-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
How Do Bacteria Know They Are on a Surface and Regulate Their Response to an Adhering State?
article has not abstract
Published in the journal: . PLoS Pathog 8(1): e32767. doi:10.1371/journal.ppat.1002440
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002440Summary
article has not abstract
Why Do Bacteria Adhere to Surfaces and Why Is Adhesion Considered a Virulence Factor?
Bacteria adhere to virtually all natural and synthetic surfaces [1], [2]. Although there are a number of different reasons as to why bacteria adhere to a surface, the summarizing answer is brief: “Adhesion to a surface is a survival mechanism for bacteria”. Nutrients in aqueous environments have the tendency to accumulate at surfaces [1], [3], giving adhering bacteria a benefit over free floating, so-called planktonic ones. This is why mountain creeks may contain crystal clear, drinkable water, while stepping stones underneath the water may be covered with a slippery film of adhering microbes. In the oral cavity, adhesion to dental hard and soft tissues is life-saving to the organisms, because microbes that do not manage to adhere and remain planktonic in saliva are swallowed with an almost certain death in the gastrointestinal tract.
Bacterial adhesion is generally recognized as the first step in biofilm formation, and for the human host, the ability of a bacterium to adhere is a definite virulence factor, especially in immunocompromised patients and in the growing number of elderly patients relying on biomaterials implants and devices for the restoration of function after (oncological) intervention surgery, trauma, or wear [4]. Well-known examples of biomaterials implants are dental implants, vascular grafts, and prosthetic hips and knee joints. Bacterial adhesion is a virulence factor, because it stimulates the organism to produce extracellular polymeric substances (EPSs), such as polysaccharides, proteins, nucleic acids, and lipids [5], through which they embed themselves in a protective matrix. This protective matrix provides mechanical stability to a biofilm and constitutes the main difference between planktonic bacteria and bacteria adhering to a surface in a so-called biofilm mode of growth. Bacteria organized in biofilms are at least ten to 1,000 times more resistant to antibiotics [6] than bacteria in a planktonic state and can cope much better with unfavorable external conditions in the host immune system than their planktonic counterparts. Not surprisingly, the fate of an infection associated with a biomaterials implant is mostly removal and replacement of the implant [7], at high costs to the health care system and great inconvenience to the patient.
What Are the Mechanisms Controlling Bacterial Adhesion to Surfaces?
Over the past decades, two mechanisms have been described to control microbial adhesion to surfaces. From a biochemical perspective, bacterial adhesion has been described in terms of specific interactions between localized, specific molecular groups. For example, Escherichia coli expresses type 1 fimbriae possessing tip-positioned adhesive protein FimH that bind specifically to mannose [8]. Sometimes, even specific forces have been categorized as a separate class of fundamental interaction forces, although such forces do not exist from a physico-chemical perspective. Adhesion, whether arising from specific, molecular, or non-specific interactions, is supposed to originate from an interplay between ever present attractive Lifshitz-Van der Waals forces, attractive or repulsive electrostatic, hydrogen bonding, and Brownian motion forces [9]. Reconciling the biochemical and physico-chemical perspective [9], [10], specific, molecular interactions are now recognized as highly directional, spatially confined interactions, operative over small distances, arising from highly specific, small stereo-chemical domains on the interacting surfaces, but arising from the above mentioned fundamental physico-chemical forces [10], [11].
Can We Measure the Forces with which a Bacterium Adheres to a Surface?
Since the introduction of atomic force microscopy [12], it has become possible to directly measure the adhesion forces between bacteria and substratum surfaces [13]. In these measurements, bacteria are attached to a cantilever. Subsequently, the bacterium-coated cantilever is manoeuvred toward a substratum surface, and the force upon approach and retraction of the bacterial probe is recorded from the cantilever deflection. Upon approach, an increasing repulsive force is measured until physical contact, while upon subsequent retraction, an attractive adhesion force is recorded until failure, which is the force value generally reported in the literature as “the adhesion force” [13].
How Does a Bacterium Know It Is on a Surface?
In the absence of visual, auditory, and olfactory perception, adhering bacteria react to membrane stresses arising from minor deformations due to the adhesion forces felt to make them aware of their adhering state on a surface and change from a planktonic to a biofilm phenotype. E. coli, for instance, are known to have mechano-sensitive channels [14].
