near half of emerging plant infectious diseases [1] and often have detrimental consequences for food production. Emergences result from complex processes leading to novel virus-vector-plant-environment interactions [2], [3]. At the ecosystem level, numerous ecological factors, often related to changes in agricultural practices [3], favour emergence by impacting the very biology of viruses and vectors. At the molecular level, evolutionary factors allow viruses to jump host species barriers. As most viruses transferred to new hosts replicate poorly, the existence of already adapted variants within virus populations is often crucial to achieve a successful jump [4]. Though high mutation rates of RNA viruses favour the existence of already adapted variants, their dynamics in the reservoir hosts also depend on the strength of natural selection and genetic drift [4], [5]. Disentangling how selection and drift shape the evolutionary dynamics of viruses is therefore required to understand emergences [2], [5]. Mathematical models, which already play an important role in scrutinising the effects of such mechanisms, are also essential for estimating the likelihood of future emergences. Their scope of applications ranges from the management of drug-resistance in infectious diseases [6] to the achievement of durable plant resistance [7], [8].
Natural selection is a deterministic process by which the frequencies of the fittest variants in a given environment increase [9]. Selective effects among virus variants differing only by one or two point mutations can be very strong. Indeed, viruses with small genomes, including RNA and ssDNA viruses infecting animals, plants and bacteria, are characterized by a high mutational sensitivity. Non-lethal mutations reduce fitness by 10–13% on average [10]. Genetic drift is a stochastic process by which frequencies of virus variants change due to random sampling effects. Its strength is usually characterized by the effective population size () which is defined as the size of a theoretical population that would drift at the same rate as the observed population [11]. Although plant virus populations can reach extremely large sizes, estimates of during colonization of plant tissues remained relatively small, ranging from units [12], [13] to a few hundreds [14]. These figures indicate that virus populations are often faced with narrow genetic bottlenecks that limit the fixation of advantageous mutations and allow slightly deleterious mutations to reach high frequencies [2].
While within-host genetic drift and selection act simultaneously, and thus jointly determine emergence, their intensities have rarely been estimated and modelled jointly from experimental data. Drift intensity was often measured using populations of pathogen variants with equal multiplicative fitness [14]–[16] and comparison of selection intensity acting on variants did not take into account genetic drift [17]–[19]. In the present work, we characterized experimentally and modelled the within-host population dynamics of four Potato virus Y (PVY) variants simultaneously submitted to genetic drift and natural selection. The four variants differ by one or two mutations that change their pathogenicity properties towards pepper genotypes carrying resistance alleles at a single locus [20]. Virus population dynamics were followed using high-throughput sequencing (HTS) [21] to track quantitatively the dynamics of PVY populations within a susceptible pepper host. Analysis of HTS data was performed with some sensible mathematical models which allowed inferring both the selection process between competing virus variants and the intensity of drift experienced by viruses during host plant colonization.
Materials and Methods
Plant and virus materials
The pepper (Capsicum annuum L.) genotype used in this study was Yolo Wonder, a bell pepper cultivar susceptible to all PVY isolates. The SON41p infectious cDNA clone [22] and three derived PVY variants were used: NN, DN, NH and DH (the latter corresponding to SON41p). They were named after the amino acids observed at positions 119 and 121 of the VPg (viral protein genome-linked) pathogenicity factor (D, H and N representing aspartic acid, histidine and asparagine, respectively) (Figure 1A). The three mutated clones of SON41p differing by one or two substitutions in the VPg cistron were constructed using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, U.S.A.) [23]. Only variant DH, also termed resistance-breaking (RB) variant, was able to infect the pepper genotype Florida VR2 which carries the pvr22 resistance gene (Figure 1A) [20].
Fig. 1. Observed intra-host dynamics of four PVY variants. 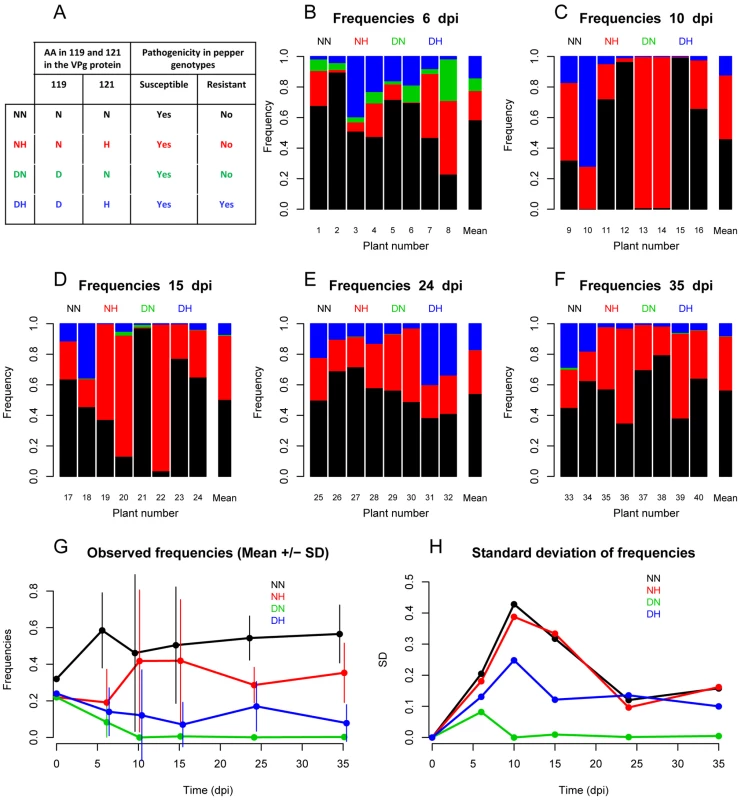
A: Description of the four PVY variants used (NN, NH, DN and DH). Variants are named according to the amino acids at positions 119 and 121 of the VPg pathogenicity factor. All variants infect the pepper genotype Yolo Wonder (YW) but only DH infects the genotype Florida VR2 which carries the pvr22 resistance gene. B–F: Frequencies of the four PVY variants in the eight plant samples collected 6, 10, 15, 24 and 35 dpi. Additionally, for each date, a bar with the mean frequencies of the variants for the eight samples is provided. G: Mean (± standard deviation) frequencies of the four PVY variants as a function of time (dpi). H: Standard deviation of the frequencies of the four PVY variants as a function of time (dpi). Plant inoculation
Inoculations were carried out under insect-proof greenhouse conditions. First, separate inoculations with the cDNA clones were realized by DNA-coated tungsten particle bombardments of juvenile Nicotiana clevelandii plants (four week old) [22]. Crude extracts of infected N. clevelandii plants were calibrated using DAS-ELISA [23], adjusted by dilution, mixed equally and then inoculated mechanically on the two cotyledons of 40 Yolo Wonder plants approximately three weeks after sowing (i.e. at two-leaf stage). The conformity of each variant was checked by direct sequencing of the RT-PCR product corresponding to the entire VPg cistron of the PVY populations present in the four plants used for the inoculum [22].
