-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
Bacterial extracellular polysaccharides are a key constituent of the extracellular matrix material of biofilms. Pseudomonas aeruginosa is a model organism for biofilm studies and produces three extracellular polysaccharides that have been implicated in biofilm development, alginate, Psl and Pel. Significant work has been conducted on the roles of alginate and Psl in biofilm development, however we know little regarding Pel. In this study, we demonstrate that Pel can serve two functions in biofilms. Using a novel assay involving optical tweezers, we demonstrate that Pel is crucial for maintaining cell-to-cell interactions in a PA14 biofilm, serving as a primary structural scaffold for the community. Deletion of pelB resulted in a severe biofilm deficiency. Interestingly, this effect is strain-specific. Loss of Pel production in the laboratory strain PAO1 resulted in no difference in attachment or biofilm development; instead Psl proved to be the primary structural polysaccharide for biofilm maturity. Furthermore, we demonstrate that Pel plays a second role by enhancing resistance to aminoglycoside antibiotics. This protection occurs only in biofilm populations. We show that expression of the pel gene cluster and PelF protein levels are enhanced during biofilm growth compared to liquid cultures. Thus, we propose that Pel is capable of playing both a structural and a protective role in P. aeruginosa biofilms.
Published in the journal: . PLoS Pathog 7(1): e32767. doi:10.1371/journal.ppat.1001264
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001264Summary
Bacterial extracellular polysaccharides are a key constituent of the extracellular matrix material of biofilms. Pseudomonas aeruginosa is a model organism for biofilm studies and produces three extracellular polysaccharides that have been implicated in biofilm development, alginate, Psl and Pel. Significant work has been conducted on the roles of alginate and Psl in biofilm development, however we know little regarding Pel. In this study, we demonstrate that Pel can serve two functions in biofilms. Using a novel assay involving optical tweezers, we demonstrate that Pel is crucial for maintaining cell-to-cell interactions in a PA14 biofilm, serving as a primary structural scaffold for the community. Deletion of pelB resulted in a severe biofilm deficiency. Interestingly, this effect is strain-specific. Loss of Pel production in the laboratory strain PAO1 resulted in no difference in attachment or biofilm development; instead Psl proved to be the primary structural polysaccharide for biofilm maturity. Furthermore, we demonstrate that Pel plays a second role by enhancing resistance to aminoglycoside antibiotics. This protection occurs only in biofilm populations. We show that expression of the pel gene cluster and PelF protein levels are enhanced during biofilm growth compared to liquid cultures. Thus, we propose that Pel is capable of playing both a structural and a protective role in P. aeruginosa biofilms.
Introduction
Biofilms are surface associated communities embedded within an extracellular matrix [1], [2], [3]. Biofilm communities exhibit enhanced antibiotic tolerance [4], [5], [6]. As a result, biofilm infections tend to be chronic and difficult to eradicate [2], [7]. This enhanced tolerance is thought to be multi-factorial, owing to biofilm-associated patterns of gene expression, slow growth rate, and reduced antimicrobial diffusion within the biofilm [4]. A focus of research has been to identify biofilm-associated factors that contribute to their antibiotic tolerance.
The opportunistic pathogen, Pseudomonas aeruginosa, is a model organism in biofilm research. P. aeruginosa is well known for the chronic infections it causes in individuals with the genetic disease, cystic fibrosis (CF) [7]. Biofilm formation within the CF airways is believed to facilitate the infection, helping the bacteria to withstand aggressive antimicrobial treatment and host defenses [8], [9].
The extracellular matrix is a distinguishing feature of biofilms, capable of functioning as both a structural scaffold and protective barrier to antimicrobials [1], [2], [10], [11], [12]. A key component of the matrix is extracellular polysaccharides [13]. Exopolysaccharides carry out a wide range of functions involving surface and cell-cell interactions, as well as protecting against antimicrobials and host defenses [3], [10], [14], [15], [16]. P. aeruginosa produces three exopolysaccharides, alginate, Pel and Psl, all of which have been implicated in biofilm development under different circumstances [11].
Pel's composition has yet to be fully elucidated. Initial carbohydrate analysis suggests Pel is a glucose-rich polysaccharide polymer although the exact structure remains unknown [17]. Pel synthesis machinery is encoded by a seven gene operon (pelA-F) originally identified in a mutagenesis screen for the loss of pellicle formation in PA14 [17]. Pel also appears to be important in static microtiter dish biofilm assays. A pel mutant strain had a defect in biofilm biomass accumulation in comparison to wild-type PA14 [17], [18]. The mechanism behind this observation remains unclear. Other studies have demonstrated that in the absence of type IV pili, Pel can play a role in attachment suggesting it can compensate as an attachment factor in the absence of other adhesins [18].
In this study, we conducted an analysis of Pel function. We focused our study on two common laboratory strains, PAO1 and PA14. PAO1 is capable of making both the Pel and Psl exopolysaccharides, while PA14 is only capable of producing Pel since three genes of the psl operon are deleted in this strain. We show that Pel is critical for maintaining cell-cell interactions in developing PA14 biofilms as well as providing protection against aminoglycoside antibiotics during biofilm growth. We also show that Pel does not appear to play any critical role in PAO1 biofilm development, where Psl appears to be the primary biofilm polysaccharide. Finally, we demonstrate that the pel operon is transcriptionally induced and PelF protein levels increase during biofilm growth. Thus, we propose that Pel can serve both as a structural and protective factor within a biofilm community.
Results
Generation of a pel-inducible overexpression strain
To initiate our study, we constructed a Pel overexpression strain. The native promoter region of pelA was replaced with the araC-PBAD promoter on the chromosome in two common laboratory strains, PAO1 and PA14, allowing arabinose-dependent expression of the pel operon (Figure S1A). PA14 is a clinical strain obtained from a burn patient that has a multi-gene truncation in the N-terminal region of the psl operon and is incapable of synthesizing the Psl polysaccharide [19]. Accordingly, PA14 serves as a useful strain to study the contribution of the Pel polysaccharide independently of Psl. In contrast, PAO1 has the necessary genes to produce both polysaccharides. The inducible strains will be referred to as PAO1PBADpel and PA14PBADpel. Quantitative RT-PCR was used to quantify pelA transcript level from log phase cells. The level of pelA transcript increased with increasing concentrations of the inducer, arabinose (Figure S1C). The dose-dependent increase in transcription level was similar between PA14 and PAO1. Addition of 0.2% arabinose led to 51 - and 61-fold increase in expression levels in PA14PBADpel and PAO1PBADpel, respectively. pelA transcript is expressed 1.8 times higher in wild-type PA14 compared to wild-type PAO1 relative to the internal control transcript, ampR (Figure S1C).
Pel overexpression contributes to aggregation, Congo red binding, pellicle formation and rugose colony morphology
We evaluated PAO1PBADpel and PA14PBADpel for the ability to conditionally produce more Pel polysaccharide with increasing pel transcription. Previous work has demonstrated that Pel synthesis is controlled at multiple levels, transcriptionally and allosterically [20], [21]. Congo red binding and liquid culture aggregation are two phenotypes associated with increased polysaccharide production in multiple bacterial species [20], [22], [23]. Addition of 1% arabinose to both PA14PBADpel and PAO1PBADpel leads to bacterial aggregation in liquid culture relative to the uninduced strain and these bacterial aggregates hyperbind Congo red (Figure S1B).
Pel expression was previously demonstrated to impact pellicle formation and colony morphology [17]. Wild-type PA14 forms a distinct pellicle after about two days of incubation at room temperature which becomes more pronounced over time. When induced, PA14PBADpel rapidly produces a thicker pellicle compared to wild-type. A top-down view reveals that the PA14PBADpel stain produces a pellicle with a highly defined wrinkly architecture that is resistant to extensive vortexing (Figure S1D, bottom panel). Consistent with previously published data, a mutation in pelB leads to a dramatic reduction in pellicle formation compared to the parental strain [17], [24]. Pellicles produced in wild-type PAO1 grown under the same conditions are less distinct than PA14 pellicles. No discernable difference in pellicle formation is seen between PAO1 and PAO1ΔpelB, but overexpressing Pel enhances pellicle formation similar to PA14 (Figure S1D). Overexpression of pel produces enhanced wrinkly colony morphology in PA14, whereas the pelB mutant grows as a smooth colony with little Congo red binding (Figure S1D, top panel). Unexpectedly, PAO1 did not produce a wrinkly colony morphology for any of the tested strains despite many attempts with varying temperature and media conditions (Figure S1D, top panel).
