-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Social Motility in African Trypanosomes
African trypanosomes are devastating human and animal pathogens that cause significant human mortality and limit economic development in sub-Saharan Africa. Studies of trypanosome biology generally consider these protozoan parasites as individual cells in suspension cultures or in animal models of infection. Here we report that the procyclic form of the African trypanosome Trypanosoma brucei engages in social behavior when cultivated on semisolid agarose surfaces. This behavior is characterized by trypanosomes assembling into multicellular communities that engage in polarized migrations across the agarose surface and cooperate to divert their movements in response to external signals. These cooperative movements are flagellum-mediated, since they do not occur in trypanin knockdown parasites that lack normal flagellum motility. We term this behavior social motility based on features shared with social motility and other types of surface-induced social behavior in bacteria. Social motility represents a novel and unexpected aspect of trypanosome biology and offers new paradigms for considering host-parasite interactions.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000739
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000739Summary
African trypanosomes are devastating human and animal pathogens that cause significant human mortality and limit economic development in sub-Saharan Africa. Studies of trypanosome biology generally consider these protozoan parasites as individual cells in suspension cultures or in animal models of infection. Here we report that the procyclic form of the African trypanosome Trypanosoma brucei engages in social behavior when cultivated on semisolid agarose surfaces. This behavior is characterized by trypanosomes assembling into multicellular communities that engage in polarized migrations across the agarose surface and cooperate to divert their movements in response to external signals. These cooperative movements are flagellum-mediated, since they do not occur in trypanin knockdown parasites that lack normal flagellum motility. We term this behavior social motility based on features shared with social motility and other types of surface-induced social behavior in bacteria. Social motility represents a novel and unexpected aspect of trypanosome biology and offers new paradigms for considering host-parasite interactions.
Introduction
Studying microbial life under conditions that promote a single-cell lifestyle has proven very effective for uncovering important aspects of microbial physiology. However, microbes are social organisms, capable of communicating with one another and engaging in cooperative behavior [1]–[3]. Well-characterized social activities include biofilm formation, social motility, fruiting body development and quorum sensing [1], [3]–[10]. Social interactions among cells in a population effectively give rise to multicellular communities having specialized functionalities and offering advantages over a unicellular lifestyle. Some of these advantages include increased protection from external antagonists, such as desiccation or host defenses, access to nutrients, exchange of genetic information and enhanced ability to colonize, penetrate and migrate on surfaces [1],[2],[11]. In bacterial and fungal pathogens, social interactions have major influences on microbial physiology and disease pathogenesis and considering multicellularity as a general property of bacteria has profoundly changed our understanding of microbiology [1]–[3],[6].
Most microorganisms, particularly pathogens, are intimately associated with surfaces in their natural environments and preferentially engage in social behavior when exposed to semisolid surfaces [2], [7], [10], [12]–[14]. Commonly, this is manifested as various forms of social motility, including swarming, gliding and twitching [12],[15]. Each of these surface-induced motilities is influenced by environmental and genetic factors and driven by overlapping yet distinct mechanisms that are not completely understood. The defining feature is cooperative movement of groups of bacteria across a surface, requiring active motility and cell-cell communication among members of the group in response to external stimuli. Once studied only in a few bacteria, such as Proteus mirabilis and Serratia marcescens, surface-induced cooperative motilities are now known to be widespread among both Gram-negative and Gram-positive bacteria, including several important pathogens, such as Salmonella and Pseudomonas spp. [13], [16]–[18]. Surface-induced social interactions have also been observed in yeasts and fungi, including the opportunistic pathogen Candida albicans [5],[6]. Thus, various types of surface-induced social behavior are widespread among microorganisms and applying this conceptual framework to studies of bacterial biology has yielded many novel insights. Surprisingly, the paradigm of social behavior has not previously been applied to parasitic protozoa.
African trypanosomes, i.e. Trypanosoma brucei and related species, are protozoan parasites that cause significant human mortality and limit economic development in sub-Saharan Africa [19]. T. brucei is transmitted to the bloodstream of a mammalian host through the bite of an infected tsetse fly vector. Parasite motility is important in both hosts and this is especially apparent in the tsetse, where parasites undergo an ordered series of directional migrations that are critical for parasite survival and completion of the life cycle [20]–[23]. Trypanosomes first colonize the midgut, then migrate into the ectoperitrophic space and advance back up the alimentary canal to the mouthparts and from there, to the salivary glands [21],[23]. Throughout this process, parasites are in intimate contact with tissue surfaces of the tsetse fly. Once in the salivary glands, epimastigotes colonize the epithelial surface, stimulating the final stage of differentiation into mammalian-infective trypomastigotes [22]–[24]. Thus, throughout the tsetse stage of its life cycle T. brucei is in intimate contact with host tissue surfaces and exhibits an implicit requirement for sensing and signaling to guide parasite migration and differentiation. Currently, little is known about how surface contact modulates trypanosome behavior or motility [25].
Here we report that T. brucei engages in social motility when cultivated on semisolid agarose surfaces. This behavior is characterized by the formation of multicellular communities that sense external stimuli and communicate with one another to coordinate movement of the population. T. brucei social motility shares features with surface-induced social behavior in other microorganisms and represents a novel form of motility and intercellular communication not previously observed in these pathogens. As such, our findings present a novel and unprecedented feature of trypanosome biology and provide new concepts for considering development and pathogenesis of parasitic protozoa.
