-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
The CD8+ T-cell is a key mediator of antiviral immunity, potentially contributing to control of pathogenic lentiviral infection through both innate and adaptive mechanisms. We studied viral dynamics during antiretroviral treatment of simian immunodeficiency virus (SIV) infected rhesus macaques following CD8+ T-cell depletion to test the importance of adaptive cytotoxic effects in clearance of cells productively infected with SIV. As previously described, plasma viral load (VL) increased following CD8+ T-cell depletion and was proportional to the magnitude of CD8+ T-cell depletion in the GALT, confirming a direct relationship between CD8+ T-cell loss and viral replication. Surprisingly, first phase plasma virus decay following administration of antiretroviral drugs was not slower in CD8+ T-cell depleted animals compared with controls indicating that the short lifespan of the average productively infected cell is not a reflection of cytotoxic T-lymphocyte (CTL) killing. Our findings support a dominant role for non-cytotoxic effects of CD8+ T-cells on control of pathogenic lentiviral infection and suggest that cytotoxic effects, if present, are limited to early, pre-productive stages of the viral life cycle. These observations have important implications for future strategies to augment immune control of HIV.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000748
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000748Summary
The CD8+ T-cell is a key mediator of antiviral immunity, potentially contributing to control of pathogenic lentiviral infection through both innate and adaptive mechanisms. We studied viral dynamics during antiretroviral treatment of simian immunodeficiency virus (SIV) infected rhesus macaques following CD8+ T-cell depletion to test the importance of adaptive cytotoxic effects in clearance of cells productively infected with SIV. As previously described, plasma viral load (VL) increased following CD8+ T-cell depletion and was proportional to the magnitude of CD8+ T-cell depletion in the GALT, confirming a direct relationship between CD8+ T-cell loss and viral replication. Surprisingly, first phase plasma virus decay following administration of antiretroviral drugs was not slower in CD8+ T-cell depleted animals compared with controls indicating that the short lifespan of the average productively infected cell is not a reflection of cytotoxic T-lymphocyte (CTL) killing. Our findings support a dominant role for non-cytotoxic effects of CD8+ T-cells on control of pathogenic lentiviral infection and suggest that cytotoxic effects, if present, are limited to early, pre-productive stages of the viral life cycle. These observations have important implications for future strategies to augment immune control of HIV.
Introduction
The capacity and limits of host immunity in containing lentiviral infection are fundamental to the understanding of Human Immunodeficiency Virus (HIV) and SIV pathogenesis yet are incompletely understood. Previous studies support effects of host immunity in modulating HIV disease progression [1],[2],[3],[4] and in driving viral evolution and escape. Concurrent with the appearance of HIV specific CD8+ T-cells following either acute HIV or SIV infection, plasma viral load falls abruptly [4], indirectly supporting a role for adaptive, cytotoxic lymphocyte responses in the control of viral replication. However, this evidence is circumstantial and inconclusive, since in most cases of natural infection, several HIV specific immune parameters vary in tandem [3],[5].
The most direct evidence for the antiviral effects of CD8+ T-cells have come from the observation of profound elevations in viral load following the depletion of CD8+ T-cells from SIV infected macaques through the use of anti-CD8 monoclonal antibodies. These studies reveal an approximate ten-fold increase in plasma VL concurrent with CD8+ T-cell depletion [6],[7],[8]. In contrast, similar maneuvers that deplete the CD20+ cells central to humoral immune responses fail to produce comparable effects on viremia [9]. Although a favored interpretation of the CD8+ T-cell depletion experiments attributes the rise in VL to loss of CTL killing [10],[11], this has not been directly demonstrated and the contribution of non-cytotoxic effects of CD8+ T-cells including the production of chemokines that block new infectious events or the elaboration of soluble factors that attenuate viral production from infected cells remain as possible alternative mechanisms [7],[12].
In classic studies of viral dynamics performed by perturbing the VL steady state using antiretroviral drugs that inhibit HIV replication, cell free virus and the infected cells producing HIV were shown to have a very short lifespan [13],[14],[15]. While the models used to explain these dynamics invoke clearance and death of productively infected cells with a half-life of only a day [16], the mechanism responsible for this rapid elimination has yet to be elucidated.
In this study, we measured VL decay during the initiation of antiretroviral therapy in SIV-infected macaques with or without depletion of CD8+ T-cells to assess whether the rise in VL upon CD8+ T-cell depletion was accompanied by an increase in the life span of productively infected cells and, conversely, to determine whether CTL killing is responsible for the short half-life of productively infected cells in vivo. Indirect evidence for immune selective pressure by CTL was assessed by comparing sequence variation in a representative early (nef) and late (gag) viral genes from samples collected immediately before and right after CD8+ T-cell depletion.
Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and local animal welfare bodies and all animal work was approved by the UC Davis Institutional Animal Care and Use Committee (IACUC).
Infection of rhesus macaques and overview of study
Animal studies were conducted in accordance with UC Davis IACUC approved protocols at the California National Primate Center. 8 healthy junvenile rhesus macaques were infected intravenously with 1000 TCID of SIVmac251 at time zero (D0). Viral load testing and immunophenotyping were performed as shown (Figure 1). At week 12 (D84), animals received mAb cMT-807, control antibody or no mAb. At week 13 (D91), animals were started on combination antitretroviral therapy consisting of PMPA (30 mg/Kg per day) and FTC (8 mg/Kg per day) given intramuscularly (IM) daily.
