-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
In a large prospective cohort study, Zhengming Chen and colleagues examine the associations between diabetes and major cardiovascular diseases in China where the prevalence of diabetes is rising rapidly.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002026
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002026Summary
In a large prospective cohort study, Zhengming Chen and colleagues examine the associations between diabetes and major cardiovascular diseases in China where the prevalence of diabetes is rising rapidly.
Introduction
Worldwide over 400 million people have diabetes, and the prevalence is increasing rapidly in both developed and developing countries [1]. Previous studies of mostly Western populations have shown that diabetes is typically associated with a 2-fold increased risk of ischaemic heart disease (IHD) [2,3]. Uncertainty remains, however, about whether similar excess risk applies to other populations, and about the strength of the association of diabetes with stroke and, particularly, stroke subtypes [2–4]. Appropriate understanding of these issues is of considerable relevance to China, where stroke rates are high [5].
Since the 1980s, there has been a rapid and substantial increase in the prevalence of diabetes in China [1,6,7], which now affects ~10% of adults [8]. Compared with those in Western populations, individuals with diabetes in China have tended to be leaner and to have worse pancreatic beta-cell function, which may result in greater susceptibility to microvascular complications and cancer than to macrovascular complications [9]. Despite the growing diabetes epidemic, there is limited evidence about the association of diabetes with cardiovascular disease in China [10–14], with previous studies limited by potentially outdated risk estimates [12], highly selected study populations [11], relatively small size [10–12], and lack of proper investigation of the relevance of diabetes duration and other modifiable factors, such as smoking, adiposity, blood pressure, and physical activity, which frequently differ between China and Western populations.
We report relevant findings from a large prospective study, the China Kadoorie Biobank (CKB), of 0.5 million adults, recruited between 25 June 2004 and 15 July 2008 across ten diverse areas of China. The aim of the present study is to examine the association of diabetes—both self-reported and screen-detected—with the risks of major cardiovascular diseases, including IHD and stroke subtypes. By applying population-attributable fractions for cardiovascular mortality from the present study to national cause-specific mortality and representative diabetes prevalence data, we also estimate the number of cardiovascular deaths attributable to diabetes in mainland China.
Methods
Ethics Statement
All participants provided written consent prior to participation, including permission for follow-up. Ethics approval was obtained from Oxford University, the Chinese Centre for Disease Control and Prevention (CDC), and the ten study areas’ local CDCs.
Study Population
Details of the CKB design, methods, and population have been reported previously [15,16]. Briefly, the 2004–2008 baseline survey took place in ten localities across China (five urban and five rural; S1 Fig) chosen to provide diversity in exposure and disease patterns, additionally taking account of logistical considerations, including population stability, death and disease registry quality, and local capacity. All residents aged 35–74 y from 100–150 administrative units (rural villages or urban residential committees) in each area were invited to attend study assessment clinics. Overall, ~30% responded [15], and 512,891 individuals were enrolled (including a few slightly outside the 35–74 y range).
Data Collection
At study assessment clinics, trained health workers administered laptop-based questionnaires that collected data on socio-demographic status, smoking, alcohol consumption, diet, physical activity (related to leisure, household work, occupation, and commuting [17]), medical history, and—among participants reporting a history of diabetes, IHD, stroke/transient ischaemic attack, and hypertension—current use of medications. Information on age at diagnosis was collected from participants reporting a history of diabetes. Health workers measured height, weight, waist and hip circumference, and blood pressure and took non-fasting venous blood samples (with the time since last food recorded) for storage and immediate on-site testing of plasma glucose level using a SureStep Plus meter (LifeScan). Participants without self-reported diabetes with a plasma glucose level of 7.8–11.0 mmol/l were invited to undergo fasting glucose testing (using the same measurement technique) the following day. Every 4–5 y, a 5%–6% random sample of surviving participants was resurveyed, collating the same data as at baseline, with certain additions.
Assessment of Diabetes Status
Participants answering “yes” to the question, “Has a doctor ever told you that you had diabetes?” at baseline were defined as having self-reported diabetes; among them, information about age at diagnosis and current medication use was collected. Screen-detected diabetes was defined as not having self-reported diabetes but having a random plasma glucose (RPG) level ≥ 7.0 mmol/l if time since last food ≥ 8 h, RPG level ≥ 11.1 mmol/l if time since last food < 8 h, or fasting plasma glucose level ≥ 7.0 mmol/l on subsequent testing [18]. Participants with screen-detected diabetes were provided with a referral letter and advised to seek formal medical consultation.