Sampling of PVY populations and HTS
Virus populations were separately sampled from eight plants at five successive dates: 6 days post-inoculation (dpi) corresponding to the 3–4 leaf stage, 10 dpi (5–6 leaf stage), 15 dpi (7–8 leaf stage), 24 dpi (11–12 leaf stage) and 35 dpi (22–23 leaf stage). Additionally, a sample of the mixed inoculum used for mechanical inoculations on pepper plants was also collected. At each date, all leaves of each plant were harvested, homogenized in a buffer (0.03 M phosphate buffer (pH 7.0) supplemented with 2% (w ∶ v) diethyldithiocarbamate; 4 mL of buffer per gram of leaves) and total RNAs were purified with the Tri Reagent kit (Molecular Research Center Inc., Cincinnati, OH, U.S.A.) from a 150 µL aliquot of each sample. RNAs were used to amplify the central part of the VPg cistron by RT-PCR with Avian myeloblastosis virus reverse transcriptase (Promega), the high-fidelity Herculase II fusion DNA polymerase (Stratagene) and primers PYRO-FOR (5′-attcatccaattcgttgatcc-3′, nucleotide positions 5930 to 5950) and PYRO-REV (5′-tgtcacaaaccttaagtggg-3′, nucleotide positions 6149 to 6168). Emulsion-PCR and high-throughput 454 sequencing were realized by GATC-Biotech (Konstanz, Germany). The genome region sequenced encompasses notably codons 101 to 123 of the VPg cistron which has been demonstrated by reverse genetics to be the only region involved in breakdown of the pvr2 resistance genes in pepper [20]. Since sampling was destructive, virus populations at the successive dates came from different plants.
In addition, in order to estimate the effective population size during leaf colonization, the eight plants sampled at 15 dpi were kept till 50 dpi and then a single newly grown leaf was sampled randomly on each plant (Figure S1). These leaves were individually crushed, total RNAs were purified and HTS of the central part of the VPg cistron was performed as described above. In the analysis, the virus populations characterized at 15 dpi constituted the “initial” populations and the ones at 50 dpi the “final” populations. In this protocol, as explained in [14], the “initial” populations defined by sampling all infected leaves at 15 dpi is likely to represent best the virus population circulating within the vascular system as infected leaves at 15 dpi have previously received (and exported) viruses from (and into) the vascular system. The “final” populations, sampled in a single systemically infected and newly grown leaf, necessarily originate from the “initial” populations regardless of the many successive stages of the systemic infection. The estimation of the effective population size arose from the comparison between the genetic variances of the “initial” and “final” populations.
Determination of PVY variant frequencies
We obtained between 209 and 930 correctly assigned sequences per virus population of each pepper plant, corresponding to a total of 24,166 sequences. Alignment was done using default parameters of the software SeqMan (DNASTAR Lasergene, Madison, U.S.A.). Because indels are the most frequent 454 pyrosequencing errors [24], a program, developed using the software R version 2.9.2 (R Development Core Team, 2009), was used to remove insertions and to replace deletions by the nucleotide present at the corresponding position in the four PVY variants. Because the program removed short sequences, the total number of cleaned sequences reduced to 20,795, ranging from 184 to 824 per virus population. Since no mutation with a significant frequency (>1%) has been observed in the sequenced region for any PVY population, the census of each variant (NN, DN, NH and DH) was determined for each sample according to the two polymorphic sites at codon positions 119 and 121 of the VPg coding region initially present in the PVY population (Table S1).
Modelling within-host virus population dynamics
General model framework
To investigate the time course of competing virus populations within host plants, we merged deterministic and stochastic aspects into a single model. The deterministic aspect relied on the assumption that the mean numbers (or densities) Vi(t) of virus variants i = 1,…,4 at time t, within a host plant behaved as a deterministic interacting system of four ordinary differential equations (ODE):(1)Model parameters have the following interpretation:
-
K is the virus carrying capacity of a plant,
-
ri is the intrinsic rate of increase of variant i,
-
βi,j is the coefficient that accounts for the competition strength exerted by genotype j on genotype i,
-
μi,j is the mutation rate from genotype j to genotype i.
Equation 1 extends Lotka-Volterra competition equations to four competitors [25] and introduces the mutation processes occurring between virus variants. We assumed that mutations occur independently. Thereby, if μ denotes the point mutation rate per replication cycle and per nucleotide, we get if variants i and j are distant from one mutation and if variants i and j are distant from two mutations.
The stochastic aspect took account of random fluctuations from the theoretical means Vi(t) due to the heterogeneity of virus populations between plants and to the nature of samples (counts of virus sequences). To be more specific, let denote the vector of numbers of sequences observed for plant p sampled at time t (dpi) corresponding to virus variants NN, DN, NH and DH. Our second main assumption states that, conditionally to the total number of sequences, the random vector resulted from a Dirichlet-multinomial (DM) distribution, i.e.:(2)where (i) is the theoretic vector parameter of variant frequencies at time t over all plants (i.e. ) and
(ii) is a scale parameter related to the intensity of virus genetic differentiation between plants at time t.