Fig. 1. Timeline of experiments. 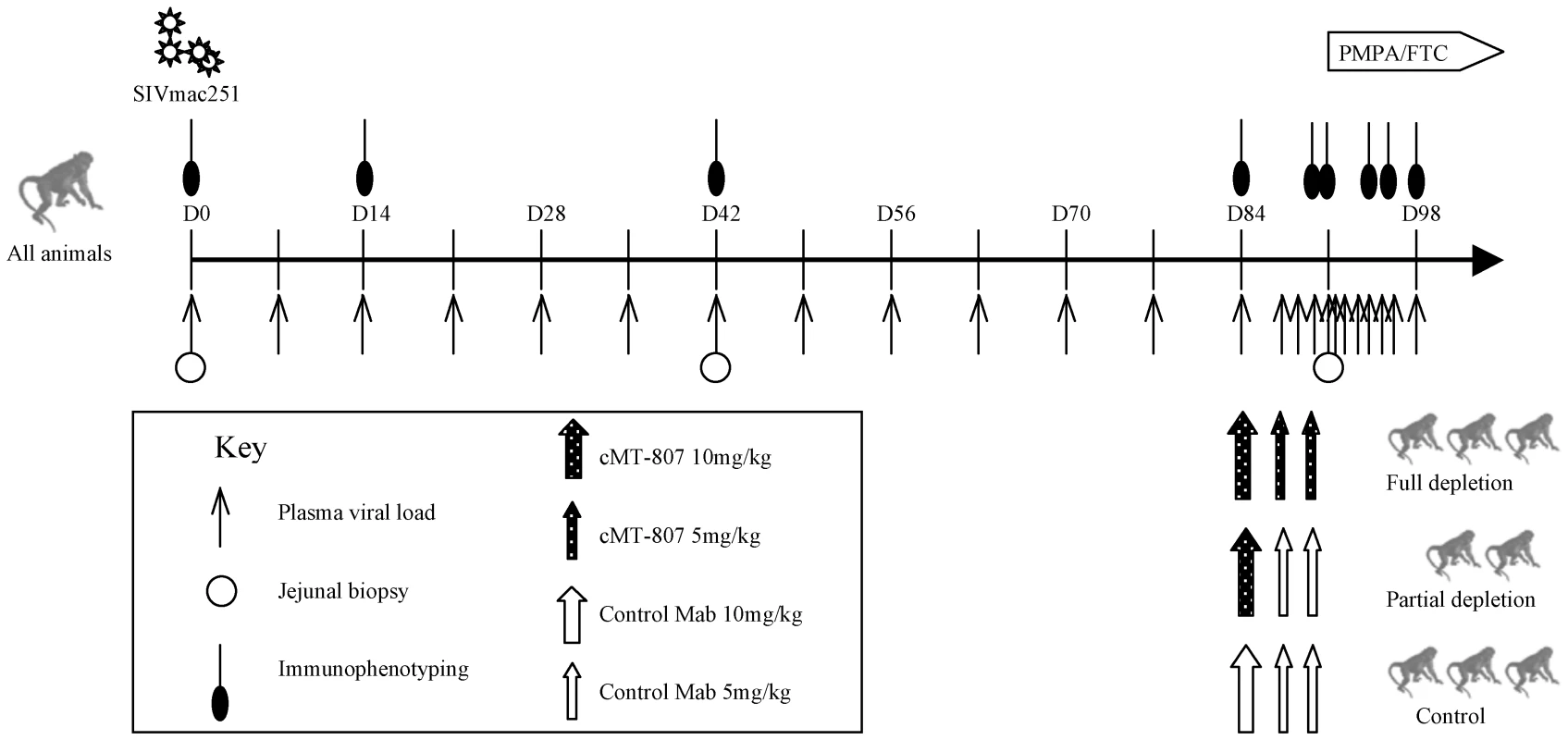
Plasma SIV RNA levels, immunophenotyping, GI biopsy and antibody treatment according to treatment group is shown. D = day post infection. Treatment phase with PMPA/FTC is indicated by the horizontal block-arrow in the right upper hand corner. CD8+ T-cell depletion
12 weeks post-infection (D84), 3 animals received full depleting doses of monoclonal antibody (mAb) cMT-807, a mouse/human chimeric monoclonal antibody, administered IM (Centocor, Horsham, PA) on D84, D87 and D91, 2 animals received partial depleting treatment with a single dose of cMT-807 followed by control antibody and 3 control animals received a series of control antibody injections (1) or no antibody treatment (2) (Figure 1).
Viral load testing and immuno-phenotyping
VL testing was performed on peripheral blood plasma using a real time PCR assay detecting a sequence in SIV gag as previously described [17],[18]. Immuno-phenotyping was performed as previously described. To assess whether the effect of epitope masking by cMT-807 might contribute to the measured depletion of CD8+ cells, a second staining mAb CD25 (DAKO, Carpinteria, CA) was used to corroborate the depletion achieved in two CD8-depleted animals and changes in proportions of CD4/CD8 double negative populations before and after treatment with cMT-807 were sought [7].
Endoscopy and gut biopsy
Jejunal pinch biopsy samples were incubated in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) and collagenase (Sigma, St. Louis, MO) at 37°C and rapidly shaken for 45 minutes and then subjected to Percoll (Sigma, St. Louis, MO) density gradient centrifugation to enrich for T-cells and eliminate tissue debris [19]. Cells were then washed with phosphate buffered saline (PBS) (Gibco/Invitrogen, Carlsbad, CA) and allowed to equilibrate over night at 37°C and 5% CO2 in complete RPMI 1640 (containing 10% fetal calf sera, penicillin and streptomyicin). PBMC were isolated by density centrifugation over Lymphocyte Separation Media (LSM) (Organon-Technica, Durham, NC). Aliquots of freshly isolated cells were then stained with fluorescently labeled antibodies for flow cytometric analysis.
Antiretroviral therapy
On D91, all animals initiated daily intramuscular injections of 30mg/kg/day of PMPA (Gilead, Foster City, CA) and 8mg/kg/day of FTC (Gilead, Foster City, CA) [19] until they were euthanized and necropsied at week 15.
Modeling viral dynamics
We based our estimates for clearance of productively infected cells on first-phase plasma virus clearance rates as proposed by Ho et al and Wei et al [13],[15]. The model employed was a basic model proposed by Perelson et al [14].
We used Maximum likelihood fits of our measured data during the first week on ART to estimate the clearance rate “δ” [14] and the calculated half life (t1/2) of productively infected cells: t1/2 = ln(2)/δ. The baseline timepoint was excluded from these fits to exclude the effect of the rapid elimination of cell-free plasma virus from the blood compartment (t1/2<1 hr.) on viral decay. We chose to limit the data for the inclusion in calculation of the first phase decay to those prior to 7 days (between 0.5 to 5 days inclusive) in order to avoid any possibility that recovery of CD8+ T-cells could affect the decay estimates. For the same reason, we have not attempted to model second phase decay. The difference in cell death rates between animals with and without depletion of CD8+ T-cells provides a measure of the effect that CTL killing has on shortening the lifespan of productively infected cells. Correlation between measures of CD8 T-cell depletion and either viral load rebound rate or death rate of productively infected cells (δ), was assessed by a Spearman rank-correlation test.
Sequencing and sequence analysis of partial gag and nef
RNA was extracted from 140µl of plasma using the Qiagen QIAamp Viral RNA Kit according to the manufacturer's protocol. cDNA was prepared using random decamers and Ambion RETROscript™ Kit (Ambion, Austin TX). Quadruplicate nested PCRs were carried out for nef using primers SIV nef5′out (TGACCTACCTACAATATGGGTG) and SIV nef3′out (TCCCCTTGTGGAAAGTCCCTGCT) and SIV nef5′in (CGTGGRGAGACTTATGGGAGACT) and SIV nef3′in (AAGGCCTCTTGCGGTTAGCCTTC). For the gag region, quadruplicate PCRs were carried out using primers SIVgag1151F (AGGAACCAACCACGACGGAG) and SIVgag2445R (AAAGGGATTGGCACTGGTGCGAGG). SuperTaq™ from Ambion (Ambion, Austin, TX) was used for all the PCR reactions. Products were proportionately pooled then cloned using the TOPO TA cloning kit. The gag PCR product was gel purified using Qiagen QIAex Gel Extraction Kit prior to being used for cloning. Clones were picked and plasmids prepared using the Qiagen Plasmid Mini Kit (Qiagen, Chatsworth CA) according to the manufacturer's protocol. Plasmids were sequenced using SIVgag1151F and SIVGag1826R (CCTGGCACTACTTCTGCTCC) as sequencing primers for gag and SIV nef5′in and SIV nef3′in for nef. Big Dye Terminator v3.1 (Applied Biosystems, Foster City, CA) sequencing mix. Sequences were aligned and edited using clustalW as executed in Sequencher and and subsequent manual editing was performed in Se-Al. Estimates of genetic diversity were calculated using Dnadist within the PHYLIP 3.6 software suite [20]. Selection intensity and dN/dS ratios were evaluated using the Synonymous-Nonsynonymous Analysis Program (SNAP), based on the methodology of Nei and Gojobori [21]. A Fisher's Exact test was used to compare site-specifc amino acid composition between timepoints. Sequences are available through GenBank, accession numbers GU366223 to GU366660.
