-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
Mohammed Ali and Venkat Narayan describe the challenge of implementing evidence-based interventions for prevention for the large number of people at increased risk of type 2 diabetes.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002084
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1002084Summary
Mohammed Ali and Venkat Narayan describe the challenge of implementing evidence-based interventions for prevention for the large number of people at increased risk of type 2 diabetes.
Diabetes is one of the most devastating and costly conditions worldwide, leading to substantial burdens of macro - and microvascular diseases, as well as other disorders. Armed with evidence from randomized controlled trials [1–3] and other data showing that progression to type 2 diabetes can be effectively [4] and cost-effectively [5] delayed among people at high risk, several countries have embarked on rolling out prevention programs to slow the growing incidence of diabetes. Such programs center on evidence-based behavior change interventions aimed at promoting healthy diets, appropriate physical activity, and modest weight loss. The most recent initiatives include the United States government’s authorization for diabetes preventive services to be covered for Medicare beneficiaries [6] and the launch by the United Kingdom’s National Health Service of a nationwide diabetes prevention program [7]. However, for these endeavors to successfully mitigate growing diabetes burdens, several important barriers to implementation of prevention strategies need to be overcome (Fig 1), and screening for dysglycemia is a key part of this process to connect demand with a growing supply of preventive services.
Fig. 1. A framework for enhancing diabetes prevention and management supply and demand. 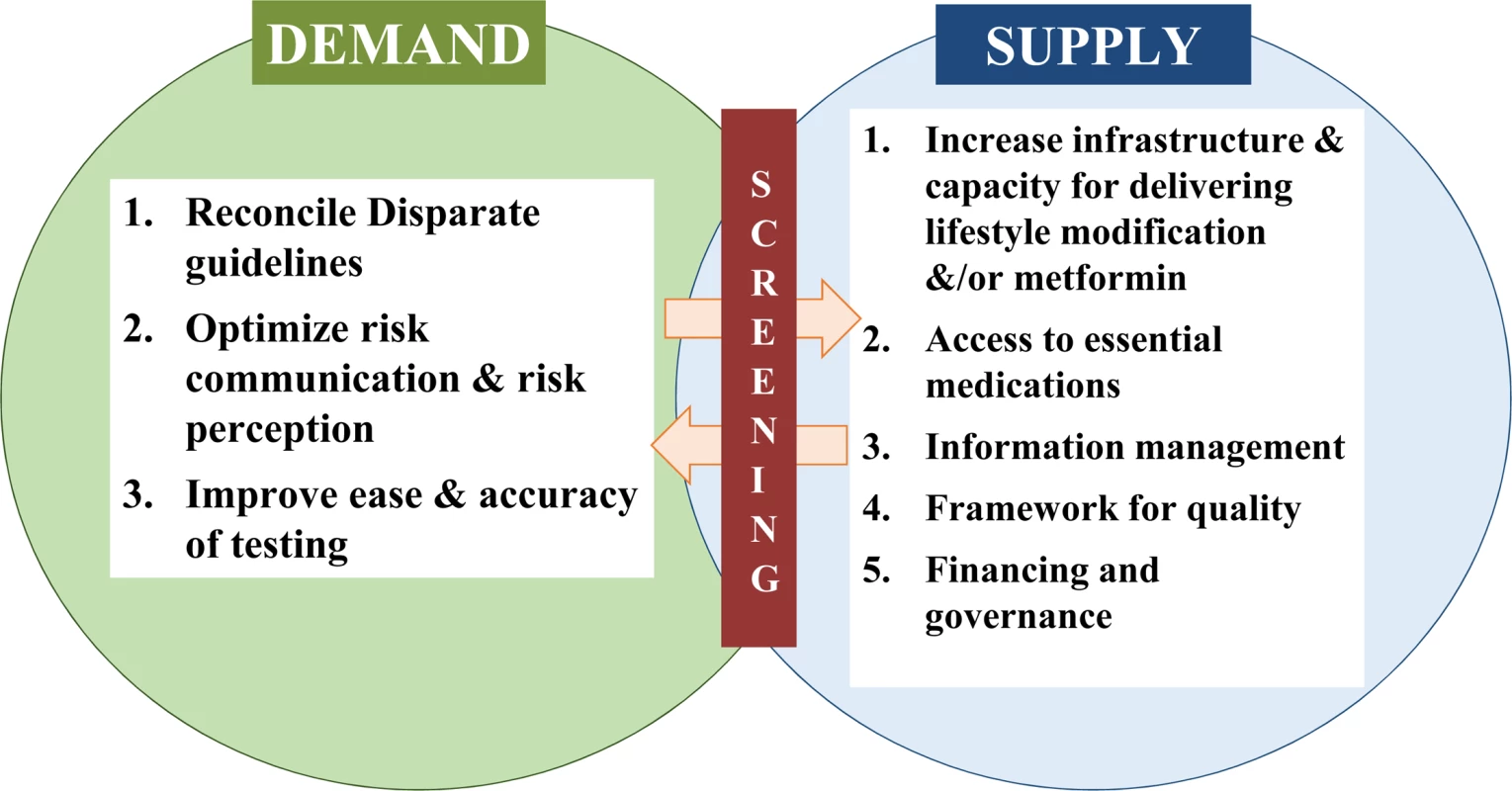
The principle that underlies screening for dysglycemia is to accurately identify risk of type 2 diabetes without causing physical or psychological harm and to motivate at-risk individuals to connect with appropriate health care and preventive services. While definitions of prediabetes vary, people with elevated fasting or 2-hour glucose, hemoglobin A1c, or any combination of these, albeit not in the diabetes range, have a 4–12 times higher annual likelihood of developing diabetes than the general, normoglycemic population [8]. Screening is therefore pertinent to identify these persons, especially since nearly 47% of the 415 million people with diabetes worldwide, and a substantial yet unquantified proportion of those with prediabetes, remain unaware of their condition [9]. Even in high-income countries like the US, over a quarter of the 29 million Americans with diabetes [10] and nearly 90% of the 86 million with prediabetes are not aware of their condition [11]. These awareness gaps are likely to impede success of evidence-based interventions to prevent diabetes and its complications among adults with both prediabetes and diabetes.