For clarity, we recall that an integer valued vector N (discarding time and plant indices t and p) is distributed if N results from a two-step random procedure. First, a non-observed random vector is drawn from a Dirichlet distribution . Then, given a total number Ntot and random frequencies Λ, N is drawn according to a multinomial . Our assumption finally reads as: sampling a plant p at time t gives rise to a non-observed random vector of variant frequencies depending on virus population differentiation θ(t) and on overall theoretic means λi(t) of the variant frequencies. The numbers of observed sequences of virus variants in plant p at time t is a multinomial . Since and , large values of θ(t) decrease the genetic differentiation among host plants. Asymptotically, if , the Dirichlet multinomial distribution converges towards a simple multinomial distribution without genetic differentiation at all, i.e. Λ is deterministic and equals λ. Besides, θ(t) can be actually related to the so-called fixation index FST [11] by [26].
Finally, the coupling between deterministic and stochastic components consisted in asserting that the overall frequencies of variants at time t were given via the ODE system as .
Within-host virus dynamics: model selection and inferences
Our goal was to determine (i) the forms of selection processes occurring between competing viruses within a host plant, which could be handled via the forms of the competition coefficients used to derive the overall theoretical frequencies λi(t) and (ii) the intensity and temporal variation of genetic drift experienced by viruses during host plant colonization, which could be investigated via the time dependence of the scale parameter θ(t).
Accordingly, six models allowing four to 14 parameters (Table 1) were considered. All models included four intrinsic rates of increase (under the constraint). Regarding the competition issue, three embedded hypotheses were proposed for the Lotka-Volterra competition coefficients. From general to particular:
-
(C1) : inverse reciprocal competition of virus variants without any prior,
-
(C2) : inverse reciprocal competition based on the ratio of intrinsic rates of increase,
-
(C3) : blind and uniform competition between virus variants.
Tab. 1. Models description and selection criteria. 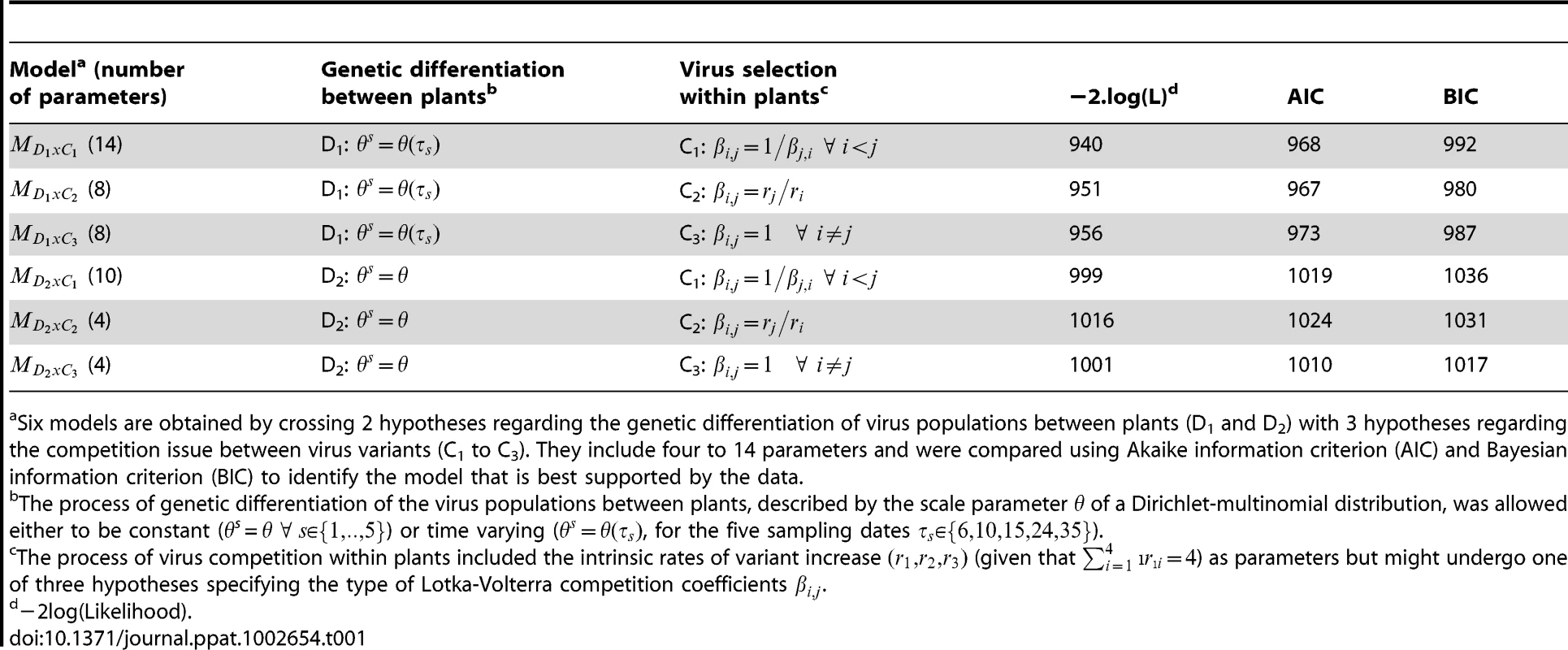
Six models are obtained by crossing 2 hypotheses regarding the genetic differentiation of virus populations between plants (D1 and D2) with 3 hypotheses regarding the competition issue between virus variants (C1 to C3). They include four to 14 parameters and were compared using Akaike information criterion (AIC) and Bayesian information criterion (BIC) to identify the model that is best supported by the data. Regarding the genetic differentiation of virus populations between plants, two embedded hypotheses were considered. From general to particular:
-
(D1) where s = 1,‥,5 are free parameters: genetic differentiation between plants is time dependent ( in dpi),
-
(D2) where s = 1,‥,5: genetic differentiation between plants is constant with time.
