-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Chronic disease concordance within Indian households: A cross-sectional study
In a cross-sectional analysis, Shivani Patel and colleagues estimate the extent of chronic disease concordance within Indian households.
Published in the journal: . PLoS Med 14(9): e32767. doi:10.1371/journal.pmed.1002395
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002395Summary
In a cross-sectional analysis, Shivani Patel and colleagues estimate the extent of chronic disease concordance within Indian households.
Introduction
Chronic conditions are now the biggest contributor to disability-adjusted life years across the globe [1], and morbidity due to these conditions has increased at a faster rate in South Asia as compared to the rest of the world over the past 20 years [2]. Alongside these major epidemiologic changes, the extended family system—in which relatives beyond the nuclear family reside with one another—remains salient in India: 50% of children reside in households with adults in addition to their parents [3], and 77% of the elderly reside with their married adult children [4]. Indians, therefore, from cradle to grave are likely to share a household environment and health-promoting resources with family members and also be exposed to one another’s lifestyle practices (e.g., tobacco use, diet). Genetically related household members—such as parents and children—may additionally share a similar hereditary predisposition to disease. Yet, most epidemiologic studies and public health interventions in India targeting chronic conditions currently focus on individuals or occasionally the community [5] and largely ignore the family unit despite its potential importance in understanding risk and designing sustainable interventions.
Indeed, the literature suggests the promise of reorienting the focus of chronic disease research from the individual to the family. Prior systematic reviews drawing largely on populations residing in high-income countries (HICs) demonstrate the concordance of cardiovascular risk factors [6,7] and mental health and health behaviors [7] among spousal dyads. Concordance of cardiometabolic conditions among parent-child (e.g., [8,9]) and sibling dyads (e.g., [9]) has also been observed in studies conducted in HICs. A small but growing body of literature in Asian countries has examined and found concordance of cardiometabolic conditions among spousal [10–12], parent-child [11–13], and sibling [12,14] dyads and concordance of health behaviors among spousal dyads [15].
Genetic, environmental, and interpersonal mechanisms are 3 types of highly plausible drivers of familial concordance of disease implicitly or explicitly considered by prior studies. Understanding the extent of genetic predisposition to certain disease conditions has been the goal of many family-based studies [8,11], although genetic explanations of disease concordance largely apply to parent-child and sibling dyads. Environmental factors can include shared household socioeconomic resources important for health [7,16], a common household diet, and the extrahousehold shared community milieu (e.g., built environment and cultural norms around physical activity). Finally, interpersonal influences include modeling of lifestyle factors such as physical activity, diet, and smoking [7,17,18]; “affective contagion” in which the moods of those around us influence our own [7]; and the stress of living with and caring for someone with a chronic condition [19–22]. In addition to these 3 mechanisms, assortative mating (largely applicable to spousal pairs) or other self-selection processes into households/families may impact the concordance of chronic disease within families.
We seek to build upon the existing literature to address 2 unresolved but important issues regarding chronic disease concordance in households. First, we are aware of no studies that have gone beyond the spousal, parent-child, and sibling pairs that comprise the nuclear family to investigate associations of chronic disease status among all household members. Most of the hypothesized pathways linking chronic conditions within families would also apply to individuals beyond the nuclear family who reside in the same household. Second, we are aware of no studies that have investigated the correspondence of different chronic conditions among household members (e.g., husband’s diabetes status and wife’s common mental disorder status). Because many chronic conditions have common behavioral and psychosocial risk factors, shared environmental or interpersonal factors that lead to the development of a specific chronic condition in 1 household member may lead to the development of another chronic condition in a coresiding household member. For example, diabetes, hypertension, obesity, and high cholesterol are cardiometabolic conditions that are impacted by physical activity and dietary intake [23], and much evidence links diabetes and depression [24]. Ignoring the correspondence between chronic conditions may underestimate the degree of household aggregation of disease. Understanding associations of shared and differing chronic conditions among all coresiding adults in households may shed light on new approaches to identify and treat chronic illness in India and other low - and middle-income countries (LMICs) where extended family households are prominent [25].
The extent to which the household as a unit may be effectively leveraged for mechanistic studies of prevention and interventions targeting chronic conditions in India will depend on whether there is indeed concordance of the same chronic conditions or correspondence of differing chronic conditions within household members. The overarching goal of this study was to test the hypothesis that living with any household member who has a chronic condition—diabetes, common mental disorder, hypertension, obesity, and/or high cholesterol—raises the risk of developing the same or another chronic condition. To explore this hypothesis, we conducted an analysis to examine whether living with someone with a chronic condition relates to one’s own chronic condition status in coresiding adults in households across 4 geographically and socioculturally diverse districts in India. In addition, we examined these associations among dyads of parents and their adult children and of spouses living in the same household.
Methods
Data source
We conducted a cross-sectional observational analysis of the baseline survey and laboratory data from the Diet and Lifestyle Interventions for Hypertension Risk Reduction through Anganwadi Workers and Accredited Social Health Activists study (DISHA study) [26]. DISHA is a community-based cluster randomized trial designed to test the effectiveness of a community health worker-led lifestyle behavior change on hypertension reduction. The baseline study was conducted in 2013 and 2014 to measure risk factors in 4 regionally and socioeconomically diverse districts located in Madhya Pradesh, Gujarat, Tamil Nadu, and Himachal Pradesh that were selected for the initial phase of the intervention. Participants were selected using a multistage cluster sampling design stratified by district. Dhar District, Madhya Pradesh (central India), is home to a predominantly indigenous (Adivasi) population and has poor road connectivity. Junagadh District, Gujarat (western India), is a rural plains setting, while the Mashobra District, Himachal Pradesh (northern India), is a rural hilly setting consisting of sparsely populated villages. Finally, the Puducherry, a union territory bordering Tamil Nadu (southern India), is an urban and coastal setting with relatively better public health infrastructure. The primary sampling units were randomly selected villages within the districts (9–12 villages per site; a total of 45 villages in the study), from which 120–150 households were randomly selected. At the household level, all adults over the age of 18 years were invited to participate in the survey. We exploited this feature of the sampling design to identify adults residing in the same household.
Of the 11,751 participants linkable to the household demographic roster, 10,703 participants had at least 1 coresiding household member enrolled in the study. Of participants with coresiding household members, 3,181 were excluded because of missing data on 1 or more outcomes. Thus, a total of 7,522 participants residing in 2,574 households with complete covariates and at least 2 sampled adults per household were analyzed in the primary analysis. We additionally examined associations of interest among adults with coresiding parents (1,660 dyads in 1,199 households) and spouses (1,598 dyads in 1,598 households). Dyads were identified through each participant’s relationship with the household head. The analysis of dyads was restricted to pairs of participants who were unambiguously identifiable as parent-adult child pairs or spouses through the participants’ relationship to the household head.