Results
Surface-induced changes to microbial motility and behavior are common among diverse bacteria and protists [2],[5],[7],[26],[27]. T. brucei spends much of its life cycle in contact with host tissue surfaces and interaction between parasite and tsetse epithelia is well documented [22],[23],[28], yet studies of T. brucei motility to date mainly utilize suspension cultures and do not provide information about how parasite behavior, e.g. motility, is affected by contact with surfaces. As part of our ongoing investigations into trypanosome motility, we thus cultivated procyclic form T. brucei on semisolid agarose plates [29]. We focused on procyclic forms because we know more about the motility apparatus and have more mutants available in this life cycle stage than in bloodstream forms and because the potential impact of parasite motility is most pronounced in this stage [30]. We found that procyclic trypanosomes formed groups of densely-packed cells within 24h post-plating (Fig. 1). The approximate doubling time on plates was 24 hours (Fig. S3), indicating that these groups did not arise simply through clonal expansion of single cells. Individuals within each group remained highly motile and actively moved out and back from the group. Interestingly, parasites were often arranged in distinct patterns on the agarose surface, with large, tightly packed groups surrounded by a zone of clearance and then a perimeter of smaller groups (Fig. 1). To investigate how these patterns arose, we established a system to monitor parasite movements over several hours using time-lapse and video microscopy (Materials and Methods). Time-lapse imaging revealed a striking behavior in which groups of hundreds to thousands of parasites moved en masse across the agarose surface, recruiting neighboring cells and enabling mergers of large groups (Fig. 1B–F, Video S1). This confirmed that the assembly of large communities was an active process and not simply the result of clonal expansion.
Fig. 1. Trypanosome communities assemble through recruitment of neighboring cells. 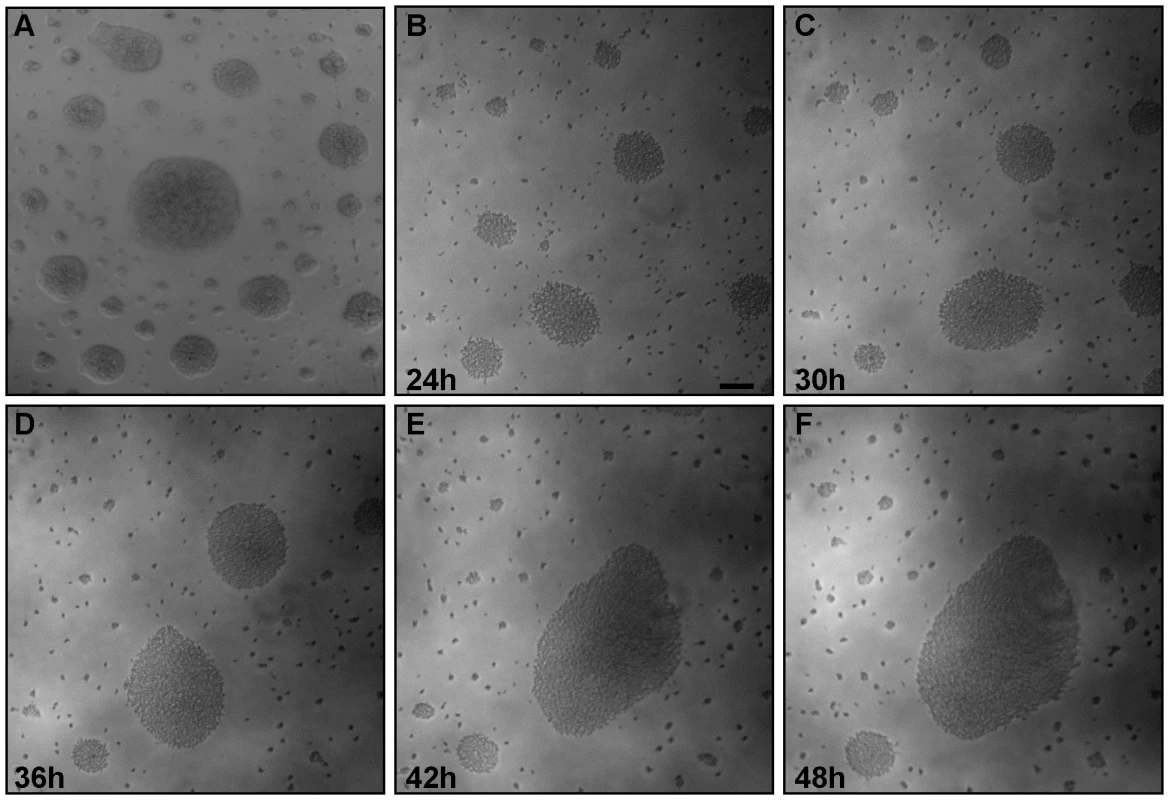
Trypanosome suspension cultures were transferred to semisolid agarose plates and monitored over time. (A) 48h post plating. (B–F) Time-lapse images show communities of parasites recruiting and merging with nearby individual cells and communities. The accompanying video (Video S1) shows groups of cells migrating en masse over the agarose surface. Scale bar is 100µm. Images are taken from Video S1 at the indicated time points post plating. The en masse movement of large groups of trypanosomes across the agarose surface suggested some form of cooperation among individuals in the population. We therefore investigated this behavior more closely using increased magnification and greater time resolution (Fig. 2, Video S2). These analyses showed that recruitment of individual parasites into a community followed a specific sequence of events as described here. Cells at the periphery of the group were highly motile and moved out and back from the community. We refer to these cells as “scouts”. When scouts came into contact with cells located adjacent to the community, they returned and induced polarized movement of the community outward at this position, forming a multicellular “pseudopod” that extended to recruit the external parasites (Fig. 2, Video S2). Mergers of large groups of cells followed essentially the same sequence of events (Fig. S1, Video S3). First, single trypanosomes advanced and returned randomly from the group periphery. Second, contact of scouts with an adjacent group biased their movement and initiated a period of reciprocal exchange. This led to formation of a multicellular “pseudopod” between the groups that intermittently broke down and reformed. Ultimately, stable contact was made and the groups merged along a path defined by the “pseudopod”. Thus, the arrangement of cells on the agarose surface resulted from the cooperative movement of parasites into groups, which then expand through recruitment of neighboring cells.
