-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: A population-based cohort study
In a population-based study, Marie-Louise Rasmussen and colleagues examine risk and recurrence of postpartum affective disorder in women with no prior psychiatric history in Denmark.
Published in the journal: . PLoS Med 14(9): e32767. doi:10.1371/journal.pmed.1002392
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002392Summary
In a population-based study, Marie-Louise Rasmussen and colleagues examine risk and recurrence of postpartum affective disorder in women with no prior psychiatric history in Denmark.
Introduction
Postpartum depression (PPD) is a nonpsychotic depressive episode occurring in the period following delivery of a child. Depending on, for example, the inclusion criteria and the quality of follow-up of women who have given birth, it is reported to affect 5%–15% of all women after childbirth [1,2], which makes it one of the most common postnatal complications of childbearing. Left untreated, the disorder can have long-term implications for both mother and child, including impairment of the child’s development [3–5] and increased risk of long-term maternal depression [6]. A number of different risk factors for PPD have been identified, of which the majority are antenatal, personal, and psychosocial factors [3,7–9]. However, such factors can account for at most a third of the variance in the diagnosis of PPD [10,11], which could indicate a genetic predisposition, as suggested in some studies [12].
Although evidence exists that there is significant heterogeneity in the timing and persistence of maternal depressive symptomatology [13], very few previous studies have distinguished between women with a prior history of psychiatric disease and women with no such history. This lack of differentiation might partly explain the divergence in findings of etiological studies of PPD and in the observed frequency of the disorder, and adds to the ongoing dispute as to whether PPD is a specific disease entity [14]. Additionally, there is a lack of population-based studies investigating the duration of treatment of PPD [15]. It is our assumption that the PPD phenotype among women with no prior psychiatric history is more homogeneous than the phenotype among women with prior psychiatric history. Thus, the main focus of this study was on women with no prior psychiatric history.
The purpose of this study was, by use of Danish national healthcare and population registers, to describe the risk of postpartum affective disorder (AD) among women with no prior psychiatric disorders, the recurrence risk, as well as the duration of treatment in this group.
Methods
In accordance with Danish law, the use of the register-based data in the study was approved by the Danish Data Protection Agency (no. 2008-54-0472). The study is reported as per STROBE guidelines (S1 STROBE Checklist). A detailed analysis plan was not written prior to the initiation of the project. However, based on the objectives of this study, we defined all basic analyses to be undertaken in meetings with all involved parties (epidemiologists, clinician, and statisticians) prior to the receipt of the registry data and before the start of the analyses. We did not depart from the analysis plan built during these meetings but added post hoc sensitivity analyses as presented. However, in response to review comments, postpartum antidepressant treatment was divided post hoc into postpartum medication and PPD hospital contact.
Study cohort
Using the Danish Civil Registration System, we established a cohort comprising all women born in Denmark who delivered their first live-born singleton child between 1 January 1996 and 31 December 2013 (n = 457,317 women). The Danish personal identification number permits complete follow-up of all persons living in Denmark and accurate linkage of individual-level information from Denmark’s many mandatory national population-based registers. The registries used for this study are described in detail in S1 Text. Women with antidepressant use (ATC: N06) registered in the Danish National Prescription Registry (DNPR) and women registered in the Psychiatric Central Research Register (PCRR) or the National Patient Registry (NPR) with mental illnesses (ICD-8 : 29, 30; ICD-10: F0–F9) any time prior to their first delivery were excluded from the cohort. Complete nationwide data on prescriptions were available in the DNPR starting in 1995, so cohort inclusion began 1 January 1996 in order to have information on antidepressant use in the year before delivery in those delivering in early 1996. Follow-up ended 31 December 2014 to include the postpartum period for women delivering in 2013.
Antidepressant treatment—In general and postpartum
In this study, we defined AD as use of antidepressant medication and/or hospital contact for depression (in - and outpatient). Episodes of postpartum AD were defined as episodes occurring within 6 months after delivery, and all other episodes were referred to as non-postpartum AD. Women with AD were identified based on information in the NPR, the PCRR, and the DNPR. Episodes in the DNPR were identified as women filling at least 1 prescription for antidepressant medication (ATC: N06). Episodes in the NPR and PCRR were identified as women having an in - or outpatient contact for a depressive episode, using main diagnoses only (ICD-8 : 2960, 2962, 2968, 2969, 2980, 3004, 3011; ICD-10: F320–F329). For simplicity, outpatients were also referred to as being admitted and discharged from hospitals, e.g., in the analysis of duration of treatment. In the analysis of the risk of postpartum AD and the duration of treatment, we did not discriminate as to whether the episodes were defined by use of medication or hospital contact. In the analysis of the recurrence of AD, we separately analyzed use of postpartum medication and PPD hospital contact after first birth. We interpreted the 2 measures as treatment for the same overall disorder—although they may represent different levels of severity of the disorder.
Initiation of treatment was defined as the date of first filled prescription for an antidepressant medication or first hospital admission date, whichever came first. Period of usage was estimated based on information in the DNPR on number of defined daily doses in the prescription. For refills of prescriptions, a gap of up to 3 months between the calculated last date of usage of a prescription and the dispensing date of the next prescription was permitted to allow for differences in drug intake and prescriber habits. If the gap between prescriptions exceeded 3 months, treatment was considered discontinued, and a new prescription after that was defined as a new incident episode. Likewise, a 3-month cutoff was applied after the discharge date of hospitalizations for defining a new incident episode.