Pel contributes to biofilm development post-attachment
We investigated the function Pel plays in biofilm development using two biofilm culturing methods, a microtiter dish assay and a flow-cell reactor. A microtiter dish assay quantifies biofilm formation on plastic during static incubation. In contrast, a flow cell bioreactor allows a microscopic analysis of live biofilms growing in dilute medium under conditions of continuous flow.
The influence of Pel on initial attachment to a plastic surface was examined. A pelB mutation in PA14 did not impact bacterial attachment (Figure 1A). However, overexpressing pel in either PA14 or PAO1 increased surface attachment (Figure 1A and 1B). Similar to PA14, no phenotype were seen in a PAO1ΔpelB mutant compared to wild-type PAO1 (Figure 1B). In contrast, but consistent with previously published work, a polar deletion in psl had a strong attachment defect in PAO1, indicating that Psl, and not Pel, is an important adhesin for surface attachment under these conditions [25], [26].
Fig. 1. Pel is not required for attachment. 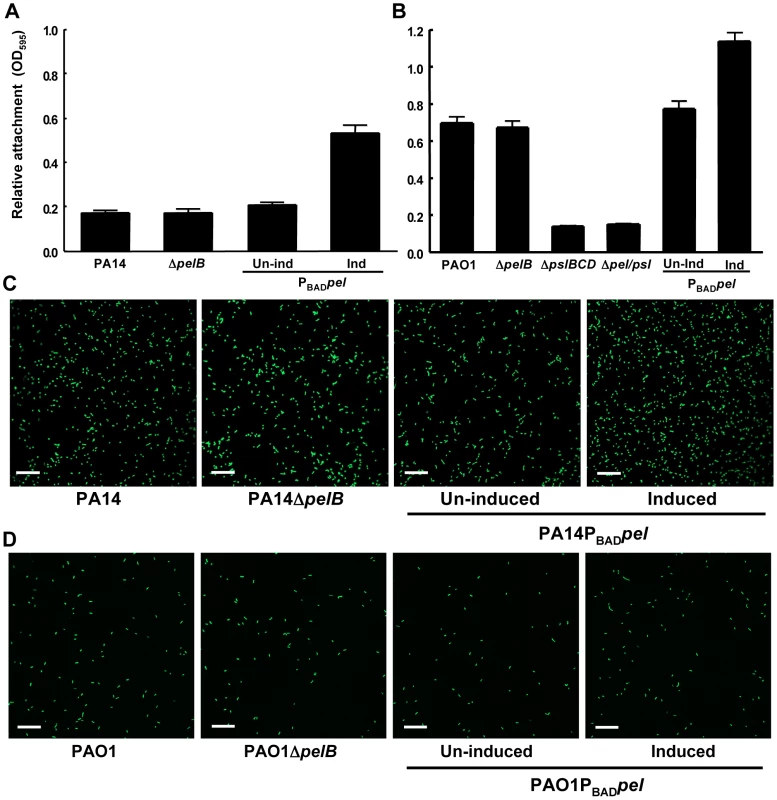
Attachment of P. aeruginosa PA14 (A) and PAO1 (B) to microtiter dish wells was measured by crystal violet binding at an OD595. Overexpressing pel by addition of arabinose increased crystal violet binding in both PA14 and PAO1, but a pelB mutant showed no defect. Attachment to glass slides was visualized in a flow cell after one hour of attachment and one hour of continuous flow. Medium was supplemented with 0.2% arabinose under inducing conditions. Representative SCLM images of PA14, PA14ΔpelB and PA14PBADpel are shown using a 40× objective (C). Representative SCLM images of PAO1, PAO1ΔpelB and PAO1PBADpel are shown using a 40× objective (D). Scale bars represent 25 µm. Error bars represent standard deviations. Crystal violet staining is an indirect measurement of bacterial attachment and thus we took a complementary, microscopic approach to evaluate Pel's role in attachment to a glass surface in a flow-cell reactor. Images were acquired by scanning confocal laser microscopy (SCLM) and analyzed by COMSTAT 1 software for surface coverage [27]. No statistical differences between PA14, PA14ΔpelB, uninduced and induced PA14PBADpel for attachment are observed (Figure 1C and S2A). Similar to PA14, no difference is observed for any of the PAO1 strains tested under non-inducing and inducing conditions (Figure 1D and S2A). These results are slightly inconsistent with our microtiter dish assay, which demonstrate a modest but clear increase in attachment for the Pel overexpression strains. However, in both PAO1 and PA14, a pel mutation did not affect attachment in either biofilm culturing method.
Unlike surface attachment, we found that Pel has a significant impact on later stages of biofilm development and this impact was found to be strain dependent. To assess effects of Pel on later stages of biofilm development, we grew strains for 24 h in a microtiter dish assay and found that the pelB mutant strain of PA14 has a significant reduction in biofilm biomass compared to the parental PA14 strain, similar to previous findings (Figure 2A) [17], [18]. The PA14ΔpelB biofilm defect was complemented by supplying Ppel in trans. Ppel contains the entire pel operon cloned into an arabinose-controlled expression plasmid, pMJT-1 (Figure 2A). Overexpressing Pel in PA14 increased biofilm biomass almost two-fold (Figure 2A). In contrast to PA14, no difference is seen between PAO1 and PAO1ΔpelB, while overexpressing Pel results in a modest increase of biofilm biomass (Figure 2B). Conversely, PAO1ΔpslBCD has a pronounced defect, suggesting that Psl is the dominant polysaccharide in PAO1 for both attachment and biofilm maintenance, as reported by Ma, et al. [26].
Fig. 2. PA14ΔpelB is arrested in the monolayer stage of biofilm development. 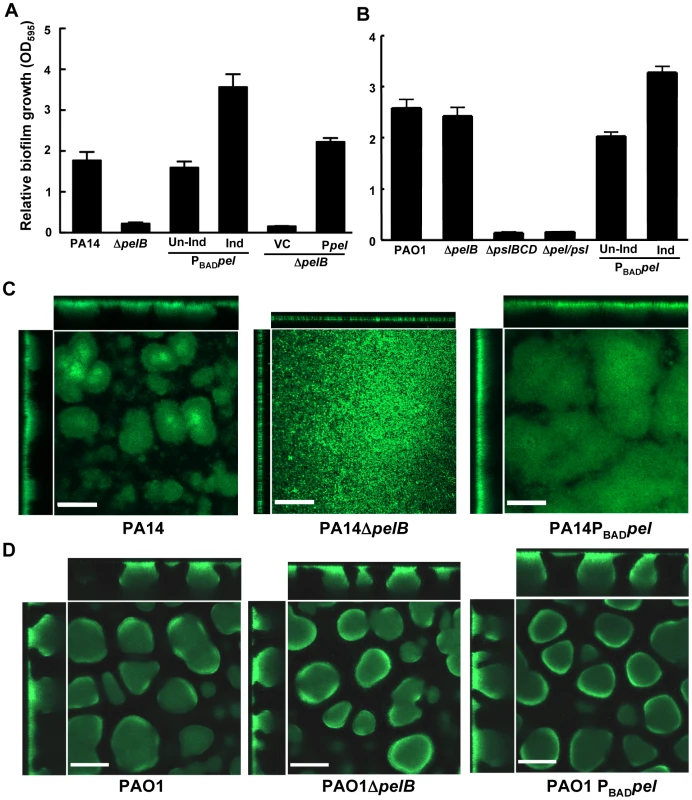
Strains PA14 (A) and PAO1 (B) were compared at 24 h for levels of biomass in microtiter dishes grown at room temperature by measuring crystal violet binding OD595. Expression of the pel operon from an arabinose-inducible plasmid pMJT1 (Ppel) but not in the vector control (VC) alleviated the crystal violet staining defect of PA14ΔpelB. Biofilm structure was visualized in a flow cell and representative top-down and side-view images are shown for PA14, PA14ΔpelB and PA14PBADpel (C) and PAO1, PAO1ΔpelB and PAO1PBADpel (D). Images were obtained using a 20× objective after four d of growth in continuous flow chambers. Scale bars represent 100 µm. Error bars represent standard deviations. Biofilm formation by these strains was monitored in a flow-cell bioreactor to allow for live imaging and structural analysis. Under these conditions, P. aeruginosa forms biofilms that contain mushroom-shaped multicellular structures. PA14 forms small microcolonies by day two that further develop into a structurally complex biofilm with large multicellular aggregates of bacteria by day four (Figure 2C). In stark contrast, PA14ΔpelB fails to form cellular aggregates. After four days of growth, PA14ΔpelB remains as a dense monolayer of cells attached to the glass surface, incapable of developing the complex three-dimensional structures typical of the wild-type strain (Figure 2C). The absence of cell aggregates in PA14ΔpelB indicates Pel may be responsible for the cell-to-cell adhesion necessary for aggregate formation. In support of this, overexpressing Pel results in larger cellular aggregates and enhanced biofilm biomass compared to wild-type PA14. Flow cell images were quantified for four properties of biofilm development using COMSTAT 1, average thickness, roughness coefficient, surface-to-volume ratio and maximum thickness (Figure S2B) [27]. Pel overexpression in PA14 affected each property by increasing the average thickness, decreasing the roughness coefficient, decreasing the surface to volume ratio and increasing the maximum thickness.
