-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
Pathogens can substantially alter gene expression within an infected host depending on metabolic or virulence requirements in different tissues, however, the effect of these alterations on host immunity are unclear. Here we visualized multiple CD4 T cell responses to temporally expressed proteins in Salmonella-infected mice. Flagellin-specific CD4 T cells expanded and contracted early, differentiated into Th1 and Th17 lineages, and were enriched in mucosal tissues after oral infection. In contrast, CD4 T cells responding to Salmonella Type-III Secretion System (TTSS) effectors steadily accumulated until bacterial clearance was achieved, primarily differentiated into Th1 cells, and were predominantly detected in systemic tissues. Thus, pathogen regulation of antigen expression plays a major role in orchestrating the expansion, differentiation, and location of antigen-specific CD4 T cells in vivo.
Published in the journal: . PLoS Pathog 8(1): e32767. doi:10.1371/journal.ppat.1002499
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002499Summary
Pathogens can substantially alter gene expression within an infected host depending on metabolic or virulence requirements in different tissues, however, the effect of these alterations on host immunity are unclear. Here we visualized multiple CD4 T cell responses to temporally expressed proteins in Salmonella-infected mice. Flagellin-specific CD4 T cells expanded and contracted early, differentiated into Th1 and Th17 lineages, and were enriched in mucosal tissues after oral infection. In contrast, CD4 T cells responding to Salmonella Type-III Secretion System (TTSS) effectors steadily accumulated until bacterial clearance was achieved, primarily differentiated into Th1 cells, and were predominantly detected in systemic tissues. Thus, pathogen regulation of antigen expression plays a major role in orchestrating the expansion, differentiation, and location of antigen-specific CD4 T cells in vivo.
Introduction
Generating vaccines for current and emerging infectious diseases remains an important goal of immunological research [1], [2]. An ideal vaccine will induce the expansion and maturation of naïve pathogen-specific lymphocyte clones that exist at low frequency in uninfected or unimmunized individuals [3]. During infection or immunization, pathogen-specific T cells expand within secondary lymphoid tissues after recognition of foreign peptides in the context of host MHC molecules. These dividing pathogen-specific T cells acquire effector capabilities that are tailored to combat different classes of microbial pathogen [4], [5]. Following the resolution of primary infection, a cohort of these expanded pathogen-specific T cells persists to provide robust secondary immunity against another encounter with the same pathogen [6].
Naïve CD4 T cells can differentiate into T helper 1 (Th1), Th2, or Th17 effector lineages depending on the instructional cues delivered during initial activation [7]. Th1 cells express the transcription factor T-bet, secrete IFN-γ, and protect the host against infection with intra-macrophage pathogens [8], [9]. In contrast, Th2 cells express GATA-3, secrete IL-4, and combat large extracellular pathogens [10], [11]. Th17 cells are a recently described effector cell lineage that express RORγt, secrete IL-17, and contribute to clearance of extracellular bacterial and fungal infections [5], [12]. A number of variables can influence CD4 T helper cell differentiation, including TCR affinity for peptide/MHC, antigen dose, costimulatory signals, and the local concentration of inflammatory cytokines [7].
In vivo analysis of CD4 T cell differentiation during infection has often involved visualization of adoptively transferred TCR transgenic T cells [13]–[19]. Although this experimental approach allows detection of pathogen-specific T cells using conventional methodologies, it can also introduce experimental variables that are not present in a natural host [20]–[22]. The development of peptide-MHC tetramer enrichment methodologies now allows direct visualization of low frequency antigen-specific CD4 T cell populations without requiring adoptive transfer of TCR transgenic cells [23]. Using this approach, infection via the intra-nasal route was shown to enhance development of Listeria-specific Th17 cells while systemic infection encouraged Th1 development [24]. Most pathogens differentially regulate their protein expression in mucosal or systemic tissues depending on local metabolic or virulence requirements [25], [26], but it is not yet clear how this might affect CD4 T cell expansion and differentiation to stage-specific antigens expressed in different tissues.