Statistical analyses
Relative risks of postpartum AD according to parity, year of birth, and mother’s age were estimated by a log-linear binomial regression model—the estimates are mutually adjusted.
The proportion of primiparous women still in treatment by the number of months since the initiation of treatment was estimated by a Kaplan–Meier analysis. Only women with a postpartum episode after their first birth were included in this analysis. Women were included from the first prescription date or first admission date, whichever came first, and were followed until the first of the following events: discontinuation of treatment, second birth, death, emigration, or end of follow-up. This means that, for example, women giving birth in 2012 were followed until the end of 2014 (end of follow-up) unless any of the other above-mentioned events occurred prior to end of follow-up.
Rates of postpartum AD after second birth and rates of non-postpartum AD (after first or second birth) in women with and without a postpartum AD episode after their first birth were estimated as number of episodes divided by the number of person-years, calculated separately based on the number of years since latest childbirth. We used a log-linear Poisson regression model to estimate rate ratios (RRs) for non-postpartum AD in (1) women with a postpartum AD episode after first birth defined by antidepressant medication and (2) women with a postpartum AD episode after first birth defined by hospital contact compared to (3) women with no postpartum AD after first birth. We compared the rate of postpartum and non-postpartum AD in second-time mothers for these same 3 groups. Women with both postpartum antidepressant medication and PPD hospital contact after first birth were classified as PPD hospital contact cases at the time of the first event, regardless of chronological order of the medication prescription and the hospital contact. The RR analyses were adjusted for year of birth and mother’s age. Primiparous women with postpartum AD were followed for non-postpartum AD after end of treatment, whereas follow-up for primiparous women with no postpartum AD episode began 6 months after the birthdate of their firstborn. If women gave birth a second time, they changed status and contributed with person-years to the second-birth analyses. Follow-up for all women continued until the first of the following events: an AD episode, a psychiatric diagnosis other than depression, death, emigration, or end of follow-up. Thus, at any point in the study, no woman, regardless of parity, had any prior history of psychiatric disorders other than a possible postpartum AD after first birth.