Results
Dynamics of acute SIV infection and establishment of cellular and viral load steady state
The SIVmac251 infection of rhesus macaques was chosen as the experimental model because viral dynamics studies in this model of AIDS have been well described [22] and the monoclonal antibody cMT-807 is effective in eliminating CD8+ T-cells in rhesus macaques [6],[23]. The overall experimental plan is shown in Figure 1. All animals were infected with 1000 TCID50 of SIVmac251 by intravenous injection and were longitudinally monitored as shown. At Week 12 (D84) post infection, 3 animals were assigned to the “full depletion group” and received 3 doses of cMT-807 at approximately twice the dosage previously used by Schmitz et al [6] in order to achieve more sustained CD8+ T-cell depletion, 2 animals received only a single dose of cMT-807 (partial treatment group) and 1 control animal received three doses of an isotypic control antibody while 2 control animals received no control antibody injections [6].
Viral infection phase
Animals exhibited high plasma viral loads in the first weeks following infection that peaked prior to D28 and declined to a “steady state” set point determined by viral and host factors including host immune responses [3],[4],[22]. The overall pattern appeared consistent with previously published data following acute infection with either SIV or HIV (Figure S1). VL set points prior to D84 were estimated to be 3.8 to 6.6 logs for the 8 animals (Figure 2 and Table 1).
Fig. 2. Plasma SIV RNA prior to, following treatment with cMT-807 antibody and over the first week after starting PMPA/FTC. 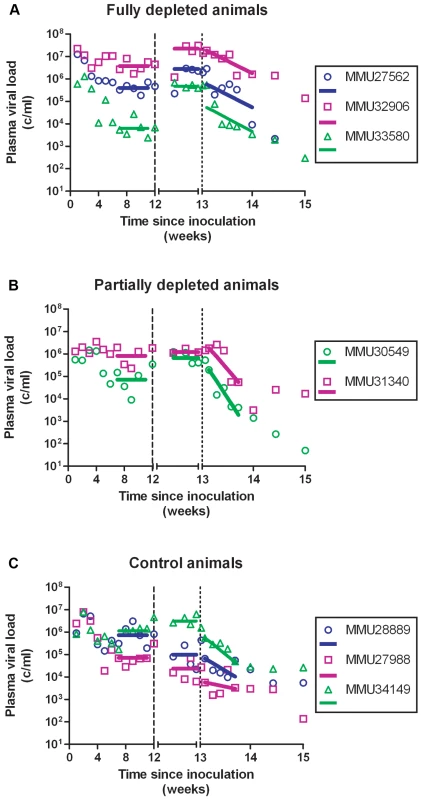
Data shown are grouped according to treatment group with fit lines for the period pre cMT-807 administration, during cMT-807 administration and during antiretroviral therapy shown as the solid line segments. Note that for the tick marks on the X-axis prior to Day 84 (Week 12) is by 21 day (3 week) increments while after Day 84, the timescale is in 4 day increments. Tab. 1. Summary of viral load dynamics and correlation to CD8 T-cell depletion. 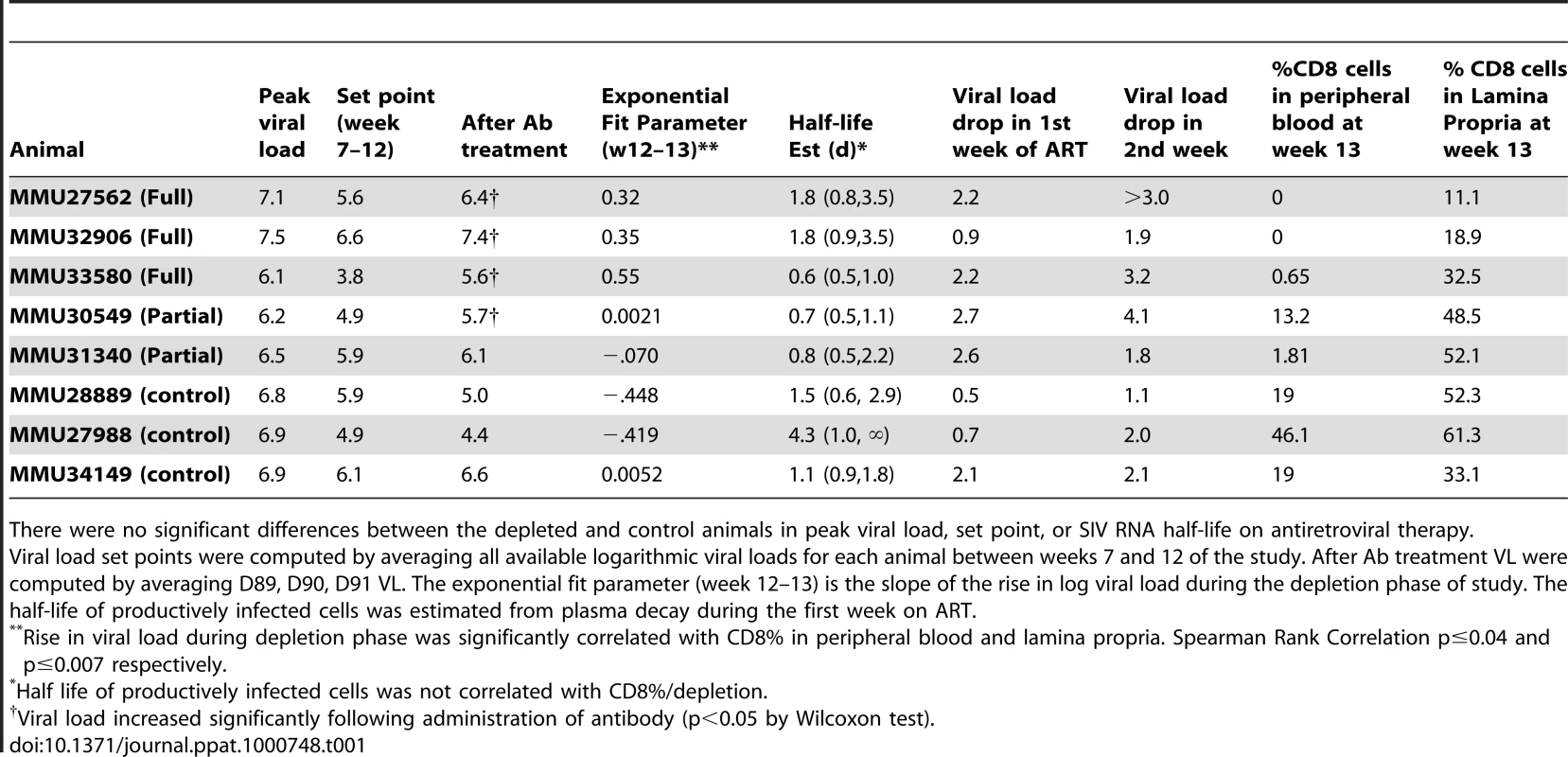
There were no significant differences between the depleted and control animals in peak viral load, set point, or SIV RNA half-life on antiretroviral therapy. After 12 weeks of SIV infection, CD4 T-cell counts in whole blood declined from a median of 807 to 693 cells/mm3 and CD8+ T-cells rose from 612 to 1247 cells/mm3 (Figures 3, 4). There were no significant differences between control and study animals in set point VL, CD4% and CD8% prior to administration of the CD8+ T-cell depleting antibody (Table 1). To the degree that these parameters reflected the equilibrium between pathologic effects of viral replication and effects of host immune response, the animals in the control and treatment arms of the study appeared comparable.