In contrast to PA14, no major visual or quantifiable difference is seen in biofilm structure after four days of growth for PAO1, PAO1ΔpelB and PAO1PBADpel (Figures 2D and S2B). However, a modest, but not statistically significant, increase in average biofilm thickness is detected for PAO1PBADpel. We subsequently assessed whether a Pel-dependent phenotype might manifest itself in older biofilms. Yet, even after nine days no significant differences were observed for PAO1, PAO1ΔpelB and PAO1PBADpel (Figure S3).
Continuous expression of pel is required for continued biofilm growth, but not for maintenance of existing biofilm structure
Continuous production of the Psl polysaccharide was recently shown to be required for both the addition of new biofilm biomass to a growing biofilm and for the maintenance of existing biofilm structure [26]. Conditional loss of Psl expression resulted in a halt of biofilm growth and an eventual erosion of the existing biofilm structure [26].
Using our conditional expression system we grew PA14PBADpel biofilms for two days in the presence of arabinose and either continued providing arabinose to the biofilm culture for an additional two days or we removed it from the growth medium (Figure 3). Interestingly, halting Pel expression by removing arabinose resulted in a biofilm that failed to increase in size, but retained the original shape and mass after two days as calculated by COMSTAT (Figure S4). The biofilm that was supplied arabinose continued to grow in size. These results suggest that continuous Pel production is important over the course of biofilm development. However, unlike Psl, continuous Pel production is not required to maintain existing biofilm structure.
Fig. 3. Continuous Pel production is required for biofilm growth, not maintenance of existing biofilm structure. 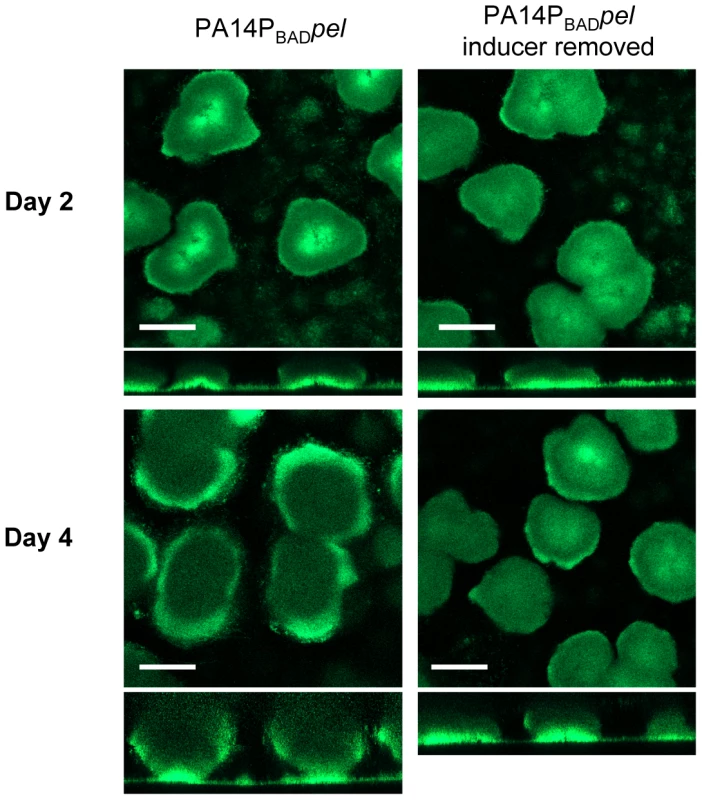
PA14PBADpel was grown in 2% TSB for two days under inducing conditions (0.2% arabiniose). Biofilm growth was continued either in the presence (left) or absence (right) of the inducer arabinose. Representative top-down and side-view SCLM images from day two and day four are shown. Scale bars represent 100 µm. Pel is critical for maintaining cell-to-cell interactions in PA14 biofilms
We hypothesized that the absence of cell aggregates in the PA14ΔpelB mutant biofilms is due to a defect in the cell-to-cell interactions necessary to hold an aggregate together. To initially test this hypothesis, we used time-lapse microscopy to analyze the behavior of biofilm cells at an early point in biofilm development. Dividing cells on the glass surface were monitored and the fate of daughter cells were separated into two categories [28]. Daughter cells that remained closely associated with the mother cell were termed “aggregate builders”. Cells that did not remain closely associated with the mother cells were designated “flyers”. We predicted if Pel were important in cell-to-cell interactions, cells incapable of Pel production would show a larger percentage of daughter cells exhibiting “flyer” behavior. Our analysis determined that Pel is a crucial determinant in daughter cell behavior in PA14 (Figure 4). As predicted, expression of Pel is related to daughter cell association with the parental cell. A pelB mutant displayed increased “flyer” behavior (88.3%) in comparison to wild-type PA14 (40.2%) and a reduced proportion of “aggregate builders” were observed (11.7%) than in PA14 (59.8%). Overexpressing pel resulted in an increased proportion of aggregate builders (82%) and relatively few flyers (18%).