Estimation in the supplementary analyses (S2 Text) was performed by the same means as in the main analyses.
Results
Risk of postpartum AD
Between 1 January 1996 and 31 December 2013, 457,317 women had a first live-born child. Of these, 273,195 women delivered a second child, and 78,556 women had 3 or more children during the follow-up period. The proportion of women with a history of postpartum AD and the proportion of women with a postpartum AD episode are given in Table 1. Overall, 0.6% (n = 4,550) of childbirths were followed by a postpartum AD episode; 2,389 of these episodes occurred in primiparous women. The proportion of postpartum AD episodes increased markedly over the study period, and the risk of an episode was significantly higher among young mothers: the relative risk for mothers <25 years versus mothers 29–31 years was 1.8 (95% CI 1.6–2.0).
Tab. 1. Risk of postpartum affective disorder (AD)—Distribution of number of births, women with prior history of postpartum AD, and postpartum AD episodes according to parity, year of birth, and age. 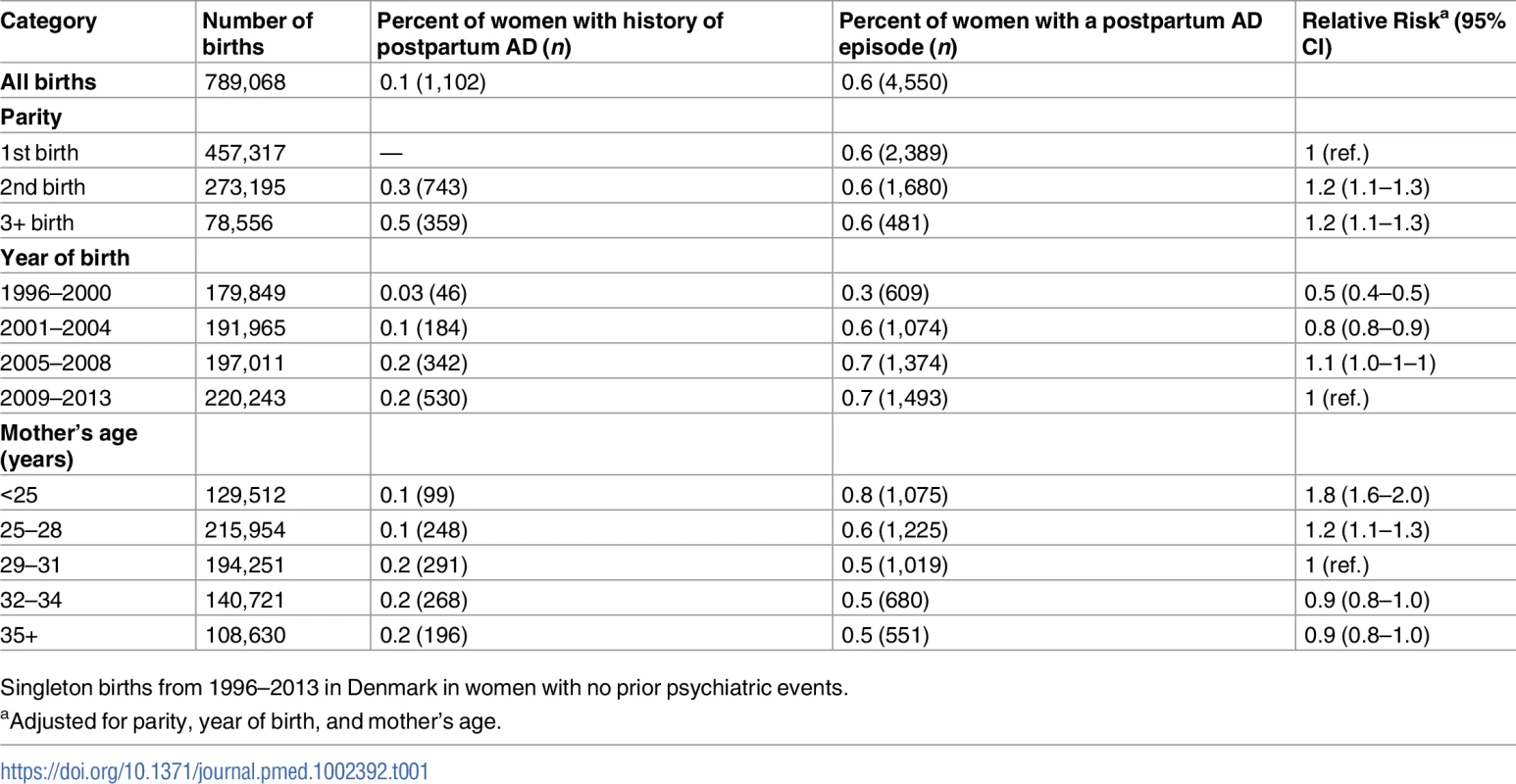
Singleton births from 1996–2013 in Denmark in women with no prior psychiatric events. Duration of treatment
Fig 1 shows the estimated proportion of women still in treatment for a postpartum episode by the number of months since the initiation of treatment among primiparous women (n = 2,389). One year after the first dispensing of antidepressants or hospital contact, 27.9% of the women were still in treatment; after 4 years, 5.4% remained in treatment. A relatively large number of women filled only 1 prescription of antidepressants or were admitted only a short period of time, causing a large decrease in treated women (23%) in the first month. In a sensitivity analysis that included only women filling at least 2 prescriptions or being hospitalized, this decrease in treated women following the first treatment period was 19%.
Fig. 1. The estimated proportion of women in antidepressant treatment by number of months since the initiation of treatment for a postpartum episode of affective disorder (AD). 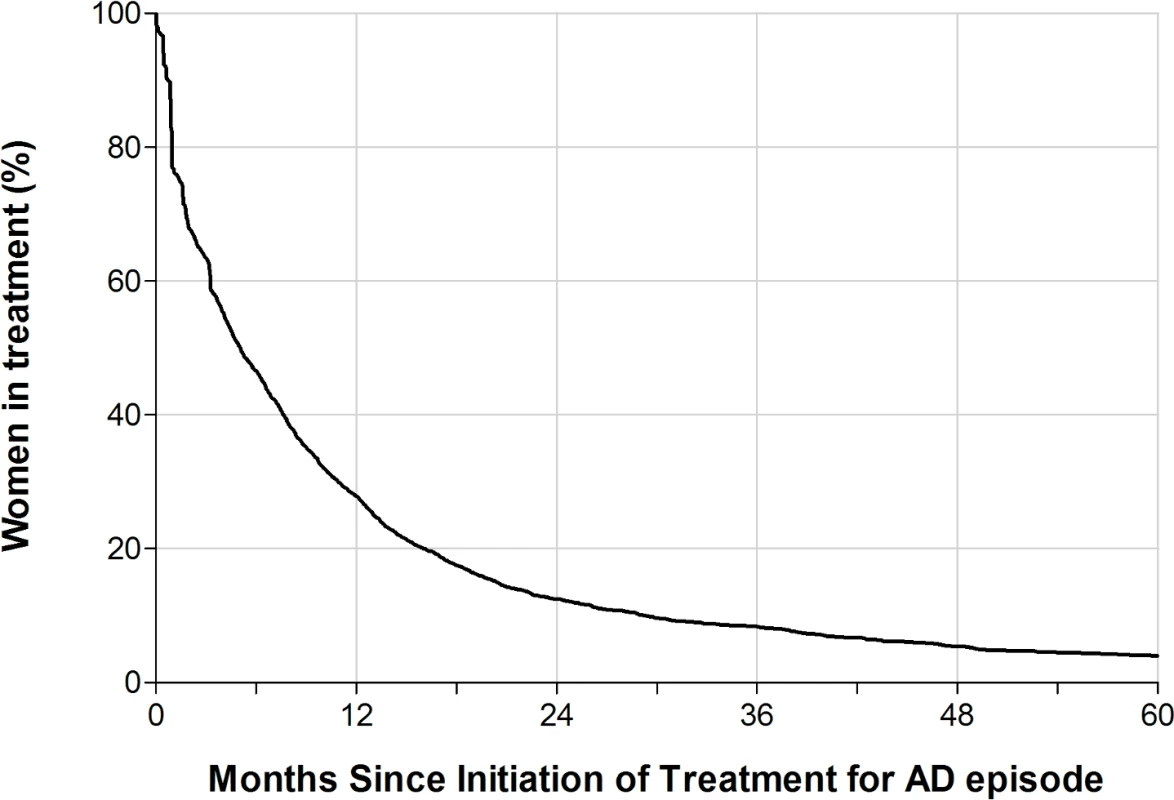
Primiparous Danish women with a postpartum AD, 1996–2013, with no prior psychiatric disorders. Recurrence risk and risk of non-postpartum AD
Fig 2 and Table 2 show the rate of non-postpartum AD in women up to 6 years following first birth, depending on postpartum AD history (postpartum antidepressant medication, PPD hospital contact, or no postpartum AD). Table 2 furthermore shows the RR of postpartum AD after second birth and of non-postpartum AD after first and second birth, depending on postpartum AD history.