Here, we examined this issue using peptide-MHC tetramers and ELISPOTs that allow simultaneous tracking of CD4 T cell responses to Salmonella flagellin and type-III-secretion system (TTSS) effector proteins. We found that flagellin-specific CD4 T cells undergo early expansion and contraction and after oral infection generate Th17 cells that localize to infected mucosal tissues. In contrast, CD4 T cells responding to TTSS effector proteins expanded and accumulated over several weeks and primarily differentiated to a Th1 lineage localized in systemic tissues. Thus, an infected host simultaneously develops distinct populations of CD4 effector lineages that detect stage-specific antigens and have divergent anatomical localization.
Results
Identification of novel class-II Salmonella epitopes
I-Ab epitopes have been identified within Salmonella flagellin [27], [28], and these remain the only confirmed class-II epitopes for examining CD4 response to Salmonella in C57BL/6 mice [29]. However, flagellin is an unusual antigen that can be directly detected by the innate immune system, and is also rapidly down-regulated as bacteria transition to intracellular growth [30]–[32]. In order to uncover antigens that are recognized by CD4 T cells during intracellular bacterial growth, we used a bioinformatic approach to identify novel Salmonella I-Ab epitopes. Based on the published structure of I-Ab in complex with human class-II invariant chain peptide (CLIP) [33], we used a positional-specific scoring matrix (PSSM) and Hidden Markov Model (HMM) to interrogate all open reading frames in the Salmonella genome for I-Ab epitopes. Although this yielded numerous epitopes that were then confirmed by peptide immunization (Table 1 and Figure 1A), none were found to be natural epitopes during murine Salmonella infection or after immunization with heat-killed Salmonella (data not shown). Since many of these candidate peptides were derived from cytoplasmic proteins that are unlikely to enter the class-II presentation pathway, we modified our approach to focus on outer membrane or secreted Salmonella proteins, (Table 2), many of which are required for bacterial persistence in vivo [34]. Using this strategy we identified natural I-Ab epitopes in two effector proteins that are encoded by Salmonella Pathogenicity Island 2 (SPI2) TTSS, SseI and SseJ (Figure 1B and Table 3).
Fig. 1. Discovery of novel Salmonella I-Ab epitopes. 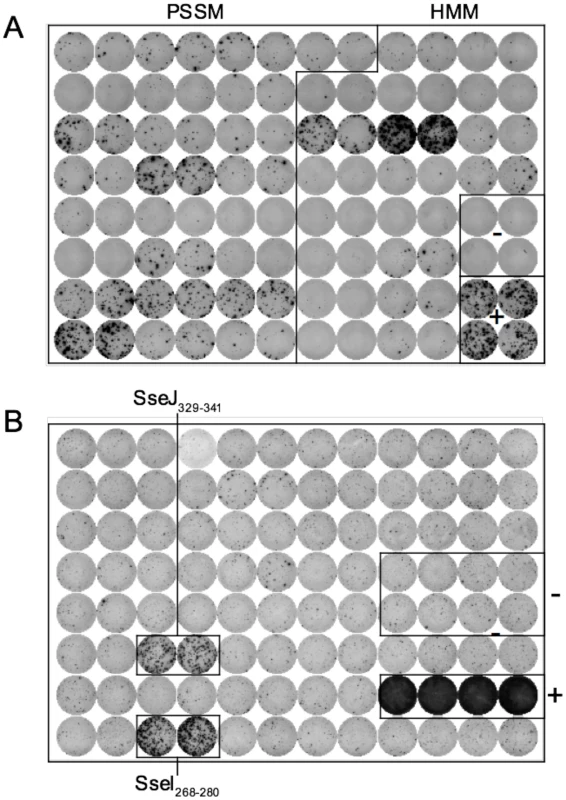
(A) C57BL/6 mice were immunized with a mixture of 4 groups of 11 peptides from Table 1 at 5 µg/peptide in CFA. Eight days later, draining lymph nodes were isolated and purified CD4 T cells restimulated with individual peptides (Table 1) in the presence of irradiated splenocytes. IFN-γ production was measured 24–36 hours later by ELISPOT. (B) 129Sv mice were orally infected with virulent 5x107 Salmonella (SL1344) and spleens harvested 11 weeks later. Purified CD4 T cells were restimulated with peptides from Table 2 for 24–36 h in the presence of irradiated splenocytes and IFN-γ production measured by ELISPOT. ConA and no peptide wells constitute the positive and negative controls respectively. Each plate is representative of 2–3 individual experiments. Tab. 1. Salmonella peptides screened for immunogenicity in C57BL/6 mice. 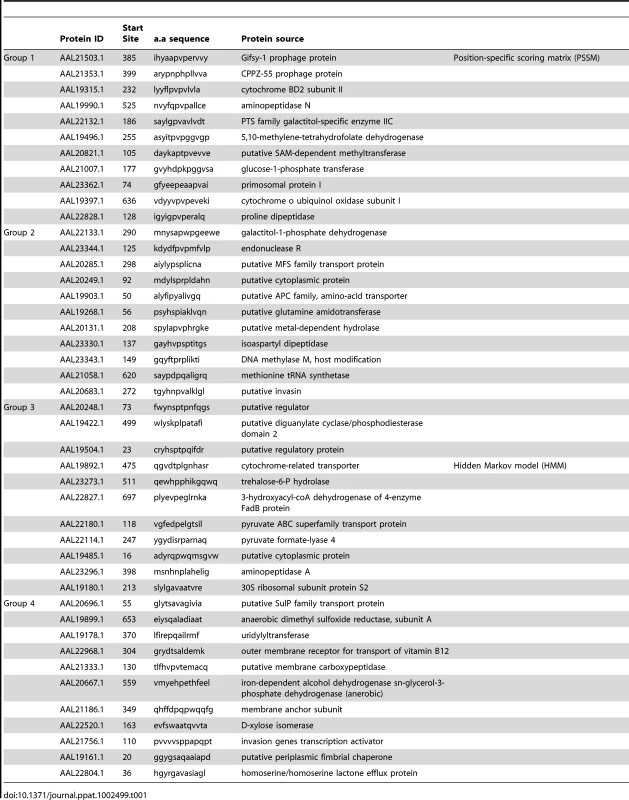
Tab. 2. List of outer membrane and secreted <i>Salmonella</i> class-II epitopes tested. 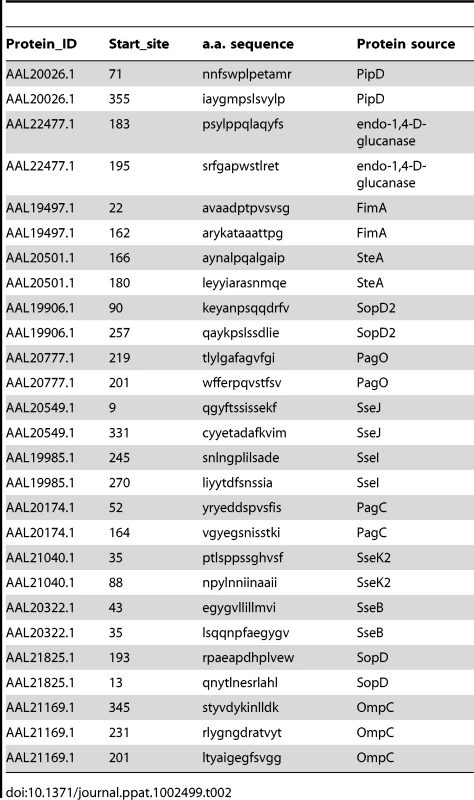
Tab. 3. Natural <i>Salmonella</i> class-II epitopes used in this study. 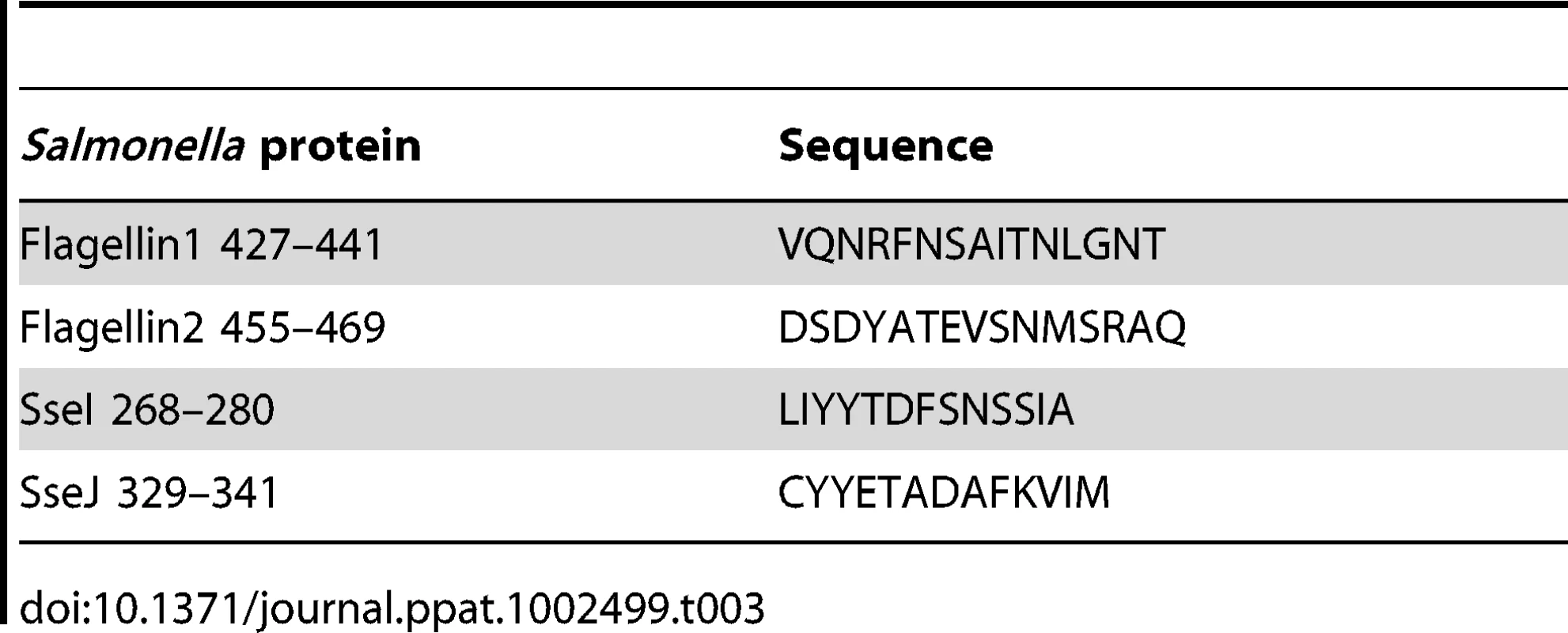
In marked contrast to flagellin, SseI and SseJ are though to be expressed during intra-macrophage replication [35]. We confirmed the differential regulation of flagellin (FliC) and SseJ by examining bacterial mRNA expression in vitro. While FliC mRNA was highly expressed under SPI1-inducing conditions, it was down-regulated under SPI2-inducing conditions which simulate the intra-macrophage environment (Figure 2). In contrast, SseJ was expressed under SPI2-inducing conditions but down-regulated under SPI1-inducing conditions (Figure 2). Thus, Salmonella differentially regulate the production of flagellin and SseJ, depending on local environmental cues. Although it was technically challenging to measure Salmonella mRNA expression in vivo, we were able to confirm the differential regulation of FliC and SseJ at early time points after infection. FliC mRNA was highly expressed at 30 minutes after infection but significantly reduced at 5 hours, while in contrast, SseJ mRNA was low at 5 minutes but expression increased at 30 minutes (Figure 2). Thus, flagellin (FliC) and SseJ represent antigens that are differentially regulated during the transition from extracellular to intracellular growth.
