-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaZebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction
article has not abstract
Published in the journal: . PLoS Pathog 8(1): e32767. doi:10.1371/journal.ppat.1002349
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002349Summary
article has not abstract
What Are the Advantages of This System Compared to Other Infection Models?
In many ways, the zebrafish represents a hybrid between mouse and invertebrate infection models. Powerful forward-genetic tools that have made invertebrates justifiably famous are not only relatively accessible in the zebrafish, but have been exploited to yield new insights into human infectious diseases, including leprosy and tuberculosis [1]. Transgenic technologies have enabled detailed, non-invasive in vivo visualization of macrophages and neutrophils in pitched battle with bacteria and fungi [2], [3]. Reverse genetics with morpholinos, vivo-morpholinos, and zinc-finger nucleases (but unfortunately not homologous recombination, which for the moment remains out of reach in this organism) enable examination of the roles of specific genes during infection. Flexible genetic systems such as Gal4-UAS and Cre-Lox permit tissue-specific transformation and ablation ([3]; Figure 1).
Fig. 1. A sampling from the zebrafish toolbox. 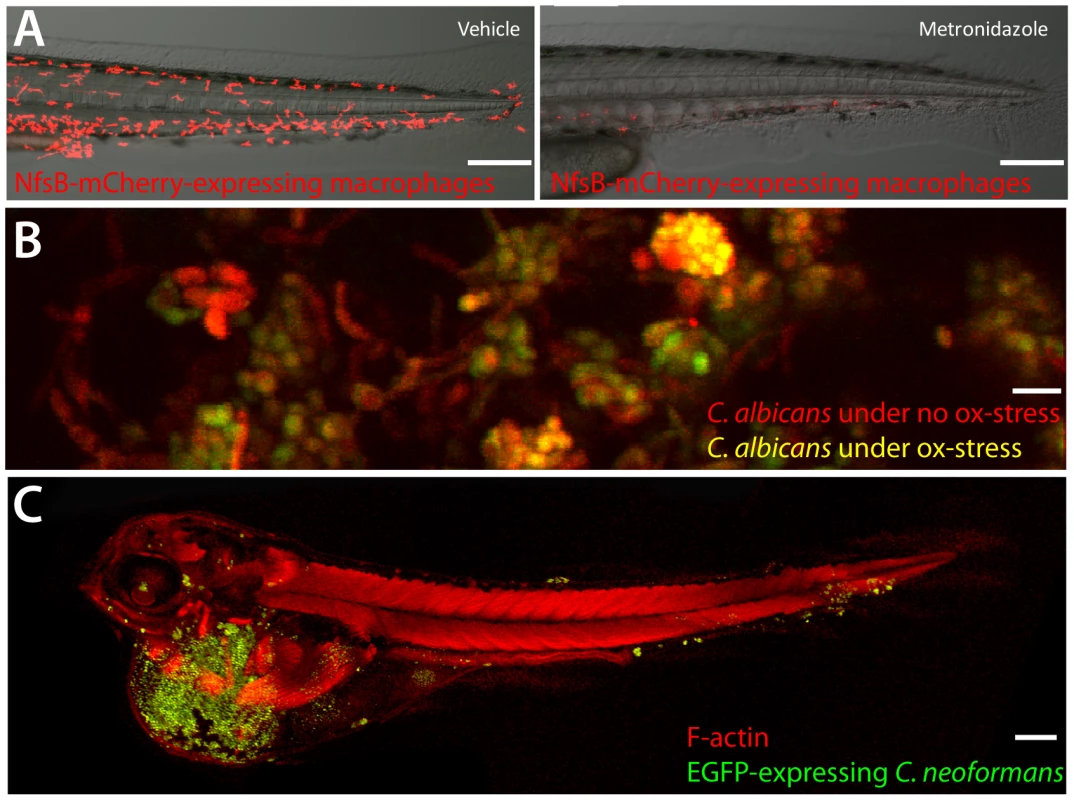
(A) Selective ablation of macrophages. Transgenic fish with macrophage-specific expression of Gal4 [2] and Gal4-dependent expression of nitroreductase-mCherry fusion protein were incubated at 3 dpf with 5 mM metronidazole or vehicle for 24 hours. Neither transgenics nor controls exposed to metronidazole had any loss of viability or developmental defects. Ablation efficiency of macrophages is >90% (R. Gratacap and R. Wheeler,unpublished data). Scale bar = 100 µm. (B) OXYellow Candida albicans reports on oxidative stress in vivo. Zebrafish larvae were infected in the hindbrain with OXYellow C. albicans (expressing mCherry constitutively and EGFP under the oxidative stress-induced catalase promoter) and imaged at 24 hours post-infection. Green/red ratio quantifies oxidative stress (K. Brothers and R. Wheeler, unpublished dat). Scale bar = 10 µm. (C) Cryptococcus neoformans infects zebrafish embryos. Zebrafish were infected with EGFP-expressing C. neoformans and imaged. Clusters of fungi are seen in the tail (S. Johnston and R. Ma, unpublished data). Scale bar = 100 µm. These technologies can be applied to hundreds of embryos in a single day. Zebrafish embryos at the one - to four-cell stage are microinjected with morpholinos to target translation or splicing of specific transcripts, or to limit microRN (mRNA) activity. This knockdown can be effective for up to 10 days post-fertilization, allowing relatively long-term imaging of infection in the background of specific gene knockdowns. Similarly, early microinjection with mRNA for the Tol2 transposase along with DNA constructs bracketed by Tol2 repeats results in remarkably efficient transgenesis. From injection to the establishment of a stable transgenic line can be less than eight weeks.