Fig. 4. Pel impacts daughter cell behavior in early PA14 biofilms. 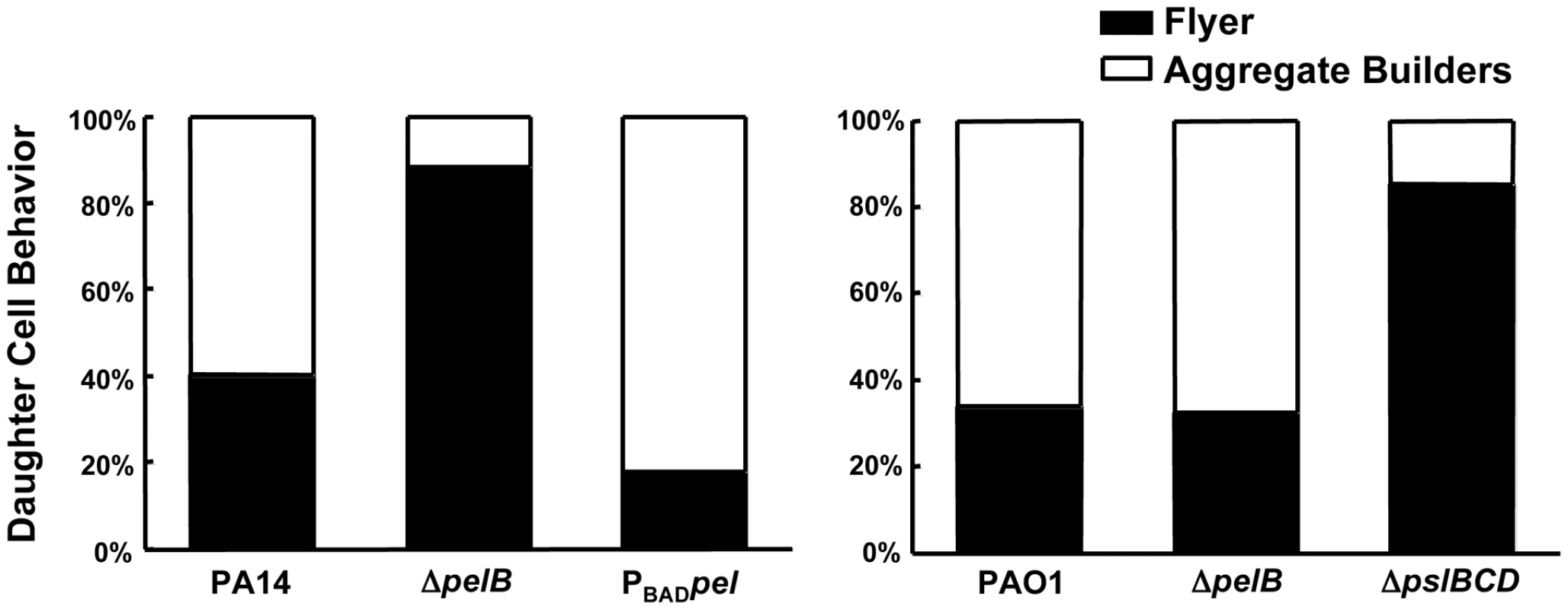
Bacterial cell divisions were monitored by time-lapse microscopy in an early-stage biofilm grown in a flow cell. Daughter cells that remained within a 15 µm diameter of the mother cell are referred to as “aggregate builders”, other cells were termed “flyers”. A minimum of 75 cell divisions was assessed for each strain. Conversely, in PAO1 Psl appears to be the primary polysaccharide involved in aggregate building. PAO1 and the PAO1Δpel mutant display indistinguishable daughter cell behavior profiles, with 33.9% and 32.6% of “flyers”, respectively. Conversely, PAO1ΔpslBCD exhibits a much larger proportion of “flyers” (85.3%) compared to PAO1 and PAO1ΔpelB (Figure 4). These data support our hypothesis that Pel contributes to aggregate formation in a PA14 biofilm by promoting retention of daughter cells within a growing aggregate, while Psl appears to be the critical polysaccharide for aggregate building in PAO1.
To complement the time-lapse microscopy study, we developed a novel assay involving infra-red laser [29]. This assay involves maintaining an optical trap in a liquid suspension of bacteria. Once bacteria enter the trap, they remain there. Initial experiments determined that continuous trapping of PA14 cells in liquid culture promoted the formation of stable aggregates. Using this technique we are able to study the effects of the Pel polysaccharide for the ability to form and maintain bacterial clusters. Since Pel was required for maintaining cell-to-cell interactions in flow cell biofilms, we predicted that Pel would be required for maintaining stable aggregates in this assay.
Wild-type PA14 forms aggregates after 20 min of trapping in all visualized fields (Figure 5A). In contrast, PA14ΔpelB did not form aggregates, even though a significant amount of free-floating bacteria entered and remained in the trap (Figure 5B). Rather, the mutant strain requires a minimum of 45 min of trapping to form aggregates (Figure 5C). Even with the extended incubation in the trap, 16% of the fields of view are absent of aggregates. Based on the differences in time for bacterial clustering to be observed, these data conclude Pel is an important component in the initiation of cellular clustering. Subsequently, we tested to see if Pel was important in maintaining clustering aggregates after an aggregate was formed. These experiments were set up similarly by allowing bacterial aggregation to occur for 20 min for wild-type PA14 and 45 min for the pelB mutant. After the designated incubation time with the laser, cluster stability was monitored by microscopy five minutes after the laser trap was disengaged. More than six aggregates in each strain were visually assessed for stability and separated into three categories as described in the figure legend (Figure 5). 85% of wild-type PA14 cell aggregates are stable five min after the release of the trap. In contrast, only 16% of the PA14ΔpelB aggregates remained after the laser trap is removed. These results further support that Pel is critical for both initiating and maintaining cell-to-cell interactions.
Fig. 5. Pel is important for cell-to-cell interactions necessary for aggregate formation. 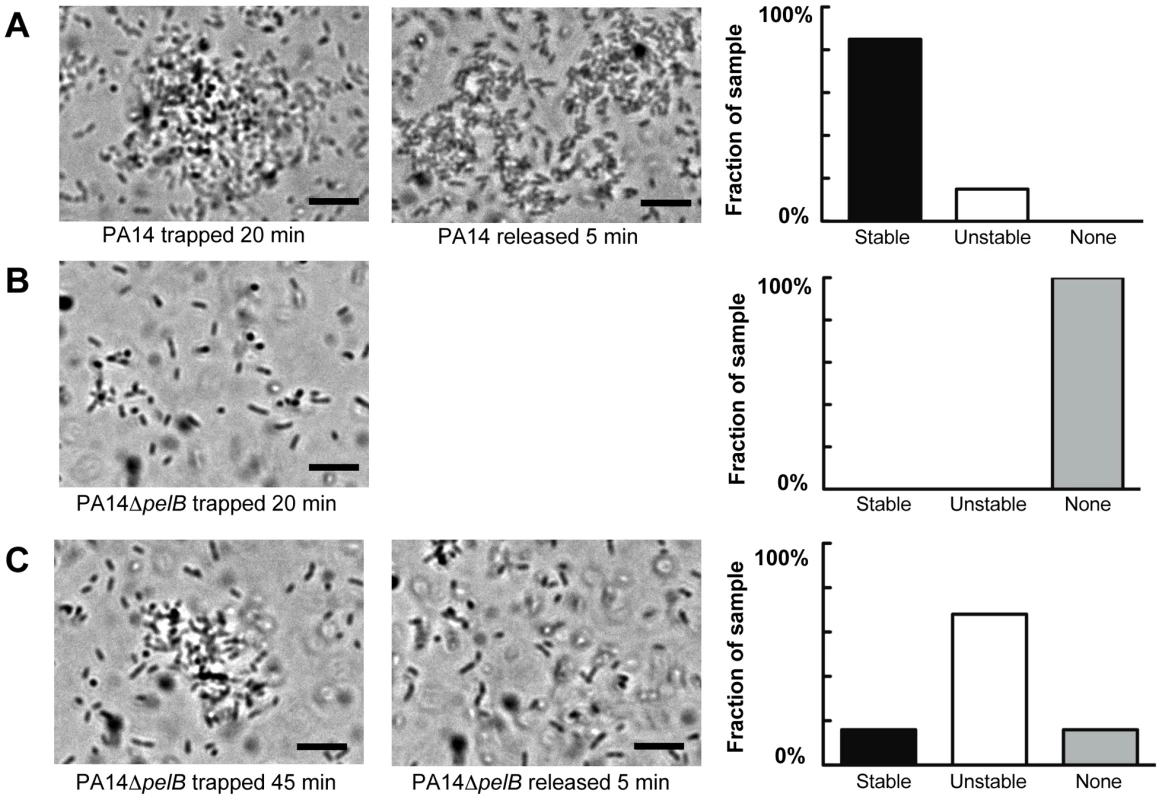
Laser tweezers were used to trap bacteria and investigate bacterial clumping phenotypes. The captured bacteria were examined visually by light microscopy for aggregation after 20 min (A) PA14 (B) PA14ΔpelB. An extended trapping time of 45 min was required to initiate aggregation in PA14ΔpelB (C). The stability of formed aggregates was visually assessed five min after the release of the laser trap (center panel A and C). Aggregate stability was classified into three categories, “stable” if the aggregate remained intact, “unstable” if the aggregate dispersed into single cells and “none” if an aggregate did not form during the allotted time (right panels). A minimum of six replicates for each strain was assessed. Scale bars represent 10 µm. Representative phase-contrast images are shown. Pel provides biofilms protection from aminoglycoside antibiotics
A primary function attributed to the extracellular matrix is protection [12]. Several well-studied polysaccharides are known to confer resistance to a range of antibiotics. In P. aeruginosa, alginate and cyclic glucans have been demonstrated to protect biofilms from aminoglycosides by directly binding these cationic antibiotics [15], [30], [31]. In addition, rugose small colony variants (RSCVs), which produce elevated levels of Pel and Psl, show increased tolerance to tobramycin, an aminoglycoside [23], [32]. Thus, we hypothesized that Pel may provide protection from antimicrobials. Therefore we tested the sensitivity of our strains to several clinically relevant antibiotics: tobramycin, gentamicin, ciprofloxacin, kanamycin, meropenem, ceftazidime, tetracycline, and carbenicillin.