Fig. 2. Differential regulation of bacterial Flagellin (FliC) and SseJ in vitro and in vivo. 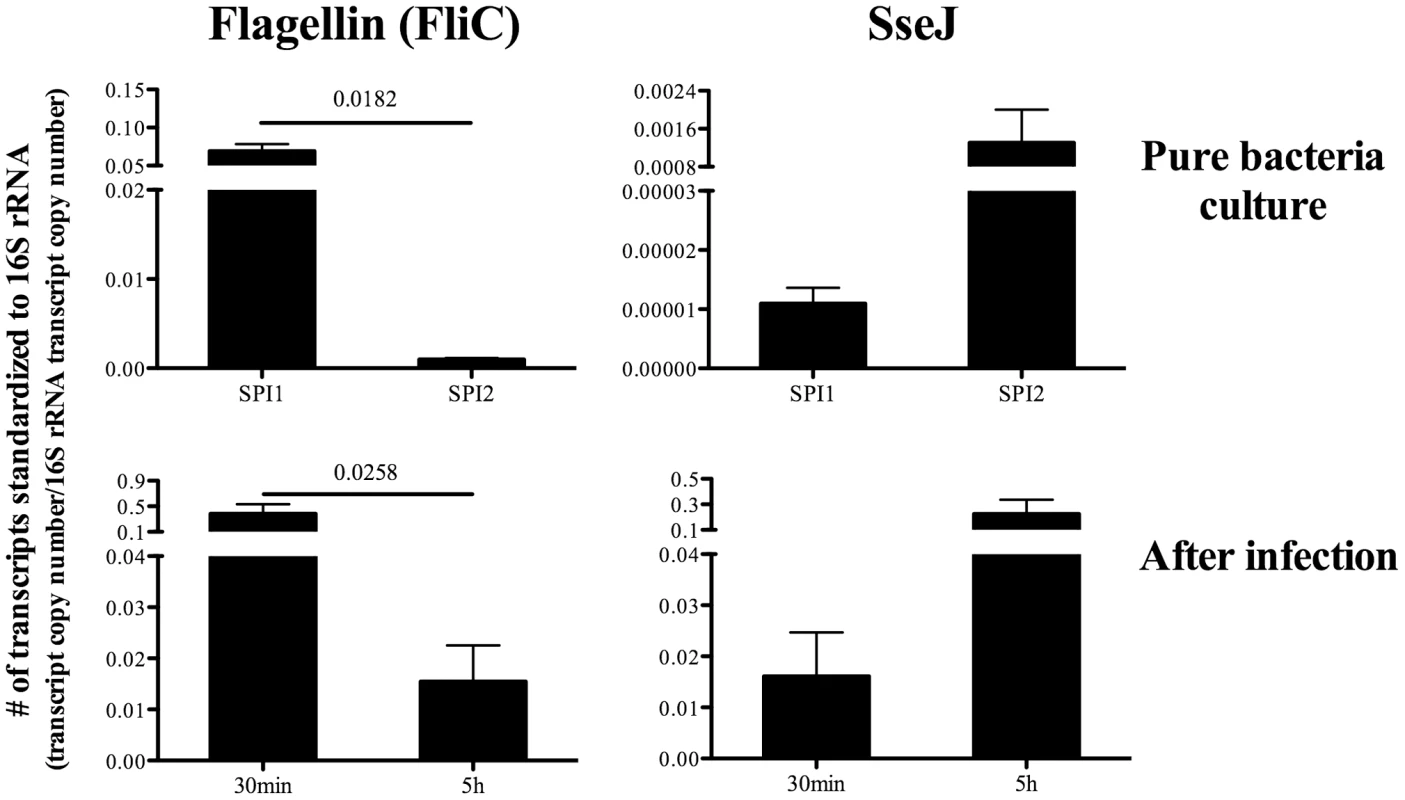
For in vitro detection of bacterial mRNA, Salmonella (BRD509) were cultured under SPI1-inducing conditions (LB broth, for 3hours) or SPI2-inducing conditions (modified N minimal medium, for 6hours) (Top). For in vivo detection, C57BL/6 mice were infected intravenously with 5X105 Salmonella (BRD509), and spleens harvested 30 min or 5 hours later (Bottom). Bacterial RNA was isolated as described in Materials and Methods. Expression of Flagellin (FliC) or SseJ mRNA was quantified by real-time qPCR. Bar graphs show the mean number ± SEM of Flagellin (FliC) or SseJ mRNA transcripts normalized to the respective amount of 16S rRNA in each sample. Data are pooled from two separate experiments (in vitro) or three separate experiments using a total of nine mice (in vivo). Numbers above indicate statistical significance and show the p value of a comparison between the groups. Construction of Salmonella-specific class-II tetramers
We constructed class-II I-Ab tetramers containing flagellin427–441 and SseJ329–341 epitopes and visualized endogenous flagellin427–441-, SseJ329–341-specific CD4 T cells using a sensitive tetramer enrichment methodology [36]. C57BL/6 mice contained low numbers of naive flagellin - and SseJ-specific CD4 T cells, but a population of expanded CD44Hi tetramer positive cells was readily detected in the draining lymph nodes after immunization with flagellin427–441 or SseJ329–341 and CFA (Figure 3). Similarly, infection with Salmonella (BRD509) allowed detection of an expanded population of flagellin - and SseJ-specific CD4 T cells (Figure 3). Salmonella-specific T cells were not detected in unbound column fractions or in CD8 T cells bound to enrichment columns (Figure 3), demonstrating the specificity of these detection regents and the efficiency of enrichment. We chose to focus on visualizing CD4 T cells responding to Salmonella strain BRD509 since this strain has been widely studied previously [37]–[39], and use of more virulent strains precludes analysis at late time points after infection of C57BL/6 mice.
Fig. 3. Expansion of endogenous flagellin427–441- or SseJ329–341-specific CD4 T cells after peptide immunization or Salmonella infection of mice. 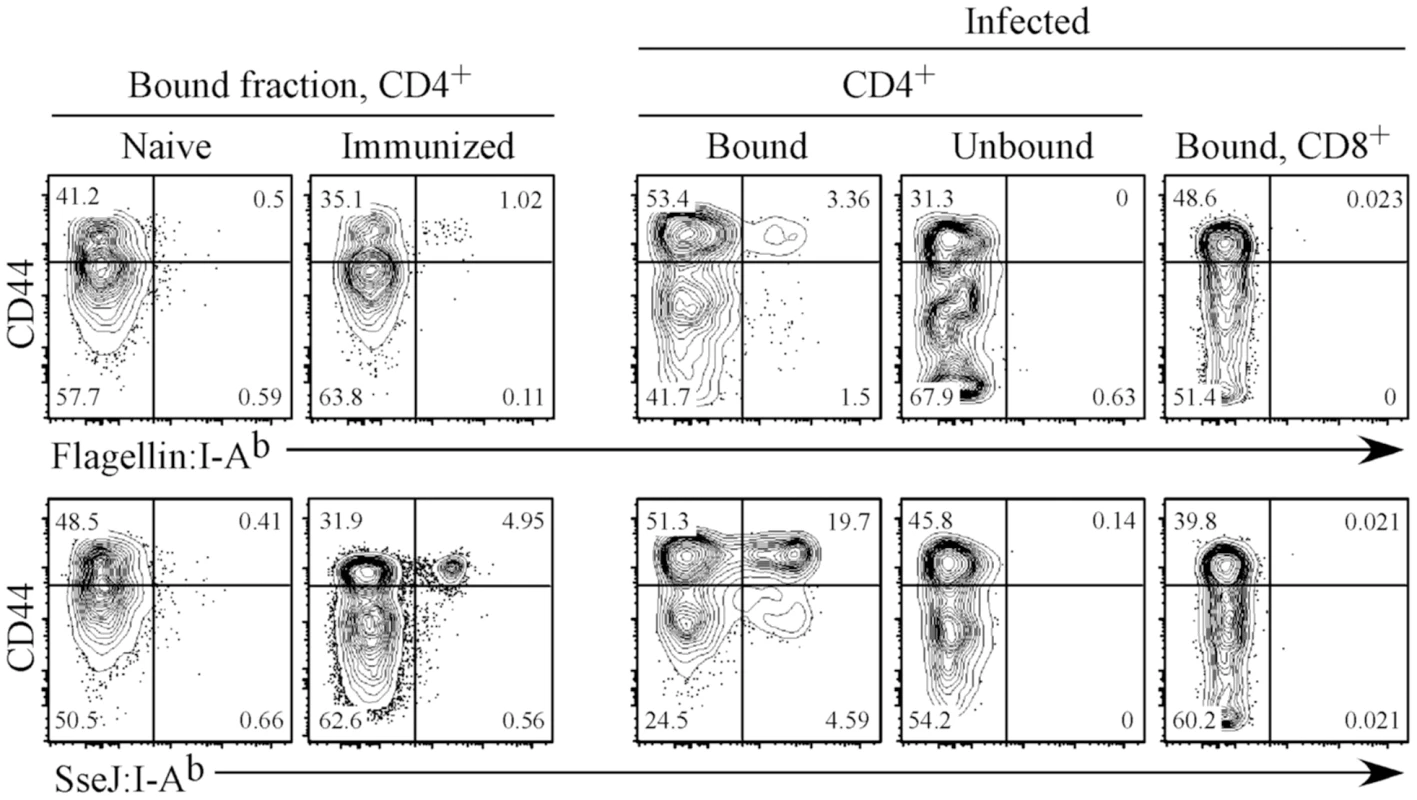
C57BL/6 mice were immunized sub-cutaneously with either 100 µg of flagellin427–441 or SseJ329–341 peptide in the presence of CFA or infected with 5x105 Salmonella (BRD509). On day 7, inguinal, axillary, and brachial lymph nodes were isolated from peptide-immunized mice (Immunized). On day 35, spleens were harvested from C57BL/6 mice infected intravenously with Salmonella BRD509 (Infected). Endogenous flagellin427–441- or SseJ329–341-specific CD4 T cells were detected using Flagellin:I-Ab or SseJ:I-Ab tetramers, enriched with anti-fluorochrome microbeads, stained with antibodies to several surface markers, and examined by flow cytometry. CD4 T cells from naive, immunized, and Salmonella-infected mice, were detected among CD11c−CD11b−F4/80−B220−CD3+ cells, and further analyzed for expression of CD44 and Flagellin:I-Ab or SseJ:I-Ab tetramer positive cells. CD4 T cells from the unbound column fraction and CD8 T cells within the bound fraction are also shown as controls. FACS plots are representative of three mice per group and three replicate experiments. Distinct kinetics of flagellin - and SseJ-specific CD4 T cell expansion following infection
We examined the kinetics of flagellin427–441-specific and SseJ329–341-specific CD4 T cell expansion after intravenous (IV) infection with Salmonella. The pooled secondary lymphoid tissues of a C57BL/6 mouse contained approximately 32 flagellin427–441-specific and 30 SseJ329–341-specific CD4 T cells (Figure 4A). After Salmonella infection, flagellin427–441-specific CD4 T cells expanded to a peak of 410 cells at day 7 (12.9 fold expansion over naïve frequency), before contracting to 88 cells by day 160 (Figure 4A). At the peak of clonal expansion, flagellin427–441-specific CD4 T cells decreased surface expression of CCR7 and CD27, indicating the development of T effector cells (Figure 4B and C). Flagellin427–441-specific T cells started to contract as early as day 10 following infection, and this coincided with a gradual increase in the percentage of cells expressing CCR7 and CD27 (Figure 4A–C). Importantly, this CD4 contraction phase occurred while bacterial burdens remained high in vivo (Figure 4D). In marked contrast, SseJ329–341-specific CD4 T cells expanded after infection and continued to accumulate in secondary lymphoid tissues until day 52 post-infection, eventually reaching a peak of 5,900 cells (210-fold expansion over naïve frequency), before decreasing to 1,800 cells by day 160 (Figure 4A). In contrast to the flagellin-specific response, SseJ329–341-specific CD4 T cells maintained low expression of CCR7 and CD27 until bacterial clearance had been achieved (Figure 4B–D). We also examined intracellular expression of transcription factors associated with T helper lineage commitment in these two populations at time points close to peak expansion. The vast majority of expanded flagellin427–441-specific and SseJ329–341-specific CD4 T cells expressed T-bet, while no staining above background levels was detected using antibodies specific for GATA-3, FoxP3, or RORγt (Figure 4E and data not shown).