Fig. 2. Parasite recruitment. 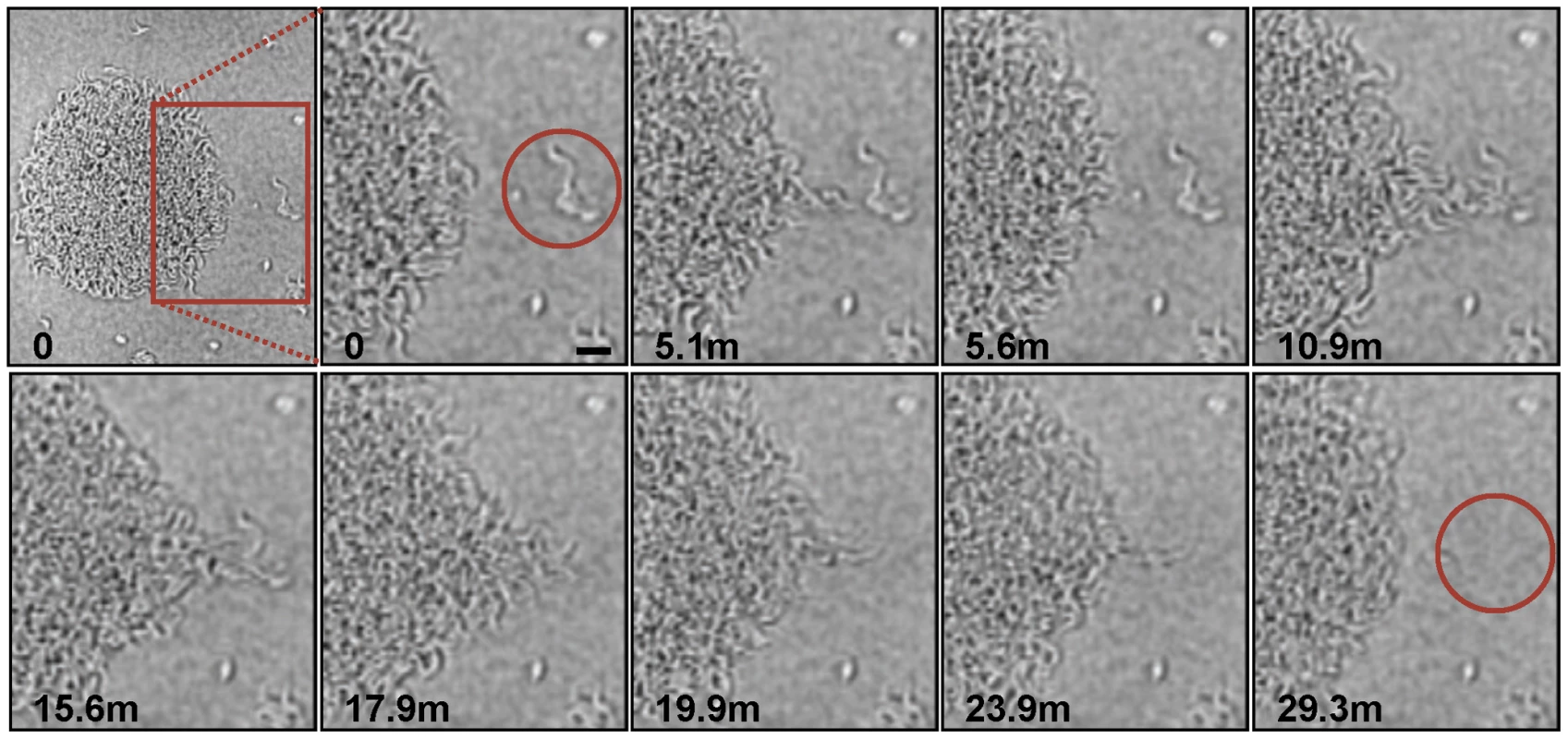
Time-lapse images show active recruitment of individual cells into larger communities. Cells at the periphery of the community (termed “scouts”) move out and back. When these scouts come into contact with a group of cells outside the community and return (time points 5.1–5.6 min), they stimulate coordinated movement of cells at the community edge outward, toward the neighboring cells, leading to their recruitment into the community (time points 10.9–29.3min). The image series was initiated approximately 24 hours after plating and time points, in minutes, of individual images are indicated. Scale bar is 20µm. Images are taken from Video S2. Long-term cultivation of social bacteria on semisolid agarose gives rise to large macro-communities that form complex patterns on the agarose surface [2],[15],[17]. T. brucei formed macrocommunities within three to six days following inoculation (Fig. 3A). A characteristic feature of this process is that parasites initially collected into small clusters that were distributed around the perimeter of the inoculation site. Parasites in these clusters then advanced outward from the site of inoculation, forming symetrical arrays of radial projections, with a median of 13 projections per inoculum. This pattern is similar to that produced during social motility in Pseudomonas aeruginosa, Myxococcus xanthus and Paenibacillus dendritiformis [7], [13], [18], [31]–[33], as shown by others (Fig. 3C). Movement of trypanosome projections was polarized, with a single leading edge that advanced at a steady rate on the order of a few microns per minute (Fig. S2, Video S4). The leading edge was characterized by a bulbous accumulation of densely packed cells, while the proximal region maintained a constant width (Fig. 3B). Cells along the lateral edge readily moved out and back (Fig. S2, Video S5), demonstrating they are not physically restrained. Therefore, polarized migration of projections is governed by parasite actions, rather than physical restrictions on parasite movement.