Fig. 3. CD8 T-cell concentrations before, following treatment with cMT-807 antibody and after starting PMPA/FTC. 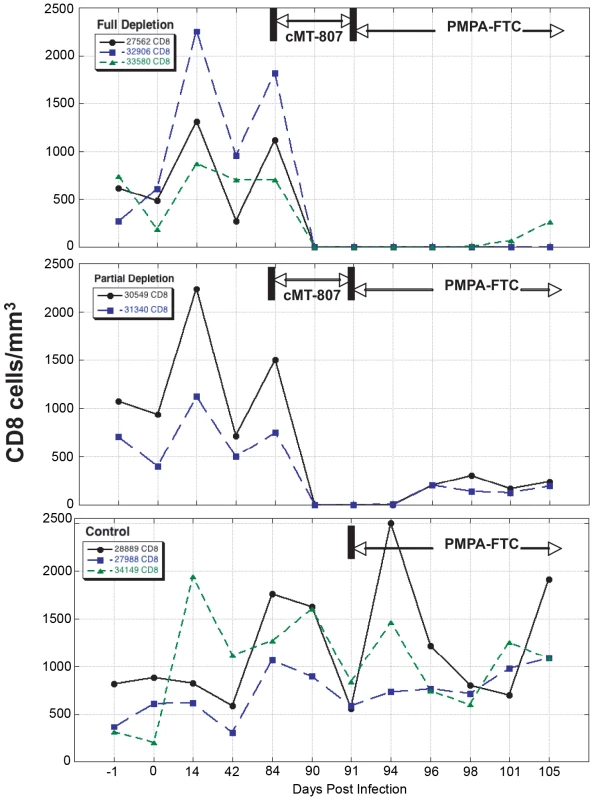
Results are displayed separately for full depletion, partial depletion and control animals. Time scales differ for each epoch shown and the x-axes are not to scale. Fig. 4. CD4 T-cell concentrations before, following treatment with cMT-807 antibody and after starting PMPA/FTC. 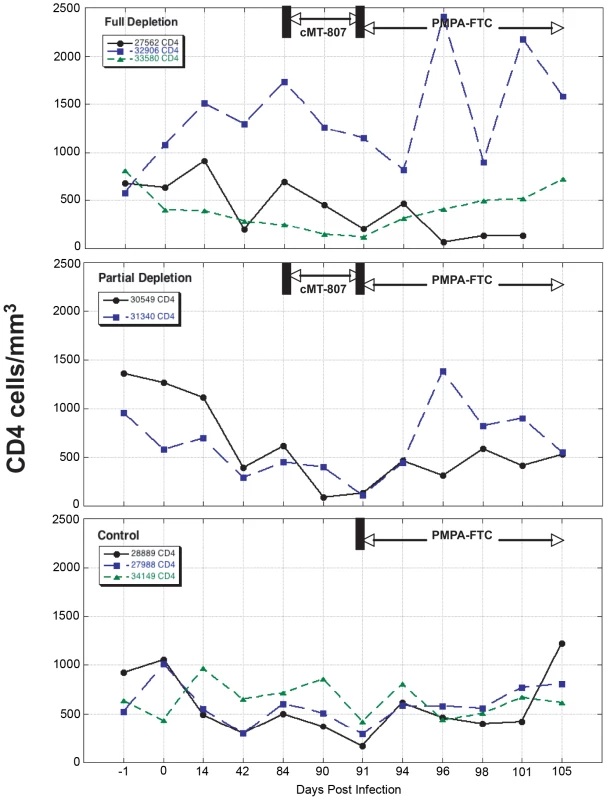
Results are displayed separately for full depletion, partial depletion and control animals. Time scales differ for each epoch shown and the x-axes are not drawn to scale. CD8+ T-cell depletion phase
Frequencies of CD8+ T-cells dropped precipitously with administration of cMT-807 in the full depletion animals, reaching undetectable levels by D91 in all 3 animals (Figure 3). This was accompanied by a 0.7 to 1.9 log10 rise in viral loads that appeared sustained, representing a new pseudo-steady state as previously described [6],[7] (Figure 2A). In the partial depletion animals, CD8+ T-cells fell initially but became detectable again by D96 (Figure 3). One partial depletion animal experienced an increase in viral load approximately 1 log above pre-antibody administration-set point at D91 (week 13) (Figure 2B). In the second partially depleted animal, plasma viral loads were unchanged between D84 (week 12) and D91 (week 13) timepoints. No consistent change in CD8+ T-cell counts was experienced by any of the three control animals including the animal receiving the isotype control antibody. CD4 numbers in the treated groups showed a mild gradual decease following administration of cMT-807 but this was also seen to a lesser extent in the control group (Figure 4).
To exclude epitope masking as a cause for the absence of detectable CD8+ T-cells in the peripheral blood by flow cytometry, data were examined for levels of CD3+ CD4/CD8 double negative cells pre and post antibody administration for each CD8+ T - cell-depleted animals as previously performed by Jin et al [7] and independent FACS was performed with DAKO clone DK25 antibody in 2 full depletion animals with sufficient sample for parallel analysis. CD3+ CD4/CD8 double-negative cells were not more frequent after CD8+ T-cell depletion nor were CD8+ T-cells detectable using the second staining antibody (data not shown).
While depletion of CD8+ T-cells in peripheral blood appeared complete in the “full depletion” group of animals, CD8+ T-cell depletion in GALT at week 13 (D91) was less extensive but nonetheless reflected a median reduction of 66% in the animals that received the full depletion protocol. In contrast, median reduction in CD8% was 30% in partial depletion animals and 0% in control animals. These findings are in keeping with the experience of other investigators that appear to confirm extensive depletion of CD8+ T-cells in peripheral blood and central lymphoid tissues, while CD8+ T-cell depletion in the GALT can be substantial but less complete.