Fig. 2. Rates of non-postpartum and postpartum affective disorder (AD), depending on postpartum AD history. 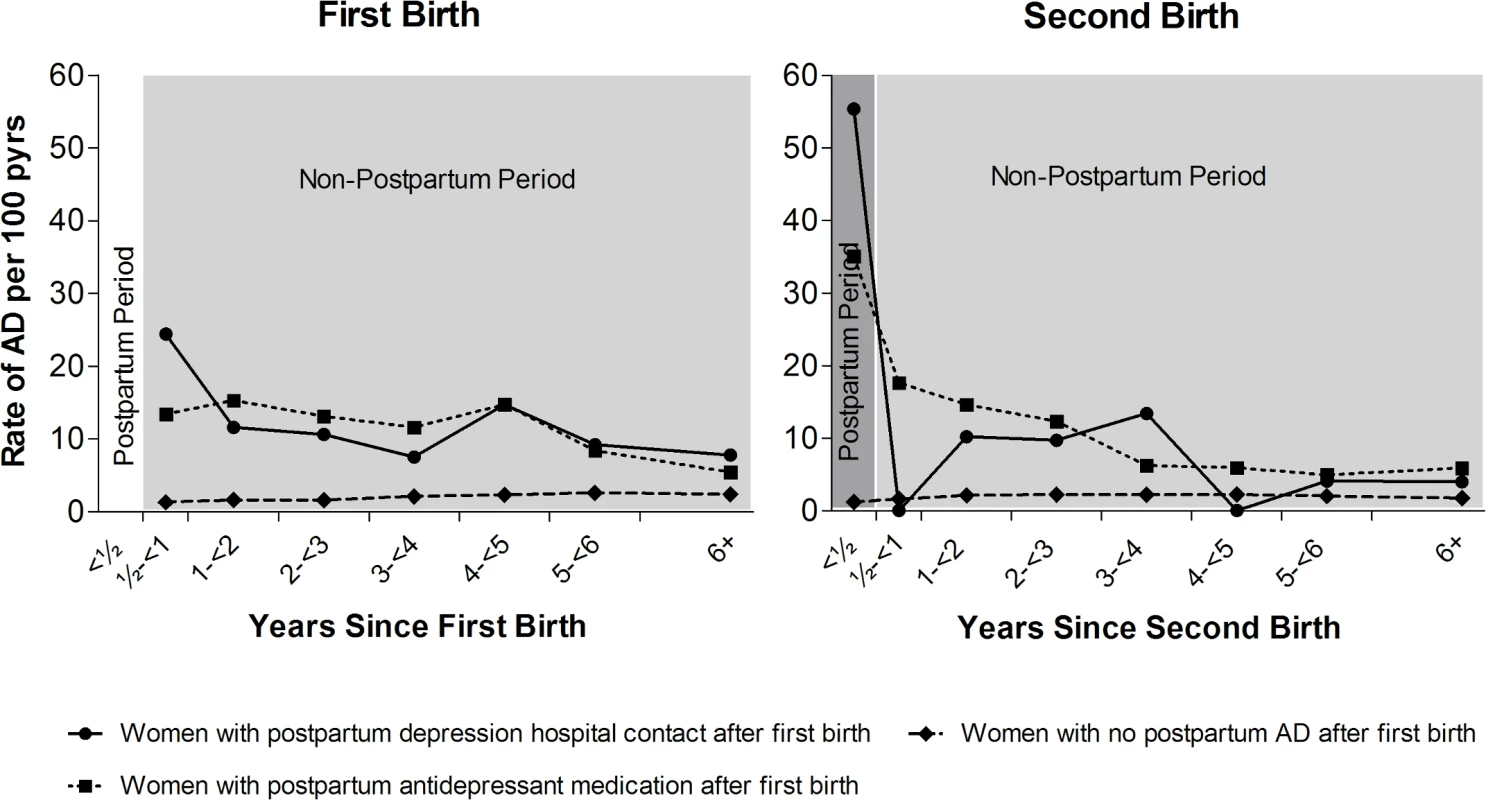
Left: First-birth rates of non-postpartum (light grey) AD, depending on postpartum AD history. Right: Second-birth rates of postpartum (dark grey) and non-postpartum (after first 6 months, light grey) AD by number of years since second birth and the women’s history of postpartum AD after first birth. Danish women, 1996–2013, with no psychiatric disorders prior to first birth. pyrs, person-years. Tab. 2. Rates and rate ratios (RRs) of non-postpartum and postpartum affective disorder (AD) after first and second birth depending on postpartum AD status after first birth. 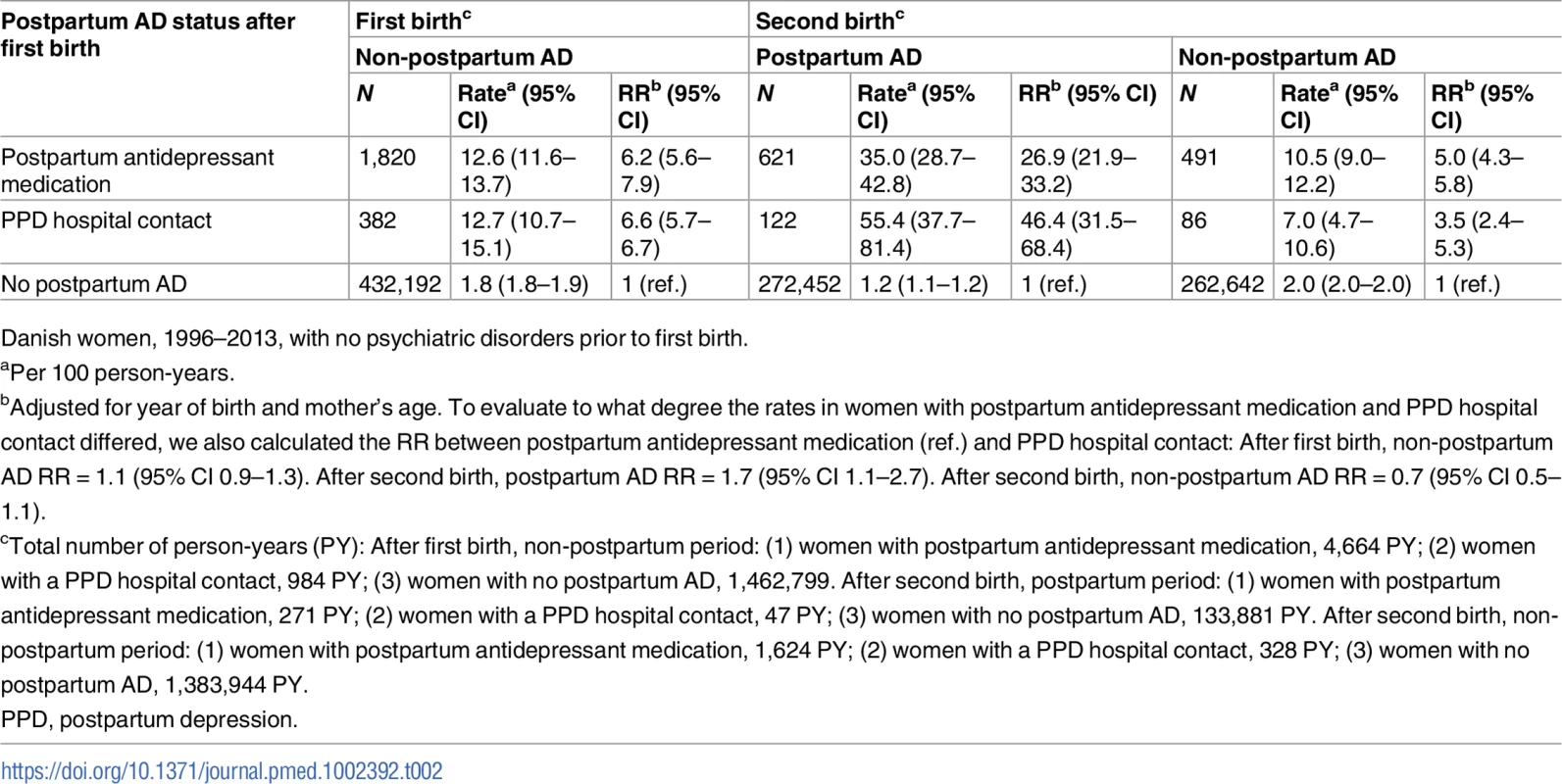
Danish women, 1996–2013, with no psychiatric disorders prior to first birth. In total, 434,394 women were eligible for follow-up for non-postpartum AD, of whom 2,202 had had a postpartum AD episode. The rate of a new AD episode did not depend on the type of postpartum AD history, i.e., among women with postpartum antidepressant medication history (n = 1,820), the rate of new AD episodes was 12.6 per 100 person-years, and for women with a PPD hospital contact (n = 382), the rate was 12.7 per 100 person-years. For women with no postpartum AD history, the corresponding rate was 1.8 per 100 person-years. Adjusted for women’s age and year of birth, and compared to women with no postpartum AD history, women with a postpartum antidepressant medication history and women with a PPD hospital contact had a 6.2 and 6.6 times higher rate of new AD episodes, respectively (see Table 2). The rate of subsequent AD was particularly high in the initial years after the first birth (women with postpartum antidepressant medication, 0.5 to <2 years: 14.6 per 100 person-years; women with PPD hospital contact, 0.5 to <2 years: 16.5 per 100 person-years). The rate decreased with the number of years since the episode (women with postpartum antidepressant medication, 6+ years: 5.4 per 100 person-years; women with PPD hospital contact, 6+ years: 7.8 per 100 person-years), whereas among women with no postpartum AD history, the rate was relatively constant.