Six models, denoted (Table 1), are obtained by crossing hypotheses Di with Cj. Under the constraint and by setting K to 106 and μ to 10−5 [27], the six models were statistically identifiable using maximum likelihood techniques. For initial values of ODE system, was arbitrarily set to 100 whereas = (0.32, 0.22, 0.22, 0.24) corresponded to the observed frequencies of virus sequences in the inoculum. A note on model identifiability and likelihood-based inferences is provided in Text S1. Computations were performed with the R software environment using “bbmle” package and “nlminb” optimization routines. Models were compared using AIC and BIC procedures (Akaike and Bayesian Information Criteria) to choose the model that is best supported by the data.
Estimation of the effective virus population size during host colonization
In order to estimate the effective population size (Ne), i.e. the number of founder infectious units initiating the systemic infection of a single leaf, we used a method based on FST statistics described in [14]. Population genetics theory asserted that, for a haploid organism, , where (resp. ) corresponds to FST value at 15 dpi (resp. 50 dpi). A 95% confidence interval was calculated for Ne with a bootstrap method by resampling data 1,000 times over plants.
Results
Intra-host virus dynamics: insights from raw data
HTS of the composite inoculum confirmed that DAS-ELISA used to calibrate PVY variants concentration was accurate. The frequencies observed a posteriori, i.e. 0.32, 0.22, 0.22 and 0.24 for the NN, DN, NH and DH variants, respectively, were quite close to the expected frequency of 0.25, although an excess of NN was noticed.
During the course of the experiment, variant NN was selected; its mean frequency increased from 0.32 in inoculum to 0.57 in the populations sampled at 35 dpi (Figure 1G). This selection took place rapidly and could be detected as soon as at 6 dpi. Selection was also observed for variant NH, whose mean frequency increased from 0.22 to 0.35. Conversely, variants DN and DH were counter-selected: their mean frequency decreased from 0.22 (resp. 0.24) in the inoculum to 0.003 (resp. 0.07) at 35 dpi (Figure 1G).
Besides these mean trends, the standard deviation of variant frequencies between plants exhibited remarkable dynamics (Figures 1B to 1F, Figure 1H). It reached a maximum at 10 dpi, except for DN which had the lowest frequency. In four out of the eight plants analyzed at 10 dpi, a single variant (NH in two plants and NN in two others) dominated (Figure 1C). This observation indicated that virus populations underwent strong stochastic variations until 10 dpi. Later, the genetic differentiation of virus populations between plants tended to decrease: two weeks later, at 24 dpi, three variants (NN, NH, DH) co-infected all the eight plants analyzed (Figure 1E).
Intra-host virus dynamics: insights from data modelling