Planktonic cultures of PAO1, PAO1ΔpelB, PAO1Δpsl, PAO1PBADpel and WFPA801 (arabinose-inducible psl strain [26]) were initially tested for antibiotic susceptibility by determining the minimum inhibitory concentration (MIC) of each strain. No difference is detected between PAO1, PAO1ΔpelB, PAO1Δpsl and WFPA801 for any of the antibiotics tested (Figure S5). However, overexpressing Pel in PAO1 slightly increases the MIC in comparison to wild-type PAO1 to gentamicin and tobramycin, two aminoglycoside antibiotics. No difference is seen in MICs between PA14, PA14ΔpelB and PA14PBADpel for any of the antibiotics tested (Figure S5).
We then assessed Pel's involvement in planktonic survival by treating log-phase cultures of our strains with both tobramycin and ciprofloxacin. Ciprofloxacin was chosen as a representative antibiotic that has the same MIC for all three strains PAO1, PAO1ΔpelB and PAO1PBADpel. Equal susceptibility is seen between the wild-type and ΔpelB mutants in both PAO1 and PA14 (Figure S6). Like the MIC experiments, overexpression of Pel in PAO1 affords a small degree of protection to killing by tobramycin and gentamycin, while Pel overexpression in PA14 does not (Figure S6). Similar killing curves are observed between PA14 and PAO1 strains during ciprofloxacin treatment (Figure S6).
We subsequently assessed Pel's role in antibiotic resistance in a biofilm model. For a valid comparison of antibiotic tolerance, the same number of cells must be challenged with the antibiotic of interest. In order to satisfy this criterion, we used a 48-h colony biofilm technique that has been demonstrated previously to capture a biofilm-specific model of antibiotic susceptibility [33]. Bacterial strains were grown on polycarbonate filters for two days, allowing complete coverage of the filter and equal colony forming units (CFUs) for all strains. The filter was then transferred to solid medium containing antibiotic and incubated for 24 hours. After treatment, the viable CFUs were quantified. In PA14, a pel mutation rendered biofilms more susceptible to the aminoglycosides tobramycin and gentamicin, while not impacting the susceptibility to ciprofloxacin (Figures 6 and S7). However, a pel mutation in PAO1 did not influence susceptibility to any antimicrobial tested.
Fig. 6. Analysis of Pel-mediated antibiotic tolerance in biofilms. 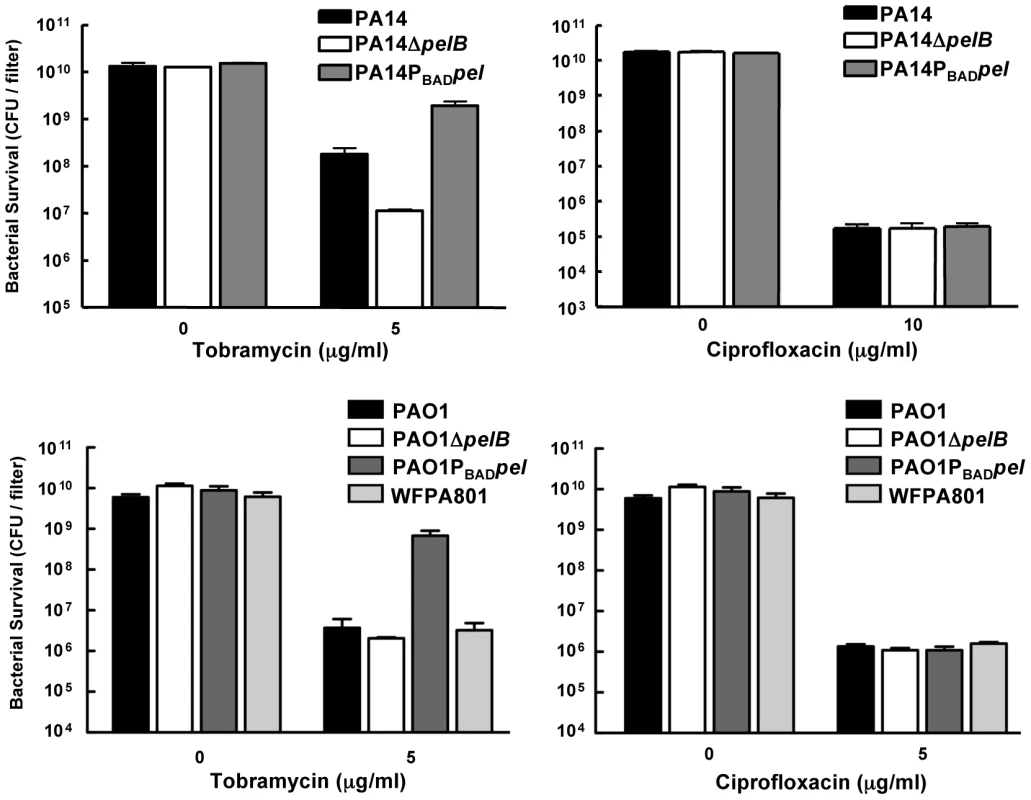
48-h filter biofilms were assessed for relative susceptibility. Biofilms were treated with tobramycin and ciprofloxacin for 24 h. No antibiotic controls are included for baseline comparison. WFPA801 over-expresses the Psl polysaccharide. Bacterial survival was measured by CFU counts. Overexpressing pel in both PAO1 and PA14 led to an elevated tolerance to tobramycin and gentamicin compared to the corresponding parental strain (Figures 6 and S7). To test whether Psl overexpression might provide similar aminoglycoside protection to PAO1, we used an arabinose-inducible Psl expression strain, WFPA801. Psl overexpression strain was found not to confer protection from tobramycin (Figure 6).
To make a more direct comparison between planktonic and biofilm cultures, we compared 24-h-old stationary phase cells with 24-h-old biofilm cells for tobramycin sensitivity. The tobramycin sensitivity profiles of stationary phase liquid cultures are identical for PAO1 and PA14 wild-type and the corresponding pelB-mutant strains (Figure 7). Interestingly, overexpression of Pel in PA14 provides protection in stationary phase cells that is not observed in log phase cells (compare Figures 7 and S6). Similar to log-phase treated cells, PAO1PBADpel provides protection to stationary phase cells. 24-h-biofilms reveal the same susceptibility profiles as the 48-h-treated biofilms shown in Figure 6, with enhanced sensitivity of PA14ΔpelB compared to wild-type (Figure 7).