Follow-Up for Mortality and Morbidity
The vital status of each participant was obtained periodically from China CDC’s Disease Surveillance Points, checked annually against local residential and health insurance records and by active confirmation through street committee or village administrators. Causes of death from official death certificates were ICD-10 coded by trained Disease Surveillance Point staff blinded to baseline information. Information on non-fatal outcomes was collected through linkage with established disease surveillance systems (for cancer, IHD, stroke, and diabetes) and, via unique national ID, with the national health insurance system, which records details of ICD-10-coded hospitalisations in all study areas [16].
By 1 January 2014, 25,488 (5.0%) participants had died, and 2,411 (0.5%) were lost to follow-up. For the present study, the primary endpoints were cardiovascular death (ICD-10 I00–I25, I27–I88, I95–I99), myocardial infarction (MI) (ICD-10 I21–I23), major coronary event (MCE) (non-fatal MI or fatal IHD; ICD-10 I20–I25), ischaemic stroke (IS) (ICD-10 I63), intracerebral haemorrhage (ICH) (ICD-10 I61), total stroke (ICD-10 I60, I61, I63, I64), and major occlusive vascular disease (MOVD) (IS or MCE).
Statistical Analysis
The main analyses excluded participants reporting prior doctor-diagnosed IHD (n = 15,472, 3.0%) or stroke/transient ischaemic attack (n = 8,884, 1.7%) at baseline. A further 1,081 (0.2%) participants with missing, implausible, or extreme values for blood pressure, height, waist circumference, hip circumference, waist-to-hip ratio, or body mass index (BMI) were excluded, leaving 488,760 individuals (199,896 men, 288,864 women) for the present analyses.
Analyses were done separately for self-reported and screen-detected diabetes, with a common reference group consisting of individuals without self-reported or screen-detected diabetes. The mean values and prevalence of certain variables were calculated by diabetes status, standardised by 5-y age group, sex, and study area. Direct standardisation was used to calculate age-, sex-, and study-area-adjusted disease incidence rates, using the total study population as the standard. Cox regression yielded hazard ratios (HRs) for diabetes versus not, stratified by age at risk (5-y age groups), study area, and, where appropriate, sex, and adjusted for education (no formal education, primary school, middle school, high school, college/university), smoking (never, occasional, ex-regular, current regular), alcohol consumption (never, occasional, ex-regular, reduced, weekly), systolic blood pressure (SBP) (nine groups), and physical activity (five groups). Sensitivity analyses were performed adjusting for age, SBP, and physical activity as continuous variables.
Comparison of HRs for the first four and subsequent years of follow-up revealed no evidence of departure from the proportional hazards assumption. Adjusted HRs were calculated across strata of other cardiovascular risk factors and duration of diabetes at baseline, and chi-square tests for trend and heterogeneity (i.e., effect modification or statistical interaction) were applied to the log HRs and their standard errors [19]. In separate analyses, risk estimates were also adjusted for adiposity. Finally, the HR for MOVD associated with the number of other presenting baseline cardiovascular risk factors (hypertension, overweight or obese, ever regular smoking, physical inactivity) among individuals with diabetes was assessed using the floating absolute risk method [20], which provides estimates of variance across all exposure categories.
Population-attributable risk is P(HR − 1)/(1 + P[HR − 1]), where P is the prevalence of diabetes. By applying age-specific HRs (which can be assumed to approximate the relative risk [21]) to nationally representative, age-specific prevalence of diabetes [8] and national cause-specific mortality data [22], we estimated the number of cardiovascular deaths attributable to diabetes. All analyses used SAS version 9.3.
Results
Among 488,760 participants without prior cardiovascular disease, the mean baseline age was 51 (standard deviation 11) y, 2.7% (n = 13,284) reported a history of doctor-diagnosed diabetes (self-reported diabetes), and a further 2.7% (n = 13,051) had screen-detected diabetes, of whom 8,554 (65.5%) were identified through RPG measurement (RPG ≥ 7.0 mmol/l and time since last food ≥ 8 h: n = 3,986; RPG ≥ 11.1 mmol/l and time since last food < 8 h: n = 4,568), 1,447 (11.1%) through fasting plasma glucose measurement, and 3,050 (23.4%) through both. For both self-reported and screen-detected diabetes, the prevalence was slightly higher in women than in men (2.9% versus 2.5% for self-reported diabetes and 2.8% versus 2.6% for screen-detected diabetes), mainly at ages > 50 y (S2 Fig), and the prevalence was higher in urban than in rural areas (3.9% versus 1.8% for self-reported diabetes and 3.4% versus 2.1% for screen-detected diabetes). Participants reporting a history of diabetes were older and less likely to be current smokers or alcohol drinkers, but more likely to be ex-smokers or ex-drinkers and to be less physically active than those without diabetes, and had an approximately 4-fold greater prevalence of a family history of diabetes (Table 1). Those with screen-detected diabetes were older than and about twice as likely to have a family history of diabetes as those without diabetes, but had comparable smoking and alcohol consumption patterns. The prevalence of overweight or obesity (BMI ≥ 25.0 kg/m2) and hypertension (SBP ≥ 140 mm Hg) were higher in participants with diabetes, particularly screen-detected diabetes. Among participants with self-reported diabetes, the median age at diagnosis was 53 (range 4 to 77) y, and the median duration of diabetes at baseline was 4 (range 0 to 56) y, with 76% (n = 8,501) reporting use of insulin or oral hypoglycaemic agents.