Fig. 3. T. brucei social motility results in polarized migration outward from the site of inoculation. 
(A) Trypanosome communities 4 days (4d), 5 days (5d) and 6 days (6d) post plating. Parasites accumulate at the periphery of the inoculation site (4d, also compare to mot+ samples in Fig. 4A). Groups at the periphery then move outward, forming characteristic radial projections (5d and 6d). (B) Close up of the radial projection leading edge, (boxed region in panel A6d) shows characteristic bulbous accumulation of densely-packed cells. (C) Comparison to social motility patterns formed by Paenibacillus vortex, as shown by Ingham and coworkers [33]. Scale bars are 1cm (panel A), 200µm (panel B). Panel C adapted from [33], with permission. To determine whether social motility requires directional motility, we employed a trypanin RNAi knockdown line that is incapable of directional motility [34]. Trypanin knockdown cells were evenly distributed at the site of inoculation and did not accumulate at the perimeter, as seen for cells having wild type motility (Fig. 4A). Moreover trypanin knockdown cells did not form radial projections (Fig. 4A–D). Cell doubling continued normally, as indicated by the roughly equivalent increase in cell density over time versus control cells (Fig. S3). Trypanin knockdown and control cells from the same plate were collected and assayed for (i) cell number, (ii) RNAi induction and (iii) motility. Both groups demonstrated an approximately equal cell doubling (Fig. S3) and trypanin protein was undetectable in the knockdown cells (Fig. 4F). Absence of directional motility in trypanin knockdowns was confirmed by direct microscopic examination (data not shown). Therefore, social motility in trypanosomes requires directional motility and is an active process.
Fig. 4. Social motility requires directional cell motility. 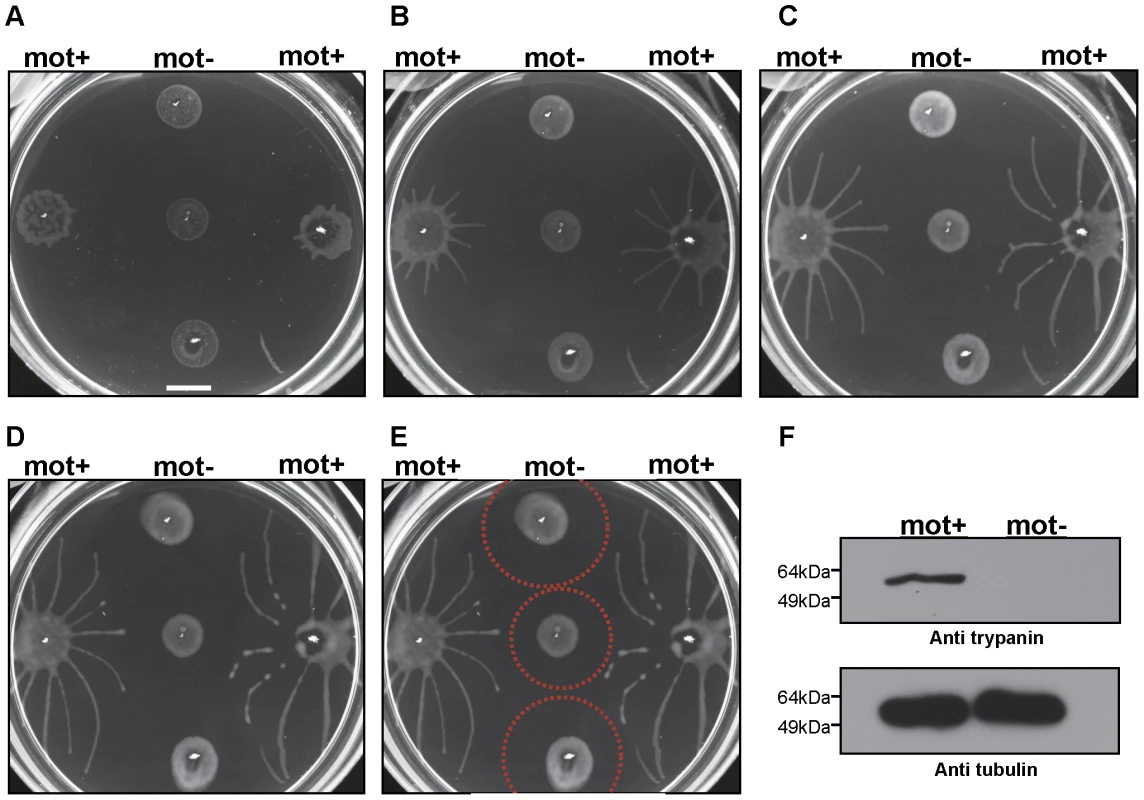
Suspension cultures of control cells (mot +) or trypanin knockdown cells (mot −) were inoculated on semisolid agarose plates and monitored over time. (A–E) Images taken 3 (A), 4 (B), 5 (C), or 6 (D, E) days post plating. (E) Indicated by red circles is the approximate zone of inhibition. (F) Western blot analysis on proteins extracted from control (mot +) and trypanin knockdown (mot −) cells grown on the same agarose plate. Tubulin is used as a loading control. Scale bar in panel a is 1cm. Radial projections advanced in parallel and did not cross paths (Fig. 4A–D). Moreover, when projections from a control group approached a non-motile group, their movement was either halted or was diverted so as to avoid contact (Fig. 4A–D). When diverted, projections did not cross, rather they continued in parallel, implying