Fig 2 and Table 2 also show the rate of postpartum and non-postpartum AD among women giving birth for the second time, dependent on whether the woman was treated for a postpartum AD episode after her first birth (postpartum antidepressant medication, PPD hospital contact, or no postpartum AD). Overall, 273,195 women had a second birth; 743 of these women had had a postpartum episode after their first birth (Table 1)—122 with a PPD hospital contact and 621 with postpartum antidepressant medication. Twenty-one percent of women with a PPD hospital contact after first birth and 15% of women with postpartum antidepressant medication after first birth experienced a recurrent postpartum episode. Out of the 272,452 women with no previous AD, 1,680 (0.6%) women had a first-time episode of postpartum AD. The rate of postpartum AD after the second birth was 1.7 times higher (95% CI 1.1–2.7) among women with a PPD hospital contact after first birth (55.4 per 100 person-years) than among women with postpartum antidepressant medication after first birth (35.0 per 100 person-years). The rate of postpartum AD after second birth for women with no history of postpartum AD was 1.2 per 100 person-years. The rate of AD in the non-postpartum period after the second birth was, except for the period 0.5 to <1 year, similar for all women with postpartum AD after first birth, regardless of treatment regime. After adjusting for year of birth and mother’s age, women with postpartum antidepressant medication after their first birth had a 26.9 times higher rate (95% CI 21.9–33.2) of recurrent postpartum AD after their second birth and a 5.0 times higher rate of AD in general (95% CI 4.3–5.8) in the years following the second birth, compared to women with no postpartum AD history. In comparison, women with a PPD hospital contact after first birth had a 46.4 times higher rate (95% CI 31.5–68.4) of recurrent postpartum AD and a 3.5 times higher rate of AD in general (95% CI 2.4–5.3).