Tab. 1. Baseline characteristics of participants by diabetes status. 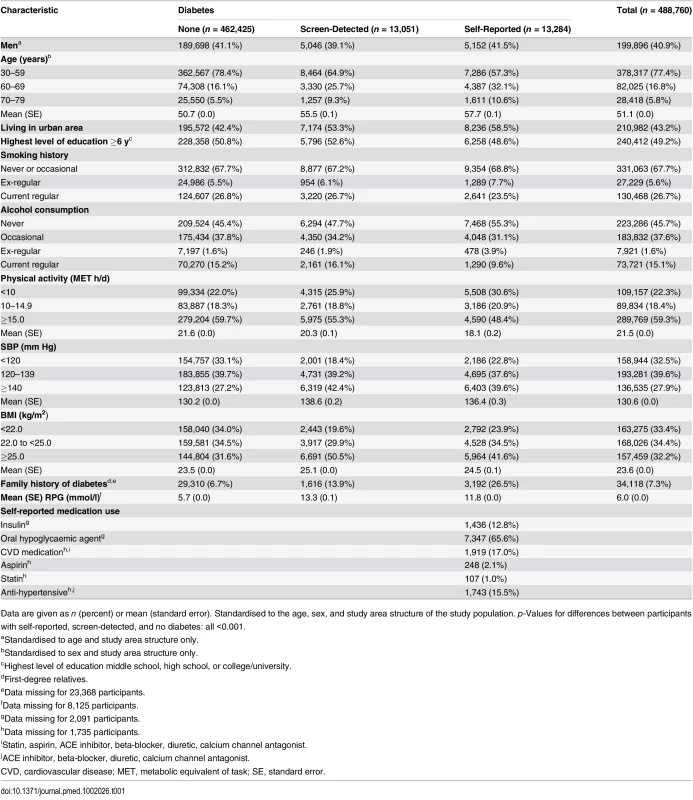
Data are given as n (percent) or mean (standard error). Standardised to the age, sex, and study area structure of the study population. p-Values for differences between participants with self-reported, screen-detected, and no diabetes: all <0.001. Self-Reported Diabetes and Cardiovascular Disease Risk
During ~3.4 million person-years (mean 7 y per person) of follow-up, 7,353 (1.5%) participants died from cardiovascular disease. Individuals with self-reported diabetes had a 2-fold increased hazard of cardiovascular mortality (HR 2.07, 95% CI 1.90–2.26) (Table 2), and the HR for cardiovascular mortality was greater in women than in men (2.37, 95% CI 2.11–2.65, versus 1.73, 95% CI 1.50–2.00) (S3 Fig). Likewise, there were significantly increased risks of MOVD (1.77, 1.69–1.86), MCE (2.44, 2.18–2.73), and IS (1.68, 1.60–1.77) among those with self-reported diabetes (Table 2). For IHD and IS, the HRs were somewhat greater for fatal than non-fatal events. For IS and MOVD (S4 Fig), the associations were stronger at younger ages (both p for trend < 0.001) and in rural areas (p for heterogeneity = 0.003 and < 0.001, respectively). The risk of MCE varied little across population subgroups.