that cells in each projection are capable of sensing each other and coordinating their movements. Avoidance of non-motile communities occurred within a radius of approximately 0.5–1 cm (Fig. 4E). To determine if this avoidance was uniquely a response to non-motile cells, we inoculated two groups of control cells on opposing sides of a culture plate and followed their development and migration over the course of several days (Fig. 5). Opposing radial projections either halted advancement, or diverted paths so as to avoid contact with one another. As a negative control, radial projections from a single community of motile cells did not divert their path of migration over time (Fig. 5F). The combined data thus indicate that T. brucei can sense and respond to external signals and that parasites in a community can sense other parasites and may choose to include them in the group or to avoid them.
Fig. 5. Trypanosomes sense nearby communities and change their course of migration. 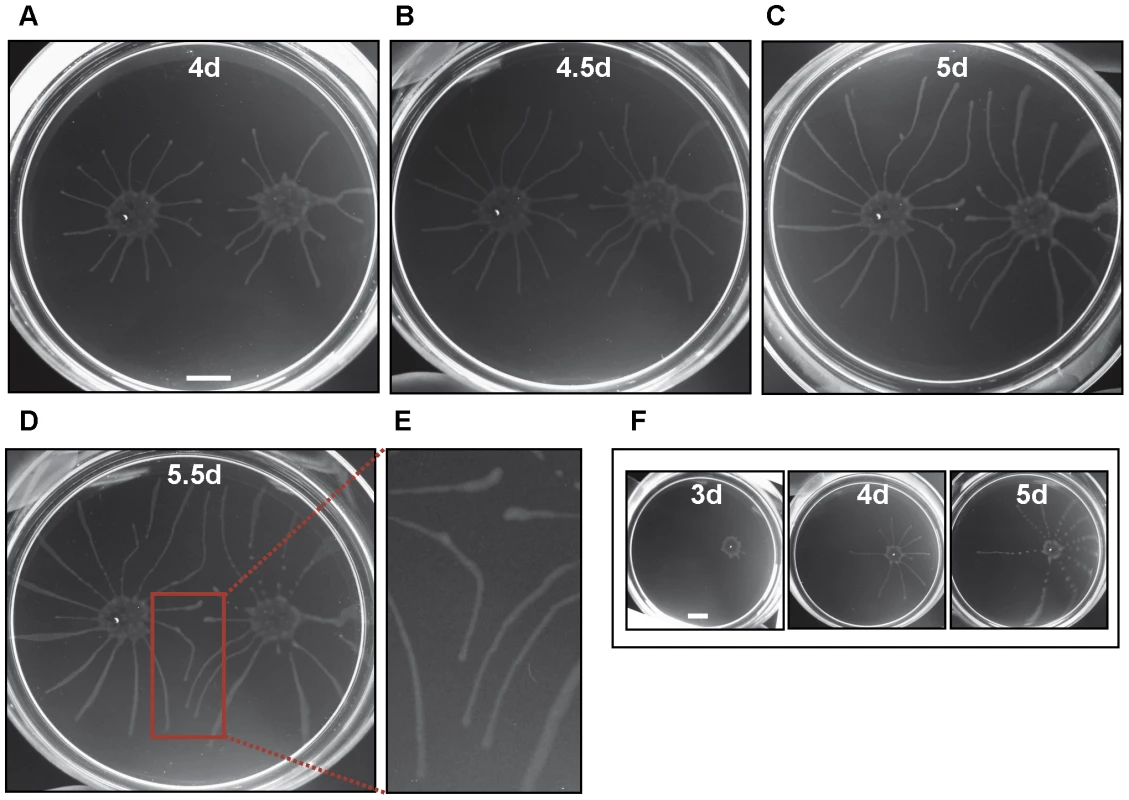
(A–D) Cells were inoculated on opposing sides of the agarose plate and monitored at the number of days post plating indicated in each panel. Projections radiating from each inoculation site advance outward in a generally straight path unless they come in proximity with opposing projections, at which point they halt progression, or redirect their movement. (E) Close up of outlined area in panel D. (F) As a control, cells from a single inoculation advance outward in a generally linear path to the edge of the culture dish. Scale bars are 1cm. Discussion
The impact of cell-cell communication and a multicellular lifestyle on the physiology and pathogenesis of bacteria is now well-established and related phenomena operate in yeast and fungi [1],[3],[5],[6],[35]. To date however, the paradigm of microbial social interactions has not been applied to parasitic protozoa. We report here that T. brucei is capable of social behavior in which parasites communicate with one another and assemble into multicellular communities with emergent properties that are not evident in single cells. This behavior manifests as groups of parasites engaging in cooperative movement across the surface of semisolid agarose and altering course in response to an external stimulus. We term this behavior social motility, based on analogy to social motility in bacteria. These results demonstrate a novel feature of trypanosome biology and reveal a level of complexity and cooperativity to trypanosome behavior that was not previously recognized. Given the widespread distribution of social interactions among other microbes, we expect our findings to have broad relevance among parasitic protozoa.