Is the Zebrafish Immune System Similar to the Human?
The short answer is yes, very similar. We share a similar developmental program, a comparable set of specialized immune cells including B and T cells, and a similar suite of immune signaling molecules. Recent studies on the monocytic phagocyte system, dendritic cells, and eosinophils show that the more we study the zebrafish immune system, the more similarities we find. Although zebrafish have both innate and adaptive arms of immunity, as in mammals, the adaptive arm takes longer to develop, and therefore innate immunity is the sole protector of young fish up to 4 weeks old. Thus, initial host–pathogen interactions can be studied in isolation in the zebrafish larva. There are some important differences, particularly in the adaptive immune response where sites of maturation differ and there are distinctIg subtypes[4], [5]. Nevertheless, zebrafish are naturally infected by many of the same classes of pathogens that affect mammals. Thus, fundamentally conserved frameworks of host–pathogen interactions can be studied in a facile model.
How Can the Transparency and Small Size of Zebrafish Be Exploited?
The most impressive feature of this model is the ability to perform non-invasive, high-resolution, long-term time-lapse and time-course experiments to visualize infection dynamics with fluorescent markers. This sets zebrafish apart from both in vitro and mammalian in vivo infection models, as summarized in Table 1. A variety of genetically encoded probes, fluorescent physiological indicator chemicals, cell type–specific fluorescent transgenes, photoactivatable proteins, and pathogen-encoded conditional reporters (for example, indicating oxidative stress or phagocytosis; Figure 1) has lit up mechanisms of bacterial, fungal, and viral pathogenesis. A particularly elegant use of the see-through fish is to photoactivate fluorescent proteins [2], prodrugs (Cre-ER; [6]), or “killer” proteins(KillerRed; [7]) to spatially restrict the desired effect. The transparency of wild-type larvae and casper mutant adults [8] provides a unique portal for observing and testing the impact of molecular perturbation on true infection dynamics in the intact host.
Tab. 1. Advantages of embryonic zebrafish model for study of innate immune-pathogen interaction. 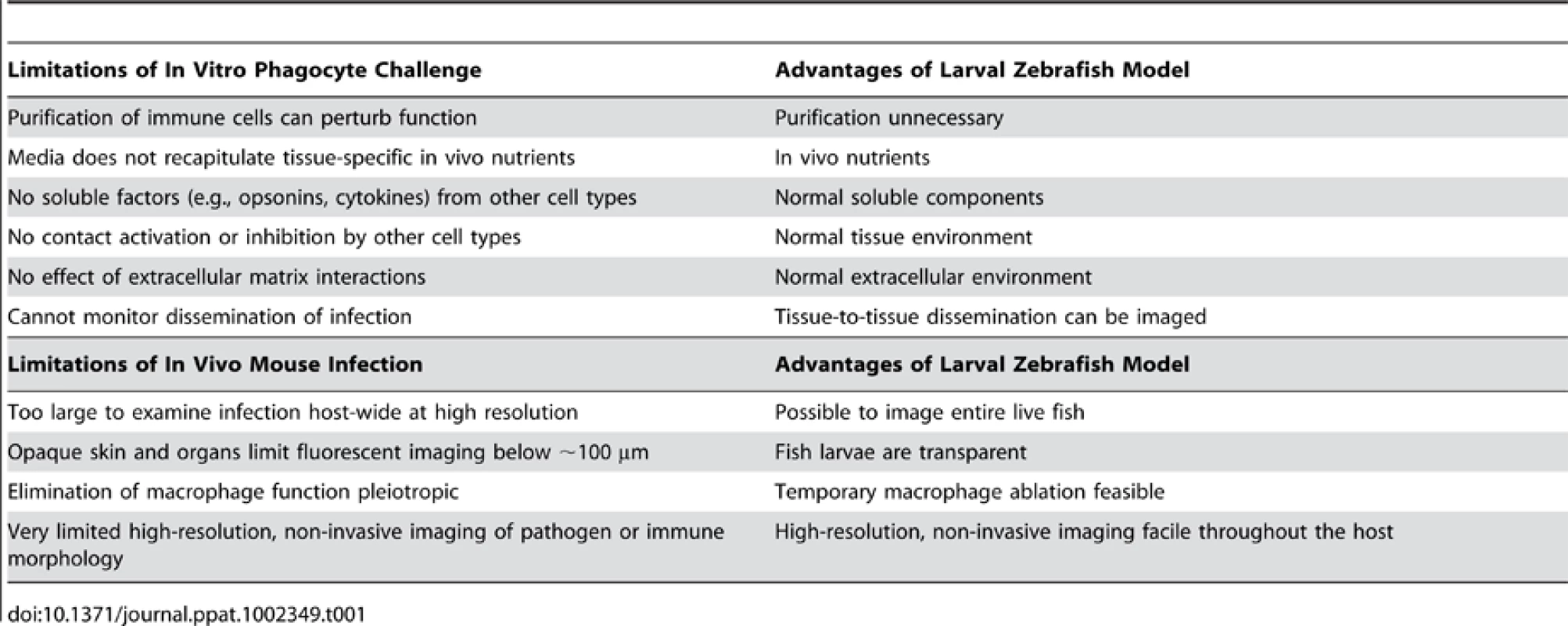
The large clutch size and the unusual ability to create gynogenetic diploids has allowed the first forward genetic screen to identify vertebrate host determinants of immunity to mycobacterial infection [1]. Other recent work demonstrates the utility of high-throughput screening to identify mycobacterial mutants with altered virulence [9], [10], whilst recent advances in automated screening now enable high-content screening of embryos [11], [12]. Embryos and young larvae are relatively permeable to small molecules, and the zebrafish embryo is small enough to develop in a well of a 384-well plate. High-throughput chemical genetic screens are made easier by direct introduction of chemicals into the water, and can be applied to identify novel antimicrobial drugs [13].