Correlation of CD8+ T-cell depletion in GALT with the increase in plasma VL
We exploited variability in CD8+ T-cell depletion in GALT resulting from our use of complete and partial depletion protocols and from biologic variability between animals as a way to gauge a “dose response” between CD8+ T-cell depletion and extent of viral load increase between D84 and D91. The residual CD8% (reflecting extent of depletion) in both peripheral blood and lamina propria lymphocytes (LPL) inversely correlated with the rise in VL observed during treatment with cMT-807 (Spearman r = −0.759, p = 0.036 and r = −0.881, p = 0.0072 respectively), as represented by the slope of the exponential rise in VL between D84 and D91 (viral rebound rate) (Table 1, Figure 5A,B). Because most SIV (and HIV) infection events are thought to take place in lymphoid tissues, the stronger correlation between CD8% of LPL and viral rebound is not surprising. These observations are consistent with a direct effect of CD8 cells in controlling HIV replication but they do not distinguish antiviral effects based on preventing new infection, CTL killing of productively infected cells or inhibition of viral transcription.
Fig. 5. Correlation between viral rebound rate and death rate δ and % CD8 remaining in peripheral blood (PBMC) and lamina propria lymphocytes (LPL). 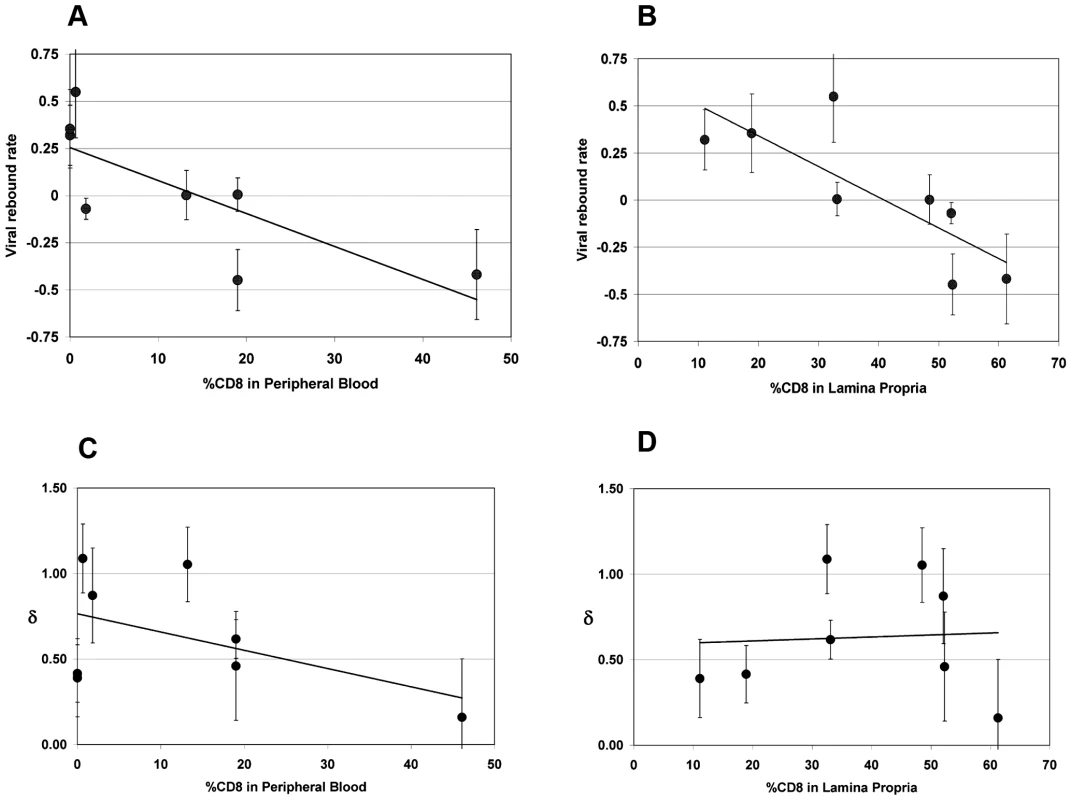
Error bars are shown for estimates. Significant negative correlations were found for viral rebound rates and CD8% in PBMC (5A, Spearman r −0.76, p = 0.036,) and CD8% in LPL (5B, Spearman r −0.88, p = 0.0072,) but not for death rate of productively infected cells, δ, and CD8% in either PBMC (5C, Spearman r = −.12, p = 0.79) or LPL (5D, Spearman r = 0.07, p = 0.88). The fit lines shown are simple regression lines and do not directly correspond to the non-parametric, Spearman correlation. Decay in plasma viremia with combination antiretroviral therapy
At Week 13 (D91), all animals received (30 mg/Kg) PMPA and (8 mg/Kg) FTC after establishment of the new VL steady state resulting from CD8+ T-cell depletion. Previous studies have established that this drug combination results in potent suppression of viral replication in SIV infected macaques as reflected in the prompt fall in plasma viremia (Figure 2). First phase plasma viral decay computed from plasma RNA measurements following initiation of PMPA/FTC was used to estimate t1/2 of productively infected cells. We used data between 0.5 and 5 days after starting antiretroviral drugs to calculate the first phase decay of plasma viremia to allow for a pharmacologic delay and to avoid potential confounding effects from CD8+ T-cell recovery however, using data including day 7 or day 10 did not change the overall observation of very similar decay characteristics between groups. The median calculated half-life for each of the three groups (Table 1) approximated the half-lives of 0.7 to 1.4 days previously reported by Nowak [22]. Unlike the significant correlation between the slope of increase in VL with extent of CD8+ T-cell depletion in blood and LPL, no similar correlation was found between extent of CD8+ T-cell depletion and clearance of productively infected cells, δ (Figure 5C, 5D). Similarly, half-life was not correlated with CD4% or VL prior to antiretroviral therapy (data not shown).
Evolution of SIV gag and nef before and after CD8+ T-cell depletion
As an independent probe for potential suppressive effects of CTL on SIV infected cells, we next examined changes in the distribution of viral variants reflected in clonal gag sequences (as a representative “late” structural gene) and clonal nef sequences (representing early, regulatory and accessory genes). Others have demonstrated that the pace of evolution of SIV immune escape epitopes in macaques following acute infection is altered by CD8+ T-cell depletion [24]. If the approximate 1 log rise in viral load was related to removal of suppressive effects of CTL clones targeting specific SIV epitopes in either Gag or Nef, we hypothesized that sequences would show evidence of positive selection (typically associated with CTL pressure) and a change in distribution of viral variants would occur after CD8+ T-cell depletion. We analyzed partial amino acid sequences of Gag and Nef that were comparable in length from CD8+ T-cell depleted and non-depleted control animals.