Social interactions among microbes are manifested in a variety of forms and represent complex behavioral responses for which the underlying molecular mechanisms are not well-understood. As is the case for bacteria [2], social motility in T. brucei requires directional motility, involves some form of cellular differentiation upon exposure to a semisolid surface and culminates in cooperative cell migration in response to external signals. At early stages parasites merge into groups, while at later stages the behavior has the added feature of groups avoiding one another. This suggests some form of differentiation and is consistent with different stages of social motility observed in some bacteria, such as Paenibacillus spp. [33]. In most cases where it has been investigated, social motility requires a combination of external and internal, i.e. genetic, factors and it is likely that this is also the case in trypanosomes. Based on our observations and what is known in other organisms, a minimum requirement for social motility in T. brucei would be directional motility, the ability to sense an external signal and to transduce this signal into a cellular response and communication between parasites in a group. Trypanosomes are certainly capable of directional motility [36],[37] and must integrate host-derived and parasite-derived signals to complete their life cycle [24], [38]–[40], although their signaling and sensory capacities are poorly understood. The trypanosome genome encodes several components of classical signal transduction pathways, as well as numerous predicted cell surface proteins of unknown function that might serve sensory and/or signaling roles [40]–[45]. The contribution of these proteins to cell-cell signaling or other sensory functions is not known and efforts to address this question have been limited by the lack of a defined in vitro assay for cell-cell signaling. Social motility assays therefore provide an opportunity to test the requirement of trypanosome signaling systems in social motility and overcome a major barrier to dissecting signaling and sensory mechanisms in trypanosomes.
Within the tsetse, close contact between parasites, as well as intimate interactions with host tissue surfaces are readily observed [20],[22],[23], indicating that surface-induced social behavior might operate in vivo. However, until appropriate mutants are available for direct investigation, we can only speculate on potential physiological roles for social motility. In this context it is informative to consider whether there are features of the parasite life cycle that might benefit from social motility or related behavior. T. brucei development within the tsetse fly requires parasite migration across and through a variety of host tissues. These migrations lead ultimately to colonization of the tsetse salivary gland epithelia, which the parasites must reach in order to complete development into mammalian-infective trypomastigotes. Trypanosomes progress through specific tsetse tissues in a well-defined order, but the mechanisms responsible for tissue tropisms are unknown. Social motility offers a system in which groups of cells coordinate their movements in response to an external stimulus and thus could provide a mechanism for parasite navigation through host tissues. In bacterial pathogens, cell-cell signaling, assembly into multicellular communities, social motility and other types of surface-induced behavior provide several advantages. Groups of bacteria feed cooperatively, resist hostile environments, prey on other microbes, exchange genetic information and develop functional specializations [1],[2]. Quorum sensing and biofilm formation induce programs of virulence gene expression, facilitate colonization of host tissues and provide resistance to immune and physical defenses [3]. We speculate that trypanosome cell-cell communication and social behavior may have similar impacts on development and pathogenesis of T. brucei. For example, assembly into groups might facilitate resistance against host defenses in the tsetse [24], as well as promote tissue colonization and invasion. Social motility might also provide a means to bring parasites together for genetic exchange [46],[47]. Finally, signaling pathways required for social motility are expected to overlap with host-parasite signaling pathways, about which very little is known. In summary, the identification of social motility in T. brucei reveals a novel and unexpected aspect of parasite biology and provides entirely new conceptual approaches for considering host-parasite interactions.
Materials and Methods
Trypanosome cell lines and suspension culture
Three procyclic T. brucei brucei cell lines, Antar 1 R5 Pro/G ITM [21], 29-13 double marker [48], and trypanin RNAi (KHTb12) [34], were used for these studies. While each experiment was not duplicated for each cell line, social motility was observed for both Antar and 29-13 lines. Suspension cultures were maintained using Cunningham's semi-defined medium (SM), supplemented with 10% heat-inactivated fetal calf serum as described previously [49]. For 29-13 cells, the medium was further supplemented with 15µg/ml G418 (Gibco) and 50µg/ml Hygromycin (Gibco). For the trypanin RNAi line, 2.5µg/ml Phleomycin, 15µg/ml G418 (Gibco) and 50µg/ml Hygromycin (Gibco) were included in the medium and RNAi was induced by adding 1µg/ml tetracycline. Cell doubling was monitored using a Z1 Coulter Particle Counter (Beckman Coulter, USA).