Although there are well-accepted, minimally-invasive glucose tests to accurately identify people with diabetes and prediabetes, and broad agreement that targeting persons at high risk for diabetes and offering glucose testing is more cost-effective and less harmful than universal testing [12], screening for dysglycemia has remained a contentious public health proposition for almost two decades. Debates about whether or not, and in whom, to encourage screening have led to disparate guidelines from influential expert committees [13,14], which may have contributed to large gaps in receipt of testing among those eligible. Indeed, recent national data from the US showed that only half of those eligible by the US Preventive Services Task Force (USPSTF) or the American Diabetes Association (ADA) guidelines reported having a glucose test in the past 3 years [15]. Harmonizing guidelines regarding glucose testing for high-risk individuals will likely be important in ensuring that people with dysglycemia are aware of their status and that appropriate services are available. The recent expansion of criteria for dysglycemia screening, to adults aged 40–70 years who are overweight or obese, by the USPSTF is a welcome step [13]. However, the criteria still exclude normal-weight people with dysglycemia, a group that may be especially prevalent in some populations, such as those of Asian or sub-Saharan African ancestry. Furthermore, since demand for and use of preventive services has been suboptimal to date, improving perception of and communication about risk in a way that motivates engagement will help to ensure that there is appropriate demand for the supply of preventive and health care services being created.
However, addressing low demand and engagement alone are not sufficient to address the problem of type 2 diabetes. Several enhancements are needed to the supply of diabetes care and preventive services. Of note, screening leads to detection of both undetected prediabetes and diabetes, and considering this continuum of risk could motivate greater integration of prevention and care—avoiding silos within diabetes would be more efficient and may promote continuity of care. In terms of diabetes care, a large proportion of people diagnosed with diabetes do not meet their care goals [10]; the absolute numbers of patients affected by disabling complications has increased; costs of care double every decade; and, although excess mortality associated with diabetes has declined for individuals in high-income countries [16], there has been an increase in the number of years lived with disability among people with diabetes. Therefore, appropriate public health policy, optimizing care services through the use of quality improvement mechanisms, information management and accountability, and patient-centered care delivery are all needed to manage the growing number of people with and costs of diabetes [17].
Supply-side concerns also include gaps in access, suboptimal organization and delivery of health care and prevention services, and workforce shortages. For prevention, for example, current infrastructure and capacity (e.g., to effectively deliver proven lifestyle interventions) are in far shorter supply in the US than the need suggests [18]. In addition to this, though data suggest that maintaining a healthy weight after initial weight loss further reduces diabetes incidence [19], most delivery programs have short-term maintenance components, and no approach has been endorsed as a minimum standard. Also, although the announcement that Medicare will finance diabetes preventive services in the US is momentous [6], the incidence of diabetes among non-Medicare-eligible young and middle-aged adults is not insubstantial; in fact, diabetes incidence among 45–64 year olds is almost the same as in those aged 65 and older [20]. Consequently, a wider array of financing options (e.g., through employers or safety nets) are needed, especially for people of lower socioeconomic status and minority populations who tend to be at the highest risk of diabetes [21]. An important consideration for countries with market-based health care like the US is who should pay for preventive interventions if the individual is unable to do so, and indeed another concern is whether lack of coverage for preventive services leads to greater health disparities.