The distribution of Gag sequence variants remained consistent pre and post CD8+ T-cell depletion for all animals (Figure S2). In contrast, several positions in Nef exhibited statistically significant differences in amino acid composition, possibly reflecting release from Nef-specific CTL pressure during the CD8+ T-cell depletion period (Figure 6A). Accordingly, Nef sequences from control animals failed to show any significant redistribution of variants during this period (Figure 7A). An excess of nonsynonymous over synonymous nucleotide substitution was observed across regions of nef, in all depleted animals (Figure 6B) and 1 of 2 control animals (Figure 7B), indicative of positive selection consistent with positive or diversifying selection typically associated with CTL pressure. Ratios of nonsynonymous to synonymous substitution (dN/dS) (Figures 6B, 7B and S3) and nucleotide sequence diversity (as represented by pairwise genetic distance) were consistently higher in nef clones than in gag clones both pre and post CD8+ T-cell depletion (data not shown). We did not have sufficient viable cell samples to directly test for the presence or absence of epitope specific CTL clones to explain the change in distribution of viral variants following CD8+ T-cell depletion. Nevertheless, although stronger functional constraints on Gag may play a role, these sequence findings are compatible with CTL activity disproportionately targeting Nef and are consistent with the observation of O'Connor that CTL driven viral evolution may be greater for early viral genes such as tat and nef.
Fig. 6. SIV nef evolution in CD8-depleted animals. 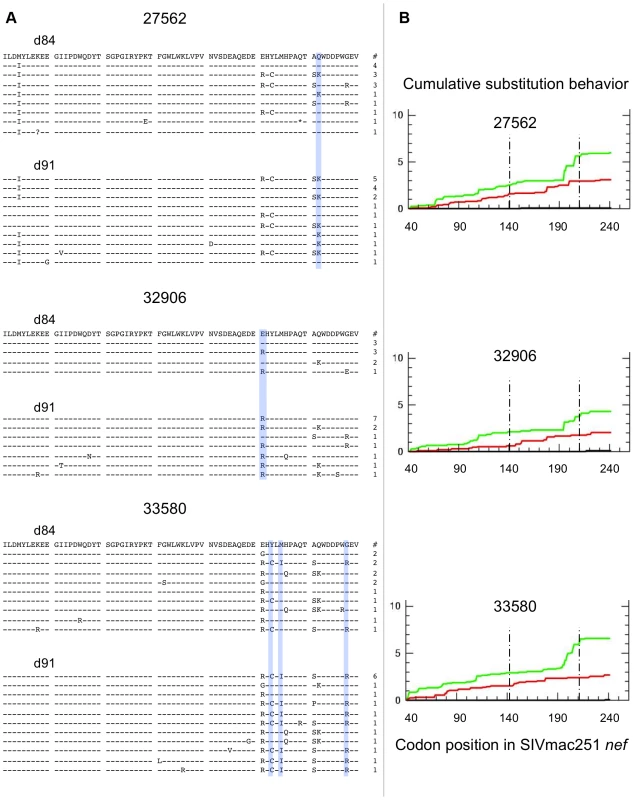
Data from 3 fully depleted animals are shown. A) Sequence alignments of SIVmac251 Nef region demonstrating greatest diversity and evolution during the depletion period (amino acids 141–210). Dashes indicate identity to SIVmac251 inoculum consensus sequence, asterisks indicate premature stop codons, and question marks represent unresolvable sites (due to nucleotide ambiguity). Blue bars highlight positions with statistically significant differences in amino acid distribution between day 84 and day 91 (based on Fisher's Exact, p<0.05). Numbers of sampled clones associated with each predicted protein sequence are listed on right. B) Cumulative behavior of synonymous and nonsynonymous substitutions across nef sequence. Green lines = nonsynonymous mutations, red lines = synonymous substitutions; area between dashed lines represents region displayed in panel A. Fig. 7. SIV nef evolution in control animals. 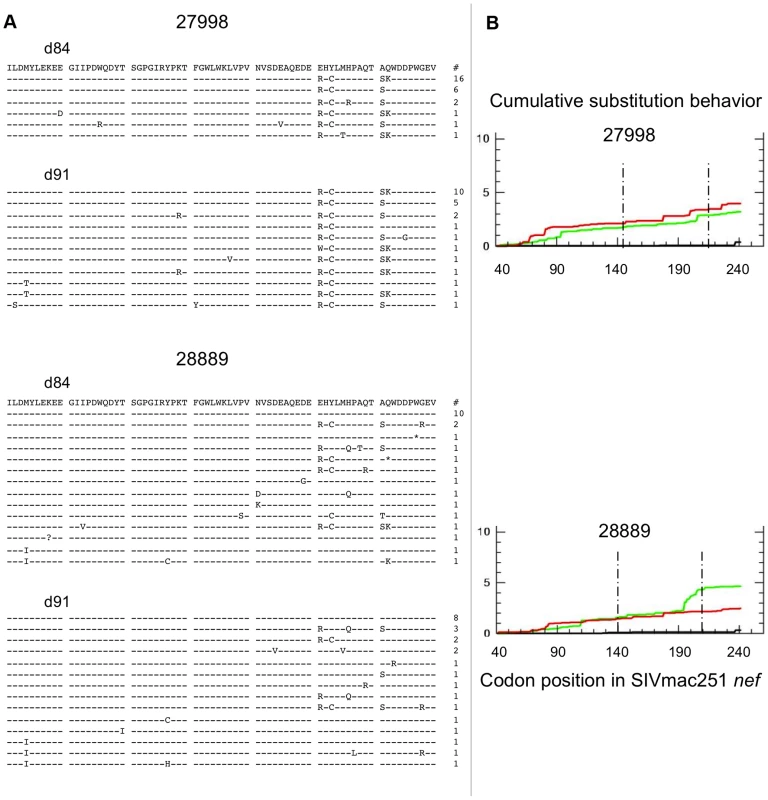
Data from 2 non-depleted control animals are shown. A) Sequence alignments of SIVmac251 Nef region demonstrating greatest diversity and evolution in depleted animals (amino acids 141–210). Dashes indicate identity to SIVmac251 inoculum consensus sequence, asterisks indicate premature stop codons, and question marks represent unresolvable sites (due to nucleotide ambiguity). There were no positions with statistically significant differences in amino acid distribution between day 84 and day 91 (based on Fisher's Exact test). Numbers of sampled clones associated with each predicted protein sequence listed on right. B) Cumulative behavior of synonymous and nonsynonymous substitutions across nef sequence. Green lines = nonsynonymous mutations, red lines = synonymous substitutions; area between dashed lines represents region displayed in panel A. Discussion
The limited success of recent HIV vaccine trials designed to elicit CTL responses to protect at risk subjects from infection underscores the need to reexamine the relative roles of adaptive and innate immunity in the control of HIV infection [25]. The current study was designed to investigate how CD8+ T-cells work to contain pathogenic lentiviral infection in vivo by examining changes to the lifespan of productively infected cells inferred from viral dynamics during antiretroviral therapy with or without CD8+ T-cell depletion using the anti-CD8 monoclonal antibody cMT-807. While our data confirmed the importance of CD8+ T-cells in viral control by demonstrating a rapid increase in VL resulting from CD8+ T-cell elimination, they unexpectedly revealed that the rate of clearance of productively infected cells is independent of the extent of CD8+ T-cell depletion. This latter finding challenges the conventional view that the principal contribution of CD8+ T-cells to antiviral immunity is through their function as CTL's that recognize and kill productively infected cells. The suppressive effects of CD8+ T-cells may instead be exerted through non-cytotoxic effects such as those affecting viral expression/production (as might be mediated by the non-cytotoxic antiviral factor described by Walker [26],[27]) or infectivity (such as chemokines that block infection [28]). Alternatively, CD8+ T-cells may suppress viral replication via CTL killing of infected cells but this may be confined to a narrow window in the pre-productive stages of infection [29]. The study by Klatt and colleagues published in this issue of PLoS Pathogens, using a different experimental protocol to deplete CD8+ T-cells reached similar conclusions [30].