Tab. 2. Adjusted hazard ratios for incident cardiovascular diseases by diabetes status. 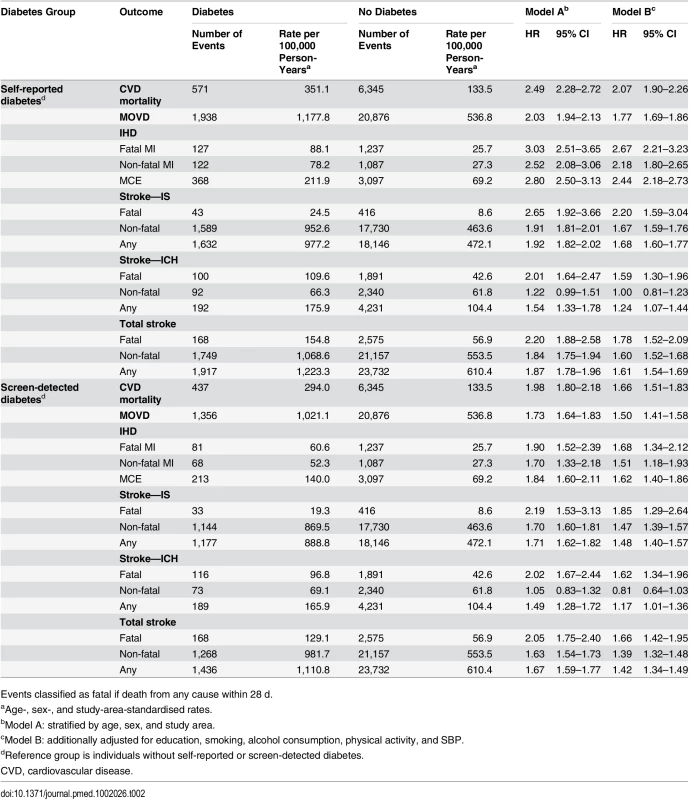
Events classified as fatal if death from any cause within 28 d. Self-reported diabetes was also associated with significant, but more modest, excess risk of ICH, with an adjusted HR of 1.24 (95% CI 1.07–1.44), more extreme for fatal (1.59, 95% CI 1.30–1.96) than for non-fatal (1.00, 95% CI 0.81–1.23) events (Table 2). The risk varied little across participant subgroups (S5 Fig). For total stroke, self-reported diabetes was associated with an adjusted HR of 1.61 (95% CI 1.54–1.69; Table 2).
Additional adjustment for waist-to-hip ratio modestly, but non-significantly, attenuated the HRs for ischaemic cardiovascular diseases, but not for ICH or cardiovascular mortality (S1 Table). In sensitivity analyses excluding participants who developed incident diabetes during follow-up (n = 8,896), HRs for all cardiovascular diseases remained largely unchanged (S2 Table). Sensitivity analyses adjusting for age, SBP, and physical activity as continuous variables also produced similar findings.
Screen-Detected Diabetes and Cardiovascular Disease Risk
Among participants with screen-detected diabetes, the excess risks were also highly significant, though somewhat less extreme compared with self-reported diabetes. For cardiovascular mortality, the adjusted HR was 1.66 (95% CI 1.51–1.83), while for MOVD, MCE, and IS the adjusted HRs were 1.50 (95% CI 1.41–1.58), 1.62 (95% CI 1.40–1.86), and 1.48 (95% CI 1.40–1.57), respectively. Similarly, for ICH the excess risk was more modest (1.17, 95% CI 1.01–1.36) (Table 2). There was little evidence of heterogeneity in the observed HRs for MCE, ICH (S6 Fig), or cardiovascular mortality (S7 Fig) across population subgroups. For IS and MOVD (S8 Fig), however, the risk was somewhat greater at younger ages (both p for trend < 0.001), in rural residents (p for heterogeneity < 0.001 and 0.003, respectively), and in more physically active individuals (p for trend 0.008 and 0.006, respectively).
Among individuals with diabetes, the risk of major ischaemic cardiovascular events increased progressively with longer duration of diabetes (p for trend < 0.001; Fig 1), but no such trend was seen for ICH (p = 0.3). Similarly, among individuals with self-reported diabetes, the risk of MOVD increased progressively with the number of other presenting cardiovascular risk factors, with adjusted HRs of 1.68 (95% CI 1.53–1.85), 2.08 (95% CI 1.93–2.23), and 2.82 (95% CI 2.59–3.06) in those with one, two, and three or more risk factors, respectively, compared with participants with self-reported diabetes who had no other cardiovascular risk factors (p for trend < 0.001; Fig 2). A similar gradient was seen for screen-detected diabetes (p for trend < 0.001; Fig 2).