The study obtained ethics approval from the Centre for Chronic Disease Control’s ethics committee (#IRB00006330), as well as ethics committees of participating sites. Participants provided written informed consent prior to being surveyed and assessed. This study is reported as per STROBE guidelines (S1 Text).
Chronic conditions
We analyzed 5 prevalent chronic conditions: diabetes (prior diagnosis by a physician, fasting plasma glucose ≥ 126 mg/dL, or taking medication [27]), common mental disorder (i.e., depressive and anxiety disorders, measured here using the General Health Questionnaire score ≥ 12 [28,29]), hypertension (prior diagnosis by a physician, blood pressure ≥ 140/90 mmHg, or taking medication [30]), obesity (body mass index ≥ 30 kg/m2 [31]), and high cholesterol (prior diagnosis by a physician, total blood cholesterol ≥ 240 mg/dL, or taking medication [32]). We also created a composite binary variable indicating the presence of at least 1 of the 5 chronic conditions.
Data collection took place at the participant’s home. Height was measured using a stadiometer with accuracy of 2 mm (Seca), weight was measured using a digital weighing scale with accuracy of 100 gm (Seca), and systolic and diastolic blood pressure was measured using an electronic blood pressure monitor (OMRON 7080). A 5-ml fasting blood sample was collected from participants reporting at least 8 hours of fasting. The sample was centrifuged in the field, and the resulting serum and plasma samples were then transported to a central laboratory in New Delhi at the Indian Council of Medical Research for biochemical analysis and storage. Fasting plasma glucose was assessed using the Enzymatic Colorimetric Assay method. The General Health Questionnaire, previously validated for detecting common mental disorders in the Indian setting [28,29], was translated into the local language.
Sociodemographic covariates
Sociodemographic and behavioral data were collected through standard survey tools. For more details, please see the DISHA methods paper [26]. Individual age (continuously specified in years), sex (binary), education (years of schooling and college), marital status (married or unmarried), and family religion (Hindu or other) were included in the analysis as correlates of chronic conditions.
Statistical analysis
The development of the statistical analysis plan is described in S2 Text. We first constructed a set of indicator variables for each participant describing whether any other individual (excluding self) in the household had a given chronic condition. We next estimated 3 sets of logistic regression models that included differing groups of participants defined by the type of relationship among household members. Each set of logistic regression models estimated the relative odds of having any chronic condition for individuals living with a household member with any chronic condition relative to individuals who were not living with a household member with a chronic condition (i.e., the odds ratio of any chronic condition associated with living with someone who has any chronic condition). In addition, we estimated the relative odds of a given chronic condition associated with living with a household member with that same chronic condition (chronic condition concordance; e.g., the odds ratio of diabetes that is associated with living with someone who has diabetes) and living with a household member with a different chronic condition (chronic condition correspondence; e.g., the odds ratio of diabetes that is associated with living with someone who has a common mental disorder).
The first set of models included data from all available household members aged 18 years and older and was agnostic to the type of relationship between the index participant and the coresiding household members. To examine whether associations were observed across all study sites, we also estimated a set of models with an interaction term between the exposure condition and the study site. We tested the statistical significance of the interaction term using generalized score tests for Type III contrasts.
A second set of models examined associations among adult children with coresiding parents. For this analysis, the exposure was the presence of a given chronic condition in either parent for whom we had data. A third set of models examined associations among spousal dyads. In the spousal analysis, the wife’s chronic condition status was modeled as the outcome, and the husband’s chronic condition status was considered the exposure because it is culturally normative for women to move to their husband’s home after marriage and presumably adopt the household diet and lifestyle practices therein.
Missing data for any single outcome ranged from 0.2%–3% for common mental disorder, obesity, and hypertension to 24%–26% for diabetes and high cholesterol. To maintain the same analytic sample for each exposure-outcome model, we were thus forced to exclude 30% of the available participants because of missing data. We applied inverse probability weighting (IPW) to address potential bias arising from the exclusion of participants with missing data. IPW weights each observation by the inverse of the probability of having complete data to create a weighted pseudopopulation that resembles the full sample with respect to observed data [33]. We constructed the missing data IPW weights using a logistic model to predict the probability of having complete covariate data. The IPW model predictors were study site, age, sex, and education. We further accounted for the uneven number of adults in a single household contributing to the analysis using an IPW approach by creating a household weight that was the inverse of the household size. All analyses were weighted by a final weight that was the product of the missing data weight and the household weight and normalized to sum to the number of individuals with complete covariate data (7,522 participants). S1 Table shows missing data by covariate and descriptive analysis of participant characteristics in the total sample, unweighted analytic sample, and weighted analytic sample.
Adjusted models included the age, sex, education, marital status, religion, and study site of the index participant whose outcome was being modeled. Supplementary tables include results from unadjusted models. Data from Madhya Pradesh were excluded from the analysis of common mental disorder because less than 0.01% of respondents reported symptoms consistent with the common mental disorder definition. All analyses were model-based and accounted for data correlation arising from sampling multiple individuals in the same household and in the same cluster through generalized estimating equations [34]. Data management and recoding were performed using STATA 13 and 14 (College Station, Texas, United States), and statistical analyses were performed using SAS 9.4 (Cary, North Carolina, US) statistical software.
Results
Table 1 shows the household - and individual-level characteristics of the weighted analytic sample. A total of 2,574 households with 7,522 individuals were analyzed. On average, we observed 2.9 individuals per household (range: 2 to 11 individuals). The mean age of participants was 39 years (range: 18 to 96 years), 46% were men, and mean years of schooling of participants was 6 years. The majority of participants were married (78%) and Hindu (94%). While 43% of individuals had at least 1 chronic condition, this proportion varied from 37% in Mashobra to 50% in Gujarat. The least common chronic condition was high cholesterol (6%), and the most common condition was hypertension (23%).