We confirmed earlier studies reporting a rapid rise in VL following administration of cMT-807 coincident with elimination of CD8+ T-cells. The CD8+ T-cell depletion maneuver could result in several indirect effects that might influence viral replication that deserve consideration. Homeostatic mechanisms that regulate total T-lymphocyte number could result in compensatory increases in CD4+ T-cell numbers. Alternatively, administration of large doses of exogenous antibody with massive cellular depletion could result in generalized T-cell activation and could increase the infectability of target cells without increasing absolute CD4+ T-cell numbers. Earlier studies observed that treatment with anti-CD8 antibodies had little effect on the availability of total CD4 T-cells [6],[7]. We observed moderate reductions in CD4+ T-cell counts in all 3 groups rather than increases in CD4+ T-cell numbers. Finally, Okoye recently demonstrated that the heightened tempo of SIV infection following CD8+ T-cell depletion occurred independently of increases in either absolute CD4+ T-cell number or their activation status [31]. Moreover, the finding that there was a strong correlation between the extent of CD8+ T-cell depletion at the putative sites of SIV replication in GALT and the rate of rise in VL after cMT-807 administration provides additional support that it is CD8+ T-cell depletion and not administration of exogenous antibody per se that was responsible for heightened viral replication. Finally, cMT-807 used in these experiments targets the CD8 molecule expressed on both CD8+ T-cells and some NK cells (CD8+, CD3−). Thus, the viral load increases following treatment with cMT-807 could have resulted from both CD8+ T-cell and partial NK cell depletion.
The observation that first phase decay of plasma viremia during treatment with potent inhibitors of viral replication is not affected by depletion of CD8+ T-cells suggests that CTL killing is not necessary to effect the rapid turnover of productively infected cells seen in this and many previous viral dynamic studies (nor does it seem that CD8+, CD3 − NK cells would be required although this was not specifically evaluated). It should be noted that the productively infected cell half-life inferred from these data represents the “functional” half-life rather than a “chronological” half-life. If for example, the average productively infected cell exhibits an exponential increase in viral transcription between days 1 and 3 post-infection, a slight shortening of the chronological lifespan of the cell could have a disproportionately large effect on viral production (burst size). However, such an effect would in fact be perceived in the model used here as a relatively large change in the functional productive lifespan of the infected cell and would be expected to alter first phase plasma RNA kinetics.
The observed one log increase of VL following CD8+ T-cell depletion would require, to first approximation in the timescale studied, a 10-fold increase in productive cell lifespan for the mathematical model used here. Such a change in lifespan is unlikely to be missed even with the study of relatively few animals. To illustrate, we can approximate the most optimistic estimate for the CTL effect on clearance of productively infected cells by determining the difference between the geometric mean of the upper bounds of the 95% confidence intervals for the death rate of the control animals (−0.796 day−1) and the geometric mean for the lower bounds of the 95% CI for death rate among the depleted animals (−.30 day−1). The mean t1/2 for the depleted animal group of 1.24 day would decrease to 0.66 day if we add the calculated upper limit of the CTL effect of −0.496 day−1. However, this approximate two-fold change in t1/2 should only account for a two-fold rise in VL following depletion and not the observed 10-fold change.
A limited role for CTL killing of productively infected cells, although at first surprising, is further supported by several intriguing published observations. For example, first phase plasma HIV RNA decay has been noted to be relatively invariant whether HIV+ patients were treated during early or late stage disease when immune responses would be expected to be robust and waning, respectively [32],[33]. Correspondingly, following structured treatment interruption of HIV suppressive antiretroviral treatment, viral rebound rates do not correlate with the magnitude of HIV specific CD8+ T-cell responses [34]. A final set of observations in accord with the current result is the similarity of viral kinetics of SIVsm following treatment of infected sooty mangabeys and macaques [35] even though in the former, the SIV specific-immune responses tend to be low [36] and CD8 T-cell depletion of sooty mangabeys does not elicit increases in viral load comparable to that seen in macaques [37].
The simultaneous conclusions that CD8+ T-cells exert strong antiviral effects in vivo but that the mechanism of viral control is not through the recognition and killing of productively infected cells raises obvious questions about the nature of the CD8+ T-cell antiviral effect(s). In the earlier study by Jin, very frequent plasma sampling immediately after administration of the CD8+ T-cell depleting antibody in two animals allowed the authors to detail the kinetics of increase in SIV VL [7]. The rapid increases in VL were found to be compatible with CD8+ T-cell effects that prevented new infections as might be expected from the elaboration of infection-blocking chemokines or effects that impaired SIV transcription as might be expected with the cell antiviral factor (CAF) [27] but not with CTL effects alone. Because we focused our study on obtaining frequent viral load measurements at week 13 and beyond to accurately define first phase plasma virus decay, we did not have the ability to perform similar immediate post-depletion analyses. Further studies are needed to better characterize and quantify these non-lytic antiviral effects.
The conclusion that CTL activity does not substantially contribute to the clearance of productively infected cells does not imply that CTL activity is irrelevant to viral control. We surveyed viral gene sequences for other markers of CTL effects that might distinguish CTL recognition and killing of infected cells prior to or during viral production. The observation of positive selection pressure acting on nef but not gag is in alignment with the lack of impact of CD8+ T-cell depletion on first phase plasma virus decay. Because the model used here to describe viral dynamics relates plasma viral decay to the turnover of cells already producing HIV virions, the half-life of productive cells need not change if CTL killing occurred through targeting of Nef (or another early HIV gene product) expressing cells prior to virion production [38],[39]. In contrast, CTL targeting Gag epitopes would be expected to affect measured half-life of productively infected cells to a greater degree. The ability of Nef to downregulate MHC class I provides one possible explanation for why the vulnerability of SIV (and HIV) infected cells to CTL attack is restricted to the early steps of the viral lifecycle (prior to production of threshold levels of Nef needed to downregulate class I and before Gag expression and virion production commence) [40],[41]. Such infected cells expressing Nef could be recognized and cleared by CTL but, because they do not yet contribute to viral production, clearance of these cells would not be reflected in the clearance/death rate (δ) of productively infected cells calculated from first phase plasma virus decay. Instead, the effect of removing these cells would be likened to a reduction in the infection rate. Recent observations that a large proportion of HIV production by infected T-lymphocytes occurs in the subset of CD4 − CD8 − double negative cells is also compatible with the hypothesis that by the time virion production commences, CD4 and by inference MHC I downregulation has already occurred [42]. Parenthetically, it appears that, in all three cases, the Nef variants that increased in proportion following CD8 T-cell depletion were variants that differed from the consensus sequence of the inoculum (Figure 4). While this suggests that the CTL selective pressure may have been greater against these earlier CTL escape forms than against the inoculum consensus strain, the lack of functional immunologic assays limits our ability to confirm this.