Plating on semisolid agarose plates
Cultivation on semi-solid agarose plates was adapted from [29]. Four percent (w/v) agarose (SeaPlaque GTG Agarose, Cambrex-LONZA, ME, USA) solution was made in MiliQ water, autoclaved and cooled to 65°C. A 1∶10 dilution of this 4% stock solution was prepared in pre-warmed (42°C for 20min) SM culture medium supplemented with the appropriate antibiotics for selection. The resulting 0.4% agarose solution was cooled to 37°C for 1h. In most cases ethanol (final concentration 1%) was added to the medium. A 13ml aliquot was poured into Petri Dishes (100×15mm), which were then dried without lids for 1.5h in a laminar flow hood at room temperature. For inoculation onto the plate, 5µl of cells from a suspension culture at a density of 1.5×107cells/ml were added on the agarose surface. For the experiments in Fig. 2 and S1, 50ul of cells were spread on the surface by gently rotating and rocking the plate. Trypanin RNAi lines were induced for 72h with 1µg/ml tetracycline in suspension culture prior to plating. Inoculated plates were dried for 3 min without lids, closed and sealed with parafilm and incubated as for suspension cultures at 27°C.
Imaging of plates
For Fig. 1A, the plate was imaged using a Zeiss Axioskop II microscope with a 2.5×LD Plan NeoFluor objective and Zeiss Axiocam camera. For Fig. 1B–F (Video S1), the plate was imaged using a Zeiss Axiovert 200M microscope with a 2.5×LD Plan NeoFluor objective and a COHU RS-170 high performance CCD camera (COHU, Inc.). Images were captured at 1 frame per 10min at room temperature using Adobe premiere Elements 1.0 (Adobe Systems). Time stamps are indicated in the panels. For Video S1, images were compiled into a movie using NIH-ImageJ (http://rsbweb.nih.gov/ij). The playback speed is 5 frames per second (3000× original speed) and elapsed time is 24h.
For Fig. 2 (Video S2) and Fig. S1 (Video S3), plates were maintained at 28°C, 5% CO2 in a CTI humidified live cell cultivation chamber equipped with heating insert and CTI 3700 controller from Zeiss, Inc. This chamber allows independent control of humidity, temperature and CO2 on the microscope stage. Plates were monitored on a Zeiss Axiovert 200M microscope, using a 10×LD Plan NeoFluor objective and a COHU RS-170 High performance CCD camera (COHU, Inc.). For Fig. 2 (Video S2), images were captured once every 5 sec and played back at 10 frames per second (fps), giving a final playback speed of 50×. For Fig. S1 (Video S3), the video was recorded in real-time using a VCR, then digitized in AVI format at 30 frames per second (fps) using an in-line Sony Handycam digital camera as an analog/digital converter and Adobe Premier Elements (Adobe Systems). Individual images were extracted at 1 fps, exported into QuickTime video format using the Sorenson TM CODEC within Adobe Premier Elements, and played back at 30 fps. The final playback speed is thus 30× real speed and elapsed time is 10 minutes 57 seconds.
For Fig. 3A, 4A–E and 5, plates were imaged at the indicated times post plating using an Olympus Stylus 770 SW digital camera and processed using Adobe Photoshop 8.0. For Fig. 3B, the plate was imaged as described above for Fig. 1B–F.
For Fig. S2 (Videos S4 and S5), time-lapse images were captured and compiled into video as described above for Fig. 1B–F. The playback speed is 6429× and elapsed time is 21.43 hours for Video S4 and 8.9h for Video S5.
Western blotting
Cells were collected in PBS from the agarose plate, counted and washed two times in PBS. The equivalent increase in opaqueness of control and trypanin RNAi communities on plates, Fig. 4, indicated that they continued doubling at equivalent rates and direct cell counting confirmed this. Protein samples were prepared and subjected to Western blot analysis as described [49], using 1×106 cell equivalents per lane. Monoclonal anti-trypanin antibody [50] was used at 1∶5000, and monoclonal anti-β-tubulin E7 hybridoma supernatant was used at 1∶5000. The anti-β-tubulin antibody was developed by Michael Klymkowsky, University of Colorado and was obtained from the Developmental Studies hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Secondary antibody was horseradish peroxidase-coupled goat anti-mouse (BioRad) used at 1∶2500.