Another remarkable opportunity of this small transparent model comes from its complex anatomy, which enables infection through multiple routes of infection in an intact host with a complex immune system. Thus, fish viruses can be inoculated through immersion or microinjection, mycobacterial infection can be modeled by localized hindbrain injection or direct injection into the bloodstream, and pseudomonad interaction can be examined in the gastrointestinal tract as well as in the hindbrain and through intravenous injection. This versatility emphasizes the unique position of this model for understanding infection dynamics.
What Limits Use of the Zebrafish to Model Infection, and How Can These Limits Be Turned into Advantages?
The use of any model host necessitates a trade-off in order to ask new experimental questions. For instance, there are some important anatomical differences between zebrafish and mammals (gills instead of lungs, hematopoesis in the anterior kidney instead of bone marrow, lack of discernable lymph nodes, and a very different reproductive system) that constrain the range of infections that can be successfully studied in the zebrafish. In comparison to traditional model systems for pathogenesis, most notably the mouse, there is a lack of antibody reagents available. Antibodies raised against well-conserved mammalian proteins often demonstrate cross-reactivity with zebrafish orthologs, and there are concerted efforts in the zebrafish community to increase the number of antibodies raised specifically against zebrafish proteins. Nonetheless, this remains a current limitation of the model. The zebrafish larva grows well at water temperatures between 22°C and 33°C and lacks adaptive immunity until approximately 1 month post-fertilization. Thus, the zebrafish is well-suited to the study of cold-adapted or broad host-range pathogens [1], whilst on the positive side the ability to rear fish at different temperatures allows manipulation of infection that is not possible with other vertebrate model hosts [14]. The natural lack of adaptive immunity early in development limits the possibility of examining innate-adaptive crosstalk in the transparent embryo. But on the other hand, this developmental feature has permitted an unprecedented elucidation of innate immune functions that regulate immunity to Mycobacterium marinum, a fish pathogen closely related to the global human pathogen Mycobacterium tuberculosis. Furthermore, if adaptive immune function is to be tested, transparent “casper” adult fish can be used to image fluorescent events non-invasively [8]. As a general rule, zebrafish are also more tolerant of serious abnormalities than mammalian models (for instance, animals with essentially no cardiac function are viable for a few days after hatching), providing a unique opportunity to study mutants that are not available in rodent models [15].