Fig. 7. Analysis of Pel-mediated antibiotic resistance to stationary phase planktonic and biofilm grown cells. 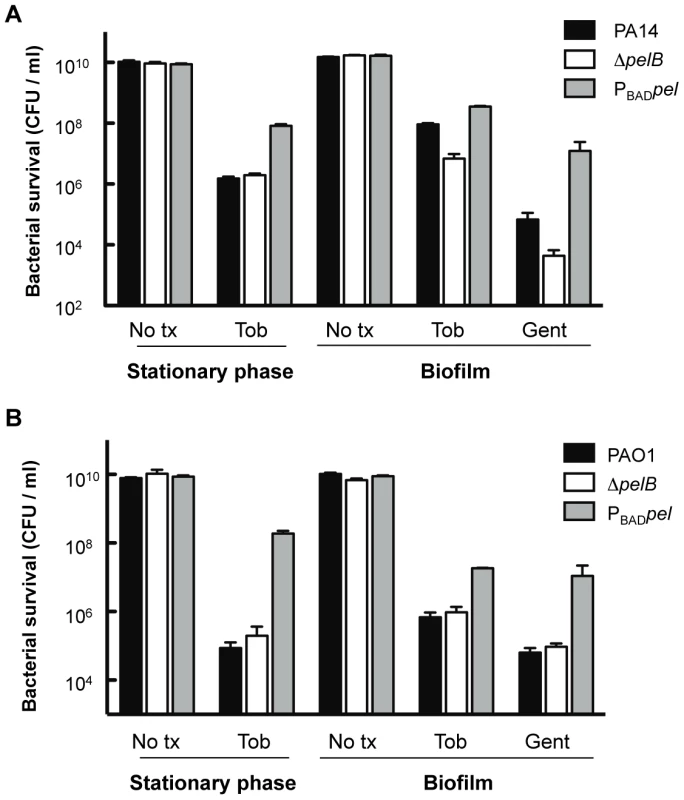
Bacterial survival was assessed for both PA14 (A) and PAO1 (B) 24 h stationary phase cultures and 24 h filter biofilms. Prior to antibiotic treatment, stationary phase planktonic cells were centrifuged and resuspended in fresh media containing no treatment (No tx) or indicated antibiotics. Biofilm cells were moved to a fresh media source containing no treatment (No tx) or antibiotics. Planktonic cultures were treated with 5 µg/ml tobramycin (Tob). Biofilm cells were treated with either 10 µg/ml of gentamicin (Gent) or tobramycin at 5 µg/ml for PAO1 and 150 µg/ml for PA14. To complement our analysis of colony biofilms, we determined the spatial distribution of tobramycin killing in flow cell biofilms. As expected, PA14 and its derivatives display a similar tobramycin resistance pattern as the filter biofilm. The pelB mutant strain produced a monolayer that is easily killed, while the PA14PBADpel strain biofilm is the least susceptible, probably in part due to the production of greater amounts of biofilm biomass than PA14 (Figure S8).
Expression of the pel operon is induced during biofilm growth
Our antimicrobial tolerance data suggest that in PA14, Pel plays a more important role in biofilm communities as compared to planktonic cultures. One explanation for this observation is that pel expression may be enhanced during biofilm growth as compared to planktonic growth. To test this, we analyzed the expression of the pel operon in planktonic and biofilm cells using quantitative RT-PCR. To generate enough biofilm biomass for RT-PCR we grew the strains on the surface of silicon tubing under constant flow. We observed that pelA transcript in PA14 is 7.2-fold higher (+/−2.0) when grown as a biofilm for 48 h than in planktonic conditions (for either logarithmic or stationary phase cells), while in PAO1 it is 5.11-fold higher (+/−3.49). The control transcripts pslA, lasR and sadC did not exhibit biofilm-specific induction (Figure 8A). The pslA transcript was chosen as a control because the Psl polysaccharide is an important structural component in biofilm development in PAO1. The lasR transcript was chosen because LasR responds to an increase in biomass and the sadC transcript was chosen because the product SadC is a diguanylate cyclase important in regulating biofilm advancement [34], [35]. Therefore, an increase in pelA transcript is specific to the pel operon and not all genes involved in biofilm formation. Even by 24 h of biofilm growth, PAO1 shows nearly a 15-fold increase in pelA transcript (Figure S9). To corroborate our transcriptional analysis, we also demonstrate PelF protein levels are elevated during biofilm growth but went undetected in stationary phase liquid cultures (Figure 8B). These data suggest a biofilm-associated role for Pel.
Fig. 8. pel expression is elevated during biofilm growth. 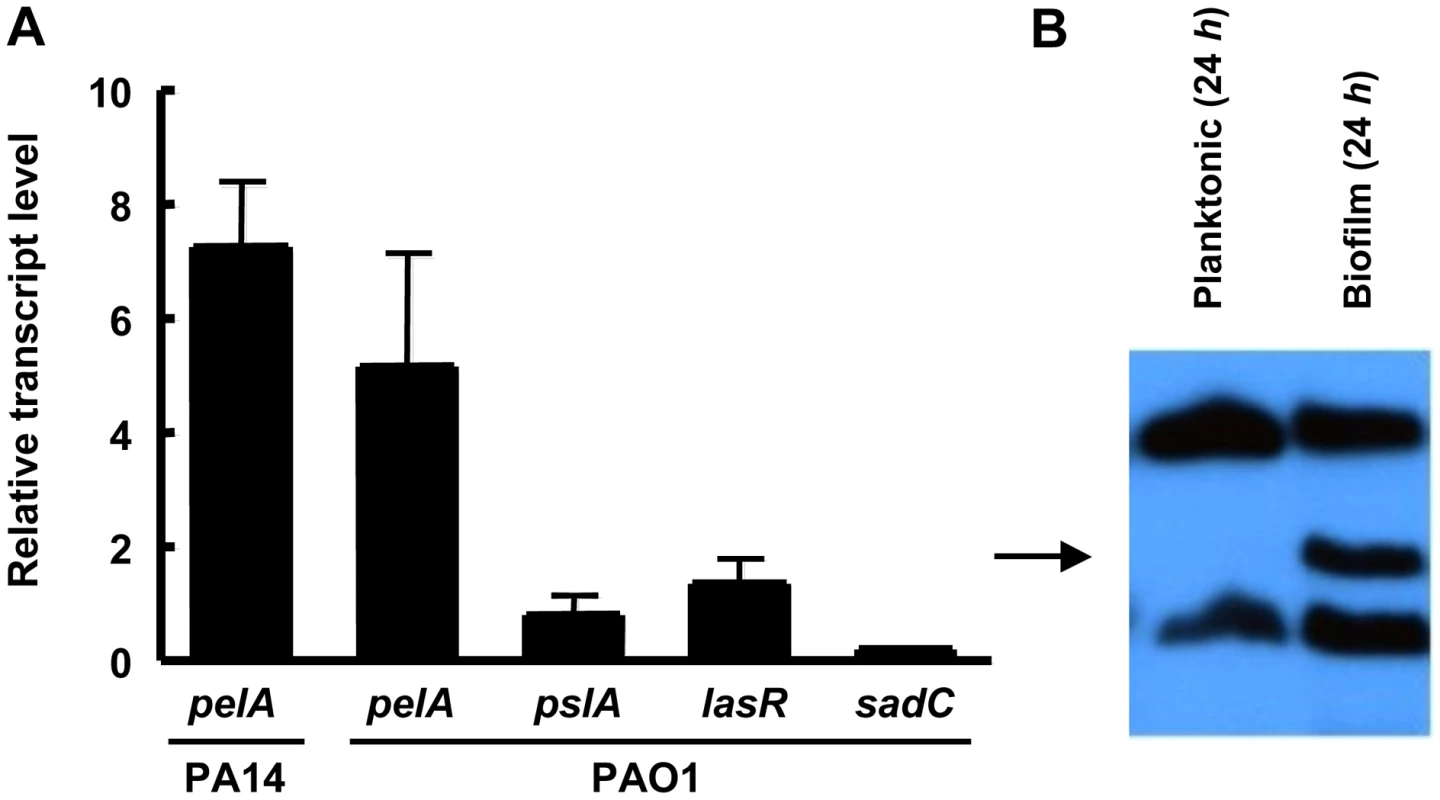
(A) Planktonic and biofilm cells are compared for transcript level by quantitative RT-PCR. In both conditions, bacteria are grown to log phase at 37°C. Planktonic cells are incubated statically in a test tube at room temperature for 30 min. Biofilm cells are grown in a tube biofilm, with an initial attachment period of 30 min followed by continuous flow for 48 h. Transcripts are normalized to ampR and then to the initial planktonic condition. Results shown are the mean of three independent experiments. Error bars represent the standard deviations. (B) Planktonic and biofilm cells were probed for PelF protein expression by western blot. A 24 h shaking liquid culture was compared to a 24 h grown tube biofilm at RT. Samples are normalized to total protein. The arrow indicates PelF expected protein size, 56 kDa. Discussion
In this study, we have identified two key biofilm-associated functions of the Pel polysaccharide. Pel is critical for initiating and maintaining cell-cell interactions. These functions have been implicated in polysaccharides in other species, such as the MDX polysaccharide of Shewanella oneidensis and colanic acid of E. coli K-12 [36], [37]. This appears to be a crucial mechanism by which parent cells retain their daughter cells in the biofilm community. In the absence of Pel, biofilm formation does not progress beyond the monolayer stage in PA14.