Fig. 4. Kinetics of endogenous Salmonella flagellin427–441-, and SseJ329–341-specific CD4 T cells after Salmonella infection. 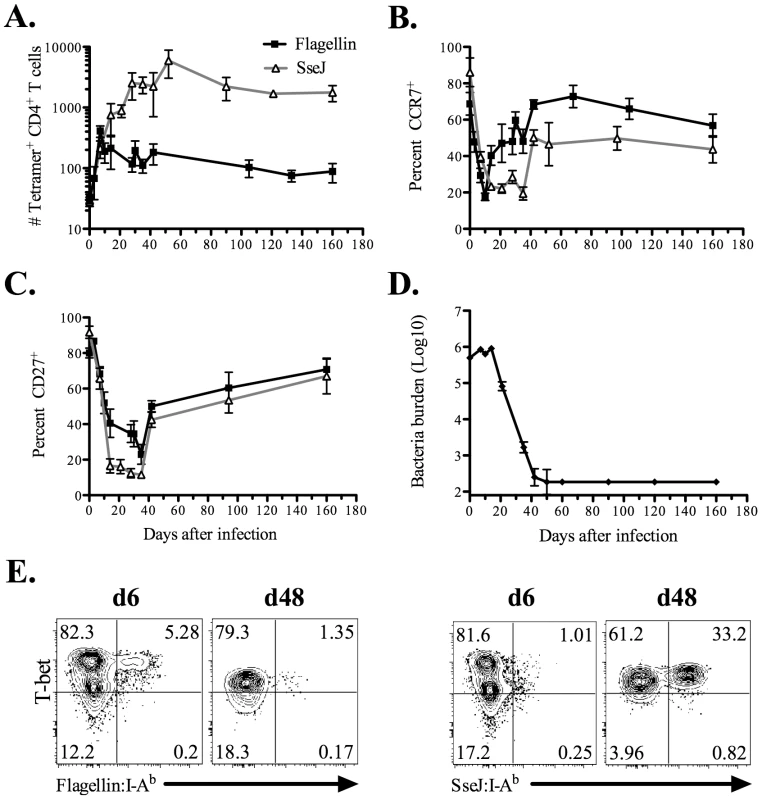
C57BL/6 mice were infected intravenously with 5×105 Salmonella (BRD509). At various time points, endogenous CD4 T cells were stained using Flagellin:I-Ab or SseJ:I-Ab tetramers, enriched using magnetic selection, and examined by flow cytometry. CD4 T cells were gated from CD11c−CD11b−F4/80−B220−CD3+ cells, and further analyzed for Flagellin:I-Ab or SseJ:I-Ab tetramer positive cells. (A) The number of endogenous flagellin427–441- or SseJ329–341-specific CD4 T cells was calculated based on FACS analysis. Graph shows the mean number ± SEM of flagellin427–441- or SseJ329–341-specific CD4 T cells. (B and C) Line graphs show the mean percentage ± SEM of tetramer specific CD4 T cells positive for CCR7 (B) or CD27 (C) expression. (D) The number of viable Salmonella determined in the spleen at various time points. These data were pooled from several experiments and represent at least 3–5 mice at each time point. (E) Endogenous flagellin427–441- or SseJ329–341-specific CD4 were enriched from day 6 or day 48 Salmonella-infected mice, and stained intracellularly with antibodies specific for T-bet. In order to confirm the distinct tempo of CD4 responses to Salmonella flagellin and TTSS effector proteins, we also examined CD4 T cell responses by ELISPOT. IFN-γ-producing CD4 T cells were detected seven days after Salmonella infection and were found to predominantly focus on flagellin, rather than TTSS effector epitopes (Figure 5). In marked contrast, 40 days after infection, larger pools of IFN-γ-producing CD4 T cells were detected responding to SseI and SseJ epitopes rather than flagellin (Figure 5). No Th2 or Th17 CD4 T cell response was detected at either of these time points after intravenous infection (data not shown). Together, these data demonstrate distinct temporal differences in the targeting of flagellin and TTSS effector proteins by Salmonella-specific CD4 T cells.
Fig. 5. Salmonella-specific Th1 cells target flagellin and T3SS effectors at different time points after infection. 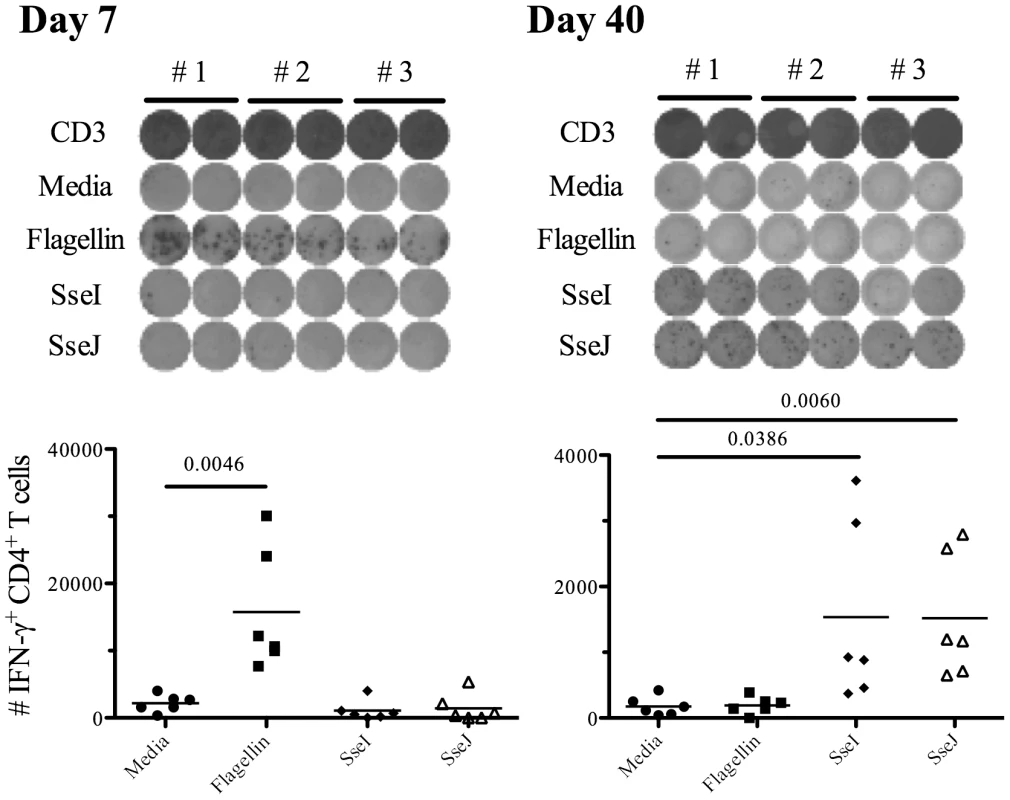
C57BL/6 mice were infected intravenously with 5×105 Salmonella (BRD509). On day 7 or day 40 post-infection, CD4 T cells from the spleens were purified and restimulated with 10 µM of various peptides (Table 3) for 16 h in the presence of irradiated splenocytes. IFN-γ production was measured by ELISPOT assay. (Top) Representative images from IFN-γ ELISPOT plates using CD4 T cells from Salmonella-infected mice at day 7 or day 40 after infection. (Bottom) Scatter graphs show the number of IFN-γ-producing CD4 T cells per infected mouse. Numbers above indicate statistical significance and show p value of a comparison between media alone (no stimulation) and peptide stimulation group. Bacterial persistence is required for accumulation of SseJ-specific T cells
We hypothesized that the temporal difference in the CD4 T cell response to flagellin and SseJ was due to the maintenance of SseJ expression in vivo by persistent bacteria. However, it was also possible that these different kinetics reflected T cell-intrinsic variables that were completely unrelated to antigen persistence. We therefore examined the clonal expansion of SseJ-specific T cells in mice that were administered antibiotics to clear bacteria, beginning five days after infection. Although SseJ-specific T cells were clearly detected in both treated and untreated mice, clonal expansion was significantly reduced in mice that had been treated with antibiotics (Figure 6). Thus, the continued accumulation of SseJ-specific CD4 T cells at later time points after infection required bacterial persistence.