Tab. 1. Characteristics of the analytic sample. 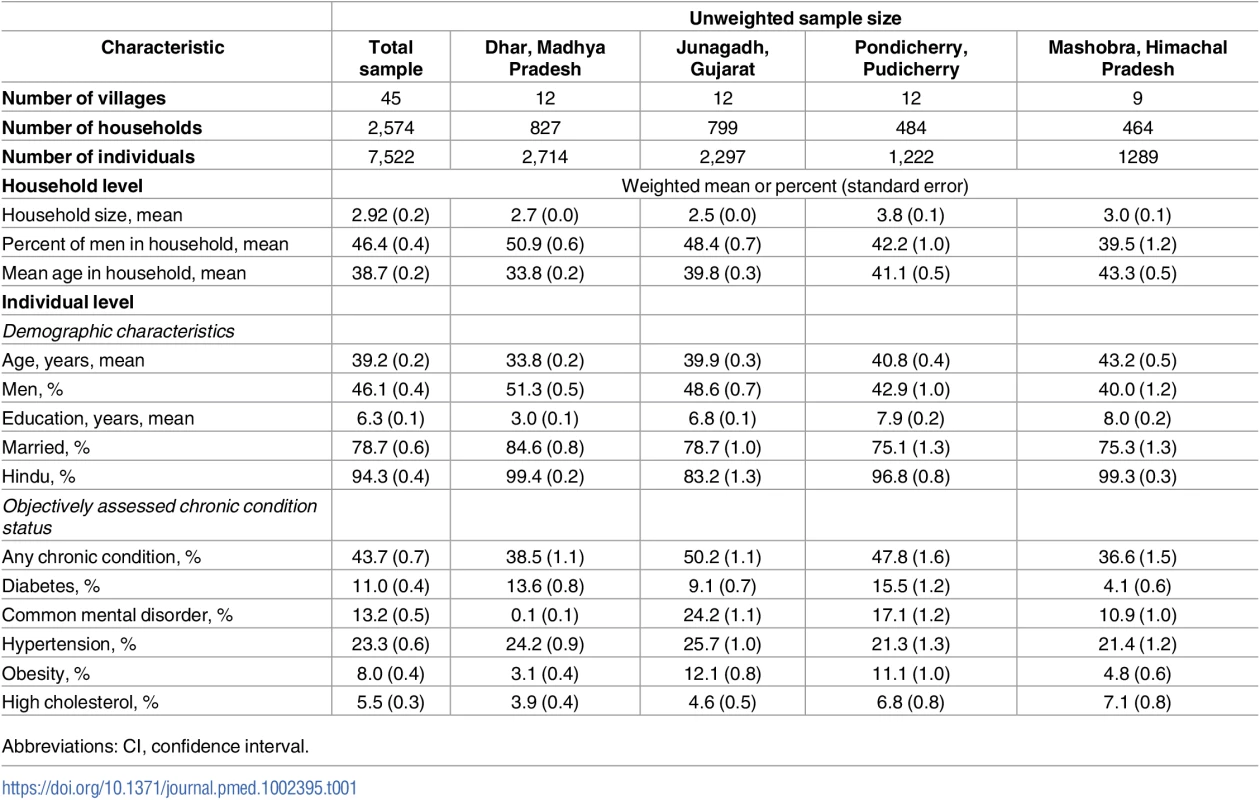
Abbreviations: CI, confidence interval. Data are from 7,522 adults in 2,574 households in the DISHA study. Chronic conditions were defined as follows: diabetes, fasting plasma glucose ≥ 126 mg/dL or taking medication; common mental disorder, General Health Questionnaire score ≥ 12; hypertension, blood pressure ≥ 140/90 mmHg or taking medication; obesity, body mass index ≥ 30 kg/m2; and high cholesterol, total blood cholesterol ≥ 240 mg/dL or taking medication. The sums of the weights in the analytic sample by site were 2,026 (Dhar), 2,039 (Junagadh), 1,957 (Pondicherry), and 1,500 (Mashobra).
Table 2 summarizes the results from separate adjusted logistic regression models estimating the odds ratio for having a given chronic condition if living with an individual with that same condition (Table 2, diagonal cells) and living with an individual with a different chronic condition (Table 2, off-diagonal cells); see S2 Table for unadjusted associations. Those who resided with another individual with any chronic condition had 29% higher adjusted relative odds of having any chronic condition themselves (adjusted odds ratio [aOR] = 1.29; 95% confidence interval [95% CI] 1.10–1.50). In general, the strongest relationships were observed in the same-condition models; positive associations were observed for diabetes (aOR = 1.60; 95% CI 1.23–2.07), common mental disorder (aOR = 2.69; 95% CI 2.12–3.42), and obesity (aOR = 1.82; 95% CI 1.33–2.50). With respect to differing conditions, the only statistically significant relationship was that between living with someone with hypertension and obesity status (aOR = 1.24; 95% CI 1.02–1.53).
Tab. 2. Adjusted association between living with someone with a given chronic condition and having that same or another chronic condition (n = 7,572). 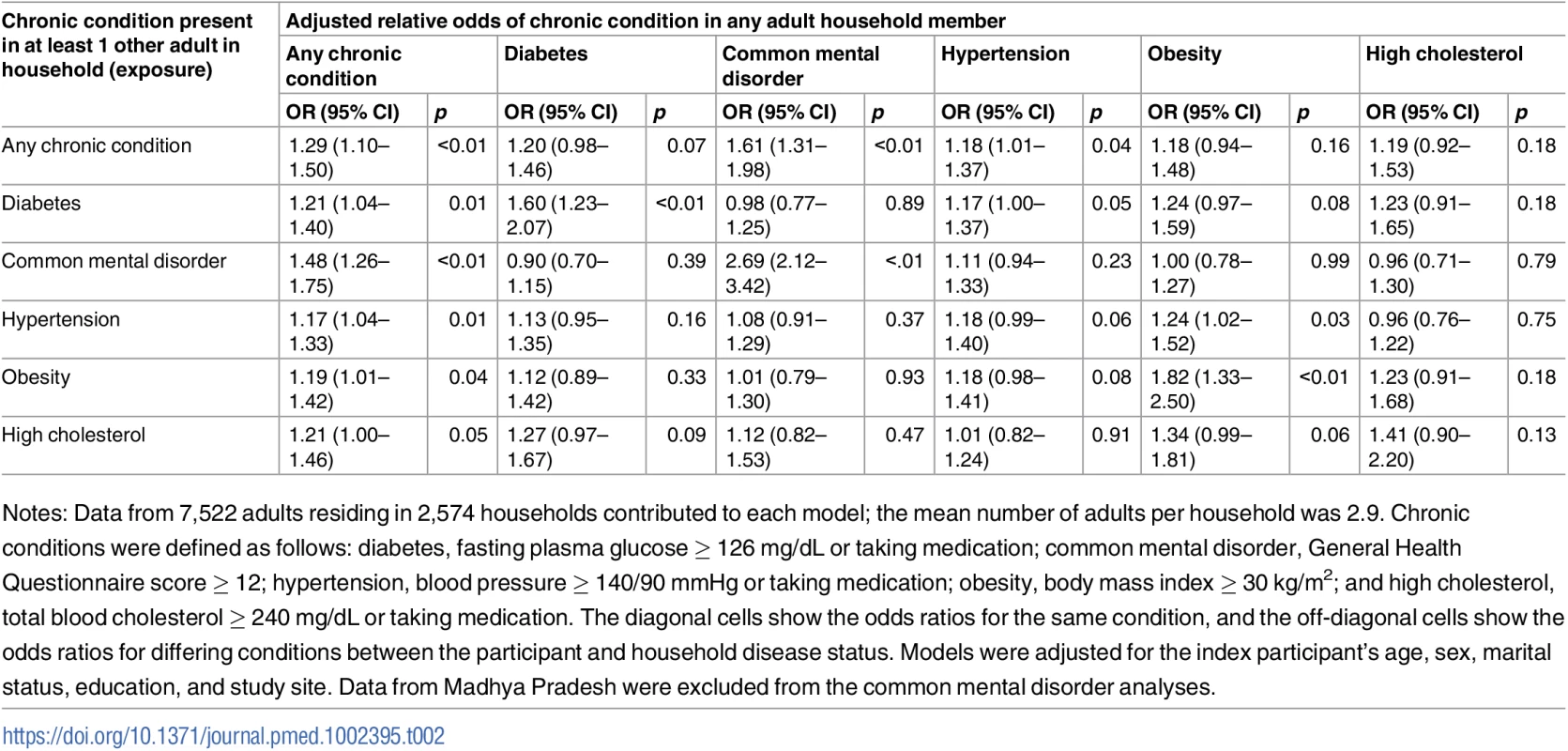
Notes: Data from 7,522 adults residing in 2,574 households contributed to each model; the mean number of adults per household was 2.9. Chronic conditions were defined as follows: diabetes, fasting plasma glucose ≥ 126 mg/dL or taking medication; common mental disorder, General Health Questionnaire score ≥ 12; hypertension, blood pressure ≥ 140/90 mmHg or taking medication; obesity, body mass index ≥ 30 kg/m2; and high cholesterol, total blood cholesterol ≥ 240 mg/dL or taking medication. The diagonal cells show the odds ratios for the same condition, and the off-diagonal cells show the odds ratios for differing conditions between the participant and household disease status. Models were adjusted for the index participant’s age, sex, marital status, education, and study site. Data from Madhya Pradesh were excluded from the common mental disorder analyses. Fig 1 shows the adjusted association