In addition, Pel appears to provide a measure of protection from aminoglycoside antibiotics. The antibiotic susceptibility experiments suggest that Pel is capable of providing protection to planktonic cells when artificially overexpressed, although there is no phenotype for the pel mutant strain in liquid culture for any of the tested conditions. However, in biofilms of PA14, both pel overexpression and a pel mutation impacted aminoglycoside sensitivities. This suggests that Pel may play an important protective role in biofilms of this strain.
The mechanism responsible for protection is not clear, but if Pel behaves similarly to other polysaccharides leading to elevated aminoglycoside resistance like alginate and ndvB-encoded glucans, it may bind or sequester the antibiotic. Both alginate and ndvB-encoded glucans have a high negative charge that is consistent with their ability to bind positively charged aminoglycosides. If this model proves to be true, Pel may be an acidic polysaccharide capable of interacting with cationic antibiotics. This hypothesis helps explain why no differences were seen in killing of planktonic or biofilm cells by ciprofloxacin, an anionic antibiotic, and why no protection was afforded by overexpressing the neutral polysaccharide, Psl (Figure 6). Another possibility is that Pel production can influence biofilm structure, which in turn may influence antimicrobial susceptibility. However, we feel this is unlikely since the structure of colony biofilms tends to be uniform.
Using PAO1 and PA14 as representative P. aeruginosa laboratory strains, we see that the role Pel plays in biofilm formation can vary drastically. In PAO1, it appears that Psl is the predominate polysaccharide of the biofilm EPS matrix, while in PA14 Pel is required. However, it appears in other strains, MJK8, PAO1ΔwspF and ZK2870, both polysaccharides contribute to biofilm and/or autoaggregation phenotypes [20], [24], [32]. Whether each polysaccharide has a distinct role in biofilm formation and/or protection, or if their functions are redundant remain to be determined. Although purely in terms of surface attachment, it appears that Psl is more important, while Pel is less so.
The enhanced expression of Pel in biofilms is noteworthy. pelA transcript levels were minimally expressed under the planktonic culturing conditions we used. Yet despite low pelA transcript in planktonic conditions, only in biofilms do we detect PelF protein expression (Figure 8). Therefore, the protection afforded to P. aeruginosa by Pel from aminoglycosides appears to be a biofilm-associated mechanism of antimicrobial tolerance. To date, only the cyclic glucans encoded by the ndvB locus has been shown to be a biofilm-specific mechanism of antimicrobial tolerance in this species [30]. Characterizing the structure of Pel and the specific mechanism behind aminoglycoside protection is underway.
Finally, the ability to prohibit PA14 biofilms from growing larger by arresting Pel expression is exciting. The biofilm does not dissipate indicating that continuous Pel is not necessary for biofilm maintenance. This result is contrary to PAO1 biofilms that require Psl to be continuously produced for biofilm maintenance [26]. Thus, manipulating Pel and Psl expression may be a central strategy for disrupting biofilms and targeting them for antibiotic therapy.
Materials and Methods
Bacterial strains, strain construction, and growth media
Strains and primers used in this study are listed in Table S1 [38], [39], [40], [41], [42], [43]. Plasmid and strain construction are described in Text S1. Unless otherwise noted, strains were grown at 37°C in LB medium. For plasmid selection, 300 µg/ml carbenicillin or 100 µg/ml gentamicin was used with P. aeruginosa, and 100 µg/ml ampicillin or 10 µg/ml gentamicin was used with Escherichia coli.
RNA purification and analysis
RNA was extracted using an RNeasy kit (QIAGEN) according to manufactures instructions. Contaminating DNA was removed with an on-column RNase-free DNase I treatment (QIAGEN) and remaining DNA was removed by an off-column DNase I treatment (Promega) as recommended. The RNA prep was confirmed to be free of DNA by PCR. cDNA was generated by SuperScript III First-Strand Synthesis System for RT-PCR using random hexamers (Invitrogen). cDNA synthesis was verified by PCR and quantitated by RT-PCR using the SYBR Green PCR Master Mix (Applied Biosystems) as the fluorescent dye. Fluorescence was measured using ABI Prism 7000 Sequence Detection and pelA transcript levels were normalized to ampR.
Microtiter dish biofilm
96-well microtiter dish experiments were performed as described previously [44]. For rapid attachment assays, 100 µl of log-phase cells were incubated at 37°C for one hour. For analysis of biofilm development, log-phase cells were incubated at room temperature for 20 h.
Pellicle formation assay
Standing cultures containing 3-ml LB broth were grown at room temperature in a glass tube. Pellicles were monitored by visual inspection between four and ten d. Complete coverage at the air-liquid interface of an opaque layer of cells is considered to be indicative of pellicle formation [17].
Congo red assays
LB liquid cultures supplemented with 40 µg/ml Congo red (Sigma-Aldrich) and incubated shaking overnight 37°C. The supernatants were measured at OD495 to assess Congo red binding. Congo red plates contained LB without NaCl, 1% agar, 40 µg/ml Congo red and 15 µg/ml brilliant blue R (Sigma-Aldrich). Cells were diluted 1/100, 10 µl spotted and incubated at room temperature for five d.
Flow cell and tube biofilm reactor
The flow cell system and tube biofilm system was assembled as described previously [32], [45], [46]. Additional information is found in Text S1.
Live/dead staining
Biofilms were grown in flow cells in 1% TSB as described in Text S1 for 4 d and subsequently treated with 1 µg/ml of tobramycin for 24 h. The MIC for tobramycin when the cells are grown in 1% TSB is 0.03 µg/ml for PAO1 and 0.06 µg/ml for PA14. This is in contrast to the MIC of 1 µg/ml seen for PA14 and PAO1 grown in full strength LB. After treatment, flow was stopped and biofilms were stained with 500 µl of propidium iodide and SYTO 9 (Invitrogen) for 10 m according to manufactures instructions. Flow resumed and images were captured after 15 m of washing.
Biofilm daughter-cell analysis
The movement and behavior of individual daughter cells of P. aeruginosa monocultures were monitored in young (<2 days old) flow cell biofilms. The flow cell setup was the same as described. Fluorescent images were taken every 45 seconds for 2 hours using the time-lapse feature of the Zeiss Axiophot microscope (Carl Zeiss). The fate of new daughter cells were visually tracked and each cell was classified as an “aggregate builder” or “flyer” [28]. From one cell division to the next, “aggregate builders” remained within a 15 µm diameter circle centered at the point of cell division, while “flyers” moved further away on the surface or were dissociated from the surface by media flow. A minimum of 75 cell divisions of each strain were tracked and classified.
Laser trap experiments
An open-top chamber for microscopy was constructed by layering five Secure-Seal Imaging Spacers 13mm in diameter (Grace Bio-Labs) onto a microscope cover slip and this chamber placed on an inverted Olympus microscope above a 40× or 60× long-working-distance objective. Bacteria were grown shaking at 37°C to OD600 1. Samples were incubated statically at room temperature for 1–7 hours. Static incubation of the cells prior to the experiment allowed for a slight increase (∼2-fold, data not shown) in pel expression, presumably for the same reasons pel expression is required for pellicle formation in static liquid cultures [17]. A 100 µL bacterial suspension was placed into the chamber. Laser trapping was done by focusing a 1064 nm laser through the microscope objective at the top of the sample at a transmitted power of ∼50mW. For PA14 wild-type bacteria, the first trap-induced clustering was seen in samples that had been in the open-top chamber for 20 min; for ΔpelB bacteria the first trap-induced clustering, if any, was seen in samples that had been in the open-top chamber for 45 min. Cluster stability was evaluated by monitoring formed bacterial aggregates for cluster dispersal five min after the laser beam was blocked.
Immunoblot analysis
Immunoblots were performed with whole-cell lysates as described with equal amounts of total protein in each lane [47]. Protein concentration was measured using the Pierce 660 nm protein assay (Thermo Scientific). Lysates were probed for PelF expression levels with a specific PelF antibody. Additional information on antisera production and immunoblot analysis are described in Text S1.
Antibiotic sensitivity assays
MIC growth curves were completed in a 96-well microtiter dish grown in LB broth at 37°C. Log phase bacteria were diluted to 105 CFU in each well. A range of concentrations was assessed for each antibiotic. Bacterial growth was measured after 24 hours of incubation using a microplate reader (OD590).