A model selection procedure was used to test hypotheses concerning the selection process occurring between competing virus variants within a host plant and the intensity of drift experienced by viruses during host plant colonization. According to both AIC and BIC criteria, the model (Table 1) was best supported by the data. It satisfactorily fitted the data with an r2 value of 0.88 between observed and predicted mean frequencies of virus variants (Figure 2A) and of 0.71 between observed and predicted standard deviations of these frequencies (Figure 2B). The root mean square error (RMSE) was 0.07. Inference was not sensitive (percentage of variations <5%) to 1000 fold ranges of variation of the mutation rate μ and of the number of inoculated viruses . Inference was also only slightly sensitive to a 20% random fluctuation of the inoculum initial frequencies (Text S1).
Fig. 2. Goodness of fit of the model that is best supported by the data (model 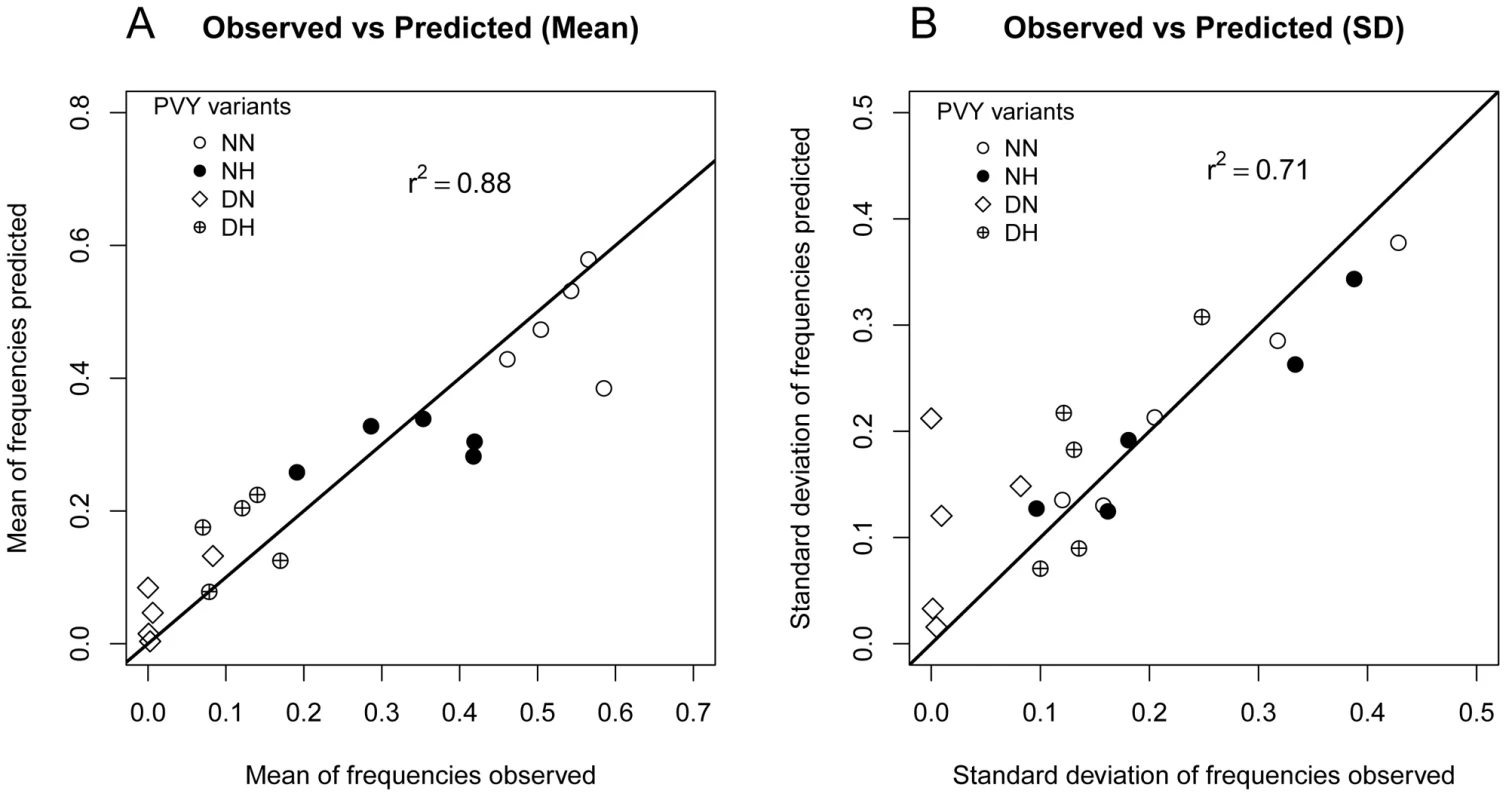
). A: Correlation between the 20 (4 variants×5 dates) observed mean frequencies of the four PVY variants and their estimated mean values. B: Correlation between the 20 observed standard deviations of the frequencies of the four PVY variants and their estimated standard deviations. The full line is the first diagonal (i.e. line y = x). Inference on intrinsic rates of increase revealed significant differences of fitness between the four variants (Figure 3A). Variants NN and NH had the highest increase rates (1.048 for NN and 1.043 for NH), significantly higher than the one of DH (0.99). Variant DN had significantly the lowest rate of increase (0.91). Moreover, the selection of the model lent support to Lotka-Volterra competition coefficients expressed as the ratio .
Fig. 3. Parameter estimates of the model that is best supported by the data (model 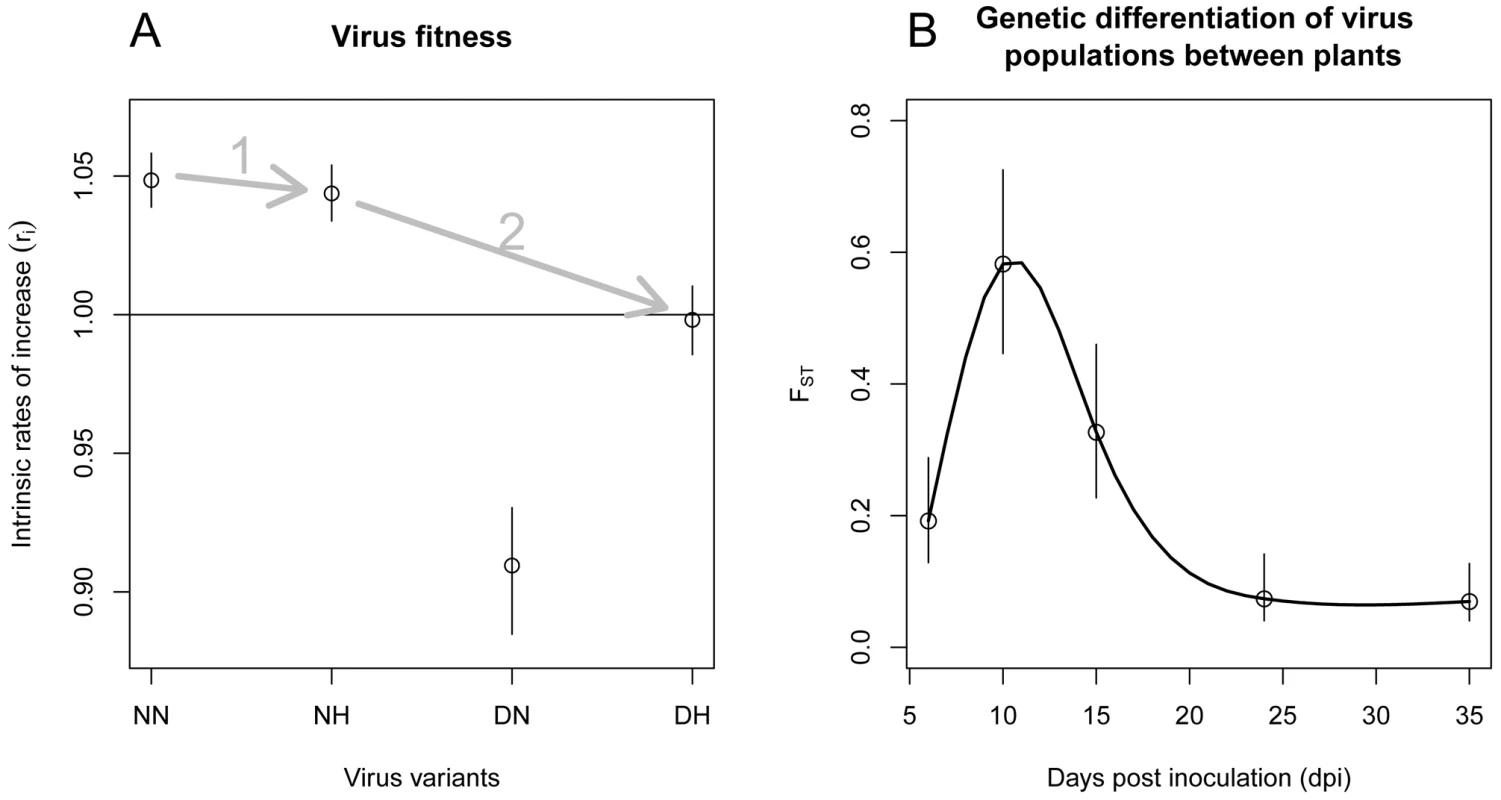
). A: Relative fitness of the four PVY variants (NN, NH, DN and DH) estimated by their intrinsic rates of increase r. The mean fitness of the population was arbitrarily set to 1 due to identifiability constraints (Text S1). In both graphs, dots indicate the mean values of the parameter whereas segments stand for the 95% confidence interval. Arrows indicate the most likely pathway leading to the resistance-breaking variant. B: FST indices as a function of time (dpi). FST characterizes the degree of genetic differentiation of the virus populations between plants. For each sampling date , was assessed as where is the scale parameter of a Dirichlet-multinomial distribution. For illustration purposes, a spline function (full line) is fitted to data. Regarding the intensity of drift experienced by viruses during plant colonization, selection of model indicated that the genetic differentiation of virus populations between plants varied significantly with time. So did the fixation indices, FST (Figure 3B). The differentiation was maximal at 10 dpi, with an FST of 0.58 and minimal at 35 dpi with an FST of 0.069.