Fig. 1. Adjusted hazard ratios for cardiovascular diseases by duration of diabetes diagnosis. 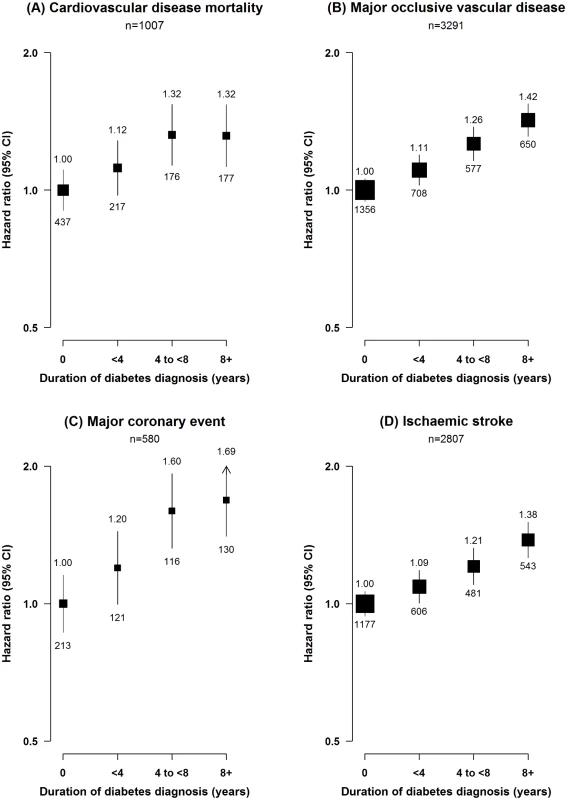
(A) Cardiovascular disease mortality; (B) MOVD; (C) MCE; (D) IS. Stratified by age, sex, and study area and adjusted for education, smoking, alcohol consumption, physical activity, and SBP. Diabetes duration defined as 0 y in individuals with screen-detected diabetes. Diabetes duration data missing or implausible for 17 participants. Squares represent the HR, with area inversely proportional to the variance of the log HR. Vertical lines represent the corresponding 95% confidence intervals. Numbers above the CIs are HRs, and numbers below the CIs are the number of participants with cardiovascular events. Fig. 2. Adjusted hazard ratios for major occlusive vascular disease by number of cardiovascular risk factors at baseline among individuals with diabetes. 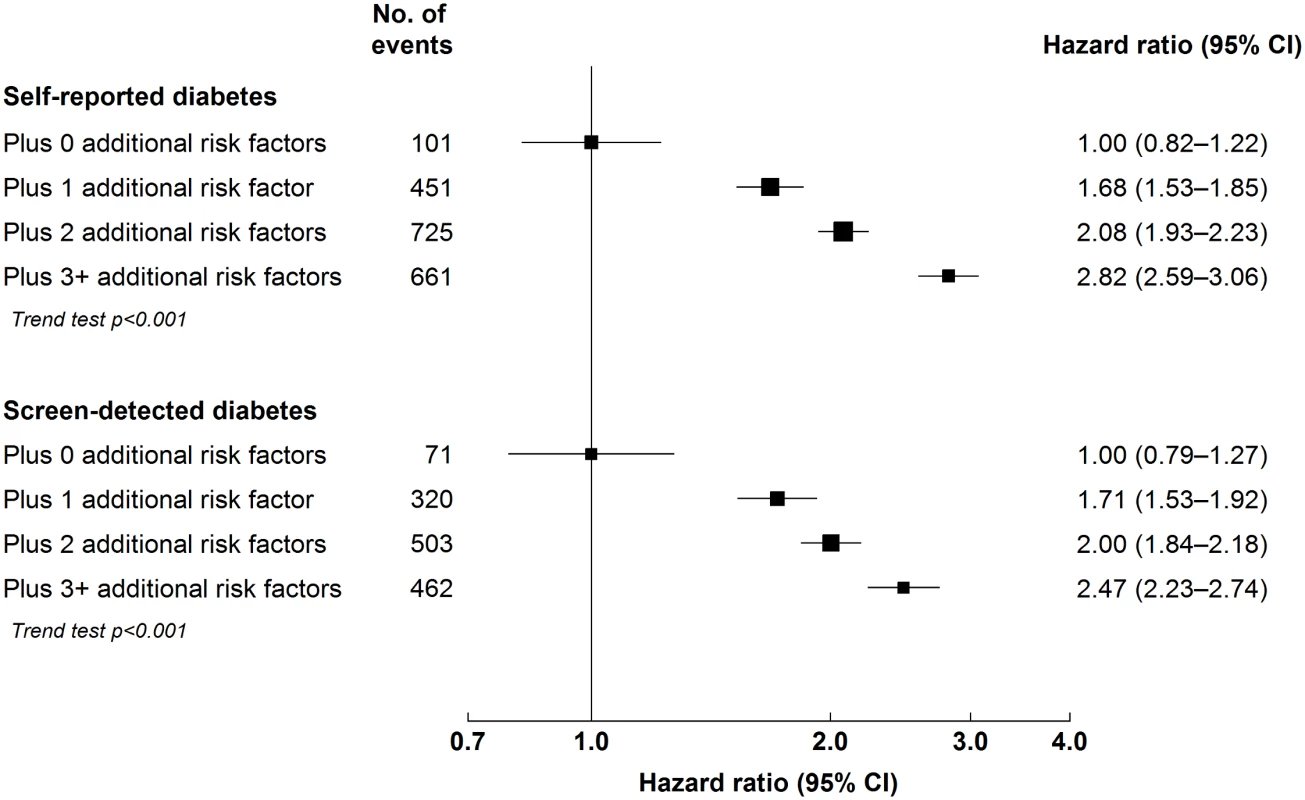
Stratified by age, sex, and study area and adjusted for education and alcohol consumption. Cardiovascular risk factors: hypertension (self-reported hypertension, mean SBP ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg), overweight or obesity (BMI ≥ 25 kg/m2), ever regular smoking, and physical inactivity (<10 metabolic equivalent of task hours/day). Squares represent the HR, with area inversely proportional to the variance of the log HR. Horizontal lines represent the corresponding 95% confidence intervals. Diabetes-Attributable Cardiovascular Mortality
By applying the age-specific population-attributable fractions for cardiovascular mortality in the present study to diabetes prevalence derived from a recent nationally representative survey [8] and the number of sex - and age-specific cardiovascular deaths in mainland China [22], it was estimated that 489,676 (95% CI 335,777–681,202) cardiovascular deaths could be attributable to diabetes in 2010.