How Do Bacteria Respond to Different Adhesion Forces?
Based on available literature, we propose three adhesion force regimes dictating the bacterial response to a substratum surface, as schematically summarized in Figure 1. Recently, a link has been described between strong adhesion forces between bacteria and substratum surfaces yielding membrane stresses and the percentage of dead cells on a surface for which the term “stress de-activation” was coined [15]. Stress de-activation may set in gradually with increasing adhesion forces, and in a first instance it has been demonstrated that bacterial generation times on a surface increase with decreasing desorption rates, i.e., increasing adhesion forces [16]. The existence of stress de-activation was further supported by the observation that an external mechanical stress on adhering bacteria enhances the antimicrobial efficacy of quaternary ammonium compounds in solution [17]. Since the great majority of bacterial strains and species possess a negative surface charge [18], strong adhesion forces can be found on positively charged surfaces, such as quaternary ammonium-coated surfaces that are known to kill bacteria upon contact [19] in this “lethal” regime of strong adhesion forces (see Figure 1). It has been suggested that such lethal effects upon adhesion require a minimum positive charge density of the substratum surface [20], [21]; for example, a positive charge density of 8.1015 per cm2 is required to kill around 108 E. coli adhering per cm2, equalling a monolayer of bacteria [21]. The positive charge density necessary for lethal effects depends on the bacterial species and is, for instance, ten times higher for Staphylococcus epidermidis than for E. coli [20].
Fig. 1. Three regimes of bacterial adhesion to substratum surfaces that dictate the bacterial response to a surface. 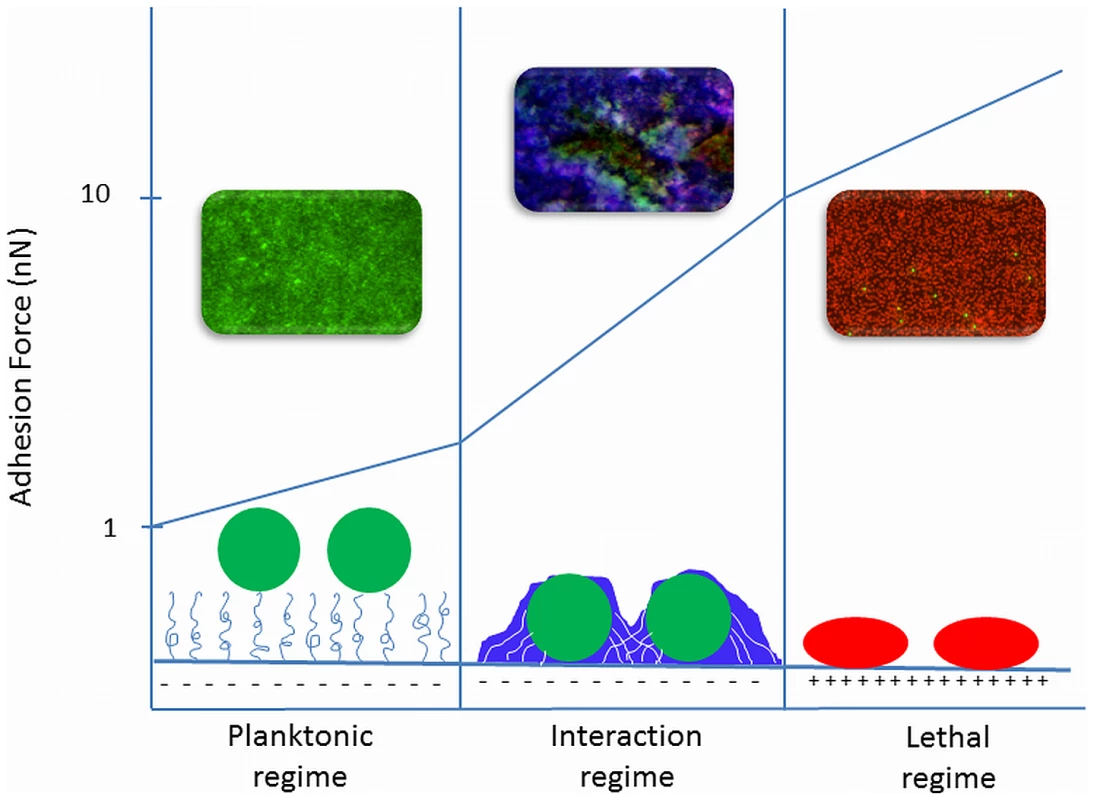
1) In the planktonic regime, adhesion forces are extremely weak as on polymer-brush coatings, and bacteria do not realize they are on a surface. Weakly adhering bacteria are mainly live (green fluorescence). This regime is called “planktonic”, because bacteria do not adapt their planktonic phenotype despite their adhering state. 2) In the “interaction” regime, bacterial responses to their adhering state increase with increasing adhesion forces, for instance by the production of EPS (blue fluorescence), encasing themselves in a protective biofilm. 3) In the “lethal regime”, strong adhesion forces, as occurring on positively charged surfaces, cause membrane deformation that causes stress de-activation of the adhering bacteria, leading to reduced growth and cell death (red fluorescence). The confocal laser scanning micrographs represent biofilms in all three regimes of adhesion forces in which bacteria were stained with Baclight LIVE/DEAD stain, rendering live bacteria green and membrane damaged or dead bacteria red. EPS was stained with calcofluor white, rendering blue fluorescence. On the lower end of the adhesion force scale are polymer brush–coated surfaces and hydrogels that exert very weak adhesion forces on adhering bacteria [18] to the extent that adhering bacteria hardly realize they are on a surface and do not change to the protected phenotype enabling them to form a biofilm with EPS encasing [22]. We propose calling this the “planktonic” regime (see Figure 1) of adhesion forces.