Fig. 6. Bacterial persistence is required for optimal expansion of SseJ-specific CD4 T cells. 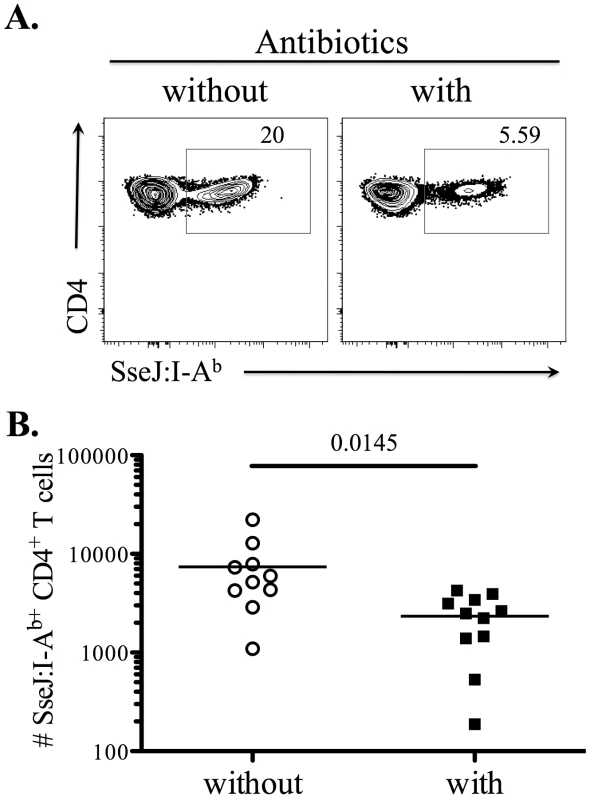
C57BL/6 mice were infected intravenously with 5×105 Salmonella (BRD509). Some mice were treated with Enrofloxacin (Baytril) (2mg/ml in drinking water), beginning 5 days post-infection. At day 30 post-infection, endogenous SseJ-specific CD4 T cells were stained with SseJ:I-Ab tetramers, enriched, and examined by flow cytometry. (A) Representative FACS plots from control (without, left) or antibiotic-treated mice (with, right). Tetramer-specific CD4 T cells were gated from CD11c−CD11b−F4/80−B220−CD3+. (B) Scatter plots show the number of SseJ329–341-specific CD4 T cells in control (without) or treatment group (with). Data are pooled from three separate experiments. Numbers above indicate statistical significance and show p value of a comparison between control and treatment group. Enrichment of flagellin-specific Th17 CD4 T cells in mucosal tissues after oral infection
Next, we used ELISPOTs to examine the CD4 T cell response to oral Salmonella infection and closely monitored Th1, Th2, and Th17 responses to flagellin and TTSS effector proteins in mucosal and systemic tissues. We used three oral doses of Salmonella (BRD509) as this was required for optimal priming of effector CD4 T cell responses. Consistent with the absence of Th2 responses after IV infection, IL-4 production was not detected in either mucosal or systemic tissues (data not shown). In contrast, IFN-γ-producing CD4 T cells were detected in intestinal (mesenteric lymph nodes, MLNs, and lamina propria, LP) and systemic (spleen and liver) tissues (Figure 7A). SseI and J-specific CD4 Th1 cells were more numerous in the spleen and liver, while flagellin-specific CD4 Th1 cells were predominantly detected in intestinal tissues (Figure 7A). Furthermore, IL-17A-producing CD4 T cells were also detected and this response was notably focused on the flagellin epitopes and occurred in mucosal tissues (Figure 7B). Indeed, flagellin-specific CD4 T cells in intestinal tissues had a higher ratio of Th17:Th1 cells, while in contrast SseI and J-specific CD4 T cells in systemic tissues had a higher ratio of Th1:Th17 cells (Figure 7C). In order to confirm the preferential generation of flagellin-specific Th17 cells in the intestine, we also examined IL-22 production by CD4 T cells recovered from the spleen or MLN of Salmonella-infected mice. Consistent with the ELISPOT data, IL-22 was detected after stimulation of CD4 T cells from the MLN but not the spleen of infected mice, and was directed towards flagellin427–441 rather than T3SS effectors (Figure 8).
Fig. 7. Detection of flagellin-specific CD4 Th17 cells in intestinal tissues. 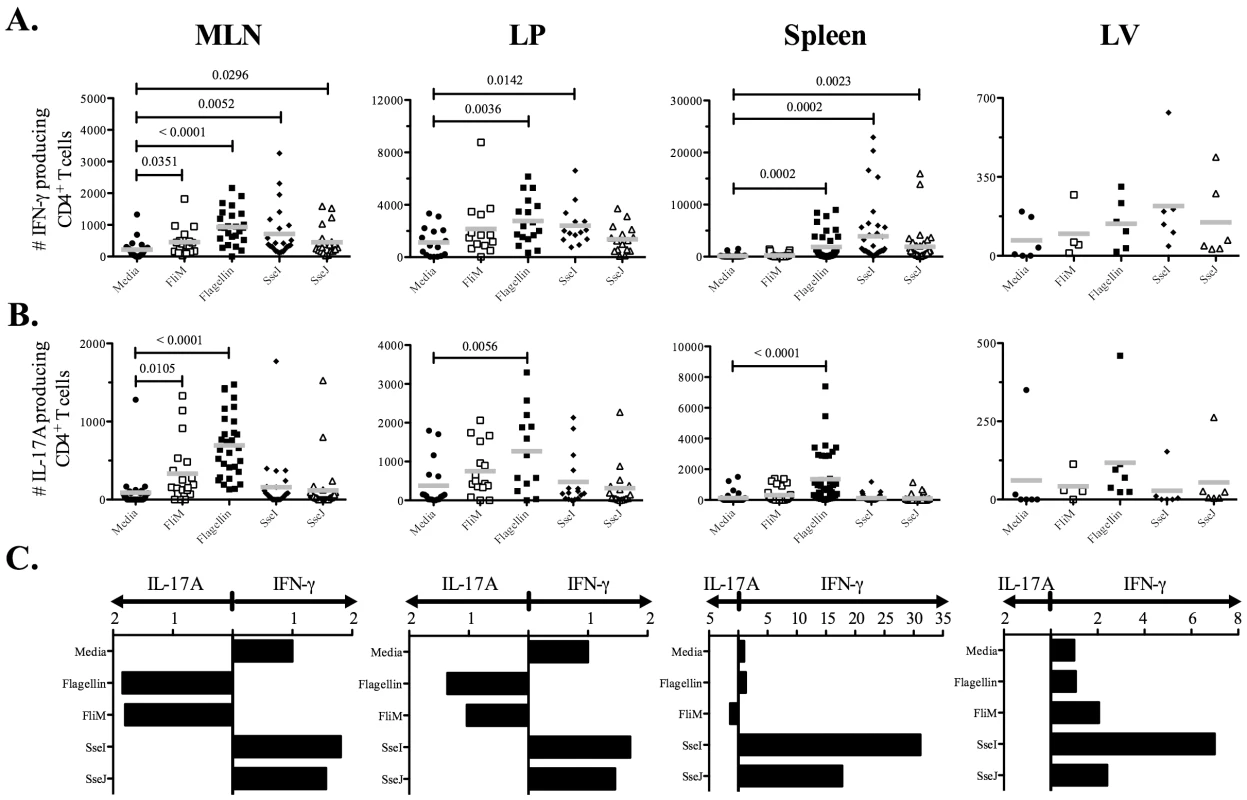
C57BL/6 mice were infected three times orally with 5×109 Salmonella (BRD509) at one-month intervals. On day 7 after the third infection, CD4 T cells from the spleens, MLN, including Peyer's patches, livers (LV), and laminar propria (LP) were purified. Isolated CD4 T cells (1×105) were restimulated with 10 µM of various peptides (Table 3) for 16 h in the presence of irradiated splenocytes. (A) IFN-γ or, (B) IL-17A production was measured by ELISPOT. Scatter graphs show the number of (A) IFN-γ or, (B) IL-17A-producing CD4 T cells in infected tissues from individual mice. (C) Bar graphs show the mean ratio of IFN-γ to IL-17 production after in vitro stimulation. Numbers above indicate statistical significance and show p value of a comparison between media alone (no stimulation) and peptide stimulation group. Fig. 8. Mucosal flagellin-specific CD4 T cells produce IL-22 upon restimulation. 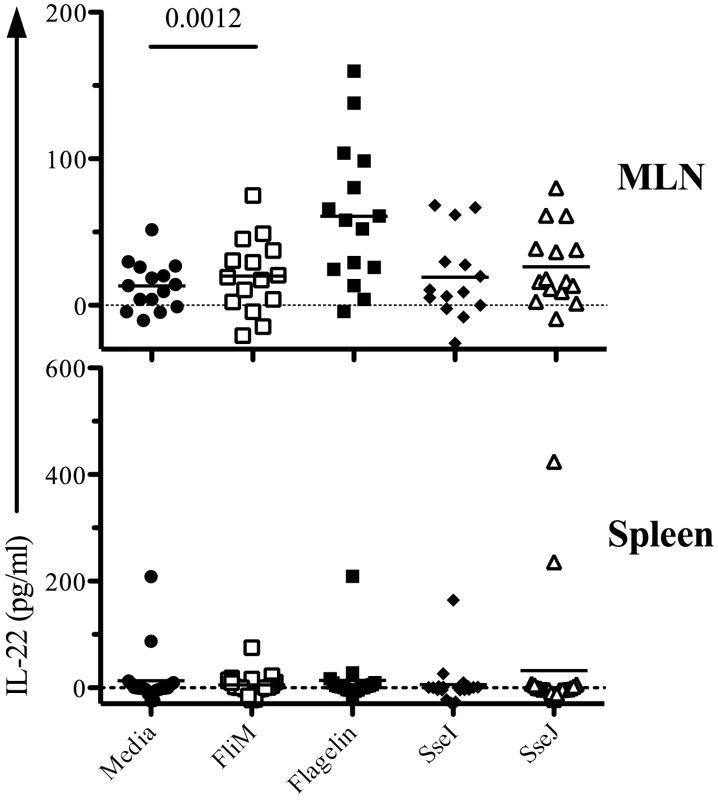
C57BL/6 mice were infected three times orally with 5×109 Salmonella (BRD509) at one-month intervals. On day 7 after the third infection, CD4 T cells from spleen and MLN, including Peyer's patches, were isolated. Purified CD4 T cells were restimulated with 10 µM peptide (Table 3) for 16 h in the presence of irradiated splenocytes. Culture supernatants were collected and IL-22 production measured by ELISA. Data show IL-22 production in culture supernatants as scatter plots representing individual mice. Data are pooled from three separate experiments. Numbers above each group indicate statistical significance and show the p value of a comparison between media alone (no stimulation) and peptide stimulation group. Discussion
This study describes the first examination of endogenous CD4 T cell responses to multiple Salmonella epitopes using tetramer and ELISPOT assays. Previous analysis of Salmonella-specific CD4 T cells has been severely limited by the lack of defined epitopes and has largely focused on the response to a single I-Ab epitope within flagellin [13], [40], [41]. Our ability to track multiple epitope-specific CD4 responses in this study has revealed that in vivo regulation of bacterial antigen expression is a critical factor in orchestrating both the tempo and effector maturation of Salmonella-specific CD4 T cells at mucosal and systemic sites. High expression of flagellin is reported from extracellular bacteria but this is rapidly decreased after infection of macrophages, and flagelin cannot be detected in the spleen of infected mice [32], [42]. In contrast, expression of SPI-2 genes is initiated within the phagolysosome of infected macrophages and is required for bacterial survival in vivo [35]. Our data confirm these findings and demonstrate differential expression of FliC and SseJ mRNA in response to environmental cues, both in vitro and in vivo. Thus, naïve Salmonella-specific CD4 T cells will encounter flagellin only during the early stage of infection within the intestine, whereas SPI-2 TTSS effector proteins accumulate as more intracellular bacteria replicate in the systemic tissues of the spleen and liver.