between living with someone with a given chronic condition and having that same chronic condition by study site in the full analytic sample. See S3 Table for point estimates, CIs, and interaction tests in table form. There were no statistically significant differences between sites, and point estimates of the odds ratios indicate positive associations for concordant conditions among coresiding household members at all sites. Point estimates of odds ratios for any chronic condition, depression, and hypertension were very comparable across sites. Although not statistically distinguishable, point estimates of odds ratios for diabetes, high cholesterol, and obesity were more variable than those observed for any chronic condition, depression, or hypertension.
Fig. 1. Site-specific adjusted relative odds (95% confidence interval) of having a chronic condition if any other member of the household has that same chronic condition (reference: no other member of the household has that same condition) and test for interaction between sites. 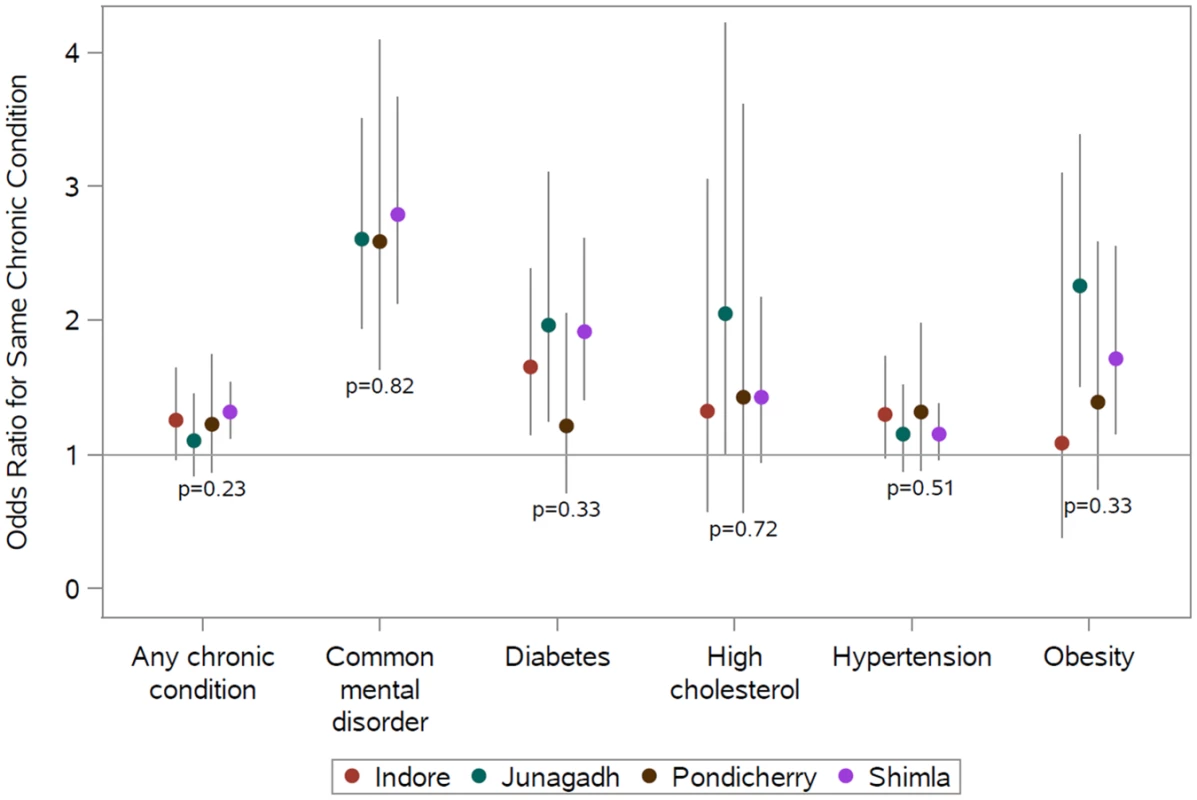
Site-specific associations were computed by including an interaction term between the site and the exposure condition. The P values shown are from generalized score tests for Type III contrasts for the site x exposure interaction term. The horizontal line marks the null value. Madhya Pradesh data were excluded from the common mental disorder analysis because of poor performance of the survey tool. Chronic conditions were defined as follows: diabetes (prior diagnosis, fasting plasma glucose ≥ 126 mg/dL, or taking medication); common mental disorder (General Health Questionnaire score ≥ 12); hypertension (prior diagnosis, blood pressure ≥ 140/90 mmHg, or taking medication); obesity (body mass index ≥ 30 kg/m2); and high cholesterol (prior diagnosis, total blood cholesterol ≥ 240 mg/dL, or taking medication). See S3 Table for these data in table form. Table 3 shows adjusted associations between the chronic condition status of parents and their adult children (sample restricted to parent-child dyads); see S4 Table for unadjusted associations. Adults coresiding with a parent who had a common mental disorder (aOR = 2.20; 95% CI 1.28–3.77), hypertension (aOR = 1.58; 95% CI 1.15–2.16), obesity (aOR = 4.99; 95% CI 2.71–9.20), or high cholesterol (aOR = 2.57; 95% CI 1.15–5.73) were more likely to have the same respective condition. We found no statistically significant association between parents and their adult children for any chronic condition or diabetes. The only statistically significant relationships between differing chronic conditions were those between parental high cholesterol and adult child diabetes (aOR = 2.02; 95% CI 1.08–3.78) and between parental diabetes and adult child hypertension (aOR = 1.97; 95% CI 1.28–3.02).