In between these two ends of the adhesion force scale is an intermediate or “interaction” regime of bacterial adhesion forces, as encountered on “” materials, such as polymers, metals, and ceramics commonly used for biomaterials implants and devices. In the interaction regime, phenotypic changes occur progressively with increasing adhesion forces. On polyethylene, for instance, with bacterial adhesion forces in the interaction regime, EPS production by adhering staphylococci was much more pronounced than on polymethylmethacrylate or stainless steel [23].
Adhesion forces at the proposed transitions between the different regimes are all approximate because adhesion forces tend to strengthen considerably during the first minutes after contact, yielding a switch from reversible to irreversible adhesion. Microbiologically, this switch has been associated with the production of EPS in response to a surface [2], but EPS production in response to adhesion likely occurs much later on during growth, as completely inert polystyrene particles also demonstrate this initial bond strengthening [24]. Upon first approach of a bacterium to a surface, it becomes attached to a layer of highly viscous water adjacent to the surface that is subsequently slowly penetrated to allow stronger contact with the surface, after which protein structures on the cell surface re-orient themselves to allow optimal binding. Since it is unlikely that metabolic processes and phenotypic changes occur within minutes, we envisage that adhesion forces after physico-chemical strengthening represent the transition forces between the three adhesion force regimes depicted in Figure 1.
The proposal of three adhesion force regimes not only sheds light on how bacteria may sense a surface and what directs their response to a surface, but the implications of these different regimes extend also to interactions between bacteria. Communication between bacteria in a biofilm is often described as being due to quorum-sensing (QS) molecules [2], but it may not be ruled out that the production of QS molecules is also dictated in a first instance by membrane stresses developing as a result of adhesion forces between adhering bacteria in a biofilm in the interaction regime. This suggests two means of bacterial communication in a biofilm: (i) initial signalling through direct physical contact during adhesion to the substratum surface over short distances according to the three regimes of adhesion forces (see Figure 1), and (ii) through QS molecules that diffuse through a biofilm and allow communication over longer distances than possible through adhesion forces, which are limited to several hundreds of nanometers.