Supplementary analyses
In additional analyses, we explored how the main results varied by age and year of giving birth (see S2 Text). We found that young (<25 years) primiparous mothers seemed be characterized by a marginally faster treatment period and a higher rate of non-postpartum AD after first birth than primiparous mothers ≥25 years. A relatively smaller proportion of women having a postpartum AD episode in the beginning of the study period (1996–2000) were still in treatment a number of years after the episode compared to women having a postpartum AD episode in the latter part of the study period (2009–2013) (1 year after initiation: 21% versus 32%).
We furthermore tested the sensitivity of the AD definition by varying the length of the postpartum period and the number of prescriptions required to define an AD episode (S2 Text). While altering the above measures obviously changed the estimated incidence of postpartum AD, it did not change the conclusions regarding duration of treatment and recurrence risk. To further examine our outcome definition of AD as a joint measure encompassing both antidepressant medication and hospital contacts, we conducted a number of subanalyses dividing these 2 groups. Overall, dividing the outcome in 3 groups (the main analyses used 2 groups) did not change the results markedly.
We also analyzed the conversion rate of postpartum AD to bipolar AD. With follow-up up to 19 years, we showed that 3.3% of the women with a postpartum AD episode after first birth converted to bipolar AD. Further details on the additional analyses can be found in S2 Text.
The main focus of this study is on women with no prior psychiatric history. However, in order to compare the proportion of women with AD in the postpartum period with existing literature, we also calculated the proportion among “all births,” i.e., the same birth cohort (singleton, first birth 1996–2013) as the main analyses but with no restriction on previous history of mental illness. Among the 920,965 births in the “all births” cohort (i.e., 789,068 births to women with no previous mental illness and 131,897 births to women with previous mental illness), we found 22,251 (2.4%) AD episodes in the postpartum period.
Discussion
In this nationwide, population-based cohort study, 0.6% of childbirths among women with no prior history of psychiatric disease resulted in a postpartum AD, defined as a prescription fill for antidepressant medication and/or hospital contact for depression during the first 6 months after birth. However, less than 1/3 of the women were still receiving treatment 1 year after treatment initiation. Compared to women with no episode of postpartum AD after their first childbirth, women with postpartum antidepressant medication and PPD hospital contact, respectively, had a 6.2 and 6.6 times increased risk of a non-postpartum AD in the years following first childbirth and a 27 and 46 times higher recurrence rate of postpartum AD following a second birth.
To our knowledge, no other study has specifically addressed the duration of antidepressant use in the postpartum period. We found that a substantial proportion of women filled only 1 prescription for antidepressants, and that less than 28% remained in treatment for 1 year or more. This could reflect that symptoms subsided faster than expected (although a maintenance therapy period of at least 6 months is recommended) [16], or that women stopped treatment due to adverse effects of the medicine or out of concern for the child if they were breastfeeding. If the latter is the case and women dropped out of treatment, we would expect women to relapse and treatment to be restarted for months or years following childbirth. In contrast, the rate of treatment diminishes with time for those who received antidepressant treatment after their first childbirth (Fig 2). Therefore, our finding most likely reflects the transitory nature of PPD. This interpretation is supported by a study that investigated different trajectories of perinatal depressive symptomatology and found 5 different classes, including a “postpartum class,” for which depressive symptomatology resolved after 12–24 months postpartum [13].
Women with a history of PPD have an increased risk of experiencing a recurrence in connection with a subsequent delivery [6,17–19]. The population-based nature of our design allowed us to quantify the recurrence risk as 15% for women with postpartum antidepressant medication after first birth and 21% for women with a PPD hospital contact after first birth, or, in other terms, 27 and 46 times higher, respectively, than for women who did not have a history of postpartum AD after first birth. Thus, women with a PPD hospital contact had an almost twice as high AD recurrence rate compared to women with postpartum antidepressant medication. To the extent that PPD hospital contact is an indicator of a more severe PPD episode compared to medication treatment, the severity of the previous episode seems to significantly influence a women’s risk of a recurrent postpartum AD episode. This finding could perhaps reflect a more proactive treatment strategy among physicians for women with a previous severe episode of PPD (hospital contact), or simply that the more severe the previous PPD episode was, the higher the risk of a recurrent postpartum AD episode—a common predisposing factor underlying the risk of developing PPD in a dose–response relationship.
In the “all births” cohort (not restricting on previous mental history), we found that 2.4% of women had a postpartum AD episode; we considered these likely to have experienced PPD. There is evidence that the majority of PPD episodes are never diagnosed and treated [20,21]. An American study found that only 15% of postpartum women who, according to interview, had experienced a mood disorder during the first year after childbirth had sought help, had been prescribed medications, or had had hospital contact because of their problem [22]. If generally applicable, a postpartum AD risk of 2.4% as measured in our study would correspond to an underlying risk of 16% of PPD, which is consistent with the 10%–15% reported in studies on PPD that have relied primarily on self-reports [1,21,23,24]. A systematic study of 6,790 Danish women who had given birth showed that only 6% had PPD according to the Edinburg Postnatal Depression Scale, so it cannot be ruled out that the prevalence of PPD is lower in Denmark and similar countries with a developed welfare system than in other countries [24,25]. Using the same assumptions for women with no previous mental history, the observed proportion of women receiving postpartum AD treatment of 0.6% corresponds to an underlying risk of 4% of PPD. Episodes captured by treatment status (i.e., medicine use and/or hospital contact) without doubt constitute a group of women likely to be at the more severe end of the PPD spectrum.