Interestingly, our tetramer data reveal a pattern of CD4 T cell expansion and contraction to flagellin and SseJ that corresponds closely to their relative temporal abundance during in vivo growth. In particular, we detected early contraction of flagellin-specific T cells, while SseJ-specific T cell responses remained elevated for several weeks. There may be T cell-intrinsic factors that determine the peak clonal expansion of FliC - and SseJ-specific T cells since SseJ-specific T cells exhibit greater clonal expansion. However, this issue does not explain the maintenance of the SseJ response for several weeks after infection. Furthermore, the elimination of bacteria using antibiotics severely dampened the CD4 T cell response to SseJ, indicating that antigen persistence is essential for sustained clonal expansion of this population. Although antibiotics can affect adaptive response indirectly via effects on commensal flora, it is more likely that the reduction in the SseJ response is due to a direct effect on bacterial persistence. Together, these data suggest that CD4 T cells are surprisingly dependent upon local antigen availability even after clonal expansion has occurred and indicate that clearer definition of in vivo protein expression by pathogens might be critical for identification of protective antigens, especially for pathogens that reside in multiple tissues or have distinct life-cycle stages. Indeed, a correlation between temporal antigen expression and in vivo activation of protective CD4 T cell responses has recently been observed during M. tuberculosis infection [43], [44]. Furthermore, a requirement for sustained antigen expression for the generation of optimal CD4 T cell responses has also been identified in other model systems [45], [46].
Our data also demonstrate divergent CD4 T cell helper development to flagellin and TTSS effector proteins. After intravenous infection, CD4 T cells responding to each of these antigens expressed T-bet and produced IFN-γ following in vitro restimulation, thus Salmonella generates distinct antigen-specific Th1 cell populations that appear at different stages of infection. This finding may help explain why Th1 cells can be detected within days of Salmonella infection but do not appear to actively participate in bacterial clearance until several week later [47]. If the majority of the early Salmonella-specific Th1 response is largely focused on antigens that are transiently expressed, such as flagellin or SPI-1 gene products, then intracellular bacteria can evade detection by the initial wave of Th1 effector cells. In contrast, a second wave of Th1 cells that are specific for highly expressed intracellular antigens such as T3SS effectors would be more likely to participate in the later stages of bacterial clearance from systemic tissues. A similar model of bacterial antigen regulation and effector T cell evasion has been proposed in mycobacterial infection [43], [48], and may be a common feature of persistent bacterial infection.
After oral infection with Salmonella, we detected Th17 cells specific for flagellin epitopes and these were particularly enriched in the intestine. TGF-β is abundant in mucosal tissues and plays an important role in the generation of both Treg and Th17 cells [5]. Intestinal IL-6 production is induced by Salmonella infection and is likely to drive Salmonella-specific differentiation towards a Th17 lineage in the intestine [49], [50]. Indeed, flagellin is a ligand for TLR5 and can directly induce IL-6 production in vitro and in vivo [38], [51]. This may explain why Th17 cells specific for flagellin epitopes found only in infected intestinal tissues while Th17 responses to T3SS effector proteins are largely absent. Th17 cells are thought to provide protective immunity during extracellular bacterial infections via upregulation of anti-bacterial mediators and neutrophil recruitment [5], [52]. Since Salmonella grow intracellularly in vivo [53], a protective role for Salmonella-specific Th17 CD4 T cells during primary infection is not immediately obvious. However, Th17 cells may be vital for mobilizing local innate defenses against secondary infection after bacteria penetrate intestinal epithelium. Indeed, it may be particularly important that these mucosal Th17 cells recognize bacterial antigens such as flagellin that are expressed by the initial bacterial burden.
In summary, this study has identified new Salmonella I-Ab epitopes in T3SS effectors and used peptide-MHC tetramers and ELIPOT assays to simultaneously track multiple CD4 T cell responses during Salmonella infection for the first time. These data show that an infected host develops distinct CD4 effector lineages that are tightly regulated by bacterial antigen expression patterns in vivo, a finding that may assist the generation of vaccines directed against microbial pathogens that replicate in mucosal and systemic tissues.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The University of Minnesota and University of California Davis are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animal experiments were approved by University of Minnesota Institutional Animal Care and Use Committee (IACUC) (Protocol numbers: 1011A93173 and 1004A80157) or UC Davis IACUC Protocol 16612.
Mice and bacterial strains
C57BL/6 and 129Sv mice were purchased from the National Cancer Institute (Frederick, MD) and used at 6–16 weeks of age. All mice were maintained in accordance with University of Minnesota or University of California Davis Research Animal Resource guidelines. Salmonella enterica serovar Typhimurium strains SL1344 and BRD509 (AroA−D−) were kindly provided by Dr. D. Xu (University of Glasgow, Glasgow, U.K).
Salmonella infection and antibiotic treatment
S. Typhimurium were cultured overnight in Luria-Bertani (LB) broth without shaking, and diluted in PBS after estimation of bacterial concentration using a spectrophotometer. Mice were infected intravenously in the lateral tail vein with 5X105 BRD509. For oral infection, mice were administered 0.1ml of 5% sodium bicarbonate to neutralize stomach pH before oral infection with 5x109 BRD509. The actual bacterial dose administered was confirmed by plating serial dilutions of the original culture onto MacConkey agar plates counting the number of colonies that grew after overnight culture at 37°C. In some experiments mice were administered Enrofloxacin (Baytril) at 2mg/ml in their drinking water, beginning on 5 days post-infection, as previously described [54].