These results and the questions they raise point to a need for more work to be done to better understand the full spectrum of CD8+ T-cell-mediated antiviral effects and the factors that limit CTL capable of targeting the productive stages of the viral life-cycle. As these data argue against CD8+ T-cell-mediated cytolytic activity as the likely mechanism for clearance of productively infected cells, one is left to speculate that viral production by an infected cell is limited by viral cytopathic effects or by as yet unappreciated or unrecognized innate or humoral immune mechanisms that either kill infected cells or drastically down-modulate viral transcription [43],[44]. Refinement of present models of viral and cellular dynamics together with focused research to track the fate of infected cells in vivo may provide new insights into these cryptic antiviral mechanisms that could be exploited to treat and prevent HIV disease in the future.
Supporting Information
Zdroje
1. BorrowP
LewickiH
WeiX
HorwitzMS
PefferN
1997 Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 3 205 211
2. BorrowP
LewickiH
HahnBH
ShawGM
OldstoneMB
1994 Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68 6103 6110
3. MooreJP
CaoY
HoDD
KoupRA
1994 Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol 68 5142 5155
4. KoupRA
SafritJT
CaoY
AndrewsCA
McLeodG
1994 Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68 4650 4655
5. OggGS
JinX
BonhoefferS
DunbarPR
NowakMA
1998 Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279 2103 2106
6. SchmitzJE
KurodaMJ
SantraS
SassevilleVG
SimonMA
1999 Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283 857 860
7. JinX
BauerDE
TuttletonSE
LewinS
GettieA
1999 Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189 991 998
8. MatanoT
ShibataR
SiemonC
ConnorsM
LaneHC
1998 Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol 72 164 169
9. SchmitzJE
KurodaMJ
SantraS
SimonMA
LiftonMA
2003 Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J Virol 77 2165 2173
10. JohnstonMI
FauciAS
2007 An HIV vaccine–evolving concepts. N Engl J Med 356 2073 2081
11. SekalyRP
2008 The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med 205 7 12
12. CastroBA
HomsyJ
LennetteE
MurthyKK
EichbergJW
1992 HIV-1 expression in chimpanzees can be activated by CD8+ cell depletion or CMV infection. Clin Immunol Immunopathol 65 227 233
13. WeiX
GhoshSK
TaylorME
JonsonVA
EminiEA
1995 Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373 117 122
14. PerelsonAS
NeumannAU
MarkowitzM
LeonardJM
HoDD
1996 HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271 1582 1586
15. HoDD
NeumannAU
PerelsonAS
ChenW
LeonardJM
1995 Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373 123 126
16. LouieM
HoganC
Di MascioM
HurleyA
SimonV
2003 Determining the relative efficacy of highly active antiretroviral therapy. J Infect Dis 187 896 900
17. LeuteneggerCM
HigginsJ
MatthewsTB
TarantalAF
LuciwPA
2001 Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res Hum Retroviruses 17 243 251
18. GeorgeMD
ReayE
SankaranS
DandekarS
2005 Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol 79 2709 2719
19. VerhoevenD
SankaranS
SilveyM
DandekarS
2008 Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J Virol 82 4016 4027
20. FelsensteinJ
1993 Phylogenetic Inference Package (Phylip) 3.5 University of Washington
21. NeiM
GojoboriT
1986 Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3 418 426
22. NowakMA
LloydAL
VasquezGM
WiltroutTA
WahlLM
1997 Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol 71 7518 7525
23. SchmitzJE
JohnsonRP
McClureHM
MansonKH
WyandMS
2005 Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J Virol 79 8131 8141
24. KimEY
VeazeyRS
ZahnR
McEversKJ
BaumeisterSH
2008 Contribution of CD8+ T cells to containment of viral replication and emergence of mutations in Mamu-A*01-restricted epitopes in Simian immunodeficiency virus-infected rhesus monkeys. J Virol 82 5631 5635
25. JohnstonMI
FauciAS
2008 An HIV vaccine–challenges and prospects. N Engl J Med 359 888 890
26. WalkerCM
LevyJA
1989 A diffusable lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology 66 628
27. WalkerCM
MoodyDJ
StitesDP
LevyJA
1986 CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234 1563
28. CocchiF
DeVicoAL
Garzino-DemoA
AryaSK
GalloRC
1995 Identification of Rantes, MIP-1a, and MIP-1b as the major HIV-suppressive factors produced by CD8+ T-Cells. Science 270 1811 1815
29. SachaJB
ChungC
RakaszEG
SpencerSP
JonasAK
2007 Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol 178 2746 2754
30. KlattNR
ShudoE
OrtizAM
EngramJC
PaiardiniM
2010 CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog 6 e1000747 doi:10.1371/journal.ppat.1000747
31. OkoyeA
ParkH
RohankhedkarM
Coyne-JohnsonL
LumR
2009 Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med 206 1575 1588
32. KlenermanP
PhillipsRE
RinaldoCR
WahlLM
OggG
1996 Cytotoxic T lymphocytes and viral turnover in HIV type 1 infection. Proc Natl Acad Sci U S A 93 15323 15328
33. KilbyJM
LeeHY
HazelwoodJD
BansalA
BucyRP
2008 Treatment response in acute/early infection versus advanced AIDS: equivalent first and second phases of HIV RNA decline. Aids 22 957 962
34. OxeniusA
McLeanAR
FischerM
PriceDA
DawsonSJ
2002 Human immunodeficiency virus-specific CD8(+) T-cell responses do not predict viral growth and clearance rates during structured intermittent antiretroviral therapy. J Virol 76 10169 10176
35. GordonSN
DunhamRM
EngramJC
EstesJ
WangZ
2008 Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol 82 3725 3735
36. DunhamR
PagliardiniP
GordonS
SumpterB
EngramJ
2006 The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108 209 217
37. BarryAP
SilvestriG
SafritJT
SumpterB
KozyrN
2007 Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J Immunol 178 8002 8012
38. KlotmanME
KimS
BuchbinderA
DeRossiA
BaltimoreD
1991 Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc Natl Acad Sci USA 88 5011 5015
39. KimS-Y
ByrnR
GroopmanJ
BaltimoreD
1989 Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. Journal of Virology 63 3708 3713
40. CollinsKL
BaltimoreD
1999 HIV's evasion of the cellular immune response. Immunol Rev 168 65 74
41. CollinsKL
ChenBK
KalamsSA
WalkerBD
BaltimoreD
1998 HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391 397 401
42. KaiserP
JoosB
NiederostB
WeberR
GunthardHF
2007 Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol 81 9693 9706
43. HuberM
FischerM
MisselwitzB
ManriqueA
KusterH
2006 Complement lysis activity in autologous plasma is associated with lower viral loads during the acute phase of HIV-1 infection. PLoS Med 3 e441
44. AlterG
AltfeldM
2006 NK cell function in HIV-1 infection. Curr Mol Med 6 621 629
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