Tab. 3. Adjusted association between living with a parent with a given chronic condition and having that same or another chronic condition (n = 1,660). 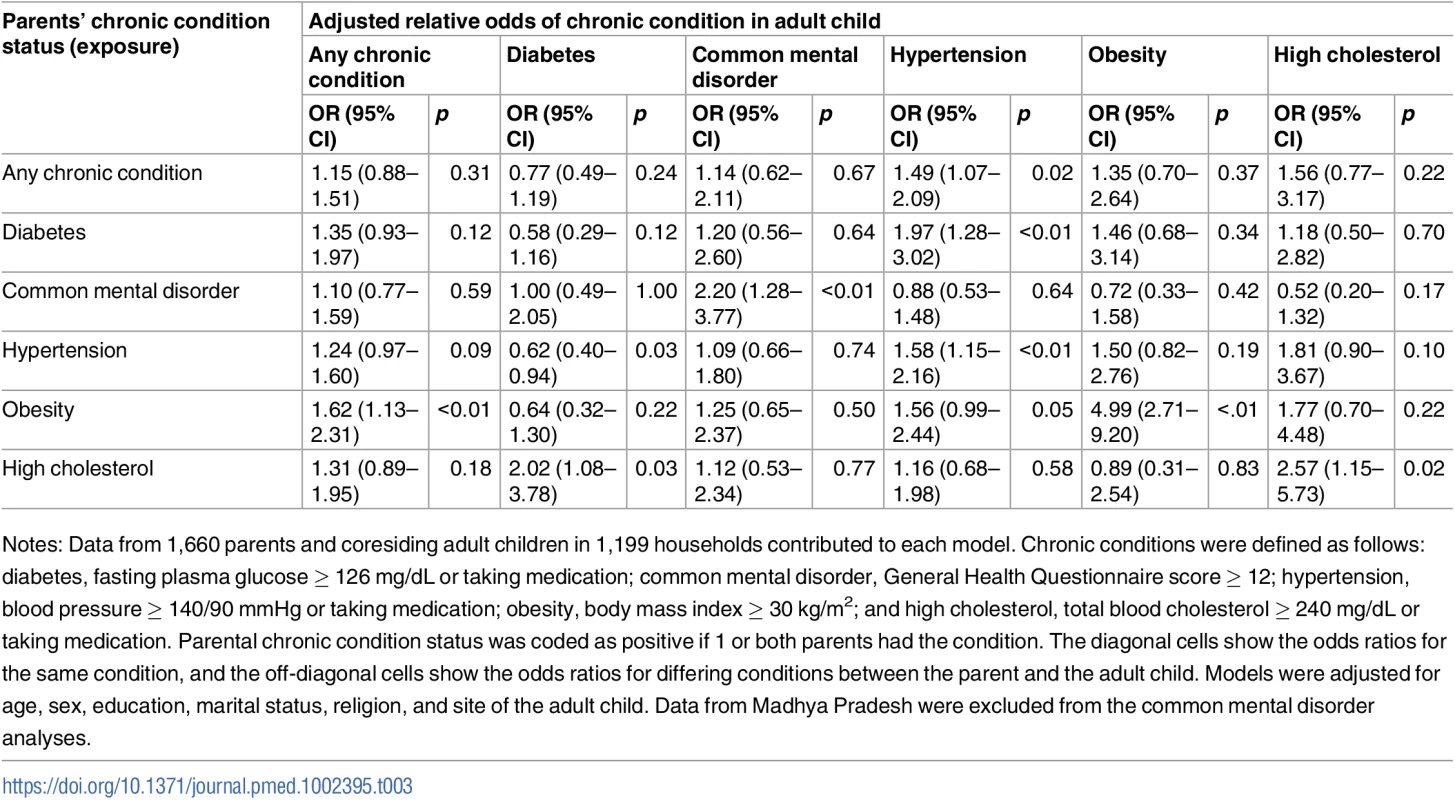
Notes: Data from 1,660 parents and coresiding adult children in 1,199 households contributed to each model. Chronic conditions were defined as follows: diabetes, fasting plasma glucose ≥ 126 mg/dL or taking medication; common mental disorder, General Health Questionnaire score ≥ 12; hypertension, blood pressure ≥ 140/90 mmHg or taking medication; obesity, body mass index ≥ 30 kg/m2; and high cholesterol, total blood cholesterol ≥ 240 mg/dL or taking medication. Parental chronic condition status was coded as positive if 1 or both parents had the condition. The diagonal cells show the odds ratios for the same condition, and the off-diagonal cells show the odds ratios for differing conditions between the parent and the adult child. Models were adjusted for age, sex, education, marital status, religion, and site of the adult child. Data from Madhya Pradesh were excluded from the common mental disorder analyses. Table 4 shows adjusted associations between chronic condition status of spousal dyads; see S5 Table for unadjusted associations. A woman had 44% higher adjusted relative odds of having a chronic condition if her husband had a chronic condition (aOR = 1.44; 95% CI 1.14–1.82). Similar to the analyses above, the strongest relationships were seen for the same condition. Concordant diabetes (aOR = 2.28; 95% CI 1.52–3.42) and common mental disorder (aOR = 3.01; 95% CI 2.01–4.52) status in husbands and wives were the only statistically significant associations among spouses.