Zdroje
1. FletcherM 1994 Bacterial biofilms and biofouling. Curr Opin Biotechnol 5 302 306
2. Hall-StoodleyLCostertonJWStoodleyP 2004 Bacterial biofilms: from the natural environment to infectious disease. Nat Rev Microbiol 2 95 108
3. Van LoosdrechtMCLyklemaJNordeWZehnderAJ 1990 Influence of interfaces on microbial activity. Microbiol Rev 54 75 87
4. BusscherHJPloegRJVan der MeiHC 2009 Snapshot: Biofilms and biomaterials: mechanisms of medical device related infections. Biomaterials 30 4247 4248
5. FlemmingHCWingenderJ 2010 The biofilm matrix. Nat Rev Microbiol 8 623 633
6. Rodriguez-MartinezJMPascualA 2006 Antimicrobial resistance in bacterial biofilms. Rev Med Microbiol 17 65 75
7. BozicKJRiesMD 2005 The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am 87 1746 1751
8. TchesnokovaVAprikianPKisielaDGoweySKorotkovaNThomasWSokurenkoE 2011 Type 1 fimbrial adhesin FimH elicits and immune response that enhances cell adhesion of Eschericia coli. Infect Immun 79 3895 3904
9. Van OssCJ 1994 Polar or Lewis acid-base interactions. Interfacial forces in aqueous media New York Marcel Dekker . 18 46
10. BosRVan der MeiHCBusscherHJ 1999 Physico-chemistry of initial microbial adhesive interactions – its mechanisms and methods for study. FEMS Microbiol Rev 23 179 230
11. BusscherHJCowanMMVan der MeiHC 1992 On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol Rev 88 119 210
12. BinnigGQuateCFGerberC 1986 Atomic force microscopy. Phys Rev Lett 56 930 933
13. DufrêneYF 2008 Towards nanomicrobiology using atomic force microscopy. Nat Rev Microbiol 6 674 680
14. IsclaIWrayRBlountP 2011 The oligomeric state of the truncated mechanosensitive channel of large conductance shows no variance in vivo. Protein Sci 20 1638 1642
15. LiuYStraussJCamesanoTA 2008 Adhesion forces between Staphylococcus epidermidis and surfaces bearing self-assembled monolayers in the presence of model proteins. Biomaterials 29 4374 4382
16. GottenbosBVan der MeiHCBusscherHJ 2000 Initial adhesion and surface growth of Staphylococcus epidermidis and Pseudomonas aeruginosa on biomedical polymers. J Biomed Mater Res 50 208 214
17. CrismaruMAsriLATWLoontjensTJAKromBPDe VriesJVan der MeiHCBusscherHJ 2011 Survival of adhering staphylococci during exposure to a quaternary ammonium compound evaluated by using atomic force microscopy imaging. Antimicrob Agents Chemother 55 5010 5017
18. JückerBAHarmsHZehnderAJB 1996 Adhesion of the positively charged bacterium Stenotrophomas (Xanthomonas) maltophilia 70401 to glass and Teflon. J Bacteriol 178 5472 5479
19. TillerJCLiaoCJLewisKKlibanovAM 2001 Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci 98 5981 5985
20. KüglerRBouloussaORondelezF 2005 Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 151 1341 1348
21. MurataHKoepselRRMatyjaszewskiKRussellA 2007 Permanent, non-leaching antibacterial surface—2: how high density cationic surfaces kill bacterial cells. Biomaterials 28 4870 4879
22. NejadnikMRVan der MeiHCNordeWBusscherHJ 2008 Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 29 4117 4121
23. NuryastutiTKromBPAmanATBusscherHJVan der MeiHC 2011 Ica-expression and gentamicin susceptibility of Staphylococcus epidermidis biofilm on orthopedic implant biomaterials. J Biomed Mater Res 96A 365 371
24. BusscherHJNordeWSharmaPKVan der MeiHC 2010 Interfacial re-arrangement in initial microbial adhesion to surfaces. Curr Opinion Coll Interf Sci 15 510 517
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- How “Humane” Is Your Endpoint?—Refining the Science-Driven Approach for Termination of Animal Studies of Chronic Infection
- Sexual Development in : Lessons from Functional Analyses
- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Historical Contingencies Modulate the Adaptability of
- Human-like PB2 627K Influenza Virus Polymerase Activity Is Regulated by Importin-α1 and -α7
- -Sialidase in Complex with a Neutralizing Antibody: Structure/Function Studies towards the Rational Design of Inhibitors
- Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction
- How Do Bacteria Know They Are on a Surface and Regulate Their Response to an Adhering State?
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- The Murine Coronavirus Hemagglutinin-esterase Receptor-binding Site: A Major Shift in Ligand Specificity through Modest Changes in Architecture
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- Sexual Development in : Lessons from Functional Analyses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