Stable equilibrium of the Lotka-Volterra system
The long term behaviour of the model that is best supported by the data (Lotka-Volterra system with and mutation process) was theoretically studied. The differential system admits a single stable equilibrium which attracts all possible trajectories (i.e. the equilibrium point does not depend on initial conditions). The detailed proof is provided in Text S2. At equilibrium, all variants co-exist but no simple analytical expression of the equilibrium could be derived. Analytical results showed that the fittest variant predominates at equilibrium point whereas the density of other variants depended largely on (i) their genetic distance from the fittest variant and (ii) the difference between the intrinsic increase rate of the fittest variant and their own increase rate. According to parameter estimates, the relative frequencies of variants NN, DN, NH and DH at equilibrium were 0.9978, 0.0021, 7.18×10−5 and 4.34×10−7, respectively.
Estimation of Ne during plant colonization
Ne during plant colonization was estimated by comparing the genetic variances of PVY populations sampled at 15 dpi (initial populations) and at 50 dpi (final populations) among the same plants. At 15 dpi, three variants were systematically detected in all plants, although with varying frequencies (Figure 1D). The final PVY populations, sampled from a single apical leaf of each plant at 50 dpi, differed largely from the initial ones. For seven out of eight plants, the frequency of variant NN was >0.85, while in the remaining plant, variant NH predominated (Table S1). This observation supported the hypothesis of large stochastic variations during the systemic infection of a newly formed leaf. Accordingly, the estimated effective population size amounted to 2.25 (Table S2) with a 95% confidence interval ranging from 1.3 to 3.38. Actually, the method did not allow to disentangle the effects of selection and genetic drift on . When applied only to the two variants NN and NH showing equal relative fitness (Figure 3A) and therefore subjected only to genetic drift, Ne was estimated to 2.14 with a 95% confidence interval ranging from 1.29 to 3.41.
Discussion
The present study investigated with HTS the intra-host dynamics of plant virus populations and their variability between plants. Data revealed a strong pattern of genetic differentiation of virus populations between plants with FST indices that increased from inoculation date to 10 dpi and then decreased until 35 dpi. From inoculation to 6 dpi, heterogeneity observed between plants could be potentially related to the very inoculation process and/or to the process of colonization of plants by viruses. Although we cannot exclude some random effects due to inoculation, we believe that most observed variance was due to within-plant colonization processes, as the four variants inoculated were detected still in all samples at 6 dpi (Figure 1B). Severe bottlenecks act on most virus populations at all the scales within infected plants, from virus loading into individual cells (MOI, multiplicity of infection, defined as the number of virus genomes that enter and effectively replicate in individual cells [15], [28]), to colonization of tissues and organs through plasmodesmata [29] and to translocation in the whole plant through the vascular system [12], [13], [30]. We strengthen the latter results for another RNA virus by showing that between one to four PVY genomes initiate the population of systemically-infected leaves. However, severe bottlenecks during host colonization are not necessarily the rule for all plant viruses. Using the same protocol, several hundred genomes of CaMV, a DNA virus, were shown to initiate infection of apical leaves [14].
The scale of our study was the set of leaves of infected plants. It represents the epidemiologically-relevant part of the virus population since it is readily accessible to vectors that ensure plant-to-plant horizontal transmission. It is possible that the narrow bottlenecks and intense genetic drift observed at larger scales (whole plant and plant organs) are direct consequences of those incurred by virus populations at the smallest scale (individual cell). Supporting this hypothesis, the time dependence of FST which we observed at the whole-plant level (Figure 3B) parallels that of the MOI in another plant virus [15]. MOI values observed by [15] were close to 2 at 14 dpi, increased up to 13 at 40 dpi and then decreased to the initial level at 70 dpi. Accordingly, we observed a FST decrease in the time period shared by both studies (from 14 to 35 dpi).
It is also possible that decreasing FST values observed at later infection times are related to the sink-source transition undergone by leaves during plant growth. Source-to-sink translocation of carbohydrates through the phloem corresponds to the direction of the systemic movement of viruses within plants [31], [32]. As the plant grows, more and more leaves behave as virus sources, a process beginning in oldest leaves. Consequently, as the plant matures, more leaves unload their viruses into the phloem sieve elements and can contribute to the colonization of new expanding leaves at the apex of the plant, hence increasing the size of the source virus population within plants and decreasing between-plant FST values. In the context of our experiments, the timing of the sink-source transition in pepper and its comparison with FST variation remain to be determined. The two above hypotheses are not mutually exclusive, since the increasing number of source leaves during infection could also increase the MOI [15], and, in turn, decrease between-plant FST values.