Tab. 4. Adjusted association between living with a spouse with a given chronic condition and having that same or another chronic condition (n = 1,598). 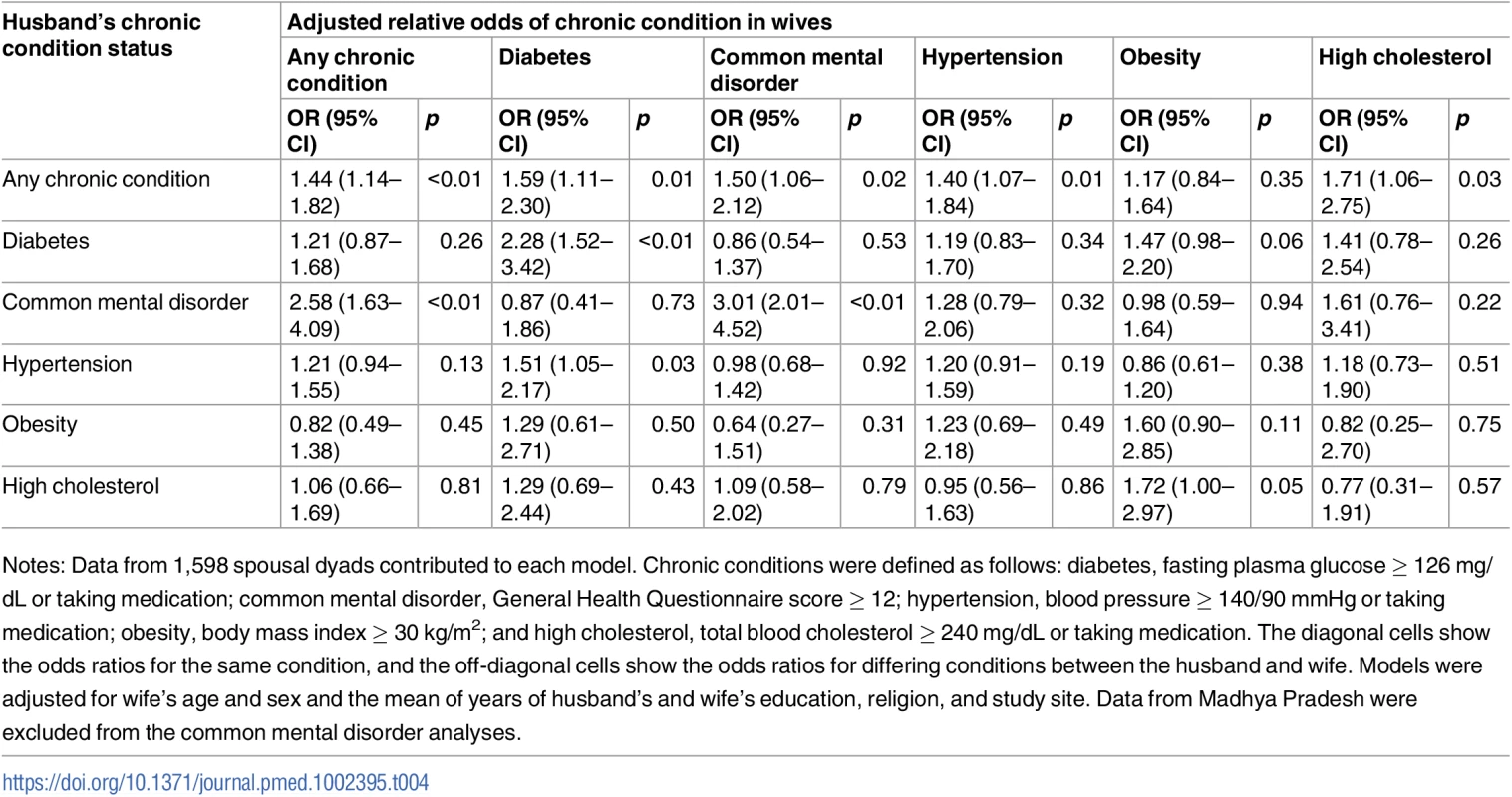
Notes: Data from 1,598 spousal dyads contributed to each model. Chronic conditions were defined as follows: diabetes, fasting plasma glucose ≥ 126 mg/dL or taking medication; common mental disorder, General Health Questionnaire score ≥ 12; hypertension, blood pressure ≥ 140/90 mmHg or taking medication; obesity, body mass index ≥ 30 kg/m2; and high cholesterol, total blood cholesterol ≥ 240 mg/dL or taking medication. The diagonal cells show the odds ratios for the same condition, and the off-diagonal cells show the odds ratios for differing conditions between the husband and wife. Models were adjusted for wife’s age and sex and the mean of years of husband’s and wife’s education, religion, and study site. Data from Madhya Pradesh were excluded from the common mental disorder analyses. Discussion
To our knowledge, this is the first study examining the relationship of 5 prevalent chronic conditions—hypertension, diabetes, obesity, common mental disorder, and high cholesterol—among coresiding adults in India. Irrespective of familial relationship, adults who resided with another adult with any chronic condition had 29% higher adjusted odds of having 1 or more chronic conditions themselves. For all of the 5 specific conditions examined, we consistently observed that adults tended to have the same chronic condition as a coresiding household member (e.g., living with someone who is obese was associated with 82% higher relative odds of obesity). Among all household members, parent-adult child dyads, and spousal dyads, common mental disorder was twice to thrice as high among individuals residing with someone with a common mental disorder. Other salient findings included the strong concordance of diabetes status among husbands and wives and concordance of obesity status among parents and their adult children. Across different disease phenotypes, we observed weaker and few statistically significant associations among household members.
In India, the past 20 years of unprecedented economic growth [35] has coincided with an increase in healthy life expectancy in men and women by 6 and 9 years, respectively [36]. Chronic diseases, however, threaten continued progress in this arena. There is a great need to reorient the existing health system in India to address the rise of chronic conditions [5,37]. The bulk of familial concordance studies have been conducted to understand the genetic influences of parents on young or adolescent children or environmental influences on health among spouses [7]. In India [4] and other LMICs [25] where extended family households are still intact, it may be particularly relevant and effective to engage the full household—irrespective of genetic ties or marital connections—in the prevention and management of chronic conditions. At the most superficial level, family history of chronic disease can be used for risk stratification [38].
Our findings are largely consistent with prior literature examining the relationship of metabolic outcomes among spouses [10–12,39] and between parents and adolescent children [11–13] within nuclear families. Specifically, the concordance of chronic condition status among spouses in our study was remarkably similar to findings published in a systematic review, reporting odds ratios between 1.2 and 1.6 for hypertension, 1.1 to 1.8 for diabetes, and 1.3 to 1.7 for obesity [6]. Additionally, there is robust concordance in these metabolic outcomes as a package (i.e., metabolic syndrome) among spouses [10–12]. Regarding phenotypic similarities between parents and their adult children, our results tended to demonstrate more statistically robust relationships compared with prior studies [11,12], likely due to a larger sample size and our restriction to adult children (versus adolescents and younger children). Previous analyses of chronic disease-related traits—such as continuously measured blood pressure and body mass index (BMI)—have also found that these traits are related in parent-child dyads and cluster within the household [8,9,13,40,41]. Across these previous studies and ours, generally, the correlation of obesity/BMI among family members was stronger than the correlation of hypertension/blood pressure. Comparable to what we found for common mental disorder, a single study examining concordance of multiple diseases among married couples estimated an aOR of 2.1 for depression and also reported that depression was the most concordant condition of the many outcomes examined in the study [39].