Other considerations in successfully rolling out diabetes preventive services include governance, a framework for quality measurement, and offering different options for prevention, including the use of metformin for people with prediabetes. The US Centers for Disease Control and Prevention have created a set of quality assurance criteria through the Diabetes Prevention Recognition Program [22], which certifies providers nationally. In addition to this, establishing a set of aspirational, yet pragmatic, quality indicators (e.g., proportion of screen-eligible people tested, proportion of people with prediabetes referred to certified lifestyle programs, and proportion of those enrolled in lifestyle programs attending a minimum number of sessions and/or achieving behavior changes), as was done successfully for diabetes care in the 1990s, may have important impacts on process and health outcomes. These measures would also help stimulate governance structures, real-time quality improvement opportunities, and systems to drive better prevention services. Indeed, with the momentum of greater political will and efforts to optimize the balance of supply and demand for lifestyle modification programs underway in the US, it is also time for the broader diabetes community to collectively support the use of metformin for prevention. There is sufficient evidence to support its use [1], with few concerns about harm that should prevent action.
In a complex, fast-changing world, diabetes prevention offers a strategic opportunity to achieve better health at lower cost for a large proportion of the population [17]. It is important to note that the goal of scaling-up supply and demand of preventive and care services for high-risk individuals should be considered complementary to—and not mutually exclusive with—society-level population-based policies that need to advance in terms of rigor of the evidence base. However, to successfully achieve the aspiration of ubiquitous diabetes prevention, we need to harness all of the evidence-based intervention options at our disposal, build the systems and governance needed to optimize delivery of care and prevention, and, most importantly, rally behind a single, aligned set of screening guidelines to identify and connect supply with demand.
Zdroje
1. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. Epub 2002/02/08. doi: 10.1056/NEJMoa012512 346/6/393 [pii]. 11832527; PubMed Central PMCID: PMC1370926.
2. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. Epub 1997/04/01. 9096977.
3. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. Epub 2001/05/03. doi: 10.1056/NEJM200105033441801 11333990.
4. Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75. Epub 2012/01/11. doi: 10.1377/hlthaff.2011.1009 22232096.
5. Li R, Qu S, Zhang P, Chattopadhyay S, Gregg EW, Albright A, et al. Economic Evaluation of Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):452–460. doi: 10.7326/M15-0469 26167962.
6. U.S. Department of Health and Human Services. Independent experts confirm that diabetes prevention model supported by the Affordable Care Act saves money and improves health HHS.gov2016 [cited 2016 April 19]. http://www.hhs.gov/about/news/2016/03/23/independent-experts-confirm-diabetes-prevention-model-supported-affordable-care-act-saves-money.html.
7. Torjesen I. NHS England rolls out world’s first national diabetes prevention programme. BMJ. 2016;352. doi: 10.1136/bmj.i1669
8. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–312. Epub 2007/07/03. S0168-8227(07)00309-9 [pii] doi: 10.1016/j.diabres.2007.05.004 17601626.
9. International Diabetes Federation. IDF Diabetes Atlas 7th Edition Brussels, Belgium 2015 [cited 2016 April]. http://www.diabetesatlas.org/
10. Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med. 2014;161(10):681–689. doi: 10.7326/M14-0019 25402511.
11. Li Y, Geiss LS, Burrows NR, Rolka DB, Albright A. Awareness of Prediabetes—United States, 2005–2010. Morbidity and Mortality Weekly. March 22, 2013;62(11):209–212.
12. Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KM. Screening for type 2 diabetes and dysglycemia. Epidemiol Rev. 2011;33(1):63–87. Epub 2011/06/01. doi: 10.1093/epirev/mxq020 21624961.
13. Siu AL. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus. Ann Intern Med. 2015;163(11):861–868. doi: 10.7326/M15-2345 26501513
14. American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39(Supplement 1):S13–S22. doi: 10.2337/dc16-S005
15. Bullard KM, Ali MK, Imperatore G, Geiss LS, Saydah SH, Albu JB, et al. Receipt of Glucose Testing and Performance of Two US Diabetes Screening Guidelines, 2007–2012. PLoS ONE. 2015;10(4):e0125249. doi: 10.1371/journal.pone.0125249 25928306; PubMed Central PMCID: PMCPMC4416019.
16. Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4(6):537–547. doi: 10.1016/S2213-8587(16)30010-9 27156051
17. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769. doi: 10.1377/hlthaff.27.3.759 18474969.
18. Konchak JN, Moran MR, O’Brien MJ, Kandula NR, Ackermann RT. The State of Diabetes Prevention Policy in the USA Following the Affordable Care Act. Curr Diab Rep. 2016;16(6):1–12. doi: 10.1007/s11892-016-0742-6
19. Penn L, White M, Lindström J, den Boer AT, Blaak E, Eriksson JG, et al. Importance of Weight Loss Maintenance and Risk Prediction in the Prevention of Type 2 Diabetes: Analysis of European Diabetes Prevention Study RCT. PLoS ONE. 2013;8(2):e57143. doi: 10.1371/journal.pone.0057143 23451166
20. Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226. doi: 10.1001/jama.2014.11494 25247518
21. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867–874. doi: 10.1016/S2213-8587(14)70161-5 25128274.
22. Centers for Disease Control and Prevention. Diabetes Prevention Recognition Program: Standards and Operating Procedures Atlanta, USA 2015 [cited 2016 April 20]. http://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