Even if major stochastic events impact the evolutionary dynamics of virus populations, natural selection remains a powerful force in virus evolution [2]. In our experiment, two variants were selected (NN and NH) and the other two counter-selected (DN and DH). The fitness effect of each mutation can be assessed from their intrinsic rates of increase. Compared to the fittest variant (NN), the fitness effects of mutations N121H and N119D amounted to −0.45% and −13.2%, respectively. These figures agree with the distribution of the mutational effect of single nucleotide substitutions. Indeed non-lethal mutations reduce virus fitness by 10–13% on average [10]. The fitness cost (4.8%) of the variant combining both mutations (DH) indicated a case of negative epistasis, as often observed for RNA viruses [2], [4]. Altogether, these data determined the most likely evolutionary pathway toward the breakdown of the pepper resistance gene pvr22 (mutation N121H followed by mutation N119D) [4], [33] (Figure 3A). In all, the RB variant is counter-selected in virus populations. When extrapolating the dynamics of the Lotka-Volterra system, the mean frequency of the RB variant would be <4% at 50 dpi. This prediction fitted our observation of ∼0.5% mean frequency of the RB variant at 50 dpi, although these results should be read with caution, as the sampling scheme differed at this date. Moreover, even if the long-time behaviour of the system indicated that all variants would co-exist at an equilibrium (which corresponds to the mutation-selection balance), the frequency of the RB variant would be very low (∼5×10−7). In natural context, the RB variant would most likely appear initially by mutation at a very low frequency in a virus population largely dominated by the fittest variants NN and NH. Note also that, although multidrug-resistant variants can be generated by recombination (e.g. [34] on HIV), its role is unlikely in the present case because the two critical amino acid positions are only two amino acids apart. Altogether these data provide an explanation of the scarcity of viruses able to overcome the resistance gene pvr22 in natural context [20].
By confronting the results of virus dynamics simulated under several Lotka-Volterra models differing by the form of the competition coefficients, we learnt about the selection process occurring between competing virus variants. These coefficients describe the interactions of several competing variants for host factors necessary for virus replication and movement within plants. Statistical model selection results lent support to the hypothesis that the competition coefficients βij exerted by variant j on variant i are equal to their fitness ratio, . The assumption was initially argued on a theoretical ground [35]. These authors showed that Eigen's model of molecular quasispecies [36] was to a large extent equivalent to the Lotka-Volterra competition equations when assuming that . However this did not imply that the virus populations studied behaved as quasispecies. In particular, the quasispecies model does not allow for stochastic changes in population structure [30] whereas, as discussed earlier, the present results evidenced strong effects of genetic drift.
Overall, this study showed that mathematical models can accurately describe both selection and genetic drift shaping the evolutionary dynamics of viruses within hosts. A similar within-host model was coupled with an epidemiological model in [37] to assess the relative effects of ten demo-genetic and epidemiological parameters on the probability of breakdown of a plant resistance. Our present results validated a posteriori this choice. More generally, the modelling framework proposed here might provide a valuable cornerstone of models linking within - and between-host scales of disease dynamics [38]. It also might provide useful tools to study the interplay between the evolutionary and epidemiological processes acting on a virus population, at the individual host scale but also at the population host scale [39], and ultimately to design some efficient control strategies of virus emergence.
Supporting Information
Zdroje
1. AndersonPKCunninghamAAPatelNGMoralesFJEpsteinPR 2004 Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol 19 535 544
2. HolmesEC 2009 The evolutionary genetics of emerging viruses. Annu Rev Ecol Evol Syst 40 353 372
3. JonesRAC 2009 Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res 141 113 130
4. ElenaSFBedhommeSCarrascoPCuevasJMde la IglesiaF 2011 The evolutionary genetics of emerging plant RNA viruses. Mol Plant Microbe Interact 24 287 293
5. HolmesECDrummondAJ 2007 The evolutionary genetics of viral emergence. ChildsJEMacKenzieJSRichtJA Wildlife and emerging zoonotic diseases: The biology, circumstances and consequences of cross-species transmission Verlag Berlin Heidelberg Springer 51 66
6. zur WieschPAKouyosREngelstädterJRegoesRRBonhoefferS 2011 Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infect Dis 11 236 247
7. GómezPRodríguez-HernándezAMMouryBArandaMA 2009 Genetic resistance for the sustainable control of plant virus diseases: Breeding, mechanisms and durability. Eur J Plant Pathol 125 1 22
8. FabreFRousseauEMailleretLMouryB 2012 Durable strategies to deploy plant resistance in agricultural landscapes. New Phytol 193 1064 1075
9. García-ArenalFFraileAMalpicaJM 2001 Variability and genetic structure of plant virus populations. Annu Rev Phytopathol 39 157 186
10. SanjuánR 2010 Mutational fitness effects in RNA and single-stranded DNA viruses: Common patterns revealed by site-directed mutagenesis studies. Phil Trans R Soc B 365 1975 1982
11. WrightS 1931 Evolution in Mendelian populations. Genetics 16 97 159
12. HallJSFrenchRHeinGLMorrisTJStengerDC 2001 Three distinct mechanisms facilitate genetic isolation of sympatric wheat streak mosaic virus lineages. Virology 282 230 236
13. SacristánSMalpicaJMFraileAGarcía-ArenalF 2003 Estimation of population bottlenecks during systemic movement of Tobacco mosaic virus in tobacco plants. J Virol 77 9906 9911