Although we did not directly or quantitatively examine contributing pathways in this study, qualitative comparisons of coefficients across models may provide a preliminary understanding for future investigation. First, odds ratios for chronic condition concordance adjusted for sociodemographic information (such as educational level and site) were generally attenuated compared with unadjusted odds ratios; the greatest attenuation after adjustment was observed in the spousal concordance analysis (Table 4 versus S5 Table). To the extent that sociodemographic background proxies living conditions, this suggests that shared living conditions are relevant to determining spousal chronic condition concordance. Second, common mental disorder was the only condition that was highly and statistically significantly concordant in models including all household members, models restricted to dyads of parents and their adult children, and models restricted to dyads of spouses, implying a potential affective contagion that affects members of a household irrespective of familial relationship. Third, concordance in hypertension and high cholesterol were only observed in parent-adult child dyads, implying a potential genetic component for observed household concordance in those conditions. Fourth, household concordance in diabetes was not observed in parent-adult child dyads, and concordance in obesity was not statistically significant among spouses. Additionally, these findings run contrary to prior findings that metabolic syndrome is correlated in parents and their children [11,12] and that weight status is correlated among spouses [7,42]. The results raise the question of whether diabetes has a stronger environmental component and obesity has a stronger genetic component in this setting. Fifth, site-specific associations indicate a general tendency towards mild to moderate concordance of the 5 chronic conditions examined here, but diabetes, high cholesterol, and obesity odds ratios varied more than other conditions. Perhaps these conditions are more impacted by extrahousehold factors. Finally, correspondence across differing phenotypes was weaker than concordance of the same condition—even among spouses—possibly suggesting specificity of mechanisms contributing to each of the disease outcomes under study. Further data are needed to determine the robustness of each of these observations.
The DISHA study provided a geographically and socioculturally diverse study population for our secondary data analysis of the relationships between chronic conditions among members of the same household, yielding a strong foundation for generalizing findings across India and possibly other LMICs where extended family households remain common [25]. A strength of this data source was our ability to objectively characterize 5 chronic conditions and subsequently examine associations among coresiding adults in a large sample of Indian households using very recent data. Not only did we report the concordance of the same chronic condition among household members, but we also reported the correspondence between different chronic conditions. Although this approach required making several comparisons, these comparisons addressed our predefined research question regarding potential heightened risk for chronic conditions across differing phenotypes, and thus, we do not adjust for multiple comparisons in the analysis [43]. Moreover, our comprehensive analysis of these relationships among all household members, parent-adult child dyads, and spousal dyads separately provides data on the concordance and correspondence between chronic conditions in genetically related and unrelated adults who share a common living environment. Site-specific analysis assures us that the findings were consistent across the heterogeneous districts.
DISHA, however, was designed not to examine family-level associations in outcomes but rather to detect a 2 mmHg mean difference in systolic blood pressure between intervention and control villages. Thus, we had relatively small samples in the dyadic analysis, and we interpret tests of statistical significance and any qualitative differences between the associations observed for spousal versus parent-child dyads with caution. We also lacked sufficient sample size to examine associations in other potential familial relationships of interest (e.g., daughters-in-law and parents-in-law or disaggregated mother-child from father-child). In addition, 25% of the DISHA participants were missing fasting blood samples, which led to a high proportion of missing data. We addressed missing data using inverse probability weighting, which may negatively impact statistical precision. Finally, we were unable to investigate the development of new chronic conditions in household members—which could provide insight into the etiology of household clustering of disease—because of the cross-sectional nature of the analysis.
Our results provide preliminary evidence that targeting households in which 1 adult has a chronic condition may be an effective way to identify other individuals with chronic conditions and potentially prevent emerging chronic conditions in India, as has been done among spouses elsewhere [44,45]. Moreover, studying the incidence of chronic disease within household members may provide new insight regarding mechanisms relevant to primordial prevention of chronic disease risk factors. It is thus critical to substantiate these early findings in ongoing prospective cohort studies to better understand the mechanisms through which coresiding household members develop chronic conditions over time. Elucidating such mechanisms can assist with designing novel interventions that cater to the needs of households across the socioeconomic spectrum in both urban and rural settings. The design and subsequent rigorous evaluation of such interventions will contribute to generate the evidence base needed to combat the rise of chronic conditions in India.
Supporting Information
Zdroje
1. Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388 : 1603–1658. doi: 10.1016/S0140-6736(16)31460-X 27733283
2. Siegel KR, Patel SA, Ali MK. Non-communicable diseases in South Asia: contemporary perspectives. Br Med Bull. 2014;111 : 31–44. doi: 10.1093/bmb/ldu018 25190759
3. Child Trends I. World family map 2014: mapping family change and child well-being outcomes [Internet]. 2014. http://worldfamilymap.ifstudies.org/2015/wp-content/themes/WorldFamilyMap/WFM-2015-ForWeb.pdf
4. Desai S, Dubey A, Joshi B, Sen M, Shariff A, Vanneman R. Human Development in India: Challenges for a Society in Transition. Oxford University Press, New Delhi; 2010.
5. Patel V, Chatterji S, Chisholm D, Ebrahim S, Gopalakrishna G, Mathers C, et al. Chronic diseases and injuries in India. The Lancet. 2011;377 : 413–428. doi: 10.1016/S0140-6736(10)61188-9
6. Castelnuovo AD, Quacquaruccio G, Donati MB, de Gaetano G, Iacoviello L. Spousal Concordance for Major Coronary Risk Factors: A Systematic Review and Meta-Analysis. Am J Epidemiol. 2009;169 : 1–8. doi: 10.1093/aje/kwn234 18845552
7. Meyler D, Stimpson JP, Peek MK. Health concordance within couples: A systematic review. Soc Sci Med. 2007;64 : 2297–2310. doi: 10.1016/j.socscimed.2007.02.007 17374552
8. Chen W, Srinivasan SR, Bao W, Berenson GS. The Magnitude of Familial Associations of Cardiovascular Risk Factor Variables between Parents and Offspring Are Influenced by Age: The Bogalusa Heart Study. Ann Epidemiol. 2001;11 : 522–528. doi: 10.1016/S1047-2797(01)00228-9 11709270