Discussion
Our study provides, to our knowledge, the first large-scale prospective evidence of the cardiovascular consequences of diabetes among adults in China. It showed that self-reported doctor-diagnosed diabetes was associated with 1.5 - to 2.5-fold increased risks of cardiovascular mortality and incident IHD and IS. Moreover, it provided strong prospective evidence of a significant, though more modest, adverse association of diabetes with ICH risk. Individuals with screen-detected diabetes also had significantly increased cardiovascular disease risks. If these associations are causal, then almost 0.5 million cardiovascular deaths a year in China can now be attributed to diabetes.
Previous studies of predominantly Western populations have shown approximate doubling of IHD risk with diabetes [2,3,10,11,23]. The comparability of our risk estimates with previous studies suggests that differences between East Asian and Western populations in the relative importance of insulin resistance and beta-cell dysfunction in the aetiology of diabetes [9] have little, if any, impact on associated IHD risk. The method of ascertainment of IHD in the CKB may misclassify a proportion of individuals with “silent” IHD, which is more prevalent among individuals with diabetes [24], leading to underestimation of diabetes-associated IHD risk. The more modest effects of screen-detected—compared to self-reported—diabetes may reflect the shorter duration of, or less severe glycaemic aberration in, screen-detected diabetes, and greater potential for misclassification.
The CKB provides reliable evidence about the association of diabetes with stroke and, in particular, stroke subtypes. The association of screen-detected diabetes with IS in our study is largely consistent with data from studies of mostly Western populations [3]; the association with self-reported diabetes was moderately weaker than in those studies [2,3] and also weaker than in a small prospective Chinese study of 30,000 men and women [10] and a study—based on routine data and adjusted only for age—in Taiwan [25]. In mainland China, computed tomography or MRI is widely used, often leading to detection of a relatively high proportion of lacunar infarcts without major, or any, apparent focal neurological deficit [26]. The relatively low IS case fatality rate in our study might explain, at least in part, the more modest association with diabetes.
Existing evidence relating ICH to diabetes is more limited [2,3,10,25,27], partly due to lower ICH rates in Western populations [2,3] and the lack of widespread imaging in earlier studies. Where available, findings have been inconsistent [2–4,10,25]. Two of the largest studies in Western populations, including 1,000 [3] and 2,500 [4] ICH events, found modest elevated risks associated with known diabetes (HR 1.56 [3] and 1.28 [4]). Our study included almost 5,000 well-characterised ICH cases and provides the most reliable evidence to date of a positive, but modest, association between self-reported diabetes and ICH, and the first opportunity, to our knowledge, to investigate effect modification beyond age and sex. The large number of well-characterised stroke events (~90% of validated stroke events were confirmed with computed tomography/MRI) is a strength of the CKB. Outcome adjudication, through review of medical records for all IHD and stroke cases, is currently underway; findings to date have shown high positive predictive values for IHD events (~85%) and for stroke (~90%). The apparent difference in excess risk between fatal and non-fatal cardiovascular disease events could reflect poorer survival following such events in individuals with diabetes [28,29] or more severe disease in fatal cases.