What Are Unexpected Findings Pioneered Using the Zebrafish System and Validated in Mammals?
The unique power of the zebrafish model has led to several breakthroughs in our understanding of infectious disease. Studies of M. marinum, in particular, have yielded novel insight into the role of specific eicosanoids in host defense [1], the role of macrophages in promoting pathogen dissemination [16], infection-induced antibiotic tolerance [17], and the role of the ESX secretion system in granuloma formation [18]. In the case of mycobacteria, conserved virulence mechanisms and host susceptibility determinants identified during zebrafish infection have been validated in M. tuberculosis and human susceptibility. Zebrafish are now being used to model infections as disparate as Leptospira and Cryptococcus (Figure 1). As new models progress past the methodology phase, we are starting to gain real-time insight into host–pathogen interactions as varied as viral-induced hemorrhaging [14], CFTR-dependent immune responses to bacteria [19], and NADPH oxidase-mediated control of fungal filamentation [20]. These, and many more studies than could be mentioned here, should shed new light on a broad range of host–pathogen interactions driving human infectious diseases.
Zdroje
1. TobinDMVaryJCJrRayJPWalshGSDunstanSJ 2010 The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140 717 730
2. EllettFPaseLHaymanJWAndrianopoulosALieschkeGJ 2011 mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117 e49 e56
3. GrayCLoynesCAWhyteMKCrossmanDCRenshawSA 2011 Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb Haemost 105 811 819
4. StachuraDLTraverD 2011 Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol 101 75
5. TredeNSLangenauDMTraverDLookATZonLI 2004 The use of zebrafish to understand immunity. Immunity 20 367 379
6. SinhaDKNeveuPGageyNAujardILe SauxT 2010 Photoactivation of the CreER T2 recombinase for conditional site-specific recombination with high spatiotemporal resolution. Zebrafish 7 199 204
7. TehCChudakovDMPoonKLMamedovIZSekJY 2010 Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev Biol 10 110
8. WhiteRMSessaABurkeCBowmanTLeBlancJ 2008 Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2 183 189
9. CarvalhoRde SonnevilleJStockhammerOWSavageNDVenemanWJ 2011 A high-throughput screen for tuberculosis progression. PLoS ONE 6 e16779 doi:10.1371/journal.pone.0016779
10. StoopEJSchipperTRosendahl HuberSKNezhinskyAEVerbeekFJ 2011 Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis Model Mech 4 526 536
11. d'AlenconCAPenaOAWittmannCGallardoVEJonesRA 2010 A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol 8 151
12. Pardo-MartinCChangTYKooBKGillelandCLWassermanSC 2010 High-throughput in vivo vertebrate screening. Nat Methods 7 634 636
13. WhiteRMCechJRatanasirintrawootSLinCYRahlPB 2011 DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471 518 522
14. LudwigMPalhaNTorhyCBriolatVColucci-GuyonE 2011 Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathog 7 e1001269 doi:10.1371/journal.ppat.1001269
15. ShinJTFishmanMC 2002 From zebrafish to human: modular medical models. Annu Rev Genomics Hum Genet 3 311 340
16. DavisJMRamakrishnanL 2009 The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136 37 49
17. AdamsKNTakakiKConnollyLEWiedenhoftHWingleeK 2011 Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145 39 53
18. VolkmanHEPozosTCZhengJDavisJMRawlsJF 2010 Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327 466 469
19. PhennicieRTSullivanMJSingerJTYoderJAKimCH 2010 Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect Immun 78 4542 4550
20. BrothersKMNewmanZRWheelerRT 2011 Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell 10 932 944
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- How “Humane” Is Your Endpoint?—Refining the Science-Driven Approach for Termination of Animal Studies of Chronic Infection
- Sexual Development in : Lessons from Functional Analyses
- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Historical Contingencies Modulate the Adaptability of
- Human-like PB2 627K Influenza Virus Polymerase Activity Is Regulated by Importin-α1 and -α7
- -Sialidase in Complex with a Neutralizing Antibody: Structure/Function Studies towards the Rational Design of Inhibitors
- Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction
- How Do Bacteria Know They Are on a Surface and Regulate Their Response to an Adhering State?
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- The Murine Coronavirus Hemagglutinin-esterase Receptor-binding Site: A Major Shift in Ligand Specificity through Modest Changes in Architecture
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- Sexual Development in : Lessons from Functional Analyses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