Few studies have assessed PPD rate by methods comparable to the present study. Two studies based on the large prospective Danish National Birth Cohort reported a PPD prevalence of 1.6% and 1.8%, respectively, based on prescriptions of antidepressant medication in the first year postpartum [26,27], whereas a Danish population-based cohort study of antidepressant drug use from 12 months prior to childbirth to 12 months postpartum reported a prevalence of 3.2% [28]. However, none of the aforementioned studies were restricted to women free of psychiatric disease prior to enrolment, and this may at least in part, along with the differences in the length of the postpartum period, explain the lower PPD incidence in our study. A population-based study from Finland on hospitalization only for a postpartum period of 6 weeks showed a prevalence of PPD of 0.1% among women with no history of depression [29]. Interestingly, a study from the UK examining the recurrence of PPD showed that for women with a de novo PPD episode, i.e., no previous depressive events, the risk of further episodes of PPD, but not non-postpartum depression, was increased compared to women for whom the PPD episode was a recurrence of depression [14].
Major strengths of this study include its population-based prospective design, its large size, and the utilization of high-quality Danish national registers. Healthcare in Denmark is free of charge, which ensures that all residents, irrespective of economic status, receive appropriate treatment. The information used was mandatorily reported to the national registries and did not rely on self-report. This allowed us to estimate absolute AD rates with little bias. To our knowledge, this is the first population-based cohort study to present risk estimates for PPD in a population initially free of psychiatric problems, and to provide figures for the duration of treatment and rate of recurrence after a second birth.
While some of the strengths of this study are based on the use of the national registers, so are some of the limitations. The use of register data on antidepressant prescriptions to define AD implies that the women in this study do not necessarily fulfill the DSM-IV diagnostic criteria for PPD, as this type of medication is often prescribed for indications other than depression, such as anxiety and obsessive-compulsive disorder, which is why we refer to these women as being treated for an AD. However, results from a subanalysis including only hospital admissions showed that the overall conclusions regarding recurrence risk were not markedly different for this subgroup (see S2 Text). Psychiatric disorders in the postpartum period, especially in the early postpartum period, may be a marker of possible underlying bipolarity [30]. In our cohort of women with no psychiatric episodes prior to postpartum AD and with a 19-year follow-up period, only 3.3% of postpartum AD episodes later converted to bipolar illness (S2 Text).
Different assumptions made in this study are debatable; we have investigated these further in supplementary analyses (S2 Text). The length of the postpartum period is debatable [31]; we chose to employ a 6-month postpartum period [32] in the main analyses, and accompany these with subanalyses using a 3-month and 12-month postpartum period. Further, we also conducted separate analyses for PPD hospital contacts only, use of antidepressant medication only, and women who filled at least 2 prescriptions of antidepressants rather than only 1. The supplementary analyses did not change the overall conclusions regarding the duration of treatment and recurrence risk (S2 Text).
The RRs of recurrence in Table 2 are all adjusted for year of birth and mother’s age, and in Table 1 also for parity. We did not have information on personal traits or sociodemographic variables. Thus, it is possible that some of the RRs for recurrence might reflect personal propensity towards AD.
To conclude, in this study, an antidepressant treatment or depression diagnosis through hospital contact within 6 months after childbirth was observed in 0.6% of childbirths among women with no previous psychiatric history. The estimated recurrence risk of postpartum AD was 15% for women with postpartum antidepressant medication after first birth and 21% for women with a PPD hospital contact after first birth, and the observed risk of treatment for depression remained increased for several years. However, treatment duration for the majority of women in the study was short. These population-based figures provide valuable guidance to physicians treating women with PPD. The study documents the existence of a group of patients who experience elevated rates of subsequent depression and PPD following an initial postpartum AD episode. It underlines the seriousness of single initial episodes and highlights the necessity of both primary and secondary preventive measures, of which several exist [33,34].
Supporting Information
Zdroje
1. O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psych. 1996;8(1):37–54.
2. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83.