9. Lee KE, Klein BEK, Klein R. Familial aggregation of components of the multiple metabolic syndrome in the Framingham Heart and Offspring Cohorts: Genetic Analysis Workshop Problem 1. BMC Genet. 2003;4 Suppl 1: S94. doi: 10.1186/1471-2156-4-S1-S94 14975162
10. Kim HC, Kang DR, Choi KS, Nam CM, Thomas GN, Suh I. Spousal Concordance of Metabolic Syndrome in 3141 Korean Couples: A Nationwide Survey. Ann Epidemiol. 2006;16 : 292–298. doi: 10.1016/j.annepidem.2005.07.052 16230025
11. Lee MH, Kim HC, Thomas GN, Ahn SV, Hur NW, Choi DP, et al. Familial concordance of metabolic syndrome in Korean population—Korean National Health and Nutrition Examination Survey 2005. Diabetes Res Clin Pract. 2011;93 : 430–436. doi: 10.1016/j.diabres.2011.06.002 21733593
12. Park HS, Park JY, Cho S-I. Familial aggregation of the metabolic syndrome in Korean families with adolescents. Atherosclerosis. 2006;186 : 215–221. doi: 10.1016/j.atherosclerosis.2005.07.019 16126214
13. Gupta S, Kapoor S. Genetic And Environmental Influences On Blood Pressure In An Urban Indian Population. J Biosoc Sci. 2013;45 : 1–11. doi: 10.1017/S0021932012000478 22892051
14. Feng Y, Zang T, Xu X, Xu X. Familial Aggregation of Metabolic Syndrome and Its Components in a Large Chinese Population. Obesity. 2008;16 : 125–129. doi: 10.1038/oby.2007.22 18223624
15. Jurj A, Wen W, Li H, Zheng W, Yang G, Xiang Y, et al. Spousal Correlations for Lifestyle Factors and Selected Diseases in Chinese Couples. Ann Epidemiol. 2006;16 : 285–291. doi: 10.1016/j.annepidem.2005.07.060 16257231
16. Smith KR, Zick CD. Linked Lives, Dependent Demise? Survival Analysis of Husbands and Wives. Demography. 1994;31 : 81. doi: 10.2307/2061909 8005344
17. Wilson SE. The health capital of families: an investigation of the inter-spousal correlation in health status. Soc Sci Med. 2002;55 : 1157–1172. 12365528
18. Umberson D. Family Status and Health Behaviors: Social Control as a Dimension of Social Integration. J Health Soc Behav. 1987;28 : 306. doi: 10.2307/2136848 3680922
19. Hirst M. Carer distress: A prospective, population-based study. Soc Sci Med. 2005;61 : 697–708. doi: 10.1016/j.socscimed.2005.01.001 15899327
20. Pearlin LI, Aneshensel CS, Leblanc AJ. The Forms and Mechanisms of Stress Proliferation: The Case of AIDS Caregivers. J Health Soc Behav. 1997;38 : 223. doi: 10.2307/2955368 9343962
21. Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. The Gerontologist. 1995;35 : 771–791. 8557205
22. Lee C, Rodríguez G, Glei DA, Weinstein M, Goldman N. Increases in Blood Glucose in Older Adults The Effects of Spousal Health. J Aging Health. 2014; 0898264314534894. doi: 10.1177/0898264314534894 24891563
23. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and Lifestyle Recommendations Revision 2006. Circulation. 2006;114 : 82–96. doi: 10.1161/CIRCULATIONAHA.106.176158 16785338
24. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and Type 2 Diabetes Over the Lifespan A meta-analysis. Diabetes Care. 2008;31 : 2383–2390. doi: 10.2337/dc08-0985 19033418
25. Ruggles S, Heggeness M. Intergenerational coresidence in developing countries. Popul Dev Rev. 2008;34 : 253–281. 21562612
26. Jeemon P, Narayanan G, Kondal D, Kahol K, Bharadwaj A, Purty A, et al. Task shifting of frontline community health workers for cardiovascular risk reduction: design and rationale of a cluster randomised controlled trial (DISHA study) in India. BMC Public Health. 2016;16 : 264. doi: 10.1186/s12889-016-2891-6 26975187
27. American Diabetes Association. Standards of Medical Care in Diabetes—2014. Diabetes Care. 2014;37: S14–S80. doi: 10.2337/dc14-S014 24357209
28. Patel V, Araya R, Chowdhary N, King M, Kirkwood B, Nayak S, et al. Detecting common mental disorders in primary care in India: a comparison of five screening questionnaires. Psychol Med. 2008;38. doi: 10.1017/S0033291707002334 18047768
29. Kuruvilla A, Pothen M, Philip K, Braganza D, Joseph A, Jacob KS. The validation of the Tamil version of the 12 item general health questionnaire. Indian J Psychiatry. 1999;41 : 217–221. 21455393
30. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (jnc 8). JAMA. 2014;311 : 507–520. doi: 10.1001/jama.2013.284427 24352797
31. NHLBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2 : 51S–209S.
32. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285 : 2486–2497. 11368702
33. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22 : 278–295. doi: 10.1177/0962280210395740 21220355
34. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73 : 13–22. doi: 10.1093/biomet/73.1.13
35. Ding D, Masha I. India’s Growth Spillovers to South Asia [Internet]. IMF Asia and Pacific Department; 2012. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2012241
36. Murray CJL, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. The Lancet. 2015; doi: 10.1016/S0140-6736(15)61340-X 26321261
37. Chatterji S, Kowal P, Mathers C, Naidoo N, Verdes E, Smith JP, et al. The Health Of Aging Populations In China And India. Health Aff (Millwood). 2008;27 : 1052–1063. doi: 10.1377/hlthaff.27.4.1052 18607041
38. Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ. Can family history be used as a tool for public health and preventive medicine? Genet Med. 2002;4 : 304–310. 12172397
39. Hippisley-Cox J, Coupland C, Pringle M, Crown N, Hammersley V. Married couples’ risk of same disease: cross sectional study. BMJ. 2002;325 : 636. doi: 10.1136/bmj.325.7365.636 12242177
40. Hu Y, He L, Wu Y, Ma G, Li L, Hu Y. Familial correlation and aggregation of body mass index and blood pressure in Chinese Han population. BMC Public Health. 2013;13 : 686. doi: 10.1186/1471-2458-13-686 23890201
41. Vaezghasemi M, Ng N, Eriksson M, Subramanian SV. Households, the omitted level in contextual analysis: disentangling the relative influence of households and districts on the variation of BMI about two decades in Indonesia. Int J Equity Health. 2016;15. doi: 10.1186/s12939-016-0388-7 27388459
42. Cobb LK, McAdams-DeMarco MA, Gudzune KA, Anderson CAM, Demerath E, Woodward M, et al. Changes in Body Mass Index and Obesity Risk in Married Couples Over 25 Years The ARIC Cohort Study. Am J Epidemiol. 2016;183 : 435–443. doi: 10.1093/aje/kwv112 26405117
43. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiol Camb Mass. 1990;1 : 43–46.
44. Pyke SD. Levels in Couples Following Lifestyle Intervention. Arch Fam Med. 1997;6 : 354–360. 9225707
45. Falba TA, Sindelar JL. Spousal Concordance in Health Behavior Change. Health Serv Res. 2008;43 : 96–116. doi: 10.1111/j.1475-6773.2007.00754.x 18211520
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis
- Preterm birth prevention—Time to PROGRESS beyond progesterone
- Women’s and men’s reports of past-year prevalence of intimate partner violence and rape and women’s risk factors for intimate partner violence: A multicountry cross-sectional study in Asia and the Pacific
- Global services and support for children with developmental delays and disabilities: Bridging research and policy gaps
- Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: A population-based cohort study
- Chronic disease concordance within Indian households: A cross-sectional study
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Sustained effectiveness and cost-effectiveness of the Healthy Activity Programme, a brief psychological treatment for depression delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: Results of the Compass pilot randomised trial
- Keeping it real: A journal editor in clinic
- Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis
- HbA1c for type 2 diabetes diagnosis in Africans and African Americans: Personalized medicine NOW!
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Keeping it real: A journal editor in clinic
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