The large urban—rural differences in excess risk associated with self-reported diabetes in the CKB, especially for IS, may reflect the higher proportion of undiagnosed diabetes cases in rural areas, such that self-reported diabetes in rural areas may represent more severe disease. More frequent use of diabetes medications in rural areas supports this hypothesis. Several studies have previously reported greater diabetes-associated IHD risk [3,23] and, less consistently, stroke risk [3,4,30] in women. We found a sex difference for cardiovascular mortality only, and this may have contributed to the higher diabetes-associated risks in never or occasional smokers and never or occasional alcohol drinkers. Diabetes, particularly type 2 diabetes, frequently coexists with other cardiovascular risk factors [31]. In our study population, the cardiovascular disease risk among those with diabetes was also dependent on the presence of other common and modifiable cardiovascular risk factors, which highlights the need for multi-factorial approaches to managing cardiovascular disease risk, even after development of diabetes. The small proportion of participants with self-reported diabetes who reported use of medications for lowering cardiovascular disease risk suggests that there is considerable scope for improvement in this regard in China. The impact of diabetes diagnosis duration on disease risk is largely consistent with the almost 2-fold greater odds of prevalent cardiovascular disease in early-onset (<40 y of age), compared to late-onset (≥40 y), diabetes in China, with the majority of the association explained by diabetes duration [32].
The CKB was not designed to be nationally representative, but given the size and diversity of the CKB study population and the CKB’s minimal loss to follow-up, this would not be expected to bias risk estimates or reduce their generalisability to the Chinese adult population [33]. Furthermore, the prevalence of diabetes in the CKB was similar to that reported in a reasonably contemporaneous representative Chinese survey in 2000–2001 [7]. More recent surveys have reported a higher rate of diabetes in China [7], with, for example, a prevalence rate of 11.6% in the 2010 survey [8]. As well as reflecting secular trends, the lower prevalence of diabetes in the CKB may reflect different study settings, sampling methods, and approaches used to identify undiagnosed diabetes. The prevalence of self-reported diabetes was, however, similar (3.5% in the 2010 survey versus 2.7% in the present CKB population and 3.2% in the CKB population including individuals with prior cardiovascular disease). Although self-reported diabetes is widely used [33], it may be subject to misclassification. However, amongst almost 30,000 participants resurveyed in the CKB, over 90% of those who reported a history of diabetes at baseline gave the same answer at resurvey. Furthermore, the mean plasma glucose level among those with self-reported diabetes was consistent with the diagnosis. We did not collect specific information on diabetes type, but, based on age at diagnosis and medication use, <1% of diabetes in the CKB would likely be type 1.
Separate examination of screen-detected diabetes should reduce bias from lifestyle changes and treatment following diagnosis, although screen-detected diabetes may be subject to greater misclassification resulting from use of RPG [34] measurement on a glucometer [35] for diagnosis. Such misclassification likely contributed to the lower screen-detected diabetes prevalence in the CKB compared to the most recent national survey [8] (2.7% in CKB versus 8.1% in the 2010 survey), which used multiple glycaemic indicators (oral glucose tolerance testing, fasting plasma glucose, and HbA1c), and would result in underestimates of diabetes-associated risks. However, excluding participants who developed new diabetes during follow-up (identified from mortality, disease surveillance, and national health insurance data) did not materially alter risk estimates. The high proportion of undiagnosed diabetes [8] and the elevated cardiovascular risk associated with it lend support to population screening for diabetes to enable more effective primary prevention of cardiovascular diseases. Data on lipids are not currently available; this may have resulted in residual confounding, although progressive adjustment for confounders in one published study suggests that this confounding would be minimal [3]. Similarly, renal function data are not currently available in the CKB; this precludes investigation of effect modification of the association of diabetes with cardiovascular diseases but would not bias our risk estimates. Additional adjustment of presented risk estimates for other potential confounding variables, including dietary intake, had minimal effect.
In China, about 10% of the adult population is estimated to have diabetes [8]. The present nationwide prospective study provides large-scale evidence from mainland China that individuals with diabetes are at significantly increased risk of major cardiovascular diseases, similar in magnitude to that observed in Western populations [2,3]. Much of the diabetes-associated excess risk is likely to be causal, accounting for almost 0.5 million cardiovascular deaths annually in China. With further adverse changes in lifestyle, the prevalence of diabetes will likely increase further in China, especially among young adults [1,7], foreshadowing an even greater diabetes-attributable burden of cardiovascular (and other) diseases.