3. Cooper PJ, Murray L. Postnatal depression. BMJ. 1998 20;316(7148):1884–6. 9632411
4. Reck C, Hunt A, Fuchs T, Weiss R, Noon A, Moehler E, et al. Interactive regulation of affect in postpartum depressed mothers and their infants: an overview. Psychopathology. 2004;37(6):272–80. doi: 10.1159/000081983 15539778
5. Wolkind S. Mothers’ depression and their children’s attendance at medical facilities. J Psychosom Res. 1985;29(6):579–82. 4087225
6. Philipps LH, O’Hara MW. Prospective study of postpartum depression: 4 1/2-year follow-up of women and children. J Abnorm Psychol. 1991;100(2):151–5. 2040765
7. O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. 2005273
8. Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–95. doi: 10.1016/j.genhosppsych.2004.02.006 15234824
9. Harlow BL, Vitonis AF, Sparen P, Cnattingius S, Joffe H, Hultman CM. Incidence of hospitalization for postpartum psychotic and bipolar episodes in women with and without prior prepregnancy or prenatal psychiatric hospitalizations. Arch Gen Psychiatry. 2007;64(1):42–8. doi: 10.1001/archpsyc.64.1.42 17199053
10. Gotlib IH, Whiffen VE, Wallace PM, Mount JH. Prospective investigation of postpartum depression: factors involved in onset and recovery. J Abnorm Psychol. 1991;100(2):122–32. 2040762
11. O’Hara MW, Neunaber DJ, Zekoski EM. Prospective study of postpartum depression: prevalence, course, and predictive factors. J Abnorm Psychol. 1984;93(2):158–71. 6725749
12. Treloar SA, Martin NG, Bucholz KK, Madden PA, Heath AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med. 1999;29(3):645–54. 10405086
13. Mora PA, Bennett IM, Elo IT, Mathew L, Coyne JC, Culhane JF. Distinct trajectories of perinatal depressive symptomatology: evidence from growth mixture modeling. Am J Epidemiol. 2009 1;169(1):24–32. doi: 10.1093/aje/kwn283 19001135
14. Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166(2):191–5. 7728362
15. O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65(12):1258–69. doi: 10.1002/jclp.20644 19827112
16. Danish National Board of Health. Reference program for unipolar depression among adults. Copenhagen: Danish National Board of Health; 2007.
17. Cooper PJ, Campbell EA, Day A, Kennerley H, Bond A. Non-psychotic psychiatric disorder after childbirth. A prospective study of prevalence, incidence, course and nature. Br J Psychiatry. 1988;152 : 799–806. 3167466
18. Wisner KL, Perel JM, Peindl KS, Hanusa BH. Timing of depression recurrence in the first year after birth. J Affect Disord. 2004;78(3):249–52. doi: 10.1016/S0165-0327(02)00305-1 15013250
19. Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161(7):1290–2. doi: 10.1176/appi.ajp.161.7.1290 15229064
20. Halbreich U. Postpartum disorders: multiple interacting underlying mechanisms and risk factors. J Affect Disord. 2005;88(1):1–7. doi: 10.1016/j.jad.2005.05.002 15996747
21. Bågedahl-Strindlund M, Börjesson KM. Postnatal depression: a hidden illness. Acta Psychiatr Scand. 1998;98(4):272–5. 9821447
22. Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805–15. doi: 10.1001/archpsyc.65.7.805 18606953
23. Banti S, Mauri M, Oppo A, Borri C, Rambelli C, Ramacciotti D, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the perinatal depression-research & screening unit study. Compr Psychiatry. 2011;52(4):343–51. doi: 10.1016/j.comppsych.2010.08.003 21683171
24. Nielsen Forman D, Videbech P, Hedegaard M, Dalby Salvig J, Secher NJ. Postpartum depression: identification of women at risk. BJOG. 2000;107(10):1210–7. 11028570
25. Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low - and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012;90 : 139G–49G. doi: 10.2471/BLT.11.091850 22423165
26. Strøm M, Mortensen EL, Halldorson TI, Osterdal ML, Olsen SF. Leisure-time physical activity in pregnancy and risk of postpartum depression: a prospective study in a large national birth cohort. J Clin Psychiatry. 2009;70 : 1707–14. doi: 10.4088/JCP.09m05012blu 20141710
27. Strøm M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. Am J Clin Nutr. 2009;90 : 149–55. doi: 10.3945/ajcn.2009.27552 19474139
28. Munk-Olsen T, Gasse C, Laursen TM. Prevalence of antidepressant use and contacts with psychiatrists and psychologists in pregnant and postpartum women. Acta Psychiatr Scand. 2011 25;125(4):318–24. doi: 10.1111/j.1600-0447.2011.01784.x 22118213
29. Räisänen S, Lehto SM, Nielsen HS, Gissler M, Kramer MR, Heinonen S. Fear of childbirth predicts postpartum depression: a population-based analysis of 511 422 singleton births in Finland. BMJ Open. 2013;3:e004047. doi: 10.1136/bmjopen-2013-004047 24293208
30. Munk-Olsen T, Laursen TM, Meltzer-Brody S, Mortensen PB, Jones I. Psychiatric disorders with postpartum onset: possible early manifestations of bipolar affective disorders. Arch Gen Psychiatry. 2011 5;69(4):428–34. doi: 10.1001/archgenpsychiatry.2011.157 22147807
31. O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):3–12. doi: 10.1016/j.bpobgyn.2013.09.002 24140480
32. Miller LJ. Postpartum depression. JAMA. 2002;287 : 762–5. 11851544
33. Dennis C-L, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013;2:CD001134.
34. di Scalea TL, Wisner KL. Pharmacotherapy of postpartum depression. Expert Opin Pharmacother. 2009;10 : 2593–607. doi: 10.1517/14656560903277202 19874247
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis
- Preterm birth prevention—Time to PROGRESS beyond progesterone
- Women’s and men’s reports of past-year prevalence of intimate partner violence and rape and women’s risk factors for intimate partner violence: A multicountry cross-sectional study in Asia and the Pacific
- Global services and support for children with developmental delays and disabilities: Bridging research and policy gaps
- Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: A population-based cohort study
- Chronic disease concordance within Indian households: A cross-sectional study
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Sustained effectiveness and cost-effectiveness of the Healthy Activity Programme, a brief psychological treatment for depression delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: Results of the Compass pilot randomised trial
- Keeping it real: A journal editor in clinic
- Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis
- HbA1c for type 2 diabetes diagnosis in Africans and African Americans: Personalized medicine NOW!
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Keeping it real: A journal editor in clinic
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