Supporting Information
Zdroje
1. ShapiroJA
1998 Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol 52 81 104
2. HarsheyRM
2003 Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol 57 249 273
3. BasslerBL
LosickR
2006 Bacterially speaking. Cell 125 237 246
4. ShaulskyG
KessinRH
2007 The cold war of the social amoebae. Curr Biol 17 R684 692
5. ReynoldsTB
FinkGR
2001 Bakers' yeast, a model for fungal biofilm formation. Science 291 878 881
6. BlankenshipJR
MitchellAP
2006 How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9 588 594
7. VelicerGJ
YuYT
2003 Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus. Nature 425 75 78
8. KaiserD
2003 Coupling cell movement to multicellular development in myxobacteria. Nat Rev Microbiol 1 45 54
9. FirtelRA
MeiliR
2000 Dictyostelium: a model for regulated cell movement during morphogenesis. Curr Opin Genet Dev 10 421 427
10. RauprichO
MatsushitaM
WeijerCJ
SiegertF
EsipovSE
1996 Periodic phenomena in Proteus mirabilis swarm colony development. J Bacteriol 178 6525 6538
11. FraserGM
HughesC
1999 Swarming motility. Curr Opin Microbiol 2 630 635
12. HenrichsenJ
1972 Bacterial surface translocation: a survey and a classification. Bacteriol Rev 36 478 503
13. RashidMH
KornbergA
2000 Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 97 4885 4890
14. NudlemanE
WallD
KaiserD
2005 Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science 309 125 127
15. ZusmanDR
ScottAE
YangZ
KirbyJR
2007 Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol 5 862 872
16. HarsheyRM
1994 Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol 13 389 394
17. VerstraetenN
BraekenK
DebkumariB
FauvartM
FransaerJ
2008 Living on a surface: swarming and biofilm formation. Trends Microbiol 16 496 506
18. KohlerT
CurtyLK
BarjaF
van DeldenC
PechereJC
2000 Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182 5990 5996
19. LegrosD
OllivierG
Gastellu-EtchegorryM
PaquetC
BurriC
2002 Treatment of human African trypanosomiasis–present situation and needs for research and development. Lancet Infect Dis 2 437 440
20. GibsonW
BaileyM
2003 The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis 2 1
21. Van Den AbbeeleJ
ClaesY
van BockstaeleD
Le RayD
CoosemansM
1999 Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology 118(Pt 5) 469 478
22. VickermanK
1985 Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull 41 105 114
23. VickermanK
TetleyL
HendryKA
TurnerCM
1988 Biology of African trypanosomes in the tsetse fly. Biol Cell 64 109 119
24. RoditiI
LehaneMJ
2008 Interactions between trypanosomes and tsetse flies. Curr Opin Microbiol 11 345 351
25. HendryKA
VickermanK
1988 The requirement for epimastigote attachment during division and metacyclogenesis in Trypanosoma congolense. Parasitol Res 74 403 408
26. Bloodgood
1981 Flagella-Dependent Gliding Motility in Chlamydomonas. Protoplasma 106 183 192
27. O'Toole
2008 How Pseudomonas aeruginosa Regulates Surface Behaviors. Microbe 3 65 71
28. VassellaE
OberleM
UrwylerS
RenggliCK
StuderE
2009 Major surface glycoproteins of insect forms of Trypanosoma brucei are not essential for cyclical transmission by tsetse. PLoS ONE 4 e4493 doi:10.1371/journal.pone.0004493
29. CarruthersVB
CrossGA
1992 High-efficiency clonal growth of bloodstream - and insect-form Trypanosoma brucei on agarose plates. Proc Natl Acad Sci U S A 89 8818 8821
30. HillKL
2003 Biology and mechanism of trypanosome cell motility. Eukaryot Cell 2 200 208
31. CaiazzaNC
ShanksRM
O'TooleGA
2005 Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 187 7351 7361
32. Be'erA
ZhangHP
FlorinEL
PayneSM
Ben-JacobE
2009 Deadly competition between sibling bacterial colonies. Proc Natl Acad Sci U S A 106 428 433
33. InghamCJ
Ben JacobE
2008 Swarming and complex pattern formation in Paenibacillus vortex studied by imaging and tracking cells. BMC Microbiol 8 36
34. HutchingsNR
DonelsonJE
HillKL
2002 Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J Cell Biol 156 867 877
35. MurilloLA
NewportG
LanCY
HabelitzS
DunganJ
2005 Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot Cell 4 1562 1573
36. RalstonKS
KabututuZP
MelehaniJH
OberholzerM
HillKL
2009 The Trypanosoma brucei flagellum: moving parasites in new directions. Annu Rev Microbiol 63 335 362
37. GingerML
PortmanN
McKeanPG
2008 Swimming with protists: perception, motility and flagellum assembly. Nat Rev Microbiol 6 838 850
38. VassellaE
ReunerB
YutzyB
BoshartM
1997 Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci 110(Pt 21) 2661 2671
39. DeanS
MarchettiR
KirkK
MatthewsKR
2009 A surface transporter family conveys the trypanosome differentiation signal. Nature 459 213 217
40. SeebeckT
SchaubR
JohnerA
2004 cAMP signalling in the kinetoplastid protozoa. Curr Mol Med 4 585 599
41. OberholzerM
BregyP
MartiG
MincaM
PeierM
2007 Trypanosomes and mammalian sperm: one of a kind? Trends Parasitol 23 71 77
42. PaindavoineP
RolinS
Van AsselS
GeuskensM
JauniauxJC
1992 A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol Cell Biol 12 1218 1225
43. ParsonsM
RubenL
2000 Pathways involved in environmental sensing in trypanosomatids. Parasitol Today 16 56 62
44. FragosoCM
Schumann BurkardG
OberleM
RenggliCK
HilzingerK
2009 PSSA-2, a membrane-spanning phosphoprotein of Trypanosoma brucei, is required for efficient maturation of infection. PLoS ONE 4 e7074 doi:10.1371/journal.pone.0007074
45. BerrimanM
GhedinE
Hertz-FowlerC
BlandinG
RenauldH
2005 The genome of the African trypanosome Trypanosoma brucei. Science 309 416 422
46. GibsonW
PeacockL
FerrisV
WilliamsK
BaileyM
2006 Analysis of a cross between green and red fluorescent trypanosomes. Biochem Soc Trans 34 557 559
47. GibsonW
PeacockL
FerrisV
WilliamsK
BaileyM
2008 The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors 1 4
48. WirtzE
LealS
OchattC
CrossGA
1999 A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol 99 89 101
49. RalstonKS
LernerAG
DienerDR
HillKL
2006 Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell 5 696 711
50. RalstonKS
HillKL
2006 Trypanin, a component of the flagellar Dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog 2 e101 doi:10.1371/journal.ppat.0020101
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