Salmonella I-Ab epitope discovery
The crystal structure of the murine I-Ab molecule in complex with human class II invariant chain-associated peptide (CLIP) [33], was used to identify potential I-Ab epitopes from S. Typhimurium. Based on the proposed alignment, the likelihood of a particular amino acid residue appearing in a specific position in the MHC class-II binding groove was assigned a numerical score. We focused on binding positions 1, 4, 6, 7, and 9 as these were the most likely to contribute to MHC class-II binding. For example, a tyrosine at position 1 was found in 30 out of 75 core residues examined and was assigned a score of 30. An overall score total was calculated by adding individual scores from each potential binding residue, with 109 being the highest possible score. This scoring approach was applied bioinformatically to each open reading frame of Salmonella using 9-mers overlapping by one (a total of 995,592 peptides). Two algorithms were then applied to this data; the first was a position-specific scoring matrix (PSSM) [55], and the second a hidden Markov model (HMM) [56]. The epitopes with highest PSSM (25 epitopes from 105 to 84) and HMM scores (19 epitopes from 82 to 43) were tested for immunogenicity using an IFN-γ ELISPOT assay. Briefly, four groups of eleven peptides were mixed in equal amounts (5 mg/peptide) emulsified in complete Freund's adjuvant (CFA) and injected subcutaneously into groups of C57BL/6 mice. Draining lymph nodes were harvested eight days later, CD4 T cells enriched by negative selection, and mixed 1∶1 with irradiated splenocytes in IFN-γ ELISPOT plates. Duplicate wells were stimulated for 36 hours with individual peptides and spot forming cells (SFC) per 1x106 total cells enumerated. Although 88% (PSSM) and 74% (HMM) of peptides induced a CD4 T cell response in HKST/CFA-immunized mice, no response to these epitopes was detected after infection with Salmonella. The approach was therefore modified using PSSM to identify the two highest scoring candidate peptides from a combination of 13 outer membrane and secreted proteins. These epitopes were tested using purified CD4 T cells from the spleen and mesenteric lymph nodes of 129Sv mice orally infected 11 weeks previously with 5x107 Salmonella (SL1344). In these peptide identification experiments, genetically resistant 129Sv mice were used so that CD4 responses to virulent (SL1344) bacteria could be examined at late time points when C57BL/6 mice would normally succumb to infection.
Quantifying Flagellin (FliC) and SseJ mRNA
For SPI1 or SPI2-inducing cultures, Salmonella BRD509 were grown overnight at 37°C in LB broth, diluted in either LB broth for SPI1-inducing conditions or modified N minimal medium [57] for SPI2-inducing conditions, and grown for either 3 hours for SPI1 or 6 hours for SPI2 conditions. For in vivo detection of mRNA expression, C57BL/6 mice were infected intravenously with 5X105 BRD509 and spleens were harvested at either 30 minutes or 5 hours after infection. Total RNA was isolated using Aurum Total RNA Mini Kit (Bio-Rad, Hercules, CA) or TRI reagent (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer's protocol. To enrich bacterial RNA from infected splenocytes, the MICROBEnrich kit (Ambion) was used, and traces of genomic DNA eliminated using DNA-free DNase treatment kit (Ambion) according to manufacturer's recommendations. To quantitate expression of Salmonella Flagellin (FliC) or SseJ by real-time qPCR, 1 µg of bacterial RNA was used as a template and cDNA synthesis by random hexamers was performed using TaqMan reverse transcription reagents (Applied Biosystems). SYBR Green (Applied Biosystems) based real-time qPCR was carried out based on published procedures [58]. The following primers were used in this study; 16S rRNA, 5′-tgttgtggttaataaccgca-3′ and 5′-gactaccagggtatctaatcc-3′ [59]; FliC, 5′-gtaacgctaacgacggtatc-3′ and 5′-atttcagcctggatggagtc-3′ [58]; SseJ, 5′-tattacgagactgccgatgc-3′ and 5′-gcccgtggtgagtataagggt-3′ (GeneScript's real-time PCR primer design tool). Data was acquired using a ViiA 7 Real-time PCR system (Applied Biosystems) and analyzed using comparative Ct method (Applied Biosystems). Transcripts of Flagellin (FliC) or SseJ were normalized to the respective amounts of 16S rRNA in each sample.
Tracking Salmonella specific endogenous CD4 T cell response using class-II tetramers
Endogenous Salmonella specific flagellin427–441 - or SseJ329–341 -CD4 T cell responses were monitored using a previously described methodology. Biotinylated I-Ab monomers with an attached peptide derived from Salmonella flagellin427–441 (Flagellin:I-Ab) or SseJ329–341 (SseJ:I-Ab) were produced in S2 Drosophila insect cells cultured using a Wave Bioreactor (GE Healthcare Biosciences, Pittsburgh, PA). Purified monomers were tetramerized using fluorochrome-conjugated streptavidin and batch-tested for optimal binding to CD4 T cells from day 7 peptide-immunized mice, as previously described [36]. Briefly, C57BL/6 mice were immunized with either 100 µg of flagellin427–441 or SseJ329–341 peptide in the presence of CFA (Sigma-Aldrich, St. Louis, MO). On day 7, the inguinal, axillary, and brachial lymph nodes were harvested from naïve or peptide-immunized mice and stained with respective class-II tetramers in the presence of Fc block (culture supernatant from the 24G2 hybridoma, 2% mouse serum, 2% rat serum, and 0.01% sodium azide). Cells were incubated with anti-fluorochrome microbeads (Stem cell technologies, Vancouver, Canada) and tetramer-specific cells enriched using a magnet. Bound and unbound fractions were stained with fluorochrome-conjugated Abs specific for CD3, CD4, CD8, CD11b, CD11c, B220, F4/80, CCR7, and CD27 (eBioscience, or BD Bioscience, San Diego, CA). For transcription factor staining, cells were surface stained, and then treated with Foxp3 staining buffer set (eBioscience). Permeabilized cells were stained with fluorochrome-conjugated Abs specific for T-bet, GATA3, RORγT, or Foxp3. Cells were then analyzed by flow cytometry using an LSR II (BD Bioscience) or FACS Canto (BD Bioscience). Endogenous tetramer specific CD4 T cells were identified using a previously described gating strategy [36]. Tetramer specificity was confirmed by the absence of tetramer binding CD8 T cells in the bound fraction and in CD4 T cells within the unbound fraction. All the data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Cell processing for ELISPOT assay
Spleen and MLN, including Peyer's patches, were crushed through nylon mesh, and then RBC in spleen was lysed with ACK lysis buffer (Lonza, Walkersville, MD). For nonlymphoid tissue processing, we followed published methods [60]. Livers were perfused, crushed through a cell strainer (BD Biosciences), and resuspended in 35% Percoll (Sigma-Aldrich). After centrifugation, pelleted cells were treated with ACK lysis buffer and then washed with 2% FBS/PBS. For Lamina propria (LP) lymphocytes, small intestine was dissected, and washed with 2% FBS/CMF (Ca2+ and Mg2+ free HBSS with 1mM HEPES, and 2.5mM NaHCO3). The intestine pieces were then stirred at 37°C for 30 min in CMF containing 10% FBS and 1mM dithioerythritol. The epithelial cells were removed by stirring the pieces in 1.3 mM EDTA/HBSS solution at 37°C for 30 min. The intestinal tissues were then treated with collagenase (Invitrogen, Carlsbad, CA) in RPMI 1640 (with 1mM CaCl2, 1mM MgCl2, and 5% FBS) at 37°C for 1 h. Cells were washed in 2% FBS/PBS, and then centrifugated on a 44%/67% Percoll gradient (GE Healthcare Biosciences). Viable cells at the interface were collected, and these cells are LP lymphocytes.
Examining Salmonella specific endogenous CD4 T cell response using ELISPOT assay
MultiscreenHTS ELISPOT plate (Millipore, Billerica, MA) was coated with either purified anti-mouse IFN-γ (BD Bioscience) or IL-17A (eBioscience). Total CD4 T cells from each tissue were isolated using EasySep Mouse CD4 T cell enrichment kit (Stem cell technologies) according to the manufacturer's protocol. The purity of CD4 T cells were measured by flow cytometry and was typically >90% for splenocytes and MLN, and >70% for non-lymphoid tissues. Purified CD4 T cells (105 cells/well) were added to ELISPOT plates in the presence of 4×105 cells irradiated splenocytes. Cells were restimulated with 10 µM peptides (Table 3) at 37°C overnight. Bound IFN-γ or IL-17A was detected by biotin-conjugated anti-mouse IFN-γ (BD Bioscience) or IL-17A (eBioscience), followed by AP-conjugated streptavidin (BD Bioscience). Bound antibodies were visualized using One-step NBT-BCIP substrate (Thermo, Rockford, IL), and plates analyzed using an ELISPOT reader ImmunoSpot (Cellular Technology, Shaker Heights, OH).