Supporting Information
Zdroje
1. International Diabetes Federation. IDF diabetes atlas. Brussels: International Diabetes Federation; 2015.
2. Spencer EA, Pirie KL, Stevens RJ, Beral V, Brown A, Liu B, et al. Diabetes and modifiable risk factors for cardiovascular disease: the prospective Million Women Study. Eur J Epidemiol. 2008;23 : 793–799. doi: 10.1007/s10654-008-9298-3 19015938
3. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375 : 2215–2222. doi: 10.1016/S0140-6736(10)60484-9 20609967
4. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3 : 105–113. doi: 10.1016/S2213-8587(14)70219-0 25466521
5. Liu M, Wu B, Wang W-Z, Lee L-M, Zhang S-H, Kong L-Z. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6 : 456–464. 17434100
6. National Collaborative Group of Diabetes Study. [A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China.] Zhonghua Nei Ke Za Zhi. 1981;20 : 678–683. 7341098
7. Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol. 2014;2 : 969–979. doi: 10.1016/S2213-8587(14)70144-5 25218728
8. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310 : 948–959. doi: 10.1001/jama.2013.168118 24002281
9. Kong AP, Xu G, Brown N, So WY, Ma RC, Chan JC. Diabetes and its comorbidities—where East meets West. Nat Rev Endocrinol. 2013;9 : 537–547. doi: 10.1038/nrendo.2013.102 23712250
10. Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, et al. Ten-year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. 2007;153 : 552–558. 17383293
11. Shen C, Schooling CM, Chan WM, Lee SY, Leung GM, Lam TH. Self-reported diabetes and mortality in a prospective Chinese elderly cohort study in Hong Kong. Prev Med. 2014;64 : 20–26.
12. Wu Y, Liu X, Li X, Li Y, Zhao L, Chen Z, et al. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114 : 2217–2225. 17088464
13. Woo J, Lau EMC. Risk factors predisposing to stroke in an elderly Chinese population—a longitudinal study. Neuroepidemiology. 1990;9 : 131–134.
14. An Y, Zhang P, Wang J, Gong Q, Gregg EW, Yang W, et al. Cardiovascular and all-cause mortality over a 23-year period among Chinese with newly diagnosed diabetes in the Da Qing IGT and Diabetes Study. Diabetes Care. 2015;38 : 1365–1371. doi: 10.2337/dc14-2498 25887356
15. Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40 : 1652–1666. doi: 10.1093/ije/dyr120 22158673
16. Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int J Epidemiol. 2005;34 : 1243–1249. 16131516
17. Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97 : 487–496. doi: 10.3945/ajcn.112.046854 23364014
18. Bragg F, Li L, Smith M, Guo Y, Chen Y, Millwood I, et al. Associations of blood glucose and prevalent diabetes with risk of cardiovascular disease in 500 000 adult Chinese: the China Kadoorie Biobank. Diabet Med. 2014;31 : 540–551. doi: 10.1111/dme.12392 24344928
19. Early Breast Cancer Trialists’ Collaborative Group. Treatment of early breast cancer. Vol. 1: worldwide evidence 1985–1990—a systematic overview of all available randomized trials of adjuvant endocrine and cytotoxic therapy. Oxford: Oxford University Press; 1990.
20. Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10 : 1025–1035. 1652152
21. Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. 2002;55 : 893–899. 12393077
22. Institute for Health Metrics and Evaluation. China Global Burden of Disease Study 2010. Results 1990–2010. Seattle: Institute for Health Metrics and Evaluation; 2013.
23. Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57 : 1542–1551. doi: 10.1007/s00125-014-3260-6 24859435
24. Scirica BM. Prevalence, incidence, and implications of silent myocardial infarctions in patients with diabetes mellitus. Circulation. 2013;127 : 965–967. doi: 10.1161/CIRCULATIONAHA.113.001180 23459575
25. Chen H-F, Lee S-P, Li C-Y. Sex differences in the incidence of hemorrhagic and ischemic stroke among diabetics in Taiwan. J Womens Health. 2009;18 : 647–654.
26. Wu B, Lin S, Hao Z, Yang J, Xu Y, Wu L, et al. Proportion, risk factors and outcome of lacunar infarction: a hospital-based study in a Chinese population. Cerebrovasc Dis. 2010;29 : 181–187. doi: 10.1159/000267277 20029187
27. Asia Pacific Cohort Studies Collaboration. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care. 2003;26 : 360–366. 12547863
28. Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W. Subtypes and one-year survival of first-ever stroke in Chinese patients: the Nanjing Stroke Registry. Cerebrovasc Dis. 2006;22 : 130–136. 16691021
29. Wannamethee G, Whincup PH, Shaper AG, Walker M, MacFarlane PW. Factors determining case fatality in myocardial infarction “who dies in a heart attack”? Br Heart J. 1995;74 : 324–331. 7547031
30. Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383 : 1973–1980. doi: 10.1016/S0140-6736(14)60040-4 24613026
31. Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28 : 88–136. 17220161
32. Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4 : 115–124. doi: 10.1016/S2213-8587(15)00508-2 26704379
33. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57 : 1096–1103. 15528061
34. Qiao Q, Keinanen-Kiukaanniemi S, Rajala U, Uusimaki A, Kivela SL. Random capillary whole blood glucose test as a screening test for diabetes mellitus in a middle-aged population. Scand J Clin Lab Invest. 1995;55 : 3–8. 7624734
35. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3 : 971–980. 20144348
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