14. MonsionBFroissartRMichalakisYBlancS 2008 Large bottleneck size in Cauliflower mosaic virus populations during host plant colonization. PLoS Pathog 4 e1000174
15. GutiérrezSYvonMThébaudGMonsionBMichalakisY 2010 Dynamics of the multiplicity of cellular infection in a plant virus. PLoS Pathog 6 e1001113
16. ZwartMPDaròsJAElenaSF 2011 One is enough: In vivo effective population size is dose-dependent for a plant RNA virus. PLoS Pathog 7 e1002122
17. CarrascoPde la IglesiaFElenaSF 2007 Distribution of fitness and virulence effects caused by single-nucleotide substitutions in Tobacco etch virus. J Virol 81 12979 12984
18. JanzacBMontarryJPalloixANavaudOMouryB 2010 A point mutation in the polymerase of Potato virus Y confers virulence toward the Pvr4 resistance of pepper and a high competitiveness cost in susceptible cultivar. Mol Plant Microbe Interact 23 823 830
19. FraileAPagánIAnastasioGSáezEGarcía-ArenalF 2011 Rapid genetic diversification and high fitness penalties associated with pathogenicity evolution in a plant virus. Mol Biol Evol 28 1425 1437
20. AymeVPetit-PierreJSoucheSPalloixAMouryB 2007 Molecular dissection of the Potato virus Y VPg virulence factor reveals complex adaptations to the pvr2 resistance allelic series in pepper. J Gen Virol 88 1594 1601
21. BrockhurstMAColegraveNRozenDE 2011 Next-generation sequencing as a tool to study microbial evolution. Mol Ecol 20 972 980
22. MouryBMorelCJohansenEGuilbaudLSoucheS 2004 Mutations in Potato virus Y genome-linked protein determine virulence toward recessive resistances in Capsicum annuum and Lycopersicon hirsutum. Mol Plant Microbe Interact 17 322 329
23. AymeVSoucheSCarantaCJacquemondMChadoeufJ 2006 Different mutations in the genome-linked protein VPg of Potato virus Y confer virulence on the pvr23 resistance in pepper. Mol Plant Microbe Interact 19 557 563
24. HuseSMHuberJAMorrisonHGSoginMLWelchDM 2007 Accuracy and quality of massively parallel DNA pyrosequencing. Genome Biol 8 R143
25. BulmerM 1994 Theoretical evolutionary ecology Sunderland, MA Sinauer 352
26. KitakadoTKitadaSKishinoHSkaugHJ 2006 An integrated-likelihood method for estimating genetic differentiation between populations. Genetics 173 2073 2082
27. SanjuánRNebotMRChiricoNManskyLMBelshawR 2010 Viral mutation rates. J Virol 84 9733 9748
28. González-JaraPFraileACantoTGarcía-ArenalF 2009 The multiplicity of infection of a plant virus varies during colonization of its eukaryotic host. J Virol 83 7487 7494
29. MiyashitaSKishinoH 2010 Estimation of the size of genetic bottlenecks in cell-to-cell movement of soil-borne Wheat mosaic virus and the possible role of the bottlenecks in speeding up selection of variations in trans-acting genes or elements. J Virol 84 1828 1837
30. FrenchRStrengerDC 2005 Population structure within lineages of Wheat streak mosaic virus derived from a common founding event exhibits stochastic variation inconsistent with the deterministic quasi-species model. Virology 343 179 189
31. TurgeonR 1989 The sink-source transition in leaves. Annu Rev Plant Physiol Plant Mol Biol 40 119 138
32. VuorinenALKelloniemiJValkonenJPT 2011 Why do viruses need phloem for systemic invasion of plants. Plant Sci 181 355 363
33. WeinreichDMWatsonRAChaoLHarrisonR 2005 Perspective: Sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59 1165 1174
34. RhodesTDNikolaitchikOChenJPowellDHuWS 2005 Genetic recombination of Human immunodeficiency virus type 1 in one round of viral replication: effects of genetic distance, target cells, accessory genes, and lack of high negative interference in crossover events. J Virol 79 1666 1677
35. SoléRVFerrerRGonzález-GarcíaIQuerJDomingoE 1999 Red queen dynamics, competition and critical points in a model of RNA virus quasispecies. J Theor Biol 198 47 59
36. EigenMMcCaskillJSchusterP 1988 Molecular quasi-species. J Phys Chem 92 6881 6891
37. FabreFBruchouCPalloixAMouryB 2009 Key determinants of resistance durability to plant viruses: Insights from a model linking within - and between-host dynamics. Virus Res 141 140 149
38. MideoNAlizonSDayT 2008 Linking within - and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol Evol 23 511 517
39. JegerMJSealSEVan den BoschF 2006 Evolutionary epidemiology of plant virus disease. ThreshJMMaramoroschKShatkinAJ Plant virus epidemiology San Diego Academic Press 163 203
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Fungal Biofilms
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge
- The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection
- Modelling the Evolutionary Dynamics of Viruses within Their Hosts: A Case Study Using High-Throughput Sequencing
- Structural Basis of Cytotoxicity Mediated by the Type III Secretion Toxin ExoU from
- Fungal Biofilms
- The Problem of Auto-Correlation in Parasitology
- T Regulatory Cells Control Susceptibility to Invasive Pneumococcal Pneumonia in Mice
- Small-Molecule Inhibitors of Dengue-Virus Entry
- The Baculovirus Uses a Captured Host Phosphatase to Induce Enhanced Locomotory Activity in Host Caterpillars
- Matrix Metalloprotease 9 Mediates Neutrophil Migration into the Airways in Response to Influenza Virus-Induced Toll-Like Receptor Signaling
- Necrotrophism Is a Quorum-Sensing-Regulated Lifestyle in
- Modeling of the N-Glycosylated Transferrin Receptor Suggests How Transferrin Binding Can Occur within the Surface Coat of
- The Role of Cofactors in Prion Propagation and Infectivity
- The Role of Mast Cells in the Defence against Pathogens
- The Accessory Genome as a Cradle for Adaptive Evolution in Pathogens
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Accessory Genome as a Cradle for Adaptive Evolution in Pathogens
- Systematic Review of Mucosal Immunity Induced by Oral and Inactivated Poliovirus Vaccines against Virus Shedding following Oral Poliovirus Challenge
- The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection
- Modelling the Evolutionary Dynamics of Viruses within Their Hosts: A Case Study Using High-Throughput Sequencing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