IL-22 ELISA
Purified CD4 T cells (1×105 cells/well) from spleens and MLN, including Peyer's patches, were restimulated with 10 µM peptides (Table 3) in the presence of 4×105 irradiated splenocytes for 16 h. IL-22 secretion was measured from culture supernatant using mouse IL-22 ELISA Ready-SET-Go kit (eBioscience). Briefly, High-protein binding plates (Costar, Corning, NY) were coated overnight with capture IL-22 antibody. After incubation in Assay Diluent for 1 hour at room temperature, plates were washed twice before culture supernatant was added. After incubation for 2 hours, plates were washed and biotin-conjugated detection IL-22 antibody added. After 1 hour, plates were washed and incubated for 30 min with avidin-HRP. Bound antibody was visualized using TMB substrate and after 15 min, the reaction stopped by adding 50 µl of 2N H2SO4. Plates were analyzed using a spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA).
Statistical analysis
Statistical differences between groups of normally distributed data were examined using Prism (GraphPad Software, La Jolla, CA). Data in each group were compared using an unpaired t test and were considered significantly different with a p<0.05.
Zdroje
1. BarouchDHKorberB 2010 HIV-1 vaccine development after STEP. Annu Rev Med 61 153 167
2. CoffmanRLSherASederRA 2010 Vaccine adjuvants: putting innate immunity to work. Immunity 33 492 503
3. JenkinsMKChuHHMcLachlanJBMoonJJ 2010 On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol 28 275 294
4. JamesonSCMasopustD 2009 Diversity in T cell memory: an embarrassment of riches. Immunity 31 859 871
5. WeaverCTHattonRDManganPRHarringtonLE 2007 IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25 821 852
6. SallustoFLanzavecchiaAArakiKAhmedR 2010 From vaccines to memory and back. Immunity 33 451 463
7. O'SheaJJPaulWE 2010 Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327 1098 1102
8. SzaboSJKimSTCostaGLZhangXFathmanCG 2000 A novel transcription factor, T-bet directs Th1 lineage commitment. Cell 100 655 669
9. RavindranRFoleyJStoklasekTGlimcherLHMcSorleySJ 2005 Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol 175 4603 4610
10. ZhouMOuyangW 2003 The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res 28 25 37
11. MaizelsRMPearceEJArtisDYazdanbakhshMWynnTA 2009 Regulation of pathogenesis and immunity in helminth infections. J Exp Med 206 2059 2066
12. KhaderSAGaffenSLKollsJK 2009 Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2 403 411
13. McSorleySJAschSCostalongaMRieinhardtRLJenkinsMK 2002 Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity 16 365 377
14. RomanEMillerEHarmsenAWileyJVon AndrianUH 2002 CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J Exp Med 196 957 968
15. RoanNRGierahnTMHigginsDEStarnbachMN 2006 Monitoring the T cell response to genital tract infection. Proc Natl Acad Sci U S A 103 12069 12074
16. BlairDALefrancoisL 2007 Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A 104 15045 15050
17. SrinivasanAMcSorleySJ 2007 Pivotal advance: exposure to LPS suppresses CD4+ T cell cytokine production in Salmonella-infected mice and exacerbates murine typhoid. J Leukoc Biol 81 403 411
18. ReileyWWCalayagMDWittmerSTHuntingtonJLPearlJE 2008 ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc Natl Acad Sci U S A 105 10961 10966
19. WolfAJDesvignesLLinasBBanaieeNTamuraT 2008 Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med 205 105 115
20. MarzoALKlonowskiKDLe BonABorrowPToughDF 2005 Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol 6 793 799
21. HatayeJMoonJJKhorutsAReillyCJenkinsMK 2006 Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312 114 116
22. BadovinacVPHaringJSHartyJT 2007 Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity 26 827 841
23. MoonJJChuHHPepperMMcSorleySJJamesonSC 2007 Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27 203 213
24. PepperMLinehanJLPaganAJZellTDileepanT 2010 Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol 11 83 89
25. TalaatAMLyonsRHowardSTJohnstonSA 2004 The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc Natl Acad Sci U S A 101 4602 4607
26. JansenAYuJ 2006 Differential gene expression of pathogens inside infected hosts. Curr Opin Microbiol 9 138 142
27. McSorleySJCooksonBTJenkinsMK 2000 Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol 164 986 993
28. BergmanMACummingsLAAlanizRCMayedaLFellnerovaI 2005 CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun 73 7226 7235
29. MoonJJMcSorleySJ 2009 Tracking the dynamics of salmonella specific T cell responses. Curr Top Microbiol Immunol 334 179 198
30. Salazar-GonzalezRMMcSorleySJ 2005 Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett 101 117 122
31. LetranSELeeSJAtifSMUematsuSAkiraS 2011 TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol 41 29 38
32. CummingsLAWilkersonWDBergsbakenTCooksonBT 2006 In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61 795 809
33. ZhuYRudenskyAYCorperALTeytonLWilsonIA 2003 Crystal structure of MHC class II I-Ab in complex with a human CLIP peptide: prediction of an I-Ab peptide-binding motif. J Mol Biol 326 1157 1174
34. LawleyTDChanKThompsonLJKimCCGovoniGR 2006 Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog 2 e11
35. AbrahamsGLHenselM 2006 Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol 8 728 737
36. MoonJJChuHHHatayeJPaganAJPepperM 2009 Tracking epitope-specific T cells. Nat Protoc 4 565 581
37. GriffinAJMcSorleySJ 2011 Generation of Salmonella-specific Th1 cells requires sustained antigen stimulation. Vaccine 29 2697 2704
38. Salazar-GonzalezRMSrinivasanAGriffinAMuralimohanGErteltJM 2007 Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol 179 6169 6175
39. SrinivasanASalazar-GonzalezRMJarchoMSandauMMLefrancoisL 2007 Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J Immunol 178 6342 6349
40. Salazar-GonzalezRMNiessJHZammitDJRavindranRSrinivasanA 2006 CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity 24 623 632
41. BobatSFlores-LangaricaAHitchcockJMarshallJLKingsleyRA 2011 Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur J Immunol 41 1606 1618
42. ErikssonSLucchiniSThompsonARhenMHintonJC 2003 Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol 47 103 118
43. BoldTDBanaeiNWolfAJErnstJD 2011 Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog 7 e1002063
44. EgenJGRothfuchsAGFengCGHorwitzMASherA 2011 Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34 807 819
45. ObstRvan SantenHMMathisDBenoistC 2005 Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 201 1555 1565
46. ObstRvan SantenHMMelamedRKamphorstAOBenoistC 2007 Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc Natl Acad Sci U S A 104 15460 15465
47. GriffinAJMcSorleySJ 2011 Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol 4 371 382
48. UrdahlKBShafianiSErnstJD 2011 Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol 4 288 293
49. RaffatelluMSantosRLVerhoevenDEGeorgeMDWilsonRP 2008 Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 421 428
50. ValdezYDiehlGEVallanceBAGrasslGAGuttmanJA 2008 Nramp1 expression by dendritic cells modulates inflammatory responses during Salmonella Typhimurium infection. Cell Microbiol 10 1646 1661
51. Eaves-PylesTMurthyKLiaudetLViragLRossG 2001 Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol 166 1248 1260
52. PeckAMellinsED 2010 Precarious balance: Th17 cells in host defense. Infect Immun 78 32 38
53. Richter-DahlforsABuchanAMJFinlayBB 1997 Murine Salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med 186 569 580
54. GriffinABaraho-HassanDMcSorleySJ 2009 Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol 183 1263 1270
55. BordnerAJ 2010 Towards universal structure-based prediction of class II MHC epitopes for diverse allotypes. PloS One 5 e14383
56. EddySR 2004 What is a hidden Markov model? Nat Biotechnol 22 1315 1316
57. GroismanEAKayserJSonciniFC 1997 Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J Bacteriol 179 7040 7045
58. WinterSEWinterMGGodinezIYangHJRussmannH 2010 A rapid change in virulence gene expression during the transition from the intestinal lumen into tissue promotes systemic dissemination of Salmonella. PLoS Pathog 6 e1001060
59. BarmanMUnoldDShifleyKAmirEHungK 2008 Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 76 907 915
60. MasopustDVezysVMarzoALLefrancoisL 2001 Preferential localization of effector memory cells in nonlymphoid tissue. Science 291 2413 2417
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- How “Humane” Is Your Endpoint?—Refining the Science-Driven Approach for Termination of Animal Studies of Chronic Infection
- Sexual Development in : Lessons from Functional Analyses
- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Historical Contingencies Modulate the Adaptability of
- Human-like PB2 627K Influenza Virus Polymerase Activity Is Regulated by Importin-α1 and -α7
- -Sialidase in Complex with a Neutralizing Antibody: Structure/Function Studies towards the Rational Design of Inhibitors
- Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction
- How Do Bacteria Know They Are on a Surface and Regulate Their Response to an Adhering State?
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- The Murine Coronavirus Hemagglutinin-esterase Receptor-binding Site: A Major Shift in Ligand Specificity through Modest Changes in Architecture
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- Sexual Development in : Lessons from Functional Analyses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