For planktonic killing curves, cells were either grown to log phase in LB or grown for 24 h to assess stationary phase susceptibility. Log phase cultures were split and one culture was treated with either tobramycin (Sigma), gentamicin (Sigma) or ciprofloxacin (Bayer Healthcare), while the control culture was untreated. Stationary phase cells were resuspending in fresh media containing antibiotics. The cultures were incubated shaking at 37°C. Bacterial survival was assessed over time by viable plate counts.
For biofilm killing, overnight liquid cultures were diluted 1/100 and five µl were spotted onto a UV-sterilized 25mm polycarbonate filter (GE Osmonics). Biofilms were grown for two d unless otherwise stated at 37°C and moved to fresh solid media each day. Biofilms were exposed to with 5 µg/ml tobramycin or 1 µg/ml ciprofloxacin for 24 h. Bacterial viability was obtained by resuspending the filter in 1 ml of PBS and serially diluting to obtain viability counts.
Supporting Information
Zdroje
1. O'TooleGA
2003 To build a biofilm. J Bacteriol 185 2687 2689
2. ParsekMR
SinghPK
2003 Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57 677 701
3. StoodleyP
SauerK
DaviesDG
CostertonJW
2002 Biofilms as complex differentiated communities. Annu Rev Microbiol 56 187 209
4. DrenkardE
2003 Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 5 1213 1219
5. MahTF
O'TooleGA
2001 Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9 34 39
6. NickelJC
RuseskaI
WrightJB
CostertonJW
1985 Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27 619 624
7. CostertonJW
StewartPS
GreenbergEP
1999 Bacterial biofilms: a common cause of persistent infections. Science 284 1318 1322
8. SinghPK
SchaeferAL
ParsekMR
MoningerTO
WelshMJ
2000 Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407 762 764
9. WagnerVE
IglewskiBH
2008 Pseudomonas aeruginosa biofilms in CF Infection. Clin Rev Allergy Immunol 35 124 134
10. OphirT
GutnickDL
1994 A Role for Exopolysaccharides in the Protection of Microorganisms from Desiccation. Appl Environ Microbiol 60 740 745
11. RyderC
ByrdM
WozniakDJ
2007 Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol 10 644 648
12. StarkeyM
GrayK
ChangS
ParsekMR
2004
O'TooleGA
A Sticky business: The extracellular polymeric substance matrix of bacterial biofilms Washington DC ASM press
13. VarkiA
CummingsR
EskoJ
HudsonF
GeraldH
1999 Essentials in Glycobiology New York Cold Springs Harbor Laboratory Press
14. StewartPS
CostertonJW
2001 Antibiotic resistance of bacteria in biofilms. Lancet 358 135 138
15. HentzerM
TeitzelGM
BalzerGJ
HeydornA
MolinS
2001 Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183 5395 5401
16. HarrisonJJ
CeriH
StremickCA
TurnerRJ
2004 Biofilm susceptibility to metal toxicity. Environ Microbiol 6 1220 1227
17. FriedmanL
KolterR
2004 Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51 675 690
18. VasseurP
Vallet-GelyI
SosciaC
GeninS
FillouxA
2005 The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 151 985 997
19. LeeDG
UrbachJM
WuG
LiberatiNT
FeinbaumRL
2006 Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol 7 R90
20. HickmanJW
TifreaDF
HarwoodCS
2005 A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A 102 14422 14427
21. LeeVT
MatewishJM
KesslerJL
HyodoM
HayakawaY
2007 A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol 65 1474 1484
22. ZogajX
NimtzM
RohdeM
BokranzW
RomlingU
2001 The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39 1452 1463
23. StarkeyM
HickmanJH
MaL
ZhangN
De LongS
2009 Pseudomonas aeruginosa rugose small colony variants have adaptations likely to promote persistence in the cystic fibrosis lung. J Bacteriol 191 3492 3503
24. FriedmanL
KolterR
2004 Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol 186 4457 4465
25. JacksonKD
StarkeyM
KremerS
ParsekMR
WozniakDJ
2004 Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol 186 4466 4475
26. MaL
JacksonKD
LandryRM
ParsekMR
WozniakDJ
2006 Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188 8213 8221
27. HeydornA
NielsenAT
HentzerM
SternbergC
GivskovM
2000 Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146 (Pt 10) 2395 2407
28. SinghPK
ParsekMR
GreenbergEP
WelshMJ
2002 A component of innate immunity prevents bacterial biofilm development. Nature 417 552 555
29. AshkinA
DziedzicJM
1989 Internal cell manipulation using infrared laser traps. Proc Natl Acad Sci U S A 86 7914 7918
30. MahTF
PittsB
PellockB
WalkerGC
StewartPS
2003 A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426 306 310
31. SadovskayaI
VinogradovE
LiJ
HachaniA
KowalskaK
2010 High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: the ndvB gene is involved in the production of highly glycerol-phosphorylated β-(1→3)-glucans, which bind aminoglycosides. Glycobiology 20 895 904
32. KirisitsMJ
ProstL
StarkeyM
ParsekMR
2005 Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 71 4809 4821
33. WaltersMC3rd
RoeF
BugnicourtA
FranklinMJ
StewartPS
2003 Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47 317 323
34. PesciEC
MilbankJB
PearsonJP
McKnightS
KendeAS
1999 Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96 11229 11234
35. MerrittJH
BrothersKM
KuchmaSL
O'TooleGA
2007 SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J Bacteriol 189 8154 8164
36. DanesePN
PrattLA
KolterR
2000 Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 182 3593 3596
37. ThormannKM
DuttlerS
SavilleR
HyodoM
ShuklaS
2006 Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilm by cyclic di-GMP. J Bacteriol 188 2681 2691
38. HollowayBW
1955 Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol 13 572 581
39. RahmeLG
StevensEJ
WolfortSF
ShaoJ
TompkinsRG
1995 Common virulence factors for bacterial pathogenicity in plants and animals. Science 268 1899 1902
40. KanekoY
ThoendelM
OlakanmiO
BritiganBE
SinghPK
2007 The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117 877 888
41. HoangTT
Karkhoff-SchweizerRR
KutchmaAJ
SchweizerHP
1998 A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212 77 86
42. DaviesDG
ParsekMR
PearsonJP
IglewskiBH
CostertonJW
1998 The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280 295 298
43. KochB
JensenLE
NybroeO
2001 A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria at a neutral chromosomal site. J Microbiol Methods 45 187 195
44. MerrittJH
KadouriDE
O'TooleGA
2005 Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1 Unit 1B.1
45. ShroutJD
ChoppDL
JustCL
HentzerM
GivskovM
2006 The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol 62 1264 1277
46. SchaeferAL
GreenbergEP
ParsekMR
2001 Acylated homoserine lactone detection in Pseudomonas aeruginosa biofilms by radiolabel assay. Methods Enzymol 336 41 47
47. E.F. FritschJS
T. Maniatis
1989 Molecular Cloning Woodbury (New York) Cold Spring Harbor Laboratory Press
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Salivary Gland NK Cells Are Phenotypically and Functionally Unique
- Genetic Epidemiology of Tuberculosis Susceptibility: Impact of Study Design
- Early Target Cells of Measles Virus after Aerosol Infection of Non-Human Primates
- Multiple Plant Surface Signals are Sensed by Different Mechanisms in the Rice Blast Fungus for Appressorium Formation
- Biofilm Development on by Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion
- Distinct Patterns of IFITM-Mediated Restriction of Filoviruses, SARS Coronavirus, and Influenza A Virus
- Molecular Basis of Increased Serum Resistance among Pulmonary Isolates of Non-typeable
- A Helminth Immunomodulator Exploits Host Signaling Events to Regulate Cytokine Production in Macrophages
- HCMV Spread and Cell Tropism are Determined by Distinct Virus Populations
- Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1
- Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Salivary Gland, following Infection with Dengue Virus
- Structural Basis for the Recognition of Cellular mRNA Export Factor REF by Herpes Viral Proteins HSV-1 ICP27 and HVS ORF57
- Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator
- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
- Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
- Imaging Single Retrovirus Entry through Alternative Receptor Isoforms and Intermediates of Virus-Endosome Fusion
- Